Myelination, remyelination and demyelination: modeling the in vitro drug discovery pipeline: Demyelination is a multifactorial event occurring in diseases primarily involving myelin forming cells (oligodendrocytes, OLs) and their precursors (oligodendrocyte precursor cells, OPCs) such as multiple sclerosis, but is also involved in the pathology of other central nervous system (CNS) injuries and diseases, such as neonatal encephalopathy, brain and spinal cord injury, and Alzheimer’s disease. In numerous conditions, myelin repair occurs spontaneously, leading to anatomical and functional restitution. Many pathological mechanisms, however, may interfere with both developmental myelination, which occurs during late gestation and post-natal periods, and myelin replacement/repair (remyelination) which occurs in the adult CNS (Butt et al., 2019), and which can lead to the failure of myelination/remyelination. Improvement of endogenous myelin forming/repair capability is currently a recognized therapeutic target in demyelinating injuries and diseases.
Due to the complexity of these processes, there is great need for a simplified and reliable in vitro method which allows the dissection of cellular and molecular mechanisms in demyelination, both for target identification and for drug discovery.
OPCs, the cells responsible for the initiation of both myelin formation and myelin repair, is a key player in these processes (Butt et al., 2019). These poorly differentiated cells residing in the white and gray matter of the CNS have the critical role of recognizing and responding to specific stimuli which induce proliferation, proper migration to the non-myelinated axon and differentiation into mature OLs, which then develop the appropriate myelin sheath based on the structural and functional characteristics of the axon. Another cell type involved in these processes is the multipotent neural stem cell (NSC), which is still widely distributed in the fetal and early postnatal brain, being restricted to the neurogenic areas (e.g., the subventricular zone of the lateral ventricle) in the adult brain. In fact, it is not only resident OPCs which are activated in the repair process, since NSCs in the subventricular zone niche can also generate new OPCs. All steps in this process are regulated by the structural and functional interaction with other cell types, such as microglia, astrocytes and neurons, and by the extracellular matrix microenvironment.
Considering the above, we envisage two major unmet needs in establishing in vitro systems for target identification and drug discovery for myelination/remyelination-enhancing therapies: i) the setup of in vitro systems mimicking the complexity of the in vivo condition, both for infancy and adulthood; and ii) the inclusion of inflammation and hypoxia/ischemia, the main pathogenetic mechanisms present in most of the demyelination conditions, in the in vitro set-up for screening myelination-enhancing therapies.
Myelination, remyelination and demyelination: the in vitro challenge: In vitro modeling is a key step in the drug screening process, acting on different sections of the pipeline, those of drug discovery, functional studies and toxicology (Li and Xia, 2019), and in this context, a reliable in vitro system is of fundamental importance in developing the required procedures.
Classical methods used to obtain large amounts of pure OLs are based on protocols involving various shaking steps, conducted on isolated mixed glial cell cultures from the neonatal cortex to give pure cultures (greater than 90%) of differentiating OPCs (O'Meara et al., 2011). Due to the low yield of OPCs obtained from adult animals, and to the high costs of the technique which usually involves immunopanning, primary OPCs are isolated from early post-natal ages (from P0 to P7). These protocols have the unquestionable advantages of producing a simple and homogenous screening platform containing only OPCs, which reach the final maturation stage (i.e., myelin basic protein (MBP) positivity) in 4–5 days following differentiation induction. This system is proposed for the screening of drugs for demyelinating conditions affecting both neonatal and adult subjects (Lariosa-Willingham et al., 2016).
The reasons for the popularity of this technique, however, also reveals its limitations: i) the system using fetal or newborn brain cells provides a high yield, but its appropriateness for adult pathologies is questionable; ii) the very fast maturation of OPCs into MBP-producing cells reduces time and costs, but misses key biological steps critical for both neonatal and adult demyelinating pathologies, such as the induction of lineage from neural stem cells, and compresses the maturation process from OPCs through preOLs to myelinating OLs into the space of a few days, and iii) the purity of the OPC culture improves reproducibility at the expense of communication with other cell types.
