Abstract
Context
Polycystic ovary syndrome (PCOS) is common and associated with metabolic syndrome. In the general population, metabolic disease varies by race and ethnicity.
Objective
This work aimed to examine in depth the interaction of race and ethnicity with PCOS-related metabolic disease in adolescent youth.
Methods
A secondary analysis was conducted of data from girls (age 12-21 years) with overweight or obesity (> 90 body mass index [BMI] percentile) and PCOS. Measurements included fasting hormone and metabolic measures, a 2-hour oral glucose tolerance test (OGTT), and magnetic resonance imaging for hepatic fat. Groups were categorized by race or ethnicity.
Results
Participants included 39 non-Hispanic White (NHW, age 15.7 ± 0.2 years; BMI 97.7 ± 0.2 percentile), 50 Hispanic (HW, 15.2 ± 0.3 years; 97.9 ± 0.3 percentile), and 12 non-Hispanic Black (NHB, 16.0 ± 0.6 years; 98.6 ± 0.4 percentile) adolescents. Hepatic markers of insulin resistance were worse in NHW, including lower sex hormone–binding globulin and higher triglycerides over high-density lipoprotein cholesterol (TGs/HDL-C) ratio (P = .002 overall, HW vs NHB [P = .009] vs NHW [P = 0.020]), although homeostasis model assessment of estimated insulin resistance was worst in NHB (P = .010 overall, NHW vs NHB P = .014). Fasting and 2-hour OGTT glucose were not different between groups, although glycated hemoglobin A1c (HbA1c) was lowest in NHW (overall P < .001, NHW 5.2 ± 0.3 vs HW 5.5 ± 0.3 P < .001 vs 5.7 ± 0.4%, P < .001). The frequency of hepatic steatosis (HW 62%, NHW 42%, NHB 25%, P = .032); low HDL-C < 40 mg/dL (HW 82%, NHW 61%, NHB 50%, P < .001) and prediabetes HbA1c 5.7% to 6.4% (NHB 50%, HW 36%, NHW 5%, P < .001) were different between the groups.
Conclusion
Adolescents with PCOS appear to show similar racial and ethnic variation to the general population in terms of metabolic disease components.
Keywords: polycystic ovary syndrome, metabolic syndrome, adolescent, race, ethnicity
Polycystic ovary syndrome (PCOS) affects between 10% and 20% of women worldwide [1]. Metabolic disease is common in women with PCOS; 50% to 90% of these women have insulin resistance (IR) and up to 60% of them will develop dysglycemia or type 2 diabetes (T2D) [1]. The annual health care cost of PCOS alone is $4.3 billion [2]. Female hyperandrogenemia (HA) is associated with the metabolic and reproductive dysfunction seen in PCOS [3]. Metabolic syndrome (MetS) and IR are known risk factors for progression to cardiovascular disease (CVD) and T2D [4]; thus, understanding racial and ethnic differences in components of MetS in women and girls with PCOS is critical for developing targeted early prevention and treatment. This has been explored in adult women with PCOS but not in adolescent girls.
Within the adult PCOS population, racial and ethnic differences have been observed [5]. Non-Hispanic Black (NHB) [6] and Hispanic White (HW) [5] women with PCOS have an increased risk for and a higher mortality due to CVD and T2D compared to non-Hispanic White (NHW) women with PCOS. Similar to women without PCOS, HW women with PCOS display more severe metabolic dysfunction and a higher prevalence of the MetS [5] than NHB and NHW women. Interestingly, NHB women with PCOS display fewer characteristics of MetS (lower triglyceride [TG] levels, higher high-density lipoprotein cholesterol [HDL-C] levels, and lower central adiposity) with less overall incidence of MetS compared to HW and NHW women, yet NHB women have high rates mortality due to CVD and T2D [5, 7].
The TG to HDL-C ratio (TG/HDL-C) has been developed as a clinical biomarker for IR, validated in NHW and HW women, and displays a higher degree of IR in HW women, but has not been found to be as robust in predicting IR in NHB women [8]. Increasing TG/HDL-C ratios have been shown to be a rising burden globally in asymptomatic nondiabetic individuals, increasing subclinical coronary atherosclerosis [9]. However, racial differences in this biomarker have not been examined in PCOS.
Within adolescent PCOS populations, studies on the effects of race and ethnicity on metabolic disease are limited [10, 11]. A retrospective meta-analysis study showed that NHB adolescents and young adults with PCOS had an increased prevalence of MetS compared with their NHW counterparts and that in contrast, there was no difference in risk of MetS between non-PCOS NHB and NHW adolescents and adult women in the National Health and Nutrition Examination Survey (NHANES) data set [6]. After adjusting for possible confounding influences of socioeconomic status and lifestyle, there were no differences in MetS prevalence among girls, although NHB girls exhibited less hypertriglyceridemia [10]. Here, we examine racial and ethnic differences in IR and MetS markers in adolescent girls who are overweight or obese with PCOS.
Materials and Methods
Study Design, Setting, and Participants
This study was a secondary analysis including participants from 3 cross-sectional cohorts with nearly identical enrollment criteria involving metabolic characterization of adolescents with PCOS: 1) Androgens and Insulin Resistance Study (AIRS, prior to National Clinical Trials [NCT], N = 76) [12-14], 2) Liver and Fat Regulation in Overweight Girls (APPLE, NCT02157974, N = 92), and 3) Post-Prandial Liver Glucose Metabolism in PCOS (PLUM, NCT03041129, N = 17). Participants were enrolled between 2012 and 2018. The participants were recruited via general endocrine clinics and lifestyle intervention obesity clinics at Children’s Hospital Colorado. AIRS inclusion criteria comprised females with obesity (body mass index [BMI] ≥ 95th percentile) or normal weight (BMI < 85th percentile), with or without PCOS, who were physically inactive (exercising less than 3.0 hours a week) and ages 12 to 21 years. Identical inclusion criteria were used for APPLE and PLUM except that the BMI range was expanded to include females with overweight or obesity (BMI ≥ 90th percentile). The National Institutes of Health (NIH) classification of PCOS (hyperandrogenism with oligomenorrhea and no use of ovary ultrasound) was used with oligomenorrhea in adolescents defined as the presence of irregular menstrual cycles for at least 1.5 years (AIRS) or 2 years (APPLE and PLUM) after menarche, consistent with 2013 Endocrine Society guidelines [15, 16]. A pediatric endocrinologist (M.C.G., K.J.N., or M.M.K.) performed a physical exam at the screening visit to determine clinical hyperandrogenism. The Ferriman-Gallwey scale (FGS) was used to rate hirsutism [17]. For all studies, exclusion criteria included diabetes (defined as glycated hemoglobin A1c [HbA1c] ≥ 6.5%), aspartate aminotransferase (AST) or alanine aminotransferase (ALT) greater than 125 IU/L, uncontrolled hypertension defined as persistent blood pressure greater than 140/80 mm Hg or medication for hypertension and weight greater than 325 pounds, because of equipment limitations. All participants were selected from the previously listed studies if they had 1) PCOS, 2) a BMI greater than or equal to the 90th percentile, and 3) were not prescribed oral contraceptives or metformin. The 2-hour oral glucose tolerance test (OGTT) was not performed in 17 AIRS participants and OGTT data from APPLE participants who received exenatide were not included in analysis (N = 10, 3 NHB, 3 HW, 4 NHW).
