Abstract
OBJECTIVE
Whereas insulin resistance is expressed as reduced glucose uptake in peripheral tissues, the relationship between insulin resistance and brain glucose metabolism remains controversial. Our aim was to examine the association of insulin resistance and brain glucose uptake (BGU) during a euglycemic hyperinsulinemic clamp in a large sample of study participants across a wide range of age and insulin sensitivity.
RESEARCH DESIGN AND METHODS
[18F]-fluorodeoxyglucose positron emission tomography (PET) data from 194 participants scanned under clamp conditions were compiled from a single-center cohort. BGU was quantified by the fractional uptake rate. We examined the association of age, sex, M value from the clamp, steady-state insulin and free fatty acid levels, C-reactive protein levels, HbA1c, and presence of type 2 diabetes with BGU using Bayesian hierarchical modeling.
RESULTS
Insulin sensitivity, indexed by the M value, was associated negatively with BGU in all brain regions, confirming that in insulin-resistant participants BGU was enhanced during euglycemic hyperinsulinemia. In addition, the presence of type 2 diabetes was associated with additional increase in BGU. On the contrary, age was negatively related to BGU. Steady-state insulin levels, C-reactive protein and free fatty acid levels, sex, and HbA1c were not associated with BGU.
CONCLUSIONS
In this large cohort of participants of either sex across a wide range of age and insulin sensitivity, insulin sensitivity was the best predictor of BGU.
Introduction
The incidence and prevalence of obesity and type 2 diabetes (T2D) have increased continuously during the past decades and have reached epidemic dimensions (1,2). Both obesity and T2D have been linked to an increased risk of several neurodegenerative disorders, including Alzheimer disease (AD) (3,4). Thus, there is a concern that the incidence of AD could increase substantially in the future with the epidemics of obesity and T2D. This association between neurologic and metabolic disorders, as well as the incomplete understanding of the pathophysiology of obesity and insulin resistance (5), has led to an increased interest in how insulin resistance affects brain metabolism.
Positron emission tomography (PET) with 18F-fluorodeoxyglucose ([18F]-FDG) is the gold standard technique for the in vivo quantification of brain glucose uptake (BGU) and, indirectly, of brain glucose metabolism. [18F]-FDG-PET has been used widely to study AD. It is now well established that AD is characterized by regionally specific glucose-uptake reductions in parietotemporal areas (6), posterior cingulate cortex (7), and medial temporal lobe (8). As the disease progresses, frontal cortices become also involved. BGU is associated with clinical disabilities in dementia (9), and clinical AD symptoms do not occur without decreases in BGU, the extent of which is related to the severity of cognitive impairment (10).
Whereas glucose uptake in the brain is mediated by the insulin-independent glucose transporters GLUT1 and GLUT3, insulin receptors are widely expressed in the brain. Regions with high density of insulin receptors are typically affected by amyloid plaque deposition in AD, and patients with mild cognitive impairment (MCI; a prodromal state of AD) have high rather than low brain glucose metabolism (11). On the basis of these findings, it has been suggested that patients in whom AD is prone to develop exhibit, at least temporarily, brain hypermetabolism to preserve cognitive function.
Insulin-resistant states such as obesity and T2D have been linked to an increased risk of AD, and central insulin resistance has been demonstrated in AD in ex vivo studies (12). Accordingly, studying the effect of systemic insulin resistance on BGU is of paramount importance for understanding metabolic and neurologic disorders. The existing data, however, do not provide a clear-cut answer to this question.
Whereas early PET studies reported no association between BGU and insulin sensitivity (13), others have reported that insulin resistance (assessed with HOMA-IR) and prediabetes/early-onset diabetes associate with cerebral hypometabolism under fasting conditions in key brain areas that are affected in AD (14,15). On the contrary, we have shown that during euglycemic hyperinsulinemia, obese individuals and patients with impaired glucose tolerance have higher BGU as compared with lean and normal glucose tolerant individuals, respectively (16,17), and this finding has been confirmed by us and others in humans and animals (18–20). Thus, the mixed findings, to some extent, may be attributable to the different metabolic conditions in which brain glucose metabolism was studied (fasting vs. clamp conditions) and to differences in the study populations.
In recent years, the statistical power of neuroimaging studies has been questioned, and there is consensus that larger samples and data pooling are needed to guard from false-positive and -negative findings (21). In this study, we applied Bayesian hierarchical modeling to estimate the effect of insulin sensitivity on BGU during an insulin clamp in a large sample of participants across different degrees of glucose tolerance. We also analyzed the effect of other anthropometric and biochemical parameters (sex, age, BMI, T2D, steady-state insulin and free fatty acid (FFA) levels, HbA1c, and C-reactive protein levels) to gain additional insight into the factors that may associate with BGU.
