Abstract
Background
Endoplasmic reticulum stress (ERS) is one of the main mechanisms of spinal cord injury (SCI) pathology and can affect the physiological state of neurons. Icariin (ICA), the main pharmacological component of Epimedium, can relieve the symptoms of patients with SCI and has obvious protective effects on neurons through ERS.
Methods
PC12 cells were induced to differentiate into neurons by nerve growth factor and identified by flow cytometry. Cell proliferation was detected by CCK8 method, cell viability was detected by SRB assay, apoptosis was detected by flow cytometry and microstructure of ER was observed by transmission electron microscope. Western blot was used to detect the protein expression of CHOP and Grp78, and qPCR was used to detect the mRNA expression of CHOP and Grp78.
Results
The results of CCK8, SRB and flow cytometry showed that ICA could relieve ERS and reduce apoptosis of PC12 cells. The results of transmission microscope showed that ICA could reduce apoptosis of PC12 cells caused by ERS. The results of Western blot and q-PCR showed that ICA could inhibit ERS by down-regulating the expression of CHOP and Grp78.
Conclusions
ICA can inhibit ERS and promote the repair of PC12 cells by down-regulating the expression of CHOP and Grp78. ICA has the potential to promote the recovery of spinal cord injury.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12906-021-03233-1.
Keywords: Endoplasmic reticulum stress, Neuron, Icariin, CHOP, Grp78
Background
Spinal cord injury (SCI) is one of the most severely damaged diseases of the central nervous system, which can lead to loss of sensory, motor function and a decline in quality of life [1]. As the population base increased, the total number of cases of SCI also increased significantly. Among them, the incidence of elderly patients increased the most, and the hospital mortality rate was still high [2]. Although recent studies have partially elucidated the pathophysiological processes after SCI, in addition to conventional SCI treatment and rehabilitation, innovative and effective treatment options have been still limited [1].
Modern pharmacological research and clinical practice have proved that Herba Epimedii and its active compound (icariin) have a wide range of pharmacological effects, especially in hormone regulation, anti-osteoporosis, immune function regulation, anti-oxidation, anti-aging [3]. Icariin (ICA) could attenuate AGE-induced oxidative stress and mitochondrial apoptosis by specifically targeting Bax and further regulating the biological function of Bax on mitochondria [4]. Some studies have shown that Icariin protected endoplasmic reticulum stress (ERS)-induced apoptosis of PC12 cells in a Synoviolin-dependent manner [5].
When cells are exposed to external stress factors and structural abnormalities, unfolded proteins accumulate in ER, triggering the ERS pathway. It can activate the pro-apoptotic factor, C/EBP homologous transcription factor protein (CHOP), which mediates programmed cell death. Glucose-regulated protein 78 (GRP78) is the molecular chaperone of ER. In order to protect cells from unfolded proteins, it can mediate the refolding of unfolded proteins [6]. ER has the function of regulating transmembrane protein and intracellular calcium concentration, synthesizing phospholipids and cholesterol, and affecting protein folding [7]. However, ERS reaction occurs in the body under the conditions of hypoxia, ischemia and trauma, which is characterized by protein folding and unfolded protein accumulation in the ER lumen [8]. ERS is one of the main mechanisms of SCI pathology [9], which can induce cell apoptosis by activating CHOP [10]. However, the three ERS sensors, inositol-requiring enzyme 1 (IRE-1), activating transcription factors 6 (ATF6) and protein kinase-like ER kinase (PERK) in the cell can alleviate ERS [11, 12], eliminate misfolded or unfolded proteins, and return the cell to steady state [13]. In normal cells, the chaperone protein Grp78 binds to the ER membrane protein PERK and blocks its activation. When unfolded proteins accumulate under ERS, Grp78 dissociates from PERK, leading to its activation, which inhibits protein synthesis and ultimately reduces the overload of misfolded proteins in ER [10, 14]. Due to the existence of internal ribosome entry sites in its mRNA, the translation of some mRNA (such as ATF4) is not inhibited, and ATF4 protein can increase the expression of genes involved in protein folding and redox control [15, 16]. If ERS reacts violently, ATF4 will increase the expression of CHOP and induce apoptosis [17].
