Abstract
Autism spectrum disorder (ASD) is a group of heterogeneous neurodevelopmental disorders characterized by impairments activities without efficient pharmacological therapies in social interaction, speech and stereotypic patterns. Clinical studies have shown the efficacy of acupuncture as an alternative therapy for autism. The effectiveness of acupuncture as an alternative treatment for autism has been demonstrated through clinical trials. However, the molecular mechanisms that underlie these effects remain unclear. Due to its profound pro-social, anxiolytic, stress management effects, and its potential use for the treatment of psychiatric disorders associated with altered socioemotional competence, oxytocin (OT) released from the hypothalamus has attracted considerable interest. In the past decade, a number of clinical and animal studies have shown that OT administration effectively reduces core symptoms of ASD, especially social behavior deficits. Recently, the endocannabinoid system has emerged as a promising target for the treatment of autism. OT was found to facilitate the endocannabinoid-mediated social reward processes in the nucleus accumbens of the mouse brain. Furthermore, serotonin and dopamine are involved in the reward response mediated by OT. In view of these findings, we conclude that acupuncture may produce therapeutic effects on autism by triggering the hypothalamic oxytocin system, which in turn activates the release of neurotransmitters such as endocannabinoids, dopamine and serotonin. This would be a valuable guide for further research on the mechanism of treatment of autism with acupuncture.
Keywords: autism spectrum disorder, oxytocin, endocannabinoid, serotonin, dopamine, nucleus accumbens, ventral tegmental area
Autism spectrum disorder (ASD) is a developmental disability, typically manifested as difficulties in social interaction, repetitive behaviors and deficiency in language communication, which seriously affects the quality of life of patients. So far, there are no effective medicines for the treatment of ASD. However, studies indicate that early intervention can improve children’s development. The cause of ASD has not been fully determined, but cumulative evidence has shown that the onset of ASD is the result of multifactor interactions, including gene mutation, environmental contamination, and abnormal development of central nervous system (CNS) [1,2]. In terms of the nervous system, the pathogenesis of ASD is closely associated with damaged neurotransmitter systems in the nervous system, such as serotonin (5-HT), norepinephrine, dopamine, and pineal–hypothalamic–pituitary–adrenal axis dysfunction, and related neuropeptides and hormones [3,4,5].
Peñagarikano et al. demonstrated that oxytocin (OT) significantly improves social behavior in a mouse model of autism, and that this effect can be sustained over a longer period of time if treated early [6]. Recently, a research group from Hamamatsu Medical University in Japan announced that they have shown a tendency to improve symptoms in people with ASD after injecting with OT (a trial of TTA-121 on autism spectrum disorder). It is known that OT is mainly produced by the giant cells in the supraoptic nucleus (SON) and paraventricular nucleus (PVN) of the hypothalamus, then transported through the nerve fibers of the hypothalamic pituitary axis to the posterior pituitary gland, and finally released into the blood, acting on various organs throughout the body, and finally exerts its important biological functions [7]. In women, OT can trigger uterine contractions in childbirth, stimulate milk secretion, and create contact between the mother and the child through affection. In addition, OT can also reduce the levels of corticosterone and other stress hormones in the body, and lower the blood pressure [8]. Overall, OT is critical for enhancing social behavior deficits associated with ASD, establishing relationships and engaging in social interaction, the abnormal concentration, or functions of OT is closely linked to autism pathogenesis.
1. OT deficiency is associated with social deficits in autism
OT is a highly conserved 9-peptide that is synthesized by neuronal cells in the PVN and SON of the hypothalamus and then released into the blood through the axonal endings of the posterior lobe of the neurohypophysis. Endogenous OT receptors (OTRs) have a broad distribution in the brain, particularly in the entire limbic system. The central neurotransmitter-like action of OT has been predominantly shown to modulate cognitive effects, regulating the development and maintenance of complex social and bonding behaviors.
