Abstract
OBJECTIVE
Both sodium–glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1RA) demonstrated cardiovascular benefits in randomized controlled trials of patients with type 2 diabetes (T2D) generally <65 years old and mostly with cardiovascular disease. We aimed to evaluate the comparative effectiveness and safety of SGLT2i and GLP-1RA among real-world older adults.
RESEARCH DESIGN AND METHODS
Using Medicare data (April 2013–December 2016), we identified 90,094 propensity score–matched (1:1) T2D patients ≥66 years old initiating SGLT2i or GLP-1RA. Primary outcomes were major adverse cardiovascular events (MACE) (i.e., myocardial infarction, stroke, or cardiovascular death) and hospitalization for heart failure (HHF). Other outcomes included diabetic ketoacidosis (DKA), genital infections, fractures, lower-limb amputations (LLA), acute kidney injury (AKI), severe urinary tract infections, and overall mortality. We estimated hazard ratios (HRs) and rate differences (RDs) per 1,000 person-years, controlling for 140 baseline covariates.
RESULTS
Compared with GLP-1RA, SGLT2i initiators had similar MACE risk (HR 0.98 [95% CI 0.87, 1.10]; RD −0.38 [95% CI −2.48, 1.72]) and reduced HHF risk (HR 0.68 [95% CI 0.57, 0.80]; RD −3.23 [95% CI −4.68, −1.77]), over a median follow-up of ∼6 months. They also had 0.7 more DKA events (RD 0.72 [95% CI 0.02, 1.41]), 0.9 more LLA (RD 0.90 [95% CI 0.10, 1.70]), 57.1 more genital infections (RD 57.08 [95% CI 53.45, 60.70]), and 7.1 fewer AKI events (RD −7.05 [95% CI −10.27, −3.83]) per 1,000 person-years.
CONCLUSIONS
Among older adults, those taking SGLT2i had similar MACE risk, decreased HHF risk, and increased risk of DKA, LLA, and genital infections versus those taking GLP-1RA.
Introduction
The aging of the U.S. population is a significant driver of the type 2 diabetes (T2D) epidemic (1). Approximately 25% of people over the age of 65 years have diabetes, and this proportion is expected to rise to >30% by 2050 (2). Patients with diabetes are at high risk for developing cardiovascular disease (CVD) and associated morbidity and mortality, and this risk increases dramatically with age, with cardiovascular events being the primary cause of morbidity and mortality in older adults with T2D (3).
In recent large cardiovascular outcome trials (CVOTs), mandated by regulators to assess the cardiovascular safety of antidiabetes medications, glucagon-like peptide 1 receptor agonists (GLP-1RA) and sodium–glucose cotransporter 2 inhibitors (SGLT2i) showed superiority to placebo in reducing the risk of major adverse cardiovascular events (MACE) (4–9), cardiovascular mortality (4,8), all-cause mortality (4,8,10), and nephropathy progression (4,5,7,11–13), with SGLT2i also reducing the risk of hospitalization for heart failure (HHF) (8,9,11). This new evidence prompted guidelines to recommend the initiation of an SGLT2i or a GLP-1RA among patients with high cardiovascular risk or patients with established atherosclerotic CVD, heart failure, or chronic kidney disease (3). However, the comparative effectiveness of these classes of medications in older adults, who represent the majority of patients with T2D, remains largely unknown, as CVOTs have not compared these agents head-to-head, and as these trials were conducted among selected populations with a mean age generally <65 years and mostly with preexisting CVD. Furthermore, as information on potential unintended harms of SGLT2i is rapidly accumulating, e.g., diabetic ketoacidosis (DKA), bone fractures, lower-limb amputations (LLA), and severe urinary tract infections (UTI) (9,14–16), it is critical to understand their safety in older individuals with diabetes. These patients have increased occurrence of multiple comorbidities, polypharmacy, reduced functional status, accelerated muscle loss, and common geriatric syndromes, such as frailty, and thus may be at greater risk of drug-related adverse events compared with younger individuals.
Our primary objective was to evaluate the cardiovascular effectiveness associated with the initiation of SGLT2i compared with GLP-1RA in a population-based cohort of older adults as treated in routine clinical care. Our secondary objective was to assess the safety of SGLT2i versus GLP-1RA with regard to outcomes relevant to older patients.
Research Design and Methods
Data Source, Study Cohort, and Drug Exposure
Data were collected from Medicare Fee-for-Service Parts A (inpatient coverage), B (outpatient coverage), and D (prescription benefits). Medicare is a U.S. federal health insurance program that provides health care to legal residents in the U.S. at least 65 years of age and patients with disabilities. This database covers ∼50 million people and contains demographic information, health plan enrollment status, longitudinal patient-level information on all reimbursed medical services, and both inpatient and outpatient diagnoses and procedures along with pharmacy-dispensing records, including information on medication start and refill, strength, quantity, and days’ supply. Information on the exact date and cause of death was available for the entire study period through linking with the National Death Index (NDI) file.
The Institutional Review Board of Partners Healthcare approved the study. A licensing agreement was in place.
The study population included patients aged 66 years or older who initiated an SGLT2i, i.e., canagliflozin, dapagliflozin, or empagliflozin, or a GLP-1RA, i.e., albiglutide, dulaglutide, exenatide, or liraglutide, between 1 April 2013 (consistent with the release of the first SGLT2i in the U.S.) and 31 December 2016. Cohort entry was the day of the first filled prescription of any of the drugs above, defined as no use of either SGLT2i or GLP-1RA in the previous year among patients who had 12 or more months of continuous enrollment prior to cohort entry. A recorded diagnosis of T2D was required during the year prior to drug initiation. Patients were excluded if they had a diagnosis of type 1 or secondary diabetes, malignancy, end-stage renal disease, HIV, organ transplant, or a nursing home admission at baseline. To address the potential for unmeasured confounding associated with the high risk for recurrence, we excluded patients with a hospitalization for acute myocardial infarction, coronary revascularization, unstable angina, ischemic or hemorrhagic stroke, a transient ischemic attack, and heart failure in the 60 days prior to cohort entry (Supplementary Table 1 and Supplementary Fig. 1).
Follow-up for study outcomes began on the day after cohort entry and continued in an “as-treated” approach until treatment discontinuation or switch to a drug in the comparator class, the occurrence of a specific study outcome, death, end of continuous health plan enrollment, or end of the study period (31 December 2016)—whichever came first. We extended the exposure effect window until 60 days after the expiration of the last prescription’s supply.