The first point to be discussed is appropriateness, since the screening platforms based on primary OPCs are based on the assumption that adult OPCs are identical to fetal/postnatal OPCs; that these cells respond in the same way to noxious stimuli, and that the post-lesion remyelination process in adulthood involves the same molecular mechanisms as developmental myelination, known as the “recapitulation hypothesis”. In our opinion, this is a misleading methodological bias in the screening of potential myelination-enhancing therapies which have OPCs as a target. In fact, even if myelination and remyelination share some mechanisms, increasing evidence indicates the huge differences between OPCs isolated from the fetal/newborn and from the adult brain, such as the different metabolic profile, and the balance between anaerobic glycolysis and oxidative phosphorylation (Baldassarro et al., 2019). The indifferent use of fetal/newborn-derived cells to test strategies for enhancing remyelination in adulthood and vice versa therefore constitutes a serious potential bias.
A possible alternative, also as second stage testing, would be to isolate OPCs from NSCs which can be obtained from both the fetal (E14, telencephalon) and the adult (the subventricular zone) brain. While these systems remove the age-related bias, they are limited in that they do not consider the resident OPC response (Baldassarro et al., 2019). Moreover, the NSC-based system permits the derivation of age-specific cells and of cells taken from pathological animals at different times following disease induction (Fernandez et al., 2016), as well as a study of the entire differentiation process, from multipotent stem cells to fully differentiated OLs over 12–14 days (Figure 1A). During the differentiation process, the OPC switches from a proliferative state to a post-mitotic fully differentiated OL stage. To achieve this, the cell passes through various intermediate stages regulated by a complex machinery, involving the most important cell cycle exit signal (i.e., the thyroid hormone), neurotransmitters, neurotrophins, and other small molecules derived from non-myelinated axons and glial cells. This is not a “black-or-white” system which jumps from one stage to the other, but reflects a multifactorial process showing gradually different shades, which can be monitored by stage-specific markers which gradually appear and disappear according to the differentiation timing (Figure 1B). In this context, cells during the process are characterized by different molecular and morphological traits, to the extent that they can be identified as different cells. Moreover, OPCs and OLs show differences in metabolism, expression of specific receptors and vulnerability. In our opinion, a reliable in vitro model for OPC differentiation should have the capacity to resemble all the differentiation stages to allow targeting of the entire process, and to allow investigation of the time/biological phase of interest.
Figure 1.
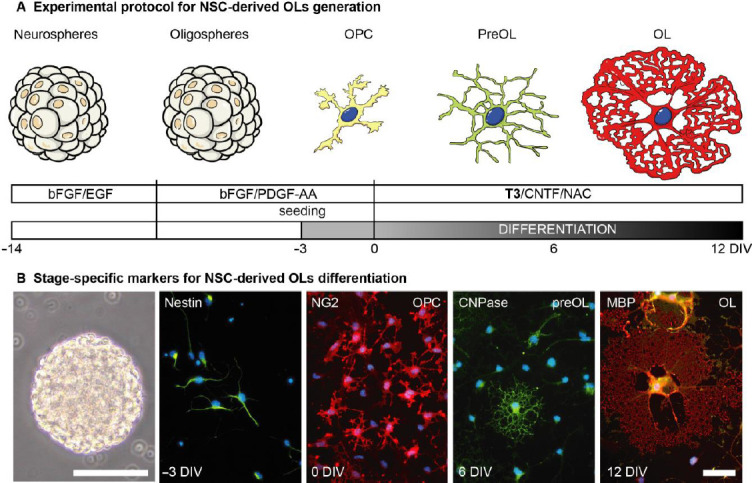
Neural stem cell-derived oligodendrocytes.
(A) Schematic representation of the culture protocol for NSC-derived OL culture generation. Following isolation, cells grow as suspension neurosphere cultures exposed to bFGF/EGF to keep them proliferating and preserving their multipotency. After the first splitting, the spheres are exposed to bFGF/PDGF-AA growth factor, to start driving lineage specification (oligospheres). After the second splitting, dissociated spheres are seeded on laminin-coated supports in the same medium. To start the differentiation process, after 3 days cells are exposed to the differentiation medium, containing thyroid hormone as the differentiation trigger. (B) The panel illustrates the changing morphology of the cells, from multipotent 3D structure (neurospheres) to the fully differentiated OL. Immunofluorescence reactions were performed as already described, using lineage-specific markers and epifluorescence microscope. Scale bar for neurosphere: 100 μm; for immunocytochemistry pictures: 20 μm. bFGF: Basic fibroblast growth factor; CNTF: ciliary neurotrophic factor; CNPase: 2′,3′-cyclic-nucleotide 3′-phosphodiesterase; DIV: day in vitro; EGF: epidermal growth factor; MBP: myelin basic protein; NAC: N-actetyl cystheine; NG2: neural/glial 2 chondroitin sulfate proteoglycan; NSCs: neural stem cells; OLs: oligodendrocytes; OPCs: oligodendrocyte precursor cells; PDGF: platelet derived growth factor; T3: triiodothyronine. Figure 1B is sourced from our unpublished data.