Accumulative evidence indicates that OT plays a putative role in social communication, and the lack or under-use of OT may be connected to disorders related to social communication. Decreased OT and its receptor may lead to low social activity and autism-like behaviors, and this shift in the OT system is commonly observed in patients with ASD. In 1998, Modahl et al. first reported that OT deficiency in autistic children could be the cause of social disorders. They found that plasma OT levels were significantly lower in autistic children than in normal children of the same age, and the decrease in OT level was significantly linked to the degree of social communication impairment in autistic children. They also found that plasma OT levels did not increase with age in autistic children, as normal children did [9]. In addition, social deficits and reduced OT immunostaining have been reported in several models of autism, such as the developmental hyperserotonemia (DHS) model [10], the Cntnap2 mouse model [6], the prenatal valproic acid (VPA) rats model [11], and Shank3b−/− mutant mice model [12]. In the Cntnap2 and VPA model, exogenous OT administration in early life was sufficient to rescue decreased OT immunoreactivity as well as social functioning, indicating that a crucial developmental time window could be required for optimal treatment [6,11]. These results indicate that a key cause to the development of autism could be the reduction or dysfunction of OT.
During long labors, the desensitization of OTR with long infusion periods of Pitocin and high infusion rates may be followed by down-regulation of OTR, which may be a cause that contributes to autism in offspring [13,14,15]. Furthermore, studies in OT [16] or OTR [17] gene knockout mice have shown abnormal social behaviors and ASD-like traits related social memory deficits. Animal experiments and clinical studies also have shown that intranasal or peripheral OT injection can obviously promote social interaction [6,18], but the therapeutic mechanism remains unclear. The possible mechanism could be linked to the excitatory–inhibitory shift of OT-mediated neuroprotective GABA during the perinatal period [19]. In ASD rodent models, the early birth GABA excitatory–inhibitory shift is disrupted, leading to greater excitatory GABA transmission and the followed autism-like behavior. The number of OT-immunoresponsive (OT-ir) cells in the SON of neonatal VPA autism rats was significantly lower than that of the control group. Early postnatal OT treatment (subcutaneous injection for 7 days from 24 h after birth) has a long-term therapeutic effect and can relieve social disorders and repetitive behavior before puberty. These behavioral changes are accompanied by an increase in the number of OT-ir cells [11].
2. OT deficiency is attributed to the stereotyped repetitive behaviors in autism
Another main characteristic of autism is repetitive and stereotypic behavior, which often occurs in other mental and psychological disorders, such as Angelman’s syndrome, Tourette’s syndrome, and obsessive-compulsive disorder. The OT system has been linked to the regulation of repetitive behavior in both animal models and human patients. For example, the OTR knockout mice showed high frequency of repetitive stereotyped behavior, such as increased self-grooming [20] and marble burying [21]. After OT treatment, these repetitive and stereotyped behaviors were substantially reduced [21,22].
In children with ASD, the plasma OT level was considerably lower than that in normal children. The correlation between OT and the repetitive stereotyped behaviors was found to be significant. Lower OT levels are often accompanied by more severe symptoms of stereotyped behavior in ASD children [23]. Furthermore, clinical trials have shown that OT administration effectively mitigated repetitive stereotyped behavior and improved comprehension of affective speech [24,25].
3. Acupuncture can cause prosocial effects of oxytocin
Although human intranasal OT studies illustrate a new hope for ASD, it should be noted that these studies had small sample sizes and some research identified relatively poor replicability, suggesting that intranasal OT studies are generally low statistical power [26]. Exogenous intranasal OT administration may also cause side effects such as nasal irritation, light headedness, drowsiness, and/or headache [27]. Bypassing the blood-brain barrier during exogenous pharmacological intervention remains a problem that needs to be tackled for a long time. Some research has turned to promoting endogenous oxytocin signaling [28].
Acupuncture is an important part of traditional Chinese medicine, which has been widely used as an alternative therapy for a variety of diseases. Acupuncture at specific acupoints regulates the neuro–immuno–endocrine network [29]. The hypothalamus is the key element of the hypothalamic–pituitary–adrenal cortex (HPA) axis, which perceives a multitude of signaling including stressful stimulations and acupuncture signals. It has been confirmed that acupuncture at specific acupoints specifically activates stress reaction neurons (SRNs) in PVN and influences the CNS through the HPA axis [30]. Several lines of evidence indicate that acupuncture treatment can improve the social and emotional responses of autistic patients characterized by impaired social behavior [31,32,33,34]. Studies have shown that acupuncture or electroacupuncture (EA) stimulation with unique frequency can promote the release of specific neurochemicals in the CNS and produce noticeable physiological effects [35]. For example, rats exposed to 30 min of 2 Hz EA treatment showed significant increase of the OT levels in plasma and in the cerebrospinal fluid (CSF) [36]. In addition, 10–20 Hz EA treatment can upregulate OT level in the SON of the hypothalamus in rats [37].