Research Design and Methods
Study Population
We pooled and reanalyzed all studies that had brain [18F]-FDG PET scans carried out during a euglycemic hyperinsulinemic clamp. All included studies were performed at Turku PET Centre, Turku, Finland, during 2005–2020. Altogether, 194 participants were included. Of them 14% had T2D, 45% had hypertension, and 35% had dyslipidemia. Twelve percent were morbidly obese patients studied before they underwent bariatric surgery. Forty percent were healthy control participants (i.e., normal BMI; absence of T2D, dyslipidemia, or hypertension; and normal biochemical results, including renal function and transaminases). None had a clinical diagnosis of neurologic disease. Patients with T2D used either metformin (1–3 g daily), or a combination of metformin and dipeptidyl peptidase-4 inhibitors. Patients receiving insulin treatment were excluded. All participants underwent a screening visit before inclusion in the study. Metformin was withheld 24–72 h and dipeptidyl peptidase-4 inhibitors 24 h before the metabolic study.
Prior to inclusion, each participant gave written consent. Each protocol included in this study was approved by the Ethics Committee of the Hospital District of Southwest Finland (Turku, Finland) and conducted in accordance with the Declaration of Helsinki. The anthropometric and metabolic characteristics of all study participants are listed by study in Supplementary Table 1.
Euglycemic Hyperinsulinemic Clamp [18F]-FDG Studies
In this cohort, BGU was quantified only during a euglycemic hyperinsulinemic clamp. The euglycemic hyperinsulinemic clamp was performed as previously described (22). In brief, a primed, continuous infusion of insulin (Actrapid; Novo Nordisk, Copenhagen, Denmark) was given at a rate of 40 mU ⋅ m−2 ⋅ min−1. During the clamp, a variable rate 20% glucose solution was infused to maintain euglycemia at ∼5 mmol/L. Plasma glucose levels were measured every 5–10 min throughout the clamp. At mean ± SD 100 ± 10 min into the clamp, [18F]-FDG (mean ± SD 187 ± 9 MBq) was injected intravenously over 15 s and the acquisition of brain radioactivity started either immediately afterward (n = 62) or ∼1 h after [18F]-FDG injection (n = 133). During the clamp, samples for plasma insulin and serum FFA measurement were taken at baseline and at 30 and 60 min, respectively, thereafter.
Quantification of Brain Glucose Uptake
Although compartmental modeling and graphical Gjedde-Patlak analysis could have been used for the “early” data (as originally planned for these experiments (16,17)), we used fractional uptake rate (FUR) for all data to homogenize the mathematical modeling across the whole data set. Of note, the Gjedde-Patlak analysis and FUR strongly correlate with each other (23). Thus, BGU (in μmol ⋅ 100 g−1 ⋅ min−1) was calculated at the voxel level as fractional uptake rate multiplied by the average plasma glucose concentration from the injection until the end of the brain scan, divided by the lumped constant for the brain (set at 0.65) (24). For the early scans, the FUR calculation was restricted between 30 and 40 min. For the late scans, all frames were included. To account for possible differences between early and late studies, we derived a regularization parameter from an ad hoc experiment, as described in the Supplementary Material.
Calculation of Insulin-Stimulated Glucose Disposal (M Value)
The M value was calculated as a measure of whole-body insulin sensitivity, as previously described (25), and expressed per kilogram of fat-free mass (μmol ⋅ kgFFM−1 ⋅ min−1), because this normalization minimizes differences due to sex, age, and body weight (26).
Neurosynth Data Set
To test whether regional effects of insulin-stimulated BGU colocalize with broad domains of cognition, we used meta-analytic functional MRI activation patterns for attention, language, executive function, and working memory retrieved from the Neurosynth database (https://www.neurosynth.org). Meta-analytic uniformity maps of the four selected key cognitive domains were downloaded. Next, the meta-analytic activation maps (from NeuroSynth) and the t value map of the association between BGU and the M value of our data set (n = 194) were correlated at the voxel level. This approach examines the extent to which M-value–dependent BGU effects correspond with cerebral localization of different cognitive functions.
