Significance
The L-type voltage-gated Ca2+ channel CaV1.2 is essential for excitation–contraction coupling in the heart. During the fight-or-flight response, CaV1.2 channel activity is augmented as a result of PKA-mediated phosphorylation, downstream of β-adrenergic receptor activation. We discovered that enhanced sarcolemmal abundance of CaV1.2 channels, driven by stimulated insertion/recycling of specific CaV1.2-containing endosomes, is essential for β-adrenergic receptor-mediated regulation of these channels in the heart. These data reveal a conceptual framework of this critical and robust pathway for on-demand tuning of cardiac excitation–contraction coupling during fight-or-flight.
Keywords: L-type calcium channel, trafficking, β-adrenergic receptor, ion channel clustering, cardiac EC-coupling
Abstract
The number and activity of Cav1.2 channels in the cardiomyocyte sarcolemma tunes the magnitude of Ca2+-induced Ca2+ release and myocardial contraction. β-Adrenergic receptor (βAR) activation stimulates sarcolemmal insertion of CaV1.2. This supplements the preexisting sarcolemmal CaV1.2 population, forming large “superclusters” wherein neighboring channels undergo enhanced cooperative-gating behavior, amplifying Ca2+ influx and myocardial contractility. Here, we determine this stimulated insertion is fueled by an internal reserve of early and recycling endosome-localized, presynthesized CaV1.2 channels. βAR-activation decreased CaV1.2/endosome colocalization in ventricular myocytes, as it triggered “emptying” of endosomal CaV1.2 cargo into the t-tubule sarcolemma. We examined the rapid dynamics of this stimulated insertion process with live-myocyte imaging of channel trafficking, and discovered that CaV1.2 are often inserted into the sarcolemma as preformed, multichannel clusters. Similarly, entire clusters were removed from the sarcolemma during endocytosis, while in other cases, a more incremental process suggested removal of individual channels. The amplitude of the stimulated insertion response was doubled by coexpression of constitutively active Rab4a, halved by coexpression of dominant-negative Rab11a, and abolished by coexpression of dominant-negative mutant Rab4a. In ventricular myocytes, βAR-stimulated recycling of CaV1.2 was diminished by both nocodazole and latrunculin-A, suggesting an essential role of the cytoskeleton in this process. Functionally, cytoskeletal disruptors prevented βAR-activated Ca2+ current augmentation. Moreover, βAR-regulation of CaV1.2 was abolished when recycling was halted by coapplication of nocodazole and latrunculin-A. These findings reveal that βAR-stimulation triggers an on-demand boost in sarcolemmal CaV1.2 abundance via targeted Rab4a- and Rab11a-dependent insertion of channels that is essential for βAR-regulation of cardiac CaV1.2.
The influx of Ca2+ through L-type Ca2+ channels (CaV1.2) is indispensable for cardiac excitation–contraction coupling (EC-coupling). These multimeric proteins consist of a pore-forming and voltage-sensing α1c subunit, and auxiliary β- and α2δ-subunits. In ventricular myocytes, CaV1.2 mainly localize to the t-tubule sarcolemma and open briefly, allowing a small amount of Ca2+ influx, in response to the wave of depolarization that travels through the conduction system of the heart from its point of origin, usually in the SA-node. This initial influx is amplified manifold though Ca2+-induced Ca2+ release (CICR) from juxtaposed type 2 ryanodine receptors (RyR2) on the junctional sarcoplasmic reticulum, a short ∼12 nm across the dyadic cleft. The synchronous opening of thousands of RyR2 generates a transient, global elevation in intracellular calcium concentration ([Ca2+]i), resulting in contraction. Reducing CaV1.2 channel current (ICa) results in less CICR, smaller [Ca2+]i transients, and less forceful contractions. Conversely, larger amplitude ICa elicits greater Ca2+ release from the sarcoplasmic reticulum, producing more forceful contractions. The level of Ca2+ influx through CaV1.2 channels therefore tunes EC-coupling.
ICa is a product of the number of channels in the sarcolemma and their open probability (Po). Consequently, there are two possible, nonmutually exclusive strategies that may be adopted to alter ICa and consequently the magnitude of EC-coupling: 1) Adjust CaV1.2 channel activity (Po) and 2) modify sarcolemmal CaV1.2 channel expression (N). The first strategy of increasing channel Po has long been associated with β-adrenergic receptor (βAR)-mediated signaling in the heart (1–3). During acute physical or emotional stress, norepinephrine spills from sympathetic varicosities onto cardiomyocytes, activating βARs. The ensuing Gαs/adenylyl cyclase/cAMP/PKA signaling cascade culminates in PKA phosphorylation of several effector proteins, including CaV1.2 [or an element of their interactome (4)], enhancing their activity to generate this positive inotropic response.
As to the second strategy to increase ICa, there remains a paucity of information regarding the mechanisms regulating CaV1.2 channel abundance in the cardiomyocyte sarcolemma. Classic secretory transport literature suggests that CaV1.2 channels are trafficked from the endoplasmic reticulum to the trans-Golgi-network and onward to their dyadic position in the sarcolemma. Underscoring the importance of faithful CaV1.2 channel trafficking, altered CaV1.2 channel density has been reported in both failing (5) and aging (6) ventricular myocytes, and impaired anterograde trafficking of CaV1.2 channels to the t-tubules of human ventricular myocytes has been linked to dilated cardiomyopathy (7). Yet, despite the importance of tight homeostatic control of CaV1.2 channel trafficking to prevent Ca2+ dysregulation, the molecular steps defining CaV1.2 channel sorting and insertion remain poorly understood. Therefore, elucidation of the trafficking pathways that regulate CaV1.2 channel abundance is critical for our understanding of the pathophysiology of heart failure and myocardial aging, and could potentially reveal new therapeutic or rejuvenation targets. Along that vein, in the treatment of cystic fibrosis, multiple drugs are in various stages of use or development to improve trafficking to, or to amplify or stabilize, CFTR channels at the apical membrane of airway epithelial cells (8).
There exist no measurements of CaV1.2 channel lifetimes in cardiomyocytes, but pulse-chase experiments in immortalized cell lines support a lifetime of plasma membrane (PM)-localized CaV1.2 of ∼3 h (9), while total cellular CaV1.2 lifetime is >20 h (10). This disparity suggests membrane-CaV1.2 turns over much more dynamically than the total cellular channel content and implies ongoing local control by endosomal trafficking. Disturbance of the equilibrium between channel insertion/recycling and internalization would be predicted to lead to alterations in sarcolemmal CaV1.2 channel abundance. Trafficking of vesicular cargo through the endosomal pathway is regulated by Rab-GTPases, a >60-member family within the larger Ras superfamily of small GTPases (11, 12). Rab5 is involved in endocytosis and control of vesicular cargo influx into early endosomes (EEs; also called sorting endosomes), while Rab4 controls efflux of cargo out of EEs and fast recycling (t1/2 ∼1 to 2 min) back to the PM (13). Rab11, expressed on recycling endosomes (RE; also called the endocytic recycling compartment or ERC), regulates slow recycling (t1/2 ∼12 min) of cargo from this compartment back to the PM (13). In cortical neurons and pancreatic β-cells, activity-dependent CaV1.2 channel internalization has been postulated to play important roles in Ca2+ homeostasis, with implications for homeostatic synaptic plasticity and insulin production, respectively (11, 14). In mouse neonatal cardiomyocytes, Rab11b has been reported to limit CaV1.2 PM expression (15), while recent studies performed in HEK and HL-1 cells reported that endocytic recycling of cardiac CaV1.2 channels, regulates their surface abundance (10, 16). Despite this crucial information from other cell-types, there has been a lack of rigorous investigations, at the molecular level, into how CaV1.2 channel recycling is regulated in cardiac myocytes.
Here, we identify a dynamic, subsarcolemmal pool of CaV1.2-cargo–carrying endosomes that are rapidly mobilized to the ventricular myocyte sarcolemma along targeted Rab4a and Rab11a GTPase-regulated recycling pathways in response to βAR-stimulation. Using electrophysiology, cell biology, total internal reflection fluorescence (TIRF), and superresolution microscopy, we report that enhanced t-tubule sarcolemmal CaV1.2 abundance via targeted, isoproterenol (ISO)-stimulated recycling of these channels is essential for βAR-regulation of cardiac CaV1.2.
Results
Internal Pools of Presynthesized CaV1.2 Channels Reside on Endosomes.
