Significance
Many studies show how a single sensory cue contributes to behaviors. To better understand external environments, however, animals use multisensory integration, referring to the enhancement of response induced by single sensory cue due to concurrent inputs from other sensory modalities. We show the proboscis extension reflex (PER), a proxy for food preference, is accomplished via multisensory integration in Drosophila. The taste-evoked PER is significantly enhanced by concurrent inputs of food odor and texture. Controlled delivery of three different sensory cues proves the simultaneous activation of three distinct sensory modalities produces a supraadditive PER. Our work shows flies employ an efficient way to find and ingest foods when the taste of the food alone is insufficient to stimulate feeding.
Keywords: multisensory integration, Drosophila, taste, olfaction, mechanosensation
Abstract
The integration of two or more distinct sensory cues can help animals make more informed decisions about potential food sources, but little is known about how feeding-related multimodal sensory integration happens at the cellular and molecular levels. Here, we show that multimodal sensory integration contributes to a stereotyped feeding behavior in the model organism Drosophila melanogaster. Simultaneous olfactory and mechanosensory inputs significantly influence a taste-evoked feeding behavior called the proboscis extension reflex (PER). Olfactory and mechanical information are mediated by antennal Or35a neurons and leg hair plate mechanosensory neurons, respectively. We show that the controlled delivery of three different sensory cues can produce a supra-additive PER via the concurrent stimulation of olfactory, taste, and mechanosensory inputs. We suggest that the fruit fly is a versatile model system to study multisensory integration related to feeding, which also likely exists in vertebrates.
Feeding is one of the most important animal behaviors for survival. Taste allows animals to select and ingest nutritious foods and prevents them from accidentally ingesting toxic substances (1). It is not just taste, however, that animals use when selecting foods. All of us, in our daily experiences with food, have noticed that our perceptions of a food do not solely depend on its taste. Instead, our perceptions are shaped by cross-modal combinations of taste, smell, texture, temperature, sights, and sounds (2). The most obvious example of cross-modal combination is the interaction between taste and olfaction. Typically tasteless odorants, such as those that smell of strawberry or vanilla, increase the perceived sweetness of sugar solutions (3). In contrast, the loss of retronasal olfactory inputs due to the common cold causes a reduced ability to distinguish various tastes (4, 5).
Despite the obvious importance of multisensory integration in food perception and feeding, many studies have focused instead on discrete sensory channels (1, 6). This is mainly due to technical difficulties in measuring the relative contributions of each sensory channel. Drosophila is an excellent model system for investigating the role multimodal interactions play in food perception and feeding (7–15). Powerful neurogenetic tools developed for use in Drosophila allow us to parse the contributions of each individual sensory channel in various aspects of fly feeding using simple behavioral assays combined with the selective silencing or activation of distinct sensory modalities.
Indeed, flies utilize multiple sensory modalities while searching for, evaluating, and ingesting various foods. Food-derived odorants are important cues in long- and short-range food searching (7, 8, 16) and also promote feeding initiation and increase food intake (10, 12). The mechanical properties of foods (e.g., hardness, viscosity, etc.) also affect food preference. Flies prefer a range of soft and viscous foods, detecting a food’s texture via taste sensilla-associated mechanosensory neurons (MNs) and multidendritic neurons in the fly labellum (9, 11, 13–15). It is noteworthy that a fly’s preference for a specific texture depends on the presence of appetitive taste cues, suggesting cross-modal interactions drive food perception and preference (11).
Here, we report that three distinct sensory channels—taste, olfaction, and mechanosensation—cooperate to enhance fly feeding initiation. In flies, the proboscis extension reflex (PER) is a proxy for food palatability and feeding (17). Activation of olfactory receptor neurons enhances the PER evoked by low concentrations of sugars, but either an increase in food viscosity or genetic perturbation of mechanosensation abolish this PER enhancement. We found that the silencing of hair plate MNs abolishes odor-induced enhancement of PER, while optogenetic activation of hair plate MNs in mechanosensory mutants recapitulates PER enhancement. Finally, we found that only concurrent inputs from the three different sensory modalities enhance PER, indicating that multisensory integration guides feeding behavior.
Results
Yeast Odor Enhances Feeding Initiation.
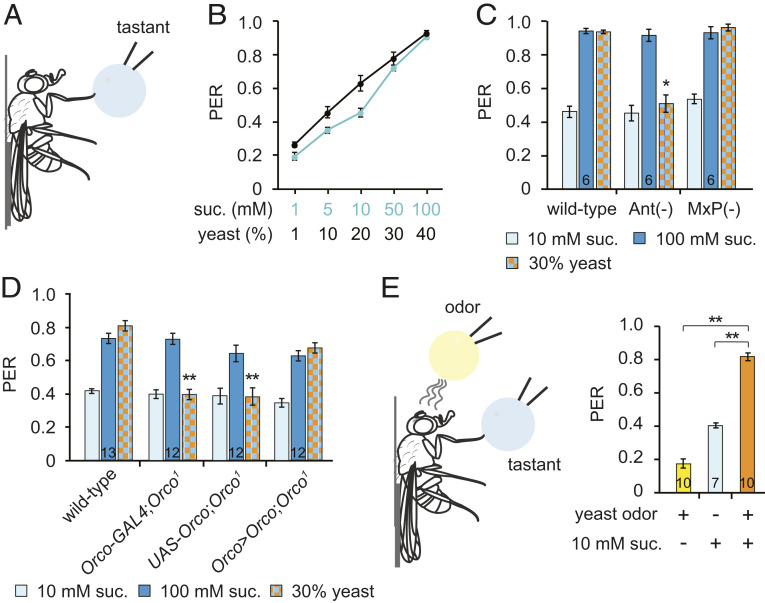
Flies eat yeast as a nutrient source for carbohydrates, protein, amino acids, and lipids (18–20) and show a stronger preference for yeast upon amino acid deprivation and also postmating (19, 21–24). Gustatory inputs are essential for yeast preference in amino acid-deprived flies (19, 21, 25). Although multiple volatile compounds emitted by yeast mediate long-range food attraction (8), their involvement in feeding is unclear. To address this question, we used the PER assay to measure food acceptance in individual animals (Fig. 1A). The detection of palatable foods by gustatory neurons in the legs or labellum elicits PER, whereas the detection of unpalatable foods inhibits PER. Stimulation of the leg gustatory receptor neurons (GRNs) of flies grown under typical laboratory conditions with yeast elicits PER in a dose-dependent manner similar to sucrose, indicating that flies consider yeast to be a palatable food source (Fig. 1 A and B). The presentation of yeast evokes a strong PER regardless of sex, mating status, age, or starvation conditions (SI Appendix, Fig. S1 A–C).
Fig. 1.
Yeast odors enhance sugar-induced PER. (A) Schematic of the PER assay. Flies were attached to a glass slide and a tastant droplet was applied to a foreleg. (B) PER evoked by sucrose or a yeast suspension. n = 8 to 17. (C) PER after ablation of the olfactory organs (third antennal segments or maxillary palps). n is indicated in the bar. (D) PER in Orco-null flies. n is indicated in the bar. (E) A modified PER assay. While a tastant droplet was applied to a foreleg, an odor source was brought near the flies without contact. n is indicated in the bar. All data are presented as means ± SEM. One-way ANOVAs with Tukey post hoc tests or Kruskal–Wallis with Mann–Whitney U tests were used for C–E. The asterisks indicate statistically significant differences from wild-type flies, unless otherwise indicated. *P < 0.01, **P < 0.001.