Statistical Modeling
We explored variables influencing BGU using Bayesian hierarchical modeling. The models were estimated with the R package BRMS that uses the Markov chain Monte Carlo sampling tools of RStan (https://mc-stan.org/users/interfaces/rstan). We estimated varying intercepts and slopes for each brain lobe and varying intercepts for the participants. To capture project-specific variation unrelated to variables of interest (e.g., effects of scanners, scan durations, source of input function, early vs. late scans), we also estimated varying intercepts for the projects. The following variables were included in the model: insulin sensitivity (as indexed by the M value), age, sex, steady-state insulin level, and presence of T2D. BMI was not included in the model, because of its high collinearity with the M value (Supplementary Fig. 1). Including all these predictors in the same model allowed us to identify the unique contribution of each of these variables while adjusting for the others. BGU values were log transformed because posterior predictive checking indicated that log transformation significantly improves model fit. For regularizing purposes, we used the standard normal distribution as the prior distribution for regression coefficients. We also provided an informative prior for the difference between early and late scans (see Supplementary Material for more details on priors and the statistical modeling). Otherwise, we used the default prior distributions of the BRMS package. In post hoc analyses, we also estimated the effects of FFAs (n = 187), C-reactive protein (n = 92), and HbA1c (n = 150). These effects were estimated by adding each of these three variables, in turn, to the main model to use maximal amount of data for each variable while adjusting for all the variables included in the main model.
Statistical Parametric Mapping Analysis
Linear regressions were performed in statistical parametric mapping (SPM12 toolbox for Matlab) to evaluate correlations between BGU and single regressors (M value, age, T2D, sex). The cluster-forming threshold was set at P < 0.05, and only statistically significant clusters (false discovery rate corrected P < 0.05) are reported.
Results
Overall Characteristics of the Euglycemic Hyperinsulinemic Clamp Studies
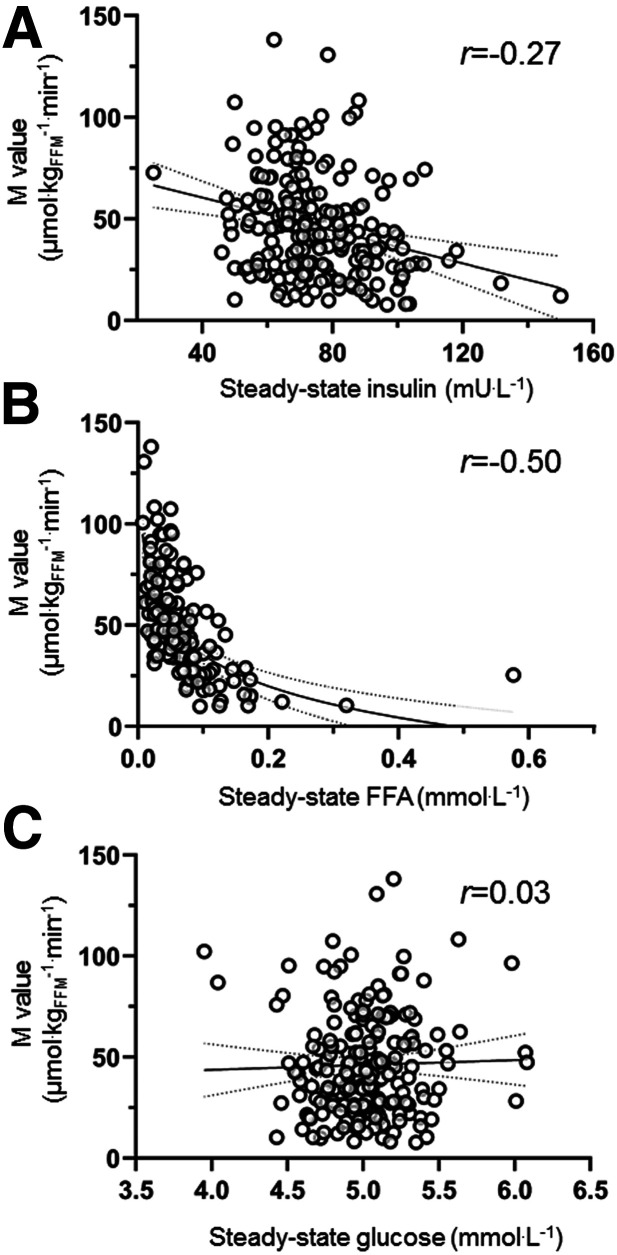
The data set comprised of 194 participants. Data on the anthropometric and metabolic characteristics of all study participants are reported as means and ranges in Table 1. During the euglycemic hyperinsulinemic clamp, median serum insulin levels were 72 (interquartile range, 23) mU/L at steady-state. Plasma glucose levels were maintained throughout the studies at mean ± SD 5.0 ± 0.3 mmol/L, FFAs were suppressed to a median steady-state FFA value of 0.05 (interquartile range, 0.05) mmol/L. Insulin sensitivity, indexed by the M value, was reciprocally related to both steady-state insulin levels (r = −0.27) and steady-state FFA levels (r = −0.48), whereas there was no correlation between the M value and steady-state plasma glucose levels during the clamp (r = 0.03) (Fig. 1).
