Abstract
Objectives:
Understanding trends in characteristics of early phase trials that allow minors with cancer to participate may inform additional efforts to improve cancer drug development for young people.
Methods:
We accessed data for oncology phase 1 or phase 1/2 trials in the United States from ClinicalTrials.gov with lower age bound for eligibility <18 years. Descriptive statistics were calculated and trends over time evaluated using logistic and multinomial logistic regression.
Results:
Six hundred twelve trials met inclusion criteria. Sixty-five percent of trials were for older adults that also allowed minors, while 9% were exclusively for patients ≤18 years of age. Eighty-three percent of trials included at least one novel agent, while 17% studied only conventional therapies. Fifty-eight percent of trials studied treatments not yet Food and Drug Administration (FDA) approved (48% if exclusively for patients ≤18 years). Fifteen percent of trials for which dose-escalation method could be determined, utilized a model-based design. Eighteen percent of all trials were industry sponsored (48% if exclusively for patients ≤18 years). Forty-nine percent of all trials were multicenter (69% if exclusively for patients ≤18 years). There was an increase in trials exclusively focused on patients with central nervous system (CNS) tumors over the study period (P ≤ .02). No other temporal trends were seen. The median times from first-in-adult to first-in-pediatric for monotherapy and combination trials were 5.7 and 3.3 years, respectively.
Conclusion:
The paucity of clear temporal trends highlights the need for innovation in early drug development for young people. Our analysis serves as a benchmark against which to evaluate initiatives to improve pediatric cancer drug development.
Keywords: adolescent, combination, monotherapy, novel, oncology, pediatric, phase 1 trials
1 |. INTRODUCTION
Five-year survival rates for children and adolescents with cancer have improved remarkably over the last 30 years. This achievement has been largely a result of cooperative clinical trials that have tested the efficacy of dose intensification of cytotoxic chemotherapy. Despite these advances, cancer remains the leading cause of death from disease in minors in the United States.1 Additionally, cytotoxic chemotherapy has remained the mainstay of treatment for the last half century, which has resulted in increased treatment-related mortality and long-term toxicity in childhood cancer survivors, highlighting the need for innovative therapeutic approaches for young people with cancer.2
Phase 1 trials have been the traditional starting point for clinical development of new therapies in pediatric oncology. Despite the prominent role these trials play in pediatric cancer drug development, the landscape of early phase trials that include minors with cancer has not been well described. This information is critical for multiple stakeholders involved in drug development for these rare diseases. For example, simulation studies comparing dose-escalation trial designs have highlighted the importance of novel trial designs that better identify target dose and require fewer patients to do so.3,4 The rate of adoption of these designs in pediatric oncology has not been well described. Likewise, position statements have emphasized the need for earlier combination testing of novel agents for young people with cancer, but the distribution of trials utilizing monotherapy versus combination therapy approaches is unknown.4 A recent analysis demonstrated a median lag time of 6.5 years from first-in-human trials to first-in-pediatric trials of U.S. Food and Drug Administration (FDA)-approved novel anticancer agents.5 Data are lacking on the lag time for agents not yet FDA approved. Recent regulatory measures, such as the Research to Accelerate Cure and Equity for Children Act (RACE Act), are attempting to stimulate earlier access to novel agents for children and adolescents with cancer.6–8 Understanding the landscape of early phase trials may inform additional efforts to improve pediatric cancer drug development while also providing baseline metrics to assess the impact of new regulatory measures.
We performed a systematic evaluation of phase 1 and phase 1/2 clinical trials that allowed patients <18 years of age in at least one center in the United States over the last 12 years using data from the ClinicalTrials.gov registry. We sought to determine (a) number and types of these trials over time; (b) drug and patient characteristics; (c) trial design elements; and (d) time between first-in-adult clinical trials for FDA-approved and unapproved agents and the analogous first-in-pediatric clinical trial.
