Abstract
Since the UK Prospective Diabetes Study (UKPDS), metformin has been considered the first-line medication for patients with newly diagnosed type 2 diabetes. Though direct evidence from specific trials is still lacking, several studies have suggested that metformin may protect from diabetes- and nondiabetes-related comorbidities, including cardiovascular, renal, neurological, and neoplastic diseases. In the past few decades, several mechanisms of action have been proposed to explain metformin’s protective effects, none being final. It is certain, however, that metformin increases lactate production, concentration, and, possibly, oxidation. Once considered a mere waste product of exercising skeletal muscle or anaerobiosis, lactate is now known to act as a major energy shuttle, redistributed from production sites to where it is needed. Through the direct uptake and oxidation of lactate produced elsewhere, all end organs can be rapidly supplied with fundamental energy, skipping glycolysis and its possible byproducts. Increased lactate production (and consequent oxidation) could therefore be considered a positive mechanism of action of metformin, except when, under specific circumstances, metformin and lactate become excessive, increasing the risk of lactic acidosis. We are proposing that, rather than considering metformin-induced lactate production as dangerous, it could be considered a mechanism through which metformin exerts its possible protective effect on the heart, kidneys, and brain and, to some extent, its antineoplastic action.
Introduction
Sixty years after its introduction in the diabetes pharmacopeia, metformin remains a milestone in the treatment of type 2 diabetes (T2D). From the UK Prospective Diabetes Study (UKPDS) (1) onward, virtually all guidelines (2,3) recommend metformin as first-line treatment for T2D. The well-known advantages of this agent include its glucose-lowering efficacy, low risk of hypoglycemia, modest body weight reduction, easy combination with almost any other glucose-lowering agent, and its low cost (2). Moreover, metformin is generally well tolerated, with diarrhea being the most common side effect. Lactic acidosis, the life-threatening condition potentially associated with the use of metformin, is a relatively rare event, usually developing in the setting of severe illness or predisposing conditions (4). Beyond its overall favorable risk-to-benefit ratio, it has been suggested that other properties of metformin may exert positive effects in T2D. Cardiovascular (CV) protection was initially reported in the UKPDS (1), while, more recently, certain studies have hypothesized a renoprotective effect (5). Discussion on the role of metformin in the prevention of cancer and cancer recurrence is still ongoing (6). Recent meta-analyses have concluded that metformin significantly improves cognitive dysfunction in patients with T2D (7). A very recent publication has reported results of a cohort study showing an association between metformin use and reduction of mortality risk and hospital readmission after a major surgical procedure (8). Finally, metformin use has been associated with reduced mortality in subjects with diabetes and coronavirus 2019 (COVID-19) (9). In spite of growing evidence for the beneficial pleiotropic effects of metformin, the true mechanism(s) accounting for all these effects remains elusive. Our aim, therefore, is to review the effects of metformin in an attempt to elucidate whether a unifying mechanism behind the multifaceted effects of the drug can be hypothesized.
Metformin and CV Protection
In the seminal UKPDS study, patients treated with metformin, compared with the conventional group, had risk reductions of 32% (95% CI 13–47, P = 0.002) for any diabetes-related end point, 42% for diabetes-related death (9–63, P = 0.017), and 36% for all-cause mortality (9–55, P = 0.011). Moreover, metformin showed a greater effect than chlorpropamide, glibenclamide, or insulin for any diabetes-related end point (P = 0.0034), all-cause mortality (P = 0.021), and stroke (P = 0.032). The CV protection was confirmed in the post-trial 10-year follow-up showing that, despite similar glycemic control in metformin-treated individuals compared with those on conventional therapy, the former retained significant risk reduction for any diabetes-related end point, diabetes-related death, all-cause death, and myocardial infarction (10). In spite of these exciting results, randomized control intervention trials designed to confirm metformin CV protection remain limited. Compared with the UKPDS, the Hyperinsulinemia: the Outcome of its Metabolic Effects (HOME) trial enrolled T2D subjects with longer disease duration in whom placebo or metformin were added to the existing insulin therapy (11). Although the trial did not show significant effects of metformin on primary composite of macro- and microvascular end points, it did show significant benefits (even after adjusting for changes in HbA1c level, daily dose of insulin, and systolic blood pressure) for the secondary macrovascular end point with a hazard ratio (HR) of 0.34 (95% CI 0.21–0.56, P = 0.001). In the study by Han et al. (12), T2D subjects with coronary artery disease randomized to either metformin or glipizide showed significant benefits for metformin on primary CV composite end points (HR 0.54, 95% CI 0.30–0.90, P = 0.026). This CV protective effect has been largely, though not universally, confirmed in observational studies. By and large, these studies have shown that metformin provides CV benefits as compared with lifestyle intervention, sulfonylureas, and acarbose (13). Beneficial effects of metformin were apparent in T2D subjects with established atherosclerotic CV disease, coronary heart disease, and elevated CV risk factors, including smoking, as well as in those with heart failure and chronic kidney disease (CKD) (13). The favorable CV effects of metformin may also apply to subjects with type 1 diabetes, as suggested by the results of the REducing with MetfOrmin Vascular Adverse Lesions (REMOVAL) trial (14). In this study, metformin did not significantly reduce the progression of mean carotid intima-media thickness (−0.005 mm per year, 95% CI −0.012 to 0.002, P = 0.1664), which was the primary end point of the study, although maximal carotid intima-media thickness (a prespecified tertiary outcome) was significantly reduced (−0.013 mm per year, −0.024 to −0.003, P = 0.0093).
