Summary
Among the large population of patients with non-alcoholic fatty liver disease (NAFLD), identifying those with advanced disease remains challenging. Many patients are diagnosed late, following the development of liver-related complications, leading to poor clinical outcomes. Accumulating evidence suggests that using non-invasive tests for liver fibrosis in patients with metabolic risk factors improves the detection of patients in need of specialised management and is cost-effective. Because of the vast number of patients requiring evaluation, the active participation of general practitioners and physicians who manage patients with metabolic disorders, such as diabetologists, is crucial; this calls for the increased awareness of NAFLD beyond liver clinics. Non-invasive case-finding strategies will need to be further validated and generalised for upcoming drug therapies to have the required impact on the worldwide burden of NAFLD.
Keywords: Liver fibrosis, Elastography, Screening, Case-finding, Patient pathway, Primary care, Type 2 diabetes mellitus, Cost-effectiveness, Awareness, FIB-4, Cirrhosis
Abbreviations: ALD, alcohol-related liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4; GP, general practitioner; NAFLD, non-alcoholic fatty liver disease; NAS, NAFLD activity score; NASH, non-alcoholic steatohepatitis; NFS, NAFLD fibrosis score; NICE, National Institute of Clinical Excellence; NIT, non-invasive test; QALY, quality-adjusted life year; T2DM, type 2 diabetes mellitus; TE, transient elastography
Key points.
-
•
The primary liver lesion to target for the case finding of at-risk patients with NAFLD is advanced fibrosis.
-
•
Diabetics and patients with multiple metabolic risk factors represent populations with an increased prevalence of advanced fibrosis and should therefore be considered in case-finding strategies.
-
•
Non-invasive tests of liver fibrosis, blood tests or elastography devices, can confidently exclude advanced liver fibrosis in low-prevalence populations.
-
•
Due to a very low rate of negative results, the NAFLD fibrosis score should not be used for the case finding of advanced liver fibrosis in diabetics.
-
•
Using FIB-4 as first-line test followed, if positive, by a specialised blood test or liver stiffness measurement seems the best strategy for the case finding of advanced liver fibrosis.
-
•
Strategies using non-invasive tests of liver fibrosis for the case finding of advanced liver disease in at-risk populations are cost-effective.
-
•
Awareness of NAFLD remains insufficient among primary care and non-liver specialists, strong effort must be made to improve the knowledge of the disease and the willingness to detect at-risk patients.
Introduction
Non-alcoholic fatty liver disease (NAFLD) has become the main cause of chronic liver disease and is associated with a significant worldwide burden.1 In some countries, NAFLD is now the primary cause of cirrhosis,2 the main cause of chronic liver disease underlying hepatocellular carcinoma,3 and the second-leading cause of liver transplantation, though its trajectory suggests that it will become the first within 10-20 years.4,5 NAFLD encompasses a wide spectrum of liver lesions, ranging from simple steatosis, to non-alcoholic steatohepatitis (NASH), fibrosis, cirrhosis and ultimately end-stage liver disease and hepatocellular carcinoma. While NASH is more a condition that promotes fibrosis progression, longitudinal studies have demonstrated that the liver-related prognosis of patients with NAFLD is mostly related to the extent of liver fibrosis,6,7 as observed for the other causes of chronic liver disease.
Currently, no treatments have been approved for NAFLD. Major efforts are being made in the research and development of new drugs able to halt the progression of this liver disease, with the aim of preventing cirrhosis and liver-related complications.8 In this context, the first positive phase III trial in 2019 gives hope that new treatments could be available in clinical practice in the near future.9 In line with the strong impact of liver fibrosis on the prognosis of NAFLD, the current consensus is that pharmacotherapy should be reserved for patients with NASH and at least significant fibrosis (stage F2 and higher).10,11 However, since these patients represent only a small proportion of the whole NAFLD population, the case finding of these patients in clinical practice is like looking for a needle in a haystack. It must also be added that targeted patients have no symptoms, their biology shows non-specific abnormalities, and that awareness of NAFLD among non-specialists is very low. These factors make the identification of patients with NAFLD at risk of progressive liver disease a tough challenge in “real life”.
Many tools are now available for the non-invasive evaluation of liver lesions in NAFLD, especially blood tests and elastography devices. With the upcoming arrival of treatments for NAFLD, there is now an urgent need to develop and validate strategies, using these tests, for the case finding of patients who will benefit from specialised management. Indeed, the accurate identification of patients in need of treatment is a prerequisite if we want new drugs to decrease the burden of NAFLD. To reach this ambitious aim, several issues still need to be addressed: in which populations should case finding be concentrated? Which liver lesions are the most relevant to target? What would be the ideal strategy? Are non-specialists aware of NAFLD and would they participate in the case finding of at-risk patients? Which strategies are cost-effective?
Which liver lesions to target
When designing case-finding strategies and pathways, an important consideration is the target condition to be diagnosed. Factors to consider when making such decisions are the pertinence of the diagnosis to the patients’ prognosis and the availability of diagnostic methods to identify the target condition.
In the general population, steatosis occurs at a prevalence of 25% and can be easily diagnosed by ultrasound. However, unselected patients with NAFLD do not have increased liver-related mortality. Moreover, it is not established whether the presence of NAFLD per se is greater than the sum of its parts (i.e. metabolic comorbidities) in terms of cardiovascular risk.12 Screening of the general population for the presence of NAFLD is therefore not recommended by learned societies.10,11,13 On the other hand, screening for NAFLD in high-risk groups remains a contentious issue, with conflicting guidance from EASL10 and AASLD.11 Concerns regarding a potential screening programme for NAFLD include the large number of tests it would involve, the lack of effective disease-specific therapies other than lifestyle measures and insufficient data on the cost-effectiveness of such an approach.
NASH is considered the progressive form of NAFLD; however, its diagnosis still requires a liver biopsy as it is notoriously difficult to diagnose with non-invasive tests (NITs). CK-18, which was initially considered promising, has limited sensitivity and cannot be used for screening purposes.14 Moreover, the presence of NASH without significant fibrosis is not associated with increased liver-related mortality,[15], [16], [17] probably because such patients have the competing mortality risks of cardiovascular disease and non-liver-related cancers. Recently, NITs that combine the diagnosis of NASH with a NAFLD activity score (NAS) of ≥4 and fibrosis stage of ≥2 were developed; these tests are based on the combination of transient elastography (TE), simple laboratory values18 and proprietary algorithms 19,20 respectively. An immediate application of these tests is for pre-screening patients for clinical trials. However, their use as triaging tests in case-finding pathways is questionable, as they would potentially miss patients with significant/advanced fibrosis without steatohepatitis, who would still be at increased risk of liver-related events.21
Advanced fibrosis in NAFLD is unequivocally associated with increased risk of liver-related mortality[15], [16], [17] and can be reliably diagnosed with a variety of NITs, such as those presented later. Therefore, the primary lesion to target in case-finding strategies is advanced fibrosis.
