Rapid implementation of remote continuous glucose monitoring (CGM) is occurring across hospitals during the coronavirus disease 2019 (COVID-19) pandemic. Despite limited experience, the U.S. Food and Drug Administration is not objecting to the inpatient use of CGM to limit the exposure of health care workers to severe acute respiratory syndrome coronavirus 2 and to reduce the waste of personal protective equipment (1). Recent efforts in non–intensive care unit (ICU) patients suggest that CGM devices are accurate in the inpatient setting and can help monitor patients remotely (2,3). In addition, two recent small trials enrolling non-ICU patients confirm the feasibility of using remote real-time CGM in the hospital (4,5).
The accuracy of sensors, however, may be affected during various conditions that have not been well studied (i.e., MRI, surgery, shock requiring vasopressor therapy, hypoxia) (1). To mitigate potential CGM inaccuracy, a hybrid approach using real-time CGM with periodic point-of-care (POC) validation has been suggested (1). We report here on the likely loss of sensor signal during cardiac surgery and potential loss of accuracy in the operating room (OR). We also report on the accuracy of sensors that recovered immediately after surgery during critical illness.
We evaluated the performance of sensors in adults without diabetes undergoing scheduled or urgent coronary artery bypass surgery (CABG). We excluded patients with severely impaired renal function, hepatic failure, or imminent risk of death. We inserted a blinded Dexcom G6 CGM (Dexcom, San Diego, CA) device in the lower abdomen preoperatively. Glycemic data were collected before, during, and after surgery. Blood glucose values were paired with concomitant sensor values for analysis. The Emory University Institutional Review Board approved the study.
Fifteen consecutive patients were included. Thirteen patients underwent open CABG (cardiopulmonary bypass n = 13, off-pump n = 2), and robotic CABG was performed in two patients. All patients received continuous insulin infusion. Patient characteristics are listed in Fig. 1A. A total of 149 paired POC-CGM measurements were used for analysis. The mean and median absolute relative differences were 12.9% and 10.5%, respectively. Clarke Error Grid analysis showed 98.6% of glucose values falling into zones A and B; 83.2% of values fell within zone A, 15.4% within zone B, and 1.3% within zone D. No values fell within zone C or E (Fig. 1). The proportion of sensor glucose values within ±15%/15 mg/dL, ±20%/20 mg/dL, and ±30%/30 mg/dL of the reference value (±15, 20, or 30% if reference BG >100 mg/dL or ± 15, 20, or 30 mg/dL if reference BG <100 mg/dL) was 69%, 82%, and 94%, respectively.
Figure 1.
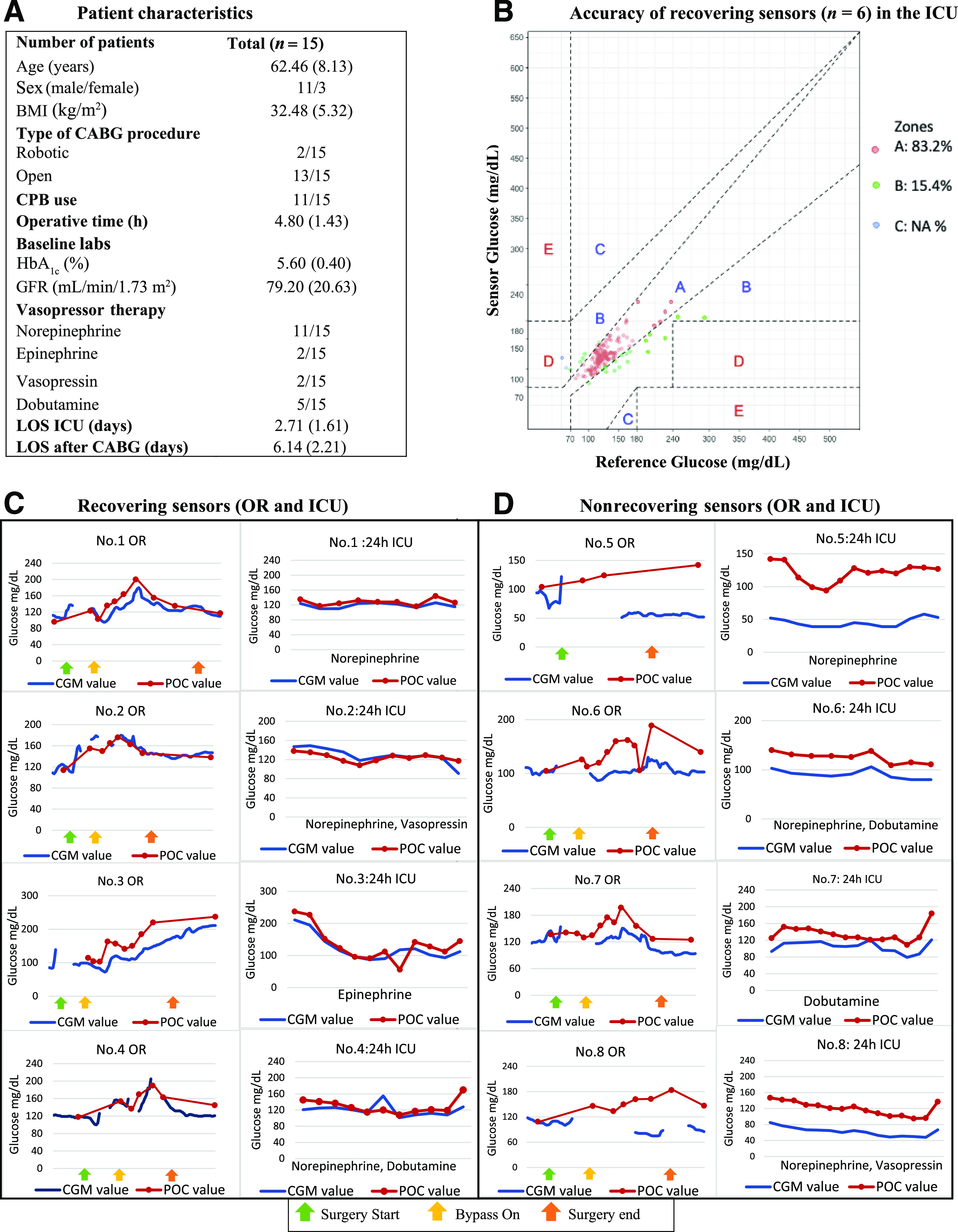
Patient characteristics (A), sensor accuracy (B), and individual-level data in patients receiving vasopressors in the cardiac ICU (C and D). CPB, cardiopulmonary bypass; GFR, glomerular filtration rate; LOS, length of stay.