To determine whether yeast-evoked PER is mediated by the taste of amino acids, we performed PER with yeast extract, which contains free amino acids and peptides. We found that yeast extract is less efficient than intact yeast at evoking PER (SI Appendix, Fig. S1D), suggesting that factors other than the taste of amino acids contribute to yeast-evoked PER.
We next wanted to identify the factors that contribute to yeast-evoked PER, so we used only well-fed male flies for subsequent experiments to eliminate any effects of starvation or effects specific to mated females. First, we asked whether yeast odors contribute to PER. We surgically removed the Drosophila olfactory organs—either the third antennal segments or the maxillary palps. We found that antenna ablation, but not maxillary palp ablation, reduces yeast-evoked PER (Fig. 1C). Furthermore, an Orco mutant, which shows a severely limited range of odor detection (26), also shows reduced yeast-evoked PER. This is rescued, however, by the reintroduction of an Orco cDNA under the control of the Orco promoter (Fig. 1D). To clarify how yeast-derived volatile compounds contribute to yeast-evoked PER, we performed a modified PER assay that separates the olfactory and gustatory stimuli (Fig. 1E). Merely bringing the yeast stimulus close enough to the fly for them to smell the yeast-derived volatile metabolites without contacting the fly, thus preventing any taste neuron activation, evokes only basal levels of PER. Flies show moderate PER to a 10-mM sucrose stimulus, but the same concentration of sucrose combined with yeast odor provokes a stronger PER than either sucrose or yeast odor alone. These data indicate that yeast-derived volatiles enhance PER evoked by gustatory stimuli.
Activation of Or35a-Expressing Neurons Is Sufficient for Yeast-Enhanced PER.
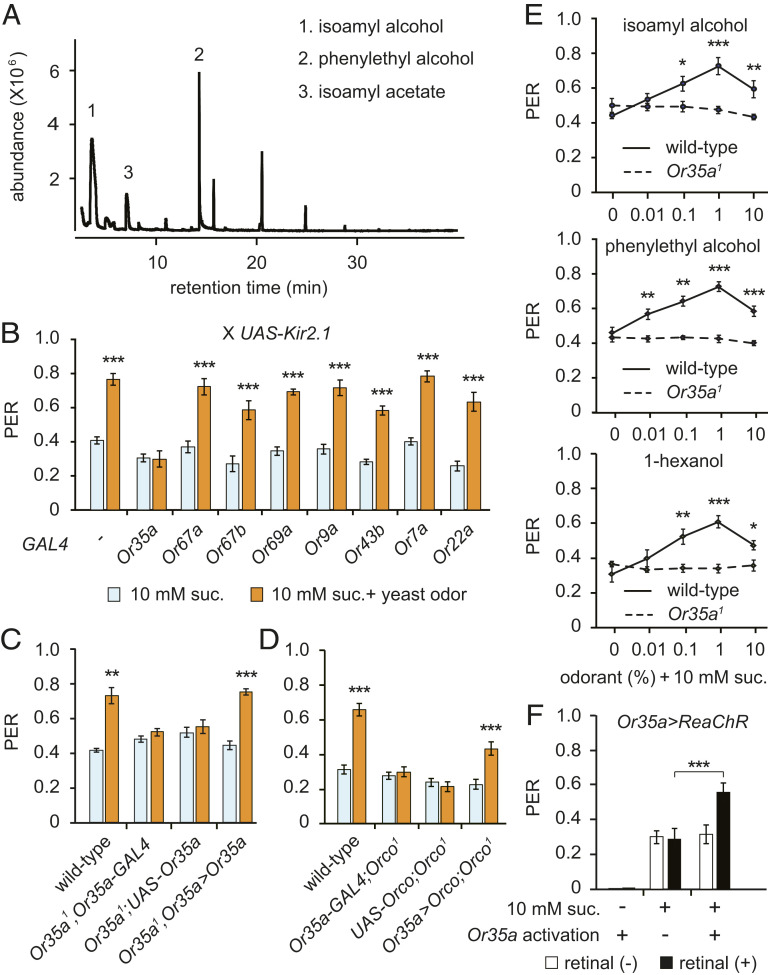
Next, we sought to identify the specific yeast-derived volatile metabolites that are capable of enhancing PER. In a gas chromatography-mass spectrometry (GC-MS) analysis, we found that isoamyl alcohol and phenylethyl alcohol are the most abundant volatile compounds produced by yeast cultures (Fig. 2A). To identify the olfactory receptor neurons (ORNs) responsible for yeast odor-enhanced PER, we screened Or-GAL4 candidate lines by crossing UAS-Kir2.1 to silence each type of ORN (27). We found that while the silencing of Or35a ORNs has no effect on PER to 10 mM sucrose, it completely prevents the enhancement of PER evoked by yeast odor. This clearly suggests the Or35a ORNs are required for yeast odor-enhanced PER (Fig. 2B).
Fig. 2.
Or35a ORNs mediating yeast odor-enhanced PER. (A) GC-MS analysis for yeast-derived volatile compounds. Volatile compounds are indicated in the order of abundance. (B) ORN screen for yeast odor-enhanced PER. The modified PER analysis was performed in flies expressing Kir2.1 in a subset of ORNs. n = 6 to 13. (C and D) Modified PER in Or35a or Orco mutant flies. The genotypes are indicated. n = 6 to 14. (E) Modified PER using volatile compounds found in yeast culture. n = 6. (F) PER upon optogenetic activation of Or35a ORNs. Or35a>ReaChR flies were tested in the absence or presence of all transretinal. n = 7 to 11. All data are presented as means ± SEM. Unpaired Student’s t tests or Mann–Whitney U tests were used for B–E. One-way ANOVAs with Tukey post hoc tests or Kruskal–Wallis with Mann–Whitney U tests were used for F. The asterisks indicate statistically significant differences from PER induced by sucrose alone, unless otherwise indicated. *P < 0.05, **P < 0.01, ***P < 0.001.
Or35a is expressed in ac3B neurons, which are located in the antennal coeloconic sensilla. These neurons are broadly tuned and express two more receptors in addition to Or35a: the olfactory coreceptor Orco and ionotropic receptor Ir76b (28). Since the combination of Or35a and Orco can detect isoamyl alcohol and phenylethyl alcohol (29, 30), we asked whether both ORs are required for yeast odor-enhanced PER. We generated flies lacking Or35a (Or35a1) using the CRISPR-Cas9 technique (SI Appendix, Fig. S2 A and B). We found the loss of either Orco or Or35a abolishes yeast odor-evoked PER enhancement, but neuron-specific introduction of the missing cDNA into the Or35a-expressing ORNs rescues this phenotype (Fig. 2 C and D). Furthermore, pure individual odors (e.g., isoamyl alcohol, phenylethyl alcohol, and the OR35a ligand 1-hexanol) produce an Or35a-dependent enhancement of sucrose-evoked PER (Fig. 2E and SI Appendix, Fig. S2C), but isoamyl acetate, another volatile compound found in yeast cultures, does not (SI Appendix, Fig. S2D). Furthermore, we found that optogenetic activation of Or35a ORNs expressing the red light-activatable channelrhodopsin (ReaChR) (31) also enhances sugar-evoked PER (Fig. 2F). These data indicate that Or35a ORN activation is necessary and sufficient for yeast odor-enhanced PER.