Study Outcomes
The primary outcomes included 1) MACE, i.e., a hospitalization for acute myocardial infarction, ischemic or hemorrhagic stroke, or cardiovascular mortality, and 2) HHF. In prior studies, the positive predictive values of claims-based algorithms for myocardial infarction, stroke, and HHF were at least 84% (17–20). Causes of death were determined through NDI linkage. Cardiovascular deaths were identified through recorded ICD-10 codes (I00–I99); only primary causes of death were considered (21). Secondary effectiveness outcomes were the individual components of the composite cardiovascular outcome, all-cause mortality, a composite outcome of MACE or HHF, and a composite of myocardial infarction, stroke, HHF, or all-cause mortality. Safety outcomes included DKA requiring hospitalization, LLA requiring surgery, nonvertebral bone fractures (fracture of humerus, wrist, or hip requiring intervention, or pelvis fracture), genital infections (inpatient or outpatient episodes), acute kidney injury (AKI) requiring hospitalization, and severe UTI, defined as those requiring hospitalization. Definitions were either validated against medical records (22–26) or previously used in pharmacoepidemiologic studies with assessment of glucose-lowering medications (27,28). (See Supplementary Table 2 for definitions.)
Patient Characteristics
Patient baseline characteristics were measured during the 12 months before and including the date of cohort entry. Covariates of interest included demographics (age, sex, and race), calendar time (in quarters and days), comorbidities, diabetes-specific complications, use of diabetes drugs, use of other medications, indicators of health care use as proxy for overall disease state, surveillance, and intensity of care. To address potential confounding by frailty, we also used a claims-based frailty index (29). Patient characteristics were defined with use of ICD-9 or ICD-10 diagnosis or procedure codes, Current Procedural Terminology, 4th Edition, procedure codes, and National Drug Code (pharmacy). A complete list of baseline patient characteristics can be found in Supplementary Table 3.
Statistical Analysis
Baseline characteristics were cross tabulated by treatment group. To control for imbalances in patient characteristics, we calculated an exposure propensity score (PS) as the predicted probability of receiving SGLT2i versus GLP-1RA treatment, conditional on 140 baseline characteristics, using a multivariable logistic regression model. All variables in Supplementary Table 3 were included, and no further selection was conducted. Patients were 1:1 PS matched by use of the nearest neighbor methodology with a maximum caliper of 0.01 of the PS (Supplementary Fig. 2). Postmatching covariate balance between treatments was assessed by the calculation of standardized differences for each covariate, with meaningful imbalances set at values >0.1 (30). For all outcomes, we calculated PS-matched numbers of events and incidence rates, as well as hazard ratios (HRs), and rate differences (RDs), each with 95% CIs. For primary end points, both overall and within strata defined by presence of CVD at baseline, we also obtained Kaplan-Meier plots of cumulative incidence and compared incidence rates between treatment groups with log-rank tests.
To address potential informative censoring, in sensitivity analyses we carried forward the exposure to the first-used medication for 365 days without considering drug discontinuation or switching, mimicking an “intention-to-treat” approach. To assess potential effect modification by CVD, we also conducted subgroup analyses for primary outcomes and selected secondary outcomes stratified by history of CVD at baseline, defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, and lower-extremity amputation. The association with primary outcomes was also assessed in subgroup analyses by age (66–74 years and 75+ years), history of chronic kidney disease, and presence of metformin at cohort entry. Within each subgroup, the PS was reestimated and PS matching was reperformed, and the presence of effect measure modification across categories was evaluated on the relative and absolute scale with the Wald test for homogeneity.
All analyses were performed with the Aetion Evidence Platform, version 3.2, with R, version 3.2 (31), and with SAS 9.4 statistical software (SAS Institute, Cary, NC).
Results
Study Cohort and Patient Characteristics
After inclusion and exclusion criteria were applied, we identified 72,900 patients initiating SGLT2i and 64,417 patients initiating GLP-1RA, resulting in 90,094 PS-matched (1:1) patients, with 45,047 in each group (Table 1 and Supplementary Fig. 1).
Table 1.
Selected baseline characteristics of patients initiating SGLT2i versus GLP-1RA before and after 1:1 PS matching
| Baseline characteristics | Before PS matching | After PS matching | ||||
|---|---|---|---|---|---|---|
| SGLT2i (N = 72,900) | GLP-1RA (N = 64,417) | St. Diff. | SGLT2i (N = 45,047)∗ | GLP-1RA (N = 45,047)∗ | St. Diff. | |
| Demographics | ||||||
| Age, mean (SD) | 71.98 (5.28) | 71.43 (4.94) | 0.11 | 71.56 (5.01) | 71.56 (5.03) | 0.00 |
| Male, n (%) | 35,982 (49.4) | 28,044 (43.5) | 0.12 | 20,541 (45.6) | 20,720 (46.0) | −0.01 |
| Race/ethnicity, n (%) | ||||||