Therefore, CHOP and GRP78 are the key factors of ERS. ICA may protect neurons and promote the recovery of SCI through this way, but the specific mechanism is still unclear, which should be further verified by experiments. PC12 is a cell line derived from a pheochromocytoma of the rat adrenal medulla, not a stem cell, that have an embryonic origin from the neural crest that has a mixture of neuroblastic cells and eosinophilic cells. So we used PC12 cells as a neuron model for experiments.
Methods
Reagents and drugs
F-12 K medium (BOSTER, USA), fetal bovine serum (Life Technology 10,099,141, USA), horse serum, DMSO (Solebao, China), penicillin/streptomycin (Yuanpei, China), trypan blue dye Liquid (Melen, China), trypsin-EDTA, BCA protein content determination kit (Jin Yibai, China), poly-L-lysine (source leaf, China), nerve growth factor (Boaosen, China), NSE, MAP 2 antibody (abcam, USA), Grp78, CHOP antibody (Santa Cruz, USA), secondary antibody (Bio-Rad, USA), thapsigargin (TG), icariin (crystal pure, China), CCK8 kit (Biosharp, China), Sulfa Rhodamine B (SRB) Kit (Beibo, China), Annexin V-FITC Apoptosis Kit (Biyuntian, China), TaKaRa PrimeScriptTM RT Master Mix Reverse Transcription Kit, TaKaRa TB GreenTM Premix Ex Taq TMII PCR kit (TaKaRa, Japan) .
Experimental grouping
The experiment was divided into 4 groups, blank group: PC12-induced differentiated neurons; DMSO group: PC12-induced differentiated neurons+ 0.1% DMSO; TG group: PC12-induced differentiated neurons+ 2 μmol/L TG (TG can induce ERS [18]); ICA group: neuronal cells of PC12 induced differentiation+ 2 μmol/L TG + 0.1 μmol/L ICA (preliminary experiments and literature review suggested that 0.1 μmol/L ICA can enhance the viability of PC12 cells [19]).
Cell culture
The PC12 cells (which can be induced to differentiate into neurons by nerve growth factor) were purchased from Jiangsu Kaiji Biotechnology Co., Ltd., melted in a water bath at 37 °C. After centrifuging, F-12 K medium containing 10% horse serum+ 5% FBS + 1% penicillin/ streptomycin was added to resuscitate PC12 cells. After counting, 1 × 104/ml cells were inoculated into 25cm2 petri dish and cultured at 37 °C in 5% CO2 incubator.
Induced differentiation and identification of cells
PC12 cells with good growth were selected and inoculated into six-well plates coated with PLL, and nerve growth factor (NGF) was used to induce differentiation. After the protuberance of PC12 cells grew, 0.25% trypsin-EDTA was used to digest for 2 min. The PBS was used to resuscitate cells which were measured. There were 2–10 × 105 cells in a EP tube, and they were divided into 2 groups with 3 tubes in each group. One group was added with 200uL NSE antibody, the other group was added with 200uL MAP 2 antibody. The mixture was gently blown and mixed, and incubated at 4 °C for 1 h. After centrifugation, 500uL cold PBS was used to wash it twice. Then the cells were transferred to the flow tube and detected by flow cytometry directly.
CCK8 assay
PC12-induced differentiated neurons were counted after digestion, centrifugation and resuspension. According to the concentration of 1 × 104/ml, 200 μL per well was inoculated into 3 96-well culture plates, and the acellular blank control group was set up parallel to the experiment. The culture plate was placed in the incubator for 24 h. After the cells adhered to the wall, each group was added to the drug-containing medium and cultured for 24 h, 48 h and 72 h, respectively. Instead of the original culture medium, F-12 K medium containing 10% horse serum+ 5% FBS + 1% penicillin/ streptomycin was added, while 10 μL CCK-8 solution was added to each well (avoid bubbles). The culture plates were placed in a static incubator for 4 h. Then the absorbance at 450 nm was determined by multi-function enzyme labeling instrument, and the difference was calculated.
SRB assay
Neuron culture was the same as above. After 24 h, 48 h and 72 h, the 96-well plate was taken out, the culture medium was absorbed and discarded, and the follow-up steps were carried out according to the instructions of SRB assay kit. After fixation, washing, dyeing and incubation, the absorbance at 515 nm was determined by enzyme labeling instrument.
