Abstract
Background:
Fine particulate matter (PM2.5) has been consistently linked to cardiovascular disease (CVD). Although studies have reported modification by income, to our knowledge no study to date has examined this relationship among adults in Medicaid, which provides health coverage to low-income and/or disabled Americans.
Methods:
We estimated the association between short-term PM2.5 exposure (average of PM2.5 on the day of hospitalization and the preceding day) and CVD admissions rates among adult Medicaid enrollees in the continental US (2000–2012) using a time-stratified case–crossover design. We repeated this analysis at PM2.5 concentrations below the World Health Organization daily guideline of 25 μg/m3. We compared the PM2.5 – CVD association in the Medicaid ≥65 years old vs. non-Medicaid-eligible Medicare enrollees (≥65 years old).
Results:
Using information on 3,666,657 CVD hospitalizations among Medicaid adults we observed a 0.9% (95%CI: 0.6, 1.1%) increase in CVD admission rates per 10 μg/m3 PM2.5 increase. The association was stronger at low PM2.5 levels (1.3%; 95%CI: 0.9, 1.6%). Among Medicaid enrollees ≥65 years old, the association was 0.9% (95%CI: 0.6, 1.3%) vs. 0.8% (95%CI: 0.6, 0.9%) among non-Medicaid-eligible Medicare enrollees ≥65 years old.
Conclusion:
We found robust evidence of an association between short-term PM2.5 and CVD hospitalizations among the vulnerable subpopulation of adult Medicaid enrollees. Importantly, this association persisted even at PM2.5 levels below the current national standards.
Keywords: Case–crossover, Cardiovascular, PM2.5, Short-term exposure, Medicaid, Medicare, Fine particles, Air pollution
Introduction
Ambient fine particulate matter pollution (PM2.5; particulate matter with aerodynamic diameter ≤ 2.5 μm) has been consistently shown to be associated with cardiovascular morbidity and mortality. 1–7 Several possible mechanisms have been proposed by which short-term exposure to PM2.5 may lead to cardiovascular disease (CVD): PM2.5, with its high alveolar penetration capacity, can trigger systemic inflammation and oxidative stress, can alter sympathetic/parasympathetic balance, and can increase clotting factors leading to CVD 1–3, one of the leading causes of mortality and morbidity globally.8,9
The Clean Air Act requires that the National Ambient Air Quality Standards (NAAQS) in the United States protect “sensitive subgroups,” including—but not limited to—socioeconomically disadvantaged individuals. Socio-economic status (SES) and access to social support are fundamental drivers of health disparity; disadvantaged individuals and communities systematically lack access to resources that protect and improve health.10 Furthermore, disadvantaged communities experience higher exposures to environmental hazards,11–14 and may be more vulnerable to the effects of such hazards.15 In the United States, environmental risk varies spatially, with documented stark racial or ethnic and socioeconomic disparities in exposure to air pollution.11–13 Socio-economically disadvantaged Americans, thus, are a particularly sensitive subgroup that the NAAQS are designed to protect.
The paucity of studies characterizing the PM2.5 – CVD association among socio-economically disadvantaged Americans motivates the focus of our study on Medicaid enrollees. Medicaid is a joint federal–state program that provides health or nursing home coverage to certain categories of low-income Americans, including children, pregnant women, parents of eligible children, people with disabilities, and elderly needing nursing home care. Although Medicaid eligibility criteria vary by state and population groups (e.g., by children, pregnant women, families of different sizes), they are based on: (a) income level in comparison to the federal poverty line; and/or (b) disability; and/or (c) substantial medical needs. Previous studies have found that those enrolled in Medicaid are at higher risk of respiratory hospitalizations from increased air pollution exposures relative to individuals with private insurance.16,17 In addition, low-income Americans have the highest prevalence of CVD.18 These reasons, in combination with the increased detrimental environmental exposures among low-SES populations,11–14 further motivate our focus on the Medicaid cohort.
To our knowledge, this is the first study to estimate the association between short-term exposure to PM2.5 and total and cause-specific CVD hospitalizations, among adult Medicaid enrollees, for the entire continental United States (2000–2012). We focused our analyses on PM2.5—a mixture of solid particles and liquid droplets that can be primarily emitted by numerous sources, including but not limited to traffic, industrial processes, wildfires, etc, and secondarily formed in the atmosphere19—as it is an indicator of the air pollution mixture at each location. Previous studies of other populations have reported how factors such as race or ethnicity, sex, and age modify the relationship between PM2.5 and CVD hospitalization rate.20,21 We have, therefore, assessed if these factors are also important effect modifiers in the adult Medicaid population. We also compared the association between short-term PM2.5 exposure and total CVD in Medicaid enrollees aged 65 years and older with that of corresponding enrollees in Medicare (a national health insurance program that provides health insurance for Americans aged 65 years and older) who are not eligible for Medicaid. Eligibility for Medicaid is based on income and/or disability status, whereas eligibility for Medicare is primarily based on age. Therefore, a side-by-side comparison of the PM2.5–CVD association in these two populations provides information about how income may modify vulnerability to air pollution exposure among the elderly. Finally, we estimated the association between PM2.5 and CVD hospitalizations in the adult Medicaid cohort at low PM2.5 concentrations (≤ 25 μg/m3), the World Health Organization (WHO) guideline for the daily average PM2.5.22
Methods
Study Population
From the Center for Medicare and Medicaid (CMS)—the two largest health insurance providers in the US—we obtained access to two nationally representative open cohorts for the period 2000–2012: all fee-for-service (FFS) full-benefit Medicaid enrollees of all ages (low-income and disabled Americans) and all FFS Medicare enrollees (aged 65 years and older). Information on Medicaid enrollees was not available for Maine from 2005–2010 and for Kansas for 2010. We have, thus, also removed this information from the Medicare dataset from our analysis to facilitate comparison.