In which populations should case finding be focused
Important information can be obtained from population-based studies evaluating the screening of liver fibrosis using TE.[22], [23], [24], [25] These works included large samples of patients in the general population and had similar design, providing the opportunity to delineate the characteristics of the subgroups with increased prevalence of liver fibrosis where the case finding of advanced liver disease (≥F3 fibrosis stage) might be most relevant. Using the 8.0 kPa threshold, the prevalence of patients at risk of significant liver fibrosis (≥F2 fibrosis stage) in the adult general population was similar across studies, around 6–7% (Table 1). Multivariate analyses aimed at identifying patient characteristics associated with liver stiffness ≥8.0 kPa found different results, mainly because different factors were tested across studies, but they also highlighted some interesting similarities. As expected, elevated serum transaminases were associated with increased risk of elevated liver stiffness in all studies, with adjusted odds ratios between 2.0 and 4.2 for serum transaminases ≥40 IU/L.22,24 However, these works also showed that serum transaminases have low sensitivity, with levels below 40 IU/L in 40–80% of patients with elevated liver stiffness.22,24
Table 1.
Population-based studies evaluating screening for liver fibrosis using transient elastography.
| Roulot 201124 | Koehler 201623 | Caballería 201822 | Abeysekara 202025 | |
|---|---|---|---|---|
| Country | France | Netherlands | Spain | United Kingdom |
| Population | Population-based | Population-based | Population-based | Population-based |
| Patients | ≥45 years | ≥45 years | 18-75 years | 22-26 years |
| Sample size (n) | 1,190 | 3,041 | 3,014 | 3,600 |
| Target | TE ≥8.0 kPa | TE ≥8.0 kPa | TE ≥8.0 kPa | TE ≥7.9 kPa |
| FibroScan probe used | M probe | M or XL probe a | M probe | M or XL probe a |
| Prevalence (%) | 7.5% | 5.6% | 5.8% | 2.7% |
| Independent predictors of the target | Diabetes ALT ≥40 IU/L Elevated WCb Age ≥57 years BMI ≥30 kg/m2 GGT ≥45 IU/L |
Diabetes ALT Age Current/former smoking HBs Ag or anti-HCV positive Liver steatosis Spleen size |
Type 2 diabetes AST and/or ALT >40 IU/L Elevated WCc Male sex Glucose ≥100 mg/dl Low HDL cholesterold Triglycerides ≥150 mg/dl |
Male sex Harmful alcohol use |
| Liver biopsy (n) | 27 | – | 60 | – |
| Fibrosis stage (n)e: | ||||
| 0 | 1 | – | 27 | – |
| 1 | 8 | – | 6 | – |
| 2 | 9 | – | 20 | – |
| 3 | 0 | – | 3 | – |
| 4 | 4 | – | 4 | – |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; IFG, impaired fasting glucose; WC, waist circumference.
According to the manufacturer recommendation.
Waist circumference ≥100 cm in men or ≥93 cm in women.
Waist circumference ≥102 cm in men or ≥88 cm in women.
HDL-cholesterol <40 mg/dl in men or <50 mg/dl in women.
according to the NASH CRN staging system, except for viral hepatitis in the Roulot study for which Metavir staging was used.
Diabetes was also consistently identified as being strongly associated with liver fibrosis. In the Rotterdam study, the prevalence of liver stiffness ≥8.0 kPa was around 9% in the subgroup of patients with diabetes but no liver steatosis, and it reached 17.2% in patients with both diabetes and liver steatosis.23 In a study by Caballería et al., among 11 factors tested, type 2 diabetes mellitus (T2DM) was the condition associated with the highest prevalence of elevated liver stiffness.22 These results were confirmed by targeted screening studies, which reported a 20% prevalence of elevated liver stiffness ≥9.6 kPa in T2DM populations from tertiary care centres (Table 2).[26], [27], [28] Using the same cut-off for TE, the Roulot study found a lower 7.3% prevalence, but this work included patients with newly diagnosed and less severe T2DM.29 Indeed, longer duration of diabetes correlates with higher prevalence of elevated liver stiffness.26 Moreover, another study testing NITs in 338 diabetic patients observed a higher prevalence of severe fibrosis with increasing T2DM severity: 28.0% in patients with T2DM, 30.4% in patients aged >50 years with T2DM, and 37.9% in patients with a past history of foot ulcer.30 Otherwise, the study of liver-related outcomes in large populations of diabetic patients also provides important information. Indeed, the increased risk of death from cancer in diabetics compared to non-diabetics is the highest for liver cancer, while chronic liver disease accounted for the third highest increase in the risk of death from non-cancer and non-vascular disease,31 supporting the case finding of advanced liver disease in this population.
Table 2.
Studies evaluating screening for liver fibrosis in diabetic patients using transient elastography.
| Kwok 201526 | Roulot 201729 | Lai 201928 | Sporea 202027 | |
|---|---|---|---|---|
| Country | China | France | Malaysia | Romania |
| Population | T2DM | Newly diagnosed T2DM | T2DM | T2DM |
| Patients | ≥18 years | ≥18 years | ≥18 years | ≥18 years |
| Sample size (n) | 1,884 | 669 | 557 | 534 |
| Target | TE ≥9.6 kPa (M probe) or ≥9.3 kPa (XL probe) | TE ≥9.6 kPa | TE ≥9.6 kPa (M probe) or ≥9.3 kPa (XL probe) | TE ≥9.7 kPa |
| FibroScan probe used | XL probe if M probe failure | XL probe if M probe failure | M or XL probe according to manufacturer recommendations | XL probe if BMI ≥30 kg/m2 or skin-to-capsule distance >25 mm |
| Prevalence (%) | 17.7% | 7.3% | 21.0% | 19.5% |
| Independent predictors of the target | Diabetes duration ALT BMI HDL-cholesterol Urine ACR |
Age ALT BMI GGT |
ALT GGT HDL-cholesterol Platelets |
AST |
| Liver biopsy (n) | 94 | 47 | 57 | – |
| Fibrosis stage (n) a: | ||||
| 0 | 5 | F0-2: 23 | – | – |
| 1 | 29 | – | – | – |
| 2 | 13 | – | – | – |
| 3 | 20 | 16 | F3-4: 23 | – |
| 4 | 27 | 8 | – | – |
ACR, albumin-creatinine ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; T2DM, type 2 diabetes mellitus.
According to the NASH CRN staging system.