Flow cytometry assay
The cells were intervened for 48 h. After digestion and centrifugation, the cells were suspended with binding buffer, mixed with MAP 2, and incubated without light for 1 h. The AnnexinV-FITC was added to incubate 10 min at room temperature without light. The cells were resuspended after washed for 3 times. The PI (final concentration was 1 μg/mL) was mixed and then flow cytometry assay was carried out.
Transmission electron microscopic observation
The cells were intervened for 48 h. After digestion and centrifugation, the cells were fixed with 2.5% glutaraldehyde. Then the cells were fixed with 1% Osmic acid. After dehydration with different gradient concentrations of ethanol, it was soaked in different proportions of acetone and entrapment solution for several hours. After 3% uranium acetate-lead citrate double staining, the microstructure of the cells was observed under transmission electron microscope, and the ER structure of the cells was observed and evaluated.
Western blot assay
The cells were intervened for 48 h. After cleavage with RIPA (including 1%PMSF), the total protein was obtained by centrifugation. After preparing separation gel and concentrated gel, electrophoresis was kept for 90 min with 100 V constant voltage. The PVDF film of appropriate size was cut, and transferred for 60 min with 100 V, 400 mA. Then the film was sealed at room temperature for 2 h by 5% skim milk. After TBST rinsing, CHOP, Grp78 and GAPDH antibodies were prepared according to the proportion of 1:1000, and incubated overnight at 4 °C. After rinsing, the second antibody was prepared according to 1:10000 and incubated at room temperature for 2 h. ECL developer was added and gel imaging system developed. The ImageJ image analysis system is used to analyze the strip and calculate the gray value of the strip.
qPCR assay
The cells were intervened for 48 h. After digestion and centrifugation, RNA was extracted by Trizol method and the concentration of RNA in each group was determined. TaKaRa reverse transcription kit was used for reverse transcription. The sequences of CHOP, Grp78 and GAPDH were found on Genbank, and primers were designed and synthesized in Shanghai Shenggong. Using rat GAPDH as internal reference, the relative quantitative analysis of CHOP and Grp78 was carried out by TaKaRa TB GreenTM Premix Ex TaqTMII PCR kit. Applied Biosystems 7500 Fast Real-Time PCR System, was used to set the conditions of fluorescence quantitative PCR amplification for PCR reaction, and the value of 2-ΔΔCt was calculated for relative quantitative analysis of the data.
Statistical analysis
SPSS 20.0 software was used for statistical analysis. The data was shown as mean ± SD. One-way ANOVA and SNK-q test were used to analyze the differences among groups. The figures were edited by GraphPad Prism 8.0.2 software. A value of P < 0.05 was considered statistically significant.
Results
PC12 cells induced by NGF had neuron-like effect
The PC12 cells induced by NGF for 7 days were observed by inverted microscope. The results showed that the induced PC12 cells had neuron-like morphology (Fig. 1a). Flow cytometry was used to detect the positive rate of MAP 2 and NSE expression. The results showed that PC12 cells induced by NGF had neuron-like effect and could be used in subsequent experiments (Fig. 1b).
Fig. 1.