Leg Mechanosensation Is Required for Yeast Odor-Enhanced PER.
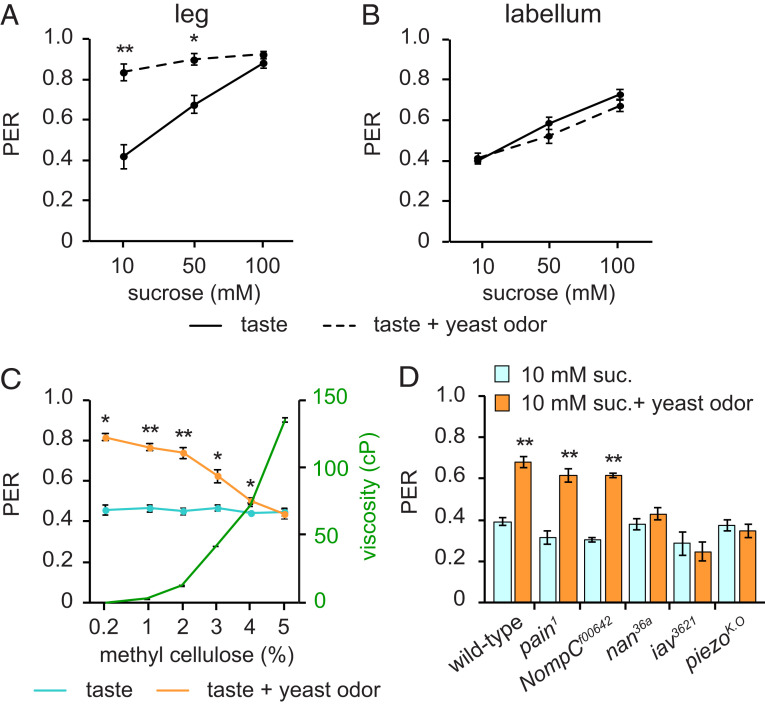
Although yeast odors clearly enhance PER, especially when combined with the stimulation of leg GRNs with low concentrations of sucrose (Fig. 3A), a similarly coupled activation of Or35a ORNs and labellar GRNs does not (Fig. 3B). To understand what makes this difference in yeast odor effects on PER, we focused on leg mechanosensation, because we and others have reported that labellar mechanosensory inputs can modulate feeding (9, 11, 14). We mixed methyl cellulose with 10 mM sucrose to produce foods with a range of viscosities (Fig. 3C). We found that stimulation of leg GRNs with 10-mM sucrose droplets of various viscosities evoked PERs of similar strength, indicating that food viscosity alone does not affect sucrose-evoked PER (Fig. 3C). Next, we performed a modified PER assay in which we stimulated fly legs with a droplet of 10 mM sucrose of varying viscosities paired with yeast odor. We found that the lower viscosity sugar solutions paired with yeast odor elicited the most robust PER, with increasing viscosities gradually reducing the yeast odor-evoked PER enhancement (Fig. 3C).
Fig. 3.
Mechanosensation is required for yeast odor-enhanced PER. (A and B) Modified PER induced by touching a foreleg (A) or the labellum (B) in conjunction with yeast odor. n = 4 to 8 and n = 8 for legs and labellum, respectively. (C) Modified PER induced by touching a foreleg with 10 mM sucrose in solutions of differing viscosity and yeast odor. Methyl cellulose was added to change viscosity. n = 6 to 9. The centipoise (cP) values for 10 mM sucrose and 30% yeast are 1.05 cP and 1.62 cP, respectively. (D) Mechanosensory screen. Modified PER was performed using 10 mM sucrose and yeast odor. n = 8 to 18. All data are presented as means ± SEM. Unpaired Student’s t tests or Mann–Whitney U tests. *P < 0.01, **P < 0.001.
To confirm the importance of mechanosensation in this phenomenon, we measured yeast odor-evoked PER enhancement in several mechanosensory mutants: Painless; pain1 (32), NompC; NompCf00642 (33), Nanchung; nan36a (34), Inactive; iav3621 (35), and Piezo; piezoK.O (36). Among these, nan36a, iav3621, and piezoK.O show no yeast odor-evoked PER enhancement (Fig. 3D), suggesting mechanosensory cues are required for yeast odor-enhanced PER. Furthermore, increasing the viscosity of the stimulation solution by adding 5% methyl cellulose does not affect the dose–response curve for sucrose-evoked PER either in wild-type or piezo mutant flies, suggesting mechanosensory cues alone do not alter sucrose-evoked PER (SI Appendix, Fig. S3 A and B).
Leg Hair Plates Are Required for Yeast-Enhanced PER.
To identify the leg mechanosensory organs required for yeast odor-enhanced PER, we screened candidate mechanosensory GAL4 lines by crossing them with UAS-Kir2.1 to silence various sets of MNs (37, 38) (SI Appendix, Fig. S4A). We found that silencing the MNs of the hair plates eliminated the yeast odor-evoked PER enhancement (R20C06-GAL4 and R48A07-GAL4) (SI Appendix, Figs. S4B and S5 A and B).
To rule out any developmental artifacts, we performed a modified PER assay using the temperature-sensitive GAL80ts to temporally restrict Kir2.1 expression (39). We found that control flies show significant yeast odor-evoked PER enhancement at both the permissive (20 °C) and restrictive temperatures (30 °C), but flies with the hair plate MNs silenced by R20C06-GAL4 and R48A07-GAL4 lose yeast odor-evoked PER enhancement at the restrictive temperature (30 °C) (SI Appendix, Fig. S5D). Yeast odor-evoked PER enhancement was also abolished by temporally restricting expression of Kir2.1 using R27E02-GAL4, which is expressed in the leg hair plates, suggesting that these are critical MNs for yeast odor-enhanced PER (SI Appendix, Fig. S5 C and D).