Table 1.
Anthropometric and biochemical characteristics of the study participants
| Men (n = 63) | Women (n = 131) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age (years) | 56 | 11 | 20–69 | 56 | 14 | 23–80 |
| BMI (kg ⋅ m−2) | 29 | 6 | 22–48 | 30 | 7 | 19–51 |
| HbA1c | ||||||
| % | 5.6 | 0.3 | 5.1–6.3 | 5.6 | 0.4 | 4.9–7.1 |
| mmol/mol | 38 | 4 | 32–45 | 38 | 8 | 30–54 |
| M value (μmol ⋅ kgFFM−1 ⋅ min−1) | 40.2 | 24.5 | 7.9–130.8 | 49.1 | 25.3 | 10.3–138.2 |
| Type 2 diabetes, n (%) | 7 (11) | 20 (15) | ||||
Figure 1.
M value correlated negatively with steady-state insulin (A) and steady-state FFA levels (B) during the clamp. No correlation was found between M value and plasma glucose levels during the clamp (C).
Predictors of BGU During Euglycemic Hyperinsulinemia
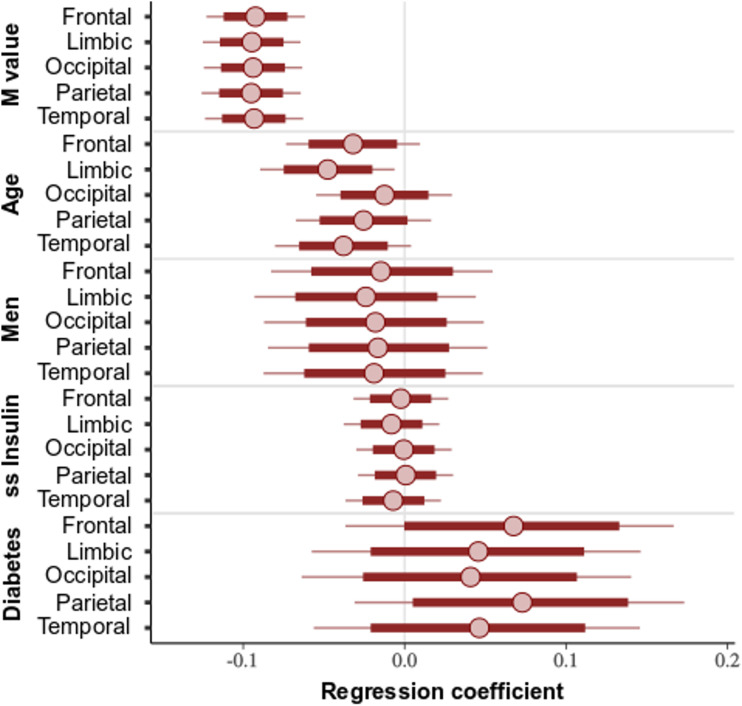
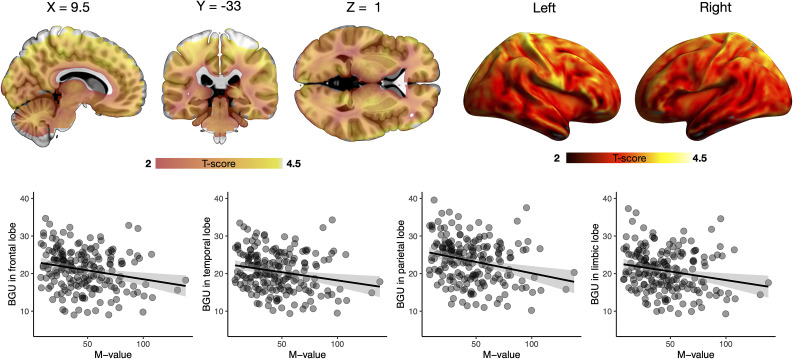
Posterior intervals (80% and 95%) for each of the parameters of interest in relation to BGU are shown in Fig. 2. BGU was negatively associated with M value and age. For M value, the effect was similar across all the brain lobes. This finding was also confirmed in the statistical parametric mapping analysis (Fig. 3). For age, however, there was regional variation: the effect was strongest in limbic and temporal lobes, whereas the frontal and parietal lobes only showed a negative trend. We could not find evidence for age dependency of BGU in the occipital lobe. Sex did not affect BGU. The data also suggest that T2D, adjusting for insulin sensitivity, is associated with elevated BGU. There is, however, uncertainty about the magnitude of the effect (as indicated by the wide posterior intervals). Steady-state insulin levels did not make an independent contribution to BGU. Similarly, the post hoc analyses revealed that C-reactive protein levels, steady-state FFA levels, and HbA1c made no unique contributions to BGU (80% posterior intervals include zero; data not shown).