2 |. METHODS
2.1 |. Data source and trial inclusion
ClinicalTrials.gov is a US registry with information on clinical trial phase, interventions, sponsorship, expected and actual enrollment, and age requirements for registered clinical trials. Trial records were accessed on June 5, 2019 using ClinicalTrials.gov filters to include only oncology trials that reported a lower bound for age of eligibility <18 years. Trials that were coded as early phase 1, phase 1, or phase 1/2 trials and that had an “interventional” study type were included. Only trials with one or more sites in the United States with actual study start dates of September 27, 2007 to May 1, 2019 were included (September 27, 2007 corresponding to date of mandatory trial reporting in ClinicalTrials.gov by Section 801 of the Food and Drug Administration Amendments Act of 2007). Trials that were withdrawn before any patient enrolled were excluded. Trials found on manual review to study a benign condition were excluded (Figure S1). Each trial was identified by its unique national clinical trial (NCT) number.
2.2 |. Variables and data extraction
Trials were defined as therapeutic trials if they included an active anticancer intervention or a posthematopoietic stem cell transplant intervention in a cancer population. Trials that included an active intervention with the intent of treating or preventing toxicity of anticancer therapy or preventing infection were defined as supportive care trials.
The cancer indication for each trial was categorized as solid, central nervous system (CNS), hematologic, or spanning multiple cancer categories based on the conditions listed on ClinicalTrials.gov. The interventions under investigation were classified as chemotherapy, novel agent, radiation, surgery, or a combination of these categories according to the definitions provided by the National Cancer Institute (NCI).9 Interventions were classified as FDA approved or not FDA approved at the time of actual study start date on ClinicalTrials.gov using the FDA drug database (https://www.accessdata.fda.gov/scripts/cder/daf/). If a combination of agents was used, the combination was coded as not FDA approved if the trial included any agent that was not approved at the time of trial registration. Age of eligibility was defined as pediatric only (maximum age of eligibility ≤18 years), child, adolescents and young adults (AYA; maximum age of eligibility ≤21 years) or pediatric and adult (lower age range <18 years; maximum age >21 years). Sponsorship was categorized as industry versus nonindustry. Trial duration was determined based on start date and primary completion date for studies that were listed as completed on ClinicalTrials.gov at the time the data were accessed. Sample size data were only used for those trials with reported actual (rather than projected) enrollment numbers available in ClinicalTrials.gov. Trials were defined as single center or multicenter, and as US only or US plus international based on the locations listed.
For dose-finding trials with multiple dose levels, study design was extracted from ClinicalTrials.gov or, if not available, from abstracts or manuscripts obtained from internet and PubMed searches. Study designs were categorized as model-based designs or rule-based designs. Model-based designs were those that used probabilistic (eg, Bayesian) distributions to estimate target toxicity. Rule-based designs were those that used predefined rules for sequential treatment of cohorts (eg, 3 +3 and rolling six designs) to titrate doses.10
The start date of the first-in-adult trial studying the intervention of interest in the corresponding trial that allowed minors was determined by searching ClinicalTrials.gov using the interventions listed for each trial.
2.3 |. Statistical analysis
To investigate temporal trends in dichotomous trial characteristics, logistic regression was used treating year of trial registration as a continuous predictor. For categorical trial characteristics with more than two categories, multinomial logistic regression was used treating year of trial registration as a continuous predictor. Two-sided P-values <.05 were considered statistically significant. Statistical analysis was performed using the mlogit package11 in R version 3.5.0.
3 |. RESULTS
3.1 |. Search outcome
Figure S1 shows the flow diagram of the trial search. Among the 717 trials that were identified, 105 trials did not meet our inclusion criteria, yielding 612 phase 1 or phase 1/2 trials that allowed patients <18 years. Of these, 24 were supportive care trials and 588 were anticancer trials.
