Neutralizing antibodies (nAbs), in the time of COVID-19, have received much attention in the lay press owing to their association with vaccine protection against severe acute respiratory syndrome coronavirus 2. The attention is well-deserved and the association of nAbs with protection is true for many viruses (1). For HIV, we eagerly await the results of the antibody-mediated protection trial investigating the ability of the HIV broadly neutralizing antibody (bnAb) VRC01 to prevent infection in humans (https://ampstudy.org/). Meanwhile, passively administered and vaccine-induced nAbs have been shown to provide protection against mucosal viral challenge in nonhuman primates (NHPs) (2). A recent analysis of a large number of NHP protection studies showed a good correlation between serum neutralization titer and protection (3). However, one set of bnAbs, those directed to the membrane proximal external region (MPER) of the HIV envelope spike, appeared to be outliers and offered greater protection at lower serum neutralizing titers. This phenomenon was first described for MPER bnAbs 2F5 and 4E10 (4) and was also tentatively noted for MPER bnAb 10E8 (5) and has been referred to colloquially as MPER bnAbs “punching above their weight.”
A very interesting related observation with regard to the phenomenon had earlier been made by Perez et al., who showed that neutralization was greatly enhanced in target cells (TZM-bl cells) engineered to express Fc receptors, particularly FcRI and to a lesser extent FcRIIb, on their surface as compared to wild-type target cells (6). The enhancement was shown to be dependent on the Fc region of the bnAbs. This result was consistent with some observations made even earlier by Holl et al. (7–9), who noted that the MPER bnAbs 2F5 and 4E10 showed hugely enhanced ability to neutralize viruses infecting mononuclear phagocytes and monocyte-derived dendritic cells and a link to FcRI expression. They likewise showed the dependence of the effect on the Fc part of the Ab molecule. A later study (10) showed that the FcR-dependent effect in TZM-bl cells was not due to phagocytosis of virus and it was proposed that a “kinetic effect” was responsible. Thus, it was suggested that bnAb bound to FcR would be prepositioned to bind more effectively to the MPER, which is probably only fully displayed once initial contact of virus with target cells has been made. Overall, the possibility then arose that an early event in infection in NHPs might involve FcRI-bearing cells, most likely mononuclear phagocytes or dendritic cells, and MPER bnAbs were thereby advantaged in protection terms. A complicating factor in this mechanism is the high serum concentration of monomeric immunoglobulin G (IgG) that will compete with specific IgG for FcRI binding, and indeed 5% serum was shown to abolish the FcRI effect for MPER bnAbs (6). However, it was argued that conditions operating in vivo may still allow the enhancement effect.
A report in PNAS (11) identifies a class of nAbs demonstrating the phenomenon of hugely enhanced neutralization of FcR-bearing target cells as compared to the corresponding cells lacking FcR. Miller et al. previously described a bnAb, D5, a number of years ago that recognizes the N-terminal heptad repeat (NHR) region of gp41 (12). The region is conserved, being a part of the HIV fusion machinery, but is only transiently exposed during the process in which the virus membrane fuses with that of the target cells preceding the transfer of viral genetic information to the target cell (13, 14). The NHR is a validated clinical target for the drug enfuviritide (15). However, D5 primarily only neutralizes highly sensitive HIV isolates (tier-1 viruses) and is very weak against more neutralization-resistant viruses (tier 2), characteristic of human infection and transmission. Therefore, interest in the antibody per se and its epitope as a vaccine target has been limited. In this report, it is demonstrated that D5 neutralizes a range of tier-2 viruses effectively in TZM-bl target cells expressing human FcRI. The enhancement effect in certain cases is estimated to be of the order of 5,000. The effect is shown to be dependent on the Fc region of the antibody. Furthermore, immunization of guinea pigs with an NHR-based immunogen generates antisera that show neutralizing activity dependent upon FcR expression on the target cells. The explanation for the results, similar to the MPER bnAbs, is that the prepositioning of the D5 and polyclonal NHR Abs on the FcR greatly increases the effective concentration of antibody when virus engages target cell receptors, leading to much more effective neutralization (Fig. 1).
