OBJECTIVE
We examined the glucose response curves (biphasic [BPh], monophasic [MPh], incessant increase [IIn]) during an oral glucose tolerance test (OGTT) and their relationship to insulin sensitivity (IS) and β-cell function (βCF) in youth versus adults with impaired glucose tolerance or recently diagnosed type 2 diabetes.
RESEARCH DESIGN AND METHODS
This was both a cross-sectional and a longitudinal evaluation of participants in the RISE study randomized to metformin alone for 12 months or glargine for 3 months followed by metformin for 9 months. At baseline/randomization, OGTTs (85 youth, 353 adults) were categorized as BPh, MPh, or IIn. The relationship of the glucose response curves to hyperglycemic clamp–measured IS and βCF at baseline and the change in glucose response curves 12 months after randomization were assessed.
RESULTS
At randomization, the prevalence of the BPh curve was significantly higher in youth than adults (18.8% vs. 8.2%), with no differences in MPh or IIn. IS did not differ across glucose response curves in youth or adults. However, irrespective of curve type, youth had lower IS than adults (P < 0.05). βCF was lowest in IIn versus MPh and BPh in youth and adults (P < 0.05), yet compared with adults, youth had higher βCF in BPh and MPh (P < 0.005) but not IIn. At month 12, the change in glucose response curves did not differ between youth and adults, and there was no treatment effect.
CONCLUSIONS
Despite a twofold higher prevalence of the more favorable BPh curve in youth at randomization, RISE interventions did not result in beneficial changes in glucose response curves in youth compared with adults. Moreover, the typical β-cell hypersecretion in youth was not present in the IIn curve, emphasizing the severity of β-cell dysfunction in youth with this least favorable glucose response curve.
Introduction
Studies in youth and adults have shown that the shape of the oral glucose tolerance test (OGTT) glucose response curve identifies physiologically distinct groups of individuals with abnormalities in insulin secretion and insulin sensitivity (IS) (1–5). Individuals with a monophasic (MPh) OGTT have lower IS and decreased β-cell function (βCF) compared with individuals with a biphasic (BPh) OGTT (1,2,4,5). A small percentage of individuals have an upward (3), or monotonous (6), or incessant increase (IIn) (7) in OGTT glucose concentrations that remain elevated at 120 min, and others have more complex (6,8) or unclassified shapes (9).
In youth and adults without diabetes, the MPh curve is the dominant phenotype, but its frequency differs between the two age-groups: 35–69% in youth (1,2,6,9) and 57–84% in adults (4,8,10–12). Although these are not head-to-head comparisons and included a heterogeneous group of participants, they do suggest a potential divergence between youth and adults in the prevalence and/or the characteristics of the glucose response curves. In the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study of 662 participants, the most frequent glucose response curve was MPh followed by IIn, and the least frequent was BPh (7). The IIn group had higher HbA1c, lower βCF relative to IS, higher glycemic failure rates, and greater declines in βCF over time compared with the MPh and BPh groups, with no difference in IS (7). Data in adults with established diabetes are somewhat limited, showing that the MPh curve is the most common (3,5,8), with (3) or without (5,8) an upward curve. Furthermore, there are no longitudinal studies of the glucose response curves in adults with diabetes.
To date, our landmark observations in the Restoring Insulin Secretion (RISE) study demonstrated that youth with dysglycemia, compared with adults, have ∼50% lower IS together with hyperresponsive β-cells across a wide range of IS (13,14). Furthermore, they had worse β-cell outcomes compared with adults in response to similar interventions (15,16). Against this RISE background and the TODAY findings, we hypothesized that 1) the BPh curve, a more favorable glucose response curve, will be less frequent in youth than adults and 2) in response to similar interventions, the change in glucose response curves will be to metabolically less favorable curve types in youth compared with adults. Therefore, we aimed to examine 1) the distribution of glucose response curves in youth compared with adults at baseline/randomization, 2) the relationship of the glucose response curves to IS and β-cell responses derived from the hyperglycemic clamp in youth compared with adults, and 3) treatment-associated (metformin for 12 months or glargine for 3 months followed by metformin for 9 months) longitudinal changes in glucose response curves and glycemia in youth compared with adults.
Research Design and Methods
The detailed description of the RISE protocol is available online (https://rise.bsc.gwu.edu/web/rise/collaborators) (17), with the primary outcome results published (15,16,18). Briefly, youth participants (n = 91) in RISE were 10–19 years old, with BMI ≥85th percentile, and adults (n = 355) were 20–65 years old with BMI >25 kg/m2. Both age-groups had impaired glucose tolerance (IGT) or recently diagnosed type 2 diabetes on the basis of a screening 75-g OGTT (17). For the baseline cross-sectional analysis, adult participants from both the RISE Medication Study (n = 267) and the RISE Surgery Study (BetaFat) (n = 88) were combined, and randomization OGTT was used for classifying the glucose response curves. Data from eight participants (six youth and two adults) were excluded because of invalid/nonfasting values for the OGTT (n = 4) or because of missing values at one of the five OGTT time points (0, 30, 60, 90, or 120 min) used in the classification of the glucose response curves (n = 4). Thus, the baseline cross-sectional analysis included 85 youth (52 with IGT and 33 with type 2 diabetes) and 353 adults (249 with IGT and 104 with type 2 diabetes). The longitudinal cohort, from baseline to month 12, included only participants with valid and nonmissing data at both baseline and month 12 who were randomized to glargine for 3 months followed by metformin for 9 months (37 youth and 61 adults) or to metformin alone for 12 months (39 youth and 84 adults). Adults who were randomized to the other medication interventions of RISE (liraglutide combined with metformin or placebo [17,18]), which were not treatments in the youth medication study (15,16), were not included in this longitudinal analysis since the aim was to compare youth with adults with similar treatments over time.
