Abstract
A major feature of neurodegeneration is disruption of central nervous system homeostasis, during which microglia play diverse roles. In the central nervous system, microglia serve as the first line of immune defense and function in synapse pruning, injury repair, homeostasis maintenance, and regulation of brain development through scavenging and phagocytosis. Under pathological conditions or various stimulations, microglia proliferate, aggregate, and undergo a variety of changes in cell morphology, immunophenotype, and function. This review presents the features of microglia, especially their diversity and ability to change dynamically, and reinterprets their role as sensors for multiple stimulations and as effectors for brain aging and neurodegeneration. This review also summarizes some therapeutic approaches for neurodegenerative diseases that target microglia.
Keywords: central nervous system, microglia, neurodegeneration, neuroinflammation, plasticity
Introduction
Disruption of central nervous system (CNS) homeostasis leads to the development of neurodegenerative diseases (Kabba et al., 2018). Microglia, often referred to as CNS macrophages, have emerged as an essential component in innate immunity and they play a vital role in CNS development, health, response to injuries, and neurodegenerative diseases. They have immense phenotypic diversity with age and in response to disease (Figure 1). During development, microglia scavenge and phagocytose foreign materials that threaten the CNS, prune synapses of neural circuits, and maintain homeostasis. They are producers and targets of neuroprotective factors that are produced under physiological and pathological conditions, thus reinforcing the microglial neuroprotective phenotype (Polazzi and Monti, 2010).
Figure 1.
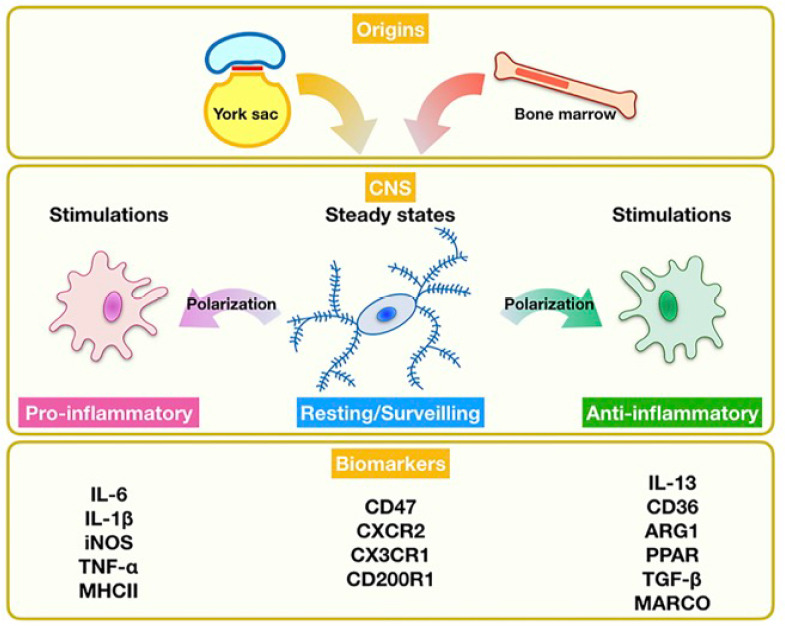
The origin and biology of microglia.
Microglia originate from the yolk sac during embryogenesis and from bone marrow during repopulation. They act as an immunological surveillant in steady states. ARG1: Arginase 1; CNS: central nervous system; CD200R1: CD200 receptor 1; CX3CR1: CX3C chemokine receptor 1; CXCR2: C-X-C motif chemokine receptor 2; IL: interleukin; iNOS: inducible nitric oxide synthase; MARCO: macrophage receptor with collagenous structure; MHCII: major histocompatibility complex II; PPAR: peroxisome proliferator-activated receptor; TGF-β: transforming growth factor-β; TNF-α: tumor necrosis factor-α.
Microglia are sensitive to many stimuli or changes in the microenvironment of the CNS. As an effector, microglia affect the development of neuronal networks and the progress of many diseases, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), Huntington’s disease, stroke, epilepsy, autism spectrum disorder, schizophrenia, and the affective disorders (Figure 2).
Figure 2.
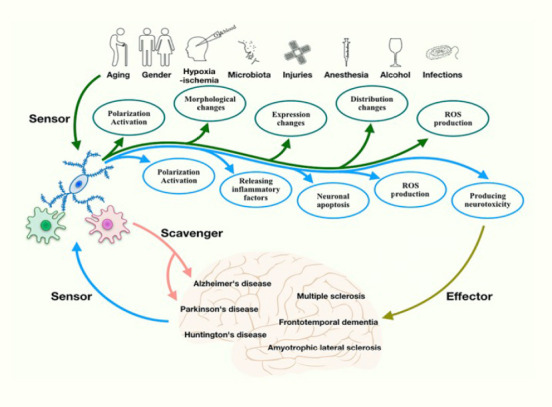
Microglia act as both sensors and effectors.
Microglia are activated, change morphology and distribution, and produce reactive oxygen species (ROS) in response to various conditions, including aging, sex, stress, injuries, infections, hypoxia-ischemia, ROS, microbiota, anesthesia and alcohol, which suggests that they can act as a sensor. Once activated, microglia can produce ROS and inflammatory factors to induce neurotoxicity and neuronal apoptosis. They can, therefore, act as an effector in neurodegenerative diseases. These diseases can further affect the function and aggregation of microglia.
All references cited in this review were retrieved by an electronic search of the PubMed database. More than 80% of all references cited were published in the past 10 years and more than 50% of them in the past 5 years. The following key search terms were used: microglia, central nervous system, neurodegeneration, neuroinflammation, plasticity, aging, hypoxia, ischemia, and therapeutic approaches.
Biology of Microglia
Origin, diversity, and relevant signaling pathways of microglia
Brain-resident microglia originate from yolk sac macrophages during embryogenesis. They then migrate to the brain (Ginhoux et al., 2010) where they account for 10% to 15% of all cells (Nayak et al., 2014) (Figure 1). The origin of repopulated microglia remained controversial until recently. It was previously believed that repopulated microglia originated from nestin-positive cells (Elmore et al., 2014). With the use of colony-stimulating factor-1 receptor (CSF1R) inhibitors, Elmore et al. (2014) observed that almost all microglia in the adult CNS were eliminated. Once these inhibitors were withdrawn, microglia rapidly repopulated, the CNS after an increase in nestin-positive cells throughout the CNS, which represent microglial progenitor cells (Elmore et al., 2014). A neuroectodermal lineage was implicated for the repopulating microglia because microglia are myeloid lineage cells and nestin-positive progenitor cells originate from the neuroectodermal lineage (Ajami et al., 2007; Ginhoux et al., 2010; Schulz et al., 2012). In contrast, Varvel et al. (2012) demonstrated that repopulated microglia are derived from myeloid progenitor cells.
However, a recent study, based on an adult mouse model, appears to disprove the above theories. Huang et al. (2018) demonstrated that repopulated microglia are not derived from blood cells, nestin-positive cells, astrocytes, oligodendrocyte precursor cells, or neurons. After selective elimination of more than 99% of microglia, the repopulated microglia originated from the proliferation of the surviving microglia (< 1%). Thus, the residual microglia are the only source of the repopulated microglia, and these newly formed microglia rapidly repopulate the whole brain (Nayak et al., 2014).
The diversity of microglia relies on genetic, epigenetic, intrinsic, and extrinsic factors (Grubman et al., 2016; Thion et al., 2018). The transcriptional profiles and motility of microglia, the effects of aging, stress, and other microenvironmental stimuli are different in various brain regions. Morphology, biomarkers, phenotypes, functions, and signaling pathways are diverse among microglia (Orihuela et al., 2016; Kierdorf and Prinz, 2017).
Microglia exhibit ‘plasticity’ and they undergo a series of morphological changes, becoming ramified, reactive, active or amoeboid, depending on their location in the brain (Lawson et al., 1990; Karperien et al., 2013; Taylor et al., 2014). Ramified microglia can also be regarded as resting microglia, with small cell bodies and long processes. Resting microglia are not passive in the CNS, rather they monitor the condition of the CNS (Karperien et al., 2013; Taylor et al., 2014; Prinz et al., 2019). Steady state microglia survey the surrounding microenvironment and maintain homeostasis via receptors for CX3C-chemokine ligand 1 (CX3CL1) (also called fractalkine), CD47, CD200, and CD22 (Nimmerjahn et al., 2005; Sierra et al., 2010; Tremblay et al., 2010; Thion et al., 2018). Microglia promote the release of inflammatory mediators, chemokines, proteases, and present antigens (Orihuela et al., 2016). The anti-inflammatory microglia express cytokines, such as interleukin (IL)-4, IL-5, IL-10, IL-13, glucocorticoids, transforming growth factor-β, and wound-healing genes, including arginase-1, CD36, CD163, macrophage receptor with collagenous structure, nerve and insulin growth factors, and peroxisome proliferator-activated receptor (Figure 2) (Mantovani et al., 2002; Nimmerjahn et al., 2005; Sierra et al., 2010; Tremblay et al., 2010; Orihuela et al., 2016; Thion et al., 2018). Advances in genome-wide expression profiling and computational biology, together with germline and epigenetic analyses, will facilitate better understanding of microglia and their crucial functions (Link et al., 2015; Romanoski et al., 2015; Amit et al., 2016; Crotti and Ransohoff, 2016).
Microglia are functionally diverse and are the primary immune defense in the CNS. They promote regrowth and remapping of damaged neural circuitry through synaptic pruning, and provide support for neurons (Gehrmann et al., 1995; Streit, 2006; Perry et al., 2010). They have an impact on the developing cerebral cortex through selective settlement in the main sub-ventricular zone and the phagocytosis of neural precursor cells as neurogenesis nears completion (Cunningham et al., 2013). Microglia continuously monitor the microenvironment by selectively responding to intercellular molecules. Resting microglia regulate their phenotypes to adapt to the microenvironment of the CNS, and are sensitive to any change in the extracellular microenvironment or pathological imbalance (Colonna and Butovsky, 2017).
Microglia are regulated and activated by various signaling pathways, including the nuclear factor kappa B (NF-κB), Toll-like receptors (TLRs), mitogen-activated protein kinases (MAPKs), Janus protein tyrosine kinase-signal transducers and activators of transcription, peroxisome proliferator-activated receptor, Notch, and the fractalkine–fractalkine receptor (CX3CL1–CX3CR1) signaling pathways (Cardona et al., 2006; Bensinger and Tontonoz, 2008; Thurston and Kitajewski, 2008; Hanamsagar et al., 2012; Frakes et al., 2014; Mathur et al., 2017; Younger et al., 2019). TLRs activate pro-IL-1β and pro-IL-18, which then develop into their active forms through the inflammasome (Kawai and Akira, 2010).