Both Medicaid and Medicare datasets provide demographic information, including age, sex, race/ethnicity, and residential ZIP Code. We focused our analyses on adult Medicaid enrollees (aged 18 years and older). For Medicaid enrollees this information is recorded at each hospitalization visit; if an enrollee suffered multiple hospitalizations, we extracted the demographic information from a random hospitalization record for that enrollee for our analyses.
Medicaid enrollees, unlike in Medicare, have to re-enroll themselves in the program on an annual basis. Therefore, individuals may leave and re-enter the program over the years. This can lead to intermittent enrollment if eligibility criteria are not satisfied every year, resulting in periods during which the health status of some individuals is not recorded in Medicaid claims.
All individuals aged 65 year and older are eligible for Medicare. However, only some are also eligible for Medicaid (Medicaid-eligible Medicare enrollees), depending on income and/or disability. Medicare is the primary payer for individuals aged 65 years and older, who are eligible for Medicaid, up to a payment limit, and Medicaid is the secondary payer.
This study was approved by the Institutional Review Board at the Harvard T.H. Chan School of Public Health.
Outcome Assessment
We defined CVD-related hospitalizations as events with a primary diagnosis (the condition chiefly responsible for the individual’s hospitalization) corresponding to an International Classification of Diseases, 9th Revision (ICD-9) codes from 390 to 495. If any individual experienced multiple hospitalizations during the study period (2000–2012), we included the first hospitalization only. We also excluded any individual who had been hospitalized for CVD in 1999. We examined associations with the following cause-specific CVD hospitalizations: ischemic heart disease (IHD; ICD-9 codes: 410–414), congestive heart failure (CHF; ICD-9 code: 428), acute myocardial infarction (AMI; ICD-9 code: 410.9) and ischemic stroke (ICD-9 code: 434.91). CVD events during which individuals were not enrolled in Medicaid are not captured in our analyses.
Exposure Assessment
We obtained daily ambient PM2.5 concentration estimates from a well-validated air pollution prediction model (2000–2012), described in detail elsewhere.23 Briefly, these estimates were derived for 1 km2 grid cells in the continental United States by integrating remote sensing, outputs from a chemical transport model, and other variables such as meteorological and land-use variables. Subsequently, PM2.5 estimates were obtained from an ensemble model that integrated multiple machine learning algorithms. Cross-validation indicated excellent overall predictive accuracy (cross-validated R2=0.86). We used the gridded predictions to estimate daily ZIP Code-level averages and subsequently linked those to the residential ZIP Codes of Medicaid and Medicare enrollees.
Covariate Information
We retrieved air and dew-point temperatures from North American Regional Reanalysis data,24 providing daily mean values for each 32 km2 grid cell in the continental United States. ZIP Code-level daily temperature values were computed by using area-weighted averages for each ZIP Code.
Ozone (O3) concentrations were derived from fitting a neural network model which integrated remote sensing, outputs from a chemical transport model, and meteorologic and land-use variables.25 Daily ambient O3 concentrations were predicted at 1 km2 grid cells for the continental United States. The cross-validated R2 for the predicted values was 0.80 for the entire study period. For O3, ZIP Code-level daily values were obtained by taking the inverse-distance mean of the four nearest grid cells to each ZIP Code’s centroid.
Statistical Analysis
We used a time-stratified case–crossover design to estimate the association between daily PM2.5 and total and cause-specific CVD hospitalizations. The case–crossover design, a variant of the case–control design, was developed to study the effects of transient exposures on acute events.26 In this design, the case days are identified as the days when the first hospitalization for CVD for a given individual occurred. For each case day—and for the same individual that experienced the event—control days are subsequently bidirectionally selected, defined as days when no hospitalization occurred, and matched on the same year, month, and day of the week as the case day. This design eliminates any confounding by factors that do not vary within an individual and month on average in the population, effectively eliminating any potential confounding bias by individual-level factors such as smoking and body mass index. This design also adjusts for measured and unmeasured confounding by seasonality and long-term trends by design.27,28 Further, by comparing the PM2.5 exposure distributions on case days with control days matched on the same day of the week within a month, we also accounted for the short-term serial correlation observed in PM2.5 variations and potential confounding by day of week. For all analyses, we used the average PM2.5 exposure on the day of and the preceding day of an individual’s CVD hospitalization (which we denote as Lag01).