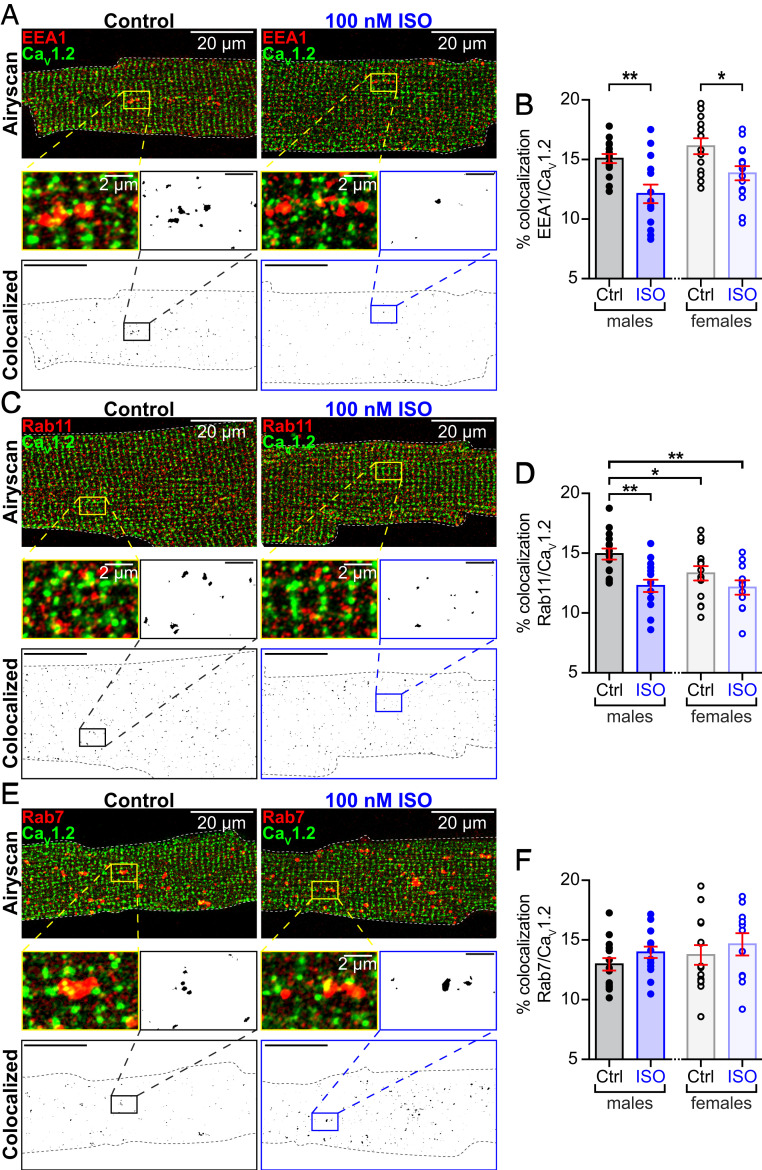
Recently, we reported that sarcolemmal CaV1.2 channel expression increases in the heart during βAR signaling (17). Activation of βARs with ISO produced a rapid, PKA-dependent augmentation of CaV1.2 channel abundance along ventricular myocyte t-tubules. We hypothesized that an endosomal pool of CaV1.2 fuels the rapid, ISO-stimulated insertion of channels into the sarcolemma of ventricular myocytes (see SI Appendix, Fig. S1A for an overview of this model). To test this, we performed an examination of the distribution of CaV1.2 channels on EEs, REs, and late endosomes (LEs) in adult mouse ventricular myocytes (AMVMs) using two- or three-color Airyscan superresolution microscopy. Immunostained CaV1.2 channel cargo was observed on 15.1 ± 0.4% of early endosome antigen-1+ (EEA1+) pixels in male ventricular myocytes and on a similar 16.1 ± 0.7% in female myocytes (P = 0.24) (Fig. 1 A and B; see also SI Appendix, Fig. S2). Prefixation stimulation with 100 nM ISO led to a significant, 15 to 20% decrease in colocalization between CaV1.2 and EEA1 (male P = 0.001, female P = 0.01). We further narrowed our analysis to Rab4+ EEs by performing triple-label experiments, costaining for CaV1.2, EEA1, and Rab4 (SI Appendix, Fig. S3). A similar trend was observed there such that 15.6 ± 1.3% of EEA1- and Rab4-coexpressing pixels were colocalized with CaV1.2, falling to 10.8 ± 0.6% in ISO-stimulated cells (P = 0.008). These data suggest that ISO activation of βARs stimulates movement of CaV1.2 out of EEs and into another cellular compartment.
Fig. 1.
Internal pools of presynthesized CaV1.2 channels reside on endosomes. (A) Two-color Airyscan superresolution images of control and 100 nM ISO-stimulated AMVMs immunostained to examine distributions of CaV1.2 and EEA1+ early endosomes. Binary colocalization maps (Bottom) display pixels in which CaV1.2 and endosomal expression precisely overlapped. (B) Histograms showing percent colocalization between EEA1 and CaV1.2 in male and female AMVMs, in control (male: n = 3, n = 15; female n = 3, n = 13) and ISO-stimulated conditions (male: n = 3, n = 14; female n = 3, n = 15). (C) Immunostaining of CaV1.2 and Rab11+ recycling endosomes and (D) accompanying histogram summarizing results from control (male: n = 4, n = 15; female n = 3, n = 14) and ISO conditions (male: n = 3, n = 15; female n = 3, n = 11). (E) Immunostaining of CaV1.2 and Rab7+ LEs and lysosomes. (F) Histogram summarizing results from control (males: n = 3, n = 15, females: n = 3, n = 14) and ISO-stimulated AMVMs (males: n = 3, n = 15, females: n = 3, n = 11). Error bars indicate SEM. Two-way ANOVA **P < 0.01; *P < 0.05. Pictured are male AMVMs; for females see SI Appendix, Fig. S1.
Cargo exiting the EE can be routed either to REs, LEs, or back to the sarcolemma via the fast-recycling pathway (SI Appendix, Fig. S1A) (12, 13). If βAR activation stimulates CaV1.2 channel trafficking from EEs into REs, then a testable prediction is that ISO should increase colocalization between CaV1.2 and Rab11 (a marker of REs). Accordingly, we examined the distribution of CaV1.2 on Rab11+ REs, and found a population of CaV1.2-cargo-carrying REs, where CaV1.2 colocalized with 14.9 ± 0.5% of Rab11+ pixels in males and with 13.3 ± 0.6% in females (Fig. 1 C and D). An 18% decrease in colocalization between CaV1.2 and Rab11 was observed in male cells treated with 100 nM ISO (P = 0.002). Data from female cardiomyocytes showed a similar downward trend in colocalization. These results do not support the hypothesis that CaV1.2 channels move from Rab4+ EEs into REs in response to ISO. We next tested the hypothesis that ISO stimulation drives the trafficking of CaV1.2 channels from EEs to LEs; however, despite identifying a population of CaV1.2 channels on Rab7+ LEs in male and female ventricular myocytes, ISO stimulation did not significantly alter the degree of Rab7/CaV1.2 colocalization (Fig. 1 E and F) (P = 0.28 in males, P = 0.39 in females). Having ruled out two of the three possibilities, we surmise that βAR stimulation drives an intracellular pool of CaV1.2 channels from Rab4+ EEs into the fast-recycling pathway, and Rab11+ REs into the slow recycling pathway.
ISO-Stimulated Enhancement of CaV1.2 Recycling Is Regulated by Rab4 and Rab11.
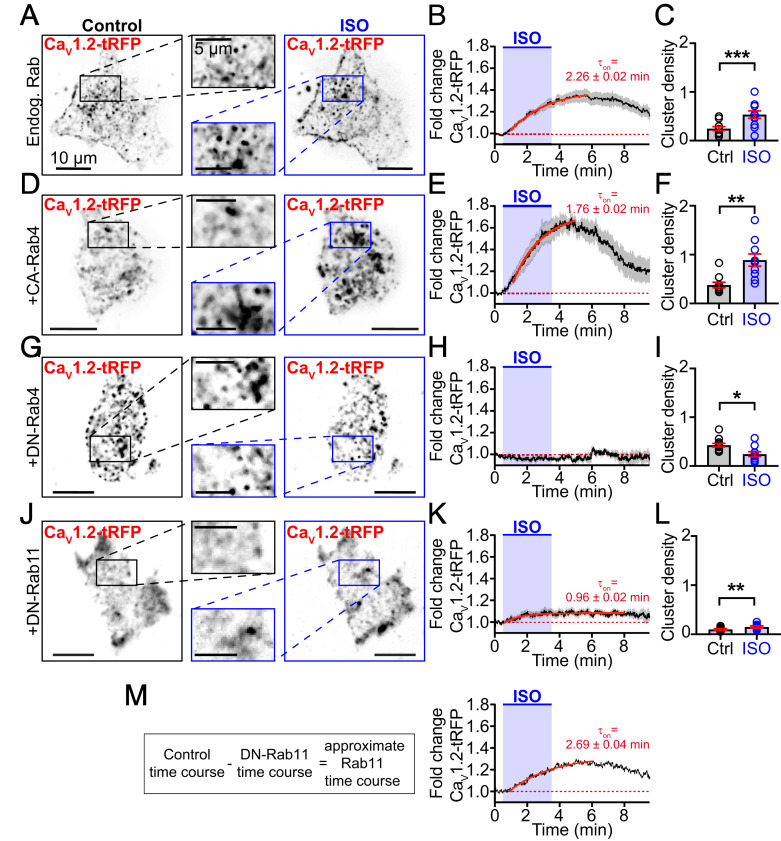
Having determined that βAR stimulation decreases the number of CaV1.2 channels on EEs and REs, we next tested if Rab4-dependent fast-, and Rab11-dependent slow-recycling pathways facilitate enhancement of CaV1.2 delivery into the PM of transiently transfected tsA201 cells. While these cells do not recapitulate all of the intricacies of signaling in cardiomyocytes, we utilized them here because: 1) They provide a reductionist framework on which to test our Rab4 and Rab11 hypotheses in the absence of other voltage-gated channels; 2) they can be easily transfected, permitting manipulation of Rab-protein complement; and 3) they endogenously express βARs (18). PM expression of CaV1.2 channels was monitored during activation of βARs with 100 nM ISO. Clusters of channels were readily identified in the TIRF footprint of the cell (Fig. 2A). Upon wash-in of ISO, CaV1.2-tagRFP intensity in the TIRF footprint increased by an average of 1.37-fold over a period of minutes (Fig. 2B) (τon = 2.26 ± 0.02 min), in close agreement with previous results showing a 44.9 ± 6.2% increase in channels in ISO-stimulated AMVM sarcolemmas (17). An increased density (number/μm2) of channel clusters in the TIRF footprint contributed to this elevation (Fig. 2C) (P < 0.0001). These results suggest that ISO stimulates enhanced recycling of CaV1.2 channels to the PM.