Figure 2.
Posterior intervals of the regression coefficients for the variables of interest predicting BGU. The thick lines represent the 80% posterior intervals, the thin lines represent the 95% posterior intervals, and the circles represent posterior means. ss, steady state.
Figure 3.
Brain clusters (as defined by false discovery rate–corrected statistical parametric mapping one-sample t test) for the association between BGU during clamp and M value and the corresponding scatterplots.
Colocalization Between M Value-Dependent BGU and Cognitive Functions
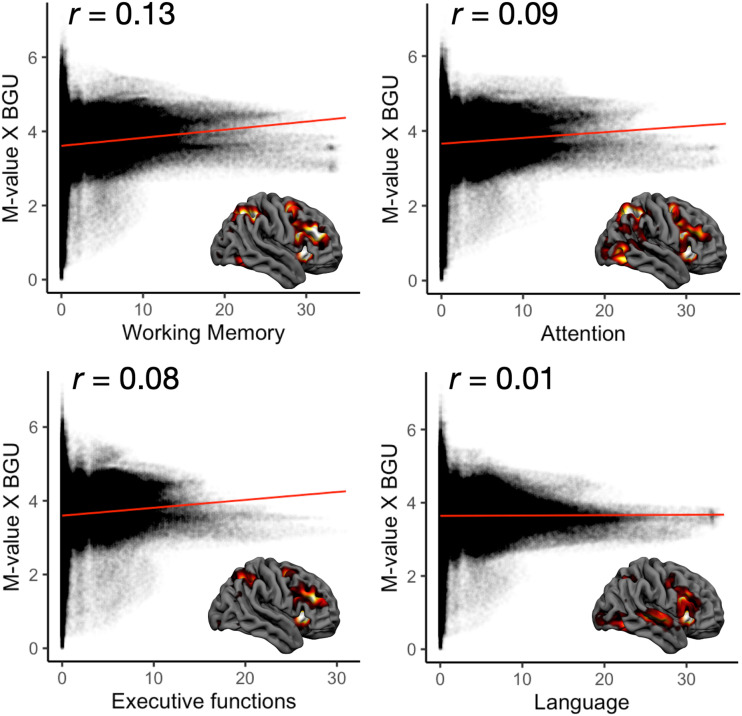
Localization of M value–dependent BGU (n = 194) was associated with localization of all the tested cognitive functions derived from NeuroSynth (Fig. 4). The strongest association was with working memory (ρ = 0.13), whereas the association was almost nonexistent with the language-related areas (ρ = 0.01).
Figure 4.
Spatial correlation between the regional M value–dependent insulin-stimulated BGU (y-axis) and meta-analytic blood oxygenation level–dependent functional MRI activation patterns for four basic cognitive functions retrieved from the Neurosynth database (https://www.neurosynth.org). These results show how well the M value–dependent BGU effects correspond with cerebral localization of different cognitive functions.
Conclusions
The main finding of our study was that insulin sensitivity, assessed using the gold standard M value from a euglycemic clamp, correlates negatively with BGU under conditions of insulin stimulation and is the strongest predictor of BGU among all parameters investigated. We also found that presence of T2D further contributes to increased BGU.
To our knowledge, the present data set comprises information on the largest cohort of people with apparently normal cognitive function whose BGU has been studied under euglycemic hyperinsulinemia, and our findings are in line with previous reports of other research groups in both humans and animals (18,19). It appears to be a consistent finding that during euglycemic hyperinsulinemia, BGU correlates inversely with insulin sensitivity. Therefore, the human brain operates in the opposite way compared with skeletal muscle and/or adipose tissue, where it is well established that insulin-stimulated glucose uptake is markedly reduced in insulin-resistant patients.