We used conditional logistic regression models to estimate the associations between short-term exposure to PM2.5 and CVD hospitalization rates. We also included the mean of the dew-point temperature and air temperature for the day of hospitalization and the day prior (Lag01)—since these factors vary within a month and could act as confounders—using natural splines with 3 degrees of freedom.
We performed analyses for all adult Medicaid enrollees and non-Medicaid-eligible Medicare enrollees. To examine whether the PM2.5 – CVD association among adult Medicaid enrollees persists at PM2.5 concentrations below the WHO guidelines, we restricted case and control days to those with PM2.5 concentrations below 25 μg/m3 and repeated the analysis. In a separate analysis, we restricted case and control days to those with PM2.5 concentrations above 25 μg/m3. Finally, we estimated the association between Lag01 PM2.5 and cause-specific CVD hospitalizations among all adult Medicaid enrollees.
We report all results as percent change in CVD admission rates (95% confidence interval [CI]) per 10 μg/m3 increase in PM2.5. We have chosen this increment for comparability with other studies examining the same association in other populations.29,30 We conducted all statistical analyses using the R Statistical Software, version 3.3.2 (Foundation for Statistical Computing, Vienna, Austria).31
Effect Modification
We assessed whether there is evidence of effect modification of the association between PM2.5 and CVD hospitalization rates among adult Medicaid enrollees by the following factors: age group (18 to 44 years, 45 to 64 years, 65 years and older), sex and race/ethnicity (White non-Hispanic, Black non-Hispanic, Hispanic, and other, as well as White vs. non-White). To test for effect modification, we ran separate conditional logistic models that included an interaction term between PM2.5 and each of the above potential effect modifiers. We used likelihood ratio tests (LRT) to compare models with and without the interaction terms.
Sensitivity Analyses
We assessed the robustness of our results to several modelling choices, such as the degrees of freedom used for confounding adjustment for air and dew-point temperature. We compared results using 3, 6 and 9 degrees of freedom. Finally, we also assessed the robustness of our results to Lag01 O3 adjustment.
Results
There were 3,779,051 first CVD hospitalizations (i.e., case days) among adult Medicaid enrollees between 2000 and 2012 in the continental United States. Of these, 3,666,657 (97%) had available information on date of birth and were aged 18 years and older. Table 1 provides summary statistics of the adult Medicaid enrollees that were hospitalized due to a CVD event. Of this population, 59.0% were female and 50.3% were White non-Hispanic.
Table 1:
Characteristics of adult Medicaid enrollees (aged 18 years and older) who experienced a CVD hospitalization during 2000 – 2012.
| Baseline Characteristics | Number | % |
|---|---|---|
| Adult Medicaid enrollees | 3,666,657 | 100.0 |
| 18 to 44 years old | 515,064 | 14.0 |
| 45 to 64 years old | 1,483,422 | 40.5 |
| 65 years and older | 1,668,171 | 45.5 |
| Sex | ||
| Females | 2,164,323 | 59.0 |
| Males | 1,502,167 | 41.0 |
| Unknown | 167 | <0.1 |
| Race/Ethnicity | ||
| White, non-Hispanic | 1,843,598 | 50.3 |