Fig. 2.
Rab4a and Rab11a regulate an ISO-stimulated boost in CaV1.2 recycling. (A) TIRF images of Cav1.2-tRFP distribution in the PM of tsA-201 cells before (Left) and after 100 nM ISO (Right; n = 18). (B) Time course and kinetics (solid red line) of the fold-change in CaV1.2-tRFP intensity in the TIRF footprint before, during, and after ISO stimulation. (C) Histogram summarizing CaV1.2 channel cluster density (number/μm2) in the TIRF footprint in control and ISO-stimulated conditions. (D–F) Same layout format in tsA-201 cells coexpressing constitutively active (GTP-locked) Rab4a (n = 10). (G–I) Same layout format in tsA-201 cells coexpressing dominant-negative (GDP-locked) Rab4a (n = 12). (J–L) Same layout format in tsA-201 cells coexpressing dominant-negative (GDP-locked) Rab11a (n = 8). (M) Calculation and resultant theoretical time-course and kinetics of Rab11a-dependent ISO-stimulated recycling. Data that passed a normality test (C and L) were analyzed with a paired t test, others (F and I) were analyzed with a nonparametric Wilcoxon test. ***P < 0.001; **P < 0.01; *P < 0.05.
We tested the role of Rab4a in this dynamic response by coexpressing a mutant Rab4a with a single amino acid substitution (Q67L) that renders it resistant to GTP-hydrolysis, locking it in a GTP-bound, constitutively active state (CA-Rab4aQ67L) (Fig. 2 D–F) (19). In these cells, addition of ISO stimulated a 1.69-fold increase in intensity, almost twice the maximal response observed in controls, and increased cluster density 2.4-fold (Fig. 2F) (P = 0.002). Interestingly, coexpression of CA-Rab4a had no effect on basal CaV1.2 cluster density before addition of ISO (P = 0.08) (Fig. 2 C and F) indicating that, even in this GTP-locked active state, ISO-stimulation is required to trigger the boost in CaV1.2 recycling. After ISO, the GTP-bound CA-Rab4a facilitates a larger, faster (1.76 ± 0.02 min) recycling response. This suggests that Rab4a plays a role in βAR-stimulated channel recycling but also implies the involvement of an upstream effector.
To support this postulate, we examined the ISO response in cells expressing a dominant-negative, GDP-locked variant of Rab4 (DN-Rab4S22N). Under these conditions, ISO-application failed to enhance CaV1.2 surface expression and instead a slight decrease in CaV1.2-tagRFP intensity and cluster density was observed in the PM over the course of the experiment (Fig. 2 G–I). These results confirm that Rab4a is part of the essential trafficking machinery that underlies the ISO-stimulated enhanced recycling of CaV1.2.
Since a subpopulation of CaV1.2 channels that localize to Rab11+ REs was also identified in AMVMs (Fig. 1 C and D), we tested the role of Rab11a in this dynamic recycling response to ISO using a dominant-negative, GDP-locked DN-Rab11aS25N. Despite impaired Rab11a function, an ISO-stimulated increase in CaV1.2-tagRFP intensity was still evident, albeit to a lesser extent than in controls (1.12-fold) (Fig. 2 J and K). This was accompanied by a 1.49-fold increase in cluster density (P = 0.009) (Fig. 2L). Accordingly, DN-Rab11a–mediated knock down of Rab11a activity generated ∼33% of the response seen in controls while knock down of Rab4a abolished the response (Fig. 2 H and M). These data suggest that upstream Rab4a activity is necessary, not only for fast recycling to the PM but also for transfer of CaV1.2 cargo from EEs to REs. Impaired Rab4a function creates a “road block” in the endosomal recycling system. The role of Rab4a in fast recycling was unmasked in cells with knocked down Rab11a activity, where τon of the stimulated recycling response was 0.96 ± 0.02 min (Fig. 2K), significantly faster than controls where both Rab4a and Rab11a were active (2.26 ± 0.02 min). A theoretical time course of the slow Rab11a-dependent contribution was calculated by subtracting the predominantly Rab4a-mediated recycling time-course in DN-Rab11a expressing cells from endogenous Rab controls (Fig. 2M). This theoretical Rab11a-dependent response displayed a 1.29-fold increase in CaV1.2-tagRFP intensity and was well-fit with a single exponential function with a τon = 2.69 ± 0.04 min that was slower than control or Rab4a-mediated responses. Based on these data, our results suggest that CaV1.2 channels stimulated to recycle to the PM in response to βAR activation are sourced approximately one-third from the fast Rab4a, and two-thirds from the slow Rab11a recycling pathways.
Actin and Microtubule Disruption Impairs ISO-Stimulated CaV1.2 Recycling.
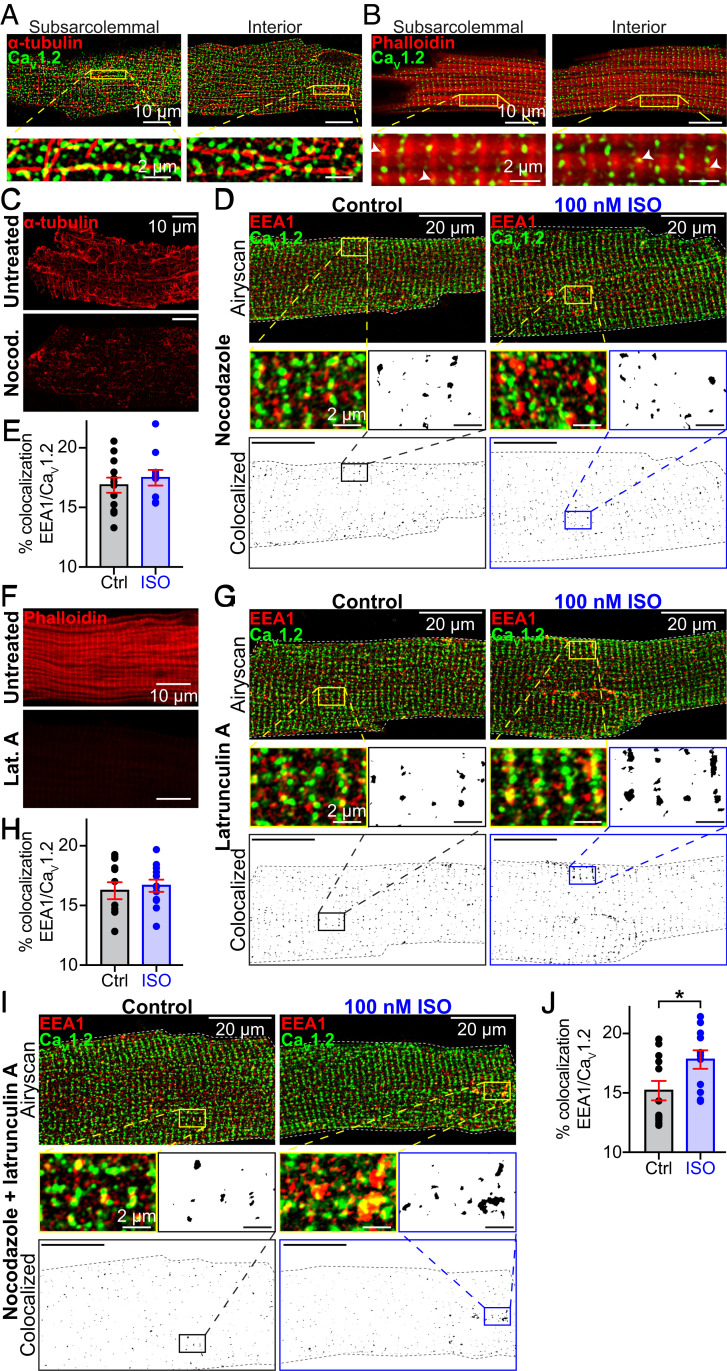
We used Airyscan superresolution microscopy to examine CaV1.2 channel proximity to microtubules (MTs) and actin filaments (Fig. 3 A and B). Immunostaining with α-tubulin revealed the extensive MT cytoskeleton with its lattice, grid-like appearance in the subsarcolemma, transitioning to a more longitudinally oriented network deeper in the cell interior (Fig. 3A). CaV1.2 channels decorate the MTs in both locations. Phalloidin-staining of the actin network revealed the periodic alignment of sarcomeric actin (Fig. 3B). It is notoriously difficult to visualize cortical actin in cardiomyocytes due to the overwhelming abundance of sarcomeric actin but in many locations CaV1.2 channels were colocalized with actin (Fig. 3B, white arrowheads). The degree of colocalization between CaV1.2 and, in particular MTs, implies a role for the cytoskeleton in regulating channel availability.
Fig. 3.
Actin and MT polymerization are essential for ISO-stimulated CaV1.2 recycling. Two-color Airyscan images of fixed AMVMs immunostained to examine the relative localization of CaV1.2 and (A) α-tubulin, or (B) phalloidin-stained actin. (C) The distribution of α-tubulin in untreated (Top) and nocodazole-treated AMVMs (Bottom). (D) Airyscan images of CaV1.2 and EEA1 distribution in nocodazole-treated control (n = 3, n = 12; Left) and ISO-stimulated (n = 3, n = 10; Right) AMVMs. (Bottom) Binary colocalization maps display pixels in which CaV1.2 and EEA1 expression precisely overlapped. (E) Histogram summarizing percent colocalization of EEA1 with CaV1.2 in nocodazole-treated cells. (F) Actin distribution in untreated (Top) and lat-A–treated cells (Bottom). (G) Airyscan images and binary colocalization maps of CaV1.2 and EEA1 distribution in lat-A–treated control (n = 3, n = 12; Left) and ISO-stimulated (n = 3, n = 12; Right) AMVMs. (H) Histogram showing percent colocalization of EEA1 with CaV1.2 in lat-A–treated AMVMs. (I) Airyscan images and binary colocalization maps of AMVMs treated with both lat-A and nocodazole under control (n = 3, n = 12) and ISO stimulated conditions (n = 3, n = 11), with accompanying summary histogram (J). Statistical analyses were unpaired t tests. *P < 0.05.