The damage of central OT system was reported for the first time in VPA autism rat model [11]. Compared with the control group, the OT mRNA level and peptide concentration in hypothalamus of the male and female VPA rats were much lower than that of the control animals, and the level of OT peptide in CSF remarkably decreased. Zhang et al. found that EA stimulation (2/15 Hz dense-disperse pulses wave) led to a selective increase in the expression of OT and arginine–vasopressin (AVP) in the hypothalamus. Furthermore, 2/15 Hz EA treatment increased the social interaction time in low socially interacting (LSI) rats. In addition, single-sequence EA stimulation can up-regulate the mRNA levels of hypothalamic OT and AVP. Similarly, repeated sequence EA stimulation can up-regulate the levels of OT and AVP mRNA and promote protein expression in SON, accompanied by a significant increase in serum AVP and enhanced sociability. The social behavior of rats was positively correlated with the mRNA levels of OT and AVP in the hypothalamus, suggesting that the socialization-promoting response observed after EA may be at least partially mediated by the upregulation of OT and AVP in the brain [38].
Based on the above studies, acupuncture may ameliorate the behavioral symptoms of autism by up-regulating the level of OT and AVP in the hypothalamus. Transcutaneous electrical acupoint stimulation (TEAS) is a non-invasive acupuncture-like technique, which is easy to apply in young children. The physiological effects of TEAS are similar to that of EA on analgesia [39] and other diseases [40]. Acupuncture intervention at an early age or during developmental critical periods may be beneficial to the facilitation of AIDRR (Activate, Integrate, Discriminate, Respond, Reward) neural loop [41]. A functional magnetic resonance imaging investigation demonstrated that acupuncture at “Fengchi” (GB 20) specifically targeting the cerebello–thalamo–cortical (CTC) circuit to alleviate the PD tremor [42]. Acupuncture may also alleviate autism symptoms through activating endogenous OT signals by stimulating specific neural circuits in the brain rather than affecting the entire brain with pharmacotherapy [28]. The clinical studies from Beijing Wucailu Autism Rehabilitation Center provide solid evidences that children in the TEAS group had better scores in Childhood Autism Rating Scale (CARS) as well as in the Autism Behavior Checklist (ABC) compared with the ASD children who only received rehabilitation training, and the levels of AVP and OT in plasma were significantly higher than those in the control group [43].
4. Oxytocin stimulates anandamide release in the nucleus accumbens
The endocannabinoids system (ECS) is an essential neuro-modulatory system that involves the regulation of neurotransmission and synaptic plasticity in a broad range of social emotional responses and cognitive functions. ECS consists of two primary cannabinoid receptors (CB1 and CB2), their endogenous lipid ligands, endocannabinoids, including N-arachidonoylethanolamine (anandamide, AEA), 2-arachidonoylglycerol (2-AG)), and their biosynthesis- and degradation-related enzymes. ECS has been shown to be implicated in social behavior regulation [44]. Recently, dysregulation of endocannabinoid signaling has been confirmed in both genetic and epigenetic models of ASD [45,46]. Pharmacological blockade of fatty acid amide hydrolase (FAAH), the main degrading enzyme of AEA, rescued ASD behavioral defects and comorbidities in different preclinical models [47]. Cannabidiol (CBD), the major non-psychoactive phytocannabinoid in cannabis is effective in improving the symptoms of ASD [48].
With respect to OT and ECS, rodent studies have suggested a complex relationship between CB1 and OT, particularly in social behavior. Chemogenetic activation of PVN OT neurons increased nucleus accumbens (NAc) endocannabinoid AEA content in an OTR-dependent manner, suggesting that the artificial activation of PVN OT neurons induced local AEA release [49]. These studies indicate that deficits in the AEA signaling mechanism driven by OT may contribute to social impairment in ASD and a correction of such deficits may provide a novel strategy for the treatment of ASD-related social impairments.
Our previous work and other animal studies have consistently shown that 2 Hz EA can increase endogenous cannabinoid AEA levels in both the peripheral skin tissues and the central nervous system [50,51]. These findings support the hypothesis that acupuncture can, on the one hand, directly up-regulate the signal of endogenous cannabinoid system to promote social interaction, and, on the other hand, by regulating OT signals to treat the behavioral symptoms of autism, it can also indirectly up-regulate the level of endogenous cannabinoid.