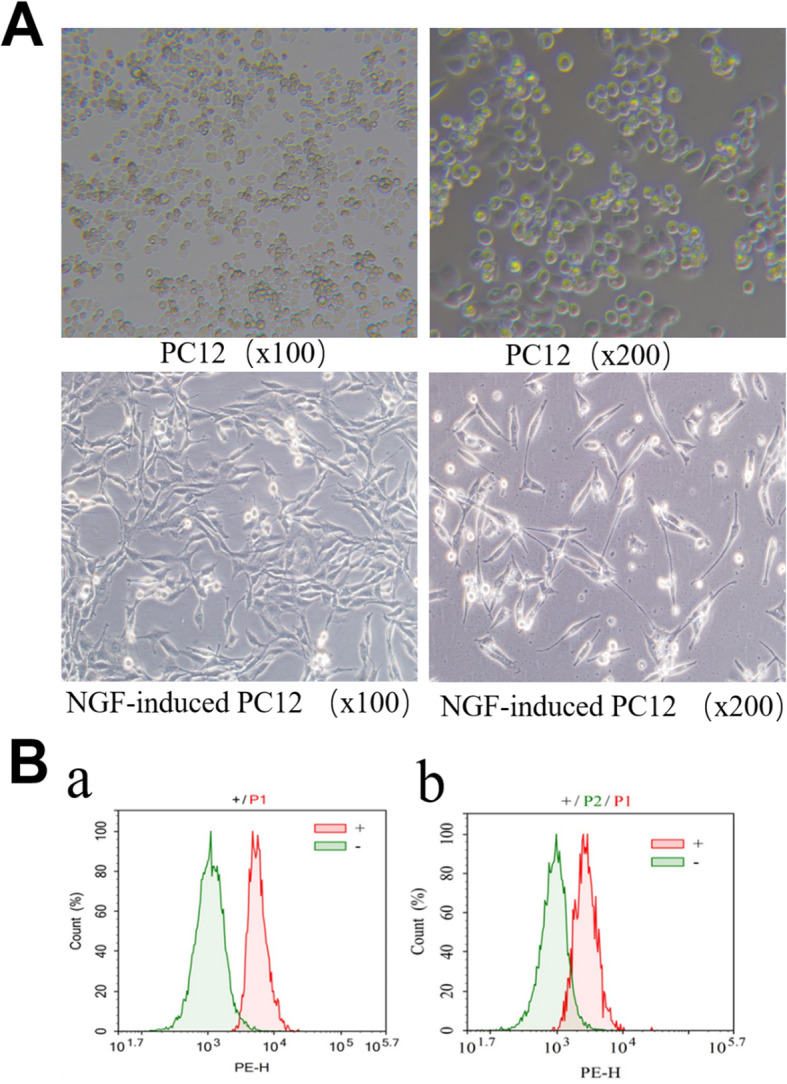
Morphology and flow cytometry identification of PC12 cells induced by NGF. a Inverted microscope was used to observe the PC12 cells and PC12 cells induced by NGF. After induction, the processes of PC12 cells became longer, and the cells grew in triangular and adherent shape. b The positive rates of MAP 2 and NSE were determined by flow cytometry. The results showed that the average positive rate of MAP 2 was 62.54% ± 1.00% (a) and the average positive rate of NSE was 93.32% ± 2.87% (b)
ICA promoted proliferation and reduced apoptosis of PC12 cells
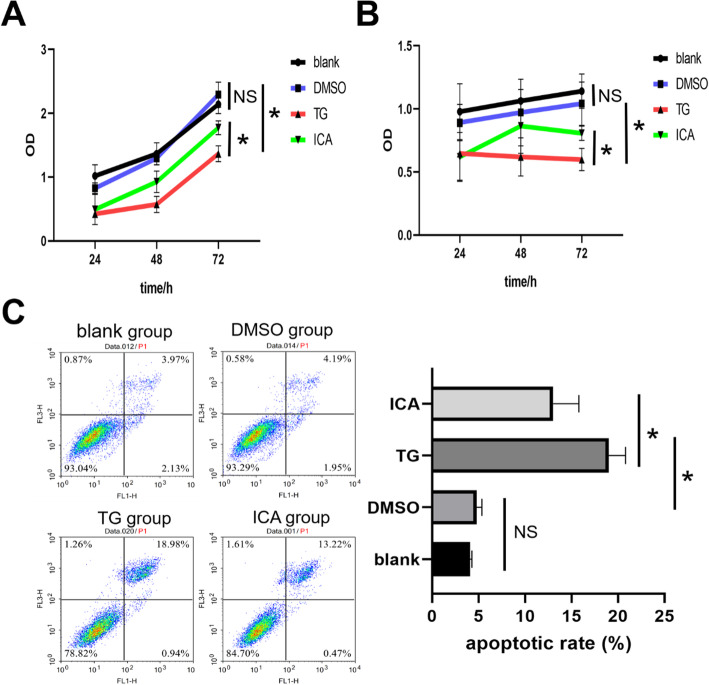
After the induced PC12 cells were cultured for 24 h, 48 h and 72 h, CCK8 assay, SRB assay and flow cytometry assay showed that DMSO had no significant effect on PC12 cells, TG could inhibit proliferation and accelerate apoptosis of PC12 cells, but ICA could promote proliferation and reduce apoptosis of PC12 cells (Fig. 2).