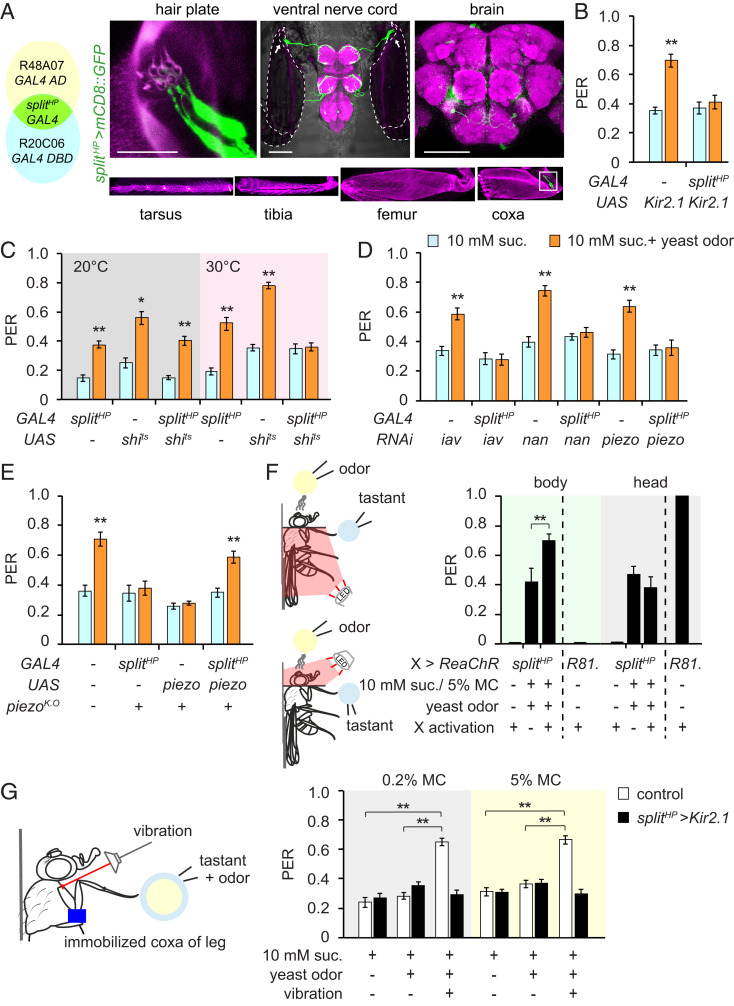
Hair plates, often situated at folds in the cuticle, are clusters of short mechanosensory hairs, each innervated by a single sensory neuron (40). In the Drosophila foreleg, there are three hair plates on the coxa of the thoraco-coxal joint, and three on the trochanter of the coxa-trochanteral joint (SI Appendix, Fig. S4A) (41). Since each of the hair plate-expressing GAL4 lines we used also show broad expression outside of the hair plate MNs, we employed Split-GAL4 (42) to restrict GAL4 expression. We found that the intersection of R20C06-GAL4.DBD and R48A07-P65.AD only labels eight MNs innervating one of the coxa hair plates (cxHP8; Fig. 4A and SI Appendix, Fig. S6 A and B). The axons of these MNs are found in the prothoracic and mesothoracic neuromeres as well as one pair of neurons in the brain (hereafter referred to as splitHP, Fig. 4A). Using this more restricted GAL4 expression pattern, we found that chronic silencing of the cxHP8 MNs using Kir2.1 (27), as well as their conditional inactivation using a temperature-sensitive dominant-negative form of dynamin (Shibirets) (43), produces a dramatic suppression of yeast odor-evoked PER enhancement (Fig. 4 B and C). Although it is unclear which mechanosensory channels are expressed in hair plate MNs, we found that at least iav, nan, and piezo are expressed in the cxHP8 MNs, as knockdown of iav, nan, or piezo in the cxHP8 MNs using splitHP-GAL4 abolishes yeast odor-evoked PER enhancement (Fig. 4D). In addition, we found that the introduction of a piezo cDNA using splitHP-GAL4 rescues the piezo mutant phenotype (Fig. 4E).
Fig. 4.
Hair plates are required for yeast odor-enhanced PER. (A) Expression pattern of splitHP-GAL4 (splitHP-GAL4 line intersection strategy on the Left). Confocal images of leg, VNC, and brain expressing mCD8::GFP driven by splitHP-GAL4. VNC and brain were stained with a rabbit anti-GFP (green) and nc82 (magenta). VNC image merged with bright field. The dashed lines and arrows indicate attached coxae and cxHP8, respectively. The hair plate image (Upper Left) is a higher magnification of the marked area on the coxa image (Bottom Right). Magenta in the leg images is cuticle autofluorescence. (Scale bars for brain, VNC, and leg, 100 μm; for hair plate, 25 μm.) (B) Modified PER upon chronic silencing of splitHP neurons. n = 6 to 9. (C) Modified PER upon acute silencing of splitHP neurons. n = 6 to 12. (D) Modified PER of flies with knockdown of mechanosensory genes in splitHP neurons. n = 7 to 10. (E) Modified PER with piezoK.O flies expressing piezo cDNA in splitHP neurons. n = 7 to 8. (F) Optogenetic activation of splitHP-GAL4-labeled neurons. Modified PER was performed using 5% methylcellulose containing 10 mM sucrose and yeast odor. Fly collars were used to prevent light leakage. A red light was applied via a glass fiber to either the body or the head. R81. (R81E10)>ReaChR flies were used as controls to confirm optogenetic activation of neurons in the brain. n = 7 to 11. (G) PER with vibration stimuli on the coxa hair plate. The coxa was immobilized using glue. PER was induced by 10 mM sucrose with isoamyl alcohol in either 0.2% or 5% methyl cellulose. Vibration was applied to activate coxa hair plate MNs. n = 8 to 13. All data are presented as means ± SEM. Unpaired Student’s t tests or Mann–Whitney U tests were used for B–E. One-way ANOVAs with Tukey post hoc tests or Kruskal–Wallis tests with Mann–Whitney U tests were used for F–G. The asterisks indicate statistically significant differences from PER evoked by sucrose alone, unless otherwise indicated. *P < 0.01, **P < 0.001.
To eliminate the potential involvement of the pair of brain neurons labeled by the splitHP-GAL4 line in yeast odor-enhanced PER, we examined the effects of artificial activation of splitHP-GAL4-labeled neurons in the brain and hair plate on yeast odor-enhanced PER. We placed splitHP>ReaChR flies in a fly collar so the light stimulus for optogenetic activation could be limited to either the body or the head (Fig. 4F). Although red light stimulation of the bodies of splitHP>ReaChR flies produces no effect alone, its combination with yeast odor and sucrose with 5% methyl cellulose produces a robust PER. In contrast, red light stimulation of the heads of splitHP>ReaChR flies neither evokes PER alone nor enhances the PER induced by yeast odor and sucrose with 5% methyl cellulose. As a control, we found that red light-induced activation of the feeding command neurons (FDG; R81E10>ReaChR flies) in the head evokes a robust PER, but the same red light stimulation of the bodies of the same flies does not evoke PER (Fig. 4F) (44, 45). This suggests red light stimulation is sufficient to activate brain neurons, that there is no leakage of light between the head and body compartments, and that the brain neurons labeled by the splitHP-GAL4 line are not involved in yeast odor-enhanced PER.
Next, we investigated the relationship between the intensity of cxHP8 MN activation and motivation to feed when the activation is combined with proper taste and olfactory inputs. We monitored PER in piezoK.O flies expressing ReaChR in cxHP8 MNs to control the degree of perceived mechanical activation using optogenetic stimulation independent of taste stimulation. We found that an increase in light intensity significantly increases PER when combined with sucrose and yeast odor (SI Appendix, Fig. S7A). We did not observe any increase in PER in controls that had not been fed retinal (SI Appendix, Fig. S7B). Thus, stronger activation of the cxHP8 MNs increases feeding motivation when combined with taste and odor, and such cxHP8 MNs are likely activated by touching low viscosity food.
Different Food Viscosity Elicits Distinct Leg Motions.
