Abstract
Parkinson’s disease is a neurodegenerative disorder characterized by a combination of severe motor and non-motor symptoms. Over the years, several factors have been discovered to play a role in the pathogenesis of this disease, in particular, neuroinflammation and oxidative stress. To date, the pharmacological treatments used in Parkinson’s disease are exclusively symptomatic. For this reason, in recent years, the research has been directed towards the discovery and study of new natural molecules to develop potential neuroprotective therapies against Parkinson’s disease. In this context, natural polyphenols have raised much attention for their important anti-inflammatory and antioxidant properties, but also for their ability to modulate protein misfolding. In this review, we propose to summarize the relevant in vivo and in vitro studies concerning the potential therapeutic role of natural polyphenols in Parkinson’s disease.
Keywords: alpha-synuclein, anti-inflammatory, antioxidants, natural molecules, neuroprotection, Parkinson’s disease, polyphenols, syntomatic effect
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease, after Alzheimer’s disease. PD is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) with consequent dopaminergic denervation of the striatum, which receives the SNc neuron projections (Blandini et al., 2007). The resulting dysfunction of the nigrostriatal pathway is responsible for the classic motor symptoms, which include resting tremor, bradykinesia, rigidity and postural instability. Scientific evidence suggests that different biological mechanism - including oxidative stress, neuroinflammation and α-synuclein (αSyn) accumulation and aggregation - play an important role in the pathogenesis of PD (Mcgeer et al., 1988; Liu et al., 2003; Blandini, 2013; Liddelow et al., 2017; Lee et al., 2019).
The term “Oxidative Stress”, coined by Denham Harman (1956) refers to a pathological process that occurs in the presence of an imbalance between production and detoxification of reactive oxygen (ROS) and nitrogen species by endogenous antioxidant systems, such as superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) (Blesa et al., 2015). This process leads to mitochondrial dysfunction and irreversible oxidation of macromolecules such as lipids, proteins and nucleic acids, compromising integrity and function of neuronal cells (Lee et al., 2009; Qin et al., 2013; Smeyne et al., 2013).
In the central nervous system, after an injury, a sequence of events take place including the production of inflammatory mediators, the recruitment of immune cells into the parenchyma and the activation of glial cells (microglia and astrocytes): all these phenomena are referred to as neuroinflammation (Lucas et al., 2006; Yong et al., 2019). At the beginning, neuroinflammation plays a defensive role against the pathological insult. However, a persistent and uncontrollable activation of this process can become detrimental (Wyss-Coray and Mucke, 2002). An abnormal intensification of glial cell activation in the brain tissue - especially in nigrostriatal area - was reported both in post-mortem brain of PD patients and PD animal models (Mcgeer et al., 1988; Liu et al., 2003; Liddelow et al., 2017). Persistent activation of both microglia and astroglia contributes to exacerbate the neurodegenerative process, mainly through the increased release of pro-inflammatory factors in the extracellular space, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-2, IL-6, interferon-γ and inducible oxide synthase (Colton et al., 2010; Blandini et al., 2013; Liddelow et al., 2017).
Alpha-Syn is a 140-amino acid protein, encoded by the SNCA gene, that accumulates in Lewy bodies, the pathologic hallmark of PD (Chen et al., 1995). Although the precise physiological function of this protein is not clear, accumulating evidence suggests that αSyn may play an important role in presynaptic dopamine recruitment, vesicle trafficking, synaptic transmission and lipid metabolism (Abeliovich et al., 2000; Murphy et al., 2000; Cabin et al., 2002; Willingham et al., 2003; Liu et al., 2004; Yavich et al., 2004). Missense mutations in the SNCA gene (A30P, E46K and A53T) as well as the overexpression of wild type αSyn causes a decrease in the release of dopamine from presynaptic terminals and increase the propensity of αSyn to form toxic intermediates and to self-aggregate (Polymeropoulos et al., 1997; Kruger et al., 1998; Singleton et al., 2003, 2004; Farrer et al., 2004; Zarranz et al., 2004). Under physiological conditions, the clearance of αSyn is mediated by both the ubiquitin/proteasome system and chaperone-mediated autophagy. However, pathological αSyn may inhibit ubiquitin/proteasome system and impair chaperone-mediated autophagy, consequently affecting autophagy (Cuervo et al., 2004; Engelender et al., 2012). Systematic database search of PubMed was performed to find articles used in this review from 2014. Main keywords related to polyphenols and PD were used.
Polyphenols
Despite extensive research efforts made in the PD field, no effective therapies able to arrest or slow down the disease are available to date. In the last decade, scientific evidence has shown that natural molecules, including polyphenols (PPH), are able to mitigate pathobiological processes involved in cancer, diabetic, cardiovascular and neurodegenerative diseases (Rodriguez-Mateos et al., 2013; Gunn et al., 2015). In particular, PPH protect neurons from ROS by increasing the activity of NAD-dependent deacetylase sirtuin-1 (SIRT1) (Anekonda et al., 2006; Albani et al., 2010) and dampen the neuroinflammatory process by modulating the signaling of nuclear factor kappa-light-chain-enhancer of activated B cells (Aquilano et al., 2008; Bhullar et al., 2013). In addition, PPH can induce neurite outgrowth, increase cellular antioxidant defense and regulate pro-survival transcription factors and gene expression by targeting different molecular pathways, such as extracellular signal-regulated kinase 1/2, phosphatidylinositol 3-kinase/Akt and mitogen activated protein kinase pathways (Lin et al., 2010). It is noteworthy that PPH also reduce toxicity induced by αSyn misfolded aggregates and amyloid β protein oligomers (Feng et al., 2009; Singh et al., 2013). All these properties strongly point to PPH compounds as potential therapeutic tools for PD. Thus, in the last decade many studies have been performed in order to investigate PPH efficacy in PD and to overcome their limitations, such as bioavailability. However, different factors potentially influencing their biotransformation are still often overlooked (Koutsos et al., 2015). Of note, gut microbiota composition significantly affects PPH biovailability, thereby modulating their effects (Visioli et al., 2011; Frolinger et al., 2019). Analogously, dietary fat can mask the health promoting effects of natural supplements (Maulik et al., 2019).
PPH may be classified into two major groups, flavonoids and non-flavonoids, based on the number of benzene rings that they contain and the structural elements that bind these rings together. Non-flavonoids contain up to two benzene rings, whereas flavonoids are compounds with more than two rings (Manach et al., 2004). This review critically appraises the main results obtained by in vivo and in vitro studies investigating the neuroprotective properties and symptomatic effects of different classes of PPH in PD.
Flavonoids
Flavonoids are widely distributed within plant kingdom. They are generally found in cereals, fruits, vegetables, legumes as well as in the most common beverages such as tea, wine and beer. Flavonoids include flavonols, flavones, flavanones, flavanols, anthocyanidins and isoflavones (Ferrazzano et al., 2011; Table 1).
Table 1.