The University of Colorado Anschutz Medical Campus institutional review board and the Children’s Hospital Colorado Research Institute approved the studies. All participants aged 18 to 21years provided written informed consent, and the parents and participants provided written consent and assent, respectively, for all participants younger than 18 years.
Data Collection
Overall study design
For APPLE and PLUM, physical exam, fasting laboratory measures, magnetic resonance imaging (MRI) and OGTT were performed within a 24-hour period. For AIRS, physical exam, fasting measurements, and MRI were performed within 24 hours, and the OGTT within 6 weeks of the fasting measures and MRI.
Waist circumference, BMI, and BMI percentile per Centers for Disease Control and Prevention BMI growth charts [18] were obtained. All fasting laboratory measurements were obtained following a monitored inpatient 12-hour fast. The OGTT included 75 g of glucola, and for APPLE and PLUM, an additional 25 g of fructose. Blood samples were collected at baseline and at 30, 60, 90, and 120 minutes from the drink.
Hepatic Fat Fraction
Hepatic fat fraction was assessed with the Dixon technique as previously described [19]. MRI imaging was obtained on a 3Tesla Magnet (Siemens Magnetom Skyra or GE Healthcare). The weighted average of the mean fat fraction was calculated for each participant. Hepatic steatosis was defined as hepatic fat fraction greater than or equal to 5.5%.
Laboratory Measurements
Analyses were performed by the University of Colorado Anschutz Research core laboratory or the Children’s Hospital Colorado clinical laboratory. Plasma total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) (Hitachi 917 autoanalyzer; Boehringer Mannheim Diagnostics) and TG (Beckman Coulter) were analyzed enzymatically. Insulin was analyzed by radioimmunoassay (Millipore). Plasma glucose was measured at the bedside using the StatStrip Hospital Glucose Monitoring System (Nova Biomedical). HbA1c was measured with the Siemens DCA Vantage (Siemens Medical Solutions). ALT and AST were determined via VITROS 5600 (Ortho Clinical Diagnostics). Sex hormone–binding globulin (SHBG) was measured using an electrochemiluminescence immunoassay (Esoterix). Total testosterone was analyzed using a liquid chromatography–tandem mass spectrometry and free testosterone with equilibrium dialysis (Esoterix).
Calculations
IR was estimated using the homeostasis model assessment of estimated insulin resistance (HOMA-IR) and calculated as (fasting plasma glucose [mg/dL] × fasting plasma insulin [mg/dL]/405) [20]. The Matsuda insulin sensitivity index (M) was calculated based on the results of the OGTT: M = 10 000/(G0 × I0 × Gmean × Imean)1/2, where G and I represent plasma glucose [mmol dl − 1] and insulin [mU l − 1] concentrations, respectively, and ‘0’ and ‘mean’ indicate fasting value and mean value during the OGTT, respectively [21]. The free androgen index (FAI) was calculated as the ratio of total testosterone divided by the SHBG (both expressed in the same units) and multiplied by 100 to yield numerical results comparable in free testosterone concentration [22, 23]. TG:HDL-C ratio was measured by dividing fasting serum TGs by the HDL-C [9]. HOMA-%B, a measurement of insulin secretion, was calculated by [24]. Fasting insulin clearance was calculated as the ratio of fasting C peptide to fasting insulin [25]. The FGS [26] was determined by examining each of the 9 body areas most sensitive to androgen, and each was assigned a score from 0 (no hair) to 4 (frankly virile). These separate scores were summed to provide a total hormonal hirsutism score.
Metabolic Syndrome Definitions
Cardiovascular risk factors were defined by American Heart Association guidelines [27] as 1) systolic blood pressure of 130 mm Hg or greater (prehypertension), HDL-C less than or equal to 40 mg/dL, and TGs greater than or equal to 150 mg/dL. Prediabetes was defined per American Diabetes Association criteria [28]: fasting glucose 100 to 125 mg/dL, 2-hour OGTT glucose 140 to 199 mg/dL, or HbA1c 5.7% to 6.4%. Thus, impaired fasting glucose (IFG) is a fasting glucose greater than or equal to 100 mg/dL, impaired glucose tolerance (IGT) is a 2-hour OGTT greater than or equal to 140 mg/dL, and an abnormal HbA1c is equal to or greater than 5.7%. MetS was defined by the version of the National Cholesterol Education Program Adult Treatment Panel III definition updated by the American Heart Association and the National Heart Lung and Blood Institute in 2005 [29]. By this definition, MetS is present if 3 or more of the following 5 criteria are met: waist circumference greater than 40 inches (men) or 35 inches (women), systolic blood pressure greater than 130 mm Hg, fasting TGs greater than 150 mg/dL, fasting HDL-C less than 40 mg/dL (men) or 50 mg/dL (women), and fasting blood sugar greater than 100 mg/dL.
Statistical Analysis
Descriptive statistics were expressed as mean ± SD (if normally distributed) and median (interquartile range, if nonnormally distributed). Analysis of variance (ANOVA) with Kruskal-Wallis post hoc testing was used to determine differences between the groups and chi-square testing was used for categorical variables. Correlations were performed with simple linear regression. Adjustments for multiple comparisons were not performed. Statistical analyses were performed using GraphPad Prism version 8.3 (GraphPad Software).
Results
Participant Characteristics
The participant characteristics are detailed in Table 1. Thirty-nine NHW, 50 HW, and 12 NHB adolescent girls with obesity or overweight and PCOS were included. Groups were similar in age and BMI percentile, although absolute BMI was higher in NHB than NHW (overall ANOVA P = .042, post hoc P = .037). Waist circumferences were larger in NHB compared to NHW (overall ANOVA P = .008, post hoc P = .007) but waist-to-hip ratios were not significantly different between groups. Systolic blood pressure was lower in NHB compared to NHW (overall ANOVA P = .011, post hoc P = .009) and HW (post hoc P = .020).