To study the role of these cytoskeletal highways in βAR-stimulated CaV1.2 mobilization from endosomes, we examined EEA1-localized CaV1.2 channel populations in AMVMs treated with cytoskeletal disruptors. Accordingly, freshly isolated myocytes were treated for 2 h with 10 μM nocodazole, a drug known to prevent addition of tubulin to dynamic MTs, and depolymerize the stable variety (20). Immunostaining with anti–α-tubulin confirmed the treatment had substantially disordered the MTs (Fig. 3C and SI Appendix, Fig. S4 A and B). In this and upcoming experimental series, we refer to cells that did not receive cytoskeletal disruptors as “untreated.” As in untreated cells (Fig. 1 A and B), CaV1.2 was observed to colocalize with a subpopulation of EEs (16.9 ± 0.6%) (Fig. 3 D and E). However, in cells stimulated with 100 nM ISO prior to fixation, the reduction in colocalization between EEA1 and CaV1.2 we had previously observed in untreated cells was absent in nocodazole-treated cells, instead remaining at 17.5 ± 0.7% (P = 0.51 compared to nocodazole-treated control). These data suggest that MT network disruption prevents the “emptying” of CaV1.2 channel cargo from the EEs into the sarcolemma and support a role for MTs and their associated motor proteins as conduits for ISO-stimulated CaV1.2 recycling.
We then examined the role of the actin cytoskeleton by disrupting it with latrunculin A (lat-A; 5 μM for 2 h), which facilitates F-actin depolymerization and prevents polymerization by sequestering actin monomers (20). Alexa Fluor 647-conjugated phalloidin staining of filamentous-actin (F-actin) was used to visually confirm lat-A–mediated actin disruption (Fig. 3F and SI Appendix, Fig. S4 C–E). In lat-A–treated myocytes, CaV1.2 and EEA1 colocalization was similar to untreated controls (16.2 ± 0.7% versus 15.1 ± 0.4%; P = 0.14). However, ISO-stimulation did not affect colocalization levels (16.7 ± 0.5%; P = 0.64) (Fig. 3 G and H). In addition, in cells cotreated with nocodazole and lat-A, ISO-stimulation actually promoted a small, but significant increase in colocalization between CaV1.2 and EEA1 (P = 0.03) (Fig. 3 I and J). These data indicate a profound alteration in the endosomal pathway where recycling and or endocytosis have been impaired, creating an “endosomal traffic jam,” leading to accumulation of cargo on the endosomes, with no cytoskeletal highways to transport the cargo to its destination.
Cytoskeletal Disruption Alters ISO-Stimulated CaV1.2 Dynamics and Recycling.
Real-time visualization and quantification of the effects of cytoskeletal disruption on channel trafficking was performed using transduced AMVMs isolated from mice that had received a retro-orbital injection of AAV9-CaVβ2a-paGFP. This auxiliary subunit of CaV1.2 binds to the pore-forming subunit with a 1:1 stoichiometry and acts in this context as a biosensor, reporting the location of the subset of CaV1.2 α1c it interacts with. This approach was previously validated by our group (17), with superresolution microscopy experiments confirming that CaVβ2a-paGFP and α1c colocalize, and unlike overexpression of α1c, at these concentrations we have found that CaVβ2a-paGFP transduction does not appreciably affect CaV1.2 α1c expression as indicated by unaltered basal channel cluster sizes. Additional validation performed for this study revealed the level of CaVβ2a-paGFP expression we achieve does not alter ICa inactivation kinetics or PKA modulation of the channels (Table 1 and SI Appendix, Fig. S5), as has been reported in previous studies with more robust overexpression (21, 22). Finally, since CaVβ2a can localize to the membrane independently of α1c (9, 21) we performed a final validation by comparing the time course of ISO-stimulated augmentation of CaVβ2a-paGFP expression in the TIRF footprint with that of ICa modulation. We fiound that the time course of the ISO-stimulated up-regulation in current density coincides with the CaVβ2a-paGFP–indicated increase in channel expression (SI Appendix, Fig. S5 G–J), supporting the use of CaVβ2a-paGFP as a proxy for α1c.
Table 1.
ISO-stimulated changes in peak ICa and voltage dependence of G/Gmax
| V1/2 (mV) | Slope factor | ||||
| Fold-change in peak ICa | Control | ISO | Control | ISO | |
| Untreated | 1.67 ± 0.19 | −7.57 ± 0.95 | −20.90 ± 1.14*** | 5.45 ± 0.84 | 4.90 ± 1.06 |
| Nocodazole | 1.18 ± 0.08* | −9.27 ± 0.42 | −15.97 ± 0.83*** | 5.00 ± 0.39 | 5.24 ± 0.71 |
| Latrunculin A | 1.39 ± 0.13 | −13.03 ± 0.87 | −18.95 ± 0.97*** | 5.51 ± 0.76 | 5.33 ± 0.88 |
| Noco + Lat.A | 1.01 ± 0.11** | −12.63 ± 1.40 | −15.16 ± 1.53 | 7.32 ± 1.24 | 7.82 ± 1.36 |
| Cavβ2a-paGFP | 2.01 ± 0.12 | 4.10 ± 1.10 | −9.55 ± 0.95*** | 10.27 ± 0.97 | 5.70 ± 0.86* |
Mean ± SEM values obtained for the fold-change peak ICa (one-way ANOVA with Dunnett’s multiple comparisons test; * indicates significant difference from the fold-change in untreated AMVMs), and for the V1/2 and slope factor of the voltage dependence of G/Gmax fits (one-way ANOVA). ***P < 0.001; **P < 0.01; *P < 0.05.
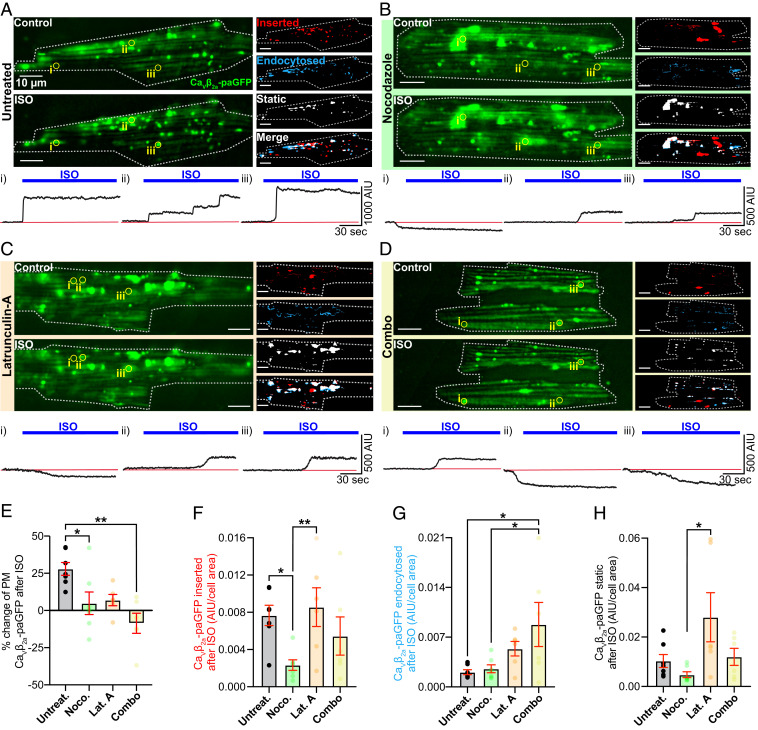
We examined the dynamic channel trafficking response to ISO in untreated AMVMs (i.e., in the absence of cytoskeletal disruptors) using TIRF microscopy. Discrete puncta of CaVβ2a-paGFP decorated the TIRF footprint of the myocyte during control frames and additional puncta/clusters were seen to appear in the TIRF footprint supplementing the initial complement after perfusion with 100 nM ISO (Fig. 4A and Movie S1). Given our endosome/CaV1.2 immunostaining results (Fig. 1), this may represent endosomal cargo mobilized in response to ISO from subsarcolemmal locations deeper within the cell. In agreement with this, Three-dimensional (3D)-plots of CaVβ2a-paGFP intensity over time and cell depth, constructed from four-dimensional (4D)-spinning disk confocal experiments performed on transduced AMVMs at 37 °C, indicated CaVβ2a-paGFP was mobilized from several microns within the cell, and moved toward the surface in response to ISO (SI Appendix, Fig. S6). At physiological temperature, the response to ISO proceeded monoexponentially with a τ = 4.12 s until CaVβ2a-paGFP intensity reached a plateau (SI Appendix, Fig. S6 C–E), presumably achieved when the endosomal pool of channels had been depleted and balance between insertion and endocytosis reached a new equilibrium.
Fig. 4.
Dynamic imaging to unmask the mobile channel population. (A) TIRF images of GFP fluorescence emission from Cavβ2a-paGFP transduced AMVMs before (Top) and after 100 nM ISO (Bottom). Images illustrating inserted, endocytosed, static, and merged channel populations are shown to the right. (Bottom) Time course of the changes in Cavβ2a-paGFP intensity in ROIs (i–iii) indicated by yellow circles on TIRF images (n = 5, n = 7). Same format for cells pretreated with (B) nocodazole (n = 5, n = 7), (C) lat-A (n = 3, n = 6), or (D) both cytoskeleton disruptors (n = 3, n = 6). (E–H) Histograms summarizing statistics for these experiments analyzed with Kruskal–Wallis tests. **P < 0.01; *P < 0.05.