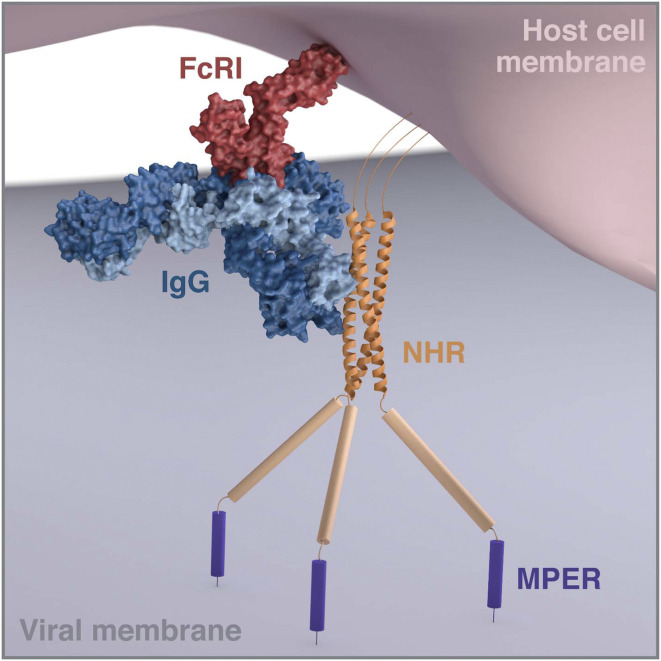
Fig. 1.
Modeling of the interaction of FcRI-bound IgG to the NHR region of gp41 of HIV envelope during viral entry (compare figure 5 of ref. 11). It is hypothesized that, during viral entry and the interaction of HIV envelope with CD4 and CCR5 on target cells, the NHR is exposed for recognition by antibody D5 complexed to FcRI on the target cells. Prepositioning of D5 in this way greatly enhances the ability of the antibody to inhibit viral entry. The modeling used known structures and interaction sites where available. The Fc portion of a human FcγR I/Fc complex (Protein Data Bank [PDB] ID code 4W4O, red) was superimposed on the Fc of human IgG (IgG b12, modified from PDB ID code 1HZH, dark and light blue) and extracted. The NHR is represented by the coordinates of a gp41 NHR trimer mimetic (PDB ID code 6R2G, orange) and the rest of gp41 is represented schematically as in figure 5 of ref. 11. To avoid structural clashes, the immunoglobulin Fab arms are bent away from the bound FcRI, and the Fc portion is lain flat parallel to the surface of the host cell membrane (pink). The figure can be compared to the tight space modeled for the interaction of an FcRIIa-bound IgG molecule bound to the envelope proteins of an alphavirus (20). Modeling and three-dimensional rendering by Christina Corbaci and Lars Hangartner.
The key question now is whether the FcR-dependent property described can be exploited either in the use of passive antibodies antivirally or in vaccine development. As proposed by the authors, an important experiment is now to determine whether D5 punches above its weight in protection experiments in NHPs—that is, does it offer protection at relatively low serum neutralizing titers to mucosal challenge? If it does, then this would suggest that D5, especially if engineered to have higher affinity for its target epitope (16), could find prophylactic and possibly therapeutic application given its breadth of neutralization. The NHR would also then be a possible attractive vaccine target. Importantly, a positive result, together with existing MPER bnAb protection, would also be suggestive of the involvement of FcRI-bearing cells, such as mononuclear phagocytes, at a crucial stage in the infection process in NHPs. As noted by the authors, the identity of the cells involved first in the very early stages of transmission is uncertain but there is evidence that FcRI-bearing cells such as macrophages and dendritic cells can transmit virus to CD4+ T cells (17, 18), which will of course become the predominant cell type infected with HIV in the developing course of infection. A positive result for D5 protection in NHPs would inspire further work in the animal model on vaccine-induced protection and the possibilities of translating findings to humans.