| Non-White | 1,496,192 | 40.8 |
| Black, non-Hispanic | 912,337 | 24.9 |
| Hispanic | 407,774 | 11.1 |
| Other | 176,081 | 4.8 |
| Unknown | 326,841 | 8.9 |
| No data | 26 | <0.1 |
| Cause-specific CVD events | ||
| AMI | 392,436 | 10.7 |
| CHF | 541,919 | 14.8 |
| IHD | 912,687 | 24.9 |
| Ischemic stroke | 304,530 | 8.3 |
| Medicare (aged 65 and older) | ||
| All Medicare enrollees | 11,252,963 | 100.0 |
| Non-Medicaid-eligible | 9,448,679 | 84.0 |
AMI: Acute Myocardial Infarction; CHF: Congestive Heart Failure; IHD: Ischemic Heart Disease
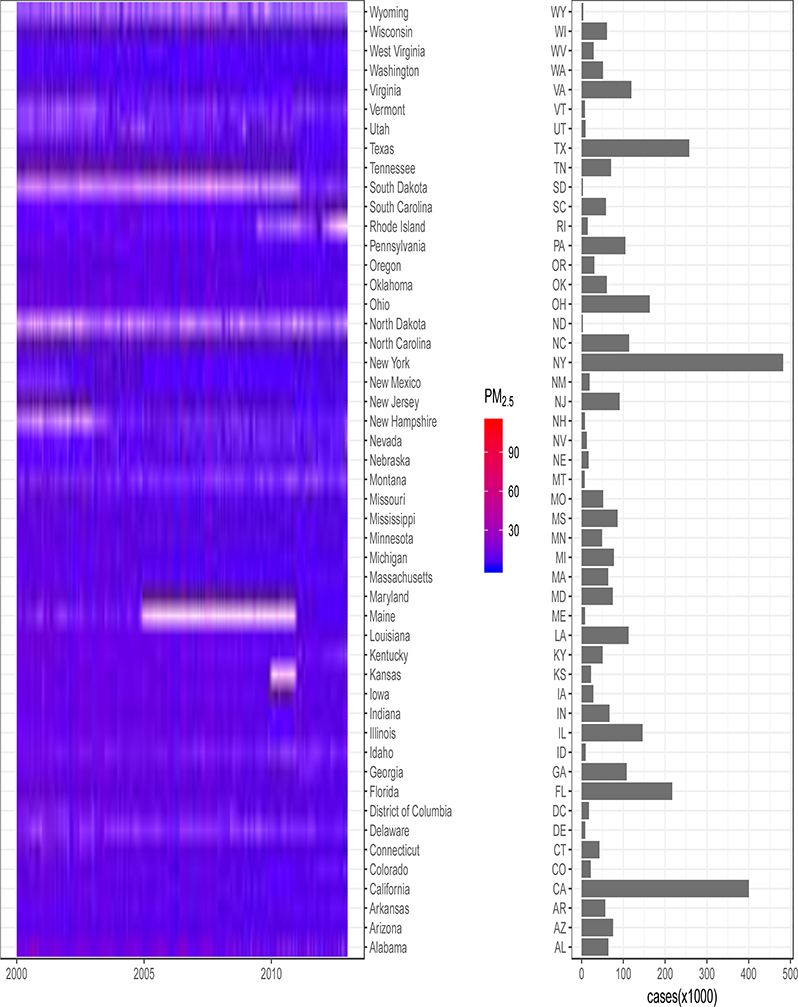
The Figure provides a summary of CVD hospitalizations and PM2.5 levels for each case day for adult Medicaid enrollees by state. IHD and CHF were the dominant causes for CVD hospitalizations, accounting for 24.9% and 14.8 % of all CVD hospitalizations, respectively. For the 3,666,657 case days, we selected 12,452,125 control days (3.4 controls per case on average). The average daily PM2.5 concentration over all case days for the study period was 11.5 μg/m3 (standard deviation 7.3 μg/m3). The daily PM2.5 for each state during the study period is shown in eFigure 1.
Figure:
Daily average PM2.5 concentrations (μg/m3) corresponding to case days in each state from 1 Jan 2000 to 31 Dec 2012, and the number of first hospitalizations for adult Medicaid enrollees, i.e., case days, in each state.
Table 2 presents all estimated effects. Overall, we found that a 10 μg/m3 increase in PM2.5 exposure was associated with a 0.9% (95%CI: 0.6, 1.1%) increase in the rate of CVD hospitalizations in the adult Medicaid population. When restricting case and control PM2.5 levels to ≤ 25 μg/m3, the association between PM2.5 and total CVD hospitalization rates in the adult Medicaid population remained elevated (1.3%; 95%CI: 0.9%, 1.6%). The association was highest for ischemic stroke hospitalizations, though the confidence intervals across all cause-specific CVD hospitalizations widely overlapped.
Table 2:
Percent change in CVD hospitalization rates per 10 μg/m3 increase in PM2.5.
| % Change | 95% CI | |
|---|---|---|
| Medicaid adults | 0.9 | 0.6, 1.1 |
| Age Groups | ||
| 18 to 44 years old | 0.4 | −0.2, 1.0 |
| 45 to 64 years old | 0.9 | 0.6, 1.3 |
| 65 years and older | 0.9 | 0.6, 1.3 |
|
Medicare enrollees (aged 65 and older) | ||
| Non-Medicaid-eligiblea | 0.8 | 0.6, 0.9 |
|
Sexb | ||
| Females | 0.8 | 0.5, 1.1 |
| Males | 0.9 | 0.6, 1.2 |
|
Race/ethnicityc | ||
| White, Non-Hispanic | 1.2 | 0.8, 1.5 |
| Black, Non-Hispanic | 0.9 | 0.4, 1.3 |
| Hispanic | 0.5 | −0.1, 1.2 |
| Other | 0.2 | −0.1, 1.1 |
|
Race/ethnicityc | ||
| White | 1.2 | 0.8, 1.5 |
| Non-White | 0.7 | 0.3, 1.0 |
| Cause-Specific CVD hospitalizations | ||
| AMI | 1.0 | 0.3, 1.7 |
| IHD | 1.1 | 0.6, 1.5 |
| CHF | 1.0 | 0.4, 1.6 |
| Ischemic Stroke | 1.2 | 0.4, 2.0 |
| PM2.5 ≤ 25 μg/m3 d | 1.3 | 0.9, 1.6 |
| PM2.5 > 25 μg/m3 e | 0.4 | −0.8, 1.7 |
Ncases = 9,448,679
Ncases = 3,666,490
Ncases = 3,339,790
Ncases = 3,514,773
Ncases = 151,884
AMI: Acute Myocardial Infarction; CHF: Congestive Heart Failure; IHD: Ischemic Heart Disease
There were 11,252,963 CVD hospitalizations among Medicare enrollees (aged 65 years and older), 84.0% of which were among non-Medicaid-eligible Medicare enrollees (Table 1). We observed a 0.8% (95%CI: 0.6, 0.9%) among non-Medicaid-eligible Medicare enrollees.
Effect Modification
All estimated associations by subgroup are presented in Table 2. Although the estimated effects were slightly lower among younger Medicaid adults (18 to 44 years old), the confidence intervals across all age groups overlapped. We observed no effect modification by sex. We detected no evidence of effect modification by race/ethnicity when we used detailed race/ethnicity categories. However, we observed an effect modification when considering adult White vs. non-White enrollees, with highest estimates among White Medicaid enrollees. Likelihood ratio tests confirmed the evidence of effect modification by the broader race or ethnicity classification.
Sensitivity Analyses
Our results were robust to confounding adjustment for air and dew-point temperature. Further adjusting for O3 also only slightly changed the association between PM2.5 and CVD hospitalizations. The results from the sensitivity analyses are presented in eTable 1.
Discussion
We conducted a nationwide analysis among adult Medicaid enrollees to estimate the association between short-term PM2.5 exposure and CVD hospitalization rates among low-income and disabled Americans. We observed harmful associations that were robust to sensitivity analyses. We did not detect evidence of effect modification by sex or age. We also did not observe meaningful differences in effect estimates among Medicaid elderly vs. non-Medicaid-eligible Medicare enrollees. However, we found evidence for higher effect estimates among adult White Medicaid enrollees. Although we observed some variability in effect estimates across cause-specific CVD hospitalizations, the confidence intervals widely overlapped. Further research is warranted to draw more conclusive evidence on potential differences in associations with PM2.5 across CVD outcomes in the Medicaid population.
Previous studies have reported effect modification by race or ethnicity, with higher estimates observed among Hispanic and Black Americans.32–34 For example, a previous study examined the association between daily PM2.5 and CVD mortality in California and reported that Hispanic residents experienced higher effect estimates.34 In our study, however, we found that the association between PM2.5 and CVD hospitalizations was highest among adult White Medicaid enrollees. Higher CVD mortality among non-White racial groups, with Black Americans experiencing the highest CVD mortality at all ages,18,35 and worse quality of and unequal access to care for racial or ethnic minorities,36 could explain the results from our multiplicative models. More frequent fatal events among non-White Americans would reduce CVD-related admissions in these racial or ethnic groups.
With access to both Medicaid and Medicare cohorts, we had the opportunity to evaluate whether enrollment in Medicaid (low-income) aggravated estimated PM2.5 effects on CVD among elderly Americans. Specifically, we compared effect estimates among Medicaid enrollees aged 65 years and older to those obtained in analyses among non-Medicaid-eligible Medicare enrollees. Although the effect estimates among elderly Medicaid enrollees were slightly higher, the confidence intervals widely overlapped. The highest association was seen in Medicaid-eligible-Medicare enrollees. As discussed previously, there is no exact overlap between the Medicaid-eligible Medicare population and the elderly Medicaid population, which would explain the differences in the estimates for the elderly Medicaid enrollees and the Medicaid-eligible Medicare enrollees. A previous study of short-term PM2.5 exposure and mortality among Medicare enrollees reported elevated effects among Medicaid-eligible enrollees.37 Although in our study we found no evidence of effect estimate heterogeneity in the Medicaid population aged 65 years and older and the non-Medicaid-eligible Medicaid cohort, further research is warranted to characterize the distinct effects of PM2.5 on CVD among low-income and elderly Americans, two particularly vulnerable subpopulations.
One of our study goals was to examine whether Medicaid enrollees exposed consistently to low PM2.5 concentrations still experience adverse PM2.5-related CVD impacts. To this end, we evaluated the association between short-term PM2.5 exposure and CVD hospitalizations among adult Medicaid enrollees defining low daily PM2.5 concentrations according to WHO guidelines (≤ 25 μg/m3). This guideline is different from the current United States Environmental Protection Agency (EPA) NAAQS for daily PM2.5 concentrations (the 98th percentile, averaged over 3 years, should not exceed 35 μg/m3).38 Which of the two standards is stricter depends on the distribution of the daily PM2.5 concentrations at each location. We chose to use the WHO guidelines, however, because using as a cut-off a guideline for daily concentrations to define low-level exposures facilitates analyses and interpretation. In our study, the average PM2.5 exposure was well below 25 μg/m3 and the majority of our data were included in the low level-restricted analysis. Conversely, only a very small fraction of our observations occurred at PM2.5>25 μg/m3 and the estimated association in this subpopulation was lower with wide confidence intervals. These two subpopulations, however, may not be directly comparable. PM2.5 levels have been steadily decreasing over the US; the events, thus, included in the analyses restricted to PM2.5>25 μg/m3 likely occurred earlier in our study period. The distribution of many other CVD risk factors that can modify the PM2.5–CVD association has also changed over the same time.39–42 Our findings of a stronger association at lower PM2.5 levels are in agreement with previous studies of both short- and long-term PM2.5 exposures and adverse health.43–45
Our study has several strengths. First, to our knowledge, this is the first nationwide study to estimate the association between short-term PM2.5 exposure and CVD among adult Medicaid enrollees. Second, we assigned daily exposures using highly accurate air pollution prediction models providing highly resolved daily PM2.5 estimates, including at areas with sparse or no monitoring. This increases the generalizability of our findings to Medicaid-eligible low-income Americans in both rural and urban areas. Third, we conducted the same statistical analysis with a side-by-side comparison of results among non-Medicaid-eligible Medicare enrollees and elderly Medicaid enrollees aged 65 years and older, which allowed us to evaluate the degree to which being enrolled in Medicaid may increase vulnerability to short-term PM2.5 exposure. Finally, we conducted sensitivity analyses for confounding adjustment and found our effect estimates to be robust.