To test the hypotheses that ISO treatment increases sarcolemmal expression of CaV1.2 by stimulating channel insertion/recycling, and that cytoskeletal highways carry these recycling channels to their destination, we performed “image math” (Materials and Methods) on TIRF time series to quantify the subpopulations of CaVβ2a-paGFP in the TIRF-footprint that were 1) inserted, 2) endocytosed, and 3) stably expressed during ISO stimulation. Responses to ISO in untreated AMVMs were compared to those in cells treated with nocodazole, lat-A, or a combination of both (Fig. 4). Live-cell time series experiments revealed a dynamic population of CaVβ2a-paGFP in all cells examined (Movies 1–4), although the dynamics were appreciably less in cells that received cytoskeletal disrupting treatments, supporting the idea that both F-actin and MTs are important conduits of this response. The number of channels at the sarcolemma at any given time, is dictated by the balance between channel insertions via the biosynthetic delivery and endosomal recycling pathways, and channel removals via endocytosis. In untreated cells, ISO stimulation heavily shifted the balance in favor of insertion, implying a stimulated insertion/recycling process (Fig. 4 A and E–G). This mismatch between insertion and endocytosis produced a 27.95 ± 4.31% increase in sarcolemmal CaVβ2a-paGFP expression (Fig. 4E). The time course of ISO-stimulated insertions of channels in untreated AMVMs can be observed in the regions-of-interest (ROIs) highlighted in Fig. 4A. Examination of these time courses revealed rapid step-like insertion profiles, suggesting that CaV1.2 appear to often insert into the sarcolemma as preformed clusters, containing many channels (Fig. 4 A, i–iii, Fig. 4 B, ii and iii, Fig. 4 C, ii and iii, and Fig. 4 D, i and ii). In some cases, a delivery hub was evident, where a succession of channel clusters appeared to insert one after the other (Fig. 4 A, ii and Fig. 4 B, iii). Channel endocytosis also appeared to involve removal of channel clusters in some instances (Fig. 4 C, i and Fig. 4 D, iii), while in other ROIs, a slower, gradual removal of potentially individual channels was more evident (Fig. 4 B, i).
Recycling of endosomal cargo back to the PM is known to rely on both MTs and actin (23). Indeed, cytoskeletal disruption reduced the magnitude of the ISO-stimulated augmentation of sarcolemmal CaVβ2a-paGFP expression compared to untreated cells (Fig. 4E). How the two elements of the cytoskeleton affected the ISO-stimulated change in sarcolemmal expression varied somewhat, as revealed by examination of insertion and endocytosis events in each AMVM cohort. Anterograde transport and targeting of CaV1.2 to the t-tubule membrane is known to occur along MTs, anchored there via the BAR-domain–containing protein, BIN1 (also known as amphiphysin II) (24). Here, our experiments were focused not on long-distance trafficking from the trans-Golgi to the membrane, but rather from the local endosome pool of channels and thus channel dynamics were observed over short 3-min periods before and after application of ISO. Our data indicate that nocodazole-mediated MT disruption reduced ISO-stimulated insertion of CaVβ2a-paGFP by an average of ∼70% compared to untreated cells (Fig. 4F). Channel internalization and the lifetime of channels in the membrane was not affected by the degree of MT disruption tested here, as both endocytosed and static channel population sizes were not significantly altered by this treatment (Fig. 4 G and H).
Actin disruption with lat-A in contrast had no significant effect on channel insertion compared to untreated cells (Fig. 4F). In neurons and HEK293 cells, interactions between CaV1.2 and the actin-interacting protein α-actinin have been found to stabilize CaV1.2 channel expression at the PM (25, 26). A notable trend toward increased endocytosis of CaVβ2a-paGFP in lat-A–treated cells was detected but this failed to reach significance assessed by a one-way ANOVA test (Fig. 4G). There was also a trending increase in the fraction of stable CaVβ2a-paGFP in the TIRF footprint that appeared to be left “stranded” there (Fig. 4H), perhaps reflective of incomplete internalization of CaVβ2a-paGFP due to the lack of actin dynamics and the disrupted cortical actin network. This effect was not statistically different from controls but was significantly different from nocodazole-treated cells. Finally, combined treatment with nocodazole and lat-A actually reduced the overall expression of CaVβ2a-paGFP in the TIRF footprint by 8.63 ± 6.76% over the course of the experiment (Fig. 4 F and G). This occurred when the balance of endocytosis and insertion/recycling shifted in favor of endocytosis. Collectively, these data suggest that MTs and actin are both important conduits of βAR-stimulated CaV1.2 recycling, with MTs playing the major role in channel insertion.
βAR Mediated ICa Regulation Is Abrogated by Cytoskeletal Disruption.
To ascertain whether ISO-stimulated recycling of CaV1.2 channels into the cardiomyocyte sarcolemma makes any functional contribution to the βAR regulation of these channels, we performed whole-cell patch-clamp recordings on freshly isolated AMVMs under various cytoskeleton disrupting conditions. Stimulation of untreated cardiomyocytes with 100 nM ISO, produced a 1.65 ± 0.19-fold enhancement of ICa (Fig. 5 A and B) and caused a 13.33 ± 1.49-mV leftward-shift in the voltage-dependence of conductance (measured as the difference between V1/2 of each fit; P < 0.0001) (Fig. 5C and Table 1). In addition, we observed an increase in the slope steepness of the Boltzmann function used to fit the G/Gmax data from 5.45 ± 0.84 in controls to 4.90 ± 1.06 in 100 nM ISO (Fig. 5C and Table 1), suggesting a potential increase in cooperative gating behavior (17).
Fig. 5.
βAR-mediated ICa enhancement is blunted by reducing dynamic channel insertion. (A) Whole-cell currents elicited from a representative AMVM during a 300-ms depolarization step from −40 mV to 0 mV before (control: black) and during application of 100 nM ISO (blue). (B) I-V plot summarizing the results from n = 7 cells (from n = 7 animals) subjected to test potentials ranging from −50 mV to +100 mV. Currents were normalized to cell capacitance to generate current density. (C) Voltage-dependence of the normalized conductance (G/Gmax) before and during ISO application, fit with Boltzmann functions. (D–F) Whole-cell currents (D), I-V (E), and G/Gmax plots (F) from AMVMs pretreated for 2 h with 10 μM nocodazole (n = 4, n = 7). (G, H) The same for cells treated with 5 μM lat-A (n = 5, n = 5). (J–L) Results from cells treated with a combination of both (n = 3, n = 6).
The impact of MT disruption on the ICa response to ISO was tested in AMVMs incubated with nocodazole. This dose and duration of treatment did not alter control ICa amplitude (compared to untreated control). Indeed, none of the cytoskeletal disrupting treatments had any significant effect on control ICa with all of them peaking between −3.78 and −4.07 pA/pF (Fig. 5 B, E, H, and K). However, nocodazole blunted the ICa response to ISO by ∼72% (1.18 ± 0.08-fold increase versus 1.65-fold change in untreated cells), halved the magnitude of leftward-shift of the voltage dependence of conductance (6.73 ± 1.38-mV versus the 13.33-mV shift in untreated cells), and eliminated the tendency toward cooperativity indicated by the slope of the Boltzmann function used to fit the G/Gmax (Fig. 5 D–F and Table 1).
We used the same experimental paradigm to test whether the actin cytoskeleton plays any role in the functional regulation of CaV1.2 by βARs. Cells treated with lat-A exhibited a 40% reduction in the ICa augmentation response to ISO compared to controls (Fig. 5 G and H and Table 1), and a similar halving of the leftward-shift in the voltage-dependence of conductance as in nocodazole-treated cells (5.92 ± 1.63 shift) (Fig. 5I and Table 1). ISO stimulation produced G/Gmax data that was well fit with a Boltzmann function that was less steep than in ISO-stimulated untreated cells but still indicated enhanced cooperativity (Fig. 5I and Table 1).
Finally, we examined the effect of ISO on ICa when both MTs and actin filaments were disrupted with a combined nocodazole and lat-A treatment. Under these conditions, βAR-mediated regulation of CaV1.2 was essentially abolished (Fig. 5 J and K), with ISO, generating only a 1.01 ± 0.11-fold increase in ICa, representing a 98% reduction in the response compared to untreated cells. The leftward-shift in the voltage-dependence of conductance was reduced to 2.54 ± 1.49 mV, equivalent to only 20% of the response seen in untreated cells. The slope factor of the Boltzmann-function used to fit the data were less steep than untreated cells in both control and ISO-stimulated conditions, indicating reduced cooperativity. These functional data collectively indicate that an intact cytoskeleton is an essential requirement for βAR-mediated regulation of CaV1.2.
Clustering of CaV1.2 Channels Is Supported by the Cytoskeleton.