5. Serotonin and dopamine are involved in acupuncture-mediated oxytocin rewarding
Both clinical and preclinical studies have confirmed the role of serotonin in the pathophysiology of autism. In addition, the imbalance of dopamine (DA) in specific brain regions may contribute autistic behaviors. These studies suggested that ASD could be closely connected to serotoninergic and dopaminergic systems [52,53].
Animal studies have shown that OT acts on serotoninergic and dopaminergic neurons and participates in many forms of social behaviors [54]. Therefore, it is conceivable that the beneficial characteristics of social interaction can be mediated by the coordinated activity of OT and serotonin (5-HT) in NAc. Walsh et al. have shown that optogenetic stimulating 5-HT release in the NAc or pharmacological activation of NAc 5-HT1b receptors rescues social deficits in 16p11.2 deletion autism mouse model [55]. In addition, autistic children have shown defects in the mesocorticolimbic dopaminergic signaling pathway, such as decreased prefrontal cortex dopamine release [56] and reduced NAc neural response [57]. PVN OT neurons project directly to the ventral tegmental area (VTA) and activate OTRs to regulate the social behavior. Consistent with OT-driven increased activity of VTA DA neurons, VTA OT administration increases DA release as well as sociability, thereby increasing DA release in the NAc, and activating PVN-VTA circuit enhances prosocial behavior in murine models [58]. It is known that EA at different frequencies can promote the release of different neurotransmitters [35]. Acupuncture at the specific acupoint (“Shenshu” (BL23)) increased NAc 5-HT release in rats [59]. Whereas 2 Hz EA in another region (“Zusanli” (ST 36)) was able to elevate both DA and 5-HT level in the rat hypothalamus [60].
These findings indicate that acupuncture may also promote social interaction by directly regulating the 5-HT or DA system, or by facilitating the release of neurotransmitters in the brain’s rewarding region through the oxytocinergic systems.
6. Conclusion
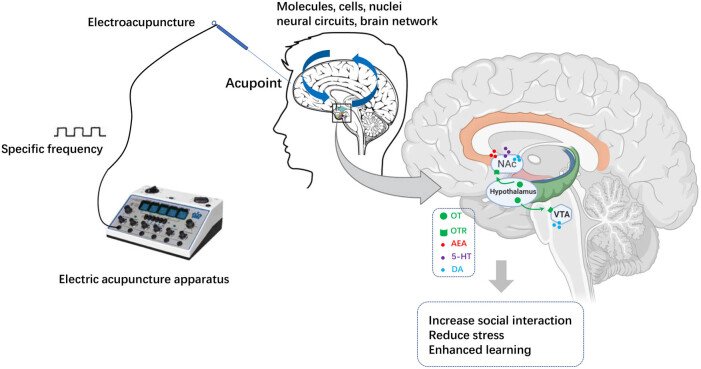
In reviewing previous and current acupuncture and ASD studies, we speculate that acupuncture may improve the behavioral symptoms of autism by directly up-regulating the level of OT in the hypothalamus and perhaps further promoting the synthesis, recruitment, and release of endogenous cannabinoid AEA, 5-HT, DA by increasing the level of OT in the key regions of the regulatory reward system in the brain (Figure 1). Future studies will uncover the underlying mechanisms of the treatment effects of acupuncture on ASD through applying neural tracing, brain mapping, calcium imaging, optogenetics, chemogenetics, and in vivo multichannel recording techniques. Human studies rely primarily on peripheral plasma OT measurement, but the correlation between central and peripheral OT has not been understood. In autistic children, plasma OT levels are lower than in age-matched controls, but many aspects of the metabolic pathways of OT are still poorly understood. It is also necessary to study whether there is insufficient use of OT in autistic children in the future. Currently, most studies are still in the preclinical stage on the relationship between ASD and OT. A thorough investigation of how acupuncture activates endogenous OT signals through affecting specific neural circuits to treat autism will be a critical step in bridging the translational gap between animal models and human therapeutics.
Figure 1.
A hypothetical model of the possible mechanism of acupuncture for the treatment of autism. Acupuncture or electric acupuncture (EA) at specific acupoints with a certain frequency alleviates impaired social behaviors by directly up-regulating the oxytocin level in the hypothalamus or implicitly activating the endogenous cannabinoid, 5-HT, or DA signal pathway. OT: oxytocin; OTR: oxytocin receptor; AEA: anandamide; NAc: nucleus accumbens; VTA: Ventral tegmental area.
Footnotes
Research funding: This study was supported by grants from the National Natural Science Foundation of China (No. 81804188, 81870932).
Conflict of interest: Authors state no conflict of interest.