| White | 58,251 (79.9) | 54,125 (84.0) | −0.11 | 37,588 (83.4) | 37,520 (83.3) | 0.00 |
| Black | 5,281 (7.2) | 4,997 (7.8) | −0.02 | 3,396 (7.5) | 3,388 (7.5) | 0.00 |
| Asian | 3,434 (4.7) | 1,433 (2.2) | 0.14 | 1,138 (2.5) | 1,177 (2.6) | −0.01 |
| Hispanic | 2,601 (3.6) | 1,719 (2.7) | 0.05 | 1,284 (2.9) | 1,300 (2.9) | 0.00 |
| Other | 3,333 (4.6) | 2,143 (3.3) | 0.06 | 1,641 (3.6) | 1,662 (3.7) | −0.01 |
| Burden of comorbidities, mean (SD) | ||||||
| Combined Comorbidity Score† | 2.94 (2.03) | 3.45 (2.32) | −0.23 | 3.16 (2.15) | 3.17 (2.15) | 0.00 |
| Frailty score (29) | 0.18 (0.06) | 0.19 (0.06) | −0.17 | 0.19 (0.06) | 0.19 (0.06) | 0.00 |
| Diabetes-related conditions, n (%) | ||||||
| Diabetic nephropathy | 6,614 (9.1) | 9,850 (15.3) | −0.19 | 5,132 (11.4) | 5,122 (11.4) | 0.00 |
| Diabetic retinopathy | 7,664 (10.5) | 7,888 (12.2) | −0.05 | 4,966 (11.0) | 4,958 (11.0) | 0.00 |
| Diabetic neuropathy | 17,187 (23.6) | 18,251 (28.3) | −0.11 | 11,721 (26.0) | 11,709 (26.0) | 0.00 |
| Diabetes with peripheral circulatory disorders | 5,841 (8.0) | 5,689 (8.8) | −0.03 | 3,708 (8.2) | 3,783 (8.4) | −0.01 |
| Diabetic foot | 1,897 (2.6) | 2,329 (3.6) | −0.06 | 1,378 (3.1) | 1,393 (3.1) | 0.00 |
| Lower-extremity amputation | 264 (0.4) | 355 (0.6) | −0.03 | 210 (0.5) | 204 (0.5) | 0.00 |
| Hypoglycemia | 3,529 (4.8) | 3,725 (5.8) | −0.04 | 2,303 (5.1) | 2,330 (5.2) | 0.00 |
| DKA | 244 (0.3) | 298 (0.5) | −0.03 | 157 (0.3) | 182 (0.4) | −0.02 |
| Diabetes treatment | ||||||
| No. of antidiabetes drugs at cohort entry, mean (SD) | 2.65 (1.00) | 2.49 (0.98) | 0.16 | 2.54 (0.98) | 2.53 (0.99) | 0.01 |
| No previous use of diabetes treatment, n (%)¶ | 2,609 (3.6) | 2,954 (4.6) | −0.05 | 1,957 (4.3) | 1,939 (4.3) | 0.00 |
| Monotherapy, n (%) | 2,332 (3.2) | 2,663 (4.1) | −0.05 | 1,768 (3.9) | 1,752 (3.9) | 0.00 |
| Long-term use of insulin, n (%)§ | 5,969 (8.2) | 9,620 (14.9) | −0.21 | 5,201 (11.5) | 5,257 (11.7) | −0.01 |
| Diabetes drug on the day of entry to the cohort, n (%) | ||||||
| Metformin | 46,049 (63.2) | 34,419 (53.4) | 0.20 | 26,190 (58.1) | 26,086 (57.9) | 0.00 |
| Sulfonylureas | 29,266 (40.1) | 23,039 (35.8) | 0.09 | 16,839 (37.4) | 16,725 (37.1) | 0.01 |
| DPP-4 inhibitors | 24,783 (34.0) | 13,425 (20.8) | 0.30 | 10,923 (24.2) | 11,004 (24.4) | 0.00 |
| Glitazones | 5,846 (8.0) | 4,853 (7.5) | 0.02 | 3,491 (7.7) | 3,518 (7.8) | 0.00 |
| Insulin | 12,742 (17.5) | 18,853 (29.3) | −0.28 | 10,847 (24.1) | 10,741 (23.8) | 0.01 |
| Lifestyle factors, n (%) | ||||||
| Obesity | 17,621 (24.2) | 21,980 (34.1) | −0.22 | 13,436 (29.8) | 13,414 (29.8) | 0.00 |
| Overweight | 3,812 (5.2) | 2,822 (4.4) | 0.04 | 2,111 (4.7) | 2,124 (4.7) | 0.00 |
| Smoking | 11,579 (15.9) | 11,207 (17.4) | −0.04 | 7,721 (17.1) | 7,692 (17.1) | 0.00 |
| Other comorbidities at baseline, n (%) | 0.00 | |||||
| CVD‖ | 31,888 (43.7) | 30,563 (47.4) | −0.07 | 20,366 (45.2) | 20,385 (45.3) | 0.00 |
| Ischemic heart disease | 23,018 (31.6) | 22,084 (34.3) | −0.06 | 14,763 (32.8) | 14,729 (32.7) | 0.00 |
| Acute myocardial infarction | 1,126 (1.5) | 1,098 (1.7) | −0.02 | 712 (1.6) | 740 (1.6) | 0.00 |
| Coronary revascularization | 587 (0.8) | 650 (1.0) | −0.02 | 395 (0.9) | 403 (0.9) | 0.00 |
| Heart failure | 7,082 (9.7) | 8,801 (13.7) | −0.12 | 5,144 (11.4) | 5,170 (11.5) | 0.00 |
| Atrial fibrillation | 7,353 (10.1) | 7,398 (11.5) | −0.05 | 4,861 (10.8) | 4,911 (10.9) | 0.00 |
| Ischemic or hemorrhagic stroke | 8,145 (11.2) | 7,549 (11.7) | −0.02 | 5,138 (11.4) | 5,114 (11.4) | 0.00 |
| Transient ischemic attack | 1,787 (2.5) | 1,666 (2.6) | −0.01 | 1,138 (2.5) | 1,163 (2.6) | −0.01 |
| Peripheral arterial disease or surgery | 9,041 (12.4) | 8,811 (13.7) | −0.04 | 5,736 (12.7) | 5,808 (12.9) | −0.01 |
| Hypertension | 66,311 (91.0) | 59,585 (92.5) | −0.05 | 41,326 (91.7) | 41,323 (91.7) | 0.00 |
| Hyperlipidemia | 65,426 (89.7) | 58,092 (90.2) | −0.02 | 40,488 (89.9) | 40,413 (89.7) | 0.01 |
| Chronic obstructive pulmonary disease | 8,524 (11.7) | 9,187 (14.3) | −0.08 | 5,848 (13.0) | 5,883 (13.1) | 0.00 |
| Pneumonia | 2,614 (3.6) | 2,902 (4.5) | −0.05 | 1,823 (4.0) | 1,824 (4.0) | 0.00 |
| Obstructive sleep apnea | 8,084 (11.1) | 10,876 (16.9) | −0.17 | 6,416 (14.2) | 6,453 (14.3) | 0.00 |
| Osteoarthritis | 21,613 (29.6) | 21,292 (33.1) | −0.08 | 14,256 (31.6) | 14,227 (31.6) | 0.00 |
| Chronic kidney disease | 9,037 (12.4) | 14,729 (22.9) | −0.28 | 7,294 (16.2) | 7,437 (16.5) | −0.01 |
| Chronic kidney disease, stage 3+ | 5,303 (7.3) | 10,773 (16.7) | −0.29 | 4,753 (10.6) | 4,784 (10.6) | 0.00 |
| AKI | 1,994 (2.7) | 3,281 (5.1) | −0.12 | 1,603 (3.6) | 1,636 (3.6) | 0.00 |
| Edema‡ | 8,391 (11.5) | 10,311 (16.0) | −0.13 | 6,160 (13.7) | 6,140 (13.6) | 0.00 |
| Fractures | 3,275 (4.5) | 3,369 (5.2) | −0.03 | 2,254 (5.0) | 2,233 (5.0) | 0.00 |
| Falls | 3,067 (4.2) | 3,246 (5.0) | −0.04 | 2,135 (4.7) | 2,110 (4.7) | 0.00 |
| Osteoporosis | 7,303 (10.0) | 6,151 (9.5) | 0.02 | 4,240 (9.4) | 4,248 (9.4) | 0.00 |
| Genital infections | 2,223 (3.0) | 2,128 (3.3) | −0.02 | 1,452 (3.2) | 1,477 (3.3) | −0.01 |
| UTIs | 11,375 (15.6) | 12,191 (18.9) | −0.09 | 7,780 (17.3) | 7,723 (17.1) | 0.01 |
| Dementia | 3,341 (4.6) | 2,987 (4.6) | 0.00 | 2,088 (4.6) | 2,025 (4.5) | 0.00 |
| Other medication use, n (%) | ||||||