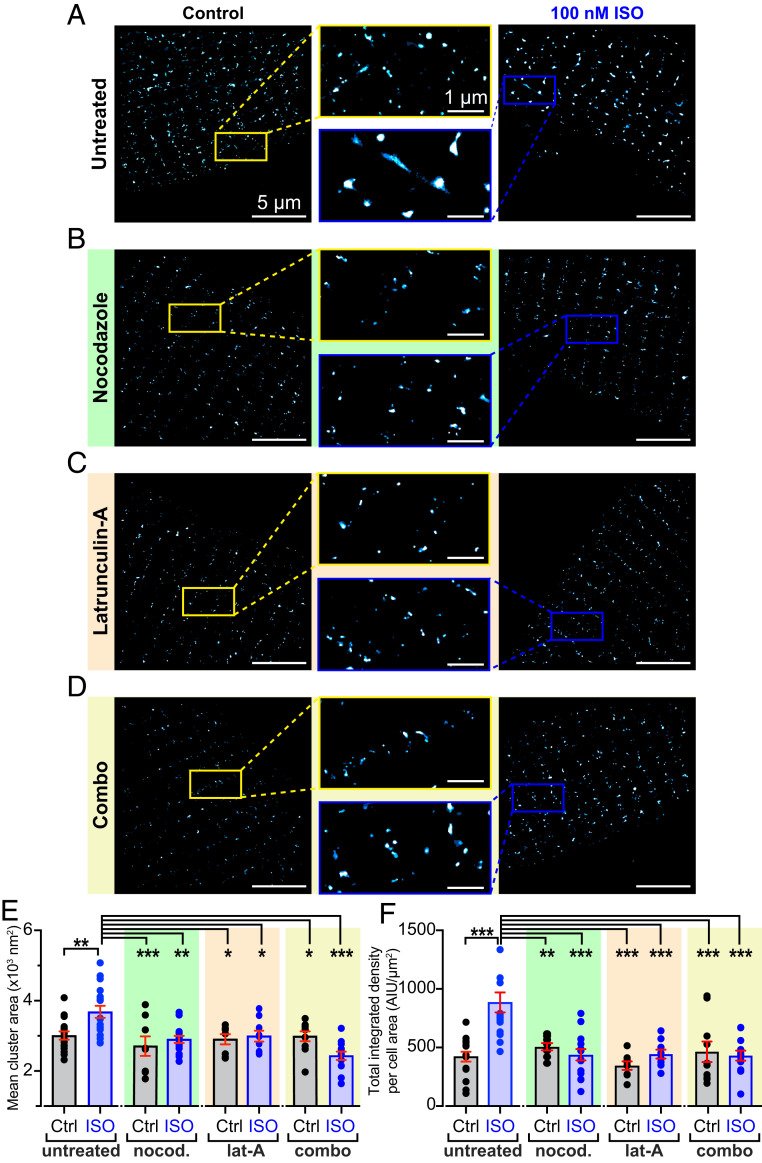
The observation that channel cooperativity was altered in AMVMs with disrupted cytoskeletal elements implies that the cytoskeleton might be important not only for βAR-mediated regulation of CaV1.2 but also for the stabilization and support of channel clusters. We tested this idea by examining CaV1.2 channel distribution in AMVMs using ground-state depletion (GSD) superresolution nanoscopy. Furthermore, since PKA is known to stimulate ICa more robustly at the t-tubules compared to the crest due to better coupling between βARs and the channels located there (27, 28), we investigated whether ISO-stimulated insertions occurred preferentially in the t-tubules by examining and comparing the CaV1.2 populations in the t-tubule and crest regions of the sarcolemma. ISO-stimulation resulted in the formation of CaV1.2 channel superclusters in AMVMs, which in the t-tubules were on average 22.5% larger (Fig. 6 A and E), and in the crest, 25.7% larger than the clusters in control AMVMs (SI Appendix, Fig. S7 A and B). Superclustering could occur due to small clusters fusing together to form larger ones, or alternatively, could reflect enhanced sarcolemmal expression of CaV1.2. If the superclusters form because of enhanced insertion/exocytosis of CaV1.2 into the sarcolemma, then a testable prediction is that the intensity of the fluorescence emission (normalized to the cell area) should be increased. In contrast, if the superclusters simply reflect fusion of existing sarcolemmal CaV1.2 clusters then the total fluorescence intensity should be similar in control and ISO-treated cells. Accordingly, in the t-tubules, normalized total integrated density was significantly larger in ISO-treated cells than controls (Fig. 6F) (P < 0.001), in agreement with the idea that βAR-activation stimulates enhanced sarcolemmal insertion and resultant superclustering of CaV1.2 channels. Interestingly, in the sarcolemmal crest, total integrated density was unaltered by ISO, suggesting small clusters there may have fused together to form larger clusters (SI Appendix, Fig. S7D). This was supported by a rightward shift of the cumulative frequency distribution of crest channel cluster areas after ISO (SI Appendix, Fig. S7G). Based on these observations, the t-tubule sarcolemma appears to be the main site of ISO-stimulated CaV1.2 insertions.
Fig. 6.
βAR-stimulated CaV1.2 superclustering and enhanced sarcolemmal expression requires an intact MT cytoskeleton. Superresolution GSD localization maps of control (Left) and 100 nm ISO‐stimulated (Right), fixed, AMVMs immunostained to examine CaV1.2 channel distribution under (A) untreated (control: n = 4, n = 16; ISO: n = 4, n = 17), (B) nocodazole-treated (control: n = 3, n = 8; ISO: (n = 3, n = 15), (C) lat-A–treated (control: n = 3, n = 8; ISO: (n = 3, n = 9), and (D) combo-treated (control: n = 2, n = 10; ISO: (n = 3, n = 13) conditions. Maps were pseudocolored “cyan hot” and received a one-pixel median filter for display purposes. Yellow (control) and blue (ISO) boxes indicate the location of the zoomed‐in regions displayed in the center. (E and F) Aligned dot plots showing mean CaV1.2 channel cluster areas and normalized total integrated density in each condition. Two-way ANOVA. ***P < 0.001; **P < 0.01; *P < 0.05.
Cytoskeletal disruption with nocodazole (Fig. 6B), lat-A (Fig. 6C), or a combination of the two (Fig. 6D), did not affect basal channel expression in the t-tubules, as indicated by similar cluster areas and total integrated density values (Fig. 6 E and F). These results validate the unaltered ICa observed in unstimulated cells from each of our four experimental groups (Fig. 5) and supports the postulate that the lifetime of these channels in the membrane is longer than the 2-h cytoskeletal disruption period. In addition, ISO-stimulation failed to induce superclustering or enhanced t-tubule membrane expression of CaV1.2 in AMVMs (Fig. 6 E and F). Altogether, these data suggest that both intact MTs and actin are necessary for the formation of CaV1.2 super clusters in response to ISO stimulation.
Discussion
The data presented in the present study provide a report of an endosomal pool of CaV1.2 channels in cardiomyocytes that undergoes rapid, targeted mobilization to the t-tubule sarcolemma in response to βAR-activation, effectively creating an “on-demand” trafficking pathway to facilitate a positive inotropic response during fight-or-flight. We present six major findings: 1) Intracellular pools of CaV1.2 channels are present on EEs, REs, and in LEs and lysosomes in AMVMs; 2) βAR-activation triggers CaV1.2 mobilization from EEs and REs to the sarcolemma via Rab4a-dependent fast, and Rab11a-dependent slow recycling pathways; 3) CaV1.2 are often inserted or removed from the sarcolemma as large multichannel clusters, rather than individual channels; 4) stimulated insertion of CaV1.2 channels occurs along MTs; 5) the endosomal pool of CaV1.2 is fueled by actin-dependent endocytosis; and finally, 6) adrenergic regulation of CaV1.2 is abrogated by cytoskeletal disruption and loss of this dynamic recycling response. On the basis of these data, we present a working model (SI Appendix, Fig. S1) for βAR-regulation of cardiac CaV1.2 channels in which stimulated recycling of CaV1.2, from subsarcolemmal pools of Rab4a+ and Rab11a+ endosomes, results in enhanced expression of CaV1.2 at the t-tubule membrane of AMVMs. Resultant superclustering and cooperative gating of CaV1.2 channels contributes to the enhanced ICa and inotropic response.
Our data revealed pools of intracellular CaV1.2 channels on EEA1 and Rab4+ EEs, on Rab11+ REs, and in Rab7+ LEs and lysosomes. In response to ISO, EE- and RE-localized channels underwent rapid recycling into the sarcolemma via the Rab4a-dependent fast-recycling pathway and the slower, Rab11a recycling pathway. While this report of stimulated recycling of a recruitable intracellular reservoir of CaV1.2 in cardiomyocytes is unique, small GTPase choreographed-recycling of endosome-localized ion channel pools are well-known to play a role in fine-tuning cellular responses to various stimuli including βAR stimulation. For example, in neurons, intracellular AMPA receptors (AMPAR) located on REs undergo Rab11-dependent recycling to the PM of dendritic spines in response to PKA-mediated phosphorylation of their GluA1 subunit at S845 downstream of β2AR stimulation (reviewed in ref. 29). In the collecting ducts of the kidney, vasopressin release initiates a Gs-coupled signaling cascade that triggers PKA-mediated phosphorylation of aquaporin-2 (AQP2) at S256 and consequent recycling of AQP2 from Rab11+ REs to the apical PM (30, 31). In the heart, acute stress initiates Rab11-dependent mobilization of endosomal reservoirs of SUR2-containing KATP channels and of KCNQ1-containing REs to the sarcolemma (32–34). Similarly, here we report an endosomal pool of CaV1.2 channels that undergoes “on-demand” stimulated recycling upon activation of βARs, providing a functional reserve that drives ventricular inotropy during sympathetic stimulation.