Population-based studies identified other factors associated with elevated liver stiffness (Table 1), most of them being related to metabolic conditions: obesity, impaired fasting glucose, low HDL-cholesterol, high triglyceride levels, and liver steatosis. NAFLD is now the leading cause of chronic liver disease worldwide,1 therefore it is not surprising that liver fibrosis is mainly related to metabolic risk factors at a population level. A recent study evaluated the targeted screening of liver fibrosis using TE in a primary care centre population with at least 1 risk factor among obesity, T2DM, or hazardous alcohol use;32 88% of the patients were aged >40 and the prevalence of elevated liver stiffness ≥8.0 kPa was 12.4%, which is higher than in the general population of the same age. Elevated liver stiffness was independently associated with obesity and T2DM, with no effect for hazardous alcohol use. The prevalence of elevated liver stiffness was 8.9% in obese patients, 10.8% in those with T2DM, and it was significantly increased to 36.7% in patients with both obesity and T2DM, demonstrating the synergistic effect of metabolic factors on the risk of advanced liver disease. Another study reported a 25.2% prevalence of elevated liver stiffness ≥8.0 kPa in 899 patients from primary care centres with at least 1 risk factor among hazardous alcohol use, T2DM or elevated serum alanine aminotransferase (ALT).33 Prevalence was 19.2% among hazardous alcohol users, 31.5% in diabetics, 45.3% in patients with raised serum transaminases, with synergic effects seen when hepatic risk factors accumulated.
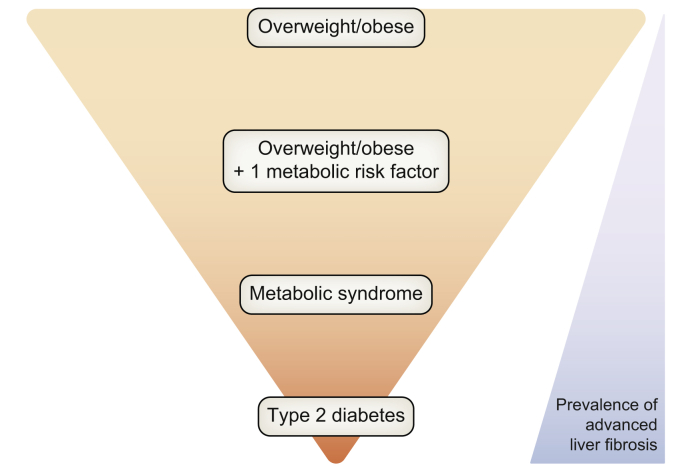
Available evidence therefore suggests that overweight/obese patients, particularly those with metabolic risk factors, and especially T2DM, might be a relevant population for the case finding of patients with significant liver disease requiring specialised management (Fig. 1). However, most of the data come from studies focused on adult patients aged >40 years. Since the 80s, we have faced an explosion of obesity and T2DM in children, the latter being more aggressive in young people with poorer response to glucose-lowering medication and greater insulin resistance.[34], [35], [36] As the length of exposure to the cause is a key factor in the development of advanced liver disease, one can suppose that the picture of chronic liver disease will change with increasing cases of advanced liver disease in young adults in the near future. In this context, a very recent population-based study in young adults aged 22–26 years reported a 2.7% prevalence of elevated liver stiffness ≥7.9 kPa.25
Fig. 1.
Populations at risk of NAFLD-related liver outcomes. NAFLD, non-alcoholic fatty liver disease.
Patients with NAFLD are at an increased risk of cardiovascular disease and recent data support pathophysiological processes linking NASH and liver fibrosis with cardiometabolic disease.37,38 A meta-analysis of studies with paired liver biopsy has shown that arterial hypertension is a strong predictor of fibrosis progression in patients with NAFLD.39 Similarly, a very recent study performed in a long-term community prospective cohort with 65 years follow-up identified arterial hypertension as the strongest predictor of chronic liver disease, followed by insulin resistance/T2DM and obesity.40 However, in a large cohort of 603 patients with biopsy-proven NAFLD, there was no independent relationship between liver lesions observed in NAFLD and cardiovascular events, the latter being driven by metabolic risk factors.41 Additional data are needed to demonstrate that patients with cardiovascular disease are at an increased risk of advanced liver disease and that screening for advanced fibrosis is relevant in this population.
Screening strategies
Which tests to use for the case finding of advanced liver fibrosis in patients with NAFLD
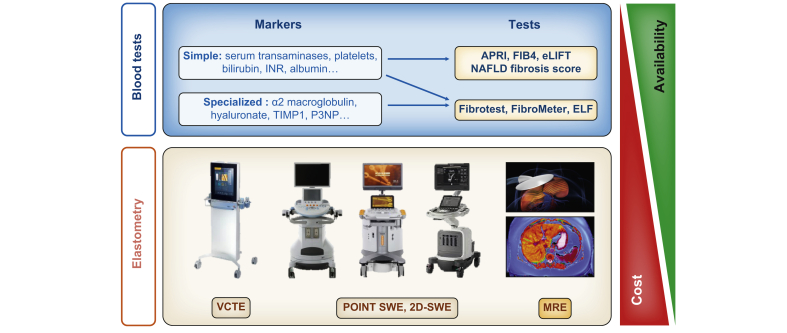
Liver biopsy remains the reference standard for the evaluation of liver lesions observed in NAFLD but, given the large population to be evaluated, this invasive procedure cannot be proposed for first-line evaluation. Several tests are now available for the non-invasive evaluation of liver fibrosis, mainly liver elastography devices and blood tests (Fig. 2). Diagnostic studies using liver biopsy as a reference have demonstrated the good sensitivity (80–90%) and specificity (90–95%) of NITs for the diagnosis of advanced liver fibrosis in NAFLD.[42], [43], [44] As these works mainly came from tertiary care centres enriched in patients with advanced liver disease, the negative predictive value is supposed to be even better in populations with a lower prevalence of liver fibrosis.
Fig. 2.
Non-invasive tests for liver fibrosis.
MRE, magnetic resonance elastography; SWE, shearwave elastography; VCTE, vibration controlled transient elastography.
Screening studies using TE have shown this device to be useful in identifying subgroups enriched in patients with liver fibrosis. Using the 8.0 kPa threshold in population-based studies, around half of the patients who underwent liver biopsy were found to have significant fibrosis (Table 1). Moreover, using the 9.6 kPa threshold in T2DM populations, 40–50% of patients were further confirmed to have advanced liver fibrosis (Table 2). However, it must be acknowledged that elevated liver stiffness was followed by histological confirmation in only 30–55% of the cases in these studies. This low rate was explained by patient refusal, but also by the fact that physicians tend to reserve liver biopsy for more severe patients. Consequently, the true prevalence of liver fibrosis in the at-risk subgroup identified by TE is probably somewhat lower than published. Currently, TE is available only in specialised centres, which is a limitation for the case finding of patients with advanced liver disease in large populations. Many Doppler-ultrasound devices now incorporate elastography technologies (point shearwave elastography, 2D-shearwave elastography),45 with similar accuracy to TE for the diagnosis of liver fibrosis in NAFLD.46,47 As these devices are more widely disseminated, they represent an interesting option to increase the availability of elastography.