3.2 |. Characteristics of phase 1 oncology trials that allowed children
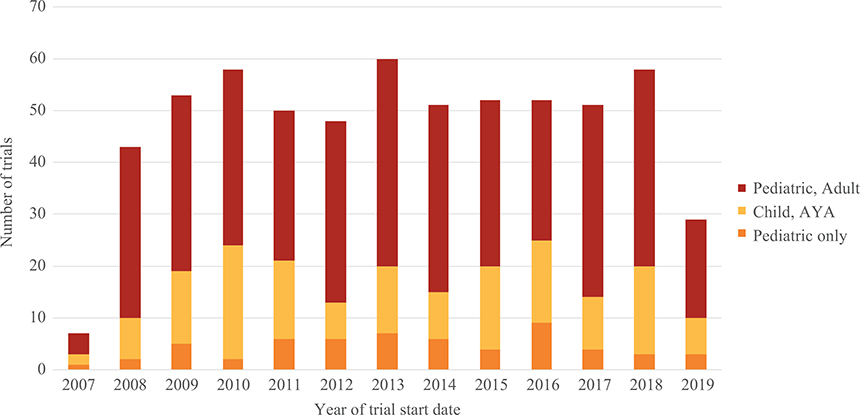
These 612 trials were categorized by the year of trial start date. There was a mean of 52 trials (range 43–60) per year from 2008 to 2018 (Figure 1).
FIGURE 1.
Analysis of the number of new phase 1 or phase 1/2 oncology trials defined by age of eligibility per year from September 27, 2007 to May 1, 2019. Data for 2007 and 2019 only include trials from September 27, 2007 to December 31, 2007, and from January 1, 2019 to May 1, 2019, respectively. Year defined by trial start date on ClinicalTrials.gov; pediatric only: maximum age ≤ 18 years; child, adolescent and young adult (AYA): maximum age ≤ 21 years; pediatric and adult: lower age range <18 years, maximum age >21 years
Figure 1 also displays trial numbers by age of eligibility of these trials over time. Only 58 (9%) trials were exclusively pediatric trials, while 156 (25%) were open to children, adolescents, and young adults. The majority of trials (65%) included older adults and also allowed patients less than 18 years old to participate (Table 1). Of these trials enrolling pediatric and older adult patients, 12% excluded patients younger than 15 years, and 14% excluded patients younger than 10 years.
TABLE 1.
Characteristics of 612 phase 1 oncology trials available to patients <18 years in the United States (September 27, 2007 to May 1, 2019)
| Trial characteristics | All trials N (%)a | Pediatric only N (%)a | Child, AYA N (%)a | Pediatric, adult N (%)a |
|---|---|---|---|---|
| FDA-approval status of agent(s) under study at the time of trial registration | ||||
| Approved | 258 (42) | 30 (52) | 65 (42) | 163 (41) |
| Not approved | 354 (58) | 28 (48) | 91 (58) | 235 (59) |
| Primary oncology indication under investigation | ||||
| Hematologic malignancy | 216 (35) | 14 (24) | 33 (21) | 169 (42) |
| Solid tumor | 194 (32) | 14 (24) | 38 (24) | 142 (36) |
| Mixed | 102 (17) | 18 (31) | 36 (23) | 48 (12) |
| CNS tumor | 100 (16) | 12 (21) | 49 (31) | 39 (10) |
| Treatment under investigation | ||||
| Conventional therapies only | 105 (17) | 14 (24) | 28 (18) | 63 (16) |
| At least one novel agent | 507 (83) | 44 (76) | 128 (82) | 335 (84) |
| Age of eligibility | ||||
| Max age ≤ 18 years | 58 (9) | 58 (100) | ||
| Max age ≤ 21 years | 156 (25) | 156 (100) | ||
| Lower age < 18; max age > 21 years | 398 (65) | 398 (100) | ||
| Trial phase | ||||
| Phase 1 | 453 (74) | 39 (67) | 129 (83) | 285 (72) |
| Phase 1/phase 2 | 159 (26) | 19 (33) | 27 (17) | 113 (28) |
| Sponsorship | ||||
| Nonindustry | 501 (82) | 30 (52) | 135 (87) | 336 (84) |
| Industry | 111 (18) | 28 (48) | 21 (13) | 62 (16) |
| Number of centers | ||||
| Single center | 315 (51) | 18 (31) | 70 (45) | 225 (57) |
| Multicenter | 297 (49) | 40 (69) | 86 (55) | 171 (43) |
| Study site | ||||
| United States sites only | 518 (85) | 36 (62) | 125 (81) | 357 (90) |
| United States + international sites | 94 (15) | 22 (38) | 31 (20) | 41 (10) |
| Study status as of June 5, 2019 | ||||
| Recruiting | 242 (40) | 23 (40) | 64 (41) | 155 (39) |
| Completed | 189 (31) | 21 (36) | 59 (38) | 109 (27) |
| Active, not recruiting | 90 (15) | 5 (9) | 12 (8) | 73 (18) |
| Terminated | 66 (11) | 8 (14) | 15 (10) | 43 (11) |
| Unknown status | 12 (2) | 0 (0) | 3 (2) | 9 (2) |
| Not yet recruiting | 9 (1) | 1 (2) | 2 (1) | 6 (2) |
| Suspended | 4 (1) | 0 (0) | 1 (1) | 3 (1) |