Metformin and Renal Protection
Several experimental data support a potential beneficial effect of metformin on the kidney. In vitro and animal studies have provided evidence that metformin reduces tubulointerstitial fibrosis and epithelial-mesenchymal transition, arrests cyst growth in models of polycystic kidney disease, and prevents nephropathy induced by gentamicin, ureteral obstruction, ischemia-reperfusion, and streptozotocin-nicotinamide (15). As far as experimental diabetic nephropathy is concerned, metformin has been shown to prevent glucose-mediated apoptosis of human podocytes (16) and to reduce urinary albumin excretion in diabetic rats (17). Preclinical data are more abundant than clinical data. In a retrospective cohort study on kidney-transplanted subjects, metformin was found to be associated with better allograft survival and reduced mortality (18). Although the UKPDS did not show any apparent effect of metformin on renal death or renal failure, the trial was conducted in newly diagnosed patients and the total number of events was very low (1). In contrast, metformin was found to reduce the risk of severe kidney failure in a large U.K. primary care database (19). Moreover, as compared with sulfonylureas, initiation of therapy with metformin was associated with a lower risk of loss of kidney function (20). In a recent study in patients with type 1 diabetes (REMOVAL), there were no cases of estimated glomerular filtration rate (eGFR) declining to <30 mL ⋅ min−1 ⋅ 1.73 m−2 (14). In the metformin group, eGFR remained more stable over time, with a between-group difference of 4.0 mL ⋅ min−1 ⋅ 1.73 m−2 (2.19 to 5.81, P < 0.001) in favor of metformin over 3 years (14). In patients with diabetes in whom metformin was withdrawn because of renal dysfunction, a worsening of glycemic control was reported, along with earlier deterioration in kidney function (21). Recently, a large (>10,000 patients) retrospective observational cohort study (22) was conducted examining data from patients with T2D and CKD followed in two tertiary Korean hospitals. Even after propensity score matching, metformin use was associated with reduced risk of all-cause mortality and incident end-stage renal disease, whatever the eGFR at metformin initiation. This difference was present even in 208 patients with eGFR <30 mL ⋅ min−1 ⋅ 1.73 m−2. A currently ongoing trial, Metformin as RenoProtector of Progressive Kidney Disease (RenoMet), expected to end in 2021, is evaluating the use of metformin versus placebo in CKD patients without diabetes. The results of this trial will shed further light on the effects of metformin on cardiorenal outcomes.
Metformin and Cancer
The association between metformin use and reduction of cancer incidence and cancer mortality has been repeatedly reported. A summary of epidemiologic studies, as well as recently completed and ongoing clinical trials, compiled by Heckman-Stoddard et al. (6) in 2017 reported a reduction in overall cancer incidence and cancer-related mortality of 10% to 40% in different meta-analyses. This confirms data published in a previous meta-analysis by the same authors (23), in which caution was suggested with respect to the actual risk reduction. Similarly, meta-analyses have suggested a reduction in cancer-related mortality among individuals with colon, lung, and prostate cancer and improved survival in other neoplastic conditions. The review by Heckman-Stoddard et al. (6) also summarizes the results of 17 clinical trials completed so far. These trials vary with respect to patients enrolled, forms of cancer, and stage of the neoplastic disease and do not yield univocal results. Nonetheless, the interest in a possible beneficial effect of metformin in subjects with and without diabetes who have neoplastic disease has not lost steam. In their review, Heckman-Stoddard et al. counted at least 27 ongoing studies registered at ClinicalTrials.gov. Since that time, this number has been increasing, with >50 trials currently registered in the same repository. These trials may help not only in lending support to the protective effects of metformin but also in determining whether specific types of cancer may respond to the drug better than others.
Metformin and Cognitive Function
Cognitive dysfunction is currently considered one of the many complications of diabetes (24). In T2D there is an increased risk of both vascular dementia and Alzheimer disease (AD). Impaired insulin signaling, oxidative stress, and chronic inflammation are a common background for traditional diabetes complications and for degenerative diseases of the central nervous system. A recent meta-analysis of three studies has shown that the prevalence of cognitive impairment is significantly lower in T2D subjects receiving metformin (OR 0.55, 95% CI 0.39–0.78), while another series of six studies reported a significant reduction of the incidence of dementia (HR 0.76, 95% CI 0.39–0.88). In a survey including 67,731 participants, use of metformin was associated with attenuation of the risk of dementia in people with diabetes. This effect was independent of glycemic control and persisted in comparison with the use of other glucose-lowering agents. Orkaby et al. (25) have recently reported that, after accounting for confounding by indication, metformin was associated with a lower risk of subsequent dementia than sulfonylurea use in veterans <75 years of age. The Sydney Memory and Ageing Study, a prospective observational study, conducted on 1,037 community-dwelling participants without dementia aged 70–90 years at baseline, found that after 6 years, patients on metformin had significantly slower decline in global cognition and executive function, similar to that observed in subjects without diabetes. On the contrary, subjects with diabetes showed increased incident dementia (odds ratio 5.29, 95% CI 1.17–23.88, P = 0.05) (26). More recently, in a pilot study, 20 subjects without diabetes with mild cognitive impairment or mild dementia due to AD were randomized to receive metformin then placebo for 8 weeks each or vice versa. Metformin was associated with improved executive functioning, and trends suggested improvement in learning/memory and attention (27). It is, however, not just the potential therapeutic effect of metformin on AD that has attracted attention. A number of other neurodegenerative conditions seem to be potentially ameliorated by the drug, including epilepsy (28). A review by Markowicz-Piasecka et al. (29) explores several preclinical studies supporting the potential protective effect of metformin with respect to impairment in cognitive function and possible mechanisms of action.
The Possible Mechanism(s) Behind the Beneficial Pleiotropic Effects of Metformin
As mentioned, the mechanism(s) through which metformin exerts its numerous beneficial effects is still largely unclear. Scholars tend to focus on the activation of adenosine monophosphate-activated protein kinase (AMPK) (30), whose core function is to maintain energy homeostasis during the fasting state. The glucose-lowering effects of metformin are mainly due to a suppression of hepatic gluconeogenesis. In the liver, metformin has been shown to inhibit the mitochondrial respiratory chain leading to AMPK activation, improving insulin sensitivity and reducing cAMP with subsequent inhibition of the expression of gluconeogenic enzymes (31), although AMPK-independent mechanisms (Table 1) have been described as well (32). Interestingly, the AMPK/mTOR pathway has also been used to explain the molecular mechanisms underlying many of the pleiotropic effects of metformin.