Blood fibrosis tests represent another attractive option as they can be prescribed by every physician. Blood fibrosis tests include simple blood tests (NAFLD fibrosis score [NFS], fibrosis-4 [FIB-4]),48,49 and specialised blood tests (Fibrotest, FibroMeter, enhanced liver fibrosis [ELF] test).[50], [51], [52] An advantage of simple blood tests is that they incorporate common and inexpensive blood markers, with free calculation available through websites and smartphone applications. Specialised blood tests are more accurate than simple blood tests,44,53 but they include more expensive specialised blood markers and they are patented with fees for test calculation. Blood fibrosis tests are mathematical models developed for the diagnosis of advanced fibrosis in cohorts of patients who underwent liver biopsy in tertiary care centres and are therefore well fitted for such populations. The following pitfalls must be avoided when using them for case finding in less selected populations.
The NFS was developed in a cohort of 480 patients with NAFLD, in whom the prevalence of diabetes was 29%.48 As diabetes is strongly associated with advanced fibrosis, this parameter was selected with age, BMI, aspartate aminotransferase (AST), ALT, platelet count, and albumin in the final diagnostic model. Two diagnostic cut-offs were calculated: -1.455 to rule-out and 0.676 to rule-in advanced fibrosis. Considering the coefficients attributed to each variable included in the blood test, the presence of diabetes implies a 1.13-point increase in the NFS result. When NFS is used for the case finding of advanced fibrosis in T2DM populations, these additional points are attributed to all patients. Consequently, 70% of patients with T2DM from primary care have an NFS result above -1.455, with advanced fibrosis only being ruled-out in 30%.54 Moreover, this rate of ruling-out dramatically decreases to 3–13% in patients from a diabetes clinic.54,55 Consequently, NFS should not be used for the case finding of advanced fibrosis in T2DM populations.
As previously said, the length of exposure to the cause is a key factor in the development of liver fibrosis in chronic liver diseases. Therefore, it is not surprising that age is a variable included in most of the blood fibrosis tests. However, these tests have been developed and therefore their coefficients for age have been calibrated in cohorts of patients aged around 45–50 years.[48], [49], [50],52 Consequently, they are less sensitive in younger people,56 which could become a matter of importance if cases of advanced liver fibrosis in young adults increase as expected due to the dramatic increase in prevalence of obesity and insulin resistance in children.25 At the other extreme, it has been well described that NFS and FIB-4 provide higher rates of false positive results in older populations. One proposal is to adapt their diagnostic cut-offs in the over 65s: 2.0 instead of 1.30 for ruling-out advanced fibrosis with FIB-4, and 0.12 for NFS instead of -1.455.56 However, such an approach induces a “threshold effect”: a FIB-4 at 1.70 does not rule-out advanced fibrosis at 60 years of age, thus requiring further investigations, whereas the patient will be ruled-out 5 years later despite 5 additional years of disease evolution. Additionally, a recent study suggested that these adapted cut-offs significantly decreased the sensitivity to only 60% in patients over 65 years of age.44
In summary, non-invasive tests of liver fibrosis provide acceptable sensitivity for the case finding of advanced liver fibrosis in at-risk populations. However, these tests have insufficient positive predictive value, especially in populations with low prevalence of the diagnostic target. Therefore, a strategy using an easy-to-obtain and cheap simple blood test as the first-line procedure followed, if positive, by a second-line confirmatory test (elastography or specialised blood test) seems the most appropriate strategy. Using such an approach, the diagnostic accuracy of the second-line test is likely to match that found in published studies. Indeed, the first-line test will select an at-risk population enriched in patients with advanced fibrosis whose prevalence will be close to that of the context where specialised fibrosis tests have been developed and validated. In the end, such a strategy will define the patient pathway: from primary care centres and diabetology clinics to liver specialists.
Algorithms combining non-invasive tests as referral pathways
Despite numerous studies on the diagnostic accuracy of NITs, there is a relative paucity of data on their applied use in referral pathways. The rationale of such pathways is the use of an inexpensive readily available first-tier NIT for all patients, followed by a second NIT in selected cases.13,57 The use of sequential NITs is more effective than single NITs in both low and high prevalence settings.58 Based on existing evidence, the best performing NIT in such scenarios is FIB-4. The main advantage of using FIB-4 for first-tier testing, is its very high negative predictive value (>95%) in unselected populations with low prevalence of advanced fibrosis, providing reassurance in up to 60% of tested patients.59 The automatic calculation and reporting of the FIB-4 score (similarly to automatic calculation of glomerular filtration rate to monitor renal function) could potentially improve the uptake of such pathways and facilitate the work up of patients in primary care or non-hepatology specialties. Uptake of referral pathways can be improved if discussed and agreed with primary care providers and adjusted to the local availability and expertise on NITs.57
Dillon and co-authors reported on the intelligent liver function testing (iLFT), which is an automated system of diagnosing and staging liver disease in primary care.60 The starting point in the system is the identification of abnormal liver tests, which triggers reflex testing for causes of liver disease and liver fibrosis staging based on simple NITs. Compared to standard of care, the diagnosis of liver disease was increased by 43%, and 80% of patients with NAFLD were appropriately staged according to their risk of fibrosis.
In a pilot study in 2 primary care practices in the UK, patients at risk of alcohol-related liver disease (ALD) or NAFLD were tested with a sequential biomarker algorithm, consisting of ALT/AST ratio in ALD or the BARD score in NAFLD followed by TE in patients with high AST/ALT ratio or BARD score. In total, 504 patients were recruited, and 98/378 had increased liver stiffness.61
To date, there is a single study that implemented and evaluated a testing and referral pathway in primary care specifically for patients with NAFLD.62 Srivastava and co-authors prospectively tested a 2-step algorithm in primary care, using FIB-4 followed by ELF in patients with indeterminate FIB-4 results. In total, over 3,000 patients were evaluated using the pathway over a 2-year period. Uptake was 48%, highlighting the difficulties of implementation in real-life scenarios. Compared to patients not evaluated using the pathway, exposure to the pathway was associated with a 4-fold improvement in the diagnosis of advanced fibrosis and cirrhosis and a 77% reduction in unnecessary referrals. Importantly, there was also a 2.5-fold increase in the number of patients coded as having NAFLD during the evaluation period, implying an increased awareness and recognition of NAFLD at the primary care level.
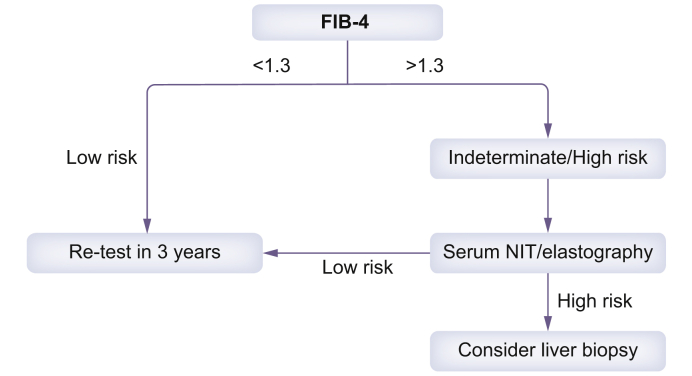
These results support the introduction of robust guidance and pathways in primary care for the management and testing of patients with NAFLD. A proposed algorithm is shown in Fig. 3.