Summary of the effects of flavonoids in PD models
| In vivo/In vitro | Model | Main findings | Duration of treatment | Dose and route of administration | References |
|---|---|---|---|---|---|
| In vivo | 6-OHDA | ↓Neurodegeneration ↑Motor performance ↑Cognitive performance ↓Oxidative stress |
28 d | Quercetin (10 and 25 mg/kg, p.o.) | Ghaffari et al., 2018 |
| In vivo | 6-OHDA | ↓Oxidative stress ↓Neuroinflammation ↑Motor performance |
14 d | Quercetin (25 mg/kg, p.o.) with piperine (2.5 mg/kg, p.o.) | Singh and Kumar, 2018 |
| In vitro | 6-OHDA | ↓Oxidative stress ↑Cell viability |
4 h | Hyperoside (0, 0.25, 0.5, 1, 2.5, 5, and 10 µM) | Kwon et al., 2019 |
| In vivo | Rotenone | ↓Oxidative stress ↓Endoplasmic reticulum stress ↓Apoptosis ↑Motor performance ↑Autophagy efficiency |
28 d | Quercetin (50 mg/kg, i.p.) | El-Horany et al., 2016 |
| In vivo | Rotenone/Iron | ↑Motor performance ↓Oxidative stress ↓Neuroinflammation ↓Neurodegeneration |
28 d | Quercetin (25 and 50 mg/kg, p.o.) | Sharma et al., 2020 |
| In vitro | αSyn | ↓Oxidative stress ↓αSyn fibrillation |
24 h | Epigallocatechin gallate (0, 10, 20, 50, 100 and 200 μM) | Zhao et al., 2017 |
| In vivo | MPTP | ↑Motor performance ↓Oxidative stress ↓ Iron levels |
7 d | Epigallocatechin gallate (25 mg/kg, p.o.) | Xu et al., 2017 |
| In vivo | MPTP | ↑Motor performance ↓Neurodegeneration ↓αSyn |
80 d | Epigallocatechin gallate (40 mg/kg, p.o.) | Chen et al., 2015a |
| In vivo | MPTP | ↓Oxidative stress ↓Neuroinflammation ↓Neurodegeneration ↓αSyn |
5 d | Naringenin (25, 50 and 100 mg/kg, p.o.) | Mani et al., 2018 |
| In vivo | MPTP | ↓Oxidative stress ↓Neuroinflammation ↑Motor performance |
5 d | Naringenin (25, 50 and 100 mg/kg, p.o.) | Sugumar et al., 2019 |
| In vivo | 6-OHDA | ↓Oxidative stress ↑Motor performance |
28 d | Vitamin E Loaded Naringenin Nanoemulsion (0.72 mg/kg, intranasal) | Gaba et al., 2019 |
| In vivo | 6-OHDA | ↓Oxidative stress ↓Neuronal damage |
15 d | Naringenin (20 and 40 mg/kg, p.o.) | Shakeel et al., 2017 |
| In vivo | 6-OHDA | ↓Neurodegeneration ↓Neuroinflammation |
7 d | Naringin (80 mg/kg, i.p.) | Kim et al. 2016 |
| In vivo | 6-OHDA | ↑Motor performance ↓Oxidative stress ↓Neuroinflammation ↓DNA fragmentation ↓Apoptosis |
7 d | Hesperetin (50 mg/kg, p.o.) | Kiasalari et al. 2015 |
| In vivo | MPTP | ↑Motor performance ↓Presynaptic glutamate release ↓Glutamatergic transmission ↑Synaptic GluR1subunit ↓Neuroinflammation |
14 d | Baicalein (10 mg/kg, i.p.) | Xue et al., 2014 |
| In vivo | MPTP | ↑Motor performance ↓Neurodegeneration |
7 d | Baicalein (1 and 10 mg/kg, i.p.) | Lee et al., 2014 |
| In vivo | MPTP | ↑Motor performance ↓Neurodegeneration ↑Neurogenesis ↑Proliferation |
7 d | Baicalein (140 and 280 mg/kg, p.o.) | Gao et al., 2015 |
| In vivo | MPTP | ↓Neurodegeneration ↑Motor performance |
9 d | Baicalein (10 mg/kg, i.p.) | Zheng et al., 2019 |
| In vivo | Transgenic | ↑Motor performance ↓Oxidative stress ↓Neurodegeneration |
24 h | Tangeritin (5, 10, and 20 µM, p.o.) | Fatima et al., 2017 |
| In vivo | Transgenic/6-OHDA | ↓αSyn ↓Neurodegeneration ↑Food-sensing behavior ↑Life span |
72 h | Irisflorentin (0, 0.1, 0.5, 2.5, and 12.5 mM, p.o.) | Chen et al., 2015c |
| In vitro | αSyn | ↓Oxidative stress ↓Apoptosis |
24 h | Genistein (20 μM) | Wu et al., 2018 |
6-OHDA: 6-Hydroxydopamine; αSyn: α-synuclein; i.p.: intraperitoneal; MPTP: methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD: Parkinson’s disease; p.o.: oral administration or per os.
Flavonols
The flavonol quercetin is widely distributed in fruits and vegetables, particularly in capers (Capparis spinosa). Ghaffari et al. (2018) demonstrated the antioxidant property of quercetin and its nanocrystals in 6-hydroxydopamine (6-OHDA) rat model of PD. These molecules restored the activity of SOD and CAT enzymes and GSH levels, as well as decreased lipid peroxidation in the hippocampus. Similarly, quercetin treatment (50 mg/kg, 4 weeks) attenuated oxidative stress in the rotenone rat model by restoring thioredoxin activity with consequent decrease of malondialdehyde (MDA) levels (El-Horany et al., 2016). The antioxidant and anti-inflammatory properties of quercetin have been recently confirmed by Sharma et al. (2020) who demonstrated that this compound restores mitochondrial complex I and IV activities and reduces pro-inflammatory marker levels in the striatum of rotenone-treated rats fed with a daily iron supplement. Quercetin has proven also effective on PD-related motor and non-motor symptoms. The administration of quercetin for 28 days after 6-OHDA injection reduced the apomorphine-induced rotational behavior and prevented the memory impairment. These findings are in line with the study of Singh and Kumar (2018), who reported antioxidant, anti-inflammatory and neuroprotective effects of pre-treatment with quercetin - alone or in combination with piperine - in association with an improvement of motor performance. El-Horany et al. (2016) also revealed that quercetin increases autophagy and reduces apoptosis.
Quercetin 3-D-galactoside, also known as hyperoside, is a natural derivative of quercetin isolated from Acer tegmentosum. This compound significantly promoted the survival of 6-OHDA-treated SH-SY5Y by activating nuclear factor erythroid 2-related factor 2, reducing lactate dehydrogenase release and dampening excessive ROS accumulation (Kwon et al., 2019).