Table 1.
Main molecular mechanism(s) of action of metformin
| AMPK activation (29) | |
| Inhibition of mitochondrial respiratory chain complex 1 (31) | |
| Increased ADP/ATP and AMP/ATP (31) | |
| Decrease in NF-κB (31) | |
| Inhibition of STAT3 (31) | |
| Inhibition of acetyl-CoA carboxylase (32) | |
| Inhibition of CPT1 (32) | |
| Inhibition of SHIP2 (41) | |
| Inhibition of mTOR pathway (5) | |
| Activation of AMPK/PGC1-α pathway (36) | |
| Activation of AMPK/eNOS pathway (36) |
In the myocardial cell, AMPK activation by metformin, through inhibition of acetyl-CoA carboxylase (ACC), reduces malonyl-CoA synthesis. Moreover, the inhibitory effect of AMPK on carnitine palmitoyl transferase 1 (CPT1) enhances fatty acid oxidation (33) with a concomitant increase in glucose uptake (32) via increased GLUT4 translocation and reduced endocytosis (34). AMPK is considered an important target for endothelial dysfunction and atherosclerosis, and available studies support a beneficial effect of AMPK activation on endothelial function due to an antioxidant effect in endothelial cells (35). Metformin may exert multiple beneficial effects also in patients with heart failure via activation of the AMPK/PGC1-α (36) and AMPK/eNOS (37) pathways and by protecting the myocardium under ischemia and ischemia-reperfusion injury conditions (38). Finally, metformin activation of AMPK has been shown to reduce chronic myocardium inflammation, cardiomyocyte apoptosis, and autophagy (39).
Recently, Ravindran et al. (5) have emphasized that the AMPK/mTOR pathway is responsible for the beneficial renal effects of metformin. AMPK also promotes autophagy, a decrease in endoplasmic reticulum stress, and the inhibition of inflammation and oxidation caused by advanced glycation end products (AGEs). The benefits of metformin use in diabetic nephropathy may, in fact, be due to AMPK’s mitigation of oxidative stress (40). It has also recently been reported that metformin directly binds to and reduces the catalytic activity of the recombinant SHIP2 phosphatase domain in vitro (41). SHIP2 is upregulated in animal models of diabetes and suppresses insulin signaling, leading to insulin resistance and decreased glucose transport (41). The reduction of SHIP2 activity has been proposed as the molecular mechanism by which metformin treatment reduces the loss of podocytes in T2D patients (41).
AMPK activation is also considered the main mechanism behind metformin’s protection against cancer, as it inhibits the mTOR pathway, reducing cell proliferation, apoptosis, and cell-cycle arrest of the neoplastic cell (42). This antineoplastic effect is likely the result of an insulin-lowering activity that may reduce proliferation in hyperinsulinemic individuals and the inhibition of mitochondrial respiration and energy reduction in the tumor cell (43).
AMPK is an important metabolic sensor in the central nervous system (44), and reduced levels have been described in neurogenerative conditions (45). Therefore, the well-known effect of metformin on AMPK activation has been used to explain its neuroprotective effect (28).
The widespread activation of AMPK induced by metformin closely mimics the imbalance between energy supply and energy consumption occurring in response to fasting and exercise. Like metformin, sodium–glucose cotransporter 2 inhibitors (SGLT2is) mimic fasting conditions (46), thus inducing an upregulation of AMPK and probably the activation of other factors that reduce cellular stress (e.g., sirtuin-1 and hypoxia inducible factors [47]), even though SGLT2i and metformin effects on gluconeogenesis are different. Typically, fasting or exercise induces an increase in alternative energy substrates such as lactate and β-hydroxybutyrate. As far as SGLT2is are concerned, Ferrannini et al. (48) hypothesized that the switch to ketones as a substrate is one of the reasons for the reduction in CV events seen with SGLT2is. With SGLT2i therapy, ketone concentration generally ranges between 0.1 mmol/L and 0.5 mmol/L (49), although under particular stress conditions, ketogenesis can be activated to the point of euglycemic ketoacidosis. Though ketone turnover during SGLT2i treatment has never been measured, ketone concentration appears too low to quantitatively represent a valid alternative to glucose as energy substrate.
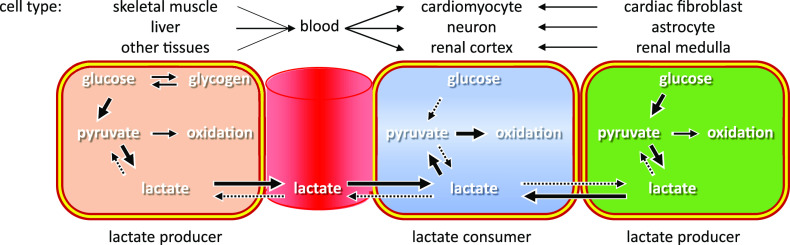
With metformin treatment, lactate concentration increases (50), and, unlike ketones, lactate concentration (∼1.0 mmol/L) is quantitatively adequate to act as an alternative substrate. The fundamental advantage of the use of lactate in heart, brain, and kidney is that it can be directly taken up and oxidized (Fig. 1) (51). We here hypothesize that at least some of the protective effect of metformin on the CV system, kidney, and brain and the antineoplastic effect may be mediated via the increased availability of lactate.
Figure 1.
Lactate shuttle promoted by metformin. Metformin promotes lactate production by virtually any tissue and cell containing glycogen and/or taking up glucose, either distal (including gut, liver, and muscles) or proximal (such as cardiac fibroblasts, astrocytes, or renal medullar cells). Cardiomyocytes, neurons, or renal cells can then uptake lactate and directly oxidize it for immediate use, bypassing glycolysis.