Another aspect that distinguishes primary OPC cultures from NSC-derived OPCs is system topography. Primary OPC-based platforms are composed of bidimensional (2D) OPC cultures in a proliferation state, and the readout when testing remyelination-enhancing molecules is usually represented by the percentage of MBP-positive cells a few days (around 5 days in vitro (DIVs)) after differentiation induction (Lariosa-Willingham and Leonoudakis, 2018).
The first steps in the set-up of NSC-derived OPC cultures, on the other hand, consist of the isolation of neurospheres and their differentiation into so-called “oligospheres”. These three-dimensional (3D) spheroid steps are fundamental for the maintenance of multipotency and unipotency respectively, as shown by the fact that the cell adhesion itself is enough to change the expression profile of the fundamental genes involved in the differentiation process (Baldassarro et al., 2019). In the 3D system, different cell types such as astrocytes interact with OPCs, and the extracellular matrix produced by the cells recreates the in vivo microenvironment, permitting study of all the intermediate steps. NSC-derived OPCs are in fact a spontaneous mixed culture with a constant presence of astrocytes (40%) throughout the entire differentiation process, and a different percentage of neurons, depending on the protocol used. In particular, at 0 DIV the majority of cells are neural/glial 2 chondroitin sulfate proteoglycan (NG2) positive (NG2-positive cells 80%; CNPase-positive cells 5%), passing through DIV 6, where CNPase cells rise in percentage (NG2-positive cells 60%; 2′,3′-cyclic-nucleotide 3′-phosphodiesterase (CNPase)-positive cells 30%), reaching the end of the differentiation (12 DIVs; Additional Figure 1A (1.1MB, tif) ) with mature OLs (CNPase/MBP-positive) representing the majority of cells (CNPase-positive cells, 70%; MBP-positive cells, 40%; Baldassarro et al., 2019). This allows the investigation of the OPC/OL response to noxious stimuli in a complex environment which includes different cell types, without losing the possibility of targeting the desired cell type, which can be captured by imaging high-throughput techniques, cell sorting and single-cell transcriptomic analysis.
On this basis, and in light of the importance of having the whole differentiation journey in a dish, new protocols to obtain OPCs and OLs for drug discovery from embryonic stem cells and induced pluripotent stem cells isolated from patients are under development, although the methods are complex and not yet fully standardized (Chanoumidou et al., 2020).
A second critical point for in vitro screening of myelination/remyelination drugs is the inclusion of challenges that mimic potential targets. For example, it is well known that inflammatory cytokines induce an OPC differentiation block both in vitro and in vivo in multiple sclerosis (Fernández et al., 2016), therefore screening of pro-myelinating agents for pathological conditions characterized by inflammation and demyelination cannot be based on spontaneous differentiation, but must include appropriate challenges, and the candidate pro-myelinating drug for inflammatory-demyelinating diseases must be able to rescue the OPC differentiation block. A second example is the investigation of the role of neurotrophins in the physiological differentiation process, as highlighted by the contrasting data on the effect of these molecules on protection from OPC/OL damage. In the 1990s and early 2000s, highly conflicting data was published on the effect of nerve growth factor and other neurotrophins on OL cultures, probably due to the heterogeneity of the cell systems and the protocols that were used (Acosta et al., 2013).
A look to the future: balancing complexity and reliability: The drug screening pipeline is based on three different models with increasing complexity which precede experimentation in human subjects: in silico, in vitro, and in vivo. The term “model” indicates its purpose: use of a simplified and highly controllable system to produce reliable data, which can be translated to the subsequent steps of the pipeline. The major risk in oversimplifying complex cell life, however, is the production of huge amount of discrepant data, together with the impossibility of translating it in vivo. The general aim in developing in vitro strategies for disease modeling and in drug screening is the production of robust and reliable data: a balancing act between simplification of the model, for ease of analysis, and the trend of making it as similar as possible to the in vivo condition.
Pure cultures of primary purified OPCs are the standard model in pro-myelinating drug screening tests, both for toxicology and for differentiation studies, in the perspective of translating the results obtained into future treatments for demyelinating diseases (Lariosa-Willingham et al., 2016). As discussed in the previous section, the complex nature of these pathologies raises doubts as to the suitability of a fetal-derived in vitro model to simulate physiological processes in the adult and to test drugs for diseases typical of adulthood characterized by a pathological microenvironment (e.g., inflammation and hypoxia/ischemia).