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
References
- [1].Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011 Nov;68(11):1095–102. [DOI] [PMC free article] [PubMed]; Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T. et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011 Nov;68(11):1095–102. doi: 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry [Internet]. 2014 Feb [cited 2021 Jan 28];4:[about 23 p.]. Available from: https://www.nature.com/articles/tp20144 [DOI] [PMC free article] [PubMed]; Rossignol DA, Genuis SJ, Frye RE.. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry. 2014 Feb doi: 10.1038/tp.2014.4. [Internet] [cited 2021 Jan 28];4:[about 23 p.]. Available from: https://www.nature.com/articles/tp20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tamada K, Takumi T. Serotonin disturbance in mouse models of autism spectrum disorders. Neuromethods. 2015;100:239–62.; Tamada K, Takumi T.. Serotonin disturbance in mouse models of autism spectrum disorders. Neuromethods. 2015;100:239–62. [Google Scholar]
- [4].Farook MF, DeCuypere M, Hyland K, Takumi T, LeDoux MS, Reiter LT. Altered serotonin, dopamine and norepinepherine levels in 15q duplication and Angelman syndrome mouse models. PLoS One [Internet]. 2012 Aug [cited 2021 Jan 28];7:[about 9 p.]. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0043030 [DOI] [PMC free article] [PubMed]; Farook MF, DeCuypere M, Hyland K, Takumi T, LeDoux MS, Reiter LT.. Altered serotonin, dopamine and norepinepherine levels in 15q duplication and Angelman syndrome mouse models. PLoS One. 2012 Aug doi: 10.1371/journal.pone.0043030. [Internet] [cited 2021 Jan 28];7:[about 9 p.]. Available from: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0043030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chamberlain RS, Herman BH. A novel biochemical model linking dysfunctions in brain melatonin, proopiomelanocortin peptides, and serotonin in autism. Biol Psychiatry. 1990 Nov;28(9):773–93. [DOI] [PubMed]; Chamberlain RS, Herman BH.. A novel biochemical model linking dysfunctions in brain melatonin, proopiomelanocortin peptides, and serotonin in autism. Biol Psychiatry. 1990 Nov;28(9):773–93. doi: 10.1016/0006-3223(90)90513-2. [DOI] [PubMed] [Google Scholar]
- [6].Peñagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med [Internet]. 2015 Jan [cited 2021 Jan 28];7:271ra8 [about 13 p.]. Available from: https://stm.sciencemag.org/content/7/271/271ra8 [DOI] [PMC free article] [PubMed]; Peñagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA. et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015 Jan doi: 10.1126/scitranslmed.3010257. [Internet] [cited 2021 Jan 28];7:271ra8 [about 13 p.]. Available from: https://stm.sciencemag.org/content/7/271/271ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brownstein MJ, Russell JT, Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan;207(4429):373–8. [DOI] [PubMed]; Brownstein MJ, Russell JT, Gainer H.. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan;207(4429):373–8. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- [8].Leake RD, Weitzman RE, Glatz TH, Fisher DA. Plasma oxytocin concentrations in men, nonpregnant women, and pregnant women before and during spontaneous labor. J Clin Endocrinol Metab. 1981 Oct;53(4):730–3. [DOI] [PubMed]; Leake RD, Weitzman RE, Glatz TH, Fisher DA.. Plasma oxytocin concentrations in men, nonpregnant women, and pregnant women before and during spontaneous labor. J Clin Endocrinol Metab. 1981 Oct;53(4):730–3. doi: 10.1210/jcem-53-4-730. [DOI] [PubMed] [Google Scholar]
- [9].Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, et al. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998 Feb;43(4):270–7. [DOI] [PubMed]; Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C. et al. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998 Feb;43(4):270–7. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- [10].McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008 Jan;1189(1189):203–14. [DOI] [PubMed]; McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM.. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008 Jan;1189(1189):203–14. doi: 10.1016/j.brainres.2007.10.063. [DOI] [PubMed] [Google Scholar]