Lactic Acid as a Mediator of Pleiotropic Effects of Metformin
Glucose is produced mainly by the liver (and to some extent the kidney), while all other tissues and cells uptake glucose. On the contrary, lactate can be produced and released by virtually any tissue and cell containing glycogen and/or taking up glucose, although it is the gut wall that seems to account for the extra lactate reaching the circulation (52), most likely due to the very high local concentration of the drug. The extra lactate released by the gut is mainly conveyed to the liver through the vena porta where it is partly converted into glucose, increasing glucose turnover after administration of metformin (53). From this point of view, it is of note that recent work has indicated that metformin increases endogenous glucose production and glucose utilization in individuals with recent-onset T2D and in control subjects without diabetes (54). Whether metformin increases basal glucose rate of disappearance by facilitating glucose uptake in other tissues, such as the intestine, remains to be further clarified. This view, however, fits in with the block of the Cori cycle proposed by Bailey et al. (52), an effect that seems to be particularly apparent after a meal resulting in a redistribution of energy substrate. Lactate can be taken up by all tissues and directly oxidized for immediate use (51). Besides the gut–liver–muscle axis of energy homeostasis, lactate represents the major “cell-to-cell shuttle’’ and ‘‘intracellular shuttle’’ for the delivery of oxidative and gluconeogenic substrates, as well as being a main player in cell signalling (55,56). Cell–cell lactate shuttles include lactate exchanges between white glycolytic muscle and red oxidative muscle fibres within working muscles, but also (Fig. 1) between skeletal muscle and heart (57), brain (58), liver and kidneys (59), astrocytes and neurons, and neurons and astrocytes (60). In summary, lactic acid, as recently summarized, can no longer be considered a mere end product of glycolysis (61). This is even more apparent when we consider that lactate is actively transported across plasma membranes via monocarboxylate transporters and is an active ligand of GPR81, a G1-protein–coupled receptor, expressed in many organs and tissues including adipose tissue, skeletal muscle (62), liver, kidney, and brain (63). Thus, lactate can be considered an alternative substrate in virtually all tissues and becomes extremely important in organs damaged by diabetes.
In normal conditions, at rest, the myocardium constantly releases and takes up lactate, and these processes increase to a greater extent during atrial pacing (57). Moreover, lactate contributes in a similar proportion to glucose in supporting myocardial work (57). The diabetic heart has a reduced capacity for glucose utilization, particularly during ischemia. This defect can be partially compensated by hyperglycemia (64). Since cardiomyocytes preferentially use lactate, an increase in lactate levels, as elicited by metformin, may provide an alternative energy substrate. More recently it has been suggested that a fibroblast-to-cardiomyocyte shuttle could operate in the heart (65). According to this cell-to-cell shuttle, glucose is taken up by fibroblasts that release lactate to be used by cardiomyocytes (Fig. 1). These authors imply that this metabolic cross talk is impaired in insulin-resistant animals and underline the need for considering this mechanism in designing therapeutic strategies for treating heart failure and diabetes-related heart conditions. It is therefore appealing to hypothesize that metformin, by improving insulin action on myocardial fibroblasts, may restore cell-to-cell lactate shuttle, thus contributing to CV protection. More recently, experimental data have shown that GPR81 activation by physiologically elevated lactate concentrations can rescue oxidative stress and reduce inflammatory cytokines in the endothelial cell (66), thus lending support to a potential antiatherosclerotic action.
Lactate is a major substrate for the kidney. It is used in part for gluconeogenesis but mostly as a readily available substrate to support the high energy requirements of renal function (67). In T2D, renal glycolysis is increased and is apparently uncoupled from mitochondrial respiration. If mitochondrial respiration is sufficient to consume the pyruvate produced and to keep glycolytic flux going, increased glycolytic flux can prevent the accumulation of the toxic glucose metabolites that occur under the hyperglycemic condition (e.g., polyols). Qi et al. (68) performed a proteomics analysis on the glomeruli of patients with and without nephropathy who had had diabetes for over 50 years. Patients without nephropathy had high levels of enzymes involved in regulating glycolysis and mitochondrial respiration; in particular, elevated pyruvate kinase M2 was identified as a protective factor in countering the development of nephropathy. If mitochondrial function is impaired, increased glycolysis can lead to an accumulation of glycolytic intermediates that overflow into the polyol pathway, where they are processed into fructose (69). Fructose is then phosphorylated by fructokinase, leading to the formation of uric acid and AGEs, one of the distinguishing features of renal damage in diabetes. Sas et al. (70) found that urinary markers of increased renal glycolysis, combined with the presence of altered mitochondrial proteins, could be predictors of nephropathy in patients with diabetes. Since lactate is taken up directly from the circulation without glycolysis in the kidneys (Fig. 1), no glycolytic metabolites are produced, and only the amount of lactate that can be oxidized is taken up. The pathophysiologic relevance of this glycolytic overflow system in kidney injury and repair was further illustrated by a study by Andres-Hernando et al. (71) that showed that fructokinase haploinsufficiency protects mice from ischemic kidney injury. Fructokinase-deficient mice had reduced ATP depletion, lower renal uric acid levels, lower markers of oxidative stress, and less kidney injury compared with wild-type mice with ischemic kidney injury (71). Lactate is therefore a clean-burning fuel (compared with glucose) providing the kidneys with energy and protecting them from the accumulation of waste products (AGEs).