| Duration of trials in years | Median (range) | Median (range) | Median (range) | Median (range) |
| Completed trials | 3.8 (0.6–10.6) | 3.7 (1.5–7.0) | 3.6 (0.9–8.7) | 4.3 (0.6–10.6) |
Abbreviations: AYA, adolescent and young adult; CNS, central nervous system; FDA, U.S. Food and Drug Administration; NIH, National Institutes of Health.
Percentages may not add to 100 due to rounding off.
Additional characteristics beyond age of eligibility of the 612 trials are also shown in Table 1. The majority of trials (58%) studied interventions or treatments that were not yet FDA approved as of the date the trial started. However, among pediatric-only studies, 52% were already FDA approved at the start of the trial. The majority of trials included patients with hematologic (35%) or solid (32%) cancers, while the minority studied patients with CNS tumors (16%) or included a range of malignancies across disease categories (17%). The majority of studies (74%) were phase 1 trials, with 26% classified as phase 1/phase 2 studies. Fifty-one percent of all trials were opened at a single center and only 15% included at least one site outside of the United States. Only 18% of studies were sponsored by the industry. Eleven percent of all studies were terminated early (Figure S2 and Table 1). Focusing on pediatric-only studies (Table 1), 69% of studies were multicenter, 48% were industry sponsoreed, and 14% were terminated early. The median time to completion for all completed trials was 3.8 years (range 0.6–10.6 years).
We next investigated potential temporal trends in trial characteristics (Table 2 and Figures S3–S9). Compared to the reference group of trials focused exclusively on patients with CNS tumors, there were significant decreases over time in trials including patients with a range of malignancies across disease categories (OR = 0.89; 95% CI = 0.820.97; P = .007) or focused exclusively on patients with solid tumors (OR = 0.92; 95% CI = 0.85–0.99; P = .02). In other words, there was 11% and 8% decrease in odds of histology agnostic trials and trials for patients with solid tumors, respectively, for every 1 year increase in trial start. There were no other statistically significant temporal trends for other trial characteristics (P ≥ .05).
TABLE 2.
Logistic/multinomial logistic regression results for testing the association of trial characteristics with year of trial registration
| Trial characteristics | Odds ratioa (95% confidence interval) | P-value |
|---|---|---|
| FDA approval status of agent(s) at the time of trial registration | ||
| Not approved vs approved | 1.04 (0.99, 1.09) | .12 |
| Primary oncology indication under investigation | ||
| Hematologic malignancy vs central nervous system | 0.94 (0.87, 1.01) | .07 |
| Mixed vs central nervous system | 0.89 (0.82, 0.97) | .007 |
| Solid vs central nervous system | 0.92 (0.85, 0.99) | .02 |
| Trial phase | ||
| Phase 1/2 vs phase 1 | 0.98 (0.93, 1.03) | .45 |
| Number of centers | ||
| Single center vs multicenter | 0.98 (0.93, 1.03) | .36 |
| Sponsorship | ||
| Nonindustry vs industry | 0.95 (0.89, 1.01) | .11 |
| Design | ||
| Rule-based vs model-based | 1.08 (0.96, 1.21) | .18 |
| Treatment type | ||
| Monotherapy vs combination therapy | 1.03 (0.98, 1.08) | .20 |
| Study site | ||
| US plus outside US vs US only | 1.00 (0.94, 1.07) | 1.00 |
Odds ratio reflects change in odds for each unit increase in the year of trial registration for a given trial to have the stated characteristic compared to reference characteristic.