Procedures
Anthropometric measurements were performed as described before (14,17). Following a 10-h overnight fast, a 3-h 75-g OGTT was performed at randomization as described before (14), with blood samples obtained at 10, 20, 30, 60, 90, 120, 150, and 180 min after glucose ingestion. Blood samples were immediately placed on ice before separation and frozen at −80°C for shipment to the central biochemistry laboratory at the University of Washington for measurement of plasma glucose, C-peptide, and insulin.
A two-step hyperglycemic clamp was performed after a 10-h overnight fast as described in detail before (13,17). Briefly, the steady-state target glucose concentration for the first step was 11.1 mmol/L, achieved with an initial intravenous bolus of 20% dextrose, followed by a variable rate infusion of 20% dextrose that was based on a computerized algorithm together with bedside blood glucose monitoring every 5–10 min. Arterialized heated-hand venous blood samples were obtained before and at 2, 4, 6, 8, 10, 100, 110, and 120 min. For the second step, the target blood glucose of >25 mmol/L was achieved using a second bolus of 20% dextrose with increased rates of the 20% dextrose infusion adjusted on the basis of bedside blood glucose monitoring every 5 min. Once the target blood glucose of >25 mmol/L was attained, a bolus of L-arginine (5 g) was administered over 1 min, with blood samples drawn at −5, −1, 2, 3, 4, and 5 min relative to the arginine injection (13,17).
Assays and Calculations
Plasma glucose (hexokinase method Cobas c501 autoanalyzer; Roche), C-peptide and insulin (two-site immunoenzymatic assay), and HbA1c (high-performance liquid chromatography) were performed at the RISE central biochemistry laboratory (Northwest Lipid Research Laboratory, University of Washington) as previously described in detail with their coefficients of variation (13,14). For plasma glucose, the interassay coefficient of variation on quality control samples with low, medium, and high concentrations was 2.1%, 1.7%, and 1.3%, respectively.
Calculations for clamp-derived IS (M/I), acute (0–10 min, first phase) C-peptide response to glucose (ACPRg), steady-state (second phase) C-peptide (SSCP), and acute C-peptide response to arginine at maximal glycemic potentiation (ACPRmax) were described in detail previously (13,17). Total area under the curve (AUC) for glucose, C-peptide, and insulin was calculated by the trapezoidal method using five time points (0, 30, 60, 90, and 120 min) over the 2 h of the OGTT (2,7).
Classification of OGTT Glucose Response Curves
At baseline, each participant’s glucose response curve was classified into one of three categories using plasma glucose concentrations over the first 2 h of the 3-h OGTT performed at randomization (1–4,6,7). An MPh curve, defined as a gradual increase in glucose concentrations between 30 and 90 min until a peak was reached followed by a subsequent decline of ≥0.25 mmol/L; a BPh curve, defined as a rise of glucose to a peak followed by a fall (as in the MPh) but then followed by a second rise of ≥0.25 mmol/L; and an upward or IIn curve, defined as a continuous increase in plasma glucose during the 2 h of the OGTT (3,6,7).
Studies in youth and adults have shown that the glucose response curves reflect different metabolic phenotypes of IS and βCF less favorably in the IIn and MPh curves and more favorably in the BPh curve (1–4,7). Therefore, for the longitudinal changes in glucose response curves, we defined improvement as change from a less favorable to a more favorable curve type (i.e., change from IIn to MPh or BPh and from MPh to BPh) and worsening as change from a more favorable to a less favorable curve type (i.e., change from MPh or BPh to IIn and from BPh to MPh); no change implies that the curve did not change from randomization to month 12 after treatment.
Statistical Methods
The χ2 test was used to evaluate differences in the distribution of the glucose response curves at baseline between youth and adults overall or within subgroups. Separate generalized linear mixed models were used to evaluate the effect of age-group (youth vs. adult) within each curve type (or the effect of curve type within each age-group) on the mean of each plasma concentration outcome over repeated time points. Baseline demographic and metabolic characteristics were compared between curve types within each age-group and between age-groups within each curve type using the Student t test or Wilcoxon rank sum test for quantitative variables and the χ2 test for categorical variables. Variables with a skewed distribution were log-transformed as appropriate. Pairwise comparisons were performed when an overall difference by curve type was found. Separate linear regression models were used to evaluate the baseline association between glucose response curves and clamp-derived measures of IS and β-cell outcomes. Linear regression models were also used to evaluate the relationship of M/I with β-cell responses at baseline within each curve type. In separate models, an interaction for M/I with age-group was added to examine whether the relationship differed by age-group. All models used natural log-transformed M/I and β-cell response variables owing to the skewed distribution of these data. Models were adjusted for sex, race/ethnicity, waist circumference, and IS (except in the IS model). For the longitudinal analysis, we first evaluated differences in the change in curve types following 12 months of treatment in all youth and adults combined and between treatment types within each age-group using χ2 tests. We next examined whether glycemic deterioration at 12 months (i.e., conversion from IGT to type 2 diabetes) differed by baseline glucose response curves using Fisher exact test. Finally, we evaluated whether the changes in HbA1c, IS, and βCF at 12 months differed by baseline glucose response curves using unadjusted linear regression models. Analyses were performed using SAS 9.4 for Windows (SAS Institute, Cary, NC). All analyses were considered exploratory, and statistical significance was defined as P < 0.05.
Results
OGTT Glucose Response Curves in Youth and Adults at Randomization
At randomization, there was a significant difference in the distribution of the glucose response curves between youth and adults (Table 1). Combining MPh and IIn curve groups, more youth had a BPh curve compared with adults (χ2 P = 0.007), with no differences in the other two curve types (Table 1). In youth and adults, the most frequent curve type was MPh; in youth, this was followed, in descending order, by BPh and IIn, and in adults, by IIn and BPh (Table 1). When analyzing the data separately for IGT and type 2 diabetes within each age-group, adults with type 2 diabetes had a higher prevalence of the IIn curve compared with IGT (P = 0.001). The trend was similar in youth between IGT and type 2 diabetes, but not significant, likely because of small numbers (Table 1).