The “appropriate” cell system, then, must be coupled with robust technology, and simple unstimulated 2D cell systems designed to save time and reduce costs might not be appropriate for modelling complex pathological processes. In this direction, the need to investigate this complexity is driving researchers to develop mixed and three-dimensional models that, even if still a long way away from being able to be used as drug screening platforms for OPCs/OLs, might provide a better balance between complexity and reproducibility/reliability, and include 3D cultures and in vitro myelinating models using mixed cultures with neurons or synthetic materials. In fact, myelination and remyelination modeling cannot be restricted to the expression of specific markers, even if they are characteristic of fully mature OLs (i.e., MBP, myelin oligodendrocyte glycoprotein, and myelin proteolipid protein). The extension and wrapping of OL protrusion to nude axons, which evolves into myelin sheath production, follows specific molecular signals directed both from the axon itself and from the microenvironment, and must be considered in an appropriate drug development pipeline. While the development of materials-based in vitro systems is an essential element of the present and future of neuroscience research, it should be coupled with a reproducible screening technique, again to avoid the issue of poor translation (Baldassarro et al., 2016).
As far as technologies are concerned, cell-based high content screening is a key technique which aims to analyze the different cell types in the whole culture in an automatic workflow, avoiding the operator bias in choosing the representative fields, and to obtain the automatic and simultaneous generation of high content meta-data of different microscopy-based parameters. However, the reliability of the high content screening technique remains highly dependent on the reliability of the cell system itself, and the disadvantages of the technique lie in its higher costs, compared to the standard assays, and the need to store and manipulate huge amounts of data (Buchser, 2014). When mixed cultures are coupled with cell-sorting and single cell transcriptome techniques, it is possible to combine the advantage of a multi-cellular system, partially reflecting the cellular crosstalk, with a separate analysis of each cell type (Additional Figure 1B (1.1MB, tif) ).
The introduction of an imaging-based high-content approach is emerging as a key step in different research fields, one which is making a fundamental contribution to all stages of the drug screening and development pipeline, from drug discovery to toxicology studies (Li and Xia, 2019).
The need to develop reliable in vitro models for OPC/OL pharmacological targeting is also highlighted by the reconsideration of the role of these cells, not only in classical demyelinating diseases such as multiple sclerosis, but also in neurodegenerative diseases (e.g., Alzheimer’s dementia; Lorenzini et al., 2020) and CNS injuries (e.g., neonatal hypoxia/ischemia, stroke, spinal cord and brain trauma). It is now clear that the vulnerability of these cells, with all the complex heterogeneity discussed above, strongly contributes to these diseases and injuries. Without a robust in vitro model, however, research in the field will continue to be slowed by technical biases, with the non-reproducibility of the results depending on the model used, and misleading results obtained from poor models.
The authors would like to thank the ARSEP Foundation (Fondation pour l'aide à la recherché sur la sclérose en plaques) for its support in developing the in NSC-derived OPC in vitro systems as part of the “Role of RXRγ in T3-mediated oligodendrocyte differentiation and remyelination” project (to LC and VAB) and fellowship (to VAB).
Additional file:
Additional Figure 1: (1.1MB, tif) Lineage-specific marker analysis and high content screening imaging.
Lineage-specific marker analysis and high content screening imaging.