Table 1.
Participant description
| NHW | HW | NHB | Overall ANOVA P | |
|---|---|---|---|---|
| Biometric | ||||
| No. of participants | 39 | 50 | 12 | |
| Age, y | 16 (15-17) | 15 (13-16.3) | 16.5 (15-17.8) | .253 |
| BMI, kg/m2 | 35.2 (31.3-36.8) | 35.1 (31.9-38.8) | 38.5 (36.5-42.0)a | .042 |
| BMI, % | 98.3 (96.8-98.7) | 98.5 (97.2-99.1) | 99.2 (97.9-99.4) | .051 |
| BMI z score | 1.99 ± 0.06 | 2.08 ± 0.06 | 2.24 ± 0.12 | .179 |
| Waist circumference, cm | 101 ± 2 | 106 ± 2 | 113 ± 3b | .008 |
| Hip circumference, cm | 116 ± 1 | 116 ± 2 | 123 ± 3 | .134 |
| Waist/Hip ratio | 0.87 (0.83-0.92) | 0.91 (0.87-0.96) | 0.92 (0.90-0.95) | .059 |
| Systolic blood pressure, mm Hg | 122 ± 9 | 120 ± 9 | 112 ± 8b,d | .011 |
| Diastolic blood pressure, mm Hg | 73 ± 9 | 72 ± 8 | 67 ± 9 | .111 |
| Measurements of hyperandrogenism | ||||
| Total testosterone, ng/dL | 43.5 (35.8-57.0) | 35.0 (28.8-48.0) | 57 (36.5-96.3)d | .009 |
| SHBG, nmol/L | 21.5 (14.9-31.9) | 15.6 (12.1-20.3)a | 18.9 (13.3-27.7) | .018 |
| FAI | 7.2 (4.9-12.1) | 8.4 (6.1-11.0) | 10.4 (5.8-14.2) | .393 |
| Free testosterone, ng/dL | 7.6 (5.8-11.3) | 7.3 (5.2-9.9) | 9.5 (6.8-14.5) | .386 |
| Hirsutism, FGS scale | 6 (2-13) | 6 (3-10) | 8 (3-12) | .840 |
Data are mean ± SD or median (25th-75th). Significant P values are in bold.
Abbreviations: ANOVA, analysis of variance; BMI, body mass index; FAI, free androgen index; FGS, Ferriman-Gallwey score; HW, Hispanic White; NHB, non-Hispanic Black; NHW, non-Hispanic White; SHBG, sex hormone–binding globulin.
a Post hoc P less than .05 to .01 compared to HW participants.
b Post hoc P less than .01 to .001 compared to NHW participants.
c Post hoc P less than .001 compared to NHW participants.
d Post hoc P less than .05 to .01 compared to HW participants.
Measurements Associated With Polycystic Ovary Syndrome
Total testosterone was higher in NHB compared to HW (overall ANOVA P = .009, post hoc P = .026), and SHBG was lower in HW compared to NHW (overall ANOVA P = .018, post hoc P = .016) (see Table 1). FAI, free testosterone, and hirsutism scores were similar between groups (see Table 1).
Measurements of Liver Function, Body Fat, Lipid, and Glycemic Parameters
There were no differences between liver fat and AST between groups. ALT was different between the groups, but none of the post hoc testing was significant. C-reactive protein was higher in NHB compared to NHW (overall ANOVA P = .004, post hoc P = .003) and HW (post hoc P = .014) (Table 2). Body composition was similar between the groups. Total cholesterol and LDL-C concentrations were similar in all 3 groups. HDL was lower in HW compared to NHW (overall ANOVA P = .004, post hoc P = .011) and NHB (post hoc P = .027), whereas TGs were lower in NHB compared to NHW (overall ANOVA P = .011, post hoc P = .026).
Table 2.
Metabolic parameters
| NHW | HW | NHB | Overall ANOVA P | |
|---|---|---|---|---|
| Liver measures | ||||
| Liver fat, % | 4.4 (2.6-8.5) | 6.3 (4.0-12.0) | 4.9 (3.5-5.7) | .057 |
| AST, IU/L | 32 (27-41) | 38 (32-52) | 37 (28-49) | .081 |
| ALT, IU/L | 35 (27-41) | 39 (30-50) | 27 (21-40) | .041 |
| hs-CRP, mg/L | 2.1 (1.0-4.1) | 2.5 (1.0-5.5) | 6.4 (4.4-9.4)b,d | .004 |
| Body composition | ||||
| Visceral fat, g | 85 ± 5 | 85 ± 4 | 78 ± 7 | .428 |
| Subcutaneous fat, g | 456 ± 18 | 468 ± 18.94 | 541 ± 43 | .672 |
| Fat mass (%) | 44 (41-46) | 43 (4-40)7 | 45 (42-48) | .719 |
| Lean mass (%) | 50 ± 1 | 49 ± 1 | 57 ± 2.4 | .966 |
| Lipid parameters | ||||
| Total cholesterol, mg/dL | 156 ± 5 | 151 ± 5 | 162 ± 9 | .999 |
| HDL-C, mg/dL | 39 ± 1 | 34 ± 1a | 39 ± 2d | .004 |
| LDL-C, mg/dL | 93 (73-127) | 83 (66-111) | 96 (79-133) | .260 |
| TGs, mg/dL | 109 (76-148) | 128 (105-165) | 95 (79-115)d | .011 |
| Glycemic parameters | ||||
| Fasting glucose, mg/dL | 84 (81-92) | 89 (84-95) | 88 (86-94) | .202 |
| Fasting insulin, mU/mL | 22 (16-30) | 29 (18-40) | 25 (19-54) | .158 |
| Fasting C peptide, mU/mL | 2.6 ± 0.1 | 3.4 ± 0.2a | 3.0 ± 0.4 | .017 |
| HbA1c, % | 5.2 (5.1-5.4) | 5.5 (4.9-5.7)c | 5.7 (5.2-6.1)c,d | < .001 |
| OGTT 2-h glucose, mg/dL | 129 (114-151) | 134 (121-158) | 132 (119-180) | .479 |
| OGTT 2-h insulin, mU/mL | 168 (92-355) | 256 (153-513) | 123 (86-275) | .076 |
Data are mean ± SD or median (25th-75th). Significant P values are in bold.
Abbreviations: ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; HbA1c, glycated hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein; HW, Hispanic White; LDL-C, low-density lipoprotein cholesterol; NHB, non-Hispanic Black; NHW, non-Hispanic White; OGTT, oral glucose tolerance test; TGs, triglycerides.
a Post hoc P less than .05 to .01 compared to HW participants.
b Post hoc P less than .01 to .001 compared to NHW participants.
c Post hoc P less than .001 compared to NHW participants.
d Post hoc P less than .05 to .01 compared to HW participants.