Table 1.
OGTT glucose response curves at baseline/randomization in youth and adult RISE participants overall and separately by IGT and diabetes
| Overall | Youth | Adults | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OGTT glucose response curve | Youth (n = 85) | Adults (n = 353) | P value | IGT (n = 52) | Diabetes (n = 33) | P value | IGT (n = 249) | Diabetes (n = 104) | P value |
| BPh | 16 (18.8) | 29 (8.2) | 0.022† | 13 (25.0) | 3 (9.1) | 0.120 | 24 (9.6) | 5 (4.8) | 0.0004‡ |
| MPh | 60 (70.6) | 273 (77.3) | 35 (67.3) | 25 (75.8) | 201 (80.7) | 72 (69.2) | |||
| IIn | 9 (10.6) | 51 (14.5) | 4 (7.7) | 5 (15.1) | 24 (9.7) | 27 (26.0) | |||
Data are n (%). P value from χ2 test evaluating differences in OGTT glucose response curves between youth and adults and between IGT and diabetes within each age-group.
Youth have more BPh curves than adults (P = 0.007), but the percentage of the other two curve types between youth and adults is similar.
Combining across MPh and BPh curves, adults with diabetes had more IIn curves compared with adults with IGT (P = 0.0001). Data from eight participants (six youth and two adults) are not included because of missing or invalid/incomplete data from the OGTT at baseline.
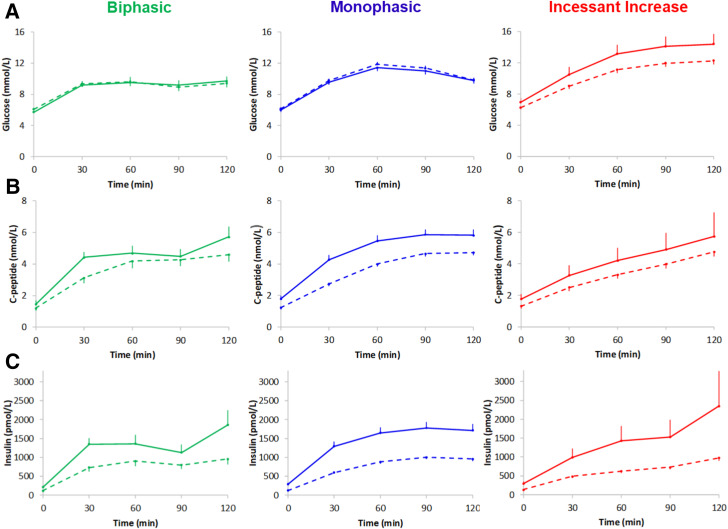
Figure 1 depicts OGTT plasma glucose (Fig. 1A), C-peptide (Fig. 1B), and insulin concentrations (Fig. 1C) in each of the three different curve types in youth and adults at randomization, and Table 2 displays OGTT AUCs for glucose, C-peptide, and insulin. The OGTT glucose AUC was higher in youth versus adults with an IIn curve (P = 0.022), with no differences in glucose AUC for the other two curves between the two age-groups (Table 2 and Fig. 1A). The OGTT C-peptide AUC was higher in youth versus adults with MPh (P < 0.0001) and BPh (P = 0.028) curves, and the OGTT insulin AUC was higher in youth versus adults for all three curve types (P < 0.0005) (Table 2 and Fig. 1C).
Figure 1.
Glucose (A), C-peptide (B), and insulin (C) concentrations by OGTT glucose response curves at randomization in youth (solid lines) and adult (dashed lines) RISE participants. Data are mean ± SE (upper or lower bar).
Table 2.
Demographic and metabolic characteristics by OGTT glucose response curve of youth and adult RISE participants at baseline/randomization
| Youth (n = 85) | Adults (n = 353) | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | BPh (n = 16) | MPh (n = 60) | IIn (n = 9) | P value for glucose response curve differences in youth | BPh (n = 29) | MPh (n = 273) | IIn (n = 51) | Glucose response curve differences in adults | Youth vs. adults within BPh | Youth vs. adults within MPh | Youth vs. adults within IIn |
| Demographic | |||||||||||
| Age (years) | 14.3 ± 1.8 | 14.4 ± 2.0 | 14.4 ± 3.2 | 0.987 | 53.5 ± 7.5 | 52.7 ± 9.3 | 51.8 ± 10.5 | 0.715 | <0.0001 | <0.00001 | <0.0001 |
| Male | 5 (31.2) | 19 (31.7) | 2 (22.2) | 0.933 | 11 (37.9) | 142 (52.0) | 19 (37.2) | 0.072 | 0.652 | 0.004 | 0.369 |
| Race/ethnicity | |||||||||||
| White | 6 (37.5) | 18 (30.0) | 1 (11.1) | 0.516 | 12 (41.4) | 135 (49.4) | 18 (35.3) | 0.284 | 0.954 | 0.0002 | 0.377 |
| Black | 5 (31.2) | 10 (16.7) | 3 (33.3) | 10 (34.5) | 70 (25.6) | 17 (33.3) | |||||
| Hispanic | 4 (25.0) | 26 (43.3) | 4 (44.4) | 6 (20.7) | 47 (17.2) | 14 (27.4) | |||||
| Other | 1 (6.2) | 6 (10.0) | 1 (11.1) | 1 (3.4) | 21 (7.7) | 2 (3.9) | |||||
| Tanner stage | |||||||||||
| 1–3 | 1 (6.2) | 11 (18.3) | 3 (33.3) | 0.218 | — | — | — | NA | NA | NA | NA |
| 4–5 | 15 (93.7) | 49 (81.7) | 6 (66.7) | — | — | — | |||||
| Weight (kg) | 91 ± 20 | 102 ± 24 | 108 ± 31 | 0.150 | 102 ± 17 | 101 ± 19 | 100 ± 16 | 0.964 | 0.063 | 0.644 | 0.242 |