Fig. 3.
Testing pathway for patients with a diagnosis of NAFLD in non-hepatology specialties.
An initial FIB-4 is followed by elastography or a patented serum non-invasive test such as ELF or FibroMeter. The target lesion is the presence of advanced fibrosis. NIT, non-invasive test.
Awareness of NAFLD by non-liver specialists
In a series of 100 patients with NAFLD-related cirrhosis from a tertiary centre, cirrhosis was not diagnosed by intent (incidental diagnosis) in 66% and the majority of these (74%) had a first diagnosis of NAFLD concomitantly.63 In this work, 80% of patients with an incidental diagnosis of cirrhosis had an NFS or FIB-4 result consistent with advanced liver fibrosis, strongly suggesting that a broader use of these tests could facilitate the early detection of patients with asymptomatic severe liver disease. Patients with NAFLD represent too large a population to be evaluated by liver specialists alone. Moreover, a lack of organisation in referrals to liver specialists will overload liver clinics with patients who do not require specialised evaluation and management, contributing to insufficient case finding of patients with advanced liver disease and ultimately increasing costs without significantly reducing the burden of NAFLD. In this context, the sequential use of NITs, starting with a simple blood test, represents a very attractive option that could define a feasible patient pathway between non-liver and liver specialists. The success of such a strategy requires the active participation of general practitioners (GPs), diabetologists and the other physicians who must therefore first and foremost be aware of the disease and the use of NITs for their patients.
GPs have the most contact with the general population and therefore manage a lot of patients at risk of advanced NAFLD. A recent evaluation of data from 18 million patients recorded in databases from primary care centres in 4 European countries (Italy, Netherlands, Spain, UK) showed that the prevalence of codes for NAFLD increased from 0.60% in 2007 to 1.85% in 2014.64 Despite this significant increase, this is way below the estimated 20–25% prevalence in the general population, showing that NAFLD remains under-recognised by GPs. From a physician’s perspective, 54% of 119 US primary care practitioners reported they did not screen for NAFLD in patients with obesity and/or diabetes, and 27% said they did in fewer than half of cases.65 When asked if they refer patients with NAFLD to a specialist, 48% reported they did not refer any and 37% would refer only some. In another survey conducted in Australia, 44% of 108 primary care clinicians stated they do not make any referral to liver specialists for an opinion regarding suspected NAFLD/NASH, 80% did not use NITs of liver fibrosis, 60% were not sure that NITs could help to identify advanced fibrosis or cirrhosis, and 70% were unlikely to refer a patient to Hepatology unless liver function tests were abnormal.66 Similar results were found among GPs from other countries.[67], [68], [69], [70] As GPs ask for more information and knowledge about NAFLD,65,67,71 an interventional study was performed in Italy with questionnaires filled in before and after a dedicated 1-day workshop about NAFLD.70 GPs indicated that this training improved their practice concerning screening of at-risk patients and their referral to specialists.
Low awareness of NAFLD is not limited to GPs. A retrospective analysis of the French hospitalisations database found a very low 0.4% prevalence of NAFLD/NASH diagnosis codes among 50 million adult patients.72 In a US survey including 246 physicians across 3 health systems from a single city, 45% of metabolic specialists did not identify NAFLD as a clinically relevant diagnosis in their patient population, and only half of them would refer suspected NAFLD cases to liver specialists.71 Patients themselves also have little awareness of NAFLD. As a relevant example, despite NAFLD awareness increasing in patients with suspected NAFLD across the different cycles of the US NHANES survey, it remained dramatically low in the 2013-2016 survey, not exceeding 3.1% of the participants.73 A survey conducted in 1,790 adult Chinese patients and their family members showed that only 30% of participants had heard of NAFLD prior to the study.74 Interestingly, after a brief 30 minute educational seminar conducted by the same team, 46% of the 420 participants achieved improvement in the knowledge about NAFLD and 93% indicated they will improve their diet and physical activity.75
All this information demonstrates the lack of awareness of NAFLD outside liver clinics. Therefore, education should be reinforced in specialties involved in the management of patients with metabolic comorbidities, and efforts should be made to directly target and inform patients with metabolic disorders to improve their knowledge of the disease.
Cost-effectiveness
The cost-effectiveness of case finding for advanced fibrosis in NAFLD has been evaluated in several models. Limiting factors in all existing models are the absence of a licensed effective treatment and our somewhat limited knowledge on the natural history of the disease. Regarding the natural history of NAFLD, the transition probabilities among fibrosis stages used in modelling approaches are probably over-estimated, as published studies so far include liver biopsies that are performed based on clinical judgment and not per protocol, with over-representation of more severe cases. These important gaps will hopefully be filled in the next few years with the results of ongoing clinical trials and registry studies.
Testing patients with NAFLD for advanced fibrosis is cost-effective compared to performing a liver biopsy.76 A cost-effectiveness study of testing for advanced fibrosis with TE, NFS or a combination of NFS and TE showed that risk stratification with NFS alone or NFS/TE are both cost-effective strategies at approximately $5,800 per quality-adjusted life year (QALY).77 The base case for the analysis was a 50-year-old patient with NAFLD and abnormal ALT. The modelling assumed that patients diagnosed with advanced fibrosis were treated with vitamin E or pioglitazone. Similarly, a UK analysis by the National Institute of Clinical Excellence (NICE) showed that ELF was cost-effective in testing patients with NAFLD for advanced fibrosis. The base case in this analysis was a 50-year-old patient with NAFLD. The modelling assumed that patients diagnosed with advanced fibrosis would be treated with vitamin E or pioglitazone and undergo screening for cirrhosis.78 The NICE analysis was criticised because of the unrealistically high diagnostic accuracy of the ELF test it used (sensitivity of 100% and specificity of 98%). Finally, a decision model to quantify the accuracy and costs of non-invasive strategies to detect cirrhosis in NAFLD concluded that the combination of FIB-4 and TE had the lowest cost and highest accuracy, followed by the combination of FIB-4 and magnetic resonance elastography, both of which outperformed liver biopsy or any NIT alone.79
Furthermore, there are now several cost-effectiveness studies that focus on testing patients in the general population/primary care. Using a hypothetical cohort of 1,000 patients with NAFLD and a 5% prevalence of advanced fibrosis, Crossan et al. showed that the sequential use of non-invasive fibrosis tests in primary care (FIB-4 followed by ELF or TE or Fibrotest) is an effective way to rationalise secondary care referrals and is associated with significant cost savings, up to 30% compared to a refer-all strategy or 100% compared to a biopsy-all strategy.59 A risk stratification pathway based on TE was more effective than standard of care for patients with NAFLD in primary care, at a cost of £2,138 per QALY gained.80 The pathway demonstrated an 85% probability of cost-effectiveness at a willingness to pay threshold of £20,000 per QALY. In a cost-effectiveness analysis study across 6 independent cohorts (5 from Europe and 1 from Asia), screening for liver fibrosis with TE was cost-effective, with incremental cost-effectiveness ratios ranging from €2,570/QALY to €6,217/QALY in patients at risk of ALD and the general population, respectively.81 The target population was >45 years old, with a history of alcohol consumption, T2DM, metabolic syndrome or any combination of the above. The number needed to screen to detect 1 case of significant fibrosis (F2 or above) ranged from 7.0 in patients with diabetes to 34.5 in the general population. A recent US study based on Markov modelling concluded that screening patients with T2DM for NAFLD was more cost-effective than no screening strategies.82 The model assumed a cohort of patients aged 55 years followed across 1-year cycles. The most cost-effective screening approach was an ultrasound (with or without ALT) followed by a Fibroscan if there was evidence of NAFLD. Finally, in a probabilistic decisional model of a cohort of patients with NAFLD in primary care, the sequential use of FIB-4 followed by ELF or TE was associated with a 25% reduction in total budget spend and a 80% reduction in unnecessary referrals. The cost per case of advanced fibrosis detected was £9,000 with the sequential strategy compared to £25,500 with standard of care.83
The aforementioned studies support the use of NITs for the initial assessment of patients at risk of NAFLD.