The underlying mechanisms for this characteristic of brain metabolism are not known. Some authors have speculated that insulin resistance does not have an effect on the expression of GLUT transporters in the brain, whereas their expression is markedly reduced in skeletal muscle in insulin resistance (19). In line with this, findings of a preclinical study indicated that whereas fasting and diabetes markedly decreased GLUT4 expression in adipose tissue, brain GLUT4 expression was only marginally affected by the same conditions (27). On the basis of recent evidence that the [18F]-FDG uptake in the brain is driven by astrocytes (28) and that a high-fat diet leads to astrocyte proliferation and activation (called astrogliosis) (29), we hypothesize that the increased BGU in insulin resistance is driven by brain inflammation. We are investigating this hypothesis in a clinical trial (Clinical trial reg. no. NCT04343469, clinicaltrials.gov). However, if astrogliosis is one part of the picture, hyperinsulinemia is a prerequisite for the higher BGU in the context of insulin resistance, because in the fasting conditions, neither we, studying humans (16), nor Bahri et al. (19), studying minipigs, found any association between BGU and insulin resistance. In turn, systemic hyperinsulinemia may either activate central circuits directly or this effect could be mediated by the periphery through retrograde signaling to the brain. Of note, it has been shown that insulin stimulates glucose uptake in cultured glial cells from brain tissue (30) and that human astrocytes, upon insulin stimulation, synthesize glycogen and proliferate (31). All in all, astrocytes represent optimal candidate cells to explain this peculiar brain characteristic regarding BGU during insulin stimulation, but more research is warranted to reveal the underlying cellular mechanisms. Even though the relevance of our findings under clamp conditions may be criticized because of their experimental nature, systemic insulin levels achieved during euglycemic hyperinsulinemic clamps were those typically seen in the postprandial state. Information about brain glucose metabolism in more physiologic conditions is scanty, but Daniele et al. (32) found that after bariatric surgery, BGU during the oral glucose tolerance test decreased, a finding which is solidly in line with our current findings.
Previous studies in patients prone to AD under fasting conditions have reported that insulin resistance associates with brain hypometabolism in key brain areas that are affected in AD (14,15). Regarding the insulin effect, seminal work by Talbot et al. (12) showed normal activation of the insulin-signaling pathway in ex vivo studies in cognitively normal brains and brains with MCI under normal and supraphysiologic insulin levels, and of insulin resistance in AD brain slices. Accumulating evidence supports the notion that AD may be considered a metabolic disease of the brain, in which brain glucose use is impaired and, whereas early brain glucose hypermetabolism (i.e., MCI) may be considered a compensatory phase to the initial neurodegenerative insult, this compensation may eventually accelerate the degenerative process and ultimately lead to brain hypometabolism (33). Similar temporal paradoxical patterns have been described in other neuroimaging studies, in which memory-related functional MRI showed hyperactivation in less-impaired patients with MCI and hypoactivation in more-impaired patients with MCI (34). More research is definitely warranted to clarify the complex pathophysiology that links systemic metabolic and central disorders. In this context, we think a cross-sectional and longitudinal comparison of brain glucose metabolism in conditions of euglycemic clamp between BMI and age-matched individuals with normal cognition, MCI, and AD could aid understanding of the present findings.
BGU decreased with advancing age, and this effect was especially evident in the limbic lobe, in line with previous studies showing that fasting BGU decreases with aging (35). Thus, we extend this finding to the insulin-stimulated state. Several other parameters were tested for their contribution to BGU. Presence of T2D seemed to further increase BGU, although there was uncertainty about the magnitude of this effect, as indicated by the wide posterior intervals. This finding is in line with the established notion that worse metabolic control is associated with more severe insulin resistance. FFAs cross the blood-brain barrier, and we have previously shown that obese patients have increased brain FFA uptake as compared with lean individuals (36). On the basis of previous studies showing that hypothalamic sensing of circulating FFAs is important in the control of nutrient intake and energy balance (37), we hypothesized that FFAs could be key players in the cross-talk between brain and peripheral tissues in the context of insulin resistance. However, our data showed that when accounting for insulin resistance, steady-state FFA levels were not an independent predictor of BGU. Likewise, we did not find any evidence for an association between plasma insulin levels and BGU. In clamp experiments, a feature of patients with insulin resistance is higher plasma insulin levels compared with insulin-sensitive patients, as also seen in our study, despite similar rates of exogenous insulin infusion. Despite being higher in patients with insulin resistance, plasma insulin levels did not correlate with BGU. In a previous study, researchers showed that whereas obese patients had increased plasma insulin levels, they had relative lower central nervous system insulin levels compared with lean individuals (38), suggesting that the central effects of insulin cannot be predicted by the peripheral plasma insulin levels.
BGU in the insulin-stimulated state was not significantly affected by sex. Previous studies regarding the effect of sex on BGU have yielded mixed results (39,40). In our data, men tended to have lower BGU across all brain regions examined. However, as shown in Fig. 2, there was wide uncertainty about the effect size of sex differences in BGU, which could be the reason for the conflicting results reported in the literature.