Fig. 2.
The results of CCK8 assay, SRB assay and flow cytometry assay. A The induced PC12 cells were cultured for 24 h, 48 h and 72 h, and CCK8 assay was carried out. The results showed that the OD value of each group increased gradually. At 72 h, there was no significant difference in OD value between DMSO group and blank group, OD value in TG group was significantly lower than that in DMSO group, and OD value in ICA group was significantly higher than that in TG group. B The induced PC12 cells were cultured for 24 h, 48 h and 72 h, and SRB assay was carried out. The results showed that the OD value of blank group and DMSO group increased gradually, but that of TG group showed a downward trend, and that of ICA group tended to be stable at 48 h. At 72 h, there was no significant difference in OD value between DMSO group and blank group, OD value in TG group was significantly lower than that in DMSO group, and OD value in ICA group was significantly higher than that in TG group. C The induced neurons were cultured for 48 h and double stained by AnnexinV-FITC and PI. The results showed that there was no significant difference in the apoptosis rate between the DMSO group and the blank group, the apoptosis rate in the TG group was significantly higher than that in the DMSO group, and the apoptosis rate in the ICA group was significantly lower than that in the TG group
ICA improved the structure of ER in PC12 cells
The neurons of each group were observed by transmission electron microscope, and the results showed that DMSO had no significant effect on the ER of PC12 cells, TG could destroy neuronal ER structure, but ICA could improve neuronal ER structure (Fig. 3).
Fig. 3.
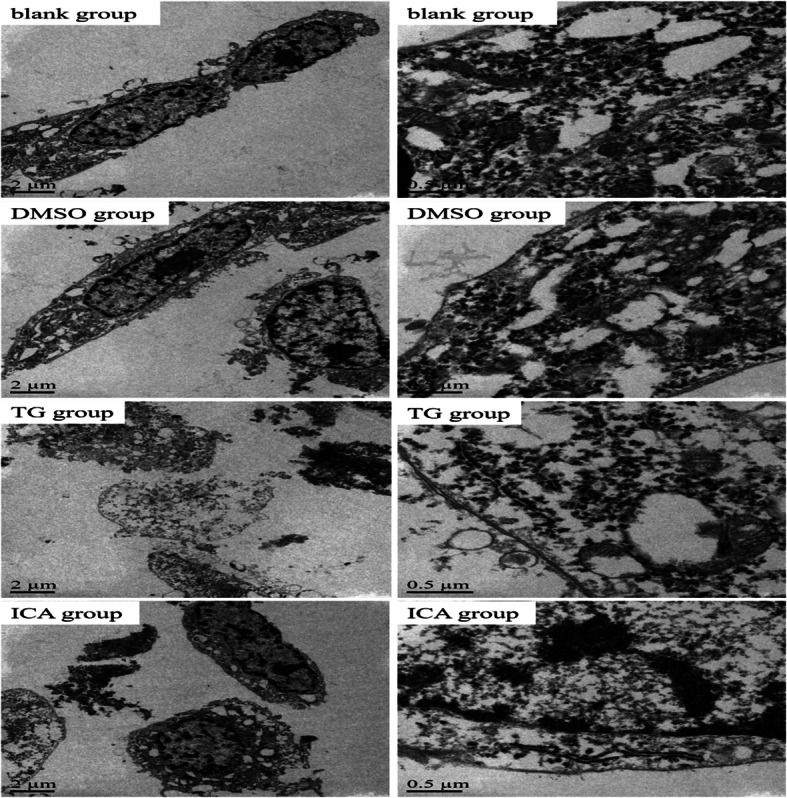
The microstructure of neuron ER. The PC12 cells of each group were observed by transmission electron microscope, and the results showed that there were abundant rough ER structures and nucleoli in blank group and DMSO group, vesicles of rough ER structure and partial nuclear fragmentation were observed in TG group, and the rough ER damage of neurons in ICA group was slighter
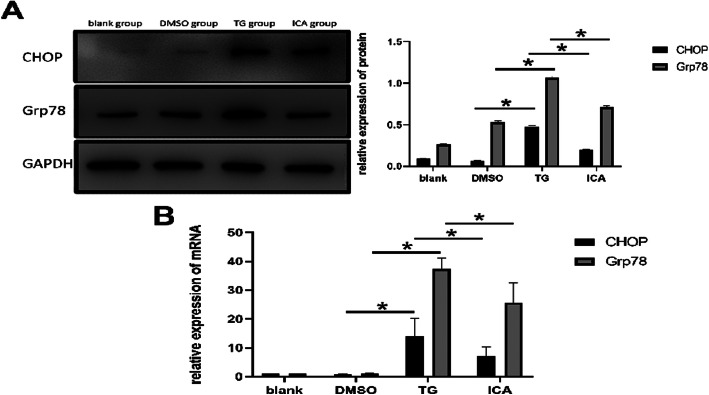
ICA reduced the expression of CHOP and Grp78 in PC12 cells
The results of Western blot and qPCR showed that TG could increase the expression of CHOP and Grp78, while ICA could decrease the expression of CHOP and Grp78, indicating that ICA could promote the recovery of PC12 cells by reducing the expression of CHOP and Grp78 (Fig. 4).