To illustrate the performance of sensors during surgery, individual-level data are presented for patients 1–8 in Fig. 1C and D. We observed that sensors were accurate before surgery; however, intermittent signal loss was common during the operative course. After surgery, some sensors maintained precision (following POC patterns in parallel [Fig. 1D]) but lost accuracy (predominantly negative bias), particularly those with longer signal gaps. Six sensors recovered accuracy after surgery (within 20% of reference values). Sensors that recovered accuracy maintained reliable readings despite vasopressor therapy (Fig. 1B). We did not observe differences in complications, length of stay, or transfusions between patients wearing the recovering devices and those wearing nonrecovering devices.
In non-ICU populations, recently Nair et al. (2) (N = 10 non–COVID-19, 178 glucose pairs) and Reutrakul et al. (3) (N = 9 COVID-19+, 105 glucose pairs) reported mean absolute relative differences of 9.4% and 9.8% with G6, respectively. We confirm and expand these findings with data from critically ill patients on vasopressor therapy. We acknowledge potential limitations: even though most POC samples are from arterial blood, there may have been intermittent capillary samples in the ICU. These findings cannot be extrapolated to the hypoglycemic range.
In summary, this information is extremely relevant because 1) we document that CGM technology is less reliable in the OR, which is likely related to electrocautery interference; 2) we show common patterns of signal loss and negative bias during surgery; however, 3) we observed that sensors that recovered immediately after surgery had adequate and sustained accuracy, even during exposure to vasopressors in the ICU. Our preliminary experience during the COVID-19 pandemic indicates that CGM use is helpful in the ICU to guide therapy in patients that require continuous insulin infusion to maintain glucose control, where hourly POC tests are extremely impractical. This information provides initial reassurance to providers that are using the technology in the sickest patients aiming at achieving better glycemic control while reducing the burden of diabetes care during the pandemic. We recommend avoiding making clinical decisions based on CGM readings after surgery until accuracy can be confirmed (e.g., within 20% of reference values) with POC testing or central laboratory tests. We do not know if calibration can improve the performance of CGM devices during or after surgery. Until we have more information, placement of a new device may be necessary.
Article Information
Funding and Duality of Interest. This study was supported by the National Institute of General Medical Sciences of the National Institutes of Health (NIH) and by an investigator-initiated study grant from Dexcom (F.J.P.). G.E.U. is partly supported by research grants from the NIH National Center for Advancing Translational Sciences (UL1 TR002378 and 1P30DK111024-05) and has received unrestricted research support from Sanofi, Novo Nordisk, and Dexcom. G.M.D. is supported by NIH under award number 1K23DK122199-01A1. F.J.P. is supported in part by NIH under award numbers 1K23GM128221-03, P30DK11102405, and P30DK111024-05S and has received research support from Merck and Dexcom. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.C.P.-G. researched the data, created the figures, and cowrote the manuscript. E.D. reviewed and edited the manuscript. S.G. and S.C. researched the data and reviewed the manuscript. A.C.-R., A.F., M.H., and G.E.U. reviewed and edited the manuscript. L.P. analyzed the data. G.M.D. participated in the study design, researched the data, and edited the manuscript. F.J.P. designed the study, obtained funding, and cowrote the manuscript. F.J.P. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Galindo RJ, Aleppo G, Klonoff DC, et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol 2020;14:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair BG, Dellinger EP, Flum DR, Rooke GA, Hirsch IB. A pilot study of the feasibility and accuracy of inpatient continuous glucose monitoring. Diabetes Care 2020;43:e168–e169 [DOI] [PubMed] [Google Scholar]
- 3.Reutrakul S, Genco M, Salinas H, et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care 2020;43:e137–e138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fortmann AL, Spierling Bagsic SR, Talavera L, et al. Glucose as the fifth vital sign: a randomized controlled trial of continuous glucose monitoring in a non-ICU hospital setting. Diabetes Care 2020;43:2873–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh LG, Satyarengga M, Marcano I, et al. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care 2020;43:2736–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]