Microglial homeostasis and dynamics
In a healthy brain, microglia constantly seek signs of damage and debris. To achieve this, they are highly mobile through their dynamic processes (Davalos et al., 2005; Nimmerjahn et al., 2005). Powerful imaging techniques enable a comprehensive understanding of microglial dynamics in vivo (Wake et al., 2009; Tremblay et al., 2010).
Time-lapse imaging techniques have been applied to investigate microglial dynamics. These studies have shown the origin of microglia and their differentiation characteristics. For example, microglia may change to a ramified form under highly dynamic conditions (Goldmann et al., 2016). Long-range non-invasive imaging of the dynamics of microglial precursors at a high spatio-temporal resolution has been especially informative. The transparent nature of the zebrafish brain makes it a perfect model to explore microglial dynamics during early development using advanced imaging techniques (Peri and Nüsslein-Volhard, 2008; Li et al., 2012). Time-lapse imaging techniques can be used in zebrafish to track the dynamic colonization of the brain by microglia. This colonization involves neuronal cell death, with lysophosphatidylcholine helping to maintain microglial homeostasis, which reduces the damage caused by the entrance of microglia precursor cells into the brain (Xu et al., 2016). The number of microglia can be increased by ultraviolet-irradiation-induced or ectopically-induced apoptosis (Casano et al., 2016). After entry into the brain, microglial proliferation in situ contributes to the expansion of the microglia population (Du and Du, 2016). During embryogenesis, CNS-associated macrophages maintain a steady state through homeostatic proliferation (Tay et al., 2017). CSF1R, binding colony stimulating factor 1, and IL-34 are vital for microglial development because they transmit intracellular signals and activate kinases that regulate extracellular signals, which are beneficial for the proliferation and survival of microglia (Greter et al., 2005; Wang et al., 2012; Guan et al., 2016).
Microglia: Active Sensors Linking the Extrinsic Environment to Central Nervous System Homeostasis
Microglia, as sensors, are sensitive to aging, sex hormones, stress, injury, infection, ROS, hypoxia, microbiota, anesthesia, alcohol, and other stimuli (Figure 2 and Table 1).
Table 1.
Microglia act as sensors to multiple stimulations
| Stimulations | Biomarkers | Functions | References |
|---|---|---|---|
| Aging | IL-1β, IL-12, IL-23, TNF-α, iNOS, CD40, MHCI, MHCII and TREM2 | Lead to telomere shortening, DNA damage, and oxidative stress; Give rise to an increase in inflammatory states in the retina; impede spatial learning; lose inhibitory ligand-receptor correlations; accumulate misfolded proteins | Vijg and Campisi, 2008; Xu et al., 2008; Perry and Holmes, 2014; Rawji et al., 2016; Scheffold et al., 2016; Niraula et al., 2017; Wolf et al., 2017 |
| Stress | IL-1β, TNF-α, IL-6, CCL2, MHCII, TLR4 and CD14 | Aggravate behavioral abnormalities and neuroimmune responses | Frank et al., 2007; Wohleb et al., 2011; Prinz and Priller, 2014; Ramirez et al., 2015; Ramirez et al., 2016 |
| Injuries | TLR, RAGE, IL-1β and TNF-α; IL-4, IL-10 and TGF | Scavenge cell debris; long-term “innate immune memory” | Nimmerjahn et al., 2005; Haynes et al., 2006; Kono and Rock, 2008; Salminen et al., 2009; Perry et al., 2010 |
| Infections | MHCII, CD163, IL-1, TNF-α, S100β, IL-8, IL-6, CCL2/MCP-1 and CCL5/RANTES | Affect the apoptosis and survival pathway of microglia | Valle et al., 2004; Schwarcz et al., 2012; Chen et al., 2017 |
| Hypoxia | IL-1β, IL-6, TNF-α and TLR4 | Promote cell death and inflammation | Pineau and Lacroix, 2009; Tschopp and Schroder, 2010; Lim and Pack, 2014; McDonough et al., 2017; Cengiz et al., 2019 |
| ROS | TNF-α, IL-1β, iNOS, prostaglandin E2, and MCP-1 | Damage cellular molecules; change the structure of membrane | Naik and Dixit, 2011; Yang et al., 2011; D’Amico et al., 2013; Qin et al., 2013; Kierdorf and Prinz, 2017 |
| Microbiota | Slight increasing CSF1R and CD31 | Maintain homeostasis | Berer et al., 2011; Kamada et al., 2013; Miron et al., 2013; Braniste et al., 2014; Yano et al., 2015; Matcovitch-Natan et al., 2016 |
CCL2: CC-chemokine ligand 2; CCL5: CC-chemokine ligand 5; CSF1R: colony-stimulating factor-1 receptor; IL: interleukin; iNOS: inducible nitric oxide synthase; MCP-1: monocyte chemoattractant protein-1; MHC: major histocompatibility complex; RAGE: advanced glycation end products; RANTES: regulated upon activation, normal T cell expressed and secreted; ROS: reactive oxygen species; S100β: S100 calcium-binding protein β; TGF: transforming growth factor; TLR: Toll-like receptor; TNF-α: tumor necrosis factor-α; TREM2: triggering receptor expressed in the myeloid cell 2.
Aging
Aging reduces the number and activity of microglia; aged microglia perform their normal functions less effectively (Rawji et al., 2016). There are fewer microglia in the aged mouse cortex compared with the young adult mouse cortex and they are smaller, less symmetrical, and more elongated with fewer ramifications (Tremblay et al., 2012). Microglia are affected by telomere shortening, DNA damage, and oxidative stress during aging. Telomeres, the ends of eukaryotic chromosomes, shorten with age and correlates with the decline in microglial self-renewal (Ajami et al., 2007; Vijg and Campisi, 2008; Scheffold et al., 2016; Niraula et al., 2017; Wolf et al., 2017). Aged microglia are less restricted from the outer retina, leading to an increase in its inflammatory state (Xu et al., 2008). Aging causes microglia to acquire intracellular auto-fluorescent lipofuscin deposits, which adversely affect vision (Xu et al., 2008). Aged microglia express increased tumor necrosis factor-α (TNF-α) and IL-6 and show dystrophic morphology, which impedes spatial learning (Niraula et al., 2017). Senescent microglia display increased levels of pro-inflammatory cytokines and reduced levels of chemokines, and decreased phagocytosis of amyloid beta (Aβ) fibrils (Niraula et al., 2017). Microglia exhibit a ‘primed’ phenotype, characterized by an augmented and out-of-control inflammatory response to an immune stimulus (Perry and Holmes, 2014).
The triggering receptor expressed on myeloid cells 2 (TREM2) pathway involves down-regulation of microglia checkpoints, which has significance for therapeutic approaches to AD and other neurodegenerative diseases (Keren-Shaul et al., 2017; Krasemann et al., 2017) that involve microglial responses triggered by energy metabolism (Ulland et al., 2017). Microglia experience distinct senescence, which inhibits the regenerative and repair response in the aging CNS (Shaw et al., 2013). Furthermore, microglial dynamics are modulated by sensory experience and/or neuronal activity (Tremblay et al., 2010; Bohlen et al., 2017). A significant association exists between microglia and late-onset AD, as demonstrated by single-cell RNA sequencing (Calderon et al., 2017; Masuda et al., 2019).
Sex
Sex through sex hormones, especially estradiol, causes differences in microglia (Villa et al., 2016; Thion et al., 2018). There are more microglia within the cortex, hippocampus, and amygdala of male than female mice in early postnatal development, an effect associated with the increased expression of CC-chemokine ligand (CCL) 20 and CCL4 because testosterone, the dominant masculinizing hormone, is aromatized to estradiol in the mouse brain. In adult female mice, microglia are thicker, with longer processes in the hippocampus, cortex, and amygdala than in male mice (Wolf et al., 2017; Thion et al., 2018). In germ-free mice, sexual dimorphism between microglia is decreased (Thion et al., 2018). The results of deep sequencing show that sex has a greater impact on microglia in adulthood than during development. Immune-related genes in the microglia of adult mice are expressed more highly in females than in males (Thion et al., 2018).
Stress
Microglia can be affected by stress from the time of embryogenesis to an aged state. Prenatal stress can lead to the elevation of IL-1β, which has an effect on microglial density and development in the embryonic brain (Meyer et al., 2006; Bittle and Stevens, 2018) and can result in an increased risk of psychiatric disorders (Bittle and Stevens, 2018).
Long-term exposure to stress results in microglial hyper-activation and morphological changes, with large amoeboid cell bodies and thicker processes (Frank et al., 2007; Wohleb et al., 2011; Xu et al., 2016). Stress increases the microglial expression of pro-inflammatory factors, including TNF-α, IL-1β, IL-6, CCL2, antigen presentation molecules including major histocompatibility complex (MHC) II, and cell surface markers including TLR4 and CD14, an effect that persists for at least 24 days after stress (Frank et al., 2007; Wohleb et al., 2011; Ramirez et al., 2015, 2016).
Stress interacts with aging; exposure to psychological stressors enhances the immune response in the aged rat brain (Niraula et al., 2017). It is also proposed that stress-induced morphological activation of microglia is different in males and females. Neither acute nor chronic stress substantially affect microglia activation. In females, stress can change the proportions of microglia showing different states of morphological activation, and can alter the expression of microglia-associated genes, especially CD40 (Bollinger et al., 2016). Psychological stress can induce aging-like sensitization of microglia and increased reactivity to secondary challenges (Niraula et al., 2017). These findings reveal the different effects of stress on microglia, depending on age and sex.
Injury
Resting microglia constantly monitor the brain parenchyma (Nimmerjahn et al., 2005) and can change into their activated state following injury. Microglia quickly respond to damage-associated molecular pattern molecules generated by the rapid degeneration of neurons and neuronal processes (Kono and Rock, 2008; Scheffold et al., 2016) and microglial processes rapidly move to the site of the injury (Davalos et al., 2005; Sieger et al., 2012). Damage-associated molecular pattern molecules are immediately detected by scavenger receptors, TLRs, and the receptor for advanced glycation end products (Haynes et al., 2006; Salminen et al., 2009), which activate microglia to express IL-1β and TNF for the scavenging of cell debris (Scheffold et al., 2016). Microglial processes can isolate lesion sites, and protect the brain from further injury (Younger et al., 2019), which can occur when microenvironmental homeostasis is disrupted (Nimmerjahn et al., 2005; Orihuela et al., 2016).
Interestingly, these microglia seem to have activated morphology, but they do not express any pro-inflammatory cytokines. Instead, they express IL-4, IL-10, and transforming growth factor for long-term ‘innate immune memory’ (Scheffold et al., 2016).