We found that optogenetic activation of the cxHP8 MNs recapitulates the effect of low viscosity foods on yeast odor-enhanced PER (Fig. 4F), suggesting that the cxHP8 MNs are activated when the distal end of the leg contacts low viscosity foods. cxHP8 is located on the proximal anterior rim of the leg and provides information regarding the position and movement of the thoraco-coxal joint of the leg (46). Therefore, we performed a video analysis to track leg movement during PER as the legs contacted foods of different viscosities. We observed that tarsal contact with low viscosity food induces a contraction of the leg back toward the body, followed by proboscis extension. Tarsal contact with high viscosity food, in contrast, is followed by a forward swing of the legs through the food without an accompanying PER (SI Appendix, Fig. S8 A and B and Movies S1 and S2). We found an identical pattern of leg movements induced by different food viscosities in piezoK.O flies, which show no PER regardless of food viscosity (SI Appendix, Fig. S8 C and D). We quantified this leg movement by measuring the maximum displacement and the degree of maximum rotation (SI Appendix, Fig. S8 E–G). This suggests that, rather than being direct sensors of food viscosity, the cxHP8 MNs are activated by the distinct leg movements themselves. We also performed the PER assay with flies whose coxa-trochanteral joints had been immobilized to prevent coxa movement. We found that tarsal stimulation of these flies with 10 mM sucrose and 1% isoamyl alcohol with either 0.2% methyl cellulose or 5% methyl cellulose evokes basal levels of PER similar to 10 mM sucrose. We also found that stimulation of the cxHP8 MNs via vibration (47) with the simultaneous presentation of taste and olfactory cues enhances PER regardless of food viscosity. We did not observe this vibration-induced enhancement, however, in flies whose hair plate MNs had been silenced by expression of Kir2.1 under the control of splitHP (Fig. 4G). These data suggest cxHP8 MNs are activated by coxal movement upon tarsal contact with low viscosity food.
Three Different Sensory Modalities Enhance PER.
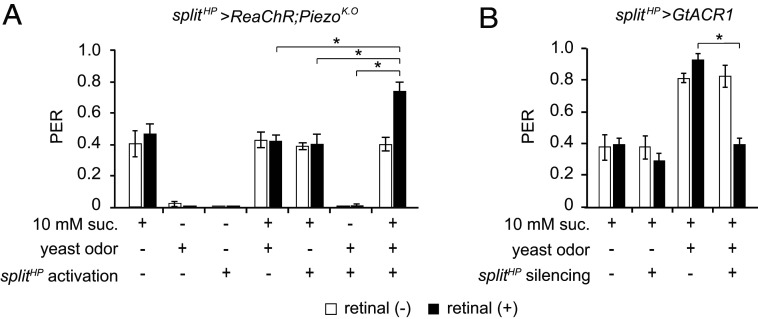
Finally, we asked whether these three different sensory modalities facilitate PER synergistically. Since contact with a food source would stimulate GRNs and hair plate MNs simultaneously, we used piezoK.O flies expressing ReaChR in cxHP8 MNs to control the perceived mechanical activation using optogenetics independent of taste stimulation. Consistent with our other results, we found that olfactory and mechanical cues, presented alone or together, do not evoke PER in these flies. We also found no increase in PER upon stimulation with a combination of either one of these sensory cues with taste. Instead, we observed PER enhancement only upon simultaneous presentation of all three different sensory modalities (Fig. 5A). We further confirmed that all three sensory modalities are required by carrying out optogenetic silencing of the cxHP8 MNs. Silencing the cxHP8 MNs by exposing splitHP>GtACR1 flies to blue light does not affect sugar-induced PER (48), but it does dramatically reduce yeast odor-enhanced PER when sucrose and yeast odor stimuli are combined (Fig. 5B). The results of this optogenetic activation and inhibition experiment confirm that simultaneous activation of all three different sensory modalities is required for PER enhancement.
Fig. 5.
Concurrent input of three different sensory cues enhances PER. (A) PER enhancement by multisensory integration. splitHP>ReaChR;piezoK.O flies were tested. 10 mM sucrose was used as a taste stimulus, yeast odor as an olfactory stimulus, and red light as a mechanical stimulus (optogenetic). n = 8 to 10. (B) Modified PER upon inhibition of hair plate neurons. splitHP neurons expressing GtACR1 were inhibited optogenetically. n = 8 to 10. All data are presented as means ± SEM. Kruskal–Wallis tests with Mann–Whitney U tests were used. The asterisks indicate statistically significant differences from PER evoked by sucrose alone, unless otherwise indicated. *P < 0.001.
Discussion
We demonstrate in this study that concurrent inputs of taste, olfactory, and mechanosensory cues facilitate food evaluation and feeding initiation in Drosophila. Once flies locate food by following its odors (7, 8, 16, 49), they must evaluate it prior to ingestion. Previous studies have shown how taste cues contribute to assessing, promoting, and sustaining the feeding of flies (50), but there may be steps preceding the use of taste sensation. Our findings suggest how these steps proceed: when the feet of the fly contact a food source sweet enough to induce PER, the fly extends its proboscis to evaluate the food with its labellar GRNs. In the case of a food source with favorable food odors and textures but lacking strong phagostimulatory taste cues, flies may utilize other food search strategies by recruiting from all three different sensory channels: taste, olfaction, and mechanosensation.
Recently, the Drosophila taste system has proven to be a useful model for studying the interactions between taste and other sensory modalities. MNs in the labellum were found to modulate food preference, suggesting food texture is an important determinant of food preference (9, 11, 13–15). Certain odors can enhance PER (12) and increase food consumption (10). We found that concurrent activation of three different sensory modalities induces supraadditive PER. In this phenomenon, the taste cue is essential for PER. While the addition of either a mechanosensory or olfactory sensory cue to the taste stimulation does not enhance PER, the synchronous addition of both a mechanosensory and an olfactory cue with the taste stimulus does enhance PER (Fig. 5A). We additionally confirmed that selective optogenetic inhibition of MNs blocks the supraadditive PER induced by the simultaneous activation of three different sensory modalities (Fig. 5B).
Shiraiwa found that odors detected through the maxillary palps enhance the PER by stimulating the labellar taste sensilla of starved animals (12). We found, however, that the antennae, rather than maxillary palps, are the major olfactory organs mediating multisensory integration, and that starvation is not required for taste-odor-mechanosensory integration and PER enhancement. It is unclear whether their observation that taste and odor can interact to enhance PER occurs only when flies are starved or whether it is a result of direct contact between odor chemicals and taste sensilla during the PER assay. Nevertheless, we found that the enhancement of PER by taste-odor-mechanosensory integration becomes more prominent at low concentrations of sucrose, suggesting it is especially relevant for survival.
Hair plates are often positioned at leg joints, such that movement of the joints or their relative positions can trigger hair deflection. In larger insects, hair plates seem to function as proprioceptors (51, 52), but their physiological function in Drosophila remains unknown. We found through several observations that touching low viscosity food leads to MN activation. First, optogenetic activation of coxa hair plate MNs enhances the PER triggered by sucrose with 5% methyl cellulose paired with yeast odor (Fig. 4F). Second, optogenetic inactivation of coxa hair plate MNs abolishes the PER triggered by sucrose in water combined with yeast odor (Fig. 5B). Third, increased optogenetic activation of coxa hair plate MNs in flies lacking piezo mimics the effect of reduced viscosity sucrose paired with yeast odor (SI Appendix, Fig. S7 A and B). It does not seem, however, that coxa hair plate MNs are the primary sensors of food texture because they do not contact food directly. Rather, tarsal contact with food induces distinct leg movements depending on food viscosity. Future studies will be necessary to determine how flies sense food viscosity, how this leads to the distinct leg motions observed, and how activation of the coxa hair plate MNs regulate feeding.