Flavanols
It is well known that metal ions such as Zn(II), Cu(II), Mn and Fe(III) are involved in the pathogenesis of neurodegenerative diseases, as they can enhance oxidative stress by generating highly toxic hydroxyl radicals through the Fenton reaction (Xu et al., 2017; Zhao et al., 2017; Maulik et al., 2019). Furthermore, metal ions can promote and accelerate the fibrillation of αSyn. Zhao et al. (2017) reported that the treatment with epigallocatechin gallate, the most abundant flavonoid in green tea (Camellia sinensis), inhibits αSyn fibrillation and reduces intracellular ROS levels, by chelating Fe(III) in wild-type αSyn-transduced PC12 cells. Xu et al. (2017) confirmed the free-radical scavenging and iron-chelating properties of epigallocatechin gallate in MPTP mice model. In this model, MPTP induces iron accumulation in the SNc, which is associated with altered expression of iron exporter ferroportin, a key regulator of cellular iron metabolism. Chronic treatment with epigallocatechin gallate counteracted MPTP-induced motor impairment and neurochemical deficits and enhanced ferroportin expression levels in the SNc (Xu et al., 2017). Interestingly, Chen et al. (2015) demonstrated neuroprotective effects of epigallocatechin gallate in MPTP-intoxicated monkeys. Epigallocatechin gallate reduced dopaminergic cell loss in the SNc and improved motor performance while reducing accumulation of neurotoxic αSyn oligomers in the striatum and hippocampus.
Flavanones
Naringenin is a flavanone found in a variety of fruits and herbs, particularly in grapefruit. naringenin possesses immunomodulatory and antioxidant effects that may be beneficial to a variety of pathological conditions, including neurodegenerative diseases (Maatouk et al., 2016; Oguido et al., 2017; Yang et al., 2019). Mani et al. (2018) showed that the naringenin treatment counteracts MPTP-induced dopaminergic degeneration and significantly upregulates DA-transporter and tyrosine hydroxylase protein expression. Oral pre-treatment with naringenin for 5 days significantly counteracted the MPTP-induced lipid peroxidation and increased GSH, SOD and CAT levels, improving the behavioral performance (Sugumar et al., 2019). Naringenin also downregulated the mRNA expression levels of pro-inflammatory mediators (i.e., TNF-α, IL-1β, and inducible oxide synthase; Sugumar et al., 2019) and inhibited αSyn fibrillation in MPTP-intoxicated animals (Mani et al., 2018). Although these results are encouraging, naringenin treatment shows several limitations due to its poor bioavailability and water insolubility, which prompted the development of new formulations and delivery routes. Recently, intranasal delivery of a naringenin-nanoemulsion proved able to enhance the uptake and bioavailability of naringenin in the brain (Gaba et al., 2019). Intranasally-administered naringenin improved the motor performance in a 6-OHDA rat model and modulated the oxidative stress response, as demonstrated by the significant increase of GSH and SOD levels, and the decrease of MDA and lipid peroxidation (Shakeel et al., 2017; Gaba et al., 2019).
Treatment with naringin, the major flavonoid glycoside in grapefruit and pummelo, protected dopaminergic neurons by reducing microglial activation and enhancing the mechanistic target of rapamycin complex-1 prosurvival pathway (Kim et al., 2016).
Hesperetin, a flavanone derived from hesperidin and found in citrus fruit, reduced MDA levels, enhanced CAT activity and GSH content in the striatum of 6-OHDA rat model; these effects were accompanied by reduced astrocyte activation and increased B-cell lymphoma 2 expression levels. Hesperetin exerted neuroprotective effects also in the SNc by preventing the loss of dopaminergic neurons and restoring motor performance (Kiasalari et al., 2016).
Flavones
Baicalein is a flavone extracted from the roots of Scutellaria baicalensis and used in traditional Chinese herbal medicine for its antibacterial, antiviral, and anti-inflammatory effects (Lee et al., 2014; Gao et al., 2015). Baicalein attenuates presynaptic glutamate release and reduce cytokines levels in the nigrostriatal pathway in mice injected with MPTP (Xue et al., 2014). Moreover, baicalein treatment - alone or in combination with levodopa - increased motor performance and prevented MPTP-induced dopaminergic cell loss (Lee et al., 2014; Gao et al., 2015; Zheng et al., 2019). This neuroprotective effect was accompanied by glial cells deactivation. In addition, baicalein suppressed the MPP+-induced nuclear translocation of nuclear factor-κB, consequently decreasing c-Jun N-terminal kinase and extracellular signal-regulated kinase activation (Lee et al., 2014). Interestingly, Gao et al. (2015) demonstrated that baicalein promotes neurogenesis, neuroblast proliferation and neurotrophin signaling pathway.
Oral treatment with Tangeritin, a flavone isolated from peels of citrus fruits, for 24 days improved motor climbing performance in a transgenic Drosophila model of PD that expresses human wild type αSyn (Fatima et al., 2017). This symptomatic effect was accompanied by an increase of dopamine and a modulation of oxidative stress markers (Fatima et al., 2017).
Isoflavones
Chen et al. (2015) demonstrated the therapeutic properties of irisflorentin, an isoflavone derived from Belamcandae Rhizoma, in two different Caenorhabditis elegans PD models. In particular, the treatment with irisflorentin prevented αSyn accumulation in the transgenic C. elegans model, whereas reduced dopaminergic neuron degeneration, and improves food-sensing behavior and life-span in 6-OHDA-induced model. According to the authors, irisflorentin may exert their effects by modulating the expression of both 26S-proteasome non-ATPase regulatory subunit 3 and Egg-laying defective protein 1, consequently enhancing the activity of the proteasome and blocking apoptosis (Chen et al., 2015c).
It was reported that genistein, a molecule present in lupin, fava beans, soybeans and kudzu, reduces rotenone-induced oxidative stress and cell apoptosis in neuroblastoma SH-SY5Y cells overexpressing A53T mutant αSyn (Wu et al., 2018). Specifically, genistein induced its cytoprotective properties by activating estrogen receptors, increasing the phosphorylation of pro-apoptotic protein BAD and modulating the levels of nuclear factor-erythroid 2-related factor. This event led to the reduction of rotenone-induced mitochondrial oxidative injury and MDA levels, and an increase of the activity of Heme Oxygenase 1, which plays a central role in neuronal protection (Wu et al., 2018).
Non-flavonoids molecules
Non-flavonoid PPH include phenolic acids, phenolic alcohol, stilbenes and lignans (Ferrazzano et al., 2011). The first two sub-classes of molecules are characterized by the presence of a single benzene ring and a carboxylic (phenolic acids) or hydroxyl (phenolic alcohols) terminal functional group. Conversely, stilbenes and lignans have both two benzene rings, but a different polymer structure: linear for stilbenes and branched for lignans (Manach et al., 2004; Table 2).
Table 2.