Lactate is also a key energy substrate in the central nervous system (72). Maran et al. (73) have shown that lactate is an alternative and ideal substrate for the brain in hypoglycemic conditions, and a growing body of literature supports its protective effect on brain tissue (74). In the brain, astrocytes take up most of the glucose supply and metabolize it to lactate, which is actively transported to neurons where it can enter the oxidative pathway (Fig. 1), exert signaling functions (72), and activate genes related to long-term memory formation (75). Therefore, lactate-induced GPR81 activation can play a critical role in learning and memory formation. As such, lactate could also reduce memory loss and cognitive impairment. These effects, coupled with the increased production of anti-inflammatory cytokines and reduction of proinflammatory ones (76), can account for the neuroprotective effects of increased lactate concentration accompanying the use of metformin. It is tempting to suggest that similar mechanisms may occur in parts of the retina. In inner retinal cells, lactate has been found to exert a protective function against glutamate excitotoxicity and neuroinflammation and to regulate cellular volume and metabolism (77). Lactate has, in particular, demonstrated a beneficial effect in promoting Müller cell function and survival (78).
Though lactate, as discussed, may exert favorable effects in the heart, kidney, and brain, its role in metformin’s antineoplastic effects may not be as simple. The high glycolytic rate of the neoplastic cell is responsible for a large production of lactate that contributes to tumor microenvironment acidosis. The increased local lactate availability has been claimed to favor tumor growth by interfering with inflammatory response and macrophage functions and by favoring angiogenesis (61). Metformin may exert antineoplastic effects through indirect (insulin-dependent) and direct (insulin-independent) mechanisms, as well as via disruption of glycolytic activity and the tricarboxylic acid cycle (79). Metabolomic studies have shown that despite promoting glucose consumption and lactate production, metformin ultimately decreases specific glycolytic intermediates as well as the levels of almost all intermediates in the tricarboxylic acid cycle (80). Therefore, though increased lactate production induced by metformin may not exert a direct effect, it is, nonetheless, a marker of the antineoplastic action of the drug.
In summary, the physiologically high concentration of lactic acid and the activation of cell-to-cell lactate shuttle occurring with the use of metformin could contribute to the many beneficial effects of the drug; the increase in lactate concentration, however, is usually seen as a forerunner of lactic acidosis.
Metformin and Lactic Acidosis
Concentrations of lactate in the blood are normally stable, as production and consumption are equivalent (51). As mentioned, one of the major effects of metformin is to increase lactate production (50), mainly by the gut wall. Since, in normal conditions, lactate clearance is proportional to its concentration, the increase in lactate during metformin treatment is usually minimal (50). Severe hyperlactatemia thus occurs mainly when clearance decreases, leading to lactic acidosis. When occurring during metformin treatment (metformin-associated lactic acidosis or MALA), lactic acidosis is classified as a type B, as it develops when clearance of lactic acid by oxidation or gluconeogenesis is impaired, although it can present with features of type A and as such hypoxemia should be considered a contraindication for its use.
Metformin is not metabolized and is eliminated unchanged primarily by the kidney (81). Metformin clearance therefore depends only on renal function and, when filtration is severely reduced, it accumulates in the plasma. The actual level of concentration of plasma metformin at which the risk of MALA increases remains a matter of debate. With the recommended dose and schedules, metformin concentrations are usually <1 mg/L and rarely exceed 5 mg/L; only persistent levels above this value seem to increase the risk of MALA (81). More recently, in metformin-treated patients admitted in an emergency context, plasma metformin concentrations ≥9.9 mg/L were strongly associated with the presence of lactic acidosis (82).
MALA is, however, an extremely rare event, with its incidence being estimated at 7.4 per 100,000 person-years (83); thus, event rates are very low and, moreover, based primarily on case reports (84). These reports rarely provide adequate details on the clinical context, doses, concomitant pathologies, or events (15). A recently published study by Lalau and colleagues (15) investigated various dose regimens for metformin in moderate and severe CKD (stages 3A/3B and 4, respectively) in terms of safety and efficacy. Levels of plasma metformin remained consistently below 2.5 mg/L in most patients. As mentioned above, a large cohort study (22) found no increase in MALA with metformin use, even in patients with eGFR <30 mL ⋅ min−1 ⋅ 1.73 m−2.
Despite the low incidence of MALA, however, there is continuing concern on behalf of health authorities and medical communities as to its use in at-risk patients. A number of health authorities and diabetes associations recommend commencing metformin in patients with eGFR >45 mL ⋅ min−1 ⋅ 1.73 m−2 and continuing with additional caution and dose reduction if the eGFR decreases to 30 to <45 mL ⋅ min−1 ⋅ 1.73 m−2 (85), as do several studies (86). Using lower doses of metformin, at 500/1,000 mg per day, could be safe even in patients with severe renal impairment (84), and patients should be advised to adopt “sick-day rules” in case of acute and severe illness. Moreover, to date, no dose-response study has demonstrated the occurrence of severe adverse events for a determined dose or eGFR, and current dose level recommendations are based on estimates dictated by caution. The relationship between metformin and lactic acidosis, therefore, is far from cut and dried, and, considering the widespread use of the drug, MALA is a rare event. Bennis et al. (82) have recently reported the association between plasma metformin concentration, lactic acidosis, and mortality in 194 consecutive patients with diabetes admitted to intensive care units (ICUs). Eighty-seven patients (44.8%) had lactic acidosis (i.e., arterial blood pH <7.35 and lactate concentration ≥4 mmol/L), and 38 of them (43.7%) died in the ICU. A metformin concentration ≥9.9 mg/L was significantly associated with lactic acidosis, but this metformin concentration was also associated with less ICU deaths, making the relationship between metformin level, lactate concentration, and mortality less clear. The benefits provided by using metformin in patients with CKD far outweigh its potential risks. Therefore, in consideration of the advantages provided by metformin in the maintenance of good glucose control and in delaying the deterioration of kidney function, MALA should be considered a manageable contraindication with a close monitoring of dosage and conditions in CKD patients. Prescribers and CKD patients on metformin should be educated to suspend metformin temporarily in case of acute severe illness, especially acute kidney injury, as lactate levels rise markedly in these circumstances (87).
