Abstract
OBJECTIVE
The empirical dietary index for hyperinsulinemia (EDIH) and empirical dietary inflammatory pattern (EDIP) scores assess the insulinemic and inflammatory potentials of habitual dietary patterns, irrespective of the macronutrient content, and are based on plasma insulin response or inflammatory biomarkers, respectively. The glycemic index (GI) and glycemic load (GL) assess postprandial glycemic potential based on dietary carbohydrate content. We tested the hypothesis that dietary patterns promoting hyperinsulinemia, chronic inflammation, or hyperglycemia may influence type 2 diabetes risk.
RESEARCH DESIGN AND METHODS
We calculated dietary scores from baseline (1993–1998) food frequency questionnaires among 73,495 postmenopausal women in the Women’s Health Initiative, followed through March 2019. We used multivariable-adjusted Cox regression to estimate hazard ratios (HRs) and 95% CIs for type 2 diabetes risk. We also estimated multivariable-adjusted absolute risk of type 2 diabetes.
RESULTS
During a median 13.3 years of follow-up, 11,009 incident cases of type 2 diabetes were diagnosed. Participants consuming the most hyperinsulinemic or proinflammatory dietary patterns experienced greater risk of type 2 diabetes; HRs (95% CI) comparing highest to lowest dietary index quintiles were EDIH 1.49 (1.32–1.68; Ptrend < 0.0001) and EDIP 1.45 (1.29–1.63; Ptrend < 0.0001). The absolute excess incidence for the same comparison was 220 (EDIH) and 271 (EDIP) cases per 100,000 person-years. GI and GL were not associated with type 2 diabetes risk: GI 0.99 (0.88–1.12; Ptrend = 0.46) and GL 1.01 (0.89–1.16; Ptrend = 0.30).
CONCLUSIONS
Our findings in this diverse cohort of postmenopausal women suggest that lowering the insulinemic and inflammatory potentials of the diet may be more effective in preventing type 2 diabetes than focusing on glycemic foods.
Introduction
In the U.S., ∼32.5 million people live with diabetes, and ∼95% of them have type 2 diabetes (1). Prospective cohort studies suggest that adherence to a healthful dietary pattern and lifestyle is associated with lower type 2 diabetes risk (2,3). However, other than raising glucose levels, which could increase β-cell inflammation, and oxidative and endoplasmic reticulum stress (4), it is unclear what specific biological mechanisms may be linking dietary factors to type 2 diabetes risk. Though the potential of diet to contribute to glycemia may have important implications for health (5), dietary glycemic potential, usually estimated with the glycemic index (GI) and glycemic load (GL) (6), has not been clearly associated with subsequent type 2 diabetes risk (7–9). Meta-analyses have reported mixed findings regarding GI or GL scores and relative type 2 diabetes risk (7–9). Also, most previous studies did not estimate the absolute risk of type 2 diabetes, making it difficult to assess the clinical utility of published relative risks.
Type 2 diabetes is characterized by hyperglycemia due to impaired insulin secretion and function and systemic inflammation (10). Although dietary carbohydrate load is important in insulin response, noncarbohydrate factors including protein and lipid food sources and quantity may also stimulate insulin secretion. A meta-analysis that included 28 randomized controlled trials found that GI and GL were not associated with circulating inflammatory biomarkers (CRP, leptin, interleukin-6, and tumor necrosis factor-α) (11) and may therefore not be indicators of dietary inflammatory potential. Our group previously developed the empirical dietary index for hyperinsulinemia (EDIH) score (12,13) and empirical dietary inflammatory pattern (EDIP) score (12). Both scores comprise food combinations that maximally predict concentrations of circulating biomarkers: C-peptide for EDIH and inflammatory markers for EDIP (13,14). These biomarkers have been implicated in the pathogenesis of several chronic diseases, including type 2 diabetes (15–18). Both scores have been extensively applied in previous studies, with robust results on health outcomes (17,18).
The current study used EDIH, EDIP, GI, and GL scores to estimate the insulinemic, inflammatory, and glycemic potentials of the diet, respectively, and compared their associations with type 2 diabetes risk in a large and diverse cohort of postmenopausal women. The effect of diet on type 2 diabetes risk can be mediated through adiposity, while diet and adiposity may interact to influence type 2 diabetes risk (19). Also, the inflammatory potential of diet differs by race/ethnicity as dietary preferences differ by culture and race (14). Therefore, we further evaluated potential differences in the associations of diet scores with type 2 diabetes risk by BMI (in kilograms per meter squared) and race/ethnicity.
Research Design and Methods
Study Population
A total of 161,808 postmenopausal women aged 50–79 years at baseline (1993–1995) enrolled in the Women’s Health Initiative (WHI) (20) in 40 clinical centers across the U.S. Participants enrolled into either an observational study (n = 93,676) or one or more of four overlapping clinical trials (n = 68,132). We sequentially excluded women with type 2 diabetes at baseline (n = 9,619); implausible energy intake (<600 kcal/day and >5,000 kcal/day; n = 4,250); extreme BMI (<15 or >50 kg/m2; n = 6,055); cardiovascular disease or prevalent cancer (except nonmelanoma skin cancer) at baseline (n = 31,589), as diet may change after disease diagnosis; no type 2 diabetes status (n = 322); and participants in the Dietary Modification Trial (n = 36,478), as it excluded women who consumed low-fat diets (<32% of energy from fat) and intervention group participants were actively changing their diets. Our final analytic sample included 73,495 women and had comparable demographic and dietary intake distributions with the excluded participants (Supplementary Table 1) and with the entire WHI cohort (Supplementary Table 2). The WHI protocol was approved by the institutional review boards at the Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center and at each clinical center.