Conclusions
The growing burden of NAFLD is changing the landscape of liver disease, with increasing number of patients presenting with cirrhosis and being listed for liver transplantation. It is very likely that we are only seeing the tip of the iceberg, as most cases are undiagnosed and under-reported in primary care/non-hepatology centres, and opportunities for early interventions are missed. Accumulating evidence supports the implementation of testing pathways for patients with an established diagnosis of NAFLD, with the target lesion being advanced fibrosis. These pathways are feasible and cost-effective, increase the awareness of the disease and lead to an increased rate of early diagnosis of advanced fibrosis and compensated cirrhosis. There are many available NITs to implement in such pathways, and the choice should be based on local availability and expertise. Further research is required on the effectiveness of case finding in patients at risk of NAFLD, with particular emphasis on the resources required and the cost-effectiveness of such approaches.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contribution
Both authors contributed equally to the manuscript.
Conflict of interest
J Boursier has consulting activities and received support for research from Echosens and Siemens. E Tsochatzis has no relevant conflicts of interest to declare.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found at https://doi.org/10.1016/j.jhepr.2020.100219.
Contributor Information
Jerome Boursier, Email: Jeboursier@chu-angers.fr.
Emmanuel A. Tsochatzis, Email: e.tsochatzis@ucl.ac.uk.
Supplementary data
References
- 1.Younossi Z.M., Stepanova M., Afendy M., Fang Y., Younossi Y., Mir H. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. e521; quiz e560. [DOI] [PubMed] [Google Scholar]
- 2.Setiawan V.W., Stram D.O., Porcel J., Lu S.C., Le Marchand L., Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016;64:1969–1977. doi: 10.1002/hep.28677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dyson J., Jaques B., Chattopadyhay D., Lochan R., Graham J., Das D. Hepatocellular cancer: the impact of obesity, type 2 diabetes and a multidisciplinary team. J Hepatol. 2014;60:110–117. doi: 10.1016/j.jhep.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Haldar D., Kern B., Hodson J., Armstrong M.J., Adam R., Berlakovich G. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European Liver Transplant Registry study. J Hepatol. 2019;71:313–322. doi: 10.1016/j.jhep.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg D., Ditah I.C., Saeian K., Lalehzari M., Aronsohn A., Gorospe E.C. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology. 2017;152:1090–1099 e1091. doi: 10.1053/j.gastro.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor R.S., Taylor R.J., Bayliss S., Hagstrom H., Nasr P., Schattenberg J.M. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–1625 e1612. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 7.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Konerman M.A., Jones J.C., Harrison S.A. Pharmacotherapy for NASH: current and emerging. J Hepatol. 2018;68:362–375. doi: 10.1016/j.jhep.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Younossi Z.M., Ratziu V., Loomba R., Rinella M., Anstee Q.M., Goodman Z. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 10.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 12.Alexander M., Loomis A.K., van der Lei J., Duarte-Salles T., Prieto-Alhambra D., Ansell D. Non-alcoholic fatty liver disease and risk of incident acute myocardial infarction and stroke: findings from matched cohort study of 18 million European adults. Bmj. 2019;367:l5367. doi: 10.1136/bmj.l5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newsome P.N., Cramb R., Davison S.M., Dillon J.F., Foulerton M., Godfrey E.M. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6–19. doi: 10.1136/gutjnl-2017-314924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cusi K., Chang Z., Harrison S., Lomonaco R., Bril F., Orsak B. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 15.Buzzetti E., Hall A., Ekstedt M., Manuguerra R., Guerrero Misas M., Covelli C. Collagen proportionate area is an independent predictor of long-term outcome in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2019;49:1214–1222. doi: 10.1111/apt.15219. [DOI] [PubMed] [Google Scholar]
- 16.Soderberg C., Stal P., Askling J., Glaumann H., Lindberg G., Marmur J. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2009;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 17.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P. Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397 e310. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newsome P.N., Sasso M., Deeks J.J., Paredes A., Boursier J., Chan W.K. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison S.A., Ratziu V., Boursier J., Francque S., Bedossa P., Majd Z. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30252-1. [DOI] [PubMed] [Google Scholar]
- 20.Boursier J., Anty R., Vonghia L., Moal V., Vanwolleghem T., Canivet C.M. Screening for therapeutic trials and treatment indication in clinical practice: MACK-3, a new blood test for the diagnosis of fibrotic NASH. Aliment Pharmacol Ther. 2018;47:1387–1396. doi: 10.1111/apt.14621. [DOI] [PubMed] [Google Scholar]
- 21.Younossi Z.M., Stepanova M., Rafiq N., Henry L., Loomba R., Makhlouf H. Nonalcoholic steatofibrosis independently predicts mortality in nonalcoholic fatty liver disease. Hepatol Commun. 2017;1:421–428. doi: 10.1002/hep4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caballeria L., Pera G., Arteaga I., Rodriguez L., Aluma A., Morillas R.M. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16:1138–1145 e1135. doi: 10.1016/j.cgh.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 23.Koehler E.M., Plompen E.P., Schouten J.N., Hansen B.E., Darwish Murad S., Taimr P. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63:138–147. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 24.Roulot D., Costes J.L., Buyck J.F., Warzocha U., Gambier N., Czernichow S. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977–984. doi: 10.1136/gut.2010.221382. [DOI] [PubMed] [Google Scholar]