Summary of the effects of non-flavonoids molecules in PD models
| In vivo/In vitro | Model | Main findings | Duration of treatment | Dose and route of administration | References |
|---|---|---|---|---|---|
| In vivo | MPTP | ↑Lifespan ↑Motor performance ↓Oxidative stress ↓Inflammation ↑Cell survival |
3 d | Resveratrol (30 and 60 mg/kg, p.o.) | Abolaji et al., 2018 |
| In vitro | MPP+ | ↓Oxidative stress ↓Apoptosis ↑Cell viability |
24 h | Resveratrol (20 and 75 nM) | Zeng et al., 2017 |
| In vitro | Parkin | ↓Oxidative stress ↑Autophagy efficiency | 24 h | Resveratrol (25 µM) | Vergara et al., 2017 |
| In vitro | Rotenone | ↓Neurotoxicity ↓Oxidative stress ↓Mitochondrial deficit ↓Apoptosis |
2 h | Resveratrol (12.5, 25, 50, and 100 µM) | Wang et al., 2018 |
| In vivo | MPTP | ↓Neurodegeneration ↑Motor performance ↓αSyn ↑Autophagy efficiency |
33 d | Resveratrol (100 mg/kg, p.o.) | Guo et al., 2016 |
| In vivo | Transgenic | ↑Motor performance ↑Cognitive performance ↓αSyn ↓Oxidative stress ↓Neuroinflammation ↓Neurodegeneration |
35 d | Resveratrol (10 and 50 mg/kg, p.o.) | Zhang et al., 2018 |
| In vivo | Rotenone | ↓Oxidative stress ↓Endoplasmic reticulum stress ↓Apoptosis ↓Neuroinflammation |
21 d | Resveratrol (20 mg/kg, p.o.) | Gaballah et al., 2016 |
| In vivo | MPTP | ↓Neurodegeneration ↓Oxidative stress ↑Motor performance |
15 d | Resveratrol (20 mg/kg, i.p.) | Da Rocha Lindner et al., 2015 |
| In vivo | Rotenone | ↓Neurodegeneration ↓Oxidative stress ↑Motor performance |
35 d | Piceid (80 mg/kg, p.o.) | Chen et al., 2015b |
| In vivo | 6-OHDA | ↓Neurodegeneration ↓Neuroinflammation ↑Motor performance |
28 d | 7-Hydroxymatairesinol (10 mg/kg, p.o.) | Giuliano et al., 2020 |
| In vivo | 6-OHDA | ↑Motor performance ↓Neuroinflammation |
10 d | Ellagic acid (50 mg/kg, i.g) | Farbood et al. 2015 |
| In vivo | αSyn | ↓aSyn ↑Life span ↓Neurodegeneration ↓Oxidative stress |
22 d | Tyrosol (1 mM, p.o.) | García-Moreno et al. 2019 |
| In vitro | αSyn | ↓aSyn toxicity ↓aSyn |
24 h | Oleuropein (0.3 mg/mL) | Mohammad-Beigi et al., 2019 |
6-OHDA: 6-Hydroxydopamine; αSyn: α-synuclein; i.p.: intraperitoneal; MPP: 1-methyl-4-phenylpyridnium; MPTP: methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PD: Parkinson’s disease; p.o.: oral administration or per os.
Stilbenes
Resveratrol is the most important compound of this sub-classe and it is found mainly in grape skin. Resveratrol modulates mitochondrial function and redox biology, more likely by its acting as gene regulator (Xia et al., 2017; Jardim et al., 2018). In a study on MPTP-treated Drosophila, resveratrol increased CAT, GSH and acetylcholinesterase activities (Abolaji et al., 2018). Resveratrol significantly alleviated MPP+-induced cytotoxicity and mitochondrial dysfunction by modulating Akt/Glycogen synthase kinase-3 pathway. Indeed, the inhibition of glycogen synthase kinase-3 activity clearly abolished the protective effects of resveratrol (Zeng et al., 2017). Analogously, resveratrol administration has been demonstrated to suppress rotenone induced-neurotoxicity in PC12 cells, whereas this compound increases heat-shock 8 and 90 protein levels and protein degradation systems in parkin-mutant skin primary fibroblasts (Vergara et al., 2017). Recent evidence has indicated that also SIRT1/Akt1 pathway is involved in the neuroprotective effect performed by resveratrol (Zhang et al., 2015; Diaz-Gerevini et., 2016). Accordingly, blocking SIRT1 or Akt1 leads to a decrease of the protective effect exerted by this compound (Wang et al., 2018). Guo et al. (2016) reported that resveratrol-induced activation of SIRT1 and subsequent LC3 deacetylation in MPTP model contribute to autophagic degradation of αSyn. Furthermore, an improvement of behavioral deficits has been reported in this model. In line with this, Zhang et al. (2018) showed that a 5-week oral treatment with resveratrol improves motor performance and alleviates cognitive deficits in the A53T αSyn mice. This effect on behavior was associated with an increase of tyrosine hydroxylase levels and a reduction of neuroinflammatory process and αSyn levels in the brain, especially phospho-Ser129-αSyn form. The anti-inflammatory effects of resveratrol have been confirmed in a rat model of rotenone-induced PD (Gaballah et al., 2016). MPTP treatment in mice also affects olfactory discrimination and social recognition memory. Administration of resveratrol for 15 days restored these behavioral and neurochemical deficits and reduced the severity of neurodegenerative process in the striatum (da Rocha Lindner et al., 2015).
Oral treatment with piceid, a non-glycosylated derivative of resveratrol, prevented oxidative damage in the rotenone rat model. In particular, Chen et al. (2015b) demonstrated that piceid restores the levels of SOD, thioredoxin and GSH and decreases MDA in the striatum. At the same time, this PPH contributed to restore proper mitochondrial function by increasing ATP levels and rescued rotenone-induced dopaminergic cell loss in the SNc.
Other non-flavonoids molecules
The lignans are a large group of PPH found in plants. In a recent study, treatment with 7-hydroxymatairesinol, a lignan extracted from the heartwood of the Norway spruce (Udani et al., 2013), induced a significant reduction of the striatal damage and improvement of motor performance in rats bearing a 6-OHDA induced nigrostriatal lesion. These effects may be ascribable to a reduction of neuroinflammatory response to 6-OHDA, induced by this compound. Indeed, fewer activated glial cells (microglia and astrocytes) and modulation of microglia phenotypes, with an important reduction of the cytotoxic phenotype, were observed in animals orally treated with this PPH. In addition, a moderate reduction of pro-inflammatory mediator mRNA levels and an increase of anti-inflammatory mediators were detected (Giuliano et al., 2020).
Similarly, the PPH ellagic acid, a phenolic acid found in blackberry, improved the motor impairment via reducing the levels of neuroinflammatory biomarkers TNF-α and IL-1β and protected the brain against free radicals-induced neural damage (Farbood et al., 2015).
García-Moreno et al. (2019) demonstrated that treatment with tyrosol, one of the main PPH in olive leaf and fruit, reduced αSyn inclusions in transgenic C. elegans that expressing human αSyn. This effect resulted in a reduced dopaminergic cell loss, lower αSyn toxicity and extended life span. Tyrosol also reduced ROS levels and promoted the expression of specific chaperones and antioxidant enzymes.
Analogously, oleuropein, a phenolic compound from olive fruit extracts belonging to the sub-classe of tyrosols, reduced αSyn toxic aggregation and, consequently, counteracted αSyn cytotoxicity in SH-SY5Y cells treated with αSyn aggregates (Mohammad-Beigi et al., 2019).
Complex Mixture of Polyphenols
Fruits and vegetables contain high levels of complex mixtures of PPH (both flavonoids and non-flavonoids) that can act synergistically to provide neuroprotective effects (Table 3).
Table 3.