- [11].Dai YC, Zhang HF, Schon M, Bockers TM, Han SP, Han JS, et al. Neonatal oxytocin treatment ameliorates autistic-like behaviors and oxytocin deficiency in valproic acid-induced rat model of autism. Front Cell Neurosci [Internet] 2018 Oct [cited 2021 Jan 28];12:355 [about 15 p.]. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2018.00355/full [DOI] [PMC free article] [PubMed]; Dai YC, Zhang HF, Schon M, Bockers TM, Han SP, Han JS. et al. Neonatal oxytocin treatment ameliorates autistic-like behaviors and oxytocin deficiency in valproic acid-induced rat model of autism. Front Cell Neurosci. 2018 Oct doi: 10.3389/fncel.2018.00355. [Internet] [cited 2021 Jan 28];12:355 [about 15 p.]. Available from: https://www.frontiersin.org/articles/10.3389/fncel.2018.00355/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Resendez SL, Namboodiri VM, Otis JM, Eckman LE, Rodriguez-Romaguera J, Ung RL, et al. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J Neurosci. 2020 Mar;40(11):2282–95. [DOI] [PMC free article] [PubMed]; Resendez SL, Namboodiri VM, Otis JM, Eckman LE, Rodriguez-Romaguera J, Ung RL. et al. Social stimuli induce activation of oxytocin neurons within the paraventricular nucleus of the hypothalamus to promote social behavior in male mice. J Neurosci. 2020 Mar;40(11):2282–95. doi: 10.1523/JNEUROSCI.1515-18.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML. Association of autism with induced or augmented childbirth in North Carolina birth record (1990–1998) and education research (1997–2007) databases. JAMA Pediatr. 2013 Oct;167(10):959–66. [DOI] [PubMed]; Gregory SG, Anthopolos R, Osgood CE, Grotegut CA, Miranda ML.. Association of autism with induced or augmented childbirth in North Carolina birth record (1990–1998) and education research (1997–2007) databases. JAMA Pediatr. 2013 Oct;167(10):959–66. doi: 10.1001/jamapediatrics.2013.2904. [DOI] [PubMed] [Google Scholar]
- [14].Weisman O, Agerbo E, Carter CS, Harris JC, Uldbjerg N, Henriksen TB, et al. Oxytocin-augmented labor and risk for autism in males. Behav Brain Res. 2015 May;284(284):207–12. [DOI] [PubMed]; Weisman O, Agerbo E, Carter CS, Harris JC, Uldbjerg N, Henriksen TB. et al. Oxytocin-augmented labor and risk for autism in males. Behav Brain Res. 2015 May;284(284):207–12. doi: 10.1016/j.bbr.2015.02.028. [DOI] [PubMed] [Google Scholar]
- [15].Oberg AS, D’Onofrio BM, Rickert ME, Hernandez-Diaz S, Ecker JL, Almqvist C, et al. Association of labori with offspring risk of autism spectrum disorders. JAMA Pediatr [Internet]. 2016 Sep [cited 2021 Jan 28];170:e160965 [about 7 p.]. Available from: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2534479 [DOI] [PMC free article] [PubMed]; Oberg AS, D’Onofrio BM, Rickert ME, Hernandez-Diaz S, Ecker JL, Almqvist C. et al. Association of labori with offspring risk of autism spectrum disorders. JAMA Pediatr. 2016 Sep doi: 10.1001/jamapediatrics.2016.0965. [Internet] [cited 2021 Jan 28];170:e160965 [about 7 p.]. Available from: https://jamanetwork.com/journals/jamapediatrics/fullarticle/2534479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000 Jul;25(3):284–8. [DOI] [PubMed]; Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT.. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000 Jul;25(3):284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- [17].Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005 Nov;102(44):16096–101. [DOI] [PMC free article] [PubMed]; Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T. et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005 Nov;102(44):16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A, et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013 Mar;23(2):123–7. [DOI] [PubMed]; Tachibana M, Kagitani-Shimono K, Mohri I, Yamamoto T, Sanefuji W, Nakamura A. et al. Long-term administration of intranasal oxytocin is a safe and promising therapy for early adolescent boys with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2013 Mar;23(2):123–7. doi: 10.1089/cap.2012.0048. [DOI] [PubMed] [Google Scholar]
- [19].Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S, et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014 Feb;343(6171):675–9. [DOI] [PubMed]; Tyzio R, Nardou R, Ferrari DC, Tsintsadze T, Shahrokhi A, Eftekhari S. et al. Oxytocin-mediated GABA inhibition during delivery attenuates autism pathogenesis in rodent offspring. Science. 2014 Feb;343(6171):675–9. doi: 10.1126/science.1247190. [DOI] [PubMed] [Google Scholar]