| ACE inhibitors | 33,901 (46.5) | 30,726 (47.7) | −0.02 | 21,199 (47.1) | 21,233 (47.1) | 0.00 |
| Angiotensin II receptor blockers | 27,012 (37.1) | 24,298 (37.7) | −0.01 | 16,850 (37.4) | 16,789 (37.3) | 0.00 |
| β-Blockers | 34,537 (47.4) | 32,856 (51.0) | −0.07 | 22,216 (49.3) | 22,153 (49.2) | 0.00 |
| Calcium channel blockers | 24,372 (33.4) | 22,530 (35.0) | −0.03 | 15,368 (34.1) | 15,335 (34.0) | 0.00 |
| Thiazides | 10,890 (14.9) | 11,390 (17.7) | −0.08 | 7,439 (16.5) | 7,463 (16.6) | 0.00 |
| Loop diuretics | 12,008 (16.5) | 16,050 (24.9) | −0.21 | 9,146 (20.3) | 9,241 (20.5) | 0.00 |
| Other diuretics | 2,532 (3.5) | 3,496 (5.4) | −0.09 | 1,970 (4.4) | 2,024 (4.5) | 0.00 |
| Nitrates | 6,163 (8.5) | 6,430 (10.0) | −0.05 | 4,053 (9.0) | 4,145 (9.2) | −0.01 |
| Digoxin | 2,027 (2.8) | 1,768 (2.7) | 0.01 | 1,214 (2.7) | 1,206 (2.7) | 0.00 |
| Statins | 56,268 (77.2) | 50,333 (78.1) | −0.02 | 34,874 (77.4) | 34,877 (77.4) | 0.00 |
| Antiplatelets | 12,197 (16.7) | 11,104 (17.2) | −0.01 | 7,476 (16.6) | 7,542 (16.7) | 0.00 |
| Anticoagulants | 6,063 (8.3) | 6,399 (9.9) | −0.06 | 4,137 (9.2) | 4,159 (9.2) | 0.00 |
| Oral corticosteroids | 19,502 (26.8) | 18,269 (28.4) | −0.04 | 12,463 (27.7) | 12,434 (27.6) | 0.00 |
| Bisphosphonates | 2,971 (4.1) | 2,267 (3.5) | 0.03 | 1,589 (3.5) | 1,586 (3.5) | 0.00 |
| Antibiotics targeting UTIs | 20,633 (28.3) | 20,628 (32.0) | −0.08 | 13,699 (30.4) | 13,711 (30.4) | 0.00 |
| Opioids | 23,530 (32.3) | 25,056 (38.9) | −0.14 | 16,301 (36.2) | 16,305 (36.2) | 0.00 |
| Benzodiazepines | 10,203 (14.0) | 9,803 (15.2) | −0.03 | 6,746 (15.0) | 6,747 (15.0) | 0.00 |
| Measures of health care use | ||||||
| Hospitalization within prior 30 days, n (%) | 717 (1.0) | 812 (1.3) | −0.03 | 501 (1.1) | 491 (1.1) | 0.00 |
| Hospitalization during prior 31–365 days, n (%) | 6,842 (9.4) | 7,762 (12.0) | −0.08 | 4,771 (10.6) | 4,768 (10.6) | 0.00 |
| No. of hospital days, mean (SD) | 0.67 (3.05) | 0.91 (3.60) | −0.07 | 0.77 (3.37) | 0.78 (3.43) | 0.00 |
| No. of emergency department visits, mean (SD) | 0.71 (1.78) | 0.86 (1.94) | −0.08 | 0.79 (1.85) | 0.79 (1.86) | 0.00 |
| No. of office visits, mean (SD) | 10.72 (7.60) | 12.16 (8.36) | −0.18 | 11.36 (7.90) | 11.36 (7.75) | 0.00 |
| Endocrinologist visit within prior 30 days, n (%) | 8,789 (12.1) | 11,963 (18.6) | −0.18 | 6,840 (15.2) | 6,829 (15.2) | 0.00 |
| Endocrinologist visit during prior 31–365 days, n (%) | 10,873 (14.9) | 12,883 (20.0) | −0.13 | 7,891 (17.5) | 7,875 (17.5) | 0.00 |
| Cardiologist visit within prior 30 days, n (%) | 7,051 (9.7) | 6,869 (10.7) | −0.03 | 4,486 (10.0) | 4,563 (10.1) | 0.00 |
| Cardiologist visit during prior 31–365 days, n (%) | 27,931 (38.3) | 27,491 (42.7) | −0.09 | 18,155 (40.3) | 18,210 (40.4) | 0.00 |
| No. of electrocardiograms, mean (SD) | 1.13 (1.91) | 1.27 (2.11) | −0.07 | 1.19 (1.98) | 1.19 (2.03) | 0.00 |
| Echocardiogram, n (%) | 15,297 (21.0) | 15,410 (23.9) | −0.07 | 9,995 (22.2) | 10,044 (22.3) | 0.00 |
| Number of distinct prescriptions, mean (SD) | 13.35 (6.03) | 14.73 (6.25) | −0.22 | 14.04 (6.24) | 14.04 (5.94) | 0.00 |
| No. of HbA1c tests ordered, mean (SD) | 2.81 (1.36) | 2.89 (1.42) | −0.06 | 2.85 (1.37) | 2.84 (1.37) | 0.01 |
DPP-4, dipeptidyl peptidase 4; HbA1c, hemoglobin A1c; St. Diff, standardized differences, i.e., the difference in means or proportions divided by the pooled SD (30).
Canagliflozin, 76.9%; dapagliflozin, 13.1%; empagliflozin, 11.1%; liraglutide, 58.7%; exenatide, 23.5%; dulaglutide, 14.8%; albiglutide, 3.0%.
Gagne et al. (32).
Defined as patients without any use of glucose-lowering medications during the 12 months prior to cohort entry.
Based on ICD coding.
Defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, or lower-extremity amputation.
Localized, generalized, or unspecified edema.
Before PS matching, compared with patients initiating a GLP-1RA, patients initiating a SGLT2i were slightly older, more frequently were male, with a lower burden of comorbidities, as measured by the Combined Comorbidity Score (32), and were slightly less frail, as measured with a claims-based frailty index (29). SGLT2i initiators were less likely to use insulin at baseline, or to have seen an endocrinologist, and more likely to be on baseline treatment with metformin and dipeptidyl peptidase 4 inhibitors. All differences in patient characteristics were well balanced (as assessed by standardized differences, <0.1) after PS matching, with 70% of GLP-1RA initiators successfully matched to an SGLT2i initiator. The average age was 72 years, 46% of study participants were male, and 83% were White; 33% had history of ischemic heart disease, 11% had history of stroke, 11% had history of heart failure, and 58% were treated with metformin and 24% with insulin during the previous year (Table 1 and Supplementary Table 3). Canagliflozin and liraglutide were the most frequently initiated agents within the SGLT2i and GLP-1RA classes (77% and 59%, respectively) (Supplementary Table 4).
After PS matching, the mean and median follow-up time on treatment were 8.5 and 5.5 months, respectively. More than 20,000 patients had follow-up time >1 year, and >5,000 patients had follow-up time >2 years. Most patients were censored due to treatment discontinuation (54%) or end of the study period, i.e., 31 December 2016 (34%) (Supplementary Table 5).