Conclusions
Metformin has been in use for more than 60 years and is still the first-choice drug for T2D. After the initial suggestion that metformin could provide CV protection, the additional data collected indicate not only that the drug can be used more liberally with respect to renal function, but that it could contribute to renal protection. Data also indicate that metformin may reduce the risk of neurodegenerative conditions, and trials are ongoing to directly assess the antineoplastic properties of the drug. Nonetheless, despite wide and long-standing experience in the clinical use of the drug, its mode of action is still not fully understood, and the protective action it may exert on the CV system, kidney, and brain and against cancer is clearly largely independent of its glucose-lowering efficacy. The molecular mechanisms of action mainly involve AMPK/mTOR pathway activation, much in line with what happens under conditions of energy restriction. These effects may be considered as being to some extent similar to those produced by SGLT2is, another class of glucose-lowering agents with proven cardiorenal protection. The metabolic effects of metformin and SGLT2is may indeed have some similarities. For instance, use of SGLT2is elicits a moderate increase in plasma concentration of ketone bodies, an alternative energy substrate that has been claimed to contribute to their CV benefit. Interestingly, metformin use is also associated with increased blood levels of another alternative fuel substrate, lactic acid. On top of this, evidence exists for a critical role of the cell-to-cell lactate shuttle, with lactate being an active ligand to specific receptors through which energy regulation, anti-inflammatory response, immune tolerance, antifibrotic action, gene plasticity, and so on, can be exerted (88). The analogy between SGLT2is and metformin becomes even more fitting if we consider the potential risk of the accumulation of the alternative substrate that may lead to an unwanted severe reaction. In the case of SGLT2is, stress conditions and relative low insulin availability can elicit excessive activation of ketogenesis with the development of euglycemic ketoacidosis (89). In the case of metformin, hypoxemic conditions can result in excessive pyruvate reduction to lactate with the development of lactic acidosis (84). Interestingly, similar recommendations exist to reduce the risk of these threatening conditions, for both treatments (87). In summary, we hypothesize that appropriate use of these drugs provides advantages, at least in part due to the physiologic increase of active substrates: ketones for SGLT2is and, possibly, lactate for metformin. This hypothesis, obviously, will require specific studies designed to establish whether the activation of the lactate shuttle, along with the elevation of circulating lactate levels, can indeed represent a potential mechanism accounting for the multiple actions metformin is believed to exert.
Article Information
Acknowledgments. The authors wish to thank Serena Rotunno (Department of Translational Medicine and Surgery, Università Cattolica del Sacro Cuore, Rome, Italy) for editing and revising the manuscript.
Duality of Interest. A.G. has undertaken ad hoc consultancy and/or sponsored lectures for Amgen, AstraZeneca, Boehringer Ingelheim, MSD, Novo Nordisk, and Sanofi and received research grants from AstraZeneca. A.S. has undertaken ad hoc consultancy and/or sponsored lectures for AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Mundipharma. S.F. reports ad hoc consultancy and/or sponsored lectures from Abbott, Eli Lilly, MSD, and Novo Nordisk. S.D.P. reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, and Novartis Pharmaceuticals and personal fees from Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk A/S, Laboratoires Servier, Sanofi, and Takeda Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
References
- 1.UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 2.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2020;63:221–228 [DOI] [PubMed] [Google Scholar]
- 3.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract 2020;26:107–139 [DOI] [PubMed] [Google Scholar]
- 4.Rajasurya V, Anjum H, Surani S. Metformin use and metformin-associated lactic acidosis in intensive care unit patients with diabetes. Cureus 2019;11:e4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravindran S, Kuruvilla V, Wilbur K, Munusamy S. Nephroprotective effects of metformin in diabetic nephropathy. J Cell Physiol 2017;232:731–742 [DOI] [PubMed] [Google Scholar]
- 6.Heckman-Stoddard BM, DeCensi A, Sahasrabuddhe VV, Ford LG. Repurposing metformin for the prevention of cancer and cancer recurrence. Diabetologia 2017;60:1639–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q-Q, Li W-S, Liu Z, Zhang H-L, Ba Y-G, Zhang R-X. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: a meta-analysis and systematic review. Medicine (Baltimore) 2020;99:e19378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reitz KM, Marroquin OC, Zenati MS, et al. Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg 2020;155:e200416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheen AJ. Metformin and COVID-19: from cellular mechanisms to reduced mortality. Diabetes Metab 2020;46:423–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 11.Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009;169:616–625 [DOI] [PubMed] [Google Scholar]
- 12.Han Y, Xie H, Liu Y, Gao P, Yang X, Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 2019;18:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zilov AV, Abdelaziz SI, AlShammary A, et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab Res Rev 2019;35:e3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrie JR, Chaturvedi N, Ford I, et al.; REMOVAL Study Group . Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2017;5:597–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Broe ME, Kajbaf F, Lalau J-D. Renoprotective effects of metformin. Nephron 2018;138:261–274 [DOI] [PubMed] [Google Scholar]
- 16.Langer S, Kreutz R, Eisenreich A. Metformin modulates apoptosis and cell signaling of human podocytes under high glucose conditions. J Nephrol 2016;29:765–773 [DOI] [PubMed] [Google Scholar]