Infections
To reveal the relationship between microglia and various infections, human immunodeficiency virus (HIV) can be used as an example. HIV-1 infection leads to disruption of microglia homeostasis through the activity of MAPK and NF-κB (Chen et al., 2017). Chronic HIV-1 infection may result in severe metabolic disorders, such as increased concentrations of kynurenine pathway metabolites, including quinolinic acid, the major CNS source of which is microglia (Valle et al., 2004; Schwarcz et al., 2012; Chen et al., 2017; Rawat and Spector, 2017). Microglia are a major reservoir of HIV-1 in the CNS (Rawat and Spector, 2017). During HIV-1 infection, microglia are morphologically activated (Rawat and Spector, 2017) and pro-inflammatory cytokines, including IL-6, IL-8, IL-10, and TNF-α, are increasingly expressed (Chen et al., 2017).
Hypoxia-ischemia
Hypoxia-ischemia can induce inflammation and death of microglia. During hypoxia-ischemia, microglia increase their expression of IL-1β, IL-6, TNF-α, and TLR4 (Pineau and Lacroix, 2009; Tschopp and Schroder, 2010; Lim and Pack, 2014; McDonough et al., 2017; Cengiz et al., 2019).
TLRs and type-1 interferon (IFN) are expressed in the brain after hypoxia-ischemia (McDonough et al., 2017). According to McDonough et al. (2017), both hypoxia-exposed and ischemia-induced microglia express increased levels of IFN-stimulated genes (ISGs), dependent on IFN-α/β receptor 1. IFN-β induces concentration-dependent secretion of ISG chemokines in cultured microglia and a significant increase in ISG expression in microglia both in vitro and in vivo. TLR4 and IFN-α/β receptor 1 help microglial ISG chemokines respond to TLR4 agonists and mediate neuroprotection pathways (McDonough et al., 2017). Once CNS homeostasis is perturbed, microglia are over-activated and synthesize pro-inflammatory factors, promoting cell death and inflammation. These mechanisms, including peripheral inflammation, indirectly activate microglia via neural transmission or direct passage of pro-inflammatory molecules across the BBB and via damage-associated molecular pattern molecules being released from adjacent injured cells. Direct microglial activation occurs by chronic intermittent hypoxia, which may be related to ROS production and may regulate and activate microglia (Pineau and Lacroix, 2009; Tschopp and Schroder, 2010; Lim and Pack, 2014).
Reactive oxygen species (oxidative stress)
ROS, including superoxide, peroxides, hydroxyl radicals and singlet oxygen, are oxygen-containing reactive chemical species (Hayyan et al., 2016), whose main sources in microglia are from nicotinamide adenine dinucleotide phosphate oxidase (NOX) and mitochondria. Oxidative stress is one of the negative effects of ROS (D'Amico et al., 2013). Oxidative stress can damage cell molecules and change the structure of the cell membrane, leading to cell death and other changes in metabolism (D'Amico et al., 2013). ROS induced by lipopolysaccharide, Aβ, and other proteins, can regulate the activation of microglia with increased expression of pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, inducible nitric oxide synthase, prostaglandin, and monocyte chemoattractant protein-1 through NF-κB, which may aggravate neurodegenerative disorders (Naik and Dixit, 2011; Yang et al., 2011; Qin et al., 2013; Kierdorf and Prinz, 2017). A recent study has demonstrated that NOX can produce sustained microglial activation, and dopaminergic neurodegeneration can inhibit the formation of ROS (Qin et al., 2013).
Microbiota
A complex gut microbiota, affected by age, sex, genes, oxygen, bile concentration, antimicrobial mediators, medicine, mental states, behavioral factors, and diet, helps maintain the homeostasis of microglia, disruption to which may promote microglial maturation, differentiation, and functional disorders (Berer et al., 2011; Kamada et al., 2013; Dorrestein et al., 2014; Erny et al., 2015; Wekerle, 2017; Thion et al., 2018). Microbiota can affect CNS biology through changes in neurotransmitter levels, and integrity of the BBB (Braniste et al., 2014; Yano et al., 2015). The impairment of remyelination in aging mice is related to decreased numbers of regulatory macrophages or microglia (Miron et al., 2013). The density of microglia is higher, with more branching processes during embryogenesis, in germ-free male mice compared with germ-free female mice (Matcovitch-Natan et al., 2016; Thion et al., 2018), while disorders normally occur during adulthood in germ-free male mice (Thion et al., 2018).
Disruption of gut microbiota leads to overexpression of α-synuclein (α-Syn), which can induce microglia activation in PD patients (Erny et al., 2015; Sampson et al., 2016; Thion et al., 2018). Moreover, the identification of key regulators that influence the homeostasis and function of microglia will contribute to the discovery of principles that govern their functions in animal models, and how they benefit or harm the brain under various circumstances (Belkaid and Hand, 2014).
Anesthesia-evoked neural responses
Exposure to sevoflurane, one of the most commonly used volatile anesthetics during surgery, may lead to a favorable microenvironment for endogenous neurogenesis through the activity of microglia (Dang et al., 2018; Yu et al., 2019). Treatment with 3% sevoflurane, 2 hours daily for 3 days, can result in an increase in calcium levels in young mice, which can generate TNF-α and IL-6 through NF-κB signaling, followed by the induction of microglial activation, which can result in the generation of more pro-inflammatory cytokines (Shen et al., 2013).
Despite the beneficial influence of microglial activation, it is also regarded as toxic to nearby neurons because of the generation of cytotoxic mediators (Burm et al., 2015; Qiu et al., 2016). Microglial brain-derived neurotrophic factor increases the phosphorylation of neuronal tropomyosin-related kinase receptor B, which is a key mediator of synaptic plasticity (Parkhurst et al., 2013). Under appropriate conditions, activated microglia secrete neuroprotective factors including brain-derived neurotrophic factor (Prinz et al., 2019).
The neuroprotective effects of propofol after traumatic brain injury appear to be mediated, in part, through the suppression of NOX (Luo et al., 2013). Propofol limits microglial activation after experimental brain trauma through inhibition of NOX, and isoflurane promotes transcription activity of NF-κB in microglia of mice, while anti-inflammatory treatment with ketorolac ameliorates sevoflurane anesthesia-induced cognitive impairment in mice (Zhang et al., 2013).
Alcohol-induced neurotoxicity
Alcohol misuse and abuse can induce various neuropsychiatric and neurological diseases. It has been reported that microglia are never activated by acute alcohol exposure in the absence of pronounced cell death (Wong et al., 2018). Analysis of the morphology and dynamics of microglia shows that developmental alcohol exposure may lead to residual impairment of neural plasticity, even in a brain region where microglia do not acutely assume or maintain an activated phenotype (Wong et al., 2018). Alcohol-treated traumatic brain injury mice exhibit increased numbers of cortical microglia. A single alcohol injection significantly increased microglial activation in the nucleus accumbens and the expression of the pro-inflammatory cytokine IL-1β after traumatic brain injury (Karelina et al., 2017, 2018). Minocycline inhibits microglial production of the pro-inflammatory cytokines IL-1b and TNF-α and increases the production of the anti-inflammatory IL-10 (Crews et al., 2013; Kobayashi et al., 2013). Alcohol-induced release of high mobility group box 1, a danger signal or ‘alarmin', activates TLR4 in microglia, neuronal apoptosis inhibitory protein, MHCII, heterokaryon incompatibility and telomerase-associated protein 1 (NACHT), the NLR family pyrin domain containing 3 (NLRP3) inflammasome, neuronal hyperexcitability, and excitotoxicity neuronal death (Wolf et al., 2017). During all stages of life, the physiological phenotype of microglia is affected by alcohol consumption, which may result in the breakdown of synaptic plasticity (Wong et al., 2018).
In maintaining CNS homeostasis, microglia simultaneously act as both sensors and effectors (Figure 2).
Microglia: A Versatile Effector in the Healthy and Pathological Brain
Physiology
Microglia play a vital role during development by promoting neural precursor cell proliferation and survival. Microglia are the major orchestrator of the brain’s inflammatory response. Microglia express a wide range of immune receptors, as well as neurotransmitters. Microglia also express pattern-recognition receptors, including TLRs and their coreceptors, nucleotide-binding oligomerization domain-like receptors, and C-type lectin receptors, to detect pathogen-associated molecular patterns (Michell-Robinson et al., 2015; Wolf et al., 2017).
Microglial checkpoint mechanisms from development to old-age ensure that the organism responds to various stimuli and any physical abnormality in the microenvironment of the CNS, but during chronic diseases and aging, microglial checkpoint mechanisms have deleterious effects on the CNS because of limitations in microglia functions (Deczkowska et al., 2018). Single-cell RNA-sequencing with high-dimensional cytometry, bulk RNA-sequencing, fate-mapping, and microscopy indicate the diversity of non-parenchymal brain macrophages, and provide a framework for understanding the interaction between host macrophages in healthy and diseased brains (Masuda et al., 2019; Van Hove et al., 2019).
Microglia also express neurotransmitters, which are very important signaling molecules in the CNS. Importantly, they can regulate the release of inflammatory cytokines (Pocock and Kettenmann, 2007). All of the receptors and neurotransmitters expressed by microglia help maintain homeostasis in the CNS.
Neurodegeneration
Microglia in AD
AD is a progressive neurodegenerative disease characterized by the formation of Aβ plaques, entangled nerve fibers, and loss of neurons, with higher morbidity compared with other types of dementia (Burns and Iliffe, 2009). An AD-associated microglia subtype is unique to AD (Keren-Shaul et al., 2017).
Immune receptors can transmit either excitatory or inhibitory signals. TYROBP, serves as a direct partner/adapter for TREM2, CD33, and CR3 (Guerreiro et al., 2013; Haure-Mirande et al., 2017). TREM2, a transmembrane glycoprotein, is able to transmit signals through DAP12 and DAP10 proteins, and is regarded as a protective factor in AD. TREM2 is a microglial Aβ receptor with the ability to transduce physiological and AD-related pathological effects related to Aβ (Colonna and Wang, 2016; Zhao et al., 2018). This can disturb immune defenses and neurotoxicity produced by the accumulation of inflammatory factors accelerates the patient’s deterioration (Lambert et al., 2013; Colonna and Wang, 2016; Hopperton et al., 2018). During Aβ accumulation, TREM2 binds to polyanions, phospholipids, sulfatides, and apolipoprotein E (APOE) (Wang et al., 2015; Yeh et al., 2016; Colonna and Butovsky, 2017) and participates in the proliferation, survival and phagocytosis of apoptotic cells (Takahashi et al., 2005; Otero et al., 2009; Yeh et al., 2017). Up-regulation of TREM2 can relieve neuropathology and spatial cognitive impairment in AD (Jiang et al., 2014).