| BMI (kg/m2) | 33.6 ± 5.6 | 37.6 ± 6.2 | 37.3 ± 6.5 | 0.076 | 36.2 ± 5.0 | 34.9 ± 5.2 | 35.8 ± 4.4 | 0.247 | 0.121 | 0.0005 | 0.391 |
| BMI z-score | 2.2 ± 0.5 | 2.4 ± 0.3 | 2.5 ± 0.4 | 0.041b | – | – | – | NA | NA | NA | NA |
| WC (cm) | 102 ± 13 | 112 ± 13 | 114 ± 14 | 0.028c | 111 ± 10 | 110 ± 13 | 110 ± 13 | 0.957 | 0.013 | 0.449 | 0.366 |
| Metabolic | |||||||||||
| HbA1c | 0.148 | 0.017d | 0.019 | 0.586 | 0.713 | ||||||
| % | 5.5 ± 0.5 | 5.7 ± 0.6 | 6.0 ± 0.8 | 5.8 ± 0.4 | 5.7 ± 0.4 | 5.9 ± 0.4 | |||||
| mmol/mol | 36.5 ± 5.6 | 38.9 ± 6.4 | 41.8 ± 8.4 | 40.1 ± 4.2 | 39.3 ± 4.3 | 41.1 ± 4.5 | |||||
| Fasting glucose (mmol/L) | 5.7 ± 1.0 | 6.0 ± 0.8 | 6.9 ± 1.3 | 0.006a | 6.1 ± 0.6 | 6.1 ± 0.6 | 6.2 ± 0.7 | 0.454 | 0.128 | 0.102 | 0.027 |
| 2-h glucose (mmol/L) | 9.7 ± 1.8 | 9.8 ± 2.2 | 14.4 ± 3.3 | <0.0001a | 9.4 ± 2.2 | 9.8 ± 2.2 | 12.3 ± 2.4 | <0.0001a | 0.667 | 0.863 | 0.022 |
| Glucose AUC (mmol/L) | 1,068 ± 185 | 1,195 ± 198 | 1,454 ± 329 | 0.0002a,b | 1,071 ± 191 | 1,231 ± 200 | 1,240 ± 205 | 0.0002c | 0.932 | 0.405 | 0.022 |
| Fasting C-peptide (nmol/L) | 1.5 ± 0.5 | 1.8 ± 0.6 | 1.8 ± 0.7 | 0.137 | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.3 ± 0.4 | 0.584 | 0.069 | <0.0001 | 0.009 |
| 2-h C-peptide (nmol/L) | 5.7 ± 2.2 | 5.8 ± 1.9 | 5.7 ± 4.3 | 0.982 | 4.6 ± 1.9 | 4.7 ± 1.5 | 4.8 ± 1.5 | 0.897 | 0.080 | <0.0001 | 0.195 |
| C-peptide AUC (nmol/L) | 516 ± 134 | 583 ± 179 | 483 ± 267 | 0.184 | 435 ± 166 | 432 ± 131 | 382 ± 116 | 0.047d | 0.028 | <0.0001 | 0.119 |
| Fasting insulin (pmol/L) | 210 ± 128 | 287 ± 175 | 292 ± 161 | 0.080 | 117 ± 77 | 125 ± 96 | 135 ± 67 | 0.199 | 0.005 | <0.0001 | 0.0001 |
| 2-h insulin (pmol/L) | 1,860 ± 1,521 | 1,716 ± 1,254 | 2,354 ± 2,733 | 0.978 | 960 ± 722 | 956 ± 712 | 977 ± 489 | 0.409 | 0.013 | <0.0001 | 0.019 |
| Insulin AUC (pmol/L) | 136,074 ± 79,563 | 159,875 ± 96,177 | 147,489 ± 124,885 | 0.382 | 82,827 ± 49,064 | 84,354 ± 51,810 | 67,095 ± 29,966 | 0.075 | 0.002 | <0.0001 | 0.0004 |
Data are mean ± SD or n (%). P values for nonnormally distributed data are based on log-transformed values (insulin). Other for self-reported race/ethnicity includes mixed, Asian, American Indian, and other. Significant comparisons (P < 0.05) across GRCs within each age-group or across age-groups within each GRC are highlighted in bold. GRC, glucose response curve; NA, not applicable; WC, waist circumference.
Pairwise comparison for GRC differences: IIn vs. MPh and BPh.
Pairwise comparison for GRC differences: BPh vs. MPh.
Pairwise comparison for GRC differences: BPh vs. MPh and IIn.
Pairwise comparison for GRC differences: IIn vs. MPh.
Demographic and Metabolic Characteristics by Glucose Response Curves in Youth and Adults
Table 2 presents the demographic and metabolic characteristics by glucose response curves in youth and adults. In youth, there was no difference in age, sex, race/ethnicity, Tanner stage, weight, BMI, HbA1c, fasting and 2-h insulin, and C-peptide concentrations by curve type. However, BMI z-score was lower in youth with BPh versus MPh curves, and waist circumference was lowest in youth with BPh compared with MPh and IIn curves (Table 2). Fasting and 2-h glucose concentrations were highest in youth with IIn versus MPh and BPh curves (Table 2). In adults, there was no difference in age, sex, race/ethnicity, weight, BMI, waist circumference, fasting glucose, fasting and 2-h insulin, and C-peptide concentrations by curve type (Table 2). However, HbA1c was significantly higher in adults with IIn versus MPh, and 2-h glucose was higher in adults with IIn versus MPh and BPh curves.