- [20].Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS 3rd, Lee HJ, et al. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav. 2012 Mar;61(3):436–44. [DOI] [PMC free article] [PubMed]; Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS, Lee HJ. et al. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Horm Behav. 2012 Mar;61(3):436–44. doi: 10.1016/j.yhbeh.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sanathara NM, Garau C, Alachkar A, Wang L, Wang Z, Nishimori K, et al. Melanin concentrating hormone modulates oxytocin-mediated marble burying. Neuropharmacology. 2018 Jan;128(128):22–32. [DOI] [PMC free article] [PubMed]; Sanathara NM, Garau C, Alachkar A, Wang L, Wang Z, Nishimori K. et al. Melanin concentrating hormone modulates oxytocin-mediated marble burying. Neuropharmacology. 2018 Jan;128(128):22–32. doi: 10.1016/j.neuropharm.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, et al. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013 Sep;72(72):187–96. [DOI] [PMC free article] [PubMed]; Teng BL, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV. et al. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013 Sep;72(72):187–96. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang CJ, Tan HP, Yang FY, Wang HP, Liu CL, He HZ, et al. The cortisol, serotonin and oxytocin are associated with repetitive behavior in autism spectrum disorder. Res Autism Spectr Disord. 2015;18:12–20.; Yang CJ, Tan HP, Yang FY, Wang HP, Liu CL, He HZ. et al. The cortisol, serotonin and oxytocin are associated with repetitive behavior in autism spectrum disorder. Res Autism Spectr Disord. 2015;18:12–20. [Google Scholar]
- [24].Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003 Jan;28(1):193–8. [DOI] [PubMed]; Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR. et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003 Jan;28(1):193–8. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- [25].Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010 Apr;67(7):692–4. [DOI] [PubMed]; Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ. et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010 Apr;67(7):692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- [26].Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol Psychiatry. 2016 Feb;79(3):251–7. [DOI] [PMC free article] [PubMed]; Walum H, Waldman ID, Young LJ. Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol Psychiatry. 2016 Feb;79(3):251–7. doi: 10.1016/j.biopsych.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011 Sep;36(8):1114–26. [DOI] [PubMed]; MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ.. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011 Sep;36(8):1114–26. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- [28].Ford CL, Young LJ. Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr Opin Neurobiol. 2020 Nov;68(68):1–8. [DOI] [PMC free article] [PubMed]; Ford CL, Young LJ.. Translational opportunities for circuit-based social neuroscience: advancing 21st century psychiatry. Curr Opin Neurobiol. 2020 Nov;68(68):1–8. doi: 10.1016/j.conb.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wu ML, Xu DS, Bai WZ, Cui JJ, Shu HM, He W, et al. Local cutaneous nerve terminal and mast cell responses to manual acupuncture in acupoint LI4 area of the rats. J Chem Neuroanat. 2015 Oct;68(68):14–21. [DOI] [PubMed]; Wu ML, Xu DS, Bai WZ, Cui JJ, Shu HM, He W. et al. Local cutaneous nerve terminal and mast cell responses to manual acupuncture in acupoint LI4 area of the rats. J Chem Neuroanat. 2015 Oct;68(68):14–21. doi: 10.1016/j.jchemneu.2015.06.002. [DOI] [PubMed] [Google Scholar]
- [30].Wang SJ, Zhang JJ, Yang HY, Wang F, Li ST. Acupoint specificity on acupuncture regulation of hypothalamic- pituitary-adrenal cortex axis function. BMC Complement Altern Med [Internet]. 2015 Mar [cited 2021 Jan 28];15:87 [about 10 p.]. Available from: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-015-0625-4. [DOI] [PMC free article] [PubMed]; Wang SJ, Zhang JJ, Yang HY, Wang F, Li ST.. Acupoint specificity on acupuncture regulation of hypothalamic- pituitary-adrenal cortex axis function. BMC Complement Altern Med. 2015 Mar doi: 10.1186/s12906-015-0625-4. [Internet] [cited 2021 Jan 28];15:87 [about 10 p.]. Available from: https://bmccomplementmedtherapies.biomedcentral.com/articles/10.1186/s12906-015-0625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chan AS, Cheung MC, Sze SL, Leung WW. Seven-star needle stimulation improves language and social interaction of children with autistic spectrum disorders. Am J Chin Med. 2009;37(3):495–504. [DOI] [PubMed]; Chan AS, Cheung MC, Sze SL, Leung WW.. Seven-star needle stimulation improves language and social interaction of children with autistic spectrum disorders. Am J Chin Med. 2009;37(3):495–504. doi: 10.1142/S0192415X09007004. [DOI] [PubMed] [Google Scholar]