Dietary Assessment and Calculation of Dietary Indices
Dietary indices were calculated using baseline dietary data, which were assessed using a self-administered semiquantitative food frequency questionnaire (FFQ) representing habitual dietary intake in the preceding 3 months (21). Nutrient intake from the FFQ was derived by linking to the University of Minnesota Nutrition Coordinating Center food and nutrient database (22). The WHI FFQ was evaluated for validity by comparing with four 24-h dietary recalls and 4-day food records (21). The development and validation of the EDIP and EDIH scores have been described, and their food components are presented in Supplementary Tables 3 and 4 (12,23). Briefly, the EDIH and EDIP are weighted sums of their respective component food groups and beverages. A GI score represents the percent incremental area under the 2-h postprandial glucose response curve for consumption of a given carbohydrate-containing food relative to a reference food (glucose or white bread) with equal amount of carbohydrates (24). The GL of each food is the product of the food’s GI and the amount of carbohydrate in that food, summed across all foods for each individual (25). EDIH and EDIP scores were calculated using baseline FFQ data, and GI and GL scores were precomputed using baseline FFQs as part of the WHI dietary database. Higher scores on all indices indicate more hyperinsulinemic, proinflammatory, or hyperglycemic dietary patterns, respectively.
Ascertainment of Type 2 Diabetes
At baseline, prevalent diabetes was assessed through self-report when participants were asked if a physician had ever told them that they had “sugar diabetes or high blood glucose” when they were not pregnant. We excluded those with prevalent diabetes at baseline. At each contact (annually in the observational study and semiannually in the clinical trials), participants were asked, “Has a doctor prescribed any of the following pills/treatments?” and chose from “pills for diabetes” or “insulin shots for diabetes” (26). During the follow-up, participants were not asked about new-onset diabetes treated with lifestyle measures alone (26). New case subjects with type 2 diabetes were ascertained if participants self-reported that they had received type 2 diabetes treatments, including pills, insulin, and diabetes diet/exercise, and/or been hospitalized for diabetes (26). Self-reported prevalent and incident diabetes were validated against medication records (27).
Statistical Analysis
Dietary scores were adjusted for total energy intake using the residual method (28). We used Cox proportional hazards regression to estimate hazard ratios (HRs) and associated 95% CIs of the associations of each dietary index and risk of developing type 2 diabetes. The lowest index quintiles served as reference. Participants were followed from enrollment until type 2 diabetes diagnosis, death, loss to follow-up, or end of study on 1 March 2019. We calculated P values for linear trend across index quintiles by entering the dietary indices as continuous variables (1-SD increment) into the models. Covariates listed in Supplementary Table 5 were included in the Cox models. For GL models, we further adjusted for total protein, dietary fiber, and total fat.
In a subset analysis among participants with available data (n = 18,187), we further adjusted models for blood glucose. In additional analyses, we included all four dietary indices in the same multivariable-adjusted model to determine associations of each index independent of the other dietary indices.
In subgroup analyses, we used the likelihood ratio test to assess potential effect modification by BMI (normal weight, 18.5–24.9 kg/m2; overweight, 25–29.9 kg/m2; and obese, 30–50 kg/m2); waist-to-hip ratio (WHR; high, >85 cm, and low, ≤85 cm), and race/ethnicity (African American, European American, Hispanic, and other). We further investigated the joint association of each dietary pattern and BMI with type 2 diabetes risk.
We calculated multivariable-adjusted incidence rates of type 2 diabetes in quintiles of each dietary index and used the residual method (28) to adjust the dietary indices for the same covariates that were adjusted in the corresponding Cox regression models. We further estimated the incidence rate associated with each dietary pattern in BMI and race/ethnicity categories.
Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC), and two-sided P < 0.05 was considered statistically significant.
Results
Participants in the highest quintiles of EDIH or EDIP dietary patterns had higher BMI, lower physical activity, and higher proportions of African American and Hispanic women compared with those in the lowest quintiles. In contrast, BMI did not vary across GI and GL quintiles (Table 1). Regarding nutrient profiles, participants who consumed hyperinsulinemic dietary patterns had low intakes of total fiber, carbohydrate, calcium, and lycopene and higher intakes of protein, cholesterol, and total fat, especially saturated fat, than those who consumed low insulinemic dietary patterns. The nutrient profile from inflammatory dietary patterns was similar. In contrast, the nutrient profile from glycemic diets, classified by GL, appeared completely inverted compared with insulinemic dietary patterns (Supplementary Table 6), which conforms to the inverse correlation between GL and EDIH (Spearman r = −0.27) (Supplementary Table 7).`
Table 1.