Absolute and Relative Hazards of Primary and Secondary Outcomes
After PS matching, the incidence rates per 1,000 person-years in SGLT2i versus GLP-1RA initiators, respectively, were 18.0 vs. 18.4 for MACE and 7.0 vs. 10.3 for HHF (Table 2). Compared with GLP-1RA, the initiation of SGLT2i was associated with a similar risk of MACE (HR 0.98 [95% CI 0.87, 1.10]) and with a 32% decreased risk of HHF (HR 0.68 [95% CI 0.57, 0.80]), corresponding to 3.2 fewer cases per 1,000 person-years (RD −3.23 [95% CI −4.68, −1.77]). Kaplan-Meier curves comparing the cumulative incidence of MACE and HHF among initiators of SGLT2i versus GLP-1RA were consistent with these findings, with the curves for HHF separating early and within the first 6 months of treatment initiation (Fig. 1A and B). The proportional hazards assumption, which was assessed by testing of the significance of the interaction term between exposure and time, was not violated. No differences in the risk of the individual components of the MACE outcome were observed between the two exposure groups (HR 0.98 [95% CI 0.84, 1.16] for myocardial infarction, HR 1.04 [95% CI 0.86, 1.27] for stroke, and HR 0.83 [95% CI 0.64, 1.07] for cardiovascular mortality), as also was the case for the risk of all-cause mortality (HR 0.95 [95% CI 0.81, 1.11]) and the composite outcome of myocardial infarction, stroke, HHF, or all-cause mortality (HR 0.92 [95% CI 0.84, 1.002]).
Table 2.
Number of events, person-time, and incidence rates for cardiovascular effectiveness outcomes in 1:1 PS-matched initiators of SGLT2i versus GLP-1RA
| SGLT2i (N = 45,047) | GLP-1RA (N = 45,047) | SGLT2i vs. GLP-1RA | ||
|---|---|---|---|---|
| HR (95% CI) | RD/1,000 PY (95% CI) | |||
| Primary outcomes | ||||
| MACE∗ | 597 (17.99) | 553 (18.37) | 0.98 (0.87, 1.10) | −0.38 (−2.48, 1.72) |
| HHF | 234 (7.02) | 309 (10.25) | 0.68 (0.57, 0.80) | −3.23 (−4.68, −1.77) |
| Secondary effectiveness outcomes | ||||
| MI | 301 (9.05) | 277 (9.18) | 0.98 (0.84, 1.16) | −0.14 (−1.63, 1.35) |
| Ischemic or hemorrhagic stroke | 214 (6.43) | 187 (6.19) | 1.04 (0.86, 1.27) | 0.24 (−1.00, 1.47) |
| Cardiovascular mortality | 115 (3.44) | 124 (4.10) | 0.83 (0.64, 1.07) | −0.65 (−1.61, 0.30) |
| All-cause mortality | 310 (9.28) | 293 (9.68) | 0.95 (0.81, 1.11) | −0.40 (−1.91, 1.12) |
| MACE or HHF† | 803 (24.24) | 820 (27.33) | 0.89 (0.80, 0.98) | −3.09 (−5.60, −0.58) |
| MI, stroke, HHF, or all-cause mortality | 985 (29.74) | 970 (32.33) | 0.92 (0.84, 1.002) | −2.59 (−5.35, 0.16) |
Data are N events (incidence rate/1,000 person-years) unless otherwise indicated. MI, myocardial infarction; PY, person-years.
Hospitalization for myocardial infarction, ischemic or hemorrhagic stroke, or cardiovascular mortality.
Hospitalization for myocardial infarction, ischemic or hemorrhagic stroke, cardiovascular mortality, or HHF.
Figure 1.
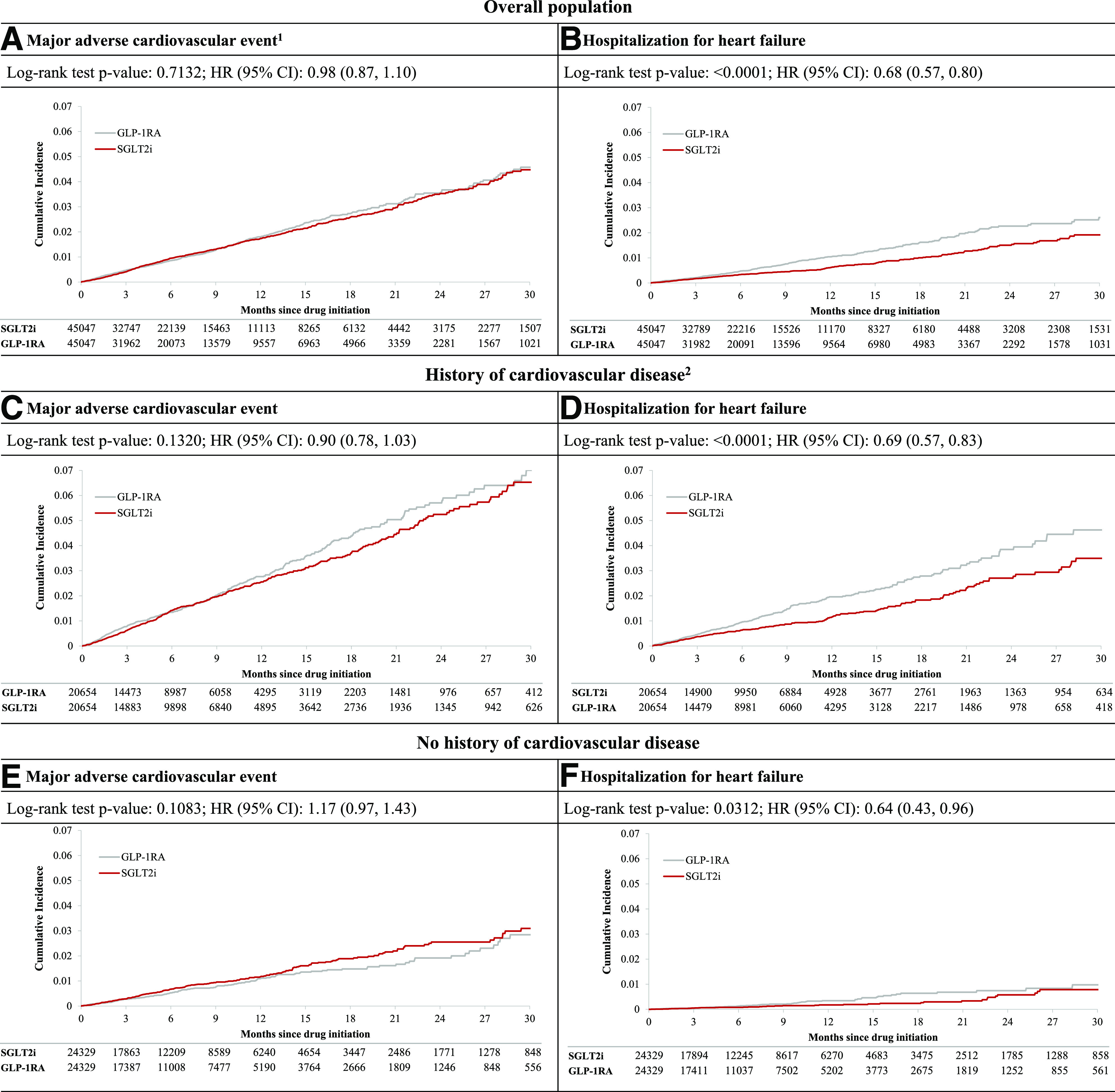
Cumulative incidence for composite cardiovascular outcome and HHF comparing PS-matched SGLT2i vs. GLP-1RA initiators overall and by history of CVD. 1Hospitalization for myocardial infarction, ischemic or hemorrhagic stroke, or cardiovascular mortality. 2History of CVD is defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, or lower-extremity amputation. Data are n unless otherwise indicated.