Fig. 4.
The results of Western blot and q-PCR assay. a The protein expression of CHOP and Grp78 in TG group was significantly higher than that in DMSO group. The protein expression of CHOP and Grp78 in ICA group was significantly lower than that in TG group. b The mRNA expression of CHOP and Grp78 in TG group was significantly higher than that in DMSO group. The mRNA expression of CHOP and Grp78 in ICA group was significantly lower than that in TG group
Discussion
With the development of high-risk occupations and the increase in major accidents, the probability of SCI due to high energy is gradually increasing. SCI is a serious central nervous system disease, with many irreversible complications, and greatly reduces the quality of life of patients, but so far there is no better treatment, so it is worth exploring drugs for the treatment of SCI [20]. In recent years, Chinese medicine has developed rapidly and has been widely recognized internationally in some fields. Epimedium belongs to the Berberis family and is harvested when the stems and leaves are lush in summer and autumn, then removed thick stems and impurities, and dried or dried in the shade. In Chinese medicine, Epimedium has the effects of nourishing kidney yang, strengthening muscles and bones, and dispelling rheumatism. ICA is an extract of Chinese herbal medicine Epimedium, which can be used to treat SCI [21]. ICA may repair nerve tissue by regulating endoplasmic reticulum stress, but the specific mechanism is not clear.
Changes in the microenvironment of the SCI site can lead to protein misfolding [22, 23], and downregulation of ERS may reduce neuronal apoptosis and promote neurological recovery [8, 24, 25]. Some studies have shown that ICA can significantly reduce malondialdehyde content, increase superoxide dismutase activity, improve spinal lipid peroxidation, spinal cord edema and histopathological damage, and promote the recovery of motor function in rats with SCI [26]; Early and continuous treatment of high-dose ICA can inhibit pro-inflammatory factors, oxidative stress and neuronal apoptosis through the mitochondrial apoptotic pathway, and significantly promote exercise recovery after SCI [27]. Therefore, the effect of ICA on the repair of damaged neurons may be related to ERS.
In this study, ICA was used to interfere with PC12 cells to verify the effectiveness and partial mechanism of ICA regulating ERS to repair damaged neurons. The results of CCK8, SRB and flow cytometry assay showed that ICA could alleviate ERS induced by TG and decrease PC12 cells apoptosis. The observation of the microstructure of ER by transmission electron microscope showed that ICA could improve the apoptosis of PC12 cells induced by ERS. The results of Western blot and qPCR showed that ICA could inhibit ERS induced by TG through down-regulating the expression of CHOP and Grp78. According to the above data, ICA can inhibit ERS by down-regulating the expression of CHOP and Grp78, and promote the repair of PC12 cells.
Some studies have shown that up-regulation of Grp78 is beneficial to the correct folding of proteins in ER and promotes cell recovery, while down-regulation of Grp78 can cause accumulation of unfolded proteins in ER, and continuous activation of ERS leads to apoptosis [28, 29]. The high expression of CHOP indicates the activation of ERS and the trend of apoptosis in cells [30]. Other studies have shown that CHOP has anti-apoptotic effects [31]. In this experiment, ICA inhibited ERS and down-regulated the expression of CHOP and Grp78 in damaged neurons, thereby preventing neuronal apoptosis. This is different from the results of some literatures, there may be other pathways that affect the expression of CHOP and Grp78, so multi-experimental verification and multi-system pathway research should be carried out. However, this experiment still has some shortcomings, such as no animal experiments, no gene knockout, no systematic pathway research, the next step should be studied.