Different CD33 variants expressed at different levels play various roles in AD. A CD33 variant, expressed at relatively high levels, increases CD33 inhibitory effects on myeloid functions and the risk of AD, while another CD33 variant, expressed at relatively low levels, reduces the inhibitory potential of CD33 and the risk of AD (Bradshaw et al., 2013). Inappropriate activation of microglia or the absence of fully differentiated disease-associated microglia are deleterious in AD (Keren-Shaul et al., 2017; Mass et al., 2017). Immunotherapy through APOE can clear Aβ deposition in the brain of AD patients, but it may damage the CNS (Salloway et al., 2014). Microglia produce APOE, which can moderate the inflammatory response (Xu et al., 2000; Cudaback et al., 2011; Terwel et al., 2011; Mandrekar-Colucci et al., 2012). The concept of homeostatic and reactive microglia suggests that the relevant risk factors for AD are associated with multifunctional and complex microglia responses to amyloid plaques (Sala Frigerio et al., 2019), which affect different branches of the phenotypic spectrum.
Microglia in PD
PD is characterized by the aggregation of α-Syn in Lewy bodies. During the early stage of the disease, microglia are activated and release ROS (Sanchez-Guajardo et al., 2013; Daher et al., 2014; Kumaran and Cookson, 2015). α-Syn can be scavenged by and activate microglia, which can aggravate inflammation to exacerbate PD (Lu et al., 2009; Sanchez-Guajardo et al., 2013). Microglia activation by α-Syn can induce increased expression of pro-inflammatory cytokines, including TNF, IL-1β, IL-6, and inducible nitric oxide synthase, the release of ROS, which further perturbs the balance of dopamine neuron survival and death, and the production of nitric oxide, which leads to the death of surrounding neurons and cells, via various signaling pathways, including p38 MAPK, NF-κB, and TLR pathways (Sanchez-Guajardo et al., 2013; Daher et al., 2014; Kumaran and Cookson, 2015). The aggregation of α-Syn leads to motor dysfunction, and is associated with gut microbiota (Sampson et al., 2016). As discussed previously, gut microbiota have a significant impact on the regulation of microglia; dysfunction of microglia is related to PD (Erny et al., 2015; Matcovitch-Natan et al., 2016; Sampson et al., 2016).
In addition to α-Syn, leucine-rich repeat kinase 2, one of the most commonly mutated genes in both idiopathic and familial PD, is highly expressed in microglia (Melrose et al., 2007), and induces microglial activation in response to inflammation through p38 MAPK and NF-κB signaling pathways. Leucine-rich repeat kinase 2 mutations contribute to PD progression by changing the microenvironment (Kim et al., 2012). TREM2 has been reported to be involved in PD. Down-regulation of TREM2 leads to the expression of pro-inflammatory factors and aggravates PD; while up-regulation of TREM2 ameliorates microglial inflammation, thereby limiting the progression of PD (Zhang et al., 2018).
In a mouse PD model produced by administration of the dopaminergic neurotoxin, 1-methyl-4-phenyl-1, 2,3, 6-tetrahydropyridine, microglia are neurotoxic owing to the lack of CX3CR1 (Paolicelli et al., 2011). CX3CL1 and CX3CL1–CX3CR1 signaling can inhibit the expression of pro-inflammatory cytokines and microglial activation to protect neurons and limit PD progression (Zujovic et al., 2000; Cardona et al., 2006).
Microglia in ALS
ALS is characterized by selective motor neuron degeneration and progressive paralysis (Kiernan et al., 2011) and is related to MHCI and aggregation-prone proteins, including mutated superoxide dismutase 1 (SOD1), chromosome 9 open reading frame 72, Tau, trans-activation response element DNA binding protein 43 (TDP-43) and heterogeneous nuclear ribonucleoproteins (Beers et al., 2006; Butovsky et al., 2012; Renton et al., 2014; Colonna and Butovsky, 2017; Nardo et al., 2018). Microglia in ALS patients express high levels of MHCI. Depletion of MHCI in microglia can reduce the neuroinflammation in SOD1G93A mice (Nardo et al., 2018). Aggregation-prone proteins can activate the microglial NLRP3 inflammasome, resulting in caspase-1 activation and IL-1β secretion, which aggravate the ALS-like phenotype of SOD1G93A mice and TDP-43Q331K mice (Deora et al., 2020). As ALS progresses, microglia, activated by misfolded SOD1 and other stress signals released by motor neurons, attack TNF-α, IL-1β, nitric oxide, ROS, and major histocompatibility complex II causing neurotoxicity, proinflammation, and the exacerbation of motor neuronal injury (Beers et al., 2006; Butovsky et al., 2012; Renton et al., 2014; Colonna and Butovsky, 2017). rNLS8 mice, an inducible mouse model of ALS used to examine the relationship between TDP-43, disease onset, progression, and neuroinflammation, express hTDP43ΔNLS in neurons in a doxycycline-regulated manner, such that hTDP43ΔNLS expression is suppressed in the presence of doxycycline 26, which results in the formation of TDP-43 aggregates (Walker et al., 2015; Spiller et al., 2016). When the proliferation of microglia is inhibited during the early recovery phase using PLX3397, a CSF1R and c-kit inhibitor, rNLS8 mice cannot acquire full motor function, indicating an important neuroprotective role for microglia (Elmore et al., 2014; Spiller et al., 2018). Additionally, an inhibitor targeting the microglial NLRP3 inflammasome and depletion of MHCI in microglia provide new therapeutic approaches to treat ALS as well as neuroinflammation during neurodegeneration (Nardo et al., 2018; Deora et al., 2020).
Microglia in MS and EAE
MS is characterized by multifocal white matter lesions and experimental autoimmune encephalomyelitis (EAE) is an animal model of inflammatory demyelinating diseases (Milo and Kahana, 2010). Microglia serve as antigen-presenting cells to invading T cells by proliferating and up-regulating MHCII (Wolf et al., 2017).
Activity of the E3 ubiquitin ligase, Peli1, which is normally important in the inactivation of microglia mediated by TLR and IL-1 signaling, leads to MS progression (Lereim et al., 2016). However, microglia also have beneficial effects in MS, one of which is the removal of apoptotic cells and myelin debris, which supports tissue regeneration and affects the maturation of oligodendrocyte progenitor cells. Microglia are very early elements involved in the onset of MS (Bogie et al., 2014; Wolf et al., 2017).
In the acute stages of MS, T cells are the first to initiate contact with resting resident microglia in the parenchyma (Heppner et al., 2005; Hirasawa et al., 2005), while activated microglia and repopulated microglia may emerge as antigen presenting cells in the chronic phase of EAE (Greter et al., 2005; McMahon et al., 2005). Although the short stimulatory impulse in the auto-aggressive effector T cells during the early stage of CNS invasion cannot cause the proliferation of T cells, it is enough to affect the overall process involved in the acute autoimmune reaction (Lodygin et al., 2013). 3H-1,2-dithiole-3-thione and its substituted derivative, 5-amino-3-thioxo-3H-(1,2) dithiole-4-carboxylic acid ethyl ester, have anti-inflammatory effects in EAE (Kuo et al., 2018). 5-Amino-3-thioxo-3H-(1,2) dithiole-4-carboxylic acid ethyl ester holds promise as a new therapeutic strategy for MS and EAE.
Therapeutic Approaches Targeting Microglia
Therapeutic approaches to the diseases discussed above include pharmacology, lifestyle changes, laboratory interventions, and gene therapy (Table 2).
Table 2.
Microglia-targeted gene therapy in neurodegeneration diseases
| Intervention | Diseases | Signaling pathways | Effects | References |
|---|---|---|---|---|
| P2RY12 and TMEM119 | MS | Lipid processing and PPARγ signaling | Therapeutic target for MS | Shemer et al., 2018 |
| IFN-β therapy | EAE | MSC immunomodulatory function | Reducing disease progression and frequency of acute exacerbations | Vigo et al., 2019 |
| Inhibition of TREM2-APOE | ALS AD |
TREM2-APOE | Restore homeostatic microglia | Krasemann et al., 2017; Pimenova et al., 2017 |
| Deletion of C1qa gene | FTD | C1qa | Decrease synaptic shearing by progranulin GRN–/– microglia; Alleviate behavioral phenotypes and premature mortality; Prevent neurodegeneration in GRN–/– mice |
Lui et al., 2016 |
| miR-124 | EAE | miR-124 | Contribute to systematically inactive macrophages reduce the activation of myelin-specific T cells | Ponomarev et al., 2011 |
| PLX3397 | AD | CSF1R and c-kit | Beneficial improves the spatial and emotional memory deficits | Elmore et al., 2014; Spiller et al., 2018 |
AD: Alzheimer’s disease; ALS: amyotrophic lateral sclerosis; APOE: apolipoprotein E; CSF1R: colony-stimulating factor-1 receptor; EAE: experimental autoimmune encephalomyelitis; FTD: frontotemporal dementia; GRN: progranulin; IFN-β: interferon-β; MS: multiple sclerosis; MSC: mesenchymal stem cell; TMEM119: transmembrane protein 119; TREM2: transmembrane protein 2.
Glucose metabolism influences inflammation by altering histone deacetylase 4 protein levels, NLRP3 inflammasome formation, and receptor for advanced glycation end product receptor activation (Manigrasso et al., 2014). Calorie restriction and a ketogenic diet can inhibit glucose utilization to reduce brain inflammation, tissue loss, and functional impairment after brain injury (Camberos-Luna and Massieu, 2020). Additionally, calorie restriction can hinder cortical-injury-induced and aging-related activation of microglia and mitigate fever and microglial activation induced by lipopolysaccharide (Radler et al., 2014). Physical exercise is associated with up-regulation of neurotrophic factors and anti-inflammatory cytokines, down-regulation of pro-inflammatory cytokines, and inhibition of microglial activation (Svensson et al., 2015). In a rat model of cerebral ischemia, exercise decreased the induction of TLR2, TLR4, myeloid differentiation primary response 88, and NF-κB (Vetreno et al., 2017). Chronic sleep insufficiency leads to microglial up-regulation of pro-inflammatory factors and further aggravates disease (Imeri and Opp, 2009). Therefore, calorie restriction, sufficient physical exercise, and sleep are beneficial for neurodegenerative diseases.
Minocycline belongs to the tetracycline class of antibiotics. It has high lipid solubility and can easily penetrate the BBB (Kriz et al., 2002; Kobayashi et al., 2013). Increased microglial TNF-α and IL-1β levels in the hippocampus were accompanied by a decrease in tumor necrosis factor receptor 2 receptor expression, which was reduced by minocycline (Mattei et al., 2014). Ginsenosides, extracted from natural ginseng, have anti-aging, anti-oxidative, and anti-apoptotic effects, and may promote neuronal regeneration by inhibiting the secretion of TNF-α and nitric oxide (Ong et al., 2015; Wolf et al., 2017).