Strengths of our study are its large size across a wide range of insulin sensitivity and age; the application of Bayesian hierarchical model for the investigation of the effects not only of insulin sensitivity but also of other potential effectors of brain glucose uptake; and the application of gold standard techniques (i.e., PET and the euglycemic hyperinsulinemic clamp). Our study has also limitations. First, the current analysis documents associations but does not explain the mechanisms underlying the observed increase in BGU in the context of insulin resistance. Second, we combined data from several projects that originally focused on different research questions, and the data, therefore, are not optimally balanced across different covariates. However, we used a large sample, analyzed all data with the same approach, and accounted for differences in the projects using statistical modeling. Whereas graphical analysis is a more accurate method of quantification, it could not be performed for the “late” studies; thus, we chose to quantify BGU using the FUR. Even though the FUR is considered a less accurate method, it correlates very well with Patlak (23) and is a valid alternative of quantification of PET data, which could be applied more often in research settings. The individuals included in the current data set had apparent normal cognitive function, but cognitive function testing was not performed. Still, we used a meta-analytic approach that showed that BGU clusters with domains of cognitive function. Unfortunately, this type of analysis does not allow an evaluation of the brain areas involved. This further underlines the need for studies to investigate how BGU is linked to cognitive function and whether an increased BGU at baseline can predict cognitive decline in the long term. Finally, due to the physics of the PET, small brain areas such as the hypothalamus cannot be examined. Even though the study of the hypothalamus is of special interest in metabolic investigation, our results demonstrate that the interplay between insulin resistance and BGU is present at the whole-brain level.
In conclusion, in a large sample of participants across a wide range of age and insulin sensitivity, we have shown that insulin-stimulated BGU correlates negatively with the degree of insulin sensitivity. Presence of T2D was also associated with enhanced BGU and, as expected, age was a negative independent predictor of BGU. As the incidence of metabolic and neurodegenerative disorders increases, there is a compelling need to identify the common pathophysiologic pathways of these conditions, which may eventually lead to efficient treatments and prevention.
Article Information
Acknowledgments. The authors thank the staff of the Turku PET Centre for performing the PET imaging.
Funding. The study was conducted within the Center of Excellence in Cardiovascular and Metabolic Diseases, supported by the Academy of Finland, the University of Turku, Turku University Hospital, Åbo Akademi University, Finnish Diabetes Foundation, Sigrid Juselius Foundation, and Finnish Cultural Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.I., K.A.V., I.H., M.L., P.N. conceived the study design. E.R., M.B., T.K., J.C.H., A.L.-R., J.H., R.P., and L.N. analyzed data and literature and drafted the manuscript. J.C.H. conducted the clinical positron emission tomography studies. M.B., T.K., V.O., and L.N. analyzed the compartmental data analyses. A.B., I.H., M.L., E.F., P.I., L.N., and P.N. reviewed the manuscript. All authors approved the final version of the manuscript. P.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the Keystone Symposium Bioenergetics and Metabolic Disease, Keystone, CO, 21–25 January 2018; and at the 54th European Association for the Study of Diabetes annual meeting, Berlin, Germany, 1–5 October 2018.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13342142.
E.R. and M.B. contributed equally to this work.
References
- 1.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes 2008;32:1431–1437 [DOI] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–321 [DOI] [PubMed] [Google Scholar]
- 3.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 1999;53:1937–1942 [DOI] [PubMed] [Google Scholar]
- 4.Ho AJ, Raji CA, Becker JT, et al.; Cardiovascular Health Study; ADNI . Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging 2010;31:1326–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz MW, Baskin DG. Leptin and the brain: then and now. J Clin Invest 2013;123:2344–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 2001;286:2120–2127 [DOI] [PubMed] [Google Scholar]
- 7.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997;42:85–94 [DOI] [PubMed] [Google Scholar]
- 8.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging 2005;32:486–510 [DOI] [PubMed] [Google Scholar]
- 9.Blass JP. Alzheimer’s disease and Alzheimer’s dementia: distinct but overlapping entities. Neurobiol Aging 2002;23:1077–1084 [DOI] [PubMed] [Google Scholar]
- 10.Mosconi L, McHugh PF. FDG- and amyloid-PET in Alzheimer’s disease: is the whole greater than the sum of the parts? Q J Nucl Med Mol Imaging 2011;55:250–264 [PMC free article] [PubMed] [Google Scholar]
- 11.Ashraf A, Fan Z, Brooks DJ, Edison P. Cortical hypermetabolism in MCI subjects: a compensatory mechanism? Eur J Nucl Med Mol Imaging 2015;42:447–458 [DOI] [PubMed] [Google Scholar]