Our findings, nonetheless, should be interpreted in light of our limitations. First, because residential information was only available at the ZIP Code level, some exposure measurement error is to be expected. Nonetheless, any error is not likely to covary with date of CVD admission within month and ZIP code, that is, we do not expect any differential exposure measurement error, given our study design that benefits from within-person contrasts. Our estimates, thus, are likely attenuated.46,47As the Medicaid eligibility criteria are different across states, the Medicaid population also differs by state. There are, for example, more Medicaid beneficiaries in states with less stringent eligibility criteria than in other states. This hinders generalizability of our results to all low-income Americans. Medicaid eligibility criteria also change over time. The largest Medicaid expansion in recent history was a result of the Affordable Care Act (ACA). The Affordable Care Act (ACA) was signed into law in 2010 and coverage under the Medicaid expansion became effective in most states as early as January 2014 (and later in other states). Our study period (2000–2012) does not cover the Medicaid population under this expansion; therefore, ACA has not influenced Medicaid eligibility in our study.
Finally, we did not adjust our main models for other pollutants. Given that PM2.5, a mixture itself partially primarily emitted from numerous sources and partially secondarily formed, is an indicator of the overall air pollution mixture at each location, adjustment for pollutants emitted from the same sources would change the interpretation of the PM2.5 effect estimates. For instance, adjustment for a traffic emissions tracer, such as nitrogen dioxide, would change the interpretation of the PM2.5 estimate—i.e., that of an overall air pollution mixture estimate—to a non-traffic air pollution estimate. Nonetheless, we adjusted for O3 in a sensitivity analysis. O3 is a secondarily formed gas through similar photochemical processes as secondary particles. Adjusting for O3 could remove some of the outcome variability that is due to secondary particles. In our data, nonetheless, O3 and PM2.5 were weakly correlated (r=0.19). The PM2.5 effect estimate, thus, did not change in the O3-adjusted model.
In conclusion, our study provides robust evidence of a harmful association between short-term PM2.5 exposure and rate of CVD hospitalizations in the United States Medicaid population. During our study period, the average PM2.5 concentration was much lower than the current daily WHO guidelines for daily PM2.5 concentrations, revealing elevated adverse PM2.5 estimated effects on CVD even at low exposures. Furthermore, the observed association remained elevated, and became even larger, when we restricted analyses to PM2.5 levels below the WHO guidelines.
Our findings are consistent with the hypothesis of adverse PM2.5 health effects in vulnerable subpopulations, such as low-income and disabled Americans, at PM2.5 concentrations well below the current standards,48 such as low-income and disabled Americans.
Supplementary Material
Acknowledgments
Sources of financial support: Funding was provided by the Health Effects Institute (HEI) grant 4953-RFA14-3/16-4, National Institute of Environmental Health Sciences (NIEHS) grants R01 ES024332, R01 ES028033, R01 ES030616, P30 ES000002 and P30 ES009089, National Institute on Minority Health and Health Disparities (NIMHD) grant P50 MD010428, R01 MD012769, and USEPA grants RD-83587201-0, RD-83615601. Robbie M Parks was supported by the Earth Institute post-doctoral research fellowship at Columbia University The contents are solely the responsibility of the grantee and do not necessarily represent the official views of the funding agencies. Further, funding agencies do not endorse the purchase of any commercial products or services related to this publication. Research described in this article was conducted under contract to the HEI, an organization jointly funded by the USEPA (Assistance Award No. R-83467701) and certain motor vehicle and engine manufacturers. The computations in this paper were run on the Research Computing Environment (RCE) supported by the Institute for Quantitative Social Science in the Faculty of Arts and Sciences at Harvard University.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Description of the process by which someone else could obtain the data and computing code required to replicate the results reported in your submission (or explanation why data or code are not available): Investigators can obtain the Medicaid claims used in this study from the Centers for Medicaid Services. The code used in this study is available on request.