IFN-β is widely used to treat patients with relapse-remission MS to reduce disease progression and frequency of deterioration, and IFN-β treatment can improve EAE in mice (Vigo et al., 2019). Importantly, it regulates key dopaminergic signaling genes, and its expression is decreased in both aged and PD post-mortem brains and in PD patients (Jakaria et al., 2019). Cytokine production and oxidative stress can be induced in microglia and astrocytes (Al-Haddad et al., 2019).
Nerve growth factor has a strong anti-inflammatory effect on microglia and guides them to neuroprotective phenotypes (Cattaneo and Capsoni, 2019). Microglia depletion and relevant reproduction are promising cell replacement therapies (Han et al., 2019). Thus, exosomes can be a powerful diagnostic tool, a promising therapeutic tool for natural nanoparticles, and also a means of disease transmission and transmission of neurodegenerative factors (Zagrean et al., 2018).
Depletion of CSF1R-mediated microglia may resolve tissue destructive inflammation. Treatment with low doses of the selective CSF1R inhibitor, PLX5562, (leading to 30% depletion) strongly reduced microglia accumulation at amyloid plaques in the 3xTg-AD mouse model (Dagher et al., 2015). Other studies with CSF1R inhibitors tested in combination with nursing or immunotherapy include the application of BLZ 945 and PRD001 (an anti-programmed cell death-1 antibody) in solid tumors, and PLX3397 combined with ozolamide and radiotherapy (Sevenich, 2018).
In the steady state, A20 has a key role in controlling the activation of microglia, including preventing the activation of NLRP3 inflammatory bodies. Targeted expression of A20 in microglia to inhibit their activation might be an important approach in against diseases (Voet et al., 2018). The molecular and functional heterogeneity of parenchymal brain macrophages highlight potential clinical implications for hematopoietic stem cell transplantation aimed to ameliorate lysosomal storage disorders, microgliopathies, or general monogenic immune deficiencies (Shemer et al., 2018). The transcription factor PU.1 plays a key role in regulating several microglial functions. PU.1 and other microglia-specific transcriptional factors should be further studied to determine possible therapeutic possibilities for neurological disorders (Yeh and Ikezu, 2019).
The receptor-mediated serine/threonine-protein kinase 1 is involved in the conversion of microglia to a disease-associated phenotype. A non-cell-death pathogenesis activates a disease-associated microglia phenotype, including the induction of an inflammatory response, a reduction in phagocytosis, and receptor-mediated serine/threonine-protein kinase 1-evoked transcription, which contributes to the etiology of AD (Ofengeim et al., 2017; Mullard, 2018).
The genetic deletion of chemokine receptor CX3CR1 and passive anti-Aβ immunization in mice to increase microglial encapsulation of plaques has been reported (Condello et al., 2015). Constitutive BRAFVE expression in microglia promotes neurodegeneration. A BRAF inhibitor prevents the phosphorylation of extracellularly regulated protein kinases and reduces microglia accumulation and astrogliosis, phagocytosis, demyelination, neuronal loss, and APP deposits (Mass et al., 2017).
The activation of APOE is dependent on TREM2, and the TREM2-APOE pathway regulates microglia phenotypes in neurodegeneration to restore homeostatic microglia (Krasemann et al., 2017; Pimenova et al., 2017). Microglia play an important role in stimulating synapse formation and circuit maturation. Knockout mice lacking complement factors display cortical excitatory hyperconnectivity, revealing a key role for microglia in the removal of excess synapses in the neocortex (Chu et al., 2010; Parkhurst et al., 2013; Weinhard et al., 2018).
Deletion of the C1qa gene dramatically decreases synaptic shearing by progranulin knockout (GRN–/–) microglia, alleviates behavioral phenotypes, and prevents neurodegeneration and premature mortality of GRN–/– mice. GRN has been shown to suppress aberrant microglia activation during aging (Lui et al., 2016).
The microRNA, miR-124, contributes to inactive macrophages and reduces the activation of myelin-specific T cells, resulting in marked inhibition of EAE. These results show that miR-124 acts both as a vital regulator of microglia quiescence and as a potential modulator of monocyte and macrophage activation (Ponomarev et al., 2011). Clearly, modification of the glial inflammatory response also has a primary role in estrogen-mediated neuroprotection (Suzuki et al., 2007).
Microglial activation and dysfunction are involved in most diseases and injuries of the CNS, so it is necessary to find therapeutic approaches targeting microglia (Prinz and Priller, 2014; Crotti and Ransohoff, 2016; Colonna and Butovsky, 2017; Wolf et al., 2017).
Conclusions and Outlook
The vital role of microglia in many neurodegenerative diseases is becoming increasingly evident. Microglia are affected by environmental stimuli as well as neurodegeneration. Microglia originate from the yolk sac and differentiate from myeloid cells during embryogenesis. However, there is some disagreement regarding postpartum repopulation of microglia. Myeloid lineage cells and nestin-positive progenitor cells used to be considered the source of repopulated microglia (Ajami et al., 2007; Ginhoux et al., 2010; Schulz et al., 2012; Elmore et al., 2014; Colonna and Butovsky, 2017). The origin, characteristics, and mechanisms involved in microglia repopulation may be clarified in the future through transcriptome, lineage, and single-cell analysis, as well as genome-editing and other techniques.
Additionally, it is uncertain whether the results of experiments in mice can be applied to humans. Many proteins are expressed to different degrees in mouse and human microglia and whether human microglia and microglia respond in the same way to drugs remains to be unequivocally established (Smith and Dragunow, 2014). In the future, we expect that more precisely targeted therapeutic approaches will be developed to eliminate the adverse effects and to potentiate the beneficial effects of microglia.
Finally, organoid techniques may be a good approach to solve the problems listed above. Organoids are three-dimensional in vitro tissue cultures derived from self-organizing stem cells that have become very popular in tumor research and have been proposed for use in new models of aging (Hu et al., 2018; Jin et al., 2018). They can be used for disease modeling and can contribute to the development of precision medicine (Jin et al., 2018). Thus, we believe that these techniques may serve as new models to investigate microglia functions.
Microglia have diverse effects on CNS homeostasis. Appropriately activated microglia can help patients recover from illness or slow down the progression of a neurodegenerative disease. They also have protective effects on the brain through their immune defense functions including the maintenance of homeostasis, phagocytosis, and synaptic pruning. However, over-activated microglia may hasten the disease process and appropriate inhibition of microglial activation can be helpful. Several studies have shown that the activation of microglia is controllable, which provides patients with diseases of the CNS with hope for better treatment, although therapeutic strategies targeting microglia for CNS disorders have yet to be developed. In addition, microglia act as effectors for many neurodegenerative disorders, but whether they can be used for screening and diagnosis remains to be determined.
Acknowledgments
We apologize to those colleagues whose important work could not be cited due to space constraints.
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interests.
Financial support: This work was supported by the National Natural Science Foundation of China, Nos. 81401279 (to ZYY), 81873740 (to ZYY); China International Medical Exchange Fund, No. 2019-anesthesia-14 (to ZYY); the Natural Science Foundation of Shanghai of China, No. 18ZR1443100 (to ZYY); Wuxin Project of International Peace Maternity and Child Health Hospital Shanghai Jiao Tong University School of Medicine of China, No. 2018-38 (to ZYY); Shanghai Jiao Tong University School of Medicine, Innovation Center of Translational Medicine Collaboration of China, No. TM201729 (to ZYY); the 12th Undergraduate Training Programs for innovation of Shanghai Jiao Tong University School of Medicine of China, No. 1218201 (to YX, MZJ and WLJ).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Natural Science Foundation of China, Nos. 81401279 (to ZYY), 81873740 (to ZYY); China International Medical Exchange Fund, No. 2019-anesthesia-14 (to ZYY); the Natural Science Foundation of Shanghai of China, No. 18ZR1443100 (to ZYY); Wuxin Project of International Peace Maternity and Child Health Hospital Shanghai Jiao Tong University School of Medicine of China, No. 2018-38 (to ZYY); Shanghai Jiao Tong University School of Medicine, Innovation Center of Translational Medicine Collaboration of China, No. TM201729 (to ZYY); the 12th Undergraduate Training Programs for innovation of Shanghai Jiao Tong University School of Medicine of China, No. 1218201 (to YX, MZJ and WLJ).