Comparison between youth and adults with similar curve types revealed that among those with the BPh curve, youth had lower waist circumference and HbA1c and higher fasting and 2-h insulin concentrations compared with their adult counterparts. Among participants with an MPh curve, youth had lower male and Non-Hispanic White prevalence and higher fasting and 2-h C-peptide and insulin concentrations compared with adults. Among participants with the IIn curve, youth had higher fasting and 2-h glucose and insulin concentrations and higher fasting C-peptide concentrations compared with adults (Table 2).
Clamp-Derived IS and β-Cell Responses by Glucose Response Curves in Youth and Adults
In youth and adults, M/I adjusted for sex, race/ethnicity, and waist circumference did not differ by glucose response curves (Fig. 2A); however, irrespective of curve type, IS was significantly lower in youth versus adults. In youth, hyperglycemic clamp–measured ACPRg (Fig. 2B), ACPRmax (Fig. 2C), and SSCP (Fig. 2D), corrected for IS, sex, race/ethnicity, and waist circumference, were lowest in the IIn curve group compared with the groups with MPh and BPh curves (P = 0.0197, P < 0.0001, and P < 0.0001, respectively). In adults, ACPRg (Fig. 2B) and SSCP (Fig. 2D) were also lowest in the IIn curve group versus the MPh and BPh curve groups (P = 0.0086 and P = 0.0011, respectively), and ACPRmax (Fig. 2C) was lower in those with IIn versus MPh curves (P = 0.0408). However, both ACPRg and ACPRmax were significantly higher in youth versus adults with BPh and MPh curves but not between the two age-groups with IIn curves (Fig. 2B and C). SSCP did not differ significantly between youth and adults for any of the three curve types (Fig. 2D). Supplementary Fig. 1 shows the inverse relationship between log-transformed M/I and log-transformed hyperglycemic clamp–measured β-cell responses in youth and adults. The slopes for youth and adults did not differ (all P > 0.05) except for the ACPRmax slopes in the IIn curve category.
Figure 2.
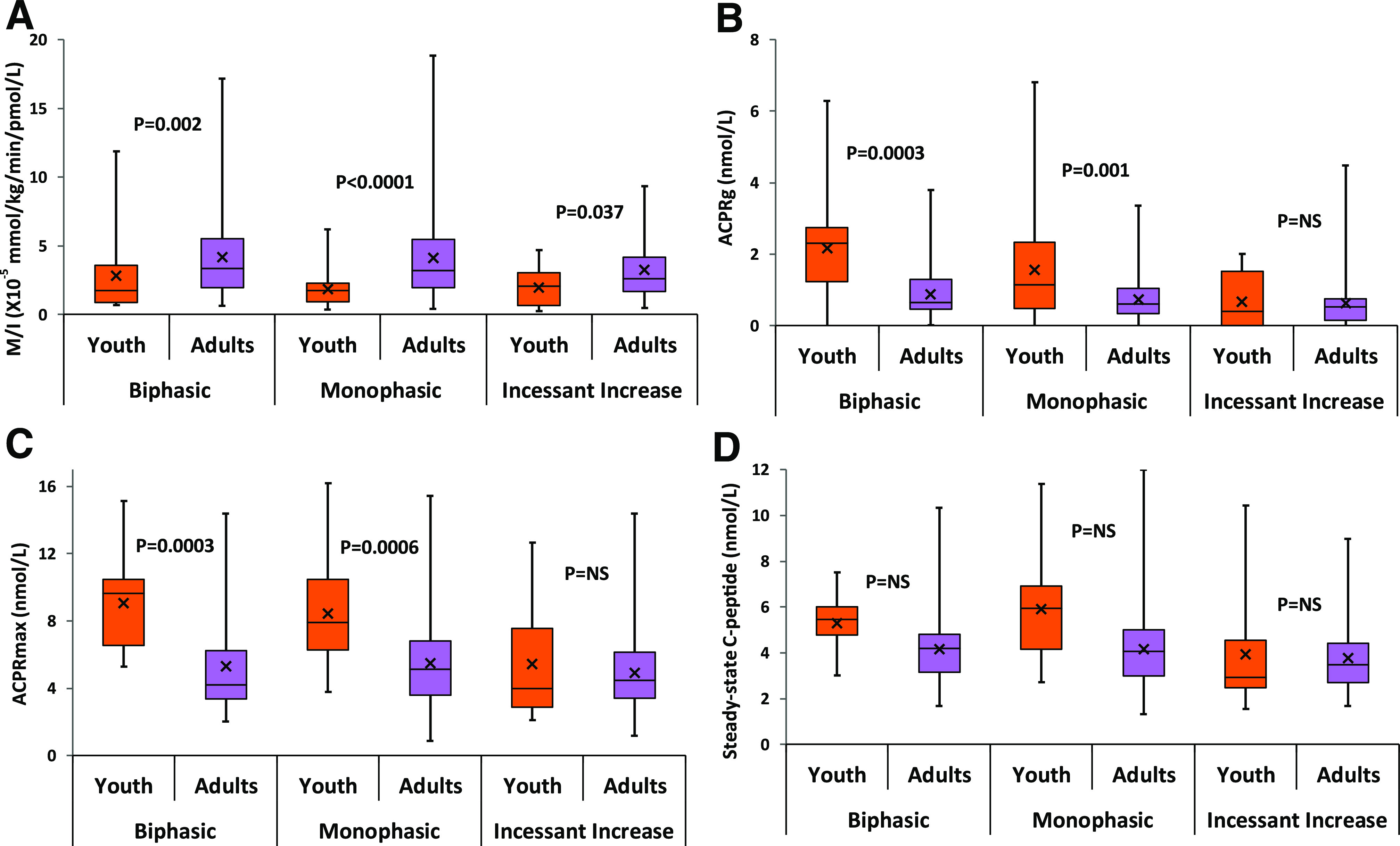
Box plots for M/I (A) and β-cell responses ACPRg (B), ACPRmax (C), and SSCP (D) by OGTT glucose response curves in youth and adults. P values from models evaluating the difference between youth and adults within each OGTT glucose response curve in adjusted models for IS (except for M/I), sex, race/ethnicity, and waist circumference.
