Reactive oxygen species (ROS) and kynurenines: Kynurenines represent a relatively heterogenous group of tryptophan metabolites (Figure 1A). The amino acid tryptophan is metabolized in the humans by the kynurenine or serotonin pathway. For a long time, the kynurenine pathway was assumed primarily to constitute the source for nicotinamide-adenine dinucleotide phosphate, one of the most utilized redox active enzyme cofactors. However, in the last years, various kynurenines were identified as important endogenous neuroactive agents. For example, quinolinic (QUIN) and kynurenic acid (KYNA) interact with the excitatory N-methyl-D-aspartate (NMDA) receptors. Additionally, KYNA can bind to α7-nicotinic receptors in high concentrations. Furthermore, several kynurenines showed apparent effects on the immune system and inflammation that is often associated with degeneration processes (Schwarcz and Stone, 2017). Since kynurenine metabolites may influence ROS concentrations, they can affect redox signal cascades by interfering with redox homeodynamic equilibrium. Within the tissue, ROS can participate in various physiological and pathological processes, such as maturation, reproduction, inflammation, and, under specific conditions, programmed cell death by ferroptosis. The function of the ferroptosis in organisms is still far from being satisfactorily understood. In this context, the accumulation of iron can contribute to the development of neurodegenerative pathogenesis in damaged brains. The senile plaques of patients suffering from Alzheimer’s disease, accumulate iron up to a concentration of about 0.9 mM, three times more than in healthy controls (0.3 mM). Usually, iron is stored in complexes with storage proteins, such as transferrin and ferritin, or occurs as cofactors of various enzymes as heme and nonheme coordination complexes. If iron coordinates other molecules than the mentioned ones, it is called “poorly liganded” iron that can catalyze uncontrolled production of highly cytotoxic hydroxyl radicals via the Fenton reaction (Kell, 2010). Again, hydroxyl radicals can increase levels of “poorly liganded” iron by oxidative destruction of the iron storage proteins.
Figure 1.
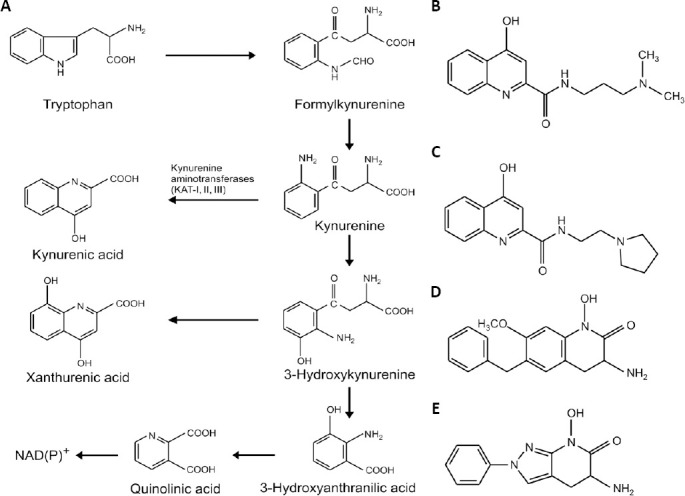
Simplified kynurenine pathway and examples of perspective currently investigated substances.
(A) Simplified kynurenine pathway. (B–E) Chemical structures of perspective compounds: (B) and (C) kynurenic acid amides showing effects on the longevity of bdelloid rotifers, (D) and (E) a chemical structure example of kynurenine aminotransferase II inhibitors.
FeII+ H2O2→ FeIII+– OH +• OH (Fenton reaction)
Additionally, the rate of the radical production can be enhanced by agents supporting iron redox cycling, for example ascorbic acid. The concentration of ascorbic acid in the brain is quite high (up to 1 mM).
Perspective of kynurenines for novel drug development: Antioxidants, such as kynurenines and their chemical analogues, can inhibit neurodegeneration. This aspect of KYNA has been extensively investigated. This 4-hydroxyquinoline derivative (Figure 1A) was identified as an endogenous anticonvulsant and neuroprotectant, which behaves as an antagonist of excitatory NMDA receptors. Recently, the general view of KYNA agonism on nicotine receptors became questionable (Stone, 2020). Furthermore, KYNA showed antioxidant and iron chelation properties (Kubicova et al., 2019). However, all these properties were only apparent in relatively high concentrations of KYNA. Accordingly, KYNA cannot disturb signal functions of ROS under normal physiological conditions, and additionally, KYNA does not decrease the bioavailability of iron ions for metalloenzymes. KYNA penetrates only very poorly through the blood-brain barrier (BBB). The natural cerebral concentrations of KYNA are low, about 150 nM. Therefore, its derivatives such as 7-chlorokynurenic acid or KYNA amide derivatives with improved abilities to pass through BBB into the brain were developed in recent decades (Li et al., 2016; Fehér et al., 2019). The substituted amides of KYNA affected electrophysiological functions investigated on hippocampus slides (Fehér et al., 2019). Furthermore, KYNA and its amides such as N-(3-(dimethylamino)propyl)-4-hydroxyquinoline-2-carboxamide (Figure 1B) or 4-hydroxy-N-(2-(1-pyrrolidinyl)ethyl)-quinoline-2-carboxamide (Figure 1C) prolonged longevity and increased reproduction as well as growth of bdelloid rotifers, microinvertebrates that recently are used in pharmacological research (Datki et al., 2019). However, elevated brain concentrations of KYNA are usually associated with schizophrenia (Stone, 2020). Therefore, kynurenine aminotransferase II inhibitors (Figure 1A) were suggested as promising novel agents against cognitive impairments or psychoses (Rossi et al., 2019). As perspective kynurenine aminotransferase II inhibitors are investigated substitute heterocyclic hydroxamates, 3-amino-3,4-dihydro-1-hydroxy-7-methoxy-6-(benzyl)-2(1H)-quinolinone (Figure 1D) and a diazole analogue (Figure 1E) (Rossi et al., 2019). Additionally, the external KYNA, a potato constituent, may affect brain functions via the gut-brain axis (Schwarcz and Stone, 2017).