Baseline characteristics of study participants across quintiles of dietary patterns scores, WHI (n = 73,495)
| EDIH*† | EDIP*† | GI‡ | GL‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Characteristic | (−9.67 to −0.89) | (−0.36 to 0.05) | (0.54–8.89) | (−11.90 to −0.89) | (−0.26 to 0.19) | (0.68–6.01) | (−12.13 to −0.58) | (−0.08 to 0.35) | (0.85–3.71) | (−8.35 to −0.46) | (0.04–0.46) | (0.98–8.06) |
| Race/ethnicity§ | ||||||||||||
| African American | 517 (3.5) | 786 (5.3) | 1,602 (11) | 398 (2.7) | 732 (5.0) | 1,891 (13) | 585 (4.0) | 755 (5.1) | 1,714 (12) | 785 (5.3) | 878 (6.0) | 1,178 (8.0) |
| American Indian or Alaskan Native | 27 (0.2) | 55 (0.4) | 66 (0.5) | 43 (0.3) | 44 (0.3) | 78 (0.5) | 61 (0.4) | 41 (0.3) | 63 (0.4) | 53 (0.4) | 55 (0.4) | 47 (0.3) |
| Hispanic American | 360 (2.4) | 486 (3.3) | 760 (5.2) | 203 (1.4) | 390 (2.6) | 1,168 (7.9) | 596 (4.0) | 491 (3.3) | 495 (3.4) | 557 (3.8) | 510 (3.5) | 490 (3.3) |
| Asian or Pacific Islander | 313 (2.1) | 486 (3.3) | 411 (2.8) | 197 (1.3) | 332 (2.3) | 806 (5.5) | 382 (2.6) | 475 (3.2) | 334 (2.3) | 259 (1.8) | 474 (3.2) | 469 (3.2) |
| European American | 13,269 (90) | 12,709 (86) | 11,633 (79) | 13,670 (93) | 12,997 (88) | 10,499 (71) | 12,854 (88) | 12,741 (87) | 11,887 (81) | 12,869 (88) | 12,552 (85) | 12,289 (84) |
| Other | 213 (1.5) | 177 (1.2) | 227 (1.5) | 188 (1.3) | 204 (1.4) | 257 (1.8) | 221 (1.5) | 196 (1.3) | 206 (1.4) | 176 (1.2) | 230 (1.6) | 226 (1.5) |
| Age, years‖ | 62.9 ± 7.2 | 63.6 ± 7.3 | 62.2 ± 7.3 | 62.7 ± 7.1 | 63.5 ± 7.3 | 62.5 ± 7.4 | 63.5 ± 7.3 | 63.2 ± 7.3 | 62.4 ± 7.2 | 63.1 ± 7.2 | 63.4 ± 7.3 | 62.4 ± 7.3 |
| BMI, kg/m2‖ | 25.6 ± 4.7 | 26.4 ± 5.1 | 28.7 ± 6.2 | 26.1 ± 4.9 | 26.5 ± 5.2 | 28.1 ± 6.1 | 26.3 ± 5.1 | 26.7 ± 5.3 | 27.5 ± 5.7 | 27.4 ± 5.8 | 26.6 ± 5.2 | 26.6 ± 5.4 |
| Under-/normal weight (15 ≤ BMI <25)§ | 7,686 (52) | 6,611 (45) | 4,454 (30) | 7,038 (48) | 6,471 (44) | 5,029 (34) | 6,801 (46) | 6,343 (43) | 5,582 (38) | 5,739 (39) | 6,389 (44) | 6,650 (45) |
| Overweight (25 ≤ BMI <30)§ | 4,797 (33) | 5,075 (34) | 4,924 (34) | 5,006 (34) | 5,136 (35) | 4,883 (33) | 5,401 (34) | 5,080 (35) | 4,953 (34) | 4,943 (34) | 5,132 (35) | 4,821 (33) |
| Obese (BMI ≥30)§ | 2,216 (15) | 3,013 (20) | 5,321 (36) | 2,655 (18) | 3,092 (21) | 4,787 (33) | 2,857 (19) | 3,276 (22) | 4,164 (28) | 4,017 (27) | 3,178 (22) | 3,228 (22) |
| WHR‖ | 0.79 ± 0.08 | 0.80 ± 0.08 | 0.82 ± 0.08 | 0.79 ± 0.08 | 0.80 ± 0.08 | 0.82 ± 0.08 | 0.80 ± 0.08 | 0.80 ± 0.08 | 0.81 ± 0.08 | 0.81 ± 0.08 | 0.80 ± 0.08 | 0.80 ± 0.08 |
| ≤0.85§ | 12,062 (82) | 11,535 (78) | 9,986 (68) | 11,833 (80) | 11,362 (77) | 10,169 (69) | 11,568 (79) | 11,307 (77) | 10,709 (73) | 10,595 (72) | 11,393 (78) | 11,452 (78) |
| >0.85§ | 2,637 (18) | 3,164 (22) | 4,713 (32) | 2,866 (20) | 3,337 (23) | 4,530 (31) | 3,131 (21) | 3,392 (23) | 3,990 (27) | 11,568 (28) | 11,307 (22) | 10,709 (22) |
| Physical activity, MET-h/week‖ | 18.1 ± 16.4 | 14.2 ± 14.1 | 9.6 ± 12.0 | 16.6 ± 15.6 | 14.1 ± 14.1 | 10.8 ± 13.1 | 17.3 ± 16.0 | 14.2 ± 14.2 | 10.3 ±12.6 | 12.0 ± 13.5 | 14.0 ± 14.1 | 16.2 ± 15.9 |
| Pack-years of smoking‖ | 10.7 ± 17.9 | 9.1 ± 17.0 | 10.6 ± 19.5 | 12.9 ± 19.9 | 9.3 ± 17.2 | 8.0 ± 16.7 | 9.9 ± 17.6 | 9.1 ± 16.8 | 10.8 ± 19.2 | 13.9 ± 21.2 | 8.6 ± 16.2 | 8.1 ± 16.2 |
| Current smoking§ | 859 (5.9) | 859 (5.9) | 1,559 (11) | 1,283 (8.9) | 900 (6.2) | 1,060 (7.3) | 828 (5.7) | 888 (6.1) | 1,531 (11) | 1,864 (13) | 801 (5.5) | 658 (4.5) |
| Aspirin/NSAIDs use§ | 1,980 (14) | 1,942 (13) | 1,891 (13) | 2,103 (14) | 2,001 (14) | 1,802 (12) | 1,994 (14) | 1,966 (13) | 1,982 (14) | 2,020 (14) | 2,013 (14) | 1,860 (13) |
| Statin use§ | 293 (2.0) | 342 (2.3) | 307 (2.1) | 270 (1.8) | 327 (2.2) | 341 (2.3) | 286 (2.0) | 314 (2.1) | 320 (2.2) | 200 (1.4) | 340 (2.3) | 395 (2.7) |
| Hypercholesterolemia§ | 1,603 (11) | 1,824 (12) | 1,806 (12) | 1,518 (10) | 1,850 (13) | 1,944 (13) | 1,601 (11) | 1,815 (12) | 1,884 (13) | 1,372 (9.3) | 1,827 (12) | 2,108 (14) |
| Educational level§ | ||||||||||||