3.3 |. Treatment characteristics
Eighty-three percent of trials included at least one novel agent per NCI definition, while 17% utilized only conventional therapies (chemotherapy, radiation, or surgery; Table 1 and Figure S10). Thirteen trials focused exclusively on radiotherapy or surgery. The remaining 599 trials evaluated interventions other than radiotherapy or surgery and were the focus of the following subsequent detailed analyses. The majority (n = 364) of these 599 trials studied combination therapies rather than single agents, with no clear temporal trends across the study period (OR = 1.03; 95% CI = 0.98–1.08; P = .20; Figure 2A). This pattern was not consistent across studies defined by age of eligibility (Figure 2B). The majority of pediatric-only trials were focused on single agents (n = 39, 71%), whereas the majority of studies that also included patients >18 years of age were focusedon combination therapies (n = 348, 64%).
FIGURE 2.
Analysis of monotherapy versus combination interventions under investigation in phase 1 oncology drug trials that allowed patients <18 years from September 27, 2007 to May 1, 2019, with year defined by trial start date on ClinicalTrials.gov. A, Proportion of drug trials evaluating combination of multiple drugs (combo) or single agents (mono) per year; n = 599. B, Treatment modalities as a function of trial ages of eligibility for enrollment; n = 599. Pediatric only: maximum age ≤ 18 years; child, adolescent and young adult (AYA): maximum age ≤ 21 years; pediatric and adult: lower age range < 18 years, maximum age > 21 years. C, Combination drug treatment modalities per year from September 27, 2007 to May 1, 2019; n = 342
We also assessed the types of combination therapies under investigation in the 342 trials that included two or more agents in the same trial (Figure 2C). Seventy-seven percent of trials included conventional cytotoxic chemotherapy as a component of the combination under investigation, including trials of chemotherapy + chemotherapy combinations and chemotherapy + novel agent combinations. Trials studying two or more novel agents in combination, without the addition of chemotherapy, were less common (23%). This pattern was consistent across studies defined by age of eligibility (Figure S11). The majority of combination trials across all age groups were focused on combinations that included chemotherapy, whereas the minority of combination studies were focused on exclusively novel + novel combination therapies.
3.4 |. Type of study designs
As dose finding is a common goal of phase 1 trials, we evaluated the design used for dose escalation, when available in ClinicalTrials.gov or in the literature (n = 231). Only 15% (35/231) of the studies used a model-based dose-escalation design (range across years = 4–35%). Evaluation of dose-escalation methods across time showed that rule-based designs have been the predominant approach without clear temporal trends (OR = 1.08; 95% CI = 0.96–1.21; P = .18; Figure 3). Analysis of actual enrollments by study design showed a median enrollment of 30.5 and 26.0 subjects per trial for model-based and rule-based designs, respectively (Figure S12). Analysis of completion length by study design showed a median time to completion of 3.5 years (range 0.6–6.6) and 4.1 years (range 1.0–10.6) for completed trials using model-based and rule-based designs, respectively.
FIGURE 3.
Analysis of dose-escalation method for phase 1 oncology trials that allowed patients <18 years from September 27, 2007 to May 1, 2019. Model-based designs include Bayesian models and variations, and rule-based designs include 3 + 3 designs and variations (eg, rolling six design). Analysis only includes those trials with dose-escalation method recorded on ClinicalTrials.gov or found in primary trial publication; n = 231
3.5 |. Timing of first-in-pediatric phase 1 oncology trials
For trials with a single agent under investigation, we evaluated the amount of time between the start date of the first trial to allow patients <18 years (first-in-pediatric) and the analogous first-in-adult trial (n = 192 agents; Figure 4A). We found a median lag time of 5.68 years between the first-in-adult trial and first-in-pediatric trial (range: −4.17 to 23.52 years; negative values indicate first-in-pediatric study occurred before first-in-adult study). We also evaluated 75 combinations being studied in trials that included patients <18 years that had also been evaluated in adults (Figure 4B). We found a median lag time of 3.29 years between the first-in-adult trial of the combination and the first-in-pediatric combination trial (range: −2.43 to 19.34 years).