- [32].Lee B, Lee J, Cheon JH, Sung HK, Cho SH, Chang GT. The efficacy and safety of acupuncture for the treatment of children with autism spectrum disorder: a systematic review and meta-analysis. Evid -Based Complementary Altern Med [Internet]. 2018 Jan [cited 2021 Jan 28];2018:1057539 [about 22 p.]. Available from: https://www.hindawi.com/journals/ecam/2018/1057539/ [DOI] [PMC free article] [PubMed]; Lee B, Lee J, Cheon JH, Sung HK, Cho SH, Chang GT.. The efficacy and safety of acupuncture for the treatment of children with autism spectrum disorder: a systematic review and meta-analysis. Evid -Based Complementary Altern Med. 2018 Jan doi: 10.1155/2018/1057539. [Internet] [cited 2021 Jan 28];2018:1057539 [about 22 p.]. Available from: https://www.hindawi.com/journals/ecam/2018/1057539/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Zhao ZQ, Jia SW, Hu S, Sun W. Evaluating the effectiveness of electro-acupuncture as a treatment for childhood autism using single photon emission computed tomography. Chin J Integr Med. 2014 Jan;20(1):19–23. [DOI] [PubMed]; Zhao ZQ, Jia SW, Hu S, Sun W.. Evaluating the effectiveness of electro-acupuncture as a treatment for childhood autism using single photon emission computed tomography. Chin J Integr Med. 2014 Jan;20(1):19–23. doi: 10.1007/s11655-014-1680-2. [DOI] [PubMed] [Google Scholar]
- [34].Liu C, Li T, Wang Z, Zhou R, Zhuang L. Scalp acupuncture treatment for children’s autism spectrum disorders: a systematic review and meta-analysis. Medicine (Baltimore) [Internet]. 2019 Mar [cited 2021 Jan 28]; 98(13):e14880 [about 8p.]. Available from: https://journals.lww.com/md-journal/Fulltext/2019/03290/Scalp_acupuncture_treatment_for_children_s_autism.12.aspx [DOI] [PMC free article] [PubMed]; Liu C, Li T, Wang Z, Zhou R, Zhuang L.. Scalp acupuncture treatment for children’s autism spectrum disorders: a systematic review and meta-analysis. Medicine. 2019 Mar doi: 10.1097/MD.0000000000014880. (Baltimore) [Internet] [cited 2021 Jan 28]; 98(13):e14880 [about 8p.]. Available from: https://journals.lww.com/md-journal/Fulltext/2019/03290/Scalp_acupuncture_treatment_for_children_s_autism.12.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Han JS. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003 Jan;26(1):17–22. [DOI] [PubMed]; Han JS.. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. 2003 Jan;26(1):17–22. doi: 10.1016/s0166-2236(02)00006-1. [DOI] [PubMed] [Google Scholar]
- [36].Uvnäs-Moberg K, Bruzelius G, Alster P, Lundeberg T. The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993 Oct;149(2):199–204. [DOI] [PubMed]; Uvnäs-Moberg K, Bruzelius G, Alster P, Lundeberg T.. The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993 Oct;149(2):199–204. doi: 10.1111/j.1748-1716.1993.tb09612.x. [DOI] [PubMed] [Google Scholar]
- [37].Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Effect of oxytocin on acupuncture analgesia in the rat. Neuropeptides. 2007 Oct;41(5):285–92. [DOI] [PubMed]; Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC.. Effect of oxytocin on acupuncture analgesia in the rat. Neuropeptides. 2007 Oct;41(5):285–92. doi: 10.1016/j.npep.2007.05.004. [DOI] [PubMed] [Google Scholar]
- [38].Zhang HF, Li HX, Dai YC, Xu XJ, Han SP, Zhang R, et al. Electro-acupuncture improves the social interaction behavior of rats. Physiol Behav. 2015 Nov;151(151):485–93. [DOI] [PubMed]; Zhang HF, Li HX, Dai YC, Xu XJ, Han SP, Zhang R. et al. Electro-acupuncture improves the social interaction behavior of rats. Physiol Behav. 2015 Nov;151(151):485–93. doi: 10.1016/j.physbeh.2015.08.014. [DOI] [PubMed] [Google Scholar]
- [39].Shi X, Yu W, Zhang W, Wang T, Battulga O, Wang L, et al. A comparison of the effects of electroacupuncture versus transcutaneous electrical nerve stimulation for pain control in knee osteoarthