Dry eye disease (DED) is a multifactorial disease characterized by a loss of homeostasis of the tear film and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles (Belmonte et al., 2017).
Interestingly, DED shares common characteristics with neuropathic pain, which is defined as pain caused by damage or disease affecting the somatosensory nervous system. Ocular pain, more commonly called corneal pain, has gained recognition due to its increasing prevalence, morbidity, and resulting social burden (Belmonte et al., 2017). To date, the management of chronic corneal pain still represents a therapeutic challenge. A better understanding of the molecular and cellular mechanisms participating in the transition from acute to chronic pain are crucial issues for developing the effective management and a therapeutic strategy to alleviate this debilitating condition. Today, much of the knowledge of neuroinflammation-neuropathic pain processing comes from data from the spinal cord, but a comparatively small number of investigations have been carried out in the corneal trigeminal pain pathway.
Corneal nociceptive pathway: from the cornea to the brain: The cornea is the most densely innervated tissue in the body, with 300–600 times the sensory innervation density of the skin (7000 nociceptors/mm2). The sensory innervation of the cornea is provided by ciliary nerves, a subdivision of the nasociliary branch, which originates from the ophthalmic branch of the trigeminal ganglion (TG) (Belmonte et al., 2017). Sensory inputs from the cornea are conveyed to the ophthalmic branch of the TG by first-order neurons, which only represent 1% to 3% of the total population of trigeminal neurons (Launay et al., 2015). Then, the central axons of corneal sensory neurons make synapses in two discrete regions of the trigeminal brainstem sensory complex: the transition region between the subnucleus interpolaris (Vi) and caudalis (Vc) and the Vc/rostral spinal cord cord junction, which constitutes the first relay of somatosensory information.
Corneal nerve damage, inflammation and peripheral sensitization after chronic dry eye: Understanding the pathophysiology of corneal neuropathic pain observed in patients suffering from DED is essential for the development of new therapeutic strategies and implies the development of relevant preclinical models that best mimic the human disease. In that context, we recently developed a model of chronic dry eye in mice, consisting of the excision of the extraorbital lachrymal gland (responsible for the aqueous constituent of the tear film) and Harderian gland (which produces lipids of the tear film). Glands removal markedly reduced tear production over time and induced corneal nerve abnormalities in the superficial epithelium (Fakih et al., 2019). The reduced number of intra-epithelial corneal nerve endings in DED mice 3 weeks after the surgery was consistent with previous animal (Kovacs et al., 2016) and clinical studies (Labbe et al., 2012; Hamrah et al., 2017). In addition to corneal nerve abnormalities, a mechanical allodynia (decreased mechanical threshold) and inflammatory responses also developed at the cornea level.
Several lines of evidence support the idea that neuroimmune and neuronal–glial interactions play a major role in the chronification of pain at both spinal and trigeminal levels (Grace et al., 2014). Indeed, the activation of immune cells participates in the peripheral sensitization mechanism, which is characterized by a change in the excitability of nociceptors (decreased threshold); an increase in spontaneous stimuli evoked the firing rate of the sensory neurons, leading to spontaneous pain and hyperalgesia.
Considering that, we further evaluated changes in the spontaneous corneal nerve fiber activity of the ciliary nerve in our preclinical model of persistent dry eye. Electrophysiological recordings of the ciliary nerve fibers revealed a 100% increase of action potential frequency between sham and dry eye mice on day 21. These data confirm that corneal inflammation and nerve damage are able to modify the characteristics of trigeminal afferent neurons, resulting in their hyperexcitability and increased ongoing activity (Acosta et al., 2013; Kovacs et al., 2016; Fakih et al., 2019) (Figure 1). These results are of importance because they link corneal nerve abnormalities, the upregulation of the ongoing activity of the corneal nerve and persistent pain. Such morphological and functional alterations of the corneal nerve may indeed explain the painful state of patients suffering from chronic DED.
Figure 1.