| ≤8 years | 238 (1.6) | 280 (1.9) | 425 (2.9) | 167 (1.1) | 232 (1.6) | 599 (4.1) | 287 (2.0) | 275 (1.9) | 392 (2.7) | 326 (2.2) | 316 (2.2) | 309 (2.1) |
| Some high school/high school/GED | 388 (2.7) | 474 (3.3) | 846 (5.8) | 376 (2.6) | 469 (3.2) | 858 (5.9) | 358 (2.5) | 476 (3.3) | 890 (6.1) | 583 (4.0) | 503 (3.4) | 531 (3.7) |
| Some college/associate degree | 2,932 (20) | 3,901 (27) | 4,967 (34) | 3,328 (23) | 3,882 (27) | 4,719 (32) | 2,977 (20) | 3,829 (26) | 5,186 (36) | 4,141 (28) | 3,953 (27) | 3,655 (25) |
| ≥4 years of college | 10,975 (76) | 9,946 (68) | 8,384 (57) | 10,687 (73) | 10,007 (69) | 8,430 (58) | 10,947 (75) | 10,016 (69) | 8,129 (56) | 9,536 (65) | 9,824 (67) | 10,065 (69) |
| Total alcohol intake, servings/week‖¶ | 5.4 ± 8.2 | 2.1 ± 4.0 | 1.7 ± 4.0 | 5.9 ± 8.5 | 2.2 ± 3.9 | 1.0 ± 2.9 | 3.8 ± 6.9 | 2.7 ± 4.7 | 1.9 ± 4.7 | 6.1 ± 8.9 | 2.0 ± 3.4 | 1.2 ± 2.8 |
GED, General Educational Development; NSAID, nonsteroidal anti-inflammatory drug.
EDIP, EDIH, GI, and GL scores were adjusted for total energy intake using the residual method. Lower EDIP indicates anti-inflammatory dietary patterns, while higher EDIP scores indicate proinflammatory dietary patterns. Lower EDIH indicates low insulinemic dietary patterns, while a higher score indicates hyperinsulinemic dietary patterns.
Food components of the EDIH and EDIP scores (servings per day) in the WHI are listed in Supplementary Tables 3 and 4.
GI and GL were calculated from total carbohydrates.
Alcohol serving was the sum of: beer (one glass, one bottle, or one can), wine (4-oz glass of red wine or white wine), and liquor (one drink or one shot whiskey, gin, etc.).
Data are means ± SD.
Data are frequency (%).
We documented 11,009 incident cases of type 2 diabetes during a median 13.3 years of follow-up. Table 2 presents the HR and 95% CI of the associations of each dietary index with type 2 diabetes risk. In multivariable-adjusted models, higher EDIH and EDIP scores were significantly associated with higher risk of type 2 diabetes. Relative to those who consumed the least, women who consumed the most hyperinsulinemic diets (EDIH) had a 49% higher risk of type 2 diabetes (quintile 5 vs. 1: HR 1.49 [95% CI 1.32–1.68]; Ptrend < 0.0001). The HR for women in highest compared with the lowest EDIP quintile was 1.45 (95% CI 1.29–1.63; Ptrend < 0.0001). In the multivariable-adjusted models, GI and GL were not associated with type 2 diabetes risk (Table 2).
Table 2.
| Dietary indices | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Per 1-SD increment | P trend§ |
|---|---|---|---|---|---|---|---|
| EDIH | |||||||
| Case subjects/noncase subjects | 1,944/12,755 | 1,988/12,711 | 2,078/12,621 | 2,267/12,432 | 2,732/11,967 | — | — |
| Age and energy adjusted | 1 (reference) | 1.07 (1.01–1.14) | 1.17 (1.10–1.24) | 1.30 (1.22–1.38) | 1.65 (1.56–1.75) | 1.19 (1.17–1.21) | <0.0001 |
| MV adjusted | 1 (reference) | 1.09 (0.96–1.25) | 1.11 (0.98–1.26) | 1.19 (1.05–1.35) | 1.49 (1.32–1.68) | 1.14 (1.10–1.18) | <0.0001 |
| MV+BMI adjusted | 1 (reference) | 1.15 (0.96–1.37) | 1.02 (0.86–1.21) | 1.17 (0.99–1.38) | 1.41 (1.20–1.65) | 1.12 (1.07–1.17) | <0.0001 |
| EDIP | |||||||
| Case subjects/noncase subjects | 1,981/12,718 | 19,91/12,708 | 2,073/12,626 | 2,268/12,431 | 2,696/12,003 | — | — |
| Age and energy adjusted | 1 (reference) | 1.03 (0.97–1.10) | 1.08 (1.01–1.14) | 1.27 (1.19–1.34) | 1.60 (1.51–1.69) | 1.18 (1.16–1.20) | <0.0001 |
| MV adjusted | 1 (reference) | 1.06 (0.94–1.21) | 1.10 (0.97–1.24) | 1.25 (1.11–1.41) | 1.45 (1.29–1.63) | 1.15 (1.11–1.19) | <0.0001 |
| MV+BMI adjusted | 1 (reference) | 1.00 (0.85–1.18) | 1.08 (0.92–1.27) | 1.27 (1.09–1.49) | 1.42 (1.22–1.65) | 1.14 (1.09–1.19) | <0.0001 |
| GI | |||||||
| Case subjects/noncase subjects | 2,143/12,556 | 2,144/12,555 | 2,187/12,512 | 2,264/12,435 | 2,271/12,428 | — | — |
| Age and energy adjusted | 1 (reference) | 1.01 (0.95–1.08) | 1.03 (0.97–1.10) | 1.10 (1.03–1.16) | 1.22 (1.15–1.30) | 1.08 (1.05–1.10) | <0.0001 |
| MV adjusted | 1 (reference) | 1.00 (0.88–1.14) | 1.03 (0.91–1.16) | 0.95 (0.84–1.07) | 0.99 (0.88–1.12) | 0.99 (0.95–1.03) | 0.46 |