Our work on live, AAV9-CaVβ2a–transduced AMVMs provides intriguing insights into CaV1.2 channel trafficking, and captures the complex dynamics of these channels. We find that these channels are often inserted into the sarcolemma as entire preformed clusters at nucleation sites. Sometimes, repetitive insertions were seen to occur at an individual site, conjuring an image of CaV1.2-carrying endosomes queued up along MTs, anchored at a sarcolemmal delivery hub. Furthermore, channel endocytosis often appeared to occur via removal of entire clusters, while in other cases, a slower, gradual removal of channels suggested ongoing removal of individual channels. Activation of βARs was observed to increase the probability of channel insertion. These scenarios were predicted in a recently published computer model designed to test the hypothesis that ion channel clustering occurs via a stochastic self-assembly process (35). Our data provide answers to the hypotheticals raised by that model, informing that model parameters with the experimental data acquired in this study would be an interesting sequel.
One well-characterized facet of cardiac CaV1.2 channel trafficking is their targeted anterograde-delivery to the t-tubule membrane along BIN1-anchored MTs via the biosynthetic delivery pathway (24). Reduced levels of BIN1 in cardiomyocytes isolated from failing human hearts are associated with impaired CaV1.2 channel delivery and slower onset calcium transients (7). Here, we find that CaV1.2 channel recycling also occurs along MTs. Three independent lines of evidence support this conclusion. First, MT disruption prevented ISO-stimulated mobilization of CaV1.2 from EE pools (Fig. 3D). Second, MT disruption significantly reduced stimulated channel insertions in AAV9-CaVβ2a-paGFP transduced AMVMs (Fig. 4F and Movie S2). Third, ISO-stimulated CaV1.2 superclustering was absent in nocodazole-treated AMVMs (Fig. 6 B and E). Our findings that basal CaV1.2 channel distribution and ICa amplitude were unaffected by 2-h nocodazole treatment suggests that channel lifetime in the membrane is longer than 2 h, so that channels delivered along intact MTs prior to depolymerization by the drug, still largely remained there (Fig. 6B). This agrees with previous measurements of PM CaV1.2 channel half-times of ∼3 h (9). However, despite negligible effects on basal channel expression and function, inhibition of MT polymerization significantly reduced ISO-stimulated responses, blunting ICa augmentation, and eliminating enhanced channel recycling and resultant superclustering. Reduced MT polymerization occurs in human heart failure where stabilized MTs form a dense network in cardiomyocytes (36, 37). The lack of polymerization and potentially enhanced MT catastrophe rates can result in traffic jams along MTs, leading to defective cargo delivery (36). Indeed, a previous study on live ventricular myocytes reported reduced delivery of KV4.2 and KV4.3 channels to the sarcolemma due to increased MT catastrophe rates upon addition of hydrogen peroxide or in the reactive oxygen species-rich postmyocardial infarction environment (38). Failing and aging myocytes display reduced CaV1.2 responsivity to ISO (39, 40), thus future studies should examine whether loss of adrenergic responsivity of CaV1.2 in heart failure occurs due to impaired channel trafficking and recycling along MTs.
Actin polymerization has also been reported to be an important determinant of cardiac ion channel trafficking, notably of Cx43 (41). Although we failed to visualize cortical F-actin because of the vast amount of sarcomeric actin in AMVMs, we found that actin disruption with lat-A led to reduced: 1) ISO-stimulated mobilization of CaV1.2 from EEs (Fig. 3H), and 2) augmentation of CaV1.2 channel expression in the PM, as indicated by superresolution GSD imaging experiments (Fig. 6C) and live-cell TIRF experiments on AAV9-CaVβ2a-paGFP–transduced AMVMs (Fig. 4E). Furthermore, lat-A significantly blunted adrenergic regulation of the channels assessed with whole-cell patch clamp (Fig. 5 G–I and Table1). The degree of apparent channel insertions in response to ISO remained at a similar level to untreated cells, suggesting that MTs, not actin, play the dominant role in channel delivery to the sarcolemma (Fig. 4F). However, the pool of channels that was mobilized to the membrane in the presence of lat-A did not appear to belong to the EE pool, since colocalization between EEA1 and CaV1.2 channels was unchanged by ISO. In the Cx43 literature, it has been proposed that Cx43 cargo on its way to the sarcolemma from the Golgi along MTs, pauses at actin “rest stops” before being handed off to additional MTs to complete its journey to the membrane (41). It is possible that a similar rest stop system exists for CaV1.2 channel delivery in cardiomyocytes and that lat-A–mediated actin disruption and ISO-stimulation releases this pool, allowing them to traffic to the sarcolemma along MTs. This intriguing hypothesis remains to be proven.
On the basis of our functional patch-clamp data, it is tempting to speculate that βAR-mediated regulation of CaV1.2 is heavily dependent on this stimulated channel recycling pathway; however, ISO-stimulation is also known to induce endocytosis and subsequent fast, actin-dependent recycling and resensitization of the receptors themselves (42, 43). It is therefore possible that the lack of functional response is simply because of a lack of sarcolemmal βAR expression, as internalized receptors cannot recycle back to the membrane (44). To investigate this possibility, we bypassed the receptors and stimulated adenylyl cyclase directly with forskolin (SI Appendix, Fig. S8). Forskolin (1 μM) produced a similar increase in sarcolemmal CaVβ2a-paGFP expression (21.60 ± 3.72%) to that observed with 100 nM ISO (27.95 ± 4.31%). Treatment of AMVMs with cytoskeletal disruptors reduced the response (SI Appendix, Fig. S8 B–E). Further examination of βAR-stimulation of cAMP production using an PM-targeted FRET-based cAMP sensor (lynICUE3) (45) revealed no significant alteration with cytoskeletal disruptors (SI Appendix, Fig. S9). These data suggest that the effects of cytoskeletal disruptors on channel responses to adrenergic stimulation are unlikely to be caused by less robust βAR-signaling and cannot explain the observed abolition of βAR-regulation of ICa (Fig. 5). It is also possible that the morphology and composition of the PM could be altered by cytoskeletal disruptors. While we cannot completely exclude this, superresolution images did not expose any overt alteration in t-tubule regularity, and capacitance measurements (SI Appendix, Fig. S5F) made during whole-cell recordings revealed no significant change in membrane area with any of the treatments. Overall, these results suggest that agonist-stimulated recycling of CaV1.2 is in fact a critical component of βAR-regulation of these channels.
The critical PKA phosphorylation site on the cardiac CaV1.2 channel complex was recently reported to be located on Rad, a member of the Rad/Rem/Rem2/Gem/Kir (RGK) family of monomeric GTP-binding proteins that interacts with the channel via the β-subunit (4). In a disinhibition process, Rad phosphorylation is proposed to dissociate from the channel complex, releasing its inhibitory hold on the channel and unveiling the hallmark larger ICa during βAR-activation. A Rad-mediated CaV1.2 disinhibition hypothesis was also proposed several years earlier by Jonathan Satin’s group (46) when they reported that Rad knockout mice are refractory to adrenergic receptor stimulation.
So how does our βAR-stimulated recycling of CaV1.2 fit into this appealing model? We do not believe the two models are mutually exclusive but instead hypothesize that Rad inhibits CaV1.2 channel function by limiting its expression at the sarcolemma, an effect that is relieved when Rad is phosphorylated by PKA. Indeed, in addition to the ability of Rad to suppress channel activity by interfering with channel Po, it has long been reported that RGK-proteins, including Rad, also reduce ICa by limiting CaV1.2 expression at the sarcolemma (47, 48). Our results in transduced AMVMs and tsA cells illustrate that ISO-stimulates enhanced transport of channels to the PM. We speculate that this transport could occur when Rad is phosphorylated and dislodges from CaVβ, releasing the channel complex and allowing more to traffic to the surface in a Rab4a- and Rab11a-dependent process. Phosphorylation of Rad may be the “upstream step” that has to occur before this recycling process is initiated, explaining why overexpression of CA-Rab4a does not raise the initial expression of CaV1.2 in the membrane in and of itself, but rather Rad phosphorylation must occur first. The resultant increase in the number of channels in the PM would be predicted to up-regulate ICa, since , where (N) is the number of channels, (Po) is their open probability, and (iCa) is their single-channel current.
We have previously reported that βAR-stimulation with ISO facilitates cooperative interactions between adjacent channels, amplifying Ca2+ influx (17). In addition, there is evidence that gating of cooperatively interacting channels is driven by the highest activity channels in the cluster (49). The relationship between ISO-stimulated channel insertions and current density augmentation thus may not be linear, since stimulated insertion of a small number of phosphorylated, Rad-dissociated, high Po channels could have a disproportionately large effect on ICa, driving associated channels to a higher Po, and generating the signature increase in ICa and Po of PKA-regulation of CaV1.2.
How PKA is anchored next to Rad on endosomes is another matter but may depend on the A-kinase–anchoring protein D-AKAP2, which has been reported to regulate recycling of transferrin receptors via interactions with Rab4 and Rab11 (50). In line with that prediction, a human functional polymorphism in D-AKAP2 (I646V) is associated with reduced heart rate variability, indicative of a heart that cannot respond well to stressors (51). D-AKAP2 itself can be phosphorylated by PKA at residue 554 (50), and this may influence its localization. We briefly tested the hypothesis that ISO-stimulation of βARs would promote enhanced colocalization between D-AKAP2 and Rab11+ endosomes finding an enhanced association (SI Appendix, Fig. S10). While this is admittedly a correlative result, resolving these mechanistic details will make for an interesting future project.
Overall, our data indicate that cardiomyocytes have an endosomal reservoir of CaV1.2 channels that is rapidly mobilized to the t-tubule sarcolemma in response to βAR-stimulation, and that this stimulated insertion is fundamentally required for βAR-regulation of these channels.