Conclusions
ICA can inhibit ERS by down-regulating the expression of CHOP and Grp78, and promote the repair of PC12 cells. This study reveals part of the mechanism of ICA in the treatment of SCI and proves that ICA has the potential to promote the recovery of SCI.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- ERS
Endoplasmic reticulum stress
- ICA
Icariin
- NGF
Nerve growth factor
- SRB
Sulforhodamine B
- CHOP
C/EBP homologous transcription factor protein
- GRP78
Glucose regulation protein 78
- IRE-1
Inositol-requiring enzyme 1
- ATF6
Activating transcription factors 6
- PERK
Protein kinase-like ER kinase
- TG
Thapsigargin
Authors’ contributions
CW and GY conceived and wrote the manuscript; YM and YG provided expert comments and edits; LW, SZ, YP and PT gave some advice; All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81704100, 81573997, 81973885), 2020 Jiangsu Province Graduate Research and Practice Innovation Project (KYCX20_1533),“Qing Lan Project” of Jiangsu University Funding Project (Su Teacher [2018] No.12), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Integration of Chinese and Western Medicine) [2018]87, which supported the study design and data extraction.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The experiment was approved by the Experimental Animal Ethics Committee at Nanjing University of Chinese Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chengjie Wu and Guanglu Yang contributed equally to this work.
Contributor Information
Yang Guo, Email: drguoyang@126.com.
Yong Ma, Email: mayong@njucm.edu.cn.
References
- 1.Hayta E, Elden H. Acute spinal cord injury: a review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2018;87:25–31. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Jain NB, Ayers GD, Peterson EN, Harris MB, Morse L, O'Connor KC, Garshick E. Traumatic spinal cord injury in the United States, 1993-2012. Jama. 2015;313(22):2236–2243. doi: 10.1001/jama.2015.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma H, He X, Yang Y, Li M, Hao D, Jia Z. The genus Epimedium: an ethnopharmacological and phytochemical review. J Ethnopharmacol. 2011;134(3):519–541. doi: 10.1016/j.jep.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhao SY, Liao LX, Tu PF, Li WW, Zeng KW. Icariin inhibits AGE-induced injury in PC12 cells by directly targeting apoptosis regulator Bax. Oxidative Med Cell Longev. 2019;2019:7940808. doi: 10.1155/2019/7940808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, Gao B, Dong H, Shi J, Fang D. Icariin induces synoviolin expression through NFE2L1 to protect neurons from ER stress-induced apoptosis. PLoS One. 2015;10(3):e0119955. doi: 10.1371/journal.pone.0119955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroiwa M, Watanabe M, Katoh H, Suyama K, Matsuyama D, Imai T, Mochida J. Effect of amiloride on endoplasmic reticulum stress response in the injured spinal cord of rats. Eur J Neurosci. 2014;40(7):3120–3127. doi: 10.1111/ejn.12647. [DOI] [PubMed] [Google Scholar]
- 7.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115(10):2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohri SS, Maddie MA, Zhang Y, Shields CB, Hetman M, Whittemore SR. Deletion of the pro-apoptotic endoplasmic reticulum stress response effector CHOP does not result in improved locomotor function after severe contusive spinal cord injury. J Neurotrauma. 2012;29(3):579–588. doi: 10.1089/neu.2011.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Li H, Ren Y, Yao Y, Hu J, Zheng M, Ding Y, Chen YY, Shen Y, Wang LL, et al. Local delivery of beta-Elemene improves Locomotor functional recovery by alleviating endoplasmic reticulum stress and reducing neuronal apoptosis in rats with spinal cord injury. Cell Physiol Biochem. 2018;49(2):595–609. doi: 10.1159/000492996. [DOI] [PubMed] [Google Scholar]