(A) Panel shows a representative four-color image marking nuclei (blue, Hoechst) and specific cell types: astrocytes (GFAP-positive), OPCs (NG2-positive) and OLs (MBP-positive) at 6 DIV (half of differentiation phase). Scale bar: 100 µm. (B) Panel shows a representative image of cell-based HCS imaging of a whole 96-well plate of an NSC-derived OL culture at the end of the differentiation phase (12 DIV). A picture of a single field with split channels is included. Scale bar: 100 µm. Immunofluorescence reactions were performed as already described, using lineage-specific markers and confocal microscope (A) or cell-based HCS (B). CNPase: 2',3'-Cyclic-nucleotide 3'-phosphodiesterase; DIV: day in vitro; GFAP: glial fibrillary acidic protein; MBP: myelin basic protein; NG2: neural/glial 2 chondroitin sulfate proteoglycan; OLs: oligodendrocytes; OPCs: oligodendrocyte precursor cells.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Acosta CMR, Cortes C, MacPhee H, Namaka MP. Exploring the role of nerve growth factor in multiple sclerosis: implications in myelin repair. CNS Neurol Disord Drug Targets. 2013;12:1242–1256. doi: 10.2174/18715273113129990087. [DOI] [PubMed] [Google Scholar]
- 2.Baldassarro VA, Dolci LS, Mangano C, Giardino L, Gualandi C, Focarete ML, Calzà L. In vitro testing of biomaterials for neural repair: focus on cellular systems and high-content analysis. Biores Open Access. 2016;5:201–211. doi: 10.1089/biores.2016.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldassarro VA, Krężel W, Fernández M, Schuhbaur B, Giardino L, Calzà L. The role of nuclear receptors in the differentiation of oligodendrocyte precursor cells derived from fetal and adult neural stem cells. Stem Cell Res. 2019;37:101443. doi: 10.1016/j.scr.2019.101443. [DOI] [PubMed] [Google Scholar]
- 4.Buchser W. Assay development guidelines for image-based high content screening, high content analysis and high content imaging assay guidance manual. PubMed. 2014;1:1–80. [Google Scholar]
- 5.Butt AM, Papanikolaou M, Rivera A, Crusio WE, Dong H, Radeke HH, Rezaei N. Advances in experimental medicine and biology. London: Springer Nature; 2019. Physiology of oligodendroglia; pp. 117–128. [DOI] [PubMed] [Google Scholar]
- 6.Chanoumidou K, Mozafari S, Baron-Van Evercooren A, Kuhlmann T. Stem cell derived oligodendrocytes to study myelin diseases. Glia. 2020;68:705–720. doi: 10.1002/glia.23733. [DOI] [PubMed] [Google Scholar]
- 7.Fernández M, Baldassarro VA, Sivilia S, Giardino L, Calzà L. Inflammation severely alters thyroid hormone signaling in the central nervous system during experimental allergic encephalomyelitis in rat: Direct impact on OPCs differentiation failure. Glia. 2016;64:1573–1589. doi: 10.1002/glia.23025. [DOI] [PubMed] [Google Scholar]
- 8.Lariosa-Willingham K, Leonoudakis D. Using acutely dissociated and purified oligodendrocyte precursor cells for high-throughput drug screening to identify compounds that promote oligodendrocyte differentiation. Curr Protoc Cell Biol. 2018;79:e49. doi: 10.1002/cpcb.49. [DOI] [PubMed] [Google Scholar]
- 9.Lariosa-Willingham KD, Rosler ES, Tung JS, Dugas JC, Collins TL, Leonoudakis D. A high throughput drug screening assay to identify compounds that promote oligodendrocyte differentiation using acutely dissociated and purified oligodendrocyte precursor cells. BMC Res Notes. 2016;9:419. doi: 10.1186/s13104-016-2220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Xia M. Review of high-content screening applications in toxicology. Arch Toxicol. 2019;93:3387–3396. doi: 10.1007/s00204-019-02593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzini L, Fernandez M, Baldassarro VA, Bighinati A, Giuliani A, Calzà L, Giardino L. White matter and neuroprotection in Alzheimer’s dementia. Molecules. 2020 doi: 10.3390/molecules25030503. doi: 103390/molecules25030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Meara RW, Ryan SD, Colognato H, Kothary R. Derivation of enriched oligodendrocyte cultures and oligodendrocyte/neuron myelinating co-cultures from post-natal murine tissues. J Vis Exp. 2011:3324. doi: 10.3791/3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lineage-specific marker analysis and high content screening imaging.
(A) Panel shows a representative four-color image marking nuclei (blue, Hoechst) and specific cell types: astrocytes (GFAP-positive), OPCs (NG2-positive) and OLs (MBP-positive) at 6 DIV (half of differentiation phase). Scale bar: 100 µm. (B) Panel shows a representative image of cell-based HCS imaging of a whole 96-well plate of an NSC-derived OL culture at the end of the differentiation phase (12 DIV). A picture of a single field with split channels is included. Scale bar: 100 µm. Immunofluorescence reactions were performed as already described, using lineage-specific markers and confocal microscope (A) or cell-based HCS (B). CNPase: 2',3'-Cyclic-nucleotide 3'-phosphodiesterase; DIV: day in vitro; GFAP: glial fibrillary acidic protein; MBP: myelin basic protein; NG2: neural/glial 2 chondroitin sulfate proteoglycan; OLs: oligodendrocytes; OPCs: oligodendrocyte precursor cells.