Summary of the effects of complex mixture of polyphenols in PD models
| In vivo/In vitro | Model | Main findings | Duration of treatment | Dose and route of administration | References |
|---|---|---|---|---|---|
| In vitro | 6-OHDA | ↓Oxidative stress ↑Cell viability |
12 h | Protocatechuic acid (0.25, 0.5, 1 mM) and Chrysin (3, 6, and 12 µM) | Zhang et al., 2015 |
| In vivo | Haloperidol and reserpine | ↑Motor performance | 0–150 min (Haloperidol) 5 d (Reserpine) | Cymbopogon citratus (100, 200, and 400 mg/kg, p.o./i.p.) | Mangrulkar and Chaple, 2019 |
| In vivo | Transgenic | ↓Mitochondrial deficit ↑Motor performance ↑Lifespan |
12 h | Grape skins (4, 8, and 16%, w/v, p.o.) | Wu et al., 2018 |
| In vitro | αSyn | ↓Oxidative stress ↓aSyn toxicity ↓aSyn ↑Autophagy efficiency |
16 h | Arbutus unedo (2 μg GAE/mL) | Macedo et al., 2018 |
| In vivo | αSyn | ↓aSyn ↑Lifespan ↑Lipid levels |
12 d | Alaskan bog blueberry (0, 100, 200 and 400 μg/mL, p.o.) | Maulik et al., 2018 |
| In vivo | MnCl2 | ↑Motor performance ↑Memory performance ↓Neurodegeneration ↓Neuroinflammation |
4 mon | Alaskan bog blueberry (5% of the total food pellet weight, p.o.) | Maulik et al., 2019 |
6-OHDA: 6-Hydroxydopamine; GAE: gallic acid eqivalents; i.p.: intraperitoneal; PD: Parkinson’s disease; p.o.: oral administration or per os.
Zhang et al. (2015) demonstrated in vitro synergistic neuroprotective effects of chrysin (flavones) and protocatechuic acid (phenolic acid), two PPH identified in the fruits of Alpinia oxyphylla. In particular, the co-treatment resulted in greater cell viability with decreased lactate dehydrogenase release in 6-OHDA-treated PC12 cells. These cytoprotective effects could be related to the decrease of kappa-light-chain-enhancer of activated B expression and an increased transcriptional activity of nuclear factor erythroid 2-related factor 2, with consequent upregulated expression of antioxidant enzymes (SOD, CAT and GSH) and reduction of MDA levels (Zhang et al., 2015).
Mangrulkar and Chaple (2019) have recently demonstrated the potential of the PPH-extract of cymbopogon citratus (composed by flavonoids, tannin and phenolic acid-rich fractions) in two different animal models of PD (haloperidol and reserpine). The treatment with this extract reduced motor deficits by restoring DA levels and improving the effectiveness of antioxidant system.
Wu et al. (2018) demonstrated the benefits of daily treatment with grape skin extract, which contains PPH such as resveratrol (stilbenes) proanthocyanidine (tannins) and quercetin (flavonols), in Drosophila melanogaster with PTEN-induced kinase 1 loss-of-function. In this latter, the loss of PTEN-induced kinase 1 function causes important motor deficit, associated with energy depletion, degeneration of dopaminergic neurons and shortened lifespan (Yang et al., 2006). Notably, consumption of this extract protected mitochondrial structure and extended lifespan with an important effect on motor function (Wu et al., 2018).
PPH-digested metabolites from arbutus unedo (including flavanols, flavonols, ellagitannins and phenolic acids) have been shown to reduce oxidative and endoplasmic reticulum stress and mitochondrial impairment in PD cell models by reducing aSyn aggregation (Macedo et al., 2018).
Similarly, Maulik et al. (2018) demonstrated the ability of the crude extract of Alaskan bog blueberry (phenolic, anthocyanin and flavonoids), from vaccinum uliginosum, to attenuate human αSyn aggregation and improve motility in a transgenic C. elegans model that expressed αSyn. Maulik et al. (2018)indicated that the reduction of gene expression of NAD-dependent protein deacetylase sir-2.1 (ortholog of mammalian Sirtuin 1) could be a potential mechanism through which blueberry exerts its beneficial effects (Maulik et al., 2018). In a more recent in vivo study, Maulik et al. (2019) evaluated the interplay between dietary fat and extract of Alaskan bog blueberry supplementation on manganese-induced neurotoxicity in mice. They demonstrated that low-fat or normal-fat diets, in combination with extract supplementation, improved motor performance and attenuated the molecular hallmarks of neurotoxicity. However, when animal are fed with a high-fat diet, the therapeutic effects of the extract were suppressed, demonstrating the importance of including PPH in low-fat diet to counteract age-related neurodegenerative disorders.
Conclusion
PD is a neurodegenerative disorder with a remarkable impact on the patient’s quality of life and on the economic and welfare burden that weighs on family caregivers. Current therapeutic strategies for PD are mainly symptomatic and hampered by important side effects. It is therefore essential to promote the development of new therapeutic strategies aimed to interrupt or, at least, slow down the neurodegenerative process and thus the onset or progression of symptoms. Compounds able to positively modulate glial cells activity, reduce ROS levels and enhance autophagy may represent a valuable innovation in the treatment of PD. The in vivo and in vitro experimental results reported in the current review suggest that several PPH molecules could be protective against the pathogenic processes implicated in PD. A distinctive trait of these compounds deserving attention is their multi-target activity. Indeed, each PPH molecule may act simultaneously on different pathomechanisms, promoting the restoration of oxidative homeostasis, the reduction of the neuroinflammatory process and the enhancement of αSyn clearance. All together, these effects bring to a reduction of the neurodegenerative process in the nigrostriatal pathway with a consequent improvement of motor performance and, apparently, without side effects (Figure 1).
Figure 1.
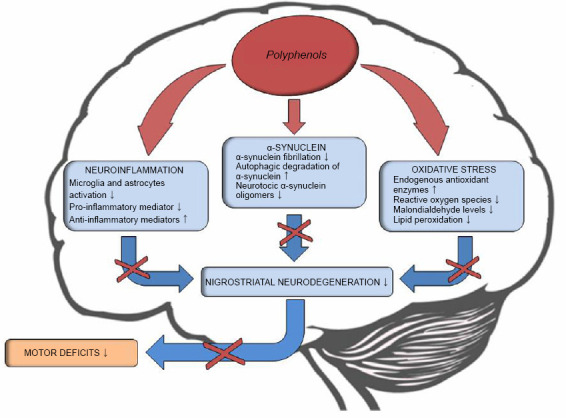
Potential effects of polyphenols in Parkinson’s disease.
Several pathobiological mechanisms, including neuroinflammation, α-synuclein accumulation and aggregation and oxidative stress play an important role in the pathogenesis of Parkinson’s disease. Polyphenols might mitigate these processes counteracting neurodegeneration within the nigrostriatal pathway.