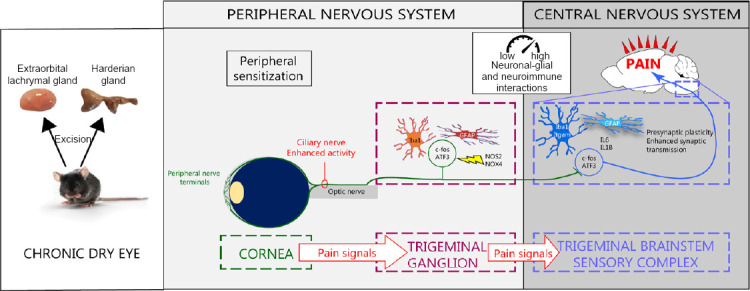
Peripheral and central sensitization under persistent dry eye pain.
After the removal of the Harderian gland and extraorbital gland in adult mice, nerve abnormalities, inflammation and sensitization of the corneal nerves developed. These mechanisms trigger the activation of primary sensory neurons, immune and glial cells in the ipsilateral trigeminal ganglion. This peripheral neuroinflammation spreads to the trigeminal brainstem sensory complex, leading to persistent ocular pain. ATF3: Activating transcription factor 3; c-fos: Fos proto-oncogene; IL: interleukin; NOS: nitric oxide synthase.
The total tear suppression observed following the excision of the extraorbital lachrymal gland and Harderian gland could be considered as a severe dry eye model. Other mild and moderate preclinical models through reducing tear volume have been developed. For example, controlled environment chamber model using exposure to low humidity and constant airflow, as well as its combination with lachrymal gland insufficiency generated by systemic application of scopolamine, are well established murine model of DED mimicking the human pathology.
Corneal inflammation spreads to the TG: Inflammatory responses and neuronal and glial activations have been reported in the ipsilateral TG (containing first-order neurons) in various models of corneal injury. For example, in an acute model of alkali burn or in mouse models of benzalkonium chloride-induced corneal neurotoxicity, corneal inflammation triggers trigeminal inflammation which is characterized by an increase of Iba1 positive (monocytes/macrophages) cells and an increase in the gene expression of proinflammatory cytokines tumor necrosis factor α and interleukin (IL)-6, substance P and its receptor NK1 (Ferrari et al., 2014; Launay et al., 2016). We also observed that the mRNA levels of neuronal activation (Fos proto-oncogene), neuronal injury (activating transcription factor 3), astrocyte (glial fibrillary acidic protein), and oxidative (inducible nitric oxide synthetase type 2 and NOX4) markers increased significantly three weeks after gland excisions in the ipsilateral TG (Fakih et al., 2019). In addition to the upregulation of the gene expression of proinflammatory mediators, persistent dry eye pain induces an upregulation of Iba1 (monocyte/macrophages), GFAP (satellite glial cells), and ATF3 (neuronal injury marker) immunoreactivity in the ipsilateral TG, revealing glial and neuronal dysregulation (Figure 1). These cumulative molecular and cellular changes also provide a much better understanding of interactions of the neuronal, glial and immune systems in the context of persistent corneal pain. In addition, there is increasing evidence that unilateral nerve injury may evoke contralateral responses and such phenomenon also occurs at the level of the eye-TG axis. In fact, clinical and preclinical studies have shown that unilateral corneal injury triggers inflammatory responses in the contralateral eye and TG (Ferrari et al., 2014; Launay et al., 2015). The release of proinflammatory mediators, such as IL-1β, tumor necrosis factor α and substance P, has been proposed to mediate these bilateral effects, although further study is required to decipher underlying mechanisms.