| MV+BMI adjusted | 1 (reference) | 1.05 (0.89–1.23) | 0.93 (0.79–1.10) | 0.92 (0.79–1.08) | 0.99 (0.85–1.16) | 0.97 (0.92–1.03) | 0.31 |
| GL | |||||||
| Case subjects/noncase subjects | 2,288/12,411 | 2,172/12,527 | 2,161/12,538 | 2,146/12,553 | 2,242/12,457 | — | — |
| Age and energy adjusted | 1 (reference) | 0.94 (0.89–1.00) | 0.93 (0.87–0.98) | 0.88 (0.83–0.93) | 0.91 (0.86–0.96) | 0.97 (0.95–0.99) | 0.0012 |
| MV adjusted | 1 (reference) | 0.97 (0.87–1.09) | 0.93 (0.83–1.05) | 0.97 (0.85–1.10) | 1.01 (0.89–1.16) | 1.02 (0.98–1.07) | 0.30 |
| MV+BMI adjusted | 1 (reference) | 0.90 (0.78–1.04) | 0.94 (0.80–1.10) | 0.94 (0.80–1.11) | 0.97 (0.81–1.15) | 0.99 (0.94–1.05) | 0.71 |
EDIP, EDIH, GI, and GL scores were adjusted for total energy intake using the residual method. The EDIH, EDIP, and GI (total carbohydrates) multivariable (MV)-adjusted models were stratified by age, hypertension, family history of type 2 diabetes, hormone use, physical activity, and further adjusted for education, race, pack-years of smoking, high cholesterol, WHI study arms, nonsteroidal anti-inflammatory drug use, statin use, and nutritional supplement use. The MV+BMI models adjusted for all covariates in the MV models and additionally for BMI. The MV-adjusted models for GL were additionally adjusted for total fat, total protein, and dietary fiber. Supplementary Tables 3 and 4 list food components of EDIH and EDIP.
Lower EDIP scores indicate anti-inflammatory dietary patterns, while higher EDIP scores indicate proinflammatory patterns. Lower EDIH indicates antihyperinsulinemic dietary patterns, while a higher score indicates prohyperinsulinemic patterns.
GI and GL were calculated using total carbohydrates and available carbohydrates. Results were similar; thus, we used only scores from total carbohydrate. Lower GI/GL scores indicate low glycemic diets, while higher scores indicate hyperglycemic diets.
P values for linear trend across dietary index quintiles were estimated using the dietary indices entered into the models as continuous variables. Models for linear trend were adjusted for all covariates listed in the corresponding models in the footnote above marked with an asterisk (*).
Regarding absolute risk, the consumption of hyperinsulinemic or proinflammatory dietary patterns resulted in an excess of 220 and 271 more cases of type 2 diabetes per 100,000 person-years, respectively, whereas consumption of hyperglycemic dietary patterns did not result in excess risk: −19 (GI) and −41 (GL) cases of type 2 diabetes per 100,000 person-years (Table 3). Results remained robust when we additionally adjusted for blood glucose in the subset analysis (Supplementary Table 8), when we mutually adjusted each index for the other three dietary indices (Supplementary Table 9), when we adjusted the associations using propensity scores (Supplementary Table 10), and when we excluded cases of type 2 diabetes diagnosed within 2 years from baseline to limit potential reverse causality (Supplementary Table 11).
Table 3.
Incidence rate of type 2 diabetes (per 100,000 person-years) in dietary index quintiles*
| Dietary indices | Type 2 diabetes incidence rate (per 100,000 person-years) | |||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | Q5 − Q1† | |
| EDIH | 1,160 | 1,195 | 1,148 | 1,161 | 1,380 | 220 |
| EDIP | 1,116 | 1,142 | 1,170 | 1,233 | 1,387 | 271 |
| GI‡ | 1,220 | 1,241 | 1,180 | 1,195 | 1,201 | −19 |
| GL‡ | 1,281 | 1,181 | 1,176 | 1,160 | 1,240 | −41 |
Each dietary score was adjusted for total energy intake, baseline age, hypertension, type 2 diabetes family history, hormone use, physical activity, education, race, pack-years of smoking, high cholesterol, WHI study arms, nonsteroidal anti-inflammatory drug use, statin use, nutritional supplement use, and BMI. The incidence rate by GL quintiles was further adjusted for total fat, total protein, and dietary fiber.
Q5 − Q1: The excess incidence due to consuming a hyperinsulinemic/proinflammatory or hyperglycemic dietary pattern.
GI and GL were calculated using total carbohydrates.