- 17.Zhai L, Gu J, Yang D, Hu W, Wang W, Ye S. Metformin ameliorates podocyte damage by restoring renal tissue nephrin expression in type 2 diabetic rats. J Diabetes 2017;9:510–517 [DOI] [PubMed] [Google Scholar]
- 18.Stephen J, Anderson-Haag TL, Gustafson S, Snyder JJ, Kasiske BL, Israni AK. Metformin use in kidney transplant recipients in the United States: an observational study. Am J Nephrol 2014;40:546–553 [DOI] [PubMed] [Google Scholar]
- 19.Hippisley-Cox J, Coupland C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. BMJ 2016;352:i1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hung AM, Roumie CL, Greevy RA, et al. Kidney function decline in metformin versus sulfonylurea initiators: assessment of time-dependent contribution of weight, blood pressure, and glycemic control. Pharmacoepidemiol Drug Saf 2013;22:623–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panossian Z, Drury PL, Cundy T. Reversible severe deterioration of glycaemic control after withdrawal of metformin treatment. Diabetologia 2012;55:267–269 [DOI] [PubMed] [Google Scholar]
- 22.Kwon S, Kim YC, Park JY, et al. The long-term effects of metformin on patients with type 2 diabetic kidney disease. Diabetes Care 2020;43:948–955 [DOI] [PubMed] [Google Scholar]
- 23.Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 2014;7:867–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simó R, Ciudin A, Simó-Servat O, Hernández C. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-the diabetologist’s perspective. Acta Diabetol 2017;54:417–424 [DOI] [PubMed] [Google Scholar]
- 25.Orkaby AR, Cho K, Cormack J, Gagnon DR, Driver JA. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 2017;89:1877–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samaras K, Makkar S, Crawford JD, et al. Metformin use is associated with slowed cognitive decline and reduced incident dementia in older adults with type 2 diabetes: the Sydney Memory and Ageing Study. Diabetes Care 2020;43:2691–2701 [DOI] [PubMed] [Google Scholar]
- 27.Koenig AM, Mechanic-Hamilton D, Xie SX, et al. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord 2017;31:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HS Nandini, Paudel YN, Krishna KL. Envisioning the neuroprotective effect of metformin in experimental epilepsy: a portrait of molecular crosstalk. Life Sci 2019;233:116686. [DOI] [PubMed] [Google Scholar]
- 29.Markowicz-Piasecka M, Sikora J, Szydłowska A, Skupień A, Mikiciuk-Olasik E, Huttunen KM. Metformin: a future therapy for neurodegenerative diseases: theme: drug discovery, development and delivery in Alzheimer’s disease guest editor: Davide Brambilla. Pharm Res 2017;34:2614–2627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017;60:1577–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol 2019;15:569–589 [DOI] [PubMed] [Google Scholar]
- 33.Ruderman NB, Park H, Kaushik VK, et al. AMPK as a metabolic switch in rat muscle, liver and adipose tissue after exercise. Acta Physiol Scand 2003;178:435–442 [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol 2007;293:H457–H466 [DOI] [PubMed] [Google Scholar]
- 35.Sambe T, Mason RP, Dawoud H, Bhatt DL, Malinski T. Metformin treatment decreases nitroxidative stress, restores nitric oxide bioavailability and endothelial function beyond glucose control. Biomed Pharmacother 2018;98:149–156 [DOI] [PubMed] [Google Scholar]
- 36.Gundewar S, Calvert JW, Jha S, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res 2009;104:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X-F, Zhang J-Y, Li L, Zhao X-Y, Tao H-L, Zhang L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol 2011;38:94–101 [DOI] [PubMed] [Google Scholar]
- 38.Ye Y, Perez-Polo JR, Aguilar D, Birnbaum Y. The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res Cardiol 2011;106:925–952 [DOI] [PubMed] [Google Scholar]
- 39.Lu Q, Li X, Liu J, et al. AMPK is associated with the beneficial effects of antidiabetic agents on cardiovascular diseases. Biosci Rep 2019;39:BSR20181995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren H, Shao Y, Wu C, Ma X, Lv C, Wang Q. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway. Mol Cell Endocrinol 2020;500:110628. [DOI] [PubMed] [Google Scholar]
- 41.Polianskyte-Prause Z, Tolvanen TA, Lindfors S, et al. Metformin increases glucose uptake and acts renoprotectively by reducing SHIP2 activity. FASEB J 2019;33:2858–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han G, Gong H, Wang Y, Guo S, Liu K. AMPK/mTOR-mediated inhibition of survivin partly contributes to metformin-induced apoptosis in human gastric cancer cell. Cancer Biol Ther 2015;16:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pollak MN. Investigating metformin for cancer prevention and treatment: the end of the beginning. Cancer Discov 2012;2:778–790 [DOI] [PubMed] [Google Scholar]
- 44.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen Z-P, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci 1999;24:22–25 [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Zhu B, Zheng F, et al. Chronic metformin treatment facilitates seizure termination. Biochem Biophys Res Commun 2017;484:450–455 [DOI] [PubMed] [Google Scholar]
- 46.Giaccari A. Sodium-glucose co-transporter inhibitors: medications that mimic fasting for cardiovascular prevention. Diabetes Obes Metab 2019;21:2211–2218 [DOI] [PubMed] [Google Scholar]
- 47.Wang S-Y, Cai G-Y, Chen X-M. Energy restriction in renal protection. Br J Nutr 2018;120:1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114 [DOI] [PubMed] [Google Scholar]
- 49.Ferrannini E, Baldi S, Frascerra S, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes 2016;65:1190–1195 [DOI] [PubMed] [Google Scholar]
- 50.Davis TME, Jackson D, Davis WA, Bruce DG, Chubb P. The relationship between metformin therapy and the fasting plasma lactate in type 2 diabetes: the Fremantle Diabetes Study. Br J Clin Pharmacol 2001;52:137–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Hall G. Lactate kinetics in human tissues at rest and during exercise. Acta Physiol (Oxf) 2010;199:499–508 [DOI] [PubMed] [Google Scholar]
- 52.Bailey CJ, Wilcock C, Scarpello JHB. Metformin and the intestine. Diabetologia 2008;51:1552–1553 [DOI] [PubMed] [Google Scholar]
- 53.Bailey CJ, Wilcock C, Day C. Effect of metformin on glucose metabolism in the splanchnic bed. Br J Pharmacol 1992;105:1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gormsen LC, Søndergaard E, Christensen NL, Brøsen K, Jessen N, Nielsen S. Metformin increases endogenous glucose production in non-diabetic individuals and individuals with recent-onset type 2 diabetes. Diabetologia 2019;62:1251–1256 [DOI] [PubMed] [Google Scholar]
- 55.Brooks GA. Cell-cell and intracellular lactate shuttles. J Physiol 2009;587:5591–5600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brooks GA. Lactate shuttles in nature. Biochem Soc Trans 2002;30:258–264 [DOI] [PubMed] [Google Scholar]
- 57.Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol 2009;587:2087–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glenn TC, Martin NA, Horning MA, et al. Lactate: brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma 2015;32:820–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol Endocrinol Metab 2000;278:E244–E251 [DOI] [PubMed] [Google Scholar]
- 60.Pellerin L, Pellegri G, Bittar PG, et al. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 1998;20:291–299 [DOI] [PubMed] [Google Scholar]
- 61.Certo M, Marone G, de Paulis A, Mauro C, Pucino V. Lactate: fueling the fire starter. Wiley Interdiscip Rev Syst Biol Med 2020;12:e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kuei C, Yu J, Zhu J, et al. Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol Pharmacol 2011;80:848–858 [DOI] [PubMed] [Google Scholar]
- 63.Lauritzen KH, Morland C, Puchades M, et al. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex 2014;24:2784–2795 [DOI] [PubMed] [Google Scholar]
- 64.Stanley WC. Rationale for a metabolic approach in diabetic coronary patients. Coron Artery Dis 2005;16(Suppl. 1):S11–S15 [DOI] [PubMed] [Google Scholar]
- 65.Gizak A, McCubrey JA, Rakus D. Cell-to-cell lactate shuttle operates in heart and is important in age-related heart failure. Aging (Albany NY) 2020;12:3388–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Z, Han Y, Song S, Chen T, Han Y, Liu Y. Activation of GPR81 by lactate inhibits oscillatory shear stress-induced endothelial inflammation by activating the expression of KLF2. IUBMB Life 2019;71:2010–2019 [DOI] [PubMed] [Google Scholar]
- 67.Cohen JJ, Little JR. Lactate metabolism in the isolated perfused rat kidney: relations to renal function and gluconeogenesis. J Physiol 1976;255:399–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi W, Keenan HA, Li Q, et al. Pyruvate kinase M2 activation may protect against the progression of diabetic glomerular pathology and mitochondrial dysfunction. Nat Med 2017;23:753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gugliucci A. Formation of fructose-mediated advanced glycation end products and their roles in metabolic and inflammatory diseases. Adv Nutr 2017;8:54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 2016;1:e86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Andres-Hernando A, Li N, Cicerchi C, et al. Protective role of fructokinase blockade in the pathogenesis of acute kidney injury in mice. Nat Commun 2017;8:14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barros LF. Metabolic signaling by lactate in the brain. Trends Neurosci 2013;36:396–404 [DOI] [PubMed] [Google Scholar]
- 73.Maran A, Crepaldi C, Trupiani S, et al. Brain function rescue effect of lactate following hypoglycaemia is not an adaptation process in both normal and type I diabetic subjects. Diabetologia 2000;43:733–741 [DOI] [PubMed] [Google Scholar]
- 74.Maran A, Cranston I, Lomas J, Macdonald I, Amiel SA. Protection by lactate of cerebral function during hypoglycaemia. Lancet 1994;343:16–20 [DOI] [PubMed] [Google Scholar]
- 75.Suzuki A, Stern SA, Bozdagi O, et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011;144:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nasi A, Fekete T, Krishnamurthy A, et al. Dendritic cell reprogramming by endogenously produced lactic acid. J Immunol 2013;191:3090–3099 [DOI] [PubMed] [Google Scholar]
- 77.Country MW. Retinal metabolism: a comparative look at energetics in the retina. Brain Res 2017;1672:50–57 [DOI] [PubMed] [Google Scholar]
- 78.Vohra R, Aldana BI, Waagepetersen H, Bergersen LH, Kolko M. Dual properties of lactate in Müller cells: the effect of GPR81 activation. Invest Ophthalmol Vis Sci 2019;60:999–1008 [DOI] [PubMed] [Google Scholar]
- 79.Gong J, Kelekar G, Shen J, Shen J, Kaur S, Mita M. The expanding role of metformin in cancer: an update on antitumor mechanisms and clinical development. Target Oncol 2016;11:447–467 [DOI] [PubMed] [Google Scholar]
- 80.Janzer A, German NJ, Gonzalez-Herrera KN, Asara JM, Haigis MC, Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. Proc Natl Acad Sci U S A 2014;111:10574–10579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lipska KJ, Bailey CJ, Inzucchi SE. Use of metformin in the setting of mild-to-moderate renal insufficiency. Diabetes Care 2011;34:1431–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bennis Y, Bodeau S, Batteux B, et al. A study of associations between plasma metformin concentration, lactic acidosis, and mortality in an emergency hospitalization context. Crit Care Med 2020;48:e1194–e1202 [DOI] [PubMed] [Google Scholar]
- 83.Eppenga WL, Lalmohamed A, Geerts AF, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care 2014;37:2218–2224 [DOI] [PubMed] [Google Scholar]
- 84.DeFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 2016;65:20–29 [DOI] [PubMed] [Google Scholar]
- 85.National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. NICE guideline [NG28]. Accessed 14 February 2020. Available from https://www.nice.org.uk/guidance/ng28/chapter/1-recommendations
- 86.Ekström N, Schiöler L, Svensson A-M, et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2012;2:e001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacCallum L, Senior PA. Safe use of metformin in adults with type 2 diabetes and chronic kidney disease: lower dosages and sick-day education are essential. Can J Diabetes 2019;43:76–80 [DOI] [PubMed] [Google Scholar]
- 88.Sun S, Li H, Chen J, Qian Q. Lactic acid: no longer an inert and end-product of glycolysis. Physiology (Bethesda) 2017;32:453–463 [DOI] [PubMed] [Google Scholar]
- 89.Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA Protocol. Diabetes Obes Metab 2019;21:2192–2202 [DOI] [PubMed] [Google Scholar]