Further studies are needed to investigate new formulations and route of administration for these molecules in order to ameliorate their bioavailability and, consequently, to maximize their biological activity in view of their potential use in PD therapy.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 2.Abolaji AO, Adedara AO, Adie MA, Vicente-Crespo M, Farombi EO. Resveratrol prolongs lifespan and improves 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced oxidative damage and behavioural deficits in Drosophila melanogaster. Biochem Biophys Res Commun. 2018;503:1042–1048. doi: 10.1016/j.bbrc.2018.06.114. [DOI] [PubMed] [Google Scholar]
- 3.Albani D, Polito L, Signorini A, Forloni G. Neuroprotective properties of resveratrol in different neurodegenerative disorders. Biofactors. 2010;36:370–376. doi: 10.1002/biof.118. [DOI] [PubMed] [Google Scholar]
- 4.Anekonda TS. Resveratrol--a boon for treating Alzheimer’s disease. Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Aquilano K, Baldelli S, Rotilio G, Ciriolo MR. Role of nitric oxide synthases in Parkinson’s disease: a review on the antioxidant and anti-inflammatory activity of polyphenols. Neurochem Res. 2008;33:2416–2426. doi: 10.1007/s11064-008-9697-6. [DOI] [PubMed] [Google Scholar]
- 6.Bhullar KS, Rupasinghe HP. Polyphenols: multipotent therapeutic agents inneurodegenerative diseases. Oxid Med Cell Longev. 2013;2013:891748. doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur J Neurosci. 2007;25:397–405. doi: 10.1111/j.1460-9568.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- 8.Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8:189–201. doi: 10.1007/s11481-013-9435-y. [DOI] [PubMed] [Google Scholar]
- 9.Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR. Oxidative stress and Parkinson’s disease. Front Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alphasynuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M, Wang T, Yue F, Li X, Wang P, Li Y, Chan P, Yu S. Tea polyphenols alleviate motor impairments, dopaminergic neuronal injury, and cerebral α-synuclein aggregation in MPTP-intoxicated parkinsonian monkeys. Neuroscience. 2015a;286:383–392. doi: 10.1016/j.neuroscience.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, de Silva HA, Pettenati MJ, Rao PN, St George-Hyslop P, Roses AD, Xia Y, Horsburgh K, Uéda K, Saitoh T. The human NACP/alpha-synuclein gene: chromosome assignment to 4q21.3-q22 and TaqI RFLP analysis. Genomics. 1995;26:425–427. doi: 10.1016/0888-7543(95)80237-g. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Zhang DQ, Liao Z. Anti-oxidant polydatin (piceid) protects against substantia nigral motor degeneration in multiple rodent models of Parkinson’s disease. Mol Neurodegener. 2015b;10:4. doi: 10.1186/1750-1326-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YM, Liu SP, Lin HL, Chan MC, Chen YC, Huang YL, Tsai MC, Fu RH. Irisflorentin improves α-synuclein accumulation and attenuates 6-OHDA-induced dopaminergic neuron degeneration, implication for Parkinson’s disease therapy. Biomedicine (Taipei) 2015c;5:4. doi: 10.7603/s40681-015-0004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 16.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 17.da Rocha Lindner G, Bonfanti Santos D, Colle D, Gasnhar Moreira EL, Daniel Prediger R, Farina M, Khalil NM, Mara Mainardes R. Improved neuroprotective effects of resveratrol-loaded polysorbate 80-coated poly(lactide) nanoparticles in MPTP-induced Parkinsonism. Nanomedicine (Lond) 2015;10:1127–1138. doi: 10.2217/nnm.14.165. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Gerevini GT, Repossi G, Dain A, Tarres MC, Das UN, Eynard AR. Beneficial action of resveratrol: How and why. Nutrition. 2016;32:174–178. doi: 10.1016/j.nut.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 19.El-Horany HE, El-Latif RN, ElBatsh MM, Emam MN. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: modulating autophagy (quercetin on experimental Parkinson’s disease) J Biochem Mol Toxicol. 2016;30:360–369. doi: 10.1002/jbt.21821. [DOI] [PubMed] [Google Scholar]
- 20.Engelender S. alpha-synuclein fate: Proteasome or autophagy. Autophagy. 2012;8:418–420. doi: 10.4161/auto.19085. [DOI] [PubMed] [Google Scholar]
- 21.Farbood Y, Sarkaki A, Dolatshahi M, Taqhi Mansouri SM, Khodadadi A. Ellagic acid protects the brain against 6-hydroxydopamine induced neuroinflam-mation in a rat model of parkinson’s disease. Basic Clin Neurosci. 2015;6:83–89. [PMC free article] [PubMed] [Google Scholar]
- 22.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek Z, Dickson D, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 23.Fatima A, Khanam S, Rahul R, Jyoti S, Naz F, Ali F, Siddique YH. Protective effect of tangeritin in transgenic Drosophila model of Parkinson’s disease. Front Biosci (Elite Ed) 2017;9:44–53. doi: 10.2741/e784. [DOI] [PubMed] [Google Scholar]
- 24.Feng Y, Wang XP, Yang SG, Wang YJ, Zhang X, Du XT, Sun XX, Zhao M, Huang L, Liu RT. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology. 2009;30:986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 25.Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: a review. Molecules. 2011;16:1486–1507. doi: 10.3390/molecules16021486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frolinger T, Sims S, Smith C, Wang J, Cheng H, Faith J, Ho L, Hao K, Pasinetti GM. The gut microbiota composition affects dietary polyphenols-mediated cognitive resilience in mice by modulating the bioavailability of phenolic acids. Sci Rep. 2019;9:3546. doi: 10.1038/s41598-019-39994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaba B, Khan T, Haider MF, Alam T, Baboota S, Parvez S, Ali J. Vitamin E loaded naringenin nanoemulsion via intranasal delivery for the management of oxidative stress in a 6-OHDA Parkinson’s disease model. Biomed Res Int. 2019;2019:2382563. doi: 10.1155/2019/2382563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaballah HH, Zakaria SS, Elbatsh MM, Tahoon NM. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem Biol Interact. 2016;251:10–16. doi: 10.1016/j.cbi.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 29.Gao L, Li C, Yang RY, Lian WW, Fang JS, Pang XC, Qin XM, Liu AL, Du GH. Ameliorative effects of baicalein in MPTP-induced mouse model of Parkinson’s disease: A microarray study. Pharmacol Biochem Behav. 2015;133:155–163. doi: 10.1016/j.pbb.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Moreno JC, Porta de la Riva M, Martínez-Lara E, Siles E, Cañuelo A. Tyrosol, a simple phenol from EVOO, targets multiple pathogenic mechanisms of neurodegeneration in a C. elegans model of Parkinson’s disease. Neurobiol Aging. 2019;82:60–68. doi: 10.1016/j.neurobiolaging.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Ghaffari F, Hajizadeh Moghaddam A, Zare M. Neuroprotective effect of quercetin nanocrystal in a 6-hydroxydopamine model of Parkinson disease: biochemical and behavioral evidence. Basic Clin Neurosci. 2018;9:317–324. doi: 10.32598/bcn.9.5.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giuliano C, Siani F, Mus L, Ghezzi C, Cerri S, Pacchetti B, Bigogno C, Blandini F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition. 2020;69:110494. doi: 10.1016/j.nut.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Gunn CA, Weber JL, McGill AT, Kruger MC. Increased intake of selected vegetables, herbs and fruit may reduce bone turnover in post-menopausal women. Nutrients. 2015;7:2499–2517. doi: 10.3390/nu7042499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo YJ, Dong SY, Cui XX, Feng Y, Liu T, Yin M, Kuo SH, Tan EK, Zhao WJ, Wu YC. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Mol Nutr Food Res. 2016;60:2161–2175. doi: 10.1002/mnfr.201600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 36.Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, de Oliveira MR. Resveratrol and brain mitochondria: a review. Mol Neurobiol. 2018;55:2085–2101. doi: 10.1007/s12035-017-0448-z. [DOI] [PubMed] [Google Scholar]