There were significant differences by WHR (Pheterogeneity < 0.0001 for both EDIH and EDIP), with stronger associations among women with high WHR (Supplementary Table 12). Similarly, intake of hyperinsulinemic and proinflammatory dietary patterns was associated with higher type 2 diabetes risk among overweight and obese women (Pheterogeneity = 0.03 for EDIP) (Supplementary Table 13). The trend was clearer in the joint associations of EDIH, EDIP, and BMI: compared with normal-weight women consuming low insulinemic or anti-inflammatory diets, those who were overweight/obese and consuming hyperinsulinemic or proinflammatory diets had 72–73% higher type 2 diabetes risk (Supplementary Fig. 1). No differences were observed across WHR or BMI subgroups for GI and GL. However, in the joint analysis, type 2 diabetes risk was elevated among overweight/obese women who also had higher GI/GL scores, though with no clear linear trend (Supplementary Fig. 1). The incidence rate of type 2 diabetes increased with higher dietary insulinemic or inflammatory potential among normal-weight individuals but more so among overweight/obese individuals (Supplementary Fig. 2A and B). No differences were observed in type 2 diabetes incidence in GI or GL quintiles (Supplementary Fig. 2C and D).
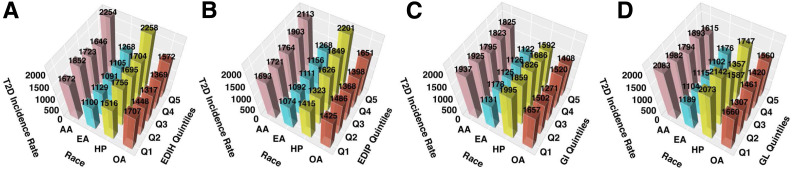
In subgroups defined by race/ethnicity (Supplementary Table 14), hyperinsulinemic and proinflammatory diets, but not hyperglycemic diets, were significantly associated with type 2 diabetes risk among European Americans and Hispanics/Latinas, and associations were strongest among Hispanic women (Pheterogeneity for EDIH = 0.12; EDIP = 0.13; GL = 0.16; and GI = 0.04). Though the risk was elevated among African Americans and other race groups, it did not attain statistical significance. Further, the absolute excess risks comparing quintile 5 versus 1 for each dietary pattern (per 100,000 person-years) were as follows: African Americans: EDIH 582, EDIP 420, GI −112, and GL −468; European Americans: EDIH 168, EDIP 194, GI −10, and GL −83; and Hispanics: EDIH 743, EDIP 786, GI −403, and GL −326 (Fig. 1).
Figure 1.
Type 2 diabetes (T2D) incidence rate per 100,000 person-years by race/ethnicity. A: EDIH. B: EDIP. C: GI. D: GL. Each dietary score was adjusted for total energy intake, baseline age, hypertension, family history of type 2 diabetes, hormone use, physical activity, education, race, pack-years of smoking, high cholesterol, WHI study arms, nonsteroidal anti-inflammatory drug use, statin use, nutritional supplement use, and BMI (continuous). The GL (total carbohydrates) was additionally adjusted for total fat, total protein, and dietary fiber. AA, African American; EA, European American; HP, Hispanic American; OA, other American; Q, quintile.
Conclusions
We used EDIH, EDIP, GI, and GL to evaluate associations of dietary insulinemic, inflammatory, and glycemic potentials with type 2 diabetes risk. Higher EDIH and EDIP, but not GI or GL, were associated with higher relative risk of developing type 2 diabetes, with a corresponding large absolute excess risk, independent of BMI, total energy intake, physical activity, and other potential confounding variables. The associations of dietary insulinemic and inflammatory potentials with type 2 diabetes were stronger among overweight and obese than normal-weight women and among Hispanics and African Americans than European Americans. The high absolute excess risk from hyperinsulinemic and proinflammatory dietary patterns among Hispanics and African Americans suggest that future type 2 diabetes prevention strategies focused on mitigating the contribution of diet to sustained insulin hypersecretion or chronic systemic inflammation may have larger benefits among these racial/ethnic subgroups. Furthermore, comparing findings across the four dietary indices suggests that focusing only on the carbohydrate content of diet may be insufficient to prevent type 2 diabetes. According to the food and beverage components of the EDIP and EDIH, a dietary pattern that is both anti-inflammatory and low insulinemic includes low intakes of red meat, processed meat, nonfatty fish, sugar-sweetened beverages, and refined grains and high intakes of green leafy vegetables, full-fat dairy, wine, and coffee.
The associations of EDIH and EDIP with type 2 diabetes risk suggest that the potential of dietary patterns to promote hyperinsulinemia and chronic systemic inflammation may partially underlie the associations of dietary patterns and type 2 diabetes risk reported in previous studies (2–4). The empirical hypothesis–oriented approach used to develop EDIH and EDIP focused on circulating biomarkers, making the derived dietary patterns independent of specific nutrients, while considering the totality of the diet without preconceived notions of healthy or unhealthy foods. Similarly, one study reported on an empirical hypothesis–oriented dietary pattern in relation to type 2 diabetes risk in the Multiethnic Cohort (29). The authors found that the dietary pattern was associated with type 2 diabetes risk in the overall study sample and in separate subsamples defined by race/ethnicity. However, this study did not calculate absolute type 2 diabetes risk estimates. In contrast, a study using data from the Aerobics Center Longitudinal Study reported no association between a literature-derived nutrient-based dietary inflammatory index and incident type 2 diabetes (30). It is, however, difficult to directly compare these findings with current study findings, as the dietary inflammatory index is mainly nutrient-based and driven by supplement use. The EDIP and EDIH are based exclusively on whole foods, thereby accounting for not only the complex interactions among foods and nutrients in the diet, but also the food matrix. The same nutrients can have different health effects depending on the food matrix in which they are consumed (31,32) (e.g., fermented dairy such as cheese and yogurt vs. butter or processed vs. unprocessed foods). Therefore, focusing on nutrients does not account for the context (e.g., food matrix and dietary pattern) in which the nutrient is consumed.