For the safety outcomes, SGLT2i initiators had a 46% increase in the risk of DKA (HR 1.46 [95% CI 1.02, 2.07]), a 44% increased risk of LLA (HR 1.44 [95% CI 1.06, 1.96]), and a 3.4-fold increase in the risk of genital infections (HR 3.34 [95% CI 3.08, 3.62]), corresponding to 0.7, 0.9, and 57.1 more events in 1,000 person-years, respectively, compared with GLP-1RA initiators (Table 3). A post hoc analysis, which evaluated the risk of genital infections among males and females separately, found that SGLT2i was associated with 27.1 more events in males and 90.3 more events in females compared with GLP-1RA (Supplementary Table 6). The risk of bone fractures and severe UTI did not differ among patients initiating SGLT2i versus GLP-1RA. There was a 15% decreased risk of AKI associated with SGLT2i (HR 0.85 [95% CI 0.79, 0.92]) compared with GLP-1RA, corresponding to 7.1 fewer events in 1,000 person-years.
Table 3.
Number of events, person-time, and incidence rates for safety outcomes in 1:1 PS-matched initiators of SGLT2i versus GLP-1RA
| Safety outcomes | SGLT2i (N = 45,047) | GLP-1RA (N = 45,047) | SGLT2i vs. GLP-1RA | |
|---|---|---|---|---|
| HR (95% CI) | RD/1,000 PY (95% CI) | |||
| DKA | 79 (2.37) | 50 (1.65) | 1.46 (1.02, 2.07) | 0.72 (0.02, 1.41) |
| Bone fracture∗ | 181 (5.43) | 175 (5.80) | 0.94 (0.76, 1.16) | −0.36 (−1.53, 0.81) |
| LLA | 104 (3.12) | 67 (2.22) | 1.44 (1.06, 1.96) | 0.90 (0.10, 1.70) |
| GIs | 2,623 (82.31) | 753 (25.23) | 3.34 (3.08, 3.62) | 57.08 (53.45, 60.70) |
| AKI | 1,268 (38.49) | 1,352 (45.54) | 0.85 (0.79, 0.92) | −7.05 (−10.27, −3.83) |
| Severe UTI† | 147 (4.41) | 161 (5.33) | 0.83 (0.67, 1.04) | −0.92 (−2.01, 0.17) |
Data are N events (incidence rate/1,000 person-years) unless otherwise indicated. GIs, genital infections; PY, person-years.
Humerus, wrist, hip, and pelvis fracture.
UTI requiring hospitalization.
Sensitivity and Subgroup Analyses
Primary and secondary findings remained consistent when we carried forward the exposure to the first-used medication for 365 days without considering drug discontinuation or switching (Supplementary Table 7).
Subgroup analyses by history of CVD showed results consistent with the primary MACE findings, though there was evidence of effect heterogeneity on both the relative and the absolute scale (Fig. 2). The initiation of SGLT2i versus GLP-1RA was associated with a similar relative risk reduction in HHF across the two subgroups, whereas a larger absolute risk reduction (corresponding to 5.9 fewer events in 1,000 person-years) was observed among patients with CVD (Fig. 1C–F and Fig. 2). In assessment of selected secondary outcomes, SGLT2i versus GLP-1RA initiation appeared to have greater benefits with respect to the risk of cardiovascular death (HR 0.67 [95% CI 0.50, 0.90]; RD −2.59 [95% CI −4.50, −0.68]) and all-cause mortality (HR 0.82 [95% CI 0.68, 0.99]; RD −2.79 [95% CI −5.61, 0.03]) among patients with CVD, whereas no difference in risk of these outcomes was noted between the two agents among patients without CVD. Results from subgroup analyses by age, history of chronic kidney disease, and presence of metformin at cohort entry were consistent with primary findings and did not show effect heterogeneity on either the relative or the absolute scale (Supplementary Fig. 3).
Figure 2.
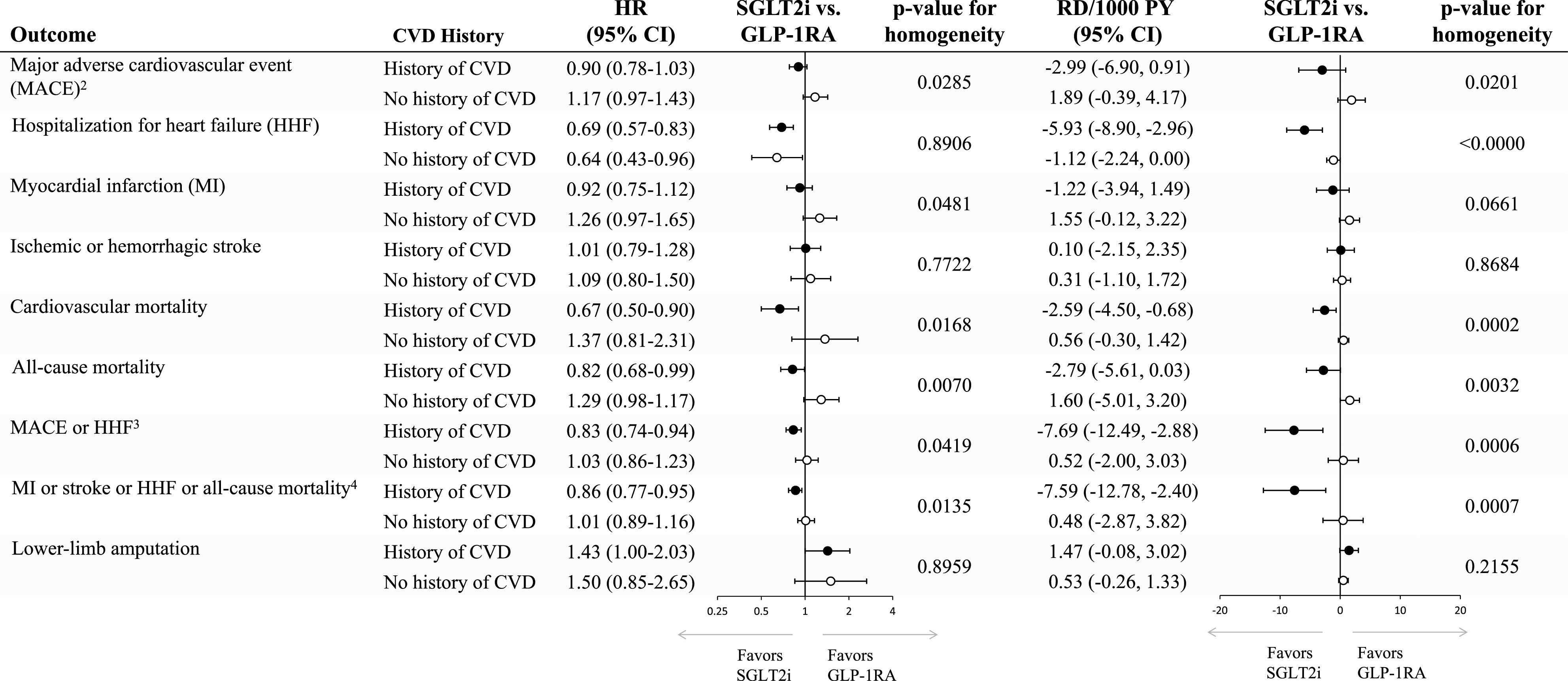
Cardiovascular effectiveness and safety outcomes in subgroups of 1:1 PS-matched initiators of SGLT2i vs. GLP-1RA by history of CVD. History of CVD is defined as history of myocardial infarction, unstable angina, coronary atherosclerosis and other forms of chronic ischemic heart disease, coronary procedure, heart failure, ischemic or hemorrhagic stroke, transient ischemic attack, peripheral arterial disease or surgery, or lower-extremity amputation. Patients with CVD, 41,308; patients without CVD, 48,658. 2Hospitalization for myocardial infarction, ischemic or hemorrhagic stroke, or cardiovascular mortality. 3Hospitalization for myocardial infarction, ischemic or hemorrhagic stroke, cardiovascular mortality, or HHF. 4Hospitalization for myocardial infarction, ischemic or hemorrhagic stroke, all-cause mortality, or HHF. PY, person-years.