Chronic corneal pain triggers neuroinflammatory responses and presynaptic changes in the central nervous system: Proinflammatory mediators are known to play an important role not only in peripheral sensitization but also in the transfer of nociceptive information from the periphery to the central nervous system (Melik Parsadaniantz et al., 2015). Indeed, it has been well described that persistent peripheral inflammation as well as peripheral nerve damage may generate extensive changes in pain processing in the central nervous system by triggering maladaptive neuroplasticity (neuroplastic changes and hyperexcitability of central nociceptive neurons). Maladaptive neuroplasticity in a chronic pain condition is referred to as central sensitization. Indeed, non-neuronal cells—i.e., glial cells in the central nervous system, namely astrocytes and microglia—become aberrantly activated in response to sustained activation under peripheral nerve injury (Grace et al., 2014). Moreover, synaptic efficacy also plays a key role in central sensitization in response to activity, inflammation, and neural injury. Although the cellular and molecular mechanisms that occur in the central nervous system in the context of pain have been elegantly described and investigated in the spinal cord, research into corneal pain has remained very patchy for a long time. Thus, our recent research works aim to decipher the neurobiological mechanisms of chronic ocular pain.
Activated microglial cells, which are known to release proinflammatory cytokines, neurotrophic factors and chemokines, contribute to neuronal excitability and central sensitization mechanism during chronic pain states (Melik Parsadaniantz et al., 2015). We found higher Iba1-immunopositive cells and CD68 and Itgam mRNA levels in the trigeminal brainstem complex, confirming the activation of microglial cells in acute (7 days) and persistent (21 days) ocular pain (Launay et al., 2016; Fakih et al., 2019). Aside from immune cell activation, astrocyte activation (astrogliosis) was also confirmed by a robust astrocyte reaction (higher levels of GFAP immunoreactivity) and the upregulation of GFAP mRNA expression in the ipsilateral trigeminal brainstem sensory complex. Proinflammatory responses also occurred in this brain structure three weeks after gland excisions; proinflammatory cytokines (IL-6 and IL-1β), oxidative stress enzyme (iNOS2) and neuronal (ATF3 and FOS) markers were increased, confirming a dysregulation in glial cells activation in the central nervous system (Fakih et al., 2019; Figure 1). Overall, this study highlights neuronal–glial and neuroinflammatory interactions which may account for the development and persistence of the ocular pain reported in DED patients.
Accumulating evidence has also suggested that chronic changes of activity in primary afferent neurons induce synaptic rearrangements in the central nervous system and lead to the functional remodeling of presynaptic sites. With regard to the synaptic mechanisms of chronic inflammatory and neuropathic pain, it has been proposed that changes in presynaptic function play an essential role; however, until now, nothing has been known about the possible presynaptic changes during a persistent ocular pain state. Piccolo, one of the components of the presynaptic zone, plays a key role in synaptic plasticity by facilitating/managing the secretion of synaptic vesicles. A significant finding in our study was the increased levels of Piccolo immunoreactivity in the trigeminal brainstem sensory complex induced by chronic dry eye pain. The upregulated expression of Piccolo demonstrates that a persistent corneal nociceptive activity triggers neuronal plasticity in the central nervous system, which may participate in the enhancement in the excitability of pain circuits.
Such cellular rearrangement (enhancement of excitatory synaptic transmission in the trigeminal brainstem complex) is in line with a previous study from Rahman et al. (2015), reporting that persistent (2-week) tear deficiency in rats caused the sensitization of ocular-responsive neurons at multiple regions of the caudal trigeminal brainstem.
In summary, the cellular modifications we reported in the context of chronic corneal pain include changes in proinflammatory gene expression, changes in cell morphology (cell activation), and a reorganization of neural nociceptive networks in the central nervous system. Many experiments are still required to further characterize the precise nature of this central neuronal plasticity linked to pain.
To conclude, a better understanding of the sequence and nature of the events that drive these neurobiological mechanisms will offer significant promise for the discovery of new approaches and targets for the management of chronic ocular pain. We predict that this will be an exciting area of new investigations. Further fundamental and clinical studies using functional and morphological magnetic resonance imaging studies may help to depict how chronic corneal pain can shape the brain and identify the morphological changes that may occur during persistent corneal pain. An elegant in vivo magnetic resonance imaging study has already reported macrophage infiltration in the TG after corneal alkali burn in mice (Ferrari et al., 2014). Although our knowledge of the mechanisms involved in corneal pain has progressed over the last decade, our efforts must be continued for the identification and validation of new therapeutic targets, which are currently sorely lacking.