- 25.Abeysekera K.W.M., Fernandes G.S., Hammerton G., Portal A.J., Gordon F.H., Heron J. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol. 2020;5:295–305. doi: 10.1016/S2468-1253(19)30419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwok R., Choi K.C., Wong G.L., Zhang Y., Chan H.L., Luk A.O. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 27.Sporea I., Mare R., Popescu A., Nistorescu S., Baldea V., Sirli R. Screening for liver fibrosis and steatosis in a large cohort of patients with type 2 diabetes using vibration controlled transient elastography and controlled attenuation parameter in a single-center real-life experience. J Clin Med. 2020;9 doi: 10.3390/jcm9041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai L.L., Wan Yusoff W.N.I., Vethakkan S.R., Nik Mustapha N.R., Mahadeva S., Chan W.K. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396–1403. doi: 10.1111/jgh.14577. [DOI] [PubMed] [Google Scholar]
- 29.Roulot D., Roudot-Thoraval F., NKontchou G., Kouacou N., Costes J.L., Elourimi G. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int. 2017;37:1897–1906. doi: 10.1111/liv.13481. [DOI] [PubMed] [Google Scholar]
- 30.de Ledinghen V., Vergniol J., Gonzalez C., Foucher J., Maury E., Chemineau L. Screening for liver fibrosis by using FibroScan((R)) and FibroTest in patients with diabetes. Dig Liver Dis. 2012;44:413–418. doi: 10.1016/j.dld.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Rao Kondapally Seshasai S., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., Sarwar N. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris R., Card T.R., Delahooke T., Aithal G.P., Guha I.N. Obesity is the most common risk factor for chronic liver disease: results from a risk stratification pathway using transient elastography. Am J Gastroenterol. 2019;114:1744–1752. doi: 10.14309/ajg.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 33.Harman D.J., Ryder S.D., James M.W., Wilkes E.A., Card T.R., Aithal G.P. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross-sectional study using transient elastography. Aliment Pharmacol Ther. 2018;47:504–515. doi: 10.1111/apt.14463. [DOI] [PubMed] [Google Scholar]
- 34.Di Cesare M., Soric M., Bovet P., Miranda J.J., Bhutta Z., Stevens G.A. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17:212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The Lancet. Type 2 diabetes: the urgent need to protect young people. Lancet. 2018;392:2325. doi: 10.1016/S0140-6736(18)33015-0. [DOI] [PubMed] [Google Scholar]
- 36.Lascar N., Brown J., Pattison H., Barnett A.H., Bailey C.J., Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- 37.Lonardo A., Nascimbeni F., Mantovani A., Targher G. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68:335–352. doi: 10.1016/j.jhep.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Singh S., Allen A.M., Wang Z., Prokop L.J., Murad M.H., Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–654. doi: 10.1016/j.cgh.2014.04.014. e641-649;quiz e639-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhanova J., O'Brien M., Minuk G., Tate R. Chronic liver disease and metabolic comorbities in healthy young males followed for 65 years: the Manitoba follow-up study. Clin Gastroenterol Hepatol. 2020 doi: 10.1016/j.cgh.2020.10.028. in press. [DOI] [PubMed] [Google Scholar]
- 41.Hagstrom H., Nasr P., Ekstedt M., Hammar U., Stal P., Askling J. Cardiovascular risk factors in non-alcoholic fatty liver disease. Liver Int. 2019;39:197–204. doi: 10.1111/liv.13973. [DOI] [PubMed] [Google Scholar]
- 42.Vali Y., Lee J., Boursier J., Spijker R., Loffler J., Verheij J. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 43.Xiao G., Zhu S., Xiao X., Yan L., Yang J., Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 44.Boursier J., Guillaume M., Leroy V., Irles M., Roux M., Lannes A. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol. 2019;71:389–396. doi: 10.1016/j.jhep.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Dietrich C.F., Bamber J., Berzigotti A., Bota S., Cantisani V., Castera L. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version) Ultraschall Med. 2017;38:e48. doi: 10.1055/a-0641-0076. [DOI] [PubMed] [Google Scholar]
- 46.Lee M.S., Bae J.M., Joo S.K., Woo H., Lee D.H., Jung Y.J. Prospective comparison among transient elastography, supersonic shear imaging, and ARFI imaging for predicting fibrosis in nonalcoholic fatty liver disease. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cassinotto C., Boursier J., de Ledinghen V., Lebigot J., Lapuyade B., Cales P. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 48.Angulo P., Hui J.M., Marchesini G., Bugianesi E., George J., Farrell G.C. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 49.Sterling R.K., Lissen E., Clumeck N., Sola R., Correa M.C., Montaner J. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 50.Cales P., de Ledinghen V., Halfon P., Bacq Y., Leroy V., Boursier J. Evaluating the accuracy and increasing the reliable diagnosis rate of blood tests for liver fibrosis in chronic hepatitis C. Liver Int. 2008;28:1352–1362. doi: 10.1111/j.1478-3231.2008.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenberg W.M., Voelker M., Thiel R., Becka M., Burt A., Schuppan D. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 52.Imbert-Bismut F., Ratziu V., Pieroni L., Charlotte F., Benhamou Y., Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 53.Guillaume M., Moal V., Delabaudiere C., Zuberbuhler F., Robic M.A., Lannes A. Direct comparison of the specialised blood fibrosis tests FibroMeter(V2G) and Enhanced Liver Fibrosis score in patients with non-alcoholic fatty liver disease from tertiary care centres. Aliment Pharmacol Ther. 2019;50:1214–1222. doi: 10.1111/apt.15529. [DOI] [PubMed] [Google Scholar]
- 54.Patel P., Hossain F., Horsfall L.U., Banh X., Hayward K.L., Williams S. A pragmatic approach identifies a high rate of nonalcoholic fatty liver disease with advanced fibrosis in diabetes clinics and at-risk populations in primary care. Hepatol Commun. 2018;2:893–905. doi: 10.1002/hep4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciardullo S., Muraca E., Perra S., Bianconi E., Zerbini F., Oltolini A. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2019-000904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McPherson S., Hardy T., Dufour J.F., Petta S., Romero-Gomez M., Allison M. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsochatzis E.A., Newsome P.N. Non-alcoholic fatty liver disease and the interface between primary and secondary care. Lancet Gastroenterol Hepatol. 2018;3:509–517. doi: 10.1016/S2468-1253(18)30077-3. [DOI] [PubMed] [Google Scholar]
- 58.Majumdar A., Campos S., Gurusamy K., Pinzani M., Tsochatzis E.A. Defining the minimum acceptable diagnostic accuracy of noninvasive fibrosis testing in cirrhosis: a decision analytic modeling study. Hepatology. 2020;71:627–642. doi: 10.1002/hep.30846. [DOI] [PubMed] [Google Scholar]
- 59.Crossan C., Majumdar A., Srivastava A., Thorburn D., Rosenberg W., Pinzani M. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: diagnostic accuracy and cost analysis. Liver Int : official J Int Assoc Study Liver. 2019;39:2052–2060. doi: 10.1111/liv.14198. [DOI] [PubMed] [Google Scholar]