References
- 1.Brook Robert D, Sanjay Rajagopalan, Arden Pope C., et al. Particulate Matter Air Pollution and Cardiovascular Disease. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- 2.Dabass A, Talbott EO, Venkat A, et al. Association of exposure to particulate matter (PM2.5) air pollution and biomarkers of cardiovascular disease risk in adult NHANES participants (2001–2008). International Journal of Hygiene and Environmental Health. 2016;219(3):301–310. doi: 10.1016/j.ijheh.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Du Y, Xu X, Chu M, Guo Y, Wang J. Air particulate matter and cardiovascular disease: the epidemiological, biomedical and clinical evidence. J Thorac Dis. 2016;8(1):E8–E19. doi: 10.3978/j.issn.2072-1439.2015.11.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between Short-Term Fine-Particulate Matter Exposure and Onset of Myocardial Infarction. Epidemiology. 2005;16(1):41–48. [DOI] [PubMed] [Google Scholar]
- 5.Milojevic A, Wilkinson P, Armstrong B, Bhaskaran K, Smeeth L, Hajat S. Short-term effects of air pollution on a range of cardiovascular events in England and Wales: case–crossover analysis of the MINAP database, hospital admissions and mortality. Heart. 2014;100(14):1093–1098. doi: 10.1136/heartjnl-2013-304963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominici F, Peng RD, Bell ML, et al. Fine Particulate Air Pollution and Hospital Admission for Cardiovascular and Respiratory Diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanobetti A, Schwartz J, Dockery DW. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environmental Health Perspectives. 2000;108(11):1071–1077. doi: 10.1289/ehp.001081071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Link BG, Phelan J. Social Conditions As Fundamental Causes of Disease. Journal of Health and Social Behavior. Published online 1995:80–94. doi: 10.2307/2626958 [DOI] [PubMed] [Google Scholar]
- 11.Bell Michelle L, Keita Ebisu. Environmental Inequality in Exposures to Airborne Particulate Matter Components in the United States. Environmental Health Perspectives. 2012;120(12):1699–1704. doi: 10.1289/ehp.1205201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tessum CW, Apte JS, Goodkind AL, et al. Inequity in consumption of goods and services adds to racial–ethnic disparities in air pollution exposure. PNAS. 2019;116(13):6001–6006. doi: 10.1073/pnas.1818859116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu H, Stuart AL. Spatiotemporal distributions of ambient oxides of nitrogen, with implications for exposure inequality and urban design. Journal of the Air & Waste Management Association. 2013;63(8):943–955. doi: 10.1080/10962247.2013.800168 [DOI] [PubMed] [Google Scholar]
- 14.Casey Joan A, Rachel Morello-Frosch, Mennitt Daniel J, Kurt Fristrup, Ogburn Elizabeth L, Peter James. Race/Ethnicity, Socioeconomic Status, Residential Segregation, and Spatial Variation in Noise Exposure in the Contiguous United States. Environmental Health Perspectives. 125(7):077017. doi: 10.1289/EHP898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gee Gilbert C, Payne-Sturges Devon C Environmental Health Disparities: A Framework Integrating Psychosocial and Environmental Concepts. Environmental Health Perspectives. 2004;112(17):1645–1653. doi: 10.1289/ehp.7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwynn RC, Thurston GD. The burden of air pollution: impacts among racial minorities. Environmental Health Perspectives. 2001;109(suppl 4):501–506. doi: 10.1289/ehp.01109s4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nauenberg E, Basu K. Effect of insurance coverage on the relationship between asthma hospitalizations and exposure to air pollution. Public Health Rep. 1999;114(2):135–148. [PMC free article] [PubMed] [Google Scholar]
- 18.Mensah George A, Mokdad Ali H, Ford Earl S, Greenlund Kurt J, Croft Janet B State of Disparities in Cardiovascular Health in the United States. Circulation. 2005;111(10):1233–1241. doi: 10.1161/01.CIR.0000158136.76824.04 [DOI] [PubMed] [Google Scholar]
- 19.Seinfeld JH, Pandis SN. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change. John Wiley & Sons; 2016. [Google Scholar]
- 20.Kioumourtzoglou M-A, Austin E, Koutrakis P, Dominici F, Schwartz J, Zanobetti A. PM2.5 and survival among older adults: Effect modification by particulate composition. Epidemiology. 2015;26(3):321–327. doi: 10.1097/EDE.0000000000000269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostro B, Malig B, Broadwin R, et al. Chronic PM2.5 exposure and inflammation: Determining sensitive subgroups in mid-life women. Environmental Research. 2014;132:168–175. doi: 10.1016/j.envres.2014.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team WHOO and EH. WHO Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide : global update 2005 : summary of risk assessment. Lignes directrices OMS relatives à la qualité de l’air : particules, ozone, dioxyde d’azote et dioxyde de soufre : mise à jour mondiale 2005 : synthèse de l’évaluation des risques. Published online 2006. Accessed June 20, 2020 https://apps.who.int/iris/handle/10665/69477 [Google Scholar]
- 23.Di Q, Amini H, Shi L, et al. An ensemble-based model of PM2.5 concentration across the contiguous United States with high spatiotemporal resolution. Environment International. 2019;130:104909. doi: 10.1016/j.envint.2019.104909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalnay E, Kanamitsu M, Kistler R, et al. The NCEP/NCAR 40-Year Reanalysis Project. Bull Amer Meteor Soc. 1996;77(3):437–472. doi: [DOI] [Google Scholar]