Regarding glycemic parameters, fasting and 2-hour OGTT glucose and insulin concentrations were similar in all 3 groups; however, fasting C peptide was higher in HW compared to NHW girls (overall ANOVA P = .017, post hoc P = .013) (see Table 2). NHB exhibited higher HbA1c than NHW (overall ANOVA P < .001, post hoc P < .001) and HW (post hoc P = .027) girls with PCOS. HbA1c was also higher in HW compared to NHW (post hoc P < .001).
Measurements of Insulin Sensitivity
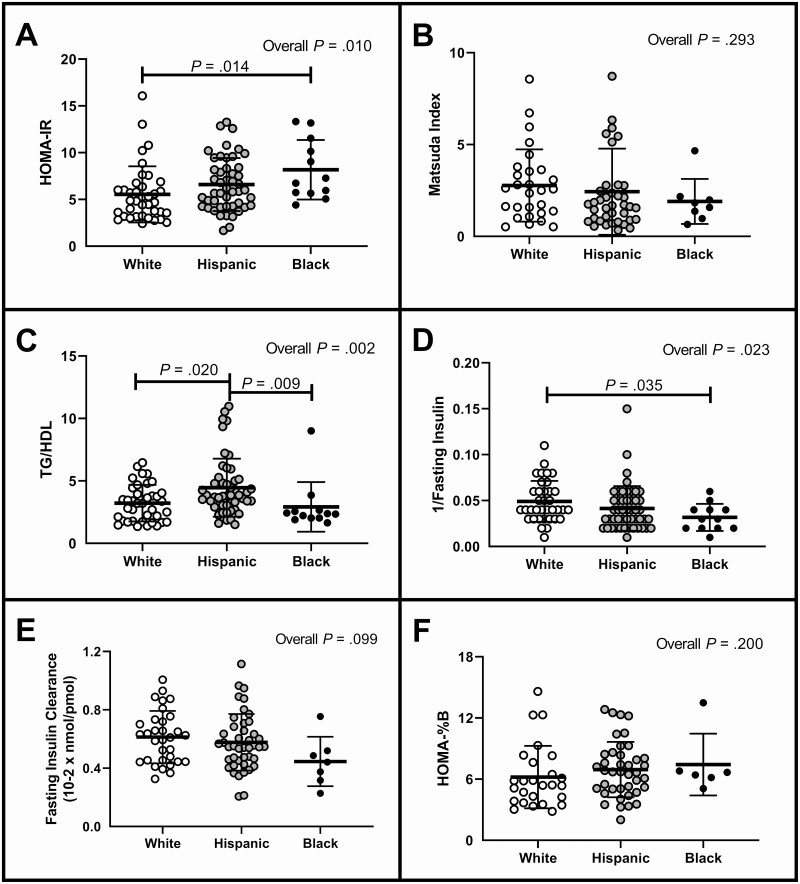
HOMA-IR (Fig. 1A) is worse (higher) in NHB compared to NHW, as was 1/fasting insulin (Fig. 1D) However, there was no difference in Matsuda scores (Fig. 1B). HW girls had a higher TG/HDL (Fig. 1C) compared to NHW and NHB girls. There were no differences between the groups in terms of fasting insulin clearance (Fig. 1E) or insulin secretion as assessed by HOMA-%B (Fig. 1F).
Figure 1.
Measurements of insulin sensitivity and secretion. Data shown compare the 3 groups of women with polycystic ovary syndrome (non-Hispanic White, Hispanic White, and non-Hispanic Black) as individual values in a scatter plots as mean ± SE bars. HOMA-IR, homeostasis model assessment of estimated insulin resistance; TG/HDL-C, triglyceride to high-density lipoprotein cholesterol ratio.
Proportion of Metabolic Syndrome Components
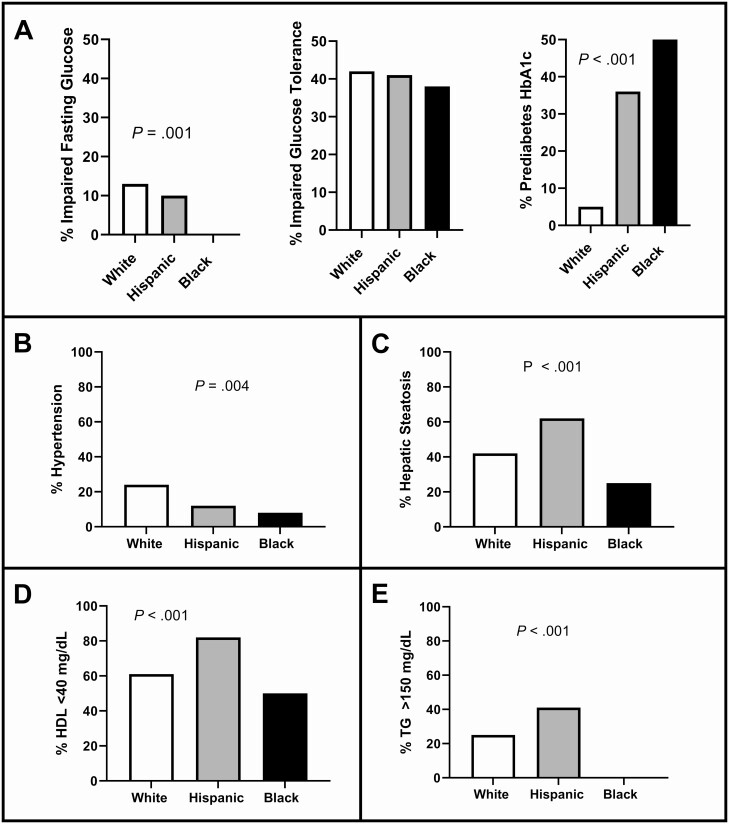
The proportion of participants who met criteria for MetS are shown in Fig. 2. Dysglycemia is shown as IFG, IGT, and HbA1c (Fig. 2A). Whereas all groups had similar rates of IGT, there was no IFG in NHB, yet NHB had a higher proportion with abnormal HbA1c. Few participants had a systolic blood pressure greater than or equal to 130 mm Hg although the frequency of hypertension was different between groups (Fig. 2B). Hepatic steatosis prevalence was different between the groups, and highest in HW (Fig. 2C). A low HDL-C was common, with differences across the groups; however, fewer NHB girls with PCOS had a low HDL-C compared to the other 2 groups (Fig. 2D). Whereas 41% of HW girls had elevated serum TGs, none of the NHB participants did (Fig. 2E). All participants had a waist circumference greater than 80 cm, and 90% had a waist greater than 90 cm, with no difference between groups (data not shown).
Figure 2.
Proportions of metabolic syndrome. Data are presented comparing the 3 groups of women with polycystic ovary syndrome (white, Hispanic, and black) as percentages graphed as a column bar graph. Because these are percentages, there are no SE bars. Statistical analyses were performed using ordinary analysis of variance tests where significance was P less than .05. According to the American Diabetes Association, a diagnosis of metabolic syndrome requires displaying 3 of the 5 following criteria: 1) dysglycemia measured by fasting glucose greater than or equal to 100 mg/dL (impaired fasting glucose), 2-hour glucose levels of 140 to 199 mg/dL (range, 7.8-11.0 mmol) on the 75-g oral glucose tolerance test (impaired glucose tolerance), and/or elevated glycated hemoglobin A1c (HbA1c) of 5.7% to 6.4% (prediabetes HbA1c); 2) blood pressure greater than or equal to 130/85 mm Hg or being treated for high blood pressure (hypertension); 3) liver fat greater than 5% (hepatic steatosis has been included in place of waist circumference because a standard waist circumference by race has yet to be fully determined); 4) high-density lipoprotein (HDL) cholesterol less than 40 mg/dL; and 5) triglycerides (TG) greater than or equal to 150 mg/dL.
Correlation Analysis
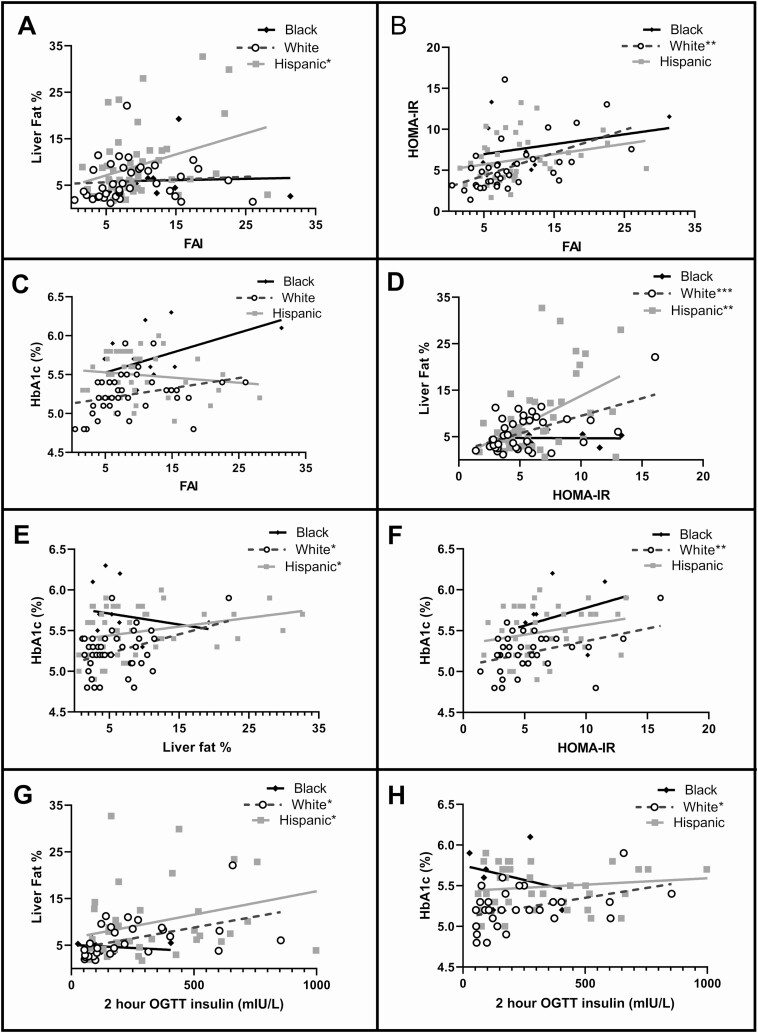
The relationship between the FAI and liver fat percentage was significant only in HW girls (Fig. 3A, r2 = 0.107, P = .023), whereas FAI and HOMA-IR were related only in NHW girls (Fig. 3B, r2 = 0.272, P = .001). HbA1c did not significantly correlate to FAI in any group, although the slope in NHB girls was the highest. Liver fat percentage related to HOMA-IR both in HW (Fig. 3D, r2 = 0.225, P = .001) and NHW girls (r2 = 0.301, P = .001), with no relationship found in NHB girls (r2 < 0.001, P = .950). HbA1c related to hepatic fat in NHW (Fig. 3E, r2 = 0.133, P = .022) and HW (r2 = 0.083, P = .042) but to HOMA-IR only in NHW girls (Fig. 3F, r2 = 0.159, P = .016). The 2-hour insulin from the OGTT related to hepatic fat in HW (Fig. 3G, r2 = 0.129, P = .027), and NHW (r2 = 0.214, P = .013) and to HbA1c only in NHW girls (Fig. 3H, r2 = 0.169, P = .030).
Figure 3.
Correlations between metabolic and androgen parameters. Statistical relationships between parameters of fat content, insulin sensitivity, and androgen concentrations were performed with Spearman correlations. FAI, free androgen index; HbA1c, glycated hemoglobin A1c; HOMA-IR, homeostasis model assessment of estimated insulin resistance; OGTT, oral glucose tolerance test.
Discussion
We found that racial and ethnic differences in IR, MetS, and glycemia displayed patterns in girls with PCOS similar to those previously reported in adolescent females in the NHANES cohort [10, 11] and adult women with PCOS [5]. We found that girls with HW ethnicity are more likely to have markers of IR, and elevated markers of hepatic and lipid dysmetabolism; whereas NHB girls have more evidence of dysglycemia. We confirmed that HW girls with PCOS have a greater prevalence of hepatic steatosis. Despite having an overall more favorable hepatic and lipid metabolic profile (lower ALT, less hepatic steatosis, higher subcutaneous fat, higher lean mass, lower TG/HDL-C), NHB girls with PCOS displayed a higher degree of IR per HOMA-IR and 1/fasting insulin along with higher HbA1c. These differences in patterns for metabolic disease risk by race and ethnicity need to be included in the consideration of treatment options for these adolescents.
Racial and ethnic differences have been examined in adult women with PCOS; however, there is a paucity of data regarding racial and ethnic differences in girls with PCOS. NHB and NHW women with PCOS who were morbidly obese (BMI > 35) had waist-hip ratio, total testosterone, and SHBG that were similar between groups [30]. Another study that compared NHW, NHB, and Hispanic adults with obesity and PCOS found no difference in waist circumference and total testosterone by race/ethnicity [5]. A meta-analysis examining metabolic disease in NHW and NHB adults with PCOS found lipid patterns similar to our data [7]. In contrast to adults, NHB girls with obesity or overweight and PCOS in our study had a larger waist than NHW and HW girls; and HW girls had the lowest total testosterone of the 3 groups. ALT followed a similar pattern in adults with obesity [30] and in our adolescents with PCOS (see Table 2), whereby NHB adolescents had lower ALT than NHW adolescents. TGs also followed similar racial and ethnic patterns in adults with obesity [5, 30] and our adolescents with PCOS (see Table 2): HW had the highest TGs and NHB the lowest between the 3 groups. In women with chronic androgen excess, plasma testosterone is positively correlated with waist circumference, an index of visceral obesity, suggesting that testosterone and visceral obesity are related [31]. Among the HW girls, androgens were highly related to the degree of central adiposity and IR [32]. HDL-C was highest in HW adults [5], but in contrast was lowest in HW adolescents, compared to other racial and ethnic groups. Interestingly, fasting glucose and fasting insulin also displayed racial and ethnic differences in adults [5, 30] but not in our sample of girls with PCOS. Differences in BMI [33, 34], physical activity [35], and sleep [36] in girls compared to adult women may contribute to the differences we observed in adolescents compared to adults with PCOS. Our data are also similar to those from the largest study describing metabolic differences by race/ethnicity in youth [10, 11, 37]. However, in both of these NHANES data sets, PCOS was not specifically excluded from the analysis, and thus it is likely that 5% to 15% of the patients described have PCOS. Additionally, lower insulin clearance and lower insulin sensitivity in youth compared to adults as shown in the RISE study [38] may contribute to the differences we observed in girls in this study compared to adults in previous findings. These latter data suggest that, as is the case for T2D, we cannot simply view adult and adolescent PCOS as identical phenomena, thus implying the need for therapeutic interventions that are tailored to an adolescent population.
Pancreatic β-cell insufficiency with dysglycemia has been demonstrated previously in Black individuals [39, 40], and this finding persists in girls with PCOS [41]. NHB girls are more likely to have dysglycemia and impaired β-cell function [42] than NHW and Hispanic girls, which is thought to underlie the increased rates of T2D in Black individuals [43]. β-cell insufficiency may be preceded by early excess insulin secretion, and mechanisms for this may vary with race. One study showed an ethnicity-specific relationship of β-cell function and pancreatic TG content [40]. In HW and NHW, high pancreatic TG levels potentially represent a risk factor for β-cell failure [40]. However, in NHB with obesity, hypersensitivity of β cells to elevated pancreatic TGs and subsequent exaggerated glucose-stimulated insulin secretion may represent risk factors for progression to β-cell failure [40]. Alternatively, greater postprandial hyperinsulinemia was observed in NHB compared to NHW premenopausal and postmenopausal women and was associated with lower hepatic insulin clearance and heightened β-cell capacity to rapid changes in glucose, but not to higher insulin secretion [44]. Moreover, racial differences including lower insulin clearance in NHB vs NHW youth have been reported [45]. We did not find differences in fasting insulin clearance or β-cell clearance when all the girls had PCOS. Clearly, further study is needed to understand race and ethnicity related differences in β-cell function prior to and through the development of dysglycemia.
Studies using HbA1c as a glycemic marker have shown that Latino Americans, African Americans, and Asian Americans have poorer control of their diabetes [46]. NHB adults have been shown to have higher HbA1c for a similar mean glucose concentration than NHW adults, in individuals both with and without diabetes [47]. This is thought to be related to genetic variants in the β chain of hemoglobin [48]. Kelsey et al and others have shown that Black youth with normal weight have higher HbA1c [47, 48] NHW adolescents. In youth aged 5 to 24 years who were part of NHANES-3, mean HbA1c was progressively higher in NHW to HW to NHB [37]. These differences have been demonstrated to persist through the lifespan [49]. Even when adjusting for adherence to prescribed therapies, in adults with T2D, HbA1c remained higher in NHB compared to NHW individuals [50]. These racial differences could be the results of higher glucose, or HbA1c may be higher in Black patients because of increased glycation of hemoglobin [51]. Other theories for the difference in HbA1c between race and ethnicity groups include differences in red blood cell turnover, hemoglobinopathies such as thalassemia or sickle cell disease [52], and genetic variation of the β chain of hemoglobin [48]. The racial difference in HbA1c has significant implications in terms of diagnosing T2D, and thus is currently a topic of debate.
The TG/HDL ratio is potentially not an ideal marker for IR when including a cohort with multiple racial ethnic backgrounds. The TG/HDL-C ratio value to predict IR differs by race and ethnicity in adults [53, 54] as shown by a study in NHW, NHB, and Mexican Americans. In NHB, TGs and TG/HDL-C were not dependable indicators of IR, as measured by the insulin sensitivity index [55]. In adolescents, we similarly found that whereas the TG/HDL-C ratio was low and would not indicate IR in NHB, HOMA-IR and Matsuda indexes for insulin sensitivity were worst in NHB individuals.
An increased risk of central obesity and IR have been well described in people of Hispanic ethnicity. In a study of more than 8000 women of various Hispanic ancestries from 4 US cities, MetS was present in 36% of the women, with a prevalence that ranged from 27% in South Americans to 41% in Puerto Ricans [56]. Abdominal obesity was present in 96% of these women, and 62% had hyperglycemia and IR [56]. In a large cohort of adults of both sexes, Hispanic participants had increased hepatic fat and serum TGs, whereas hepatic and visceral fat was low in Black individuals, as were serum TGs [57]. Similar trends of differences in hepatic fat were reported in adolescents with obesity [58]. However, we did not find that Hispanics had a large waist circumference, greater visceral, or total percentage of body fat when all participants had PCOS. Rather, we found that NHB girls had a larger waist circumference, although their absolute BMI was also great, and this may just be a reflection of this.