C-Editors: Zhao M, Yu J, Li CH; L-Editors: Yu J, Song LP; T-Editor: Jia Y Review
References
- 1.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 2.Al-Haddad BJS, Oler E, Armistead B, Elsayed NA, Weinberger DR, Bernier R, Burd I, Kapur R, Jacobsson B, Wang C, Mysorekar I, Rajagopal L, Adams Waldorf KM. The fetal origins of mental illness. Am J Obstet Gynecol. 2019;221:549–562. doi: 10.1016/j.ajog.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 4.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU. 1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454:470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 7.Berer K, Mues M, Koutrolos M, Rasbi ZA, Boziki M, Johner C, Wekerle H, Krishnamoorthy G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature. 2011;479:538–541. doi: 10.1038/nature10554. [DOI] [PubMed] [Google Scholar]
- 8.Bittle J, Stevens HE. The role of glucocorticoid, interleukin-1β, and antioxidants in prenatal stress effects on embryonic microglia. J Neuroinflammation. 2018;15:44. doi: 10.1186/s12974-018-1079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogie JF, Stinissen P, Hendriks JJ. Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol. 2014;128:191–213. doi: 10.1007/s00401-014-1310-2. [DOI] [PubMed] [Google Scholar]
- 10.Bohlen CJ, Bennett FC, Tucker AF, Collins HY, Mulinyawe SB, Barres BA. Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures. Neuron. 2017;94:759–773e8. doi: 10.1016/j.neuron.2017.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bollinger JL, Bergeon Burns CM, Wellman CL. Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun. 2016;52:88–97. doi: 10.1016/j.bbi.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, Rosenkrantz LL, Imboywa S, Lee M, Von Korff A, Morris MC, Evans DA, Johnson K, Sperling RA, Schneider JA, Bennett DA, De Jager PL. CD33 Alzheimer’s disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16:848–850. doi: 10.1038/nn.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009759. 263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burm SM, Zuiderwijk-Sick EA, t Jong AE, van der Putten C, Veth J, Kondova I, Bajramovic JJ. Inflammasome-induced IL-1β secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J Neurosci. 2015;35:678–687. doi: 10.1523/JNEUROSCI.2510-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 16.Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122:3063–3087. doi: 10.1172/JCI62636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calderon D, Bhaskar A, Knowles DA, Golan D, Raj T, Fu AQ, Pritchard JK. Inferring relevant cell types for complex traits by using single-cell gene expression. Am J Hum Genet. 2017;101:686–699. doi: 10.1016/j.ajhg.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camberos-Luna L, Massieu L. Therapeutic strategies for ketosis induction and their potential efficacy for the treatment of acute brain injury and neurodegenerative diseases. Neurochem Int. 2020;133:104614. doi: 10.1016/j.neuint.2019.104614. [DOI] [PubMed] [Google Scholar]
- 19.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 20.Casano AM, Albert M, Peri F. Developmental apoptosis mediates entry and positioning of microglia in the zebrafish brain. Cell Rep. 2016;16:897–906. doi: 10.1016/j.celrep.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 21.Cattaneo A, Capsoni S. Painless nerve growth factor: A TrkA biased agonist mediating a broad neuroprotection via its actions on microglia cells. Pharmacol Res. 2019;139:17–25. doi: 10.1016/j.phrs.2018.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Cengiz P, Zafer D, Chandrashekhar JH, Chanana V, Bogost J, Waldman A, Novak B, Kintner DB, Ferrazzano PA. Developmental differences in microglia morphology and gene expression during normal brain development and in response to hypoxia-ischemia. Neurochem Int. 2019;127:137–147. doi: 10.1016/j.neuint.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen NC, Partridge AT, Sell C, Torres C, Martín-García J. Fate of microglia during HIV-1 infection: From activation to senescence. Glia. 2017;65:431–446. doi: 10.1002/glia.23081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, Prince DA. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colonna M, Wang Y. TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat Rev Neurosci. 2016;17:201–207. doi: 10.1038/nrn.2016.7. [DOI] [PubMed] [Google Scholar]
- 26.Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol. 2017;35:441–468. doi: 10.1146/annurev-immunol-051116-052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condello C, Yuan P, Schain A, Grutzendler J. Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun. 2015;6:6176. doi: 10.1038/ncomms7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity. 2016;44:505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 30.Cudaback E, Li X, Montine KS, Montine TJ, Keene CD. Apolipoprotein E isoform-dependent microglia migration. FASEB J. 2011;25:2082–2091. doi: 10.1096/fj.10-176891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunningham CL, Martínez-Cerdeño V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–4233. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amico E, Factor-Litvak P, Santella RM, Mitsumoto H. Clinical perspective on oxidative stress in sporadic amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;65:509–527. doi: 10.1016/j.freeradbiomed.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, West BL, Green KN. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J Neuroinflammation. 2015;12:139. doi: 10.1186/s12974-015-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daher JP, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of α-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A. 2014;111:9289–9294. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang DD, Saiyin H, Yu Q, Liang WM. Effects of sevoflurane preconditioning on microglia/macrophage dynamics and phagocytosis profile against cerebral ischemia in rats. CNS Neurosci Ther. 2018;24:564–571. doi: 10.1111/cns.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 37.Deczkowska A, Amit I, Schwartz M. Microglial immune checkpoint mechanisms. Nat Neurosci. 2018;21:779–786. doi: 10.1038/s41593-018-0145-x. [DOI] [PubMed] [Google Scholar]
- 38.Deora V, Lee JD, Albornoz EA, McAlary L, Jagaraj CJ, Robertson AAB, Atkin JD, Cooper MA, Schroder K, Yerbury JJ, Gordon R, Woodruff TM. The microglial NLRP3 inflammasome is activated by amyotrophic lateral sclerosis proteins. Glia. 2020;68:407–421. doi: 10.1002/glia.23728. [DOI] [PubMed] [Google Scholar]
- 39.Dorrestein PC, Mazmanian SK, Knight R. Finding the missing links among metabolites, microbes and the host. Immunity. 2014;40:824–832. doi: 10.1016/j.immuni.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du XF, Du JL. A death trap for microglia. Dev Cell. 2016;38:120–121. doi: 10.1016/j.devcel.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, Keren-Shaul H, Mahlakoiv T, Jakobshagen K, Buch T, Schwierzeck V, Utermöhlen O, Chun E, Garrett WS, McCoy KD, Diefenbach A, Staeheli P, Stecher B, Amit I, Prinz M. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, Ladner KJ, Bevan AK, Foust KD, Godbout JP, Popovich PG, Guttridge DC, Kaspar BK. Microglia induce motor neuron death via the classical NF-κB pathway in amyotrophic lateral sclerosis. Neuron. 2014;81:1009–1023. doi: 10.1016/j.neuron.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Gehrmann J, Matsumoto Y, Kreutzberg GW. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev. 1995;20:269–287. doi: 10.1016/0165-0173(94)00015-h. [DOI] [PubMed] [Google Scholar]
- 46.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldmann T, Wieghofer P, Jordão MJ, Prutek F, Hagemeyer N, Frenzel K, Amann L, Staszewski O, Kierdorf K, Krueger M, Locatelli G, Hochgerner H, Zeiser R, Epelman S, Geissmann F, Priller J, Rossi FM, Bechmann I, Kerschensteiner M, Linnarsson S, et al. Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol. 2016;17:797–805. doi: 10.1038/ni.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 49.Grubman A, Kanninen KM, Malm T. Multitasking microglia and Alzheimer’s disease: diversity, tools and therapeutic targets. J Mol Neurosci. 2016;60:390–404. doi: 10.1007/s12031-016-0825-5. [DOI] [PubMed] [Google Scholar]
- 50.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, Guan AK, Evans-Reinsch Z, Braz J, Devor M, Abboud-Werner SL, Lanier LL, Lomvardas S, Basbaum AI. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JS, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert JC, Amouyel P, Goate A, Rademakers R, Morgan K, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han J, Zhu K, Zhang XM, Harris RA. Enforced microglial depletion and repopulation as a promising strategy for the treatment of neurological disorders. Glia. 2019;67:217–231. doi: 10.1002/glia.23529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanamsagar R, Hanke ML, Kielian T. Toll-like receptor (TLR) and inflammasome actions in the central nervous system. Trends Immunol. 2012;33:333–342. doi: 10.1016/j.it.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haure-Mirande JV, Audrain M, Fanutza T, Kim SH, Klein WL, Glabe C, Readhead B, Dudley JT, Blitzer RD, Wang M, Zhang B, Schadt EE, Gandy S, Ehrlich ME. Deficiency of TYROBP, an adapter protein for TREM2 and CR3, receptors, is neuroprotective in a mouse model of early Alzheimer’s pathology. Acta Neuropathol. 2017;134:769–788. doi: 10.1007/s00401-017-1737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 56.Hayyan M, Hashim MA, AlNashef IM. Superoxide ion: generation and chemical implications. Chem Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 57.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 58.Hirasawa T, Ohsawa K, Imai Y, Ondo Y, Akazawa C, Uchino S, Kohsaka S. Visualization of microglia in living tissues using Iba1-EGFP transgenic mice. J Neurosci Res. 2005;81:357–362. doi: 10.1002/jnr.20480. [DOI] [PubMed] [Google Scholar]
- 59.Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, Bazinet RP. Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: a systematic review. Mol Psychiatry. 2018;23:177–198. doi: 10.1038/mp.2017.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu JL, Todhunter ME, LaBarge MA, Gartner ZJ. Opportunities for organoids as new models of aging. J Cell Biol. 2018;217:39–50. doi: 10.1083/jcb.201709054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, Wang J, Zhao L, Liang YX, Wu T, Lu Z, Humayun MS, So KF, Pan Y, Li N, Yuan TF, Rao Y, Peng B. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci. 2018;21:530–540. doi: 10.1038/s41593-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 62.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakaria M, Haque ME, Cho DY, Azam S, Kim IS, Choi DK. Molecular insights into NR4A2(Nurr1): an emerging target for neuroprotective therapy against neuroinflammation and neuronal cell death. Mol Neurobiol. 2019;56:5799–5814. doi: 10.1007/s12035-019-1487-4. [DOI] [PubMed] [Google Scholar]