Although yeast odor-enhanced PER is strongly affected by both the knockdown and loss of Iav, Nan, and Piezo in hair plate MNs, it remains unclear how multiple mechanotransduction channels function together in these cells. In Drosophila hearing organs, TRPV and TRPN have distinct roles in Drosophila hearing transduction. TRPV channels (Iav and Nan) form the hearing transduction complex, which is then modulated by TRPN (NompC) (53). It is possible that Nan/Iav heteromultimeric channels and Piezo play distinct roles in the same pathway in hair plate MNs, but it is also possible that these channels function independently in a parallel manner.
In conclusion, we demonstrate that flies employ odor-taste-mechanosensory integration to obtain a clearer picture of their external environment and make more informed behavioral decisions. Our results indicate that combinations of relevant sensory pathways play essential roles in feeding behavior and suggest that novel behavior paradigms should be developed to parse out the roles of each sensory pathway.
Materials and Methods
Fly Stocks.
Flies were raised on standard cornmeal-molasses-agar medium and maintained at 25 °C and 60% humidity with a 12 h/12 h light/dark cycle. We obtained the following lines from the Bloomington Stock Center: Orco-GAL4 (BL 26818), Or35a-GAL4 (BL 9967), Or67a-GAL4 (BL 23894), Or67b-GAL4 (BL 9995), Or69a-GAL4 (BL 9999), Or9a-GAL4 (BL 23918), Or43b-GAL4 (BL 23894), Or7a-GAL4 (BL 23907), Or22a-GAL4 (BL 9952), Orco1 (BL 23129), UAS-Or35a (BL 76041), UAS-Orco (BL 23145), nan36a (BL 24902), iav3621 (BL 24768), piezoK.O (BL 58770), Painless1 (BL 27895), R20C06-GAL4-DBD (BL 69841), R48A07-P65-AD (BL 71070), UAS-Kir2.1 (BL 6595), UAS-shits (BL 44222), UAS-ReaChR (BL 53741), UAS-piezo (BL 58773), Tub-GAL80ts (BL 7108), R14F12-GAL4 (BL 48654), R20C06-GAL4 (BL 48884) (37), R39D08-GAL4 (BL 50049), R95A11-GAL4 (BL 48834), R41A08-GAL4 (BL 50108), R46H11-GAL4 (BL 50284), R86D09-GAL4 (BL 40459), R27E02-GAL4 (BL 49222) (37), R48A07-GAL4 (BL 50340) (38), and R38B08-GAL4 (BL 49541). iav RNAi (v100701), nan RNAi (v100090), and piezo RNAi (v105132) were from the Vienna Drosophila Resource Center. nompCf00642 was provided by Exelixis at Harvard Medical School. UAS-GtACR1 was a gift from A. Claridge-Chang, Duke-NUS Medical School, Singapore. In this study, we use and refer to w1118 as wild-type controls. All mutant lines and transgenic lines were backcrossed for five generations to the w1118 control flies.
Yeast Preparation.
Yeast (Saccharomyces cerevisiae; BY4741) were grown in yeast extract–peptone–dextrose (YPD) media. Yeast were incubated at 30 °C overnight with shaking (∼200 rpm). The yeast culture was washed with distilled water (DW) three times. After washing, the weight of the yeast pellet was measured and the pelleted yeast were resuspended by adding 1 mL DW per 1 g yeast pellet. Yeast extract (7184A, Neogen-Acumedia), Bacto peptone (0118, BD-Difco), and D-glucose (0188, Amresco) were used to make the YPD media.
PER Assay.
Unless otherwise indicated, we used nonstarved 4- to 6-d-old male flies. The flies were anesthetized on ice and glued on a glass slide in groups of 20 to 24 per genotype using melted 1-tetradecanol (185388, Sigma-Aldrich). We allowed the flies to recover for 1 to 2 h in a humidified chamber. We then satiated the flies with water before the PER assay. A 1-mL syringe with a 32-gauge needle was used to apply a droplet of sucrose or yeast to the foreleg or labellum. We calculated the PER index by dividing the number of responding flies by the total number of flies tested per group. Each PER data point is the result of a single experiment that tested 20 to 24 flies (n = 1). The average of these data is plotted with the SEM, which was calculated as the variance of each data point divided by the square root of the total trials (n). In the modified PER assay, while taste stimuli were applied to the fly legs or labellum, either volatile chemicals or a yeast suspension were brought near the flies without contact. Both stimuli were applied simultaneously. CO2-anesthetized flies were allowed to recover for 2 d prior to behavioral experiments. For olfactory organ removal, third antennal segments or maxillary palps from newly eclosed flies were removed under CO2 anesthesia, and the flies were allowed to recover for 4 to 6 d before behavioral assays. For the temporal inhibition of neurons, we used UAS-shits and Tub-GAL80ts;UAS-Kir2.1 flies. For Shibirets experiments, flies were raised at 20 °C. After being glued to a glass slide, the flies were kept at 30 °C for 1 to 2 h before subsequent behavioral experiments were performed at 30 °C. The control flies for the Shibirets experiments were always kept at 20 °C, including during the PER assays. Tub-GAL80ts;UAS-Kir2.1 flies were grown at 20 °C before shifting them to 30 °C for 2 to 3 d to induce the expression of Kir2.1. PER assays were then performed at room temperature. The controls for the Tub-GAL80ts;UAS-Kir2.1 flies were kept at 20 °C, and the PER assay was performed at room temperature.
To stimulate the hair plates by vibration, we glued flies on a glass slide and used more glue (TB1773E, Three Bond) to attach the trochanter of the foreleg to the body to prevent coxa movement. Droplets of sucrose containing 1% isoamyl alcohol mixed with 0.2% or 5% methyl cellulose were used to touch the tip of the tarsus. The flies that responded to the droplet were counted. To stimulate the hair plate, we attached one end of a glass probe to a speaker membrane (MAX50-002-N, Max Technology) and touched the other end of the glass probe to the coxa of the thoraco-coxal joint. We then applied one to two trials of vibration stimuli to the hair plate, while simultaneously presenting the droplet to the flies. The vibration frequency of the speaker we used was roughly 100 Hz.
GC-MS Analysis.
Volatile compounds in yeast cultures were analyzed by GC-MS. Samples were analyzed on a GC (7890B, Agilent Technologies) connected to a MS (5977A, Agilent Technologies) with a Combi-pal-headspace. The MS was run in electron impact (EI) mode at 70 eV and set to a scan range of 10 to 600 mz. The solvent delay time was 0.5 min. All samples were injected in splitless mode. We prepared 0.1-g samples in 20-mL headspace vials to measure the volatile components of a yeast pellet. The GC-MS was equipped with a Cyclosil-B cyclodextrin column (30-m length, 0.25-mm inner diameter, 0.25-μm film thickness). Helium was used as a carrier gas with a constant linear velocity of 1 mL/min. The initial 40 °C temperature of the GC oven was maintained for 3 min and then raised by 5 °C/min to a maximum of 300 °C. The yeast volatile components were then identified by comparing their mass spectra and retention times with a synthetic reference compound database.
Generation of Or35a1 Mutant Flies.