- 37.Kiasalari Z, Khalili M, Baluchnejadmojarad T, Roghani M. Protective effect of oral hesperetin against unilateral striatal 6-hydroxydopamine damage in the rat. Neurochem Res. 2016;41:1065–1072. doi: 10.1007/s11064-015-1796-6. [DOI] [PubMed] [Google Scholar]
- 38.Kim HD, Jeong KH, Jung UJ, Kim SR. Naringin treatment induces neuroprotective effects in a mouse model of Parkinson’s disease in vivo, but not enough to restore the lesioned dopaminergic system. J Nutr Biochem. 2016;28:140–146. doi: 10.1016/j.jnutbio.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 39.Koutsos A, Tuohy KM, Lovegrove JA. Apples and cardiovascular health—Is the gut microbiota a core consideration. Nutrients. 2015;7:3959–3998. doi: 10.3390/nu7063959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, Przuntek H, Epplen JT, Schöls L, Riess O. Ala30Pro mutation in the gene encoding alphasynuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 41.Kwon SH, Lee SR, Park YJ, Ra M, Lee Y, Pang C, Kim KH. Suppression of 6-hydroxydopamine-induced oxidative stress by hyperoside via activation of Nrf2/HO-1 signaling in dopaminergic neurons. Int J Mol Sci. 2019 doi: 10.3390/ijms20235832. doi: 103390/ijms20235832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee E, Park HR, Ji ST, Lee Y, Lee J. Baicalein attenuates astroglial activation in the 1-methyl-4-phenyl-1, 2, 3, 4-tetrahydropyridine-induced Parkinson’s disease model by downregulating the activations of nuclear factor-κB, ERK, and JNK. J Neurosci Res. 2014;92:130–139. doi: 10.1002/jnr.23307. [DOI] [PubMed] [Google Scholar]
- 43.Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson’s disease. J Neuroimmune Pharmacol. 2009;4:419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee Y, Lee S, Chang SC, Lee J. Significant roles of neuroinflammation in Parkinson’s disease: therapeutic targets for PD prevention. Arch Pharm Res. 2019;42:416–425. doi: 10.1007/s12272-019-01133-0. [DOI] [PubMed] [Google Scholar]
- 45.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L. Cytotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin CW, Wu MJ, Liu IY, Su JD, Yen JH. Neurotrophic and cytoprotective action of luteolin in PC12 cells through ERK-dependent induction of Nrf2-driven HO-1 expression. J Agric Food Chem. 2010;58:4477–4486. doi: 10.1021/jf904061x. [DOI] [PubMed] [Google Scholar]
- 47.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinfl ammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S, Ninan I, Antonova I, Battaglia F, Trinchese F, Narasanna A, Kolodilov N, Dauer W, Hawkins RD, Arancio O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147:S232–240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maatouk M, Elgueder D, Mustapha N, Chaaban H, Bzéouich IM, Loannou I, Kilani S, Ghoul M, Ghedira K, Chekir-Ghedira L. Effect of heated naringenin on immunomodulatory properties and cellular antioxidant activity. Cell Stress Chaperones. 2016;21:1101–1109. doi: 10.1007/s12192-016-0734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macedo D, Jardim C, Figueira I, Almeida AF, McDougall GJ, Stewart D, Yuste JE, Tomás-Barberán FA, Tenreiro S, Outeiro TF, Santos CN. (Poly)phenol-digested metabolites modulate alpha-synuclein toxicity by regulating proteostasis. Sci Rep. 2018;8:6965. doi: 10.1038/s41598-018-25118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 53.Mangrulkar S, Chaple D. Pharmacological assessments of polyphenolic extract of Cymbopogon citratus leaves in rodent model of parkinson’s disease. Drug Deliv Transl Res. 2019;9:311–315. [Google Scholar]
- 54.Mani S, Sekar S, Barathidasan R, Manivasagam T, Thenmozhi AJ, Sevanan M, Chidambaram SB, Essa MM, Guillemin GJ, Sakharkar MK. Naringenin decreases α-synuclein expression and neuroinflammation in MPTP-induced Parkinson’s disease model in mice. Neurotox Res. 2018;33:656–670. doi: 10.1007/s12640-018-9869-3. [DOI] [PubMed] [Google Scholar]
- 55.Maulik M, Mitra S, Hunter S, Hunstiger M, Oliver SR, Bult-Ito A, Taylor BE. Sir-2, 1 mediated attenuation of α-synuclein expression by Alaskan bog blueberry polyphenols in a transgenic model of Caenorhabditis elegans. Sci Rep. 2018;8:10216. doi: 10.1038/s41598-018-26905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maulik M, Mitra S, Sweeney M, Lu B, Taylor BE, Bult-Ito A. Complex interaction of dietary fat and Alaskan bog blueberry supplementation influences manganese mediated neurotoxicity and behavioral impairments. J Funct Foods. 2019;53:306–317. doi: 10.1016/j.jff.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 58.Mohammad-Beigi H, Aliakbari F, Sahin C, Lomax C, Tawfike A, Schafer NP, Amiri-Nowdijeh A, Eskandari H, Møller IM, Hosseini-Mazinani M, Christiansen G, Ward JL, Morshedi D, Otzen DE. Oleuropein derivatives from olive fruit extracts reduce α-synuclein fibrillation and oligomer toxicity. J Biol Chem. 2019;294:4215–4232. doi: 10.1074/jbc.RA118.005723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murphy DD, Rueter SM, Trojanowski JQ, Lee VM. Synucleins are developmentally expressed, and alphasynuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oguido APMT, Hohmann MSN, Pinho-Ribeiro FA, Crespigio J, Domiciano TP, Verri WA, Jr, Casella AMB. Naringenin eye drops inhibit corneal neovascularization by anti-inflammatory and antioxidant mechanisms. Invest Ophthalmol Vis Sci. 2017;58:5764–5776. doi: 10.1167/iovs.16-19702. [DOI] [PubMed] [Google Scholar]
- 61.Polymeropoulos C, Lavedan E, Leroy SE, Ide A, Dehejia A, Dutra B, Pike H, Root J, Rubenstein R, Boyer ES, Stenroos S, Chandrasekharappa A, Athanassiadou T, Papapetropoulos WG, Johnson AM, Lazzarini RC, Duvoisin G, Di Iorio LI, Golbe RL Nussbaum. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 62.Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative, stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodriguez-Mateos A (2013) Intake and time dependence of blueberry favonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am J Clin Nutr. 98:1179–1191. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 64.Shakeel S, Rehman MU, Tabassum N, Amin U, Mir MUR. Effect of naringenin (A naturally occurring flavanone) against pilocarpine-induced status epilepticus and oxidative stress in mice. Pharmacogn Mag. 2017;13:S154–S160. doi: 10.4103/0973-1296.203977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma S, Raj K, Singh S. Neuroprotective effect of quercetin in combination with piperine against rotenone- and iron supplement-induced Parkinson’s disease in experimental rats. Neurotox Res. 2020;37:198–209. doi: 10.1007/s12640-019-00120-z. [DOI] [PubMed] [Google Scholar]