Conclusions
In a large population-based cohort study of ∼90,000 older adults with T2D in the U.S. with mean age of 72 years, we found similar risk of MACE, and a 32% decreased risk of HHF, among SGLT2i initiators compared with GLP-1RA initiators. SGLT2i initiation was associated with a 46% increased risk of DKA, a 44% increased risk of amputations, and a 3.3-fold increased risk of genital infections, corresponding to 0.7, 0.9, and 57.1 more cases per 1,000 person-years. Conversely, SGLT2i initiators had a 15% decreased risk of AKI (7.1 fewer events per 1,000 person-years) and no difference in the risks of fractures or severe UTI.
We observed some evidence of heterogeneity in the comparative effectiveness of SGLT2i versus GLP-1RA depending on the presence of baseline CVD. Among patients with history of CVD, the initiation of SGLT2i was associated with reductions in the risk of HHF (5.9 fewer events per 1,000 person-years), cardiovascular death (2.6 fewer events per 1,000 person-years), and all-cause mortality (2.8 fewer events per 1,000 person-years) compared with GLP-1RA. Among those without history of CVD, SGLT2i versus GLP-1RA initiation produced reductions in HHF, though with a benefit of substantially lower magnitude (1.1 fewer events per 1,000 person-years) and no benefit in the risk of the other effectiveness outcomes.
This study complements available information from published CVOTs (4–11), including two recent meta-analyses of trials evaluating SGLT2i and GLP-1RA, showing a more marked benefit of SGLT2i in prevention of HHF (33,34). Our study focuses on routinely treated patients on average 10 years older than the patients included in these trials, providing information on a subset of the population with diabetes underrepresented in CVOTs. It includes patients across a broader spectrum of CVDs at baseline, allowing for the exploration of drug effectiveness among patients with and without established CVD. Our study identifies a population of patients 5 to 10 times larger than the populations included in trials, allowing for the investigation of adverse events associated with the use of these classes of medications with greater precision and in an older population particularly vulnerable to them. Finally, it provides comprehensive information on the comparative effectiveness and safety of the two drug classes currently recommended among T2D patients with atherosclerotic CVD, heart failure, or chronic kidney disease through a direct contrast of these agents as used in routine care and with use of data collected prior to the large dissemination of the evidence demonstrating their cardiovascular or renal benefits, thus limiting chances of channeling bias.
While a direct comparison of the SGLT2i and GLP-1RA class with regard to cardiovascular effectiveness outcomes and mortality has not been addressed in a population-based investigation, information on their comparative safety has been reported for selected outcomes in previous 1:1 PS-matched analyses involving adult patients (28,35,36). In spite of the difference in mean age between our population and these cohorts (72 vs. 54–61 years), our results for DKA, fractures, LLA, and severe UTI are consistent with the findings from these studies. Two studies recently reported on the association between SGLT2i and LLA (37,38); while the former study confirmed an increased risk of LLA among older canagliflozin initiators with baseline CVD, the latter did not observe an association between SGLT2i and LLA. The lack of an association in the latter investigation may be due to the different representation of canagliflozin in the population of SGLT2i users, the younger age, or the difference in study design, i.e., prevalent new-user design. Nevertheless, our study, which included >45,000 older patients exposed to SGLT2i, allowed us to quantify the risk of fractures and severe UTI in a vulnerable older population while also reporting precise estimates of the increased risk of DKA and LLA, and a reduction in the risk of AKI, compared with GLP-1RA. Our study also quantified the risk of genital infections among older patients routinely treated with SGLT2i vs. GLP-1RA; while this is an adverse event of lesser severity compared with other safety outcomes, e.g., DKA, its occurrence may deter patients from continuing SGLT2i therapy despite its potential benefits.
This study has limitations. First, while we balanced 140 baseline characteristics between treatment groups through PS matching, residual confounding by some unmeasured (e.g., hemoglobin A1c level, diabetes duration, BMI, estimated glomerular filtration rate, ejection fraction) or not fully measured (e.g., obesity) characteristic(s) in claims, cannot be entirely ruled out. However, a previous new-user active comparator cohort study with use of claims data linked to electronic health records showed sufficient balance in many of these characteristics after adjustment for claims-based proxies of diabetes severity and duration (39). Second, due to the limited use of SGLT2i or GLP-1RA agents besides canagliflozin and liraglutide, respectively, in the U.S. during the study period, we were unable to perform a real-world evaluation of the effectiveness and safety of more recently marketed SGLT2i or GLP-1RA agents. Third, as this investigation was based on the use of SGLT2i or GLP-1RA in routine care, the mean study follow-up (i.e., time on treatment) was shorter compared with CVOTs, which have substantial measures in place to improve medication adherence. Randomized controlled trials generally require long follow-up to accumulate sufficient events for powered analyses. The size of our study population (∼90,000 patients) allowed us to generate results with high precision, despite a shorter duration of follow-up. Assuming no time-varying hazards, as confirmed in our analyses, these results should be generalizable to longer-term findings. Nevertheless, long-term clinical trials powered to assess cardiovascular events would be needed to confirm the risk-benefit profile of SGLT2i versus GLP-1RA. Fourth, the current study did not specifically address the safety signals associated with the use of GLP-1RA, including acute pancreatitis and acute gallbladder or biliary disease (40), which may also be taken into consideration in the assessment of the benefit-risk balance of SGLT2i and GLP-1RA. Lastly, our study population did not include older commercially insured or Medicare Advantage patients, who are more likely to have differential socioeconomic status, medication adherence, and risk factors for outcomes. However, the biological effect of SGLT2i on health outcomes is unlikely to differ by insurance status; thus, our results may generalize to other older populations besides Medicare Fee-for-Service.