FIGURE 4.
Analysis of time between the start date of phase 1 oncology trials that allowed patients <18 years from September 27, 2007 to May 1, 2019 and the analogous first-in-adult trial. Negative values indicate first-in-pediatric study occurred before first-in-adult study. A, Analysis includes only those phase 1 trials with a single-agent intervention for which an analogous adult trial could be found; n = 192. Each line represents a trial; median value 5.68 years. B, Analysis includes only those phase 1 trials with multiple-agent interventions for which an analogous adult trial could be found; n = 75. Each line represents a trial; median value 3.29 years
4 |. DISCUSSION
We present a comprehensive analysis of 612 phase 1 oncology trials in the United States that allowed patients <18 years over the last 12 years, using a publicly available registry. Our study provides new insights into temporal trends regarding the prevalence and characteristics of early phase trials available to minors with cancer. The lack of a clear change in most metrics over time is noteworthy and highlights the need for innovation in early drug development relevant to young people with cancer. Additionally, we found that the majority of phase 1 trials open to minors with cancer are adult studies with expanded enrollment for patients <18 years. However, the potential for a patient <18 years of age to actually access one of these trials remains unknown. We have also confirmed a substantial lag time between first-in-pediatric and first-in-adult trials of both approved and nonapproved monotherapies and combinations. Our analysis serves as a baseline against which to assess the impact of new regulatory measures in the coming years, including the RACE Act. Under the RACE Act, the FDA will have the authority to mandate evaluation of oncology products in pediatrics if the molecular target of the drug is considered relevant to the growth or progression of pediatric cancer.
Several findings are cause for concern for key stakeholders focused on pediatric cancer drug development. A recent publication reported on 7897 phase 1 or phase 1/2 oncology clinical trials open exclusively to adults over a similar timeframe to our analysis.12 In this context, the 612 trials in our study indicate that a small fraction of phase 1 or phase 1/2 trials allows minors with cancer to participate and most of these trials are adult trials with age of eligibility extended to <18 years. The proportion of patients <18 years that actually enrolled to these trials is not provided in the registry, and there are clinical and regulatory issues that may have limited pediatric participation, such as competition for slots and need to ensure that a pediatric investigator is included on the study team.8 In addition, many of these trials explicitly excluded younger pediatric patients from participation. Therefore, the extent to which data derived from patients <18 years who participated in these trials can inform safety and dosing decisions for younger pediatric patients is not clear. A focus on single-center studies may further reduce efficiency in the context of rare diseases and may have contributed to the median of nearly 4 years needed to complete these trials. An 11% rate of early trial termination is another area of concern, though it is not clear if design considerations, agent availability, or other factors contributed to decisions to terminate each trial.13 The 14% termination rate in pediatric-only trials suggests additional feasibility issues, such as very rare target populations for this age group or frequency of single-center studies. While there has been much interest in novel + novel drug combinations, we observed an overall paucity of such studies and a heavy reliance on chemotherapy combinations.