We found no significant association of GI/GL prospectively with type 2 diabetes risk in the overall sample and in subgroup analyses. In contrast with EDIH and EDIP scores, higher GI and/or GL scores reflecting hyperglycemic diets were associated with higher whole-grain and fiber (in GL) intake and lower branched-chain amino acid intake. Higher intake of whole grain and fiber were inversely associated with type 2 diabetes risk (33), while higher isoleucine status was associated with higher type 2 diabetes incidence (34), which may partly explain the lack of associations for GI/GL. Additionally, other macronutrients such as protein and fat that may influence levels of circulating inflammatory and insulin resistance biomarkers (35,36) are not considered in GI and GL. Interestingly, GL had a low correlation with EDIP and an inverse correlation with EDIH. The inverse correlation was also evident in the inverted distributions of foods and nutrients across quintiles of the EDIH and GL, suggesting that these dietary indices are not assessing the same biological construct in the diet. Whereas GI/GL assess the postprandial (short-term) glucose response of the meal, and therefore an indirect assessment of insulin response to the glucose rise, EDIH directly assesses the dietary contribution to insulinemia (37). Properties of dietary patterns, such as the ability to directly contribute to sustained hyperinsulinemia, insulin resistance, and chronic inflammation—constructs assessed by EDIH and EDIP—may explain why these scores are more strongly predictive of type 2 diabetes risk than GI and GL. Furthermore, some previous studies found that higher GI/GL scores were associated with higher type 2 diabetes risk (HRQ5vs1 18% [95% CI 6–31) (38). However, the incidence rate revealed an underestimate of excess incidence (−33 cases of type 2 diabetes/100,000 person-years) (38), which is in line with current study findings on absolute risk.
Diet has a complex relationship with adiposity (19). In previous studies, higher EDIP and EDIH scores were associated with long-term weight gain (39); therefore, the models adjusting for BMI may highlight the adiposity-mediated association of EDIP and EDIH on type 2 diabetes risk, though we observed only slightly attenuated associations when additionally adjusting for BMI. Although the association of EDIP and EDIH with type 2 diabetes risk may be partly mediated through adiposity, these empirical hypothesis–oriented dietary indices have direct associations with type 2 diabetes, independent of adiposity, as shown in the findings from BMI and WHR subgroups or in the BMI–dietary pattern joint analyses, which were additionally adjusted for continuous BMI. Normal-weight women who consumed hyperinsulinemic or proinflammatory diets were at significantly higher type 2 diabetes risk compared with normal-weight women consuming low insulinemic or anti-inflammatory diets.
Our study has several strengths, including the use of novel food-based empirical hypothesis–oriented dietary patterns in a well-characterized cohort. The large sample size allowed us to conduct multiple subgroup analyses. We estimated absolute type 2 diabetes risk in addition to the relative risks, providing insights on the clinical utility of the dietary patterns. However, our study is not without limitations. Ideally, blood glucose should have been measured on all participants; however, sensitivity analysis further adjusting for glucose in a large subsample showed comparable results. Diet may have been measured with error, though the FFQ was previously evaluated for measurement characteristics (21). Though we adjusted for a large number of potential confounding variables in the estimation of both the relative and absolute risk, the possibility of residual confounding or confounding by unmeasured variables remains. Although WHI sample selection may limit generalizability of our findings, the overall WHI sample closely represented the U.S. population distribution by race/ethnicity in 1996 (midpoint of WHI recruitment) (40). In addition, our included sample was comparable to the overall WHI sample. Though we estimated associations of dietary index quintiles with type 2 diabetes risk assuming a linear relationship, this may not always be true; however, associations using the continuous scores aligned well with the quintile results. Also, our sample was restricted to postmenopausal women; thus, future studies among men and younger women are warranted.
In this large cohort of postmenopausal women in the U.S., hyperinsulinemic and proinflammatory dietary patterns were associated with subsequent risk of type 2 diabetes, with stronger associations among overweight and obese women and among Hispanic and African American women. In contrast, dietary glycemic potential was not associated with type 2 diabetes risk. Our findings suggest that lowering the insulinemic and inflammatory potential of the diet may be effective in reducing type 2 diabetes risk, especially among overweight/obese women and among Hispanic and African American women, and that focusing on traditional glycemic foods may be insufficient to prevent type 2 diabetes.
Article Information
Funding. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. F.K.T. was responsible for concept and design. All of the authors were responsible for acquisition, analysis, or interpretation of data. Q.J. wrote the original manuscript draft. All authors contributed to the critical revision of the manuscript. Q.J. was responsible for statistical analysis. F.K.T. supervised the project. Q.J. and F.K.T. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented in abstract form at the Nutrition 2020 Conference, 1–4 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13312649.