- 66.Singh PK, Kotia V, Ghosh D, Mohite GM, Kumar A, Maji SK. Curcumin modulates a-synuclein aggregation and toxicity. ACS Chem Neurosci. 2013;4:393–407. doi: 10.1021/cn3001203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Singh S, Kumar P. Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: Biochemical and neurochemical evidences. Neurosci Res. 2018;133:38–47. doi: 10.1016/j.neures.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Singleton K, Gwinn-Hardy Y, Sharabi ST, Li C, Holmes R, Dendi J, Hardy A, Crawley DS, Goldstein Association between cardiac denervation and parkinsonism caused by alpha-synuclein gene triplication. Brain. 2004;127:768–772. doi: 10.1093/brain/awh081. [DOI] [PubMed] [Google Scholar]
- 69.Singleton M, Farrer J, Johnson A, Singleton S, Hague J, Kachergus M, Hulihan T, Peuralinna A, Dutra R, Nussbaum S, Lincoln A, Crawley M, Hanson D, Maraganore C, Adler MR, Cookson M, Muenter M, Baptista D, Miller J, Blancato J, et al. alpha-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 70.Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sugumar M, Sevanan M, Sekar S. Neuroprotective effect of naringenin against MPTP-induced oxidative stress. Int J Neurosci. 2019;129:534–539. doi: 10.1080/00207454.2018.1545772. [DOI] [PubMed] [Google Scholar]
- 72.Udani JK, Brown DJ, Tan MO, Hardy M. Pharmacokinetics and bioavailability of plant lignan 7-hydroxymatairesinol and effects on serum enterolactone and clinical symptoms in postmenopausal women: a single-blinded, parallel, dose-comparison study. J Am Coll Nutr. 2013;32:428–435. doi: 10.1080/07315724.2013.849578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vergara D, Gaballo A, Signorile A, Ferretta A, Tanzarella P, Pacelli C, Di Paola M, Cocco T, Maffia M. Resveratrol modulation of protein expression in parkin-mutant human skin fibroblasts: a proteomic approach. Oxid Med Cell Longev. 2017;2017:2198243. doi: 10.1155/2017/2198243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Visioli F, De La Lastra CA, Andres-Lacueva C, Aviram M, Calhau C, Cassano A, D'Archivio M, Faria A, Favé G, Fogliano V, Llorach R, Vitaglione P, Zoratti M, Edeas M. Polyphenols and human health: a prospectus. Crit Rev Food Sci Nutr. 2011;51:524–546. doi: 10.1080/10408391003698677. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Dong X, Liu Z, Zhu S, Liu H, Fan W, Hu Y, Hu T, Yu Y, Li Y, Liu T, Xie C, Gao Q, Li G, Zhang J, Ding Z, Sun J. Resveratrol suppresses rotenone-induced neurotoxicity through activation of SIRT1/Akt1 signaling pathway. Anat Rec (Hoboken) 2018;301:1115–1125. doi: 10.1002/ar.23781. [DOI] [PubMed] [Google Scholar]
- 76.Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ. Yeast genes that enhance the toxicity of a mutant huntingtin fragment or alpha-synuclein. Science. 2003;302:1769–1772. doi: 10.1126/science.1090389. [DOI] [PubMed] [Google Scholar]
- 77.Wu HC, Hu QL, Zhang SJ, Wang YM, Jin ZK, Lv LF, Zhang S, Liu ZL, Wu HL, Cheng OM. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen Res. 2018;13:1375–1383. doi: 10.4103/1673-5374.235250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu Z, Wu A, Dong J, Sigears A, Lu B. Grape skin extract improves muscle function and extends lifespan of a Drosophila model of Parkinson’s disease through activation of mitophagy. Exp Gerontol. 2018;113:10–17. doi: 10.1016/j.exger.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease – a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 80.Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Q, Langley M, Kanthasamy AG, Reddy MB. Epigallocatechin gallate has a neurorescue effect in a mouse model of Parkinson disease. J Nutr. 2017;147:1926–1931. doi: 10.3945/jn.117.255034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xue X, Liu H, Qi L, Li X, Guo C, Gong D, Qu H. Baicalein ameliorated the upregulation of striatal glutamatergic transmission in the mice model of Parkinson’s disease. Brain Res Bull. 2014;103:54–59. doi: 10.1016/j.brainresbull.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 83.Yang Y, Gehrke S, Imai Y, Huang Z, Ouyang Y, Wang JW, Yang L, Beal MF, Vogel H, Lu B. Mitochondrial pathology and muscle and dopaminergic neuron degeneration caused by inactivation of Drosophila Pink1 is rescued by Parkin. Proc Natl Acad Sci U S A. 2006;103:10793–10798. doi: 10.1073/pnas.0602493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Z, Kuboyama T, Tohda C. Naringenin promotes microglial M2 polarization and Aβ degradation enzyme expression. Phytother Res. 2019;33:1114–1121. doi: 10.1002/ptr.6305. [DOI] [PubMed] [Google Scholar]
- 85.Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of alpha-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yong HYF, Rawji KS, Ghorbani S, Xue M, Yong VW. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol Immunol. 2019;16:540–546. doi: 10.1038/s41423-019-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zarranz J, Alegre JC, Gomez-Esteban E, Lezcano R, Ros I, Ampuero L, Vidal J, Hoenicka O, Rodriguez B, Atares V, Llorens E, Gomez Tortosa T, del Ser DG, Munoz JG, de Yebenes The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 88.Zeng W, Zhang W, Lu F, Gao L, Gao G. Resveratrol attenuates MPP(+)-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3β pathway in SN4741 cells. Neurosci Lett. 2017;637:50–56. doi: 10.1016/j.neulet.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 89.Zhang LF, Yu XL, Ji M, Liu SY, Wu XL, Wang YJ, Liu RT. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018;9:6414–6426. doi: 10.1039/c8fo00964c. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y, Chen ML, Zhou Y, Yi L, Gao YX, Ran L, Chen SH, Zhang T, Zhou X, Zou D, Wu B, Wu Y, Chang H, Zhu JD, Zhang QY, Mi MT. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr Food Res. 2015;59:1443–1457. doi: 10.1002/mnfr.201500016. [DOI] [PubMed] [Google Scholar]
- 91.Zhang Z, Li G, Szeto SSW, Chong CM, Quan Q, Huang C, Cui W, Guo B, Wang Y, Han Y, Michael Siu KW, Yuen Lee SM, Chu IK. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic Biol Med. 2015;84:331–343. doi: 10.1016/j.freeradbiomed.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 92.Zhao J, Xu L, Liang Q, Sun Q, Chen C, Zhang Y, Ding Y, Zhou P. Metal chelator EGCG attenuates Fe(III)-induced conformational transition of α-synuclein and protects AS-PC12 cells against Fe(III)-induced death. J Neurochem. 2017;143:136–146. doi: 10.1111/jnc.14142. [DOI] [PubMed] [Google Scholar]
- 93.Zheng ZV, Cheung CY, Lyu H, Chan HY, Li Y, Bian ZX, Wang KKW, Poon WS. Baicalein enhances the effect of low dose Levodopa on the gait deficits and protects dopaminergic neurons in experimental Parkinsonism. J Clin Neurosci. 2019;4:242–251. doi: 10.1016/j.jocn.2019.02.005. [DOI] [PubMed] [Google Scholar]