Pediatric phase 1 trials most commonly start after initial testing in adults, which provides baseline knowledge about the toxicity profile, dosing, and pharmacokinetics of a novel agent. This often results in an expected delay in access to innovative therapeutics for children and adolescents with cancer. We describe a median lag time of 5.68 years for monotherapy trials and 3.29 years for combination therapy trials. These metrics are shorter than a previously reported median of 6.5 years in a prior analysis focused exclusively on FDA-approved anticancer agents.3 These differences may reflect different methodologies between analyses, though the shorter lag time for combination testing may reflect greater experience with individual novel agents given as monotherapy and therefore less reluctance to embark upon pediatric clinical testing. New drug development paradigms that allow nearly simultaneous testing in children, adolescents, and adults with cancer with shared biology may reduce these lag times.14,15 Prioritizing use of scientifically based and clinically relevant eligibility criteria is necessary to expand access to trials and lessen this delay.16 Encouraging more trials that span an eligibility age of 18 years has the potential to enhance access to novel agents, though a substantial proportion of such trials excluded younger children.8,17
Efficient trial designs that maximally inform subsequent trials are critical in the conduct of early phase trials in rare diseases. A common starting dose in pediatric phase 1 cancer trials has been approximately 80% of the adult maximum tolerated dose (MTD).18 However, it has been demonstrated that the pediatric MTD often corresponds to the adult phase 1 MTD.19 To spur pediatric oncology drug development, new study designs that will decrease the number of dose levels are needed.19,20 Recent studies have shown that model-based dose-escalation designs can maximize the efficiency of early phase trials.4,21,22 Despite this, our research suggests that the overwhelming majority of phase 1 clinical trials that allowed patients <18 years have used rule-based escalation designs. These designs were developed for cytotoxic drugs with a clear dose-toxicity relationship and aimed to limit severe toxicities by only exposing a small group of patients to increasing doses of the agent. As fewer trials are now focused solely on conventional chemotherapy, trial methodology should also evolve.
We acknowledge several limitations in our study. Phase 1 clinical trials are not subject to the registration and results submission requirements of the Public Health Service Act. Trials included in our analysis were therefore those posted on ClinicalTrials.gov voluntarily by trial sponsors. We also relied on data in ClinicalTrials.gov for assessing start dates of trials for the purposes of calculating the time between first-in-pediatric and first-in-adult trials. Since not all phase 1 trials were required to be posted, we acknowledge that earlier trials may have not been registered. Further, our data are largely based upon available data posted on ClinicalTrials.gov, and therefore depended upon sponsors to provide updated data. For example, less than half of the studies included dose-escalation design, though we supplemented these data with additional information obtained from literature and internet searches. We aimed to describe the landscape of trials available to patients <18 years with cancer, but our findings may not be representative of trials designed specifically for a pediatric oncology population. The particular pediatric facilities and expertise at centers where adult trials with expanded eligibility to include patients <18 years remains unknown based upon available data in ClinicalTrials.gov. We likewise acknowledge that the number of patients <18 years who actually enrolled to these largely adult trials is not reported, and is a critical point since barriers may exist for younger patients to actually access this type of trial. Finally, we chose to focus on studies open in the United States, and it is therefore not clear to what extent our findings will generalize to trials conducted in the rest of the world. However, we do note that the European Paediatric Regulation went into effect in 2007, which aligns well with the start date for our analysis. As pediatric cancer drug development is increasingly getting global in nature, it is important to acknowledge that regulatory changes in the European Union may impact trials available in the United States and vice versa.
Under the RACE Act, pediatric studies for oncology products will no longer be exempted based on orphan drug status or lack of specific adult cancer histologies in a pediatric population.7 These changes will help to advance drug development in this population. Our analysis over the last 12 years serves as a necessary benchmark to evaluate the effects of this new regulation. Improving systems to facilitate enrollment of children and adolescents to trials that allow patients <18 years of age to participate will also enhance access to trials already being conducted. Implementation of modern study designs, novel + novel combination therapy and rationally designed eligibility criteria in multicenter early phase trials will complement this new law, and help accelerate drug development for children and adolescents with cancer.