The Or35a-null allele was generated using the CRISPR-Cas9 system (54) with homologous recombination. Briefly, we selected two gRNAs specific to Or35a using TargetFinder (http://tools.flycrispr.molbio.wisc.edu/targetFinder); ATGGGGCCGATATCTGGACAAGG and AGGACAGATTGGTTCCGACTTGG, where the protospacer-adjacent motifs are underlined. We synthesized two oligomers and subcloned them into pU6-BbsI-chiRNA (#45946, Addgene). To generate the donor plasmid for homologous recombination, the mini-white cassette from the PW35 plasmid was subcloned into the pBluescriptsKSII (+) vector using NotI and KpnI. The 1-kb genomic DNA fragments at the 5′ and 3′ ends of the two gRNA sites were amplified from w1118 and then subcloned into pBluescriptsKSII (+) along with the miniwhite cassette. The primer sequences for the left homology arm were 5′-GACATACCGCGGCCACTATCTCCAGTGCGGTC-3′ and 5′-GACATAGCGGCCGCCCAGATATCGGCCCCATTTC-3′. Those for the right homology arm were 5′-GACATAGGATCCACTTGGCCTTTACTGTATGC-3′ and 5′-GACATAGGTACCAAGATAGAGACAGCCTAAGC-3′. The two pU6-BbsI-chiRNA plasmids containing the gRNA and the donor plasmid were coinjected into nos-Cas9 embryos at 500 ng/μL at the Korean Drosophila Resource Center. Progeny were crossed to w1118 to obtain red-eyed progeny. We verified the replacement of the Or35a coding sequence with miniwhite by genomic PCR using the following primers; p1, 5′-CAATGGCCAACTGCAAGTCC-3′; p2, 5′-TGCGCTTCGAGGCAAGTAAT-3′; and p3, 5′-GCAACGAGAATAGAGTGCCG-3′; p4, 5′-GTTGTCATTGTCGTGACAGG-3′.
Chemicals.
Sucrose (S9378), isoamyl alcohol (309435, CAS 123-51-3), phenylethyl alcohol (PHR1122, CAS 60-12-8), 1-hexanol (471402), isoamyl acetate (306967, CAS 123-92-2), and methyl cellulose (M7140) were purchased from Sigma-Aldrich.
Measurement of Food Viscosity.
Food viscosity was measured using a rotational type viscometer (DV-3 Rheometer, Brookfield). Samples were placed in sample containers and held for 5 min to reach a constant temperature (25 °C). Then, the viscosity was measured at a shear rate of 20 to 40.
Optogenetics.
Newly eclosed male flies were placed in vials containing fresh food with 400 μM all-trans-retinal (R2500, Sigma-Aldrich) for 7 to 10 d in the dark. The control flies were kept under the same conditions without all-trans-retinal. A red LED (627 nm, SR-05-D2050, Luxeon-Star-LEDs or 617 nm, M617F2, Thorlabs) was used to stimulate ReaChR (Or35a ORNs, 5.2 μW/mm2; hair plate MNs, 20.4 μW/mm2 otherwise indicated). A blue LED (470 nm, SR-05-B0040, Luxeon-Star-LEDs) was used to stimulate GtACR1 (hair plate MNs, 16.1 μW/mm2). The light intensity was measured with a photodiode power sensor (S130VC, Thorlabs). The LED lights were turned on for 3 s and presented with the other indicated stimuli. A second stimulation was applied after a 5-s rest period. We counted flies as responders when they showed PER in at least one of the two trials. For the selective activation of splitHP neurons, flies were placed in fly collars and the LEDs were presented through glass fibers (M617F2, Thorlabs) directed at either the body or the head. The fly collars were constructed by taping two single-edged blades together (DN52, Dorco), with the edges in between creating a slit of 0.3 mm to place on the neck of the fly as a barrier between its head and body. Additionally, aluminum foil was used to prevent unwanted light from passing through the slit. Ten flies were used per trial (n = 1) for the PER assay combined with optogenetics.
Immunohistochemistry.
Brains and ventral nerve cords (VNCs) were dissected and fixed for 15 min in 4% paraformaldehyde (PF) in phosphate-buffered saline (PBS) with 0.2% Triton X-100 (PBS-T) at room temperature. The samples were washed at least three times for 5 min each in 1× PBS-T and blocked for 1 h in PBS-T with 5% heat-inactivated goat serum (blocking solution). The tissues were incubated with primary antibodies diluted in blocking solution overnight at 4 °C. The primary antibodies were mouse nc82 (1:50, Developmental Study Hybridoma Bank) and rabbit anti-GFP (1:1,000, A11122, Invitrogen). The samples were washed three times for 10 min each with PBS-T and incubated with secondary antibodies diluted in blocking solution for 2 h at room temperature. The secondary antibodies were Alexa Fluor plus 488 goat anti-rabbit (1:250, A32731, Invitrogen) and Alexa Fluor 568 goat anti-mouse (1:250, A11031, Invitrogen). After three washes, each sample was mounted on a glass slide using Vectashield Mounting Medium (H-1000, Vector Laboratories). The legs were dissected and fixed for 20 min in 4% PF in PBS-T at room temperature. The samples were washed at least three times for 10 min each with PBS-T. Then, each sample was mounted on a glass slide with Focus Clear (CelExplorer). The mounted samples were visualized using a Zeiss LSM 880 confocal microscope (Carl Zeiss).
Scanning Electron Microscope Analysis.
To expose the coxa hair plate located on the anterior rim of the thoraco-coxa joint, the coxa was rotated backward slightly through the dorsal axis and attached with glue to the abdomen. These flies were mounted on a metallic stub. Mounted samples were sputter coated under vacuum with an electrically conductive layer of white gold of 100 nm (E-1010, Hitachi). The coxa hair plate was then imaged using a scanning electron microscope (S-3000N, Hitachi).
Tracking Leg Movement.
To demonstrate the differences in the movement of the leg between low and high viscosity stimuli, we performed a modified PER assay with a high-speed digital camera (HAS-U2, Ditect) at 500 frames per second and at a resolution of 640 × 480 pixels. A 3.3× macro zoom lens (MLM3X-MP) was installed on the camera. The flies, with all of their legs immobilized except for their forelegs, were mounted onto a glass slide. The samples were surrounded by an illuminating light source (CL 1500 ECO, Carl Zeiss) and LEDs to provide suitable contrast. They were recorded in a dark room to reduce the influence of environmental stimuli. To exclude the variability in leg movements caused by different droplet positions, we placed the droplet just above each fly’s head using a micromanipulator (DC-3K, Marzhauser). Leg movement was monitored with the HAS-xViewer program, and frame-by-frame positions of the legs were tracked using the Tracker program (https://physlets.org/tracker/).
Statistics.
All data are presented as means ± SEM. Normality and homoscedasticity were tested using the Shapiro–Wilk and Levene tests, respectively. For comparing two groups of data, unpaired Student’s t tests and Mann–Whitney U tests were used, depending on the result of the normality test. For multiple comparisons, one-way ANOVAs with Tukey post hoc tests were used. In these cases, when the normality test failed, the Kruskal–Wallis test with Mann–Whitney U tests combined with Bonferroni corrections were used. The details of these statistical analyses are shown in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
We thank the Bloomington Stock Center, the Vienna Drosophila RNAi Center, Exelixis at Harvard Medical School, and Dr. A. Claridge-Chang for providing fly stocks. We thank the Korea Food Research Institute for measuring the viscosity of methyl cellulose. We thank the Korean Drosophila Resource Center for the generation of mutant flies by microinjection. We thank Dr. Tanimura and the members of the Wang laboratory at University of California San Diego for helpful comments and advice. We also thank Drs. Ji-Man Park and Dongwoo Chae for helpful comments on our video analyses. This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean Government (NRF-2016R1A5A2008630 and NRF-2018R1A2B3001668 to S.J.M.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004523118/-/DCSupplemental.
Data Availability.
All study data are included in the article and/or supporting information.
References
- 1.Liman E. R., Zhang Y. V., Montell C., Peripheral coding of taste. Neuron 81, 984–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spence C., Multisensory flavor perception. Cell 161, 24–35 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Frank R. A., Byram J., Taste smell interactions are tastant and odorant dependent. Chem. Senses 13, 445–455 (1988). [Google Scholar]
- 4.Paradis S., Sweeney S. T., Davis G. W., Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron 30, 737–749 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Rozin P., “Taste-smell confusions” and the duality of the olfactory sense. Percept. Psychophys. 31, 397–401 (1982). [DOI] [PubMed] [Google Scholar]
- 6.Scott K., Gustatory processing in Drosophila melanogaster. Annu. Rev. Entomol. 63, 15–30 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Becher P. G., Bengtsson M., Hansson B. S., Witzgall P., Flying the fly: Long-range flight behavior of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 36, 599–607 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Dweck H. K., et al. , Olfactory channels associated with the Drosophila maxillary palp mediate short- and long-range attraction. eLife 5, e14925 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong Y. T., et al. , Mechanosensory neurons control sweet sensing in Drosophila. Nat. Commun. 7, 12872 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reisenman C. E., Scott K., Food-derived volatiles enhance consumption in Drosophila melanogaster. J. Exp. Biol. 222, jeb202762 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-Alcañiz J. A., Zappia G., Marion-Poll F., Benton R., A mechanosensory receptor required for food texture detection in Drosophila. Nat. Commun. 8, 14192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiraiwa T., Multimodal chemosensory integration through the maxillary palp in Drosophila. PLoS One 3, e2191 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S. F., Ja Y. L., Zhang Y. J., Yang C. H., Sweet neurons inhibit texture discrimination by signaling TMC-expressing mechanosensitive neurons in Drosophila. eLife 8, e46165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y. V., Aikin T. J., Li Z., Montell C., The basis of food texture sensation in Drosophila. Neuron 91, 863–877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Y., Cao L. H., Sui X. W., Guo X. Q., Luo D. G., Mechanosensory circuits coordinate two opposing motor actions in Drosophila feeding. Sci. Adv. 5, eaaw5141 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riffell J. A., Lei H., Hildebrand J. G., Neural correlates of behavior in the moth Manduca sexta in response to complex odors. Proc. Natl. Acad. Sci. U.S.A. 106, 19219–19226 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dethier V. G., The Hungry Fly: A Physiological Study of the Behavior Associated with Feeding (Commonwealth Fund book, Harvard Univ. Press, Cambridge, 1976). [Google Scholar]
- 18.Piper M. D., et al. , A holidic medium for Drosophila melanogaster. Nat. Methods 11, 100–105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steck K., et al. , Internal amino acid state modulates yeast taste neurons to support protein homeostasis in Drosophila. eLife 7, e31625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatum E. L., Nutritional requirements of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 25, 490–497 (1939). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganguly A., et al. , A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep. 18, 737–750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro C., Dickson B. J., Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr. Biol. 20, 1000–1005 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Vargas M. A., Luo N., Yamaguchi A., Kapahi P., A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 20, 1006–1011 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Z., et al. , A post-ingestive amino acid sensor promotes food consumption in Drosophila. Cell Res. 28, 1013–1025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toshima N., Tanimura T., Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J. Exp. Biol. 215, 2827–2832 (2012). [DOI] [PubMed] [Google Scholar]
- 26.Larsson M. C., et al. , Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Baines R. A., Uhler J. P., Thompson A., Sweeney S. T., Bate M., Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 21, 1523–1531 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B., Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hallem E. A., Carlson J. R., Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Yao C. A., Ignell R., Carlson J. R., Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 25, 8359–8367 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inagaki H. K., et al. , Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nat. Methods 11, 325–332 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tracey W. D. Jr, Wilson R. I., Laurent G., Benzer S., painless, a Drosophila gene essential for nociception. Cell 113, 261–273 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Walker R. G., Willingham A. T., Zuker C. S., A Drosophila mechanosensory transduction channel. Science 287, 2229–2234 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Kim J., et al. , A TRPV family ion channel required for hearing in Drosophila. Nature 424, 81–84 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Gong Z., et al. , Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J. Neurosci. 24, 9059–9066 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S. E., Coste B., Chadha A., Cook B., Patapoutian A., The role of Drosophila Piezo in mechanical nociception. Nature 483, 209–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramdya P., et al. , Mechanosensory interactions drive collective behaviour in Drosophila. Nature 519, 233–236 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tuthill J. C., Wilson R. I., Parallel transformation of tactile signals in central circuits of Drosophila. Cell 164, 1046–1059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire S. E., Mao Z., Davis R. L., Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci. STKE 2004, pl6 (2004). [DOI] [PubMed] [Google Scholar]
- 40.PRINGLE J. W. S., Proprioception in insects. J. Exp. Biol. 15, 114–131 (1938). [Google Scholar]
- 41.Smith S. A., Shepherd D., Central afferent projections of proprioceptive sensory neurons in Drosophila revealed with the enhancer-trap technique. J. Comp. Neurol. 364, 311–323 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Luan H., Peabody N. C., Vinson C. R., White B. H., Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitamoto T., Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J. Neurobiol. 47, 81–92 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Flood T. F., et al. , A single pair of interneurons commands the Drosophila feeding motor program. Nature 499, 83–87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pool A. H., et al. , Four GABAergic interneurons impose feeding restraint in Drosophila. Neuron 83, 164–177 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuenzi F., Burrows M., Central connections of sensory neurones from a hair plate proprioceptor in the thoraco-coxal joint of the locust. J. Exp. Biol. 198, 1589–1601 (1995). [DOI] [PubMed] [Google Scholar]
- 47.Trimarchi J. R., Jin P., Murphey R. K., Controlling the motor neuron. Int. Rev. Neurobiol. 43, 241–264 (1999). [DOI] [PubMed] [Google Scholar]
- 48.Mohammad F., et al. , Optogenetic inhibition of behavior with anion channelrhodopsins. Nat. Methods 14, 271–274 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Giang T., He J., Belaidi S., Scholz H., Key odorants regulate food attraction in Drosophila melanogaster. Front. Behav. Neurosci. 11, 160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pool A. H., Scott K., Feeding regulation in Drosophila. Curr. Opin. Neurobiol. 29, 57–63 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson K. G., Wong R. K., Fourtner C. R., Connexions between hair-plate afferents and motoneurones in the cockroach leg. J. Exp. Biol. 64, 251–266 (1976). [DOI] [PubMed] [Google Scholar]
- 52.Wong R. K., Pearson K. G., Properties of the trochanteral hair plate and its function in the control of walking in the cockroach. J. Exp. Biol. 64, 233–249 (1976). [DOI] [PubMed] [Google Scholar]
- 53.Lehnert B. P., Baker A. E., Gaudry Q., Chiang A. S., Wilson R. I., Distinct roles of TRP channels in auditory transduction and amplification in Drosophila. Neuron 77, 115–128 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gratz S. J., et al. , Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194, 1029–1035 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or supporting information.