Conclusion
Overall, in a large population-based cohort study of ∼90,000 older adults with T2D, the initiation of SGLT2i versus GLP-1RA was associated with a similar occurrence of MACE, 3.2 fewer HHF events, 0.7 more DKA, 0.9 more LLA, 57.1 more genital infection, and 7.1 fewer AKI events over 1,000 person-years. SGLT2i appeared to be associated with greater cardiovascular benefit than GLP-1RA among older patients with history of CVD, with less benefit in those without history of CVD.
Article Information
Funding. This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. E.P. was supported by a career development grant from the National Institute on Aging (K08AG055670)
Duality of Interest. E.P. is co-investigator of an investigator-initiated grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not directly related to the topic of the submitted work. R.J.G. reports grants from Pfizer, Novartis, and Kowa outside the submitted work. M.N.M. reports consultant fees from Sanofi and Lilly. S.S. is co-investigator of investigator-initiated grants to the Brigham and Women’s Hospital from Boehringer Ingelheim unrelated to the topic of this study and a consultant to Aetion, a software manufacturer of which he owns equity; his interests were declared, reviewed, and approved by the Brigham and Women’s Hospital and Partners HealthCare System in accordance with their institutional compliance policies. D.J.W. reports serving on Data Monitoring Committees for Novo Nordisk not related to the topic of this work. S.C.K. reports grants from Pfizer, AbbVie, Bristol-Myers Squibb, and Roche, outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. E.P. and S.C.K. were involved in all parts of the study. A.P. and L.G.B. were involved in data analysis and revising the manuscript. D.H.K., S.S., R.J.G., M.N.M., D.J.W., and C.D. were involved in designing the study and revising the manuscript. E.P. and S.C.K. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13476855.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narayan KM, Boyle JP, Geiss LS, Saaddine JB, Thompson TJ. Impact of recent increase in incidence on future diabetes burden: U.S., 2005-2050. Diabetes Care 2006;29:2114–2116 [DOI] [PubMed] [Google Scholar]
- 3.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020;43:487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marso SP, Bain SC, Consoli A, et al.; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 6.Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 7.Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 8.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 10.Holman RR, Bethel MA, Mentz RJ, et al.; EXSCEL Study Group . Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 12.Wanner C, Inzucchi SE, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–334 [DOI] [PubMed] [Google Scholar]
- 13.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration . FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections, 2015. Accessed 15 June 2020. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about
- 15.U.S. Food and Drug Administration . FDA revises label of diabetes drug canagliflozin (Invokana, Invokamet) to include updates on bone fracture risk and new information on decreased bone mineral density, 2015. Accessed 15 June 2020. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-label-diabetes-drug-canagliflozin-invokana-invokamet
- 16.U.S. Food and Drug Administration . FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR), 2017. Accessed 15 June 2020. Available from https://www.fda.gov/media/104870/download
- 17.Kiyota Y, Schneeweiss S, Glynn RJ, Cannuscio CC, Avorn J, Solomon DH. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J 2004;148:99–104 [DOI] [PubMed] [Google Scholar]
- 18.Wahl PM, Rodgers K, Schneeweiss S, et al. Validation of claims-based diagnostic and procedure codes for cardiovascular and gastrointestinal serious adverse events in a commercially-insured population. Pharmacoepidemiol Drug Saf 2010;19:596–603 [DOI] [PubMed] [Google Scholar]
- 19.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke 2002;33:2465–2470 [DOI] [PubMed] [Google Scholar]
- 20.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf 2012;21(Suppl. 1):129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olubowale OT, Safford MM, Brown TM, et al. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the national death index: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc 2017;6:e004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobo WV, Cooper WO, Epstein RA Jr., Arbogast PG, Mounsey J, Ray WA. Positive predictive value of automated database records for diabetic ketoacidosis (DKA) in children and youth exposed to antipsychotic drugs or control medications: a Tennessee Medicaid Study. BMC Med Res Methodol 2011;11:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol 1999;52:199–207 [DOI] [PubMed] [Google Scholar]
- 24.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol 1992;45:703–714 [DOI] [PubMed] [Google Scholar]
- 25.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high--a systematic review. J Clin Epidemiol 2013;66:278–285 [DOI] [PubMed] [Google Scholar]
- 26.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol 2006;17:1688–1694 [DOI] [PubMed] [Google Scholar]
- 27.Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab 2019;21:434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dave CV, Schneeweiss S, Kim D, Fralick M, Tong A, Patorno E. Sodium-glucose cotransporter-2 inhibitors and the risk for severe urinary tract infections: a population-based cohort study. Ann Intern Med 2019;171:248–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring frailty in Medicare data: development and validation of a claims-based frailty index. J Gerontol A Biol Sci Med Sci 2018;73:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang SV, Verpillat P, Rassen JA, Patrick A, Garry EM, Bartels DB. Transparency and reproducibility of observational cohort studies using large healthcare databases. Clin Pharmacol Ther 2016;99:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol 2011;64:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 34.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39 [DOI] [PubMed] [Google Scholar]
- 35.Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ 2018;363:k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E. Fracture risk after initiation of use of canagliflozin: a cohort study. Ann Intern Med 2019;170:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fralick M, Kim SC, Schneeweiss S, Everett BM, Glynn RJ, Patorno E. Risk of amputation with canagliflozin across categories of age and cardiovascular risk in three US nationwide databases: cohort study. BMJ 2020;370:m2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu OHY, Dell’Aniello S, Shah BR, et al.; Canadian Network for Observational Drug Effect Studies (CNODES) Investigators . Sodium-glucose cotransporter 2 inhibitors and the risk of below-knee amputation: a multicenter observational study. Diabetes Care 2020;43:2444–2452 [DOI] [PubMed] [Google Scholar]
- 39.Patorno E, Gopalakrishnan C, Franklin JM, et al. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes Obes Metab 2018;20:974–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monami M, Nreu B, Scatena A, et al. Safety issues with glucagon-like peptide-1 receptor agonists (pancreatitis, pancreatic cancer and cholelithiasis): data from randomized controlled trials. Diabetes Obes Metab 2017;19:1233–1241 [DOI] [PubMed] [Google Scholar]