Finally, the enhanced neutralization described by bringing nAbs in close proximity to the membrane of target cells may have utility in terms of bifunctional antibody constructs combining specificity for HIV with specificity for a target cell antigen. Indeed, one such “CrossMAb” incorporating the MPER bnAb 10E8 and anti-CD4 has been described and is now under clinical evaluation (19). The anti-CD4 specificity in that construct is as an active inhibitor of viral entry but also tethers the MPER Ab to the target cell surface at precisely the location for viral entry on the cell membrane. A similar result is found for 10E8 linked to an anti-CCR5 specificity. Following the studies described here, more tethering constructs could be readily envisaged and evaluated.
Acknowledgments
I thank Lars Hangartner and Christina Corbaci for help with the figure. I acknowledge the financial support of the National Institute of Allergy and Infectious Diseases, the Bill and Melinda Gates Foundation, the International AIDS Vaccine Initiative, and the James B. Pendleton Charitable Trust.
Footnotes
The author declares no competing interest.
See companion article, “The high-affinity immunoglobulin receptor FcγRI potentiates HIV-1 neutralization via antibodies against the gp41 N-heptad repeat,” 10.1073/pnas.2018027118.
References
- 1.Klasse P. J., Neutralization of virus infectivity by antibodies: Old problems in new perspectives. Adv. Biol. 2014, 157895 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hessell A. J., Malherbe D. C., Haigwood N. L., Passive and active antibody studies in primates to inform HIV vaccines. Expert Rev. Vaccines 17, 127–144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pegu A., et al. , A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe 26, 336–346.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hessell A. J., et al. , Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84, 1302–1313 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pegu A., et al. , Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci. Transl. Med. 6, 243ra88 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez L. G., Costa M. R., Todd C. A., Haynes B. F., Montefiori D. C., Utilization of immunoglobulin G Fc receptors by human immunodeficiency virus type 1: A specific role for antibodies against the membrane-proximal external region of gp41. J. Virol. 83, 7397–7410 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holl V., et al. , Involvement of Fc gamma RI (CD64) in the mechanism of HIV-1 inhibition by polyclonal IgG purified from infected patients in cultured monocyte-derived macrophages. J. Immunol. 173, 6274–6283 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Holl V., et al. , Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80, 6177–6181 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holl V., et al. , Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107, 4466–4474 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez L. G., Zolla-Pazner S., Montefiori D. C., Antibody-dependent, FcγRI-mediated neutralization of HIV-1 in TZM-bl cells occurs independently of phagocytosis. J. Virol. 87, 5287–5290 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montefiori D. C., et al. , The high-affinity immunoglobulin receptor FcγRI potentiates HIV-1 neutralization via antibodies against the gp41 N-heptad repeat. Proc. Natl. Acad. Sci. U.S.A., 10.1073/pnas.2018027118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller M. D., et al. , A human monoclonal antibody neutralizes diverse HIV-1 isolates by binding a critical gp41 epitope. Proc. Natl. Acad. Sci. U.S.A. 102, 14759–14764 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan D. C., Kim P. S., HIV entry and its inhibition. Cell 93, 681–684 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Eckert D. M., Kim P. S., Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70, 777–810 (2001). [DOI] [PubMed] [Google Scholar]
- 15.LaBonte J., Lebbos J., Kirkpatrick P., Enfuvirtide. Nat. Rev. Drug Discov. 2, 345–346 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Rubio A. A., et al. , A derivative of the D5 monoclonal antibody that targets the gp41 N-heptad repeat of HIV-1 with broad tier-2 neutralizing activity. bioRxiv [Preprint] (2020). https://www.biorxiv.org/content/10.1101/2020.10.23.352526v1 (Accessed 24 October 2020). [DOI] [PMC free article] [PubMed]
- 17.Loré K., Smed-Sörensen A., Vasudevan J., Mascola J. R., Koup R. A., Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4+ T cells. J. Exp. Med. 201, 2023–2033 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groot F., Welsch S., Sattentau Q. J., Efficient HIV-1 transmission from macrophages to T cells across transient virological synapses. Blood 111, 4660–4663 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Huang Y., et al. , Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell 165, 1621–1631 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burton D. R., Antibody barriers to going viral. J. Exp. Med. 216, 2226–2228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]