Our study has several strengths and limitations. Notable strengths include the fact that our data were collected in a rigorous research environment with monitored fasting, used the strictest NIH PCOS criteria, and included gold-standard MRI measures of hepatic steatosis and hormone assays. Limitations include the fact that the exclusion criteria of the parent protocols included hypertension or treatment for hypertension, which may have reduced the prevalence of hypertension in this population. Although some participants were recruited from the community, others were recruited from specialty clinics at a tertiary care regional referral center, which may have increased overall rates of metabolic disease. Our race and ethnicity distribution in our cohort is similar to that in our surrounding demographic area, leading to a smaller NHB cohort size than would be ideal. This potential underrepresentation of Black girls and overall moderate sample size is another limitation, particularly given the number of comparisons, and could be an alternative explanation for differences from reported data in adults. Finally, we do not have a local non-PCOS control population, but rather are comparing to published rates of disease. With the exception of smaller studies in which PCOS was excluded in a control group, information from large data sets such as NHANES did not specifically exclude a diagnosis of PCOS and thus 5% to 15% of these girls likely have PCOS. Conversely, 85% to 95% will not have PCOS, and thus these groups are still acceptable as a comparison reference.
In conclusion, we have demonstrated that there are racial differences in metabolic outcomes in girls with obesity or overweight and PCOS. While some of these are similar to those described in women with and without PCOS, other factors in our youth differed from data reported in adults. Moreover, whereas TG/HDL-C may be a good clinical biomarker for IR and MetS in NHW and HW girls, this ratio has a lower utility in NHB girls. However, NHB girls with obesity or overweight and PCOS are just as likely to have IR as HW girls, and are known to be at higher risk for T2D than NHW girls, and thus should be followed closely for the development of T2D. Our initial findings need to be replicated in a larger racially and ethnically balanced cohort and studied across the lifespan to develop more accurate patient-specific screening and treatment algorithms. These studies will help build cultural competency for improved PCOS care and prevention of T2D and CVD and their complications.
Acknowledgments
The authors would like to thank the participants and their families.
Financial Support: This work was supported by the American Heart Association (grant No. 13CRP 14120015 to M.C.G.), a Thrasher Pediatric Research Foundation Mentored Pilot Grant, National Institutes of Health/National Center for Research Resources (NIH/NCRR) Colorado CTSI Co-Pilot Grant (No. TL1 RR025778), a Pediatric Endocrinology Society Fellowship, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK grant Nos. T32 DK063687, and NIDDK K23DK107871), the Doris Duke Foundation (grant No. 2015212), the NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award (CTSA grant No. UL1 TR001082), and the Office of Research on Women’s Health (ORWH) at the National Institutes of Health (NIH) (grant No. BIRCWH K12HD057022).
Clinical Trial Information: Clinical trial registration numbers NCT02157974 (registered June 6, 2014) and NCT03041129 (registered February 2, 2017).
Author Contributions: S.A. researched data and co-wrote the manuscript, S.A. researched data and edited the manuscript, Y.G.R. researched data and edited the manuscript, L.P. reviewed statistical analysis and edited the manuscript, M.M.K., K.J.N. and M.C.G. designed the study, researched data, contributed to discussion, and edited the manuscript.
Glossary
Abbreviations
- AIRS
Androgens and Insulin Resistance Study
- ALT
alanine aminotransferase
- ANOVA
analysis of variance
- APPLE
Liver and Fat Regulation in Overweight Girls
- AST
aspartate aminotransferase
- BMI
body mass index
- CVD
cardiovascular disease
- FAI
free androgen index
- FGS
Ferriman-Gallwey scale
- HA
hyperandrogenemia
- HbA1c
glycated hemoglobin A1c
- HDL-C
high-density lipoprotein cholesterol
- HOMA-IR
homeostasis model assessment of estimated insulin resistance
- HW
Hispanic White
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- IR
insulin resistance
- LDL-C
low-density lipoprotein cholesterol
- MetS
metabolic syndrome
- MRI
magnetic resonance imaging
- NHANES
National Health and Nutrition Examination Survey
- NHB
non-Hispanic Black
- NHW
non-Hispanic White
- NIH
National Institutes of Health
- OGTT
oral glucose tolerance test
- PCOS
polycystic ovary syndrome
- PLUM
Post-Prandial Liver Glucose Metabolism in PCOS
- SHBG
sex hormone–binding globulin
- T2D
type 2 diabetes
- TGs
triglycerides
- TG/HDL-C
higher triglyceride to high-density lipoprotein cholesterol ratio.
Additional Information
Disclosures: M.C.G. has received product from AminoCorp and served on an advisory board on pediatric obesity for NovoNordisk. The other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 2. Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90(8):4650-4658. [DOI] [PubMed] [Google Scholar]
- 3. Huang CC, Tien YJ, Chen MJ, Chen CH, Ho HN, Yang YS. Symptom patterns and phenotypic subgrouping of women with polycystic ovary syndrome: association between endocrine characteristics and metabolic aberrations. Hum Reprod. 2015;30(4):937-946. [DOI] [PubMed] [Google Scholar]
- 4. Hanley AJ, Karter AJ, Williams K, et al. Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation. 2005;112(24):3713-3721. [DOI] [PubMed] [Google Scholar]
- 5. Engmann L, Jin S, Sun F, et al. ; Reproductive Medicine Network . Racial and ethnic differences in the polycystic ovary syndrome metabolic phenotype. Am J Obstet Gynecol. 2017;216(5):493.e1-493.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hillman JK, Johnson LN, Limaye M, Feldman RA, Sammel M, Dokras A. Black women with polycystic ovary syndrome (PCOS) have increased risk for metabolic syndrome and cardiovascular disease compared with white women with PCOS [corrected]. Fertil Steril. 2014;101(2):530-535. [DOI] [PubMed] [Google Scholar]
- 7. Kazemi M, Kim JY, Parry SA, Azziz R, Lujan ME. Disparities in cardio-metabolic risk between Black and White women with polycystic ovary syndrome: a systematic review and meta-analysis. Am J Obstet Gynecol. Published online December 11, 2020. doi:10.1016/j.ajog.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 8. Borrayo G, Basurto L, González-Escudero E, et al. TG/HDL-C ratio as cardio-metabolic biomarker even in normal weight women. Acta Endocrinol (Buchar). 2018;14(2):261-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patil S, Rojulpote C, Gonuguntla K, et al. Association of triglyceride to high density lipoprotein ratio with global cardiac microcalcification to evaluate subclinical coronary atherosclerosis in non-diabetic individuals. Am J Cardiovasc Dis. 2020;10(3):241-246. [PMC free article] [PubMed] [Google Scholar]
- 10. Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2012;22(2):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gurka MJ, Ice CL, Sun SS, Deboer MD. A confirmatory factor analysis of the metabolic syndrome in adolescents: an examination of sex and racial/ethnic differences. Cardiovasc Diabetol. 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cree-Green M, Bergman BC, Coe GV, et al. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity (Silver Spring). 2016;24(11):2399-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cree-Green M, Newcomer BR, Coe G, et al. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab. 2015;308(9):E726-E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cree-Green M, Rahat H, Newcomer BR, et al. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc. 2017;1(7):931-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teede HJ, Misso ML, Costello MF, et al.Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2018;89(3):251-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21(11):1440-1447. [DOI] [PubMed] [Google Scholar]
- 18. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1-190. [PubMed] [Google Scholar]
- 19. Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153(1):189-194. [DOI] [PubMed] [Google Scholar]
- 20. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487-1495. [DOI] [PubMed] [Google Scholar]
- 21. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 22. Wilke TJ, Utley DJ. Total testosterone, free-androgen index, calculated free testosterone, and free testosterone by analog RIA compared in hirsute women and in otherwise-normal women with altered binding of sex-hormone-binding globulin. Clin Chem. 1987;33(8):1372-1375. [PubMed] [Google Scholar]
- 23. Wheeler MJ. The determination of bio-available testosterone. Ann Clin Biochem. 1995;32(Pt 4):345-357. [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. [DOI] [PubMed] [Google Scholar]
- 25. Castillo MJ, Scheen AJ, Letiexhe MR, Lefèbvre PJ. How to measure insulin clearance. Diabetes Metab Rev. 1994;10(2):119-150. [DOI] [PubMed] [Google Scholar]
- 26. Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140(7):815-830. [DOI] [PubMed] [Google Scholar]
- 27. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177-e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yudkin JS. “Prediabetes”: are there problems with this label? Yes, the label creates further problems! Diabetes Care. 2016;39(8):1468-1471. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Crit Pathw Cardiol. 2005;4(4):198-203. [DOI] [PubMed] [Google Scholar]
- 30. Ladson G, Dodson WC, Sweet SD, et al. Racial influence on the polycystic ovary syndrome phenotype: a Black and White case-control study. Fertil Steril. 2011;96(1):224-229.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Evans DJ, Barth JH, Burke CW. Body fat topography in women with androgen excess. Int J Obes. 1988;12(2):157-162. [PubMed] [Google Scholar]
- 32. Navarro G, Allard C, Xu W, Mauvais-Jarvis F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity (Silver Spring). 2015;23(4):713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang LY, Chyen D, Lee S, Lowry R. The association between body mass index in adolescence and obesity in adulthood. J Adolesc Health. 2008;42(5):512-518. [DOI] [PubMed] [Google Scholar]
- 34. The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304(18):2042-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Azevedo MR, Araújo CL, Cozzensa da Silva M, Hallal PC. Tracking of physical activity from adolescence to adulthood: a population-based study. Rev Saude Publica. 2007;41(1):69-75. [DOI] [PubMed] [Google Scholar]
- 36. Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: changes in affect. Emotion. 2010;10(6):831-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saaddine JB, Fagot-Campagna A, Rolka D, et al. Distribution of HbA1c levels for children and young adults in the U.S.: Third National Health and Nutrition Examination Survey. Diabetes Care. 2002;25(8):1326-1330. [DOI] [PubMed] [Google Scholar]
- 38. Utzschneider KM, Tripputi MT, Kozedub A, et al. β-Cells in youth with impaired glucose tolerance or early type 2 diabetes secrete more insulin and are more responsive than in adults. Pediatr Diabetes. 2020;21(8):1421-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ladwa M, Hakim O, Amiel SA, Goff LM. A systematic review of beta cell function in adults of black African ethnicity. J Diabetes Res. 2019;2019:7891359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szczepaniak LS, Victor RG, Mathur R, et al. Pancreatic steatosis and its relationship to β-cell dysfunction in humans: racial and ethnic variations. Diabetes Care. 2012;35(11):2377-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michaliszyn SF, Lee S, Bacha F, et al. Differences in β-cell function and insulin secretion in Black vs. White obese adolescents: do incretin hormones play a role? Pediatr Diabetes. 2017;18(2):143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marcinkevage JA, Alverson CJ, Narayan KM, Kahn HS, Ruben J, Correa A. Race/ethnicity disparities in dysglycemia among U.S. women of childbearing age found mainly in the nonoverweight/nonobese. Diabetes Care. 2013;36(10):3033-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bacha F, Gungor N, Lee S, Arslanian SA. Type 2 diabetes in youth: are there racial differences in β-cell responsiveness relative to insulin sensitivity? Pediatr Diabetes. 2012;13(3):259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chung ST, Galvan-De La Cruz M, Aldana PC, et al. Postprandial insulin response and clearance among Black and White women: the Federal Women’s Study. J Clin Endocrinol Metab. 2019;104(1):181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arslanian S, El Ghormli L, Bacha F, et al. ; TODAY Study Group . Adiponectin, insulin sensitivity, β-cell function, and racial/ethnic disparity in treatment failure rates in TODAY. Diabetes Care. 2017;40(1):85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu WC, Yoon HH, Gavin JR III, Wright EE Jr, Cabellero AE, Tenzer P. Building cultural competency for improved diabetes care: introduction and overview. J Fam Pract. 2007;56(9 Suppl Building):S11-S14. [PubMed] [Google Scholar]
- 47. Kirk JK, D’Agostino RB Jr, Bell RA, et al. Disparities in HbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2006;29(9):2130-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kelsey MM, Zeitler PS, Drews K, Chan CL. Normal hemoglobin A1c variability in early adolescence: adult criteria for prediabetes should be applied with caution. J Pediatr. 2020;216:232-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Smalls BL, Ritchwood TD, Bishu KG, Egede LE. Racial/ethnic differences in glycemic control in older adults with type 2 diabetes: United States 2003-2014. Int J Environ Res Public Health. 2020;17(3):950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adams AS, Trinacty CM, Zhang F, et al. Medication adherence and racial differences in HbA1c control. Diabetes Care. 2008;31(5):916-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bergenstal RM, Gal RL, Connor CG, et al. ; T1D Exchange Racial Differences Study Group . Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95-102. [DOI] [PubMed] [Google Scholar]
- 52. Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97(4):1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196(2):696-703. [DOI] [PubMed] [Google Scholar]
- 54. Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol. 2008;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans. Arch Intern Med. 2005;165(12):1395-1400. [DOI] [PubMed] [Google Scholar]
- 56. Heiss G, Snyder ML, Teng Y, et al. Prevalence of metabolic syndrome among Hispanics/Latinos of diverse background: the Hispanic Community Health Study/Study of Latinos. Diabetes Care. 2014;37(8):2391-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49(3):791-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liska D, Dufour S, Zern TL, et al. Interethnic differences in muscle, liver and abdominal fat partitioning in obese adolescents. PLoS One. 2007;2(6):e569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.