- 12.Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012;122:1316–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eastman RC, Carson RE, Gordon MR, et al. Brain glucose metabolism in noninsulin-dependent diabetes mellitus: a study in Pima Indians using positron emission tomography during hyperinsulinemia with euglycemic glucose clamp. J Clin Endocrinol Metab 1990;71:1602–1610 [DOI] [PubMed] [Google Scholar]
- 14.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med 2014;55:759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol 2015;72:1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuulari JJ, Karlsson HK, Hirvonen J, et al. Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes 2013;62:2747–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirvonen J, Virtanen KA, Nummenmaa L, et al. Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes 2011;60:443–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boersma GJ, Johansson E, Pereira MJ, et al. Altered glucose uptake in muscle, visceral adipose tissue, and brain predict whole-body insulin resistance and may contribute to the development of type 2 diabetes: a combined PET/MR study. Horm Metab Res 2018;50:627–639 [DOI] [PubMed] [Google Scholar]
- 19.Bahri S, Horowitz M, Malbert CH. Inward glucose transfer accounts for insulin-dependent increase in brain glucose metabolism associated with diet-induced obesity. Obesity (Silver Spring) 2018;26:1322–1331 [DOI] [PubMed] [Google Scholar]
- 20.Latva-Rasku A, Honka MJ, Stančáková A, et al.; T2D-GENES Consortium . A partial loss-of-function variant in AKT2 is associated with reduced insulin-mediated glucose uptake in multiple insulin-sensitive tissues: a genotype-based callback positron emission tomography study. Diabetes 2018;67:334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013;14:365–376 [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 23.Prando S, Carneiro CG. Comparison of different quantification methods for 18F-fluorodeoxyglucose-positron emission tomography studies in rat brains. Clinics (Sao Paulo) 2019;74:e1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu HM, Bergsneider M, Glenn TC, et al. Measurement of the global lumped constant for 2-deoxy-2-[18F]fluoro-D-glucose in normal human brain using [15O]water and 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography imaging: a method with validation based on multiple methodologies. Mol Imaging Biol 2003;5:32–41 [DOI] [PubMed] [Google Scholar]
- 25.Iozzo P, Gastaldelli A, Järvisalo MJ, et al. 18F-FDG assessment of glucose disposal and production rates during fasting and insulin stimulation: a validation study. J Nucl Med 2006;47:1016–1022 [PubMed] [Google Scholar]
- 26.Gastaldelli A, Casolaro A, Pettiti M, et al. Effect of pioglitazone on the metabolic and hormonal response to a mixed meal in type II diabetes. Clin Pharmacol Ther 2007;81:205–212 [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi M, Nikami H, Morimatsu M, Saito M. Expression and localization of insulin-regulatable glucose transporter (GLUT4) in rat brain. Neurosci Lett 1996;213:103–106 [DOI] [PubMed] [Google Scholar]
- 28.Zimmer ER, Parent MJ, Souza DG, et al. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci 2017;20:393–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarke DW, Boyd FT Jr., Kappy MS, Raizada MK. Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain. J Biol Chem 1984;259:11672–11675 [PubMed] [Google Scholar]
- 31.Heni M, Hennige AM, Peter A, et al. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS One 2011;6:e21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniele G, Dardano A, Volterrani D, et al. 1817-P Metabolic surgery affects brain glucose metabolism, cognitive function, and neuroplasticity in humans. Diabetes 2019:68(Suppl. 1):1817-P [Google Scholar]
- 33.Iozzo P, Guzzardi MA. Imaging of brain glucose uptake by PET in obesity and cognitive dysfunction: life-course perspective. Endocr Connect 2019;8:R169–R183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Celone KA, Calhoun VD, Dickerson BC, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci 2006;26:10222–10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goyal MS, Vlassenko AG, Blazey TM, et al. Loss of brain aerobic glycolysis in normal human aging. Cell Metab 2017;26:353–360.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebelos E, Hirvonen J, Bucci M, et al. Brain free fatty acid uptake is elevated in morbid obesity and is irreversible 6 months after bariatric surgery: a positron emission tomography study. Diabetes Obes Metab 2020;22:1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pocai A, Lam TK, Obici S, et al. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 2006;116:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern W, Benedict C, Schultes B, et al. Low cerebrospinal fluid insulin levels in obese humans. Diabetologia 2006;49:2790–2792 [DOI] [PubMed] [Google Scholar]
- 39.Baxter LR Jr., Mazziotta JC, Phelps ME, Selin CE, Guze BH, Fairbanks L. Cerebral glucose metabolic rates in normal human females versus normal males. Psychiatry Res 1987;21:237–245 [DOI] [PubMed] [Google Scholar]
- 40.Gur RC, Mozley LH, Mozley PD, et al. Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995;267:528–531 [DOI] [PubMed] [Google Scholar]