We apologize to all colleagues and contributors to this field who were not included or cited due to space limitations.
This work was completed with the support of the Programme Investissements d'Avenir IHU FOReSIGHT (ANR-18-IAHU-01).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Acosta MC, Luna C, Quirce S, Belmonte C, Gallar J. Changes in sensory activity of ocular surface sensory nerves during allergic keratoconjunctivitis. Pain. 2013;154:2353–2362. doi: 10.1016/j.pain.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Belmonte C, Nichols JJ, Cox SM, Brock JA, Begley CG, Bereiter DA, Dartt DA, Galor A, Hamrah P, Ivanusic JJ, Jacobs DS, McNamara NA, Rosenblatt MI, Stapleton F, Wolffsohn JS. TFOS DEWS II pain and sensation report. Ocul Surf. 2017;15:404–437. doi: 10.1016/j.jtos.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fakih D, Zhao Z, Nicolle P, Reboussin E, Joubert F, Luzu J, Labbe A, Rostene W, Baudouin C, Melik Parsadaniantz S, Reaux-Le Goazigo A. Chronic dry eye induced corneal hypersensitivity, neuroinflammatory, responses, and synaptic plasticity in the mouse trigeminal brainstem. J Neuroinflammation. 2019;16:268. doi: 10.1186/s12974-019-1656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari G, Bignami F, Giacomini C, Capitolo E, Comi G, Chaabane L, Rama P. Ocular surface injury induces inflammation in the brain: in vivo and ex vivo evidence of a corneal-trigeminal axis. Invest Ophthalmol Vis Sci. 2014;55:6289–6300. doi: 10.1167/iovs.14-13984. [DOI] [PubMed] [Google Scholar]
- 5.Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamrah P, Qazi Y, Shahatit B, Dastjerdi MH, Pavan-Langston D, Jacobs DS, Rosenthal P. Corneal nerve and epithelial cell alterations in corneal allodynia: an in vivo confocal microscopy case series. Ocul Surf. 2017;15:139–151. doi: 10.1016/j.jtos.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs I, Luna C, Quirce S, Mizerska K, Callejo G, Riestra A, Fernandez-Sanchez L, Meseguer VM, Cuenca N, Merayo-Lloves J, Acosta MC, Gasull X, Belmonte C, Gallar J. Abnormal activity of corneal cold thermoreceptors underlies the unpleasant sensations in dry eye disease. Pain. 2016;157:399–417. doi: 10.1097/j.pain.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labbe A, Alalwani H, Van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–4931. doi: 10.1167/iovs.11-8708. [DOI] [PubMed] [Google Scholar]
- 9.Launay PS, Godefroy D, Khabou H, Rostene W, Sahel JA, Baudouin C, Melik-Parsadaniantz S, Reaux-Le Goazigo A. Combined 3DISCO clearing method, retrograde tracer and ultramicroscopy to map corneal neurons in a whole adult mouse trigeminal ganglion. Exp Eye Res. 2015;139:136–143. doi: 10.1016/j.exer.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Launay PS, Reboussin E, Liang H, Kessal K, Godefroy D, Rostene W, Sahel JA, Baudouin C, Melik Parsadaniantz S, Reaux Le Goazigo A. Ocular inflammation induces trigeminal pain, peripheral and central neuroinflammatory mechanisms. Neurobiol Dis. 2016;88:16–28. doi: 10.1016/j.nbd.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Melik Parsadaniantz S, Rivat C, Rostene W, Reaux-Le Goazigo A. Opioid and chemokine receptor crosstalk: a promising target for pain therapy. Nat Rev Neurosci. 2015;16:69–78. doi: 10.1038/nrn3858. [DOI] [PubMed] [Google Scholar]
- 12.Rahman M, Okamoto K, Thompson R, Katagiri A, Bereiter DA. Sensitization of trigeminal brainstem pathways in a model for tear deficient dry eye. Pain. 2015;156:942–950. doi: 10.1097/j.pain.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]