References
- 1.Centers for Disease Control and Prevention (Ed.) . National Diabetes Statistics Report 2020. Atlanta, GA, Centers for Disease Control and Prevention, 2020. (publ. no. CS 314227-A) [Google Scholar]
- 2.Cespedes EM, Hu FB, Tinker L, et al. Multiple healthful dietary patterns and type 2 diabetes in the Women’s Health Initiative. Am J Epidemiol 2016;183:622–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–1182 [DOI] [PubMed] [Google Scholar]
- 4.Burgos-Morón E, Abad-Jiménez Z, Marañón AM, et al. Relationship between oxidative stress, ER stress, and inflammation in type 2 diabetes: the battle continues. J Clin Med 2019;8:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu S, Chou EL. Dietary glycemic load and type 2 diabetes: modeling the glucose-raising potential of carbohydrates for prevention. Am J Clin Nutr 2010;92:675–677 [DOI] [PubMed] [Google Scholar]
- 6.Willett WC, Liu S. Carbohydrate quality and health: distilling simple truths from complexity. Am J Clin Nutr 2019;110:803–804 [DOI] [PubMed] [Google Scholar]
- 7.Greenwood DC, Threapleton DE, Evans CE, et al. Glycemic index, glycemic load, carbohydrates, and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Diabetes Care 2013;36:4166–4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livesey G, Taylor R, Livesey HF, et al. Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients 2019;11:1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019;393:434–445 [DOI] [PubMed] [Google Scholar]
- 10.Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008;31(Suppl. 2):S262–S268 [DOI] [PubMed] [Google Scholar]
- 11.Milajerdi A, Saneei P, Larijani B, Esmaillzadeh A. The effect of dietary glycemic index and glycemic load on inflammatory biomarkers: a systematic review and meta-analysis of randomized clinical trials. Am J Clin Nutr 2018;107:593–606 [DOI] [PubMed] [Google Scholar]
- 12.Tabung FK, Wang W, Fung TT, et al. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr 2016;116:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabung FK, Balasubramanian R, Liang L, et al. Identifying metabolomic profiles of insulinemic dietary patterns. Metabolites 2019;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabung FK, Giovannucci EL, Giulianini F, et al. An empirical dietary inflammatory pattern score is associated with circulating inflammatory biomarkers in a multi-ethnic population of postmenopausal women in the United States. J Nutr 2018;148:771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JD, Kang SJ, Lee MK, et al. C-peptide-based index is more related to incident type 2 diabetes in non-diabetic subjects than insulin-based index. Endocrinol Metab (Seoul) 2016;31:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaccardi F, Webb DR, Yates T, Davies MJ. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J 2016;92:63–69 [DOI] [PubMed] [Google Scholar]
- 17.Tabung FK, Wang W, Fung TT, et al. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr 2018;108:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma W, Jovani M, Nguyen LH, et al. Association between inflammatory diets, circulating markers of inflammation, and risk of diverticulitis. Clin Gastroenterol Hepatol 2020;18:2279–2286.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovannucci E. A framework to understand diet, physical activity, body weight, and cancer risk. Cancer Causes Control 2018;29:1–6 [DOI] [PubMed] [Google Scholar]
- 20.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials 1998;19:61–109 [DOI] [PubMed] [Google Scholar]
- 21.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol 1999;9:178–187 [DOI] [PubMed] [Google Scholar]
- 22.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc 1988;88:1268–1271 [PubMed] [Google Scholar]
- 23.Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr 2016;146:1560–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinhold CL, Dodd KW, Jiao L, et al. Available carbohydrates, glycemic load, and pancreatic cancer: is there a link? Am J Epidemiol 2010;171:1174–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr 2000;71:1455–1461 [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med 2007;167:1676–1685 [DOI] [PubMed] [Google Scholar]
- 27.Margolis KL, Lihong Qi, Brzyski R, et al.; Women Health Initiative Investigators . Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials 2008;5:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(Suppl.):1220S–1228S; discussion 1229S–1231S [DOI] [PubMed] [Google Scholar]
- 29.Jacobs S, Kroeger J, Schulze MB, et al. Dietary patterns derived by reduced rank regression are inversely associated with type 2 diabetes risk across 5 ethnic groups in the Multiethnic Cohort. Curr Dev Nutr 2017;1:e000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guinter MA, Merchant AT, Tabung FK, et al. Adiposity does not modify the effect of the dietary inflammatory potential on type 2 diabetes incidence among a prospective cohort of men. J Nutr Intermed Metab 2019;16:100095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feeney EL, Barron R, Dible V, et al. Dairy matrix effects: response to consumption of dairy fat differs when eaten within the cheese matrix-a randomized controlled trial. Am J Clin Nutr 2018;108:667–674 [DOI] [PubMed] [Google Scholar]
- 32.Benedé S, López-Expósito I, Molina E, López-Fandiño R. Egg proteins as allergens and the effects of the food matrix and processing. Food Funct 2015;6:694–713 [DOI] [PubMed] [Google Scholar]
- 33.Parker ED, Liu S, Van Horn L, et al. The association of whole grain consumption with incident type 2 diabetes: the Women’s Health Initiative Observational Study. Ann Epidemiol 2013;23:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chailurkit LO, Paiyabhroma N, Sritara P, et al. Independent and opposite associations between branched-chain amino acids and lysophosphatidylcholines with incident diabetes in Thais. Metabolites 2020;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sears B, Perry M. The role of fatty acids in insulin resistance. Lipids Health Dis 2015;14:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rietman A, Schwarz J, Tomé D, Kok FJ, Mensink M. High dietary protein intake, reducing or eliciting insulin resistance? Eur J Clin Nutr 2014;68:973–979 [DOI] [PubMed] [Google Scholar]
- 37.Lee DH, Giovannucci EL, Tabung FK. Insulin-related dietary indices predict 24-h urinary C-peptide in adult men. Br J Nutr. 19 June 2020 [Epub ahead of print]. DOI: 10.1017/S0007114520002184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 2014;100:218–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabung FK, Satija A, Fung TT, Clinton SK, Giovannucci EL. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J Nutr 2019;149:804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Women’s Health Initiative . Diversity in WHI: guidance for authors and investigators on how to address in manuscripts and proposals, 2020. Accessed 6 December 2020. Available from https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Diversity-Task-Force-Guide-for-Authors.1.27.20.pdf