- 64.Jiang T, Tan L, Zhu XC, Zhang QQ, Cao L, Tan MS, Gu LZ, Wang HF, Ding ZZ, Zhang YD, Yu JT. Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2014;39:2949–2962. doi: 10.1038/npp.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin MZ, Han RR, Qiu GZ, Ju XC, Lou G, Jin WL. Organoids: An intermediate modeling platform in precision oncology. Cancer Lett. 2018;414:174–180. doi: 10.1016/j.canlet.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 66.Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T. Microglia: Housekeeper of the central nervous system. Cell Mol Neurobiol. 2018;38:53–71. doi: 10.1007/s10571-017-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 68.Karelina K, Nicholson S, Weil ZM. Minocycline blocks traumatic brain injury-induced alcohol consumption and nucleus accumbens inflammation in adolescent male mice. Brain Behav Immun. 2018;69:532–539. doi: 10.1016/j.bbi.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karelina K, Gaier KR, Prabhu M, Wenger V, Corrigan TED, Weil ZM. Binge ethanol in adulthood exacerbates negative outcomes following juvenile traumatic brain injury. Brain Behav Immun. 2017;60:304–311. doi: 10.1016/j.bbi.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 70.Karperien A, Ahammer H, Jelinek HF. Quantitating the subtleties of microglial morphology with fractal analysis. Front Cell Neurosci. 2013;7:3. doi: 10.3389/fncel.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 72.Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169:1276–1290e17. doi: 10.1016/j.cell.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 73.Kierdorf K, Prinz M. Microglia in steady state. J Clin Invest. 2017;127:3201–3209. doi: 10.1172/JCI90602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 75.Kim B, Yang MS, Choi D, Kim JH, Kim HS, Seol W, Choi S, Jou I, Kim EY, Joe EH. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS One. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, Hirakawa A, Takeuchi H, Suzumura A, Ishiguro N, Kadomatsu K. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525. doi: 10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47:566–581e9. doi: 10.1016/j.immuni.2017.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 80.Kumaran R, Cookson MR. Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum Mol Genet. 2015;24:R32–44. doi: 10.1093/hmg/ddv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuo PC, Brown DA, Scofield BA, Paraiso HC, Wang PY, Yu IC, Yen JH. Dithiolethione ACDT suppresses neuroinflammation and ameliorates disease severity in experimental autoimmune encephalomyelitis. Brain Behav Immun. 2018;70:76–87. doi: 10.1016/j.bbi.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 82.Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, Russo G, Thorton-Wells TA, Jones N, Smith AV, Chouraki V, Thomas C, Ikram MA, Zelenika D, Vardarajan BN, Kamatani Y, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 84.Lereim RR, Oveland E, Xiao Y, Torkildsen Ø, Wergeland S, Myhr KM, Sun SC, Berven FS. The brain proteome of the ubiquitin ligase Peli1 knock-out mouse during experimental autoimmune encephalomyelitis. J Proteomics Bioinform. 2016;9:209–219. doi: 10.4172/jpb.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 86.Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev. 2014;18:35–48. doi: 10.1016/j.smrv.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Link VM, Gosselin D, Glass CK. Mechanisms underlying the selection and function of macrophage-specific enhancers. Cold Spring Harb Symp Quant Biol. 2015;80:213–221. doi: 10.1101/sqb.2015.80.027367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lodygin D, Odoardi F, Schläger C, Körner H, Kitz A, Nosov M, van den Brandt J, Reichardt HM, Haberl M, Flügel A. A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity. Nat Med. 2013;19:784–790. doi: 10.1038/nm.3182. [DOI] [PubMed] [Google Scholar]
- 89.Lu JQ, Fan Y, Mitha AP, Bell R, Metz L, Moore GR, Yong VW. Association of alpha-synuclein immunoreactivity with inflammatory activity in multiple sclerosis lesions. J Neuropathol Exp Neurol. 2009;68:179–189. doi: 10.1097/NEN.0b013e318196e905. [DOI] [PubMed] [Google Scholar]
- 90.Lui H, Zhang J, Makinson SR, Cahill MK, Kelley KW, Huang HY, Shang Y, Oldham MC, Martens LH, Gao F, Coppola G, Sloan SA, Hsieh CL, Kim CC, Bigio EH, Weintraub S, Mesulam MM, Rademakers R, Mackenzie IR, Seeley WW, et al. Progranulin deficiency promotes circuit-specific synaptic pruning by microglia via complement activation. Cell. 2016;165:921–935. doi: 10.1016/j.cell.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo T, Wu J, Kabadi SV, Sabirzhanov B, Guanciale K, Hanscom M, Faden J, Cardiff K, Bengson CJ, Faden AI. Propofol limits microglial activation after experimental brain trauma through inhibition of nicotinamide adenine dinucleotide phosphate oxidase. Anesthesiology. 2013;119:1370–1388. doi: 10.1097/ALN.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 92.Mandrekar-Colucci S, Karlo JC, Landreth GE. Mechanisms underlying the rapid peroxisome proliferator-activated receptor-γ-mediated amyloid clearance and reversal of cognitive deficits in a murine model of Alzheimer’s disease. J Neurosci. 2012;32:10117–10128. doi: 10.1523/JNEUROSCI.5268-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manigrasso MB, Juranek J, Ramasamy R, Schmidt AM. Unlocking the biology of RAGE in diabetic microvascular complications. Trends Endocrinol Metab. 2014;25:15–22. doi: 10.1016/j.tem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 95.Mass E, Jacome-Galarza CE, Blank T, Lazarov T, Durham BH, Ozkaya N, Pastore A, Schwabenland M, Chung YR, Rosenblum MK, Prinz M, Abdel-Wahab O, Geissmann F. A somatic mutation in erythro-myeloid progenitors causes neurodegenerative disease. Nature. 2017;549:389–393. doi: 10.1038/nature23672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Masuda T, Sankowski R, Staszewski O, Böttcher C, Amann L, Sagar, Scheiwe C, Nessler S, Kunz P, van Loo G, Coenen VA, Reinacher PC, Michel A, Sure U, Gold R, Grün D, Priller J, Stadelmann C, Prinz M. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566:388–392. doi: 10.1038/s41586-019-0924-x. [DOI] [PubMed] [Google Scholar]
- 97.Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, Ben-Yehuda H, David E, Zelada González F, Perrin P, Keren-Shaul H, Gury M, Lara-Astaiso D, Thaiss CA, Cohen M, Bahar Halpern K, Baruch K, Deczkowska A, Lorenzo-Vivas E, Itzkovitz S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353:aad8670. doi: 10.1126/science.aad8670. [DOI] [PubMed] [Google Scholar]
- 98.Mathur V, Burai R, Vest RT, Bonanno LN, Lehallier B, Zardeneta ME, Mistry KN, Do D, Marsh SE, Abud EM, Blurton-Jones M, Li L, Lashuel HA, Wyss-Coray T. Activation of the STING-dependent type I interferon response reduces microglial reactivity and neuroinflammation. Neuron. 2017;96:1290–1302e6. doi: 10.1016/j.neuron.2017.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mattei D, Djodari-Irani A, Hadar R, Pelz A, de Cossío LF, Goetz T, Matyash M, Kettenmann H, Winter C, Wolf SA. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav Immun. 2014;38:175–184. doi: 10.1016/j.bbi.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 100.McDonough A, Lee RV, Noor S, Lee C, Le T, Iorga M, Phillips JLH, Murphy S, Möller T, Weinstein JR. Ischemia/reperfusion induces interferon-stimulated gene expression in microglia. J Neurosci. 2017;37:8292–8308. doi: 10.1523/JNEUROSCI.0725-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 102.Melrose HL, Kent CB, Taylor JP, Dachsel JC, Hinkle KM, Lincoln SJ, Mok SS, Culvenor JG, Masters CL, Tyndall GM, Bass DI, Ahmed Z, Andorfer CA, Ross OA, Wszolek ZK, Delldonne A, Dickson DW, Farrer MJ. A comparative analysis of leucine-rich repeat kinase 2 (Lrrk2) expression in mouse brain and Lewy body disease. Neuroscience. 2007;147:1047–1058. doi: 10.1016/j.neuroscience.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 103.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michell-Robinson MA, Touil H, Healy LM, Owen DR, Durafourt BA, Bar-Or A, Antel JP, Moore CS. Roles of microglia in brain development, tissue maintenance and repair. Brain. 2015;138:1138–1159. doi: 10.1093/brain/awv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–394. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, Ffrench-Constant C. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mullard A. Microglia-targeted candidates push the Alzheimer drug envelope. Nat Rev Drug Discov. 2018;17:303–305. doi: 10.1038/nrd.2018.65. [DOI] [PubMed] [Google Scholar]
- 108.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J Exp Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nardo G, Trolese MC, Verderio M, Mariani A, de Paola M, Riva N, Dina G, Panini N, Erba E, Quattrini A, Bendotti C. Counteracting roles of MHCI and CD8(+) T cells in the peripheral and central nervous system of ALS SOD1(G93A) mice. Mol Neurodegener. 2018;13:42. doi: 10.1186/s13024-018-0271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 112.Niraula A, Sheridan JF, Godbout JP. Microglia priming with aging and stress. Neuropsychopharmacology. 2017;42:318–333. doi: 10.1038/npp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ofengeim D, Mazzitelli S, Ito Y, DeWitt JP, Mifflin L, Zou C, Das S, Adiconis X, Chen H, Zhu H, Kelliher MA, Levin JZ, Yuan J. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2017;114:E8788–E8797. doi: 10.1073/pnas.1714175114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ong WY, Farooqui T, Koh HL, Farooqui AA, Ling EA. Protective effects of ginseng on neurological disorders. Front Aging Neurosci. 2015;7:129. doi: 10.3389/fnagi.2015.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Orihuela R, McPherson CA, Harry GJ. Microglial M1/M2 polarization and metabolic states. Br J Pharmacol. 2016;173:649–665. doi: 10.1111/bph.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 118.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Peri F, Nüsslein-Volhard C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell. 2008;133:916–927. doi: 10.1016/j.cell.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 120.Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- 121.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 122.Pimenova AA, Marcora E, Goate AM. A tale of two genes: Microglial Apoe and Trem2. Immunity. 2017;47:398–400. doi: 10.1016/j.immuni.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 123.Pineau I, Lacroix S. Endogenous signals initiating inflammation in the injured nervous system. Glia. 2009;57:351–361. doi: 10.1002/glia.20763. [DOI] [PubMed] [Google Scholar]
- 124.Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 125.Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92:293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 126.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU. 1 pathway. Nat Med. 2011;17:64–70. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- 128.Prinz M, Jung S, Priller J. Microglia biology: One century of evolving concepts. Cell. 2019;179:292–311. doi: 10.1016/j.cell.2019.08.053. [DOI] [PubMed] [Google Scholar]
- 129.Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative, stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61:855–868. doi: 10.1002/glia.22479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qiu LL, Ji MH, Zhang H, Yang JJ, Sun XR, Tang H, Wang J, Liu WX, Yang JJ. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain Behav Immun. 2016;51:109–118. doi: 10.1016/j.bbi.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 131.Radler ME, Hale MW, Kent S. Calorie restriction attenuates lipopolysaccharide (LPS)-induced microglial activation in discrete regions of the hypothalamus and the subfornical organ. Brain Behav Immun. 2014;38:13–24. doi: 10.1016/j.bbi.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 132.Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ramirez K, Shea DT, McKim DB, Reader BF, Sheridan JF. Imipramine attenuates neuroinflammatory signaling and reverses stress-induced social avoidance. Brain Behav Immun. 2015;46:212–220. doi: 10.1016/j.bbi.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rawat P, Spector SA. Development and characterization of a human microglia cell model of HIV-1 infection. J Neurovirol. 2017;23:33–46. doi: 10.1007/s13365-016-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rawji KS, Mishra MK, Michaels NJ, Rivest S, Stys PK, Yong VW. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139:653–661. doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Renton AE, Chiò A, Traynor BJ. State of play in amyotrophic lateral sclerosis genetics. Nat Neurosci. 2014;17:17–23. doi: 10.1038/nn.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Romanoski CE, Link VM, Heinz S, Glass CK. Exploiting genomics and natural genetic variation to decode macrophage enhancers. Trends Immunol. 2015;36:507–518. doi: 10.1016/j.it.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen WT, Woodbury ME, Srivastava G, Möller T, Hudry E, Das S, Saido T, Karran E, Hyman B, Perry VH, Fiers M, De Strooper B. The major risk factors for Alzheimer’s disease: Age, sex, and genes modulate the microglia response to Aβ plaques. Cell Rep. 2019;27:1293–1306e6. doi: 10.1016/j.celrep.2019.03.