- 60.Dillon J.F., Miller M.H., Robinson E.M., Hapca A., Rezaeihemami M., Weatherburn C. Intelligent liver function testing (iLFT): a trial of automated diagnosis and staging of liver disease in primary care. J Hepatol. 2019;71:699–706. doi: 10.1016/j.jhep.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 61.Harman D.J., Ryder S.D., James M.W., Jelpke M., Ottey D.S., Wilkes E.A. Direct targeting of risk factors significantly increases the detection of liver cirrhosis in primary care: a cross-sectional diagnostic study utilising transient elastography. BMJ open. 2015;5 doi: 10.1136/bmjopen-2014-007516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Srivastava A., Gailer R., Tanwar S., Trembling P., Parkes J., Rodger A. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepatol. 2019;71:371–378. doi: 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 63.Bertot L.C., Jeffrey G.P., Wallace M., MacQuillan G., Garas G., Ching H.L. Nonalcoholic fatty liver disease-related cirrhosis is commonly unrecognized and associated with hepatocellular carcinoma. Hepatol Commun. 2017;1:53–60. doi: 10.1002/hep4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander M., Loomis A.K., Fairburn-Beech J., van der Lei J., Duarte-Salles T., Prieto-Alhambra D. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med. 2018;16:130. doi: 10.1186/s12916-018-1103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Said A., Gagovic V., Malecki K., Givens M.L., Nieto F.J. Primary care practitioners survey of non-alcoholic fatty liver disease. Ann Hepatol. 2013;12:758–765. [PubMed] [Google Scholar]
- 66.Patel P.J., Banh X., Horsfall L.U., Hayward K.L., Hossain F., Johnson T. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med J. 2018;48:144–151. doi: 10.1111/imj.13667. [DOI] [PubMed] [Google Scholar]
- 67.van Asten M., Verhaegh P., Koek G., Verbeek J. The increasing burden of NAFLD fibrosis in the general population: time to bridge the gap between hepatologists and primary care. Hepatology. 2017;65:1078. doi: 10.1002/hep.28940. [DOI] [PubMed] [Google Scholar]
- 68.Blais P., Husain N., Kramer J.R., Kowalkowski M., El-Serag H., Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110:10–14. doi: 10.1038/ajg.2014.134. [DOI] [PubMed] [Google Scholar]
- 69.Kallman J.B., Arsalla A., Park V., Dhungel S., Bhatia P., Haddad D. Screening for hepatitis B, C and non-alcoholic fatty liver disease: a survey of community-based physicians. Aliment Pharmacol Ther. 2009;29:1019–1024. doi: 10.1111/j.1365-2036.2009.03961.x. [DOI] [PubMed] [Google Scholar]
- 70.Grattagliano I., D'Ambrosio G., Palmieri V.O., Moschetta A., Palasciano G., Portincasa P. Improving nonalcoholic fatty liver disease management by general practitioners: a critical evaluation and impact of an educational training program. J Gastrointestin Liver Dis. 2008;17:389–394. [PubMed] [Google Scholar]
- 71.Wieland A.C., Quallick M., Truesdale A., Mettler P., Bambha K.M. Identifying practice gaps to optimize medical care for patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2013;58:2809–2816. doi: 10.1007/s10620-013-2740-8. [DOI] [PubMed] [Google Scholar]
- 72.Boursier J., Shreay S., Fabron C., Torreton E., Fraysse J. Hospitalization costs and risks of mortality in adults with nonalcoholic steatohepatitis: analysis of a French national hospital database. EClinicalMedicine. 2020;25(100445) doi: 10.1016/j.eclinm.2020.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A., Dhaliwal A.S., Singh S., Kumar A., Lopez R., Gupta M. Awareness of nonalcoholic fatty liver disease is increasing but remains very low in a representative US cohort. Dig Dis Sci. 2020;65:978–986. doi: 10.1007/s10620-019-05700-9. [DOI] [PubMed] [Google Scholar]
- 74.Chen S., Chao S., Konerman M., Zhang W., Rao H., Wu E. Survey of nonalcoholic fatty liver disease knowledge, nutrition, and physical activity patterns among the general public in Beijing, China. Dig Dis Sci. 2019;64:3480–3488. doi: 10.1007/s10620-019-05709-0. [DOI] [PubMed] [Google Scholar]
- 75.Zhang W., Chao S., Chen S., Rao H., Huang R., Wei L. Awareness and knowledge of nonalcoholic fatty liver disease among office employees in Beijing, China. Dig Dis Sci. 2019;64:708–717. doi: 10.1007/s10620-018-5389-5. [DOI] [PubMed] [Google Scholar]
- 76.Crossan C., Tsochatzis E.A., Longworth L., Gurusamy K., Davidson B., Rodriguez-Peralvarez M. Cost-effectiveness of non-invasive methods for assessment and monitoring of liver fibrosis and cirrhosis in patients with chronic liver disease: systematic review and economic evaluation. Health Technol Assess (Winchester, England) 2015;19:1–409. doi: 10.3310/hta19090. v-vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tapper E.B., Sengupta N., Hunink M.G., Afdhal N.H., Lai M. Cost-effective evaluation of nonalcoholic fatty liver disease with NAFLD fibrosis score and vibration controlled transient elastography. Am J Gastroenterol. 2015;110:1298–1304. doi: 10.1038/ajg.2015.241. [DOI] [PubMed] [Google Scholar]
- 78.(NICE) NIfHaCE . 2016. Non-alcoholic fatty liver disease (NAFLD): assessment and management. London. [Google Scholar]
- 79.Vilar-Gomez E., Lou Z., Kong N., Vuppalanchi R., Imperiale T.F., Chalasani N. Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Clin Gastroenterol Hepatol : official Clin Pract J Am Gastroenterological Assoc. 2020;18:2305–2314. doi: 10.1016/j.cgh.2020.04.017. e2312. [DOI] [PubMed] [Google Scholar]
- 80.Tanajewski L., Harris R., Harman D.J., Aithal G.P., Card T.R., Gkountouras G. Economic evaluation of a community-based diagnostic pathway to stratify adults for non-alcoholic fatty liver disease: a Markov model informed by a feasibility study. BMJ open. 2017;7 doi: 10.1136/bmjopen-2016-015659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serra-Burriel M., Graupera I., Torán P., Thiele M., Roulot D., Wai-Sun Wong V. Transient elastography for screening of liver fibrosis: cost-effectiveness analysis from six prospective cohorts in Europe and Asia. J Hepatol. 2019;71:1141–1151. doi: 10.1016/j.jhep.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 82.Noureddin M., Jones C., Alkhouri N., Gomez E.V., Dieterich D.T., Rinella M.E. Screening for non-alcoholic fatty liver disease in persons with type 2 diabetes in the U.S. Is cost effective: a comprehensive cost-utility analysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.07.050. [DOI] [PubMed] [Google Scholar]
- 83.Srivastava A., Jong S., Gola A., Gailer R., Morgan S., Sennett K. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019;19:122. doi: 10.1186/s12876-019-1039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.