Supplementary Material
Abbreviations:
- AYA
adolescents and young adults
- CNS
central nervous system
- FDA
Food and Drug Administration
- MTD
maximum tolerated dose
- NCI
National Cancer Institute
- RACE Act
Research to Accelerate Cure and Equity for Children Act
Footnotes
CONFLICT OF INTEREST
Steven G. DuBois reports travel expenses from Loxo Oncology, Roche, and Salarius, and consulting fee from Bayer and Loxo Oncology.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in ClinicalTrials.gov at https://clinicaltrials.gov/ct2/results?type=Intr&cond=Cancer&cntry=US&age=0&phase=04&strd_s=09%2F27%2F2007&strd_e=05%2F01%2F2019&down_fmt=csv&down_flds=all.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32(12):1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doussau A, Geoerger B, Jiménez I, Paoletti X. Innovations for phase I dose-finding designs in pediatric oncology clinical trials. Contemp Clin Trials. 2016;47:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno L, Pearson ADJ, Paoletti X, et al. Early phase clinical trials of anticancer agents in children and adolescents - an ITCC perspective. Nat Rev Clin Oncol. 2017;14(8):497–507. [DOI] [PubMed] [Google Scholar]
- 5.Neel DV, Shulman DS, DuBois SG. Timing of first-in-child trials of FDA-approved oncology drugs. Eur J Cancer. 2019;112:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shulman DS, DuBois SG. Winning the RACE: expanding pediatric cancer drug approvals. Pediatr Blood Cancer. 2019;66(8): e27705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barone A, Casey D, McKee AE, Reaman G. Cancer drugs approved for use in children: impact of legislative initiatives and future opportunities. Pediatr Blood Cancer. 2019;66(8):e27809. [DOI] [PubMed] [Google Scholar]
- 8.Chuk MK, Mulugeta Y, Roth-Cline M, Mehrotra N, Reaman GH. Enrolling adolescents in disease/target-appropriate adult oncology clinical trials of investigational agents. Clin Cancer Res. 2017;1: 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Targeted Cancer Therapies https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet.
- 10.Skolnik J, Barrett J, Jayaraman B, Patel D, Adamson P. Shortening the timeline of pediatric phase 1 trials: the rolling six design. J Clin Oncol. 2008;26(2):190–195. [DOI] [PubMed] [Google Scholar]
- 11.Croissant Yves. mlogit:Multinomial Logit Models. R Package Version 1.0–1; 2019. https://CRAN.R-project.org/package=mlogit. [Google Scholar]
- 12.Neel DV, Shulman DS, Ma C, Bourgeois F, DuBois SG. Sponsorship of oncology clinical trials in the United States according to age of eligibility. Cancer Med. 2020;9(13):4495–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rees C, Pica N, Monuteaux M, Bourgeois F. Noncompletion and non-publication of trials studying rare diseases: a cross-sectional analysis. PLoS Med. 2019;16(11):e1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laetsch TW, DuBois SG, Mascarenhas L, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol. 2018;19(5):705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drilon A, Laetsch T, Kummar S, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim ES, Bernstein D, Hilsenbek SG, et al. Modernizing eligibility criteria for molecularly driven trials. J Clin Oncol. 2015;33:2815–2820. [DOI] [PubMed] [Google Scholar]
- 17.Gore L, Ivy SP, Balis FM, et al. Modernizing clinical trial eligibility: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Minimum Age Working Group. J Clin Oncol. 2017;35:3781–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M, Bernstein M, Bleyer WA, et al. Conduct of phase 1 trials in children with cancer. J Clin Oncol. 1998;16:966–978. [DOI] [PubMed] [Google Scholar]
- 19.Lee D, Skolnik J, Adamson P. Pediatric phase I trials in oncology: an analysis of study conduct efficiency. J Clin Oncol. 2005;23:8431–8441. [DOI] [PubMed] [Google Scholar]
- 20.Paoletti X, Geoerger B, Doz F, Baruchel A, Lokiec F, Le Tourneau C. A comparative analysis of pediatric dose-finding trials of molecularly targeted agent with adults’ trials. Eur J Cancer. 2013;49(10):2392–2402. [DOI] [PubMed] [Google Scholar]
- 21.Onar-Thomas A, Xiong Z. A simulation-based comparison of the traditional method, Rolling-6 design and a frequentist version of the continual reassessment method with special attention to trial duration in pediatric Phase 1 oncology trials. Contemp Clin Trials. 2010;31:259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao L, Lee J, Mody R, Braun TM. The superiority of the time-to-event continual reassessment method to the rolling six design in pediatric oncology Phase 1 trials. Clin Trials. 2011;8(4):361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.