Materials and Methods
Detailed methods can be found in SI Appendix. Briefly, AMVMs were enzymatically isolated using standard Langendorff technique as described previously (17). Fixed, immunostained AMVMs were imaged on a Zeiss Airyscan confocal microscope or a Leica 3D-GSD-SR microscope to assess the distribution of CaV1.2 channels and various endosome populations. Live-cell TIRF imaging experiments of AAV9-CaVβ2a-paGFP–transduced AMVMs or transiently transfected tsA-201 cells were performed on an Olympus IX-83 inverted microscope with a Cell-TIRF MITICO module. Live-cell 4D-imaging of transduced AMVMs was performed at 37 °C on an Andor W-1 Spinning Disk confocal microscope. Rab mutant plasmids used for transient transfection of tsA-201 cells were gifts from Nipavan Chiamvimonvat, University of California, Davis, CA, and Jose A. Esteban, Centro de Biología Molecular ‘Severo Ochoa’, Consejo Superior de Investigaciones Científicas-Universidad Autónoma, Madrid, Spain.
Supplementary Material
Acknowledgments
We thank Dr. Luis Fernando Santana for the use of his Ground-State Depletion microscope, and for reading and commenting on this manuscript; and Dr. Yang K. Xiang for the use of his FRET system. This work was supported by NIH National Institute on Aging Grant R01AG063796 and American Heart Association Grant 15SDG25560035 (to R.E.D.); National Institute of General Medical Sciences Grant R01GM127513 (to E.J.D.); and National Heart, Lung, and Blood Institute Grants R01HL121059 and R01HL149127 (to M.F.N.). T.L.V. and H.C.S. were supported by a National Institute of General Medical Sciences-funded Pharmacology Training Program (T32GM099608).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2017937118/-/DCSupplemental.
Data Availability.
All study data are included in the article and supporting information.
References
- 1.Reuter H., Scholz H., The regulation of the calcium conductance of cardiac muscle by adrenaline. J. Physiol. 264, 49–62 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yue D. T., Herzig S., Marban E., Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc. Natl. Acad. Sci. U.S.A. 87, 753–757 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperelakis N., Schneider J. A., A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am. J. Cardiol. 37, 1079–1085 (1976). [DOI] [PubMed] [Google Scholar]
- 4.Liu G., et al. , Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J., et al. , Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovasc. Res. 49, 298–307 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Josephson I. R., Guia A., Stern M. D., Lakatta E. G., Alterations in properties of L-type Ca channels in aging rat heart. J. Mol. Cell. Cardiol. 34, 297–308 (2002). [DOI] [PubMed] [Google Scholar]
- 7.Hong T. T., et al. , BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm 9, 812–820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pranke I., Golec A., Hinzpeter A., Edelman A., Sermet-Gaudelus I., Emerging therapeutic approaches for cystic fibrosis. From gene editing to personalized medicine. Front. Pharmacol. 10, 121 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien A. J., et al. , Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J. Biol. Chem. 270, 30036–30044 (1995). [DOI] [PubMed] [Google Scholar]
- 10.Conrad R., et al. , Rapid turnover of the cardiac L-type CaV1.2 channel by endocytic recycling regulates its cell surface availability. iScience 7, 1–15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buda P., et al. , Eukaryotic translation initiation factor 3 subunit e controls intracellular calcium homeostasis by regulation of cav1.2 surface expression. PloS One 8, e64462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenmark H., Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Maxfield F. R., McGraw T. E., Endocytic recycling. Nat. Rev. Mol. Cell Biol. 5, 121–132 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Green E. M., Barrett C. F., Bultynck G., Shamah S. M., Dolmetsch R. E., The tumor suppressor eIF3e mediates calcium-dependent internalization of the L-type calcium channel CaV1.2. Neuron 55, 615–632 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Best J. M., et al. , Small GTPase Rab11b regulates degradation of surface membrane L-type Cav1.2 channels. Am. J. Physiol. Cell Physiol. 300, C1023–C1033 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh D., et al. , Dynamic L-type CaV1.2 channel trafficking facilitates CaV1.2 clustering and cooperative gating. Biochim. Biophys. Acta Mol. Cell Res. 1865, 1341–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito D. W., et al. , β-Adrenergic-mediated dynamic augmentation of sarcolemmal CaV 1.2 clustering and co-operativity in ventricular myocytes. J. Physiol. 597, 2139–2162 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atwood B. K., Lopez J., Wager-Miller J., Mackie K., Straiker A., Expression of G protein-coupled receptors and related proteins in HEK293, AtT20, BV2, and N18 cell lines as revealed by microarray analysis. BMC Genomics 12, 14 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cormont M., et al. , Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol. Cell Biol. 16, 6879–6886 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calaghan S. C., Le Guennec J. Y., White E., Cytoskeletal modulation of electrical and mechanical activity in cardiac myocytes. Prog. Biophys. Mol. Biol. 84, 29–59 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Takahashi S. X., Mittman S., Colecraft H. M., Distinctive modulatory effects of five human auxiliary β2 subunit splice variants on L-type calcium channel gating. Biophys. J. 84, 3007–3021 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miriyala J., Nguyen T., Yue D. T., Colecraft H. M., Role of CaVbeta subunits, and lack of functional reserve, in protein kinase A modulation of cardiac CaV1.2 channels. Circ. Res. 102, e54–e64 (2008). [DOI] [PubMed] [Google Scholar]
- 23.Grant B. D., Donaldson J. G., Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong T. T., et al. , BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 8, e1000312 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall D. D., et al. , Competition between α-actinin and Ca2+-calmodulin controls surface retention of the L-type Ca2+ channel Ca(V)1.2. Neuron 78, 483–497 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng P. Y., et al. , α-Actinin promotes surface localization and current density of the Ca2+ channel CaV1.2 by binding to the IQ region of the α1 subunit. Biochemistry 56, 3669–3681 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chase A., Colyer J., Orchard C. H., Localised Ca channel phosphorylation modulates the distribution of L-type Ca current in cardiac myocytes. J. Mol. Cell. Cardiol. 49, 121–131 (2010). [DOI] [PubMed] [Google Scholar]
- 28.Balijepalli R. C., Foell J. D., Hall D. D., Hell J. W., Kamp T. J., Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for β(2)-adrenergic regulation. Proc. Natl. Acad. Sci. U.S.A. 103, 7500–7505 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diering G. H., Huganir R. L., The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nedvetsky P. I., et al. , A Role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 8, 110–123 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Fushimi K., Sasaki S., Marumo F., Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J. Biol. Chem. 272, 14800–14804 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Bao L., Hadjiolova K., Coetzee W. A., Rindler M. J., Endosomal KATP channels as a reservoir after myocardial ischemia: A role for SUR2 subunits. Am. J. Physiol. Heart Circ. Physiol. 300, H262–H270 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., et al. , [Ca2+]i elevation and oxidative stress induce KCNQ1 protein translocation from the cytosol to the cell surface and increase slow delayed rectifier (IKs) in cardiac myocytes. J. Biol. Chem. 288, 35358–35371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seebohm G., et al. , Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ. Res. 100, 686–692 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Sato D., et al. , A stochastic model of ion channel cluster formation in the plasma membrane. J. Gen. Physiol. 151, 1116–1134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caporizzo M. A., Chen C. Y., Prosser B. L., Cardiac microtubules in health and heart disease. Exp. Biol. Med. 244, 1255–1272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C. Y., et al. , Suppression of detyrosinated microtubules improves cardiomyocyte function in human heart failure. Nat. Med. 24, 1225–1233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drum B. M., et al. , Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J. Mol. Cell. Cardiol. 93, 32–43 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouadid H., Albat B., Nargeot J., Calcium currents in diseased human cardiac cells. J. Cardiovasc. Pharmacol. 25, 282–291 (1995). [DOI] [PubMed] [Google Scholar]
- 40.Strait J. B., Lakatta E. G., Aging-associated Cardiovascular changes and their relationship to heart failure. Heart Failure Clinics 8, 143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth J. W., et al. , Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ. Res. 110, 978–989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanyaloglu A. C., von Zastrow M., Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu. Rev. Pharmacol. Toxicol. 48, 537–568 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Yudowski G. A., Puthenveedu M. A., Henry A. G., von Zastrow M., Cargo-mediated regulation of a rapid Rab4-dependent recycling pathway. Mol. Biol. Cell 20, 2774–2784 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Millman E. E., et al. , Rapid recycling of beta-adrenergic receptors is dependent on the actin cytoskeleton and myosin Vb. Traffic 9, 1958–1971 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DiPilato L. M., Cheng X., Zhang J., Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. U.S.A. 101, 16513–16518 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manning J. R., et al. , Rad GTPase deletion increases L-type calcium channel current leading to increased cardiac contraction. J. Am. Heart Assoc. 2, e000459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Béguin P., et al. , Nuclear sequestration of beta-subunits by Rad and Rem is controlled by 14-3-3 and calmodulin and reveals a novel mechanism for Ca2+ channel regulation. J. Mol. Biol. 355, 34–46 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Béguin P., et al. , Regulation of Ca2+ channel expression at the cell surface by the small G-protein kir/Gem. Nature 411, 701–706 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Dixon R. E., Yuan C., Cheng E. P., Navedo M. F., Santana L. F., Ca2+ signaling amplification by oligomerization of L-type Cav1.2 channels. Proc. Natl. Acad. Sci. U.S.A. 109, 1749–1754 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eggers C. T., Schafer J. C., Goldenring J. R., Taylor S. S., D-AKAP2 interacts with Rab4 and Rab11 through its RGS domains and regulates transferrin receptor recycling. J. Biol. Chem. 284, 32869–32880 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neumann S. A., et al. , AKAP10 (I646V) functional polymorphism predicts heart rate and heart rate variability in apparently healthy, middle-aged European-Americans. Psychophysiology 46, 466–472 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and supporting information.