Change in Glucose Response Curves, HbA1c, IS, βCF, and Glycemic Deterioration at Month 12 After Randomization in Youth Versus Adults
In the subset of participants randomized to glargine for 3 months followed by metformin for 9 months or to metformin alone for 12 months, the change in glucose response curves (defined in the classification of ogtt glucose response curves section) after 12 months of treatment did not differ between youth and adults (improvement 14.5% vs. 19.3%, worsening 21.0% vs. 20.0%, no change 64.5% vs. 60.7%). Moreover, there was no association between treatment type and the change in curve type in youth and adults (Supplementary Table 1).
In the longitudinal cohort of youth (n = 76) and adults (n = 145) combined, 67% (148 of 221) had IGT at screening. Of these participants with IGT, 25.0% (n = 4) with IIn, 33.3% (n = 38) with MPh, and 11.1% (n = 2) with BPh glucose response curves at baseline converted to diabetes (by OGTT criteria) by month 12 (P = 0.15 by Fisher exact test). The numbers are too small to test separately in youth and adults (data not shown). In the longitudinal cohort of youth and adults combined and in each age-group separately, HbA1c and IS at 12 months, and the change in them from baseline to 12 months, did not differ by baseline glucose response curves (Supplementary Table 2). Likewise, the change in ACPRg, ACPRmax, and SSCP from baseline to 12 months did not differ by baseline glucose response curves. However, 12-month ACPRg, ACPRmax, and SSCP were significantly different and coherent with baseline data, being highest in BPh compared with MPh and IIn curves (Supplementary Table 2).
Conclusions
The present investigation of glucose response curves in the RISE study of youth and adults with IGT and recently diagnosed type 2 diabetes demonstrates that 1) youth at randomization had a higher prevalence of the more favorable BPh curve compared with adults; 2) while insulin sensitivity did not differ across glucose response curves in youth and adults, youth had lower IS than adults, regardless of curve type; 3) across the three curve types, clamp-measured β-cell responses were lowest in the least favorable, IIn curve in youth and adults; 4) the upregulated β-cell responses in youth compared with adults was present in those with BPh and MPh curves but not with the IIn curve; 5) following intervention, the change in glucose response curves at month 12 did not differ between youth and adults, and there were no differences between the two treatment interventions; and 6) the 12-month progression from IGT to type 2 diabetes, and the change in HbA1c, IS, and βCF, did not differ by baseline glucose response curves. Previously, in the RISE study, youth had ∼50% lower IS than adults measured during hyperglycemic clamp (13) and OGTT (14). Likewise, in another study, obese youth had ∼40–50% lower peripheral and hepatic IS and twofold higher fasting insulin concentrations compared with adults of similar percent body fat (19). Furthermore, the RISE study showed that across a wide range of IS, youth had hyperresponsive β-cells compared with adults, as manifested by higher C-peptide and insulin concentrations during hyperglycemic clamp and OGTT (13,14). In another report, primary insulin hypersecretion, independent of insulin resistance, was associated with worse clinical and metabolic phenotypes in adolescents and adults and predicted deterioration in glucose control over time (20). Taken collectively, these observations imply that youth are worse off than adults because of their greater insulin resistance and their insulin hypersecretion across a wide range of IS. Therefore, we hypothesized that youth in the RISE study would manifest less favorable glucose response curves compared with adults. Contrary to our theory, however, the prevalence of the more favorable BPh curve was more common in youth than adults at randomization.
Adolescents without diabetes (1,2,6,9) exhibit higher rates of the BPh curve compared with reports in adults (4,8,11,12). It is possible that this is age driven because the BPh curve in adults is associated with younger age compared with the MPh curve (4,5). This was also the case in the European Group for the Study of Insulin Resistance (EGIR) of healthy adults, where individuals with the most favorable glucose response curves were the youngest (21). The mechanisms responsible for this youth-adult, age-related divergence in glucose response curves remain unknown. A potential explanation could be that since the pattern of the glucose response curve is defined by the increase and/or decrease in glucose concentrations (1,2,4,5,7), the hyperinsulinemia in youth might modulate this glucose response to be consistent with a BPh pattern. As can be seen in Table 2, the OGTT C-peptide and insulin AUCs were higher in youth with BPh curves compared with adults, while the OGTT glucose AUC was not different.
Our current observation that the MPh curve is the dominant curve in youth (70.6%) and adults (77.3%) is consistent with prior studies showing a dominant prevalence ranging from 34 to 69% in youth (1,2,6,9) and 57 to 87% in adults (4,5,8,10–12). With respect to the pathophysiological components of type 2 diabetes, studies in youth (1,2,6,9) and adults without diabetes (4,5,8,11,12) show that the MPh glucose response curve harbors worse metabolic parameters, with lower IS and lower βCF, than the BPh curve or more complex curves. Furthermore, adults with MPh OGTT have an increased risk of future impaired fasting glucose (IFG) (22) and type 2 diabetes (10). Data in adults with established diabetes are somewhat limited and without longitudinal observations. One study showed that the prevalence of the MPh curve was the highest, followed by the prevalence of an upward curve, followed by the BPh curve (3). In another study of women with prior gestational diabetes mellitus, the MPh curve was the most common and manifested worse metabolic parameters (8). In recent retrospectively analyzed OGTT data of adults with normal glucose tolerance, prediabetes (IFG, IGT, IFG + IGT), and type 2 diabetes, the MPh curve was more frequent in prediabetes and diabetes and manifested worse βC (5).
With respect to the pathophysiological components of type 2 diabetes, obese adolescents without diabetes who had MPh glucose response curves had lower hepatic and peripheral IS (hyperinsulinemic-euglycemic clamp) with inadequate compensation in first- and second-phase insulin secretion (hyperglycemic clamp) and a lower disposition index compared with those with BPh curves (2). Other studies using fasting or OGTT-derived estimates of IS and insulin secretion showed similar findings (1,6,9). In the TODAY study of youth with established type 2 diabetes, the most frequent glucose response curve was MPh (68.6%), followed by IIn (21.7%) and BPh (9.7%) (7). The IIn group had similar IS to the other two groups but significantly lower OGTT-derived βCF (7). These data are consistent with our current data showing that IS did not differ across glucose response curves in youth and adults. On the other hand, hyperglycemic clamp–measured ACPRg and ACPRmax, corrected for IS, declined across the three curve types from BPh to MPh to IIn in both youth and adults (Fig. 2B and C). Moreover, consistent with our prior RISE data of hyperresponsive β-cells in youth (13,14), both ACPRg and ACPRmax were significantly higher in youth versus adults with BPh and MPh curves (Fig. 2B and C). This, however, was not the case in those with the IIn curve, implying that in this curve type, which is associated with worst βCF, the youth-adult contrast in β-cell hyperresponsiveness is no more evident. A similar pattern is evident in Supplementary Fig. 1 in which across a wide range of IS, β-cell responses are higher in youth versus adults except within the IIn curve. Further evidence that the IIn curve is a metabolically deleterious glucose response curve comes from longitudinal observations in TODAY (7). Youth with IIn curves in TODAY had significantly higher glycemic failure rates (58.3%) versus the MPh group (42.3%) versus the BPh group (39.1%) (7). Moreover, the 6-month decline in βCF was greatest in the IIn group versus the MPh and BPh groups independent of diabetes duration and treatment assignment (7). Contrary to these results, the present RISE study shows that the 12-month progression from IGT to type 2 diabetes, and the change in HbA1c, IS, and β-cell responses, did not differ by baseline glucose response curves. These contrasting findings could be due to differing patient populations, youth with type 2 diabetes of longer disease duration in TODAY versus youth and adults with recently diagnosed type 2 diabetes and IGT in RISE, and differing sample sizes.
Following the RISE interventions, the change in glucose response curves at 12 months did not differ between youth and adults, and there were no differences between the two treatment interventions (Supplementary Table 1). This is consistent with TODAY data showing that 6 months after randomization there was no shift from one curve pattern to another in each treatment group, implying that no one treatment was better or worse than the other in improving or worsening the glucose response curves. In the EGIR cohort of clinically healthy adults, the glucose patterns identified at the 3-year observational follow-up were similar to those at baseline (21). This stability of the glucose response curves over time, with or without treatment, could be due to the heritability of the patterns, as shown in twin studies where genetic modeling revealed a heritability estimate between 45 and 67% (23). In the current study, the progression from IGT to type 2 diabetes, and the change in HbA1c, IS, and βCF at month 12 after treatment, did not differ by baseline glucose response curves. However, consistent with the baseline data, ACPRg, ACPRmax, and SSCP remained low in those who had IIn glucose response curves at baseline (Supplementary Table 2). There is no literature to compare and contrast with our findings because our study is the first to address longitudinal changes in adults with type 2 diabetes and IGT.
The strengths of the present investigation include 1) a first-time evaluation of the glucose response curves in adults with established type 2 diabetes, 2) a first-time head-to-head comparison of glucose response curves between youth and adults against the backdrop that youth are worse off than adults with respect to type 2 diabetes (13–15,19,24), 3) an assessment of the relationship between glucose response curves and IS and βCF measured by identical hyperglycemic clamps in youth and adults, and 4) an evaluation of longitudinal changes in glucose response curves with two different interventions. Limitations are that the glucose response curve was determined by a single OGTT, which may have limited reproducibility (25,26). A study in adults found inadequate reproducibility of the glucose response curves (27), but such data do not exist in pediatrics. Another limitation is the small sample size in youth. However, contrary to adults, the prevalence of youth with IGT and type 2 diabetes continues to be limited, making larger sample sizes more challenging to obtain.
In summary, youth compared with adults had a twofold higher prevalence of the BPh glucose response curves at randomization in the RISE study. While IS did not differ across glucose response curves, βCF declined from BPh to MPh to IIn curves in youth and adults. The typical β-cell hypersecretion in youth compared with adults was lost in those with IIn glucose response curves, emphasizing the severity of β-cell dysfunction in youth with this least favorable glucose response curve. Finally, the higher prevalence of the more favorable BPh curve in youth at randomization did not translate to beneficial changes in glucose response curves after treatment in youth compared with adults.
Article Information
Acknowledgments. The RISE Consortium acknowledges the support and input of the RISE data and safety monitoring board; Barbara Linder, the National Institute of Diabetes and Digestive and Kidney Diseases program official for RISE; and Ellen Leschek, the National Institute of Diabetes and Digestive and Kidney Diseases project scientist for RISE. The consortium is also grateful to the participants who, by volunteering, are furthering our ability to reduce the burden of diabetes.
Funding and Duality of Interest. RISE is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (U01-DK-094406, U01-DK-094430, U01-DK-094431, U01-DK-094438, U01-DK-094467), the National Institutes of Health (P30-DK-017047, P30-DK-020595, P30-DK-045735, P30-DK-097512, UL1-TR-000430, UL1-TR-001082, UL1-TR-001108, UL1-TR-001855, UL1-TR-001857, UL1-TR-001858, UL1-TR-001863), the Department of Veterans Affairs, and Kaiser Permanente Southern California. Additional financial and material support from the American Diabetes Association, Allergan Corporation, Apollo Endosurgery, Abbott Laboratories, and Novo Nordisk A/S is gratefully acknowledged. S.A.A. and S.E.K. serve as paid consultants on advisory boards for Novo Nordisk. S.A.A. is a participant in a Novo Nordisk–sponsored clinical trial. K.J.M. held an investigator-initiated research grant from Novo Nordisk. At the time of publication, K.J.M. was an employee of Eli Lilly and Company. His involvement in the research underlying this manuscript preceded this employment, and his participation in data review and preparation of the final report was independent of his employment at Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. Members of the RISE Consortium recruited participants and collected study data. S.A.A. proposed the analysis, interpreted data, and wrote and edited the manuscript, which was also reviewed and edited by members of the writing group. The RISE Steering Committee reviewed and edited the manuscript and approved its submission. L.E.g. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
A complete list of the RISE Consortium can be found in the supplementary material online.