- 25.Di Q, Rowland S, Koutrakis P, Schwartz J. A hybrid model for spatially and temporally resolved ozone exposures in the continental United States. Journal of the Air & Waste Management Association. 2017;67(1):39–52. doi: 10.1080/10962247.2016.1200159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maclure M The Case–crossover Design: A Method for Studying Transient Effects on the Risk of Acute Events. Am J Epidemiol. 1991;133(2):144–153. doi: 10.1093/oxfordjournals.aje.a115853 [DOI] [PubMed] [Google Scholar]
- 27.Janes H, Sheppard L, Lumley T. Case–crossover Analyses of Air Pollution Exposure Data: Referent Selection Strategies and Their Implications for Bias. Epidemiology. 2005;16(6):717–726. [DOI] [PubMed] [Google Scholar]
- 28.Bateson TF, Schwartz J. Selection Bias and Confounding in Case–crossover Analyses of Environmental Time-Series Data. Epidemiology. 2001;12(6):654–661. [DOI] [PubMed] [Google Scholar]
- 29.Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmospheric Environment. 2011;45(35):6267–6275. doi: 10.1016/j.atmosenv.2011.08.066 [DOI] [Google Scholar]
- 30.Lee B-J, Kim B, Lee K. Air Pollution Exposure and Cardiovascular Disease. Toxicol Res. 2014;30(2):71–75. doi: 10.5487/TR.2014.30.2.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Team RC. RA Language and Environment for Statistical Computing. Versión 3.4. 3, Vienna, Austria, R Foundation for Statistical Computing; 2017. [Google Scholar]
- 32.Qiu X, Wei Y, Wang Y, et al. Inverse probability weighted distributed lag effects of short-term exposure to PM2.5 and ozone on CVD hospitalizations in New England Medicare participants - Exploring the causal effects. Environmental Research. 2020;182:109095. doi: 10.1016/j.envres.2019.109095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sebhat Erqou, Clougherty Jane E, Oladipupo Olafiranye, et al. Particulate Matter Air Pollution and Racial Differences in Cardiovascular Disease Risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2018;38(4):935–942. doi: 10.1161/ATVBAHA.117.310305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostro BD, Feng W-Y, Broadwin R, Malig BJ, Green RS, Lipsett MJ. The impact of components of fine particulate matter on cardiovascular mortality in susceptible subpopulations. Occupational and Environmental Medicine. 2008;65(11):750–756. doi: 10.1136/oem.2007.036673 [DOI] [PubMed] [Google Scholar]
- 35.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/Ethnicity, Income, Major Risk Factors, and Cardiovascular Disease Mortality. Am J Public Health. 2005;95(8):1417–1423. doi: 10.2105/AJPH.2004.048165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jolly S, Vittinghoff E, Chattopadhyay A, Bibbins-Domingo K. Higher Cardiovascular Disease Prevalence and Mortality among Younger Blacks Compared to Whites. The American Journal of Medicine. 2010;123(9):811–818. doi: 10.1016/j.amjmed.2010.04.020 [DOI] [PubMed] [Google Scholar]
- 37.Dickman SL, Himmelstein DU, Woolhandler S. Inequality and the health-care system in the USA. The Lancet. 2017;389(10077):1431–1441. doi: 10.1016/S0140-6736(17)30398-7 [DOI] [PubMed] [Google Scholar]
- 38.US EPA O. NAAQS Table. US EPA; Published April 10, 2014. Accessed June 20, 2020 https://www.epa.gov/criteria-air-pollutants/naaqs-table [Google Scholar]
- 39.Ezzati M, Obermeyer Z, Tzoulaki I, Mayosi BM, Elliott P, Leon DA. The contributions of risk factor trends and medical care to cardiovascular mortality trends. Nat Rev Cardiol. 2015;12(9):508–530. doi: 10.1038/nrcardio.2015.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. The Lancet. 2011;378(9793):815–825. doi: 10.1016/S0140-6736(11)60814-3 [DOI] [PubMed] [Google Scholar]
- 41.Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nature Reviews Endocrinology. 2013;9(1):13–27. doi: 10.1038/nrendo.2012.199 [DOI] [PubMed] [Google Scholar]
- 42.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KMV, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. The Lancet Diabetes & Endocrinology. 2014;2(11):867–874. doi: 10.1016/S2213-8587(14)70161-5 [DOI] [PubMed] [Google Scholar]
- 43.Papadogeorgou G, Kioumourtzoglou M-A, Braun D, Zanobetti A. Low Levels of Air Pollution and Health: Effect Estimates, Methodological Challenges, and Future Directions. Curr Envir Health Rpt. 2019;6(3):105–115. doi: 10.1007/s40572-019-00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnett R, Chen H, Szyszkowicz M, et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. PNAS. 2018;115(38):9592–9597. doi: 10.1073/pnas.1803222115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Q, Dai L, Wang Y, et al. Association of Short-term Exposure to Air Pollution With Mortality in Older Adults. JAMA. 2017;318(24):2446–2456. doi: 10.1001/jama.2017.17923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kioumourtzoglou M-A, Spiegelman D, Szpiro AA, et al. Exposure measurement error in PM2.5 health effects studies: A pooled analysis of eight personal exposure validation studies. Environ Health. 2014;13(1):2. doi: 10.1186/1476-069X-13-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environmental Health Perspectives. 2000;108(5):419–426. doi: 10.1289/ehp.00108419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.The Need for a Tighter Particulate-Matter Air-Quality Standard. New England Journal of Medicine. 2020;0(0):null. doi: 10.1056/NEJMsb2011009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.