Xanthurenic acid (XA) represents a further, often investigated kynurenine metabolite with quinoline structure (Figure 1A). Contrary to KYNA, XA can penetrate the BBB probably utilizing a transporter mechanism. Therefore, XA can be accumulated in the brain up to the concentrations of 1 μM. Still, kynurenines concentrations depend on metabolic conditions (Schwarcz and Stone, 2017). For example, pyridoxine (vitamin B6) hypovitaminosis can lead to elevated KYNA and XA concentrations. Although the cerebral concentrations of XA are higher than KYNA, XA physiological functions are not fully known. However, in the brains of patients suffering from schizophrenia, XA concentrations are unusually low (Rossi et al., 2019). Additionally, XA shows more effective antioxidant and iron chelating abilities than KYNA. Compared to KYNA, the better chelating capabilities of XA are caused by the 8-hydroxy group in the XA molecule (Kubicova et al., 2019). 8-Hydroxyquinoline is well known as an excellent chelator of transition metals, included iron, and as very efficient ROS scavenger (Chobot et al., 2018). Therefore, 8-hydroxyquinoline derivatives are candidates for neurodegenerative diseases treatment.
Another extensively investigated kynurenine metabolite is QUIN that has been identified as an agonist of NMDA receptors, primarily in the neocortex and hippocampus (Figure 1A). Additionally, QUIN can affect inflammation that accompanies most degenerative diseases (Schwarcz and Stone, 2017). Furthermore, QUIN is a potent iron chelator but has no redox properties. It can influence hydroxyl radical production in the Fenton reaction by affecting iron catalytic activities. However, these effects depend on the acid concentration and the overall reaction milieu (Kubicova et al., 2013). The role of QUIN in pathological processes of psychiatric and neurological diseases is often discussed. QUIN and other kynurenines are involved in pre-natal brain development. Moreover, kynurenines can freely penetrate through the fetal BBB (Schwarcz and Stone, 2017). In the adult brain, physiological concentrations of QUIN are lower than 100 nM but pathological levels can increase up to 10–40 μM.
The antioxidant and iron chelation treatment is often voiced as a promising cure approach of neurodegenerative diseases. Especially, plant polyphenols are extensively discussed in this context and their beneficial effects on human health are well proven (Dhakal et al., 2019). However, the plant polyphenol bioavailability from the human gut and their passing through the BBB are in many cases low. Contrary to plant polyphenols, kynurenines are endogenous metabolites of brain tissue. Moreover, kynurenines can affect the brain processes by interactions with receptors and by modulation of ROS and “poorly liganded” iron concentrations as a part of a pre-receptor chemistry. Therefore, the manipulation of kynurenine concentrations in the target brain tissue seems to represent a promising neurodegenerative disease treatment.
Our investigations showed that a redox homeodynamic equilibrium stabilization requires the appropriate antioxidant concentrations. An antioxidant can demonstrate the expected activity if it is present in the proper concentration, the right time, and the correct compartment of the tissue. In case of lower or higher antioxidant agent concentration, the antioxidant effects can disappear or be even changed to a dangerous pro-oxidant activity (Chobot et al., 2014). Consequently, oxidative stress increases and tissue damages become more evident.
Furthermore, the presence of other redox active substances or competing chelators (e.g., ascorbic acid, other kynurenines, cysteine, tyrosine, tocopherols) merits attention. For example, the presence of ascorbic acid in combination with another chelator in reaction mixtures can modulate antioxidant activities of kynurenic and quinolinic acid substantially (Kubicova et al., 2013, 2019).
ROS concentration is a further factor that affects antioxidant activity. We use hydrogen peroxide to simulate oxidative stress in damaged tissue. In the range of tested concentrations, KYNA was antioxidant in presence of hydrogen peroxide. However, the antioxidant effects of QUIN depended on its concentration and the dose-response curve was U shaped. Compared to KYNA and QUIN, XA showed excellent antioxidant properties in all tested reaction milieus (Kubicova et al., 2019). This antioxidant variability seems to depend on different factors, including the reversibility of the chemical reactions and the occurrence of other simultaneously running chemical reactions. Therefore, the predictability of the antioxidant effects is very difficult. Nevertheless, the in vitro assays and electrochemical experiments can offer some insights into possible pro- or antioxidant activities of the tested substances. The in vitro experiments, albeit limited in interpretation, may help to understand the results of in vivo experiments. The chemical complexity of the cell protoplast varies in every microcompartment. Without in-depth knowledge of the chemical properties of the tested substances, differentiation between cause and consequences is often impossible. For example, the pathologically elevated concentrations of KYNA, XA or QUIN can be interpreted as a part of natural antioxidant defence in a damaged organism.
Processes associated with neurodegenerative diseases or aging are far from being satisfactorily understood. The pathogenesis of neurodegenerative diseases involves function modifications of various receptors, ROS generation often catalyzed by “poorly liganded” iron, increased permeability of BBB, and affected inflammatory processes. The current treatment approaches are mainly just palliative. One glimmer of hope is treatment by manipulation of kynurenine metabolites that can decrease the rate of cognitive function destruction in patients suffering from such diseases causing them. Kynurenines, especially KYNA, XA and QUIN, can affect many of these pathogenic processes by their antioxidant and iron chelation properties, interactions with various receptors and stimulatory effects on immune system. Furthermore, the aim of the treatment therapy should be to restore the redox equilibria in general and also the equilibria among kynurenines, other redox active agents, and neurotransmitters, in the brain tissue. In this context, the chemical analogs or derivatives of KYNA, XA and QUIN become very perspective and important in the development of novel drugs.
This work was funded by Austrian Science Fund (FWF), grant No. P 24630-B21 (to VC).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Chobot V, Hadacek F, Kubicova L. Effects of selected dietary secondary metabolites on reactive oxygen species production caused by iron(II) autoxidation. Molecules. 2014;19:20023–20033. doi: 10.3390/molecules191220023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chobot V, Hadacek F, Bachmann G, Weckwerth W, Kubicova L. Antioxidant properties and the formation of iron coordination complexes of 8-hydroxyquinoline. Int J Mol Sci. 2018;19:3917. doi: 10.3390/ijms19123917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Datki Z, Galik-Olah Z, Bohar Z, Zadori D, Fulop F, Szatmari I, Galik B, Kalman J, Vecsei L. Kynurenic acid and its analogs are beneficial physiologic attenuators in bdelloid rotifers. Molecules. 2019;24:2171. doi: 10.3390/molecules24112171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhakal S, Kushairi N, Phan CW, Adhikari B, Sabaratnam V, Macreadie I. Dietary polyphenols: A multifactorial strategy to target Alzheimer’s disease. Int J Mol Sci. 2019;20:5090. doi: 10.3390/ijms20205090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fehér E, Szatmári I, Dudás T, Zalatnai A, Farkas T, Lorinczi B, Fülöp F, Vécsei L, Toldi J. Structural evaluation and electrophysiological effects of some kynurenic acid analogs. Molecules. 2019;24:3502. doi: 10.3390/molecules24193502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kell DB. Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, Alzheimer’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol. 2010;84:825–889. doi: 10.1007/s00204-010-0577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kubicova L, Hadacek F, Chobot V. Quinolinic acid: Neurotoxin or oxidative stress modulator. Int J Mol Sci. 2013;14:21328–21338. doi: 10.3390/ijms141121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubicova L, Hadacek F, Bachmann G, Weckwerth W, Chobot V. Coordination complex formation and redox properties of kynurenic and xanthurenic acid can affect brain tissue homeodynamics. Antioxidants. 2019;8:476. doi: 10.3390/antiox8100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li CF, Chen XM, Chen SM, Mu RH, Liu BB, Luo L, Liu XL, Geng D, Liu Q, Yi LT. Activation of hippocampal BDNF signaling is involved in the antidepressant-like effect of the NMDA receptor antagonist 7-chlorokynurenic acid. Brain Res. 2016;1630:73–82. doi: 10.1016/j.brainres.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Rossi F, Miggiano R, Ferraris DM, Rizzi M. The synthesis of kynurenic acid in mammals: An updated kynurenine aminotransferase structural KATalogue. Front Mol Biosci. 2019;6:7. doi: 10.3389/fmolb.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarcz R, Stone TW. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology. 2017;112:237–247. doi: 10.1016/j.neuropharm.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone TW. Does kynurenic acid act on nicotinic receptors. An assessment of the evidence. J Neurochem. 2020;152:627–649. doi: 10.1111/jnc.14907. [DOI] [PMC free article] [PubMed] [Google Scholar]