K.J.M. is currently employed by Eli Lilly and Company.
Clinical trial reg. nos. NCT01779362, NCT01779375, NCT01763346, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13373708.
Contributor Information
Collaborators: The RISE Consortium, David A. Ehrmann, Karla A. Temple, Abby Rue, Elena Barengolts, Babak Mokhlesi, Eve Van Cauter, Susan Sam, M. Annette Miller, Steven E. Kahn, Karen M. Atkinson, Jerry P. Palmer, Kristina M. Utzschneider, Tsige Gebremedhin, Abigail Kernan-Schloss, Alexandra Kozedub, Brenda K. Montgomery, Emily J. Morse, Kieren J. Mather, Tammy Garrett, Tamara S. Hannon, Amale Lteif, Aniket Patel, Robin Chisholm, Karen Moore, Vivian Pirics, Linda Pratt, Kristen J. Nadeau, Susan Gross, Philip S. Zeitler, Jayne Williams, Melanie-Cree Green, Yesenia Garcia Reyes, Krista Vissat, Silva A. Arslanian, Kathleen Brown, Nancy Guerra, Kristin Porter, Sonia Caprio, Mary Savoye, Bridget Pierpont, Thomas A. Buchanan, Anny H. Xiang, Enrique Trigo, Elizabeth Beale, Fadi N. Hendee, Namir Katkhouda, Krishan Nayak, Mayra Martinez, Cortney Montgomery, Xinhui Wang, Sharon L. Edelstein, John M. Lachin, Ashley N. Hogan, Santica Marcovina, Jessica Harting, John Albers, Dave Hill, Peter J. Savage, and Ellen W. Leschek
References
- 1.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012;35:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JY, Michaliszyn SF, Nasr A, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care 2016;39:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchigami M, Nakano H, Oba K, Metori S. Oral glucose tolerance test using a continuous blood sampling technique for analysis of the blood glucose curve. Nihon Ronen Igakkai Zasshi 1994;31:518–524 [in Japanese] [DOI] [PubMed] [Google Scholar]
- 4.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003;26:1026–1033 [DOI] [PubMed] [Google Scholar]
- 5.Cheng X, Yang N, Li Y, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds β-cell function in a large Chinese population. BMC Endocr Disord 2019;19:119–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolfe G, Spreghini MR, Sforza RW, Morino G, Manco M. Beyond the morphology of the glucose curve following an oral glucose tolerance test in obese youth. Eur J Endocrinol 2012;166:107–114 [DOI] [PubMed] [Google Scholar]
- 7.Arslanian S, El Ghormli L, Young Kim J, et al.; TODAY Study Group . The shape of the glucose response curve during an oral glucose tolerance test: forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care 2019;42:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011;300:R941–R948 [DOI] [PubMed] [Google Scholar]
- 9.Bervoets L, Mewis A, Massa G. The shape of the plasma glucose curve during an oral glucose tolerance test as an indicator of beta cell function and insulin sensitivity in end-pubertal obese girls. Horm Metab Res 2015;47:445–451 [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010;26:280–286 [DOI] [PubMed] [Google Scholar]
- 11.Kanauchi M, Kimura K, Kanauchi K, Saito Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int J Clin Pract 2005;59:427–432 [DOI] [PubMed] [Google Scholar]
- 12.Trujillo-Arriaga HM, Román-Ramos R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput Biol Med 2008;38:185–195 [DOI] [PubMed] [Google Scholar]
- 13.RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.RISE Consortium . Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018;41:1707–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.RISE Consortium; RISE Consortium Investigators . Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019;68:1670–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RISE Consortium . Restoring Insulin Secretion (RISE): design of studies of β-cell preservation in prediabetes and early type 2 diabetes across the life span. Diabetes Care 2014;37:780–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.RISE Consortium . Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes 2019;42:1742–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arslanian S, Kim JY, Nasr A, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes 2018;19:205–211 [DOI] [PubMed] [Google Scholar]
- 20.Tricò D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight 2018;3:e124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hulman A, Witte DR, Vistisen D, et al. Pathophysiological characteristics underlying different glucose response curves: a latent class trajectory analysis from the prospective EGIR-RISC study. Diabetes Care 2018;41:1740–1748 [DOI] [PubMed] [Google Scholar]
- 22.Manco M, Nolfe G, Pataky Z, et al. Shape of the OGTT glucose curve and risk of impaired glucose metabolism in the EGIR-RISC cohort. Metabolism 2017;70:42–50 [DOI] [PubMed] [Google Scholar]
- 23.Dalgård C, Möller S, Kyvik KO. Heritability of curve patterns in oral glucose tolerance test. Twin Res Hum Genet 2020;23:39–44 [DOI] [PubMed] [Google Scholar]
- 24.Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 25.Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med 2007;167:1545–1551 [DOI] [PubMed] [Google Scholar]
- 26.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 2008;93:4231–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kramer CK, Vuksan V, Choi H, Zinman B, Retnakaran R. Emerging parameters of the insulin and glucose response on the oral glucose tolerance test: reproducibility and implications for glucose homeostasis in individuals with and without diabetes. Diabetes Res Clin Pract 2014;105:88–95 [DOI] [PubMed] [Google Scholar]