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-beta oligomers trigger innate immunity defence via pattern recognition receptors. Prog Neurobiol. 2009;87:181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 141.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M. Neuroimmunological processes in Parkinson’s disease and their relation to á-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro. 2013;5:113–139. doi: 10.1042/AN20120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Scheffold A, Holtman IR, Dieni S, Brouwer N, Katz SF, Jebaraj BM, Kahle PJ, Hengerer B, Lechel A, Stilgenbauer S, Boddeke EW, Eggen BJ, Rudolph KL, Biber K. Telomere shortening leads to an acceleration of synucleinopathy and impaired microglia response in a genetic mouse model. Acta Neuropathol Commun. 2016;4:87. doi: 10.1186/s40478-016-0364-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 145.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sevenich L. Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front Immunol. 2018;9:697. doi: 10.3389/fimmu.2018.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shemer A, Grozovski J, Tay TL, Tao J, Volaski A, Süß P, Ardura-Fabregat A, Gross-Vered M, Kim JS, David E, Chappell-Maor L, Thielecke L, Glass CK, Cornils K, Prinz M, Jung S. Engrafted parenchymal brain macrophages differ from microglia in transcriptome, chromatin landscape and response to challenge. Nat Commun. 2018;9:5206. doi: 10.1038/s41467-018-07548-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–515. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sieger D, Moritz C, Ziegenhals T, Prykhozhij S, Peri F. Long-range Ca2+ waves transmit brain-damage signals to microglia. Dev Cell. 2012;22:1138–1148. doi: 10.1016/j.devcel.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 151.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Smith AM, Dragunow M. The human side of microglia. Trends Neurosci. 2014;37:125–135. doi: 10.1016/j.tins.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 153.Spiller KJ, Cheung CJ, Restrepo CR, Kwong LK, Stieber AM, Trojanowski JQ, Lee VM. Selective motor neuron resistance and recovery in a new inducible mouse model of TDP-43 proteinopathy. J Neurosci. 2016;36:7707–7717. doi: 10.1523/JNEUROSCI.1457-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spiller KJ, Restrepo CR, Khan T, Dominique MA, Fang TC, Canter RG, Roberts CJ, Miller KR, Ransohoff RM, Trojanowski JQ, Lee VM. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nat Neurosci. 2018;21:329–340. doi: 10.1038/s41593-018-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Streit WJ. Microglial senescence: does the brain’s immune system have an expiration date. Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 156.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Svensson M, Lexell J, Deierborg T. Effects of physical exercise on neuroinflammation, neuroplasticity, neurodegeneration ,and behavior: What we can learn from animal models in clinical settings. Neurorehabil Neural Repair. 2015;29:577–589. doi: 10.1177/1545968314562108. [DOI] [PubMed] [Google Scholar]
- 158.Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201:647–657. doi: 10.1084/jem.20041611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tay TL, Mai D, Dautzenberg J, Fernández-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, Staszewski O, Margineanu A, Sporbert A, Steinmetz LM, Pospisilik JA, Jung S, Priller J, Grün D, Ronneberger O, Prinz M. A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017;20:793–803. doi: 10.1038/nn.4547. [DOI] [PubMed] [Google Scholar]
- 160.Taylor SE, Morganti-Kossmann C, Lifshitz J, Ziebell JM. Rod microglia: a morphological definition. PLoS One. 2014;9:e97096. doi: 10.1371/journal.pone.0097096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Terwel D, Steffensen KR, Verghese PB, Kummer MP, Gustafsson J, Holtzman DM, Heneka MT. Critical role of astroglial apolipoprotein E and liver X receptor-α expression for microglial Aβ phagocytosis. J Neurosci. 2011;31:7049–7059. doi: 10.1523/JNEUROSCI.6546-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thion MS, Low D, Silvin A, Chen J, Grisel P, Schulte-Schrepping J, Blecher R, Ulas T, Squarzoni P, Hoeffel G, Coulpier F, Siopi E, David FS, Scholz C, Shihui F, Lum J, Amoyo AA, Larbi A, Poidinger M, Buttgereit A, et al. Microbiome influences prenatal and adult microglia in a sex-specific manner. Cell. 2018;172:500–516e16. doi: 10.1016/j.cell.2017.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thurston G, Kitajewski J. VEGF and delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer. 2008;99:1204–1209. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tremblay M, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tremblay M, Zettel ML, Ison JR, Allen PD, Majewska AK. Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia. 2012;60:541–558. doi: 10.1002/glia.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production. Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 167.Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M. TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell. 2017;170:649–663e13. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Valle M, Price RW, Nilsson A, Heyes M, Verotta D. CSF quinolinic acid levels are determined by local HIV infection: cross-sectional analysis and modelling of dynamics following antiretroviral therapy. Brain. 2004;127:1047–1060. doi: 10.1093/brain/awh130. [DOI] [PubMed] [Google Scholar]
- 169.Van Hove H, Martens L, Scheyltjens I, De Vlaminck K, Pombo Antunes AR, De Prijck S, Vandamme N, De Schepper S, Van Isterdael G, Scott CL, Aerts J, Berx G, Boeckxstaens GE, Vandenbroucke RE, Vereecke L, Moechars D, Guilliams M, Van Ginderachter JA, Saeys Y, Movahedi K. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci. 2019;22:1021–1035. doi: 10.1038/s41593-019-0393-4. [DOI] [PubMed] [Google Scholar]
- 170.Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, Garaschuk O, Ransohoff RM, Jucker M. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vetreno RP, Patel Y, Patel U, Walter TJ, Crews FT. Adolescent intermittent ethanol reduces serotonin expression in the adult raphe nucleus and upregulates innate immune expression that is prevented by exercise. Brain Behav Immun. 2017;60:333–345. doi: 10.1016/j.bbi.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Vigo T, La Rocca C, Faicchia D, Procaccini C, Ruggieri M, Salvetti M, Centonze D, Matarese G, Uccelli A. IFNβ enhances mesenchymal stromal (stem) cells immunomodulatory function through STAT1-3 activation and mTOR-associated promotion of glucose metabolism. Cell Death Dis. 2019;10:85. doi: 10.1038/s41419-019-1336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37:372–402. doi: 10.1210/er.2016-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Voet S, Mc Guire C, Hagemeyer N, Martens A, Schroeder A, Wieghofer P, Daems C, Staszewski O, Vande Walle L, Jordao MJC, Sze M, Vikkula HK, Demeestere D, Van Imschoot G, Scott CL, Hoste E, Gonçalves A, Guilliams M, Lippens S, Libert C, et al. A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat Commun. 2018;9:2036. doi: 10.1038/s41467-018-04376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Walker AK, Spiller KJ, Ge G, Zheng A, Xu Y, Zhou M, Tripathy K, Kwong LK, Trojanowski JQ, Lee VM. Functional recovery in new mouse models of ALS/FTLD after clearance of pathological cytoplasmic TDP-43. Acta Neuropathol. 2015;130:643–660. doi: 10.1007/s00401-015-1460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang Y, Szretter KJ, Vermi W, Gilfillan S, Rossini C, Cella M, Barrow AD, Diamond MS, Colonna M. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, Gilfillan S, Krishnan GM, Sudhakar S, Zinselmeyer BH, Holtzman DM, Cirrito JR, Colonna M. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Weinhard L, di Bartolomei G, Bolasco G, Machado P, Schieber NL, Neniskyte U, Exiga M, Vadisiute A, Raggioli A, Schertel A, Schwab Y, Gross CT. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat Commun. 2018;9:1228. doi: 10.1038/s41467-018-03566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wekerle H. Brain autoimmunity and intestinal microbiota: 100 trillion game changers. Trends Immunol. 2017;38:483–497. doi: 10.1016/j.it.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 182.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Wolf SA, Boddeke HW, Kettenmann H. Microglia in physiology and disease. Annu Rev Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 184.Wong EL, Lutz NM, Hogan VA, Lamantia CE, McMurray HR, Myers JR, Ashton JM, Majewska AK. Developmental alcohol exposure impairs synaptic plasticity without overtly altering microglial function in mouse visual cortex. Brain Behav Immun. 2018;67:257–278. doi: 10.1016/j.bbi.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu H, Chen M, Manivannan A, Lois N, Forrester JV. Age-dependent accumulation of lipofuscin in perivascular and subretinal microglia in experimental mice. Aging Cell. 2008;7:58–68. doi: 10.1111/j.1474-9726.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 186.Xu J, Wang T, Wu Y, Jin W, Wen Z. Microglia colonization of developing zebrafish midbrain is promoted by apoptotic neuron and lysophosphatidylcholine. Dev Cell. 2016;38:214–222. doi: 10.1016/j.devcel.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 187.Xu Q, Li Y, Cyras C, Sanan DA, Cordell B. Isolation and characterization of apolipoproteins from murine microglia. Identification of a low density lipoprotein-like apolipoprotein J-rich but E-poor spherical particle. J Biol Chem. 2000;275:31770–31777. doi: 10.1074/jbc.M002796200. [DOI] [PubMed] [Google Scholar]
- 188.Yang G, Meng Y, Li W, Yong Y, Fan Z, Ding H, Wei Y, Luo J, Ke ZJ. Neuronal MCP-1 mediates microglia recruitment and neurodegeneration induced by the mild impairment of oxidative metabolism. Brain Pathol. 2011;21:279–297. doi: 10.1111/j.1750-3639.2010.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yeh FL, Hansen DV, Sheng M. TREM2, microglia, and neurodegenerative diseases. Trends Mol Med. 2017;23:512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 191.Yeh FL, Wang Y, Tom I, Gonzalez LC, Sheng M. TREM2 binds to apolipoproteins, including APOE and, CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91:328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 192.Yeh H, Ikezu T. Transcriptional and epigenetic regulation of microglia in health and disease. Trends Mol Med. 2019;25:96–111. doi: 10.1016/j.molmed.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Younger D, Murugan M, Rama Rao KV, Wu LJ, Chandra N. Microglia receptors in animal models of traumatic brain injury. Mol Neurobiol. 2019;56:5202–5228. doi: 10.1007/s12035-018-1428-7. [DOI] [PubMed] [Google Scholar]
- 194.Yu X, Zhang F, Shi J. Effect of sevoflurane treatment on microglia activation, NF-kB and MAPK activities. Immunobiology. 2019;224:638–644. doi: 10.1016/j.imbio.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 195.Zagrean AM, Hermann DM, Opris I, Zagrean L, Popa-Wagner A. Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic Implications. Front Neurosci. 2018;12:811. doi: 10.3389/fnins.2018.00811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth 110 Suppl. 2013;1:i82–91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang Y, Feng S, Nie K, Li Y, Gao Y, Gan R, Wang L, Li B, Sun X, Wang L, Zhang Y. TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun. 2018;499:797–802. doi: 10.1016/j.bbrc.2018.03.226. [DOI] [PubMed] [Google Scholar]
- 198.Zhao Y, Wu X, Li X, Jiang LL, Gui X, Liu Y, Sun Y, Zhu B, Piña-Crespo JC, Zhang M, Zhang N, Chen X, Bu G, An Z, Huang TY, Xu H. TREM2 is a receptor for β-amyloid that mediates microglial function. Neuron. 2018;97:1023–1031e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zujovic V, Benavides J, Vigé X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. [PubMed] [Google Scholar]