- 10.Hood KN, Zhao J, Redell JB, Hylin MJ, Harris B, Perez A, Moore AN, Dash PK. Endoplasmic reticulum stress contributes to the loss of newborn hippocampal neurons after traumatic brain injury. J Neurosci. 2018;38(9):2372–2384. doi: 10.1523/JNEUROSCI.1756-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science (New York, NY) 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Davis RJ. Cell Signaling and Stress Responses. Cold Spring Harb Perspect Biol. 2016;8(10):a006072. doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 14.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 15.He CH, Gong P, Hu B, Stewart D, Choi ME, Choi AM, Alam J. Identification of activating transcription factor 4 (ATF4) as an Nrf2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001;276(24):20858–20865. doi: 10.1074/jbc.M101198200. [DOI] [PubMed] [Google Scholar]
- 16.Wek RC, Cavener DR. Translational control and the unfolded protein response. Antioxid Redox Signal. 2007;9(12):2357–2371. doi: 10.1089/ars.2007.1764. [DOI] [PubMed] [Google Scholar]
- 17.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5(5):897–904. doi: 10.1016/S1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 18.Sari FR, Watanabe K, Widyantoro B, Thandavarayan RA, Harima M, Kodama M, Aizawa Y. Sex differences play a role in cardiac endoplasmic reticulum stress (ERS) and ERS-initiated apoptosis induced by pressure overload and thapsigargin. Cardiovasc Pathol. 2011;20(5):281–290. doi: 10.1016/j.carpath.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Mo ZT, Li WN, Zhai YR, Gao SY. The effects of icariin on the expression of HIF-1alpha, HSP-60 and HSP-70 in PC12 cells suffered from oxygen-glucose deprivation-induced injury. Pharm Biol. 2017;55(1):848–852. doi: 10.1080/13880209.2017.1281968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckert MJ, Martin MJ. Trauma: spinal cord injury. Surg Clin North Am. 2017;97(5):1031–1045. doi: 10.1016/j.suc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Tohda C, Nagata A. Epimedium koreanum extract and its constituent Icariin improve motor dysfunction in spinal cord injury. Evid Based Complement Alternat Med. 2012;2012:731208. doi: 10.1155/2012/731208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Y, Yang H, Chi J, Xu Q, Zhao L, Yang W, Liu W, Yang W. Hydrogen gas attenuates myocardial ischemia reperfusion injury independent of Postconditioning in rats by attenuating endoplasmic reticulum stress-induced autophagy. Cell Physiol Biochem. 2017;43(4):1503–1514. doi: 10.1159/000481974. [DOI] [PubMed] [Google Scholar]
- 23.Chong WC, Shastri MD, Eri R. Endoplasmic Reticulum Stress and Oxidative Stress: A Vicious Nexus Implicated in Bowel Disease Pathophysiology. Int J Mol Sci. 2017;18(4):771. doi: 10.3390/ijms18040771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102(4):1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 25.Bi Y, Zhu Y, Zhang M, Zhang K, Hua X, Fang Z, Zhou J, Dai W, Cui Y, Li J, et al. Effect of Shikonin on spinal cord injury in rats via regulation of HMGB1/TLR4/NF-kB signaling pathway. Cell Physiol Biochem. 2017;43(2):481–491. doi: 10.1159/000480474. [DOI] [PubMed] [Google Scholar]
- 26.Ren XS, Ding W, Yang XY. Icariin alleviates lipid peroxidation after spinal cord injury in rats. Nan fang yi ke da xue xue bao. 2018;38(6):711–715. doi: 10.3969/j.issn.1673-4254.2018.06.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zhang X, Zhu X, Qi X, Lin K, Cheng L. The effects of Icariin on enhancing motor recovery through attenuating pro-inflammatory factors and oxidative stress via mitochondrial apoptotic pathway in the mice model of spinal cord injury. Front Physiol. 2018;9:1617. doi: 10.3389/fphys.2018.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. J Cell Physiol. 2015;230(7):1413–1420. doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao RV, Bredesen DE. Misfolded proteins, endoplasmic reticulum stress and neurodegeneration. Curr Opin Cell Biol. 2004;16(6):653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr Mol Med. 2016;16(6):533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Southwood CM, Garbern J, Jiang W, Gow A. The unfolded protein response modulates disease severity in Pelizaeus-Merzbacher disease. Neuron. 2002;36(4):585–596. doi: 10.1016/S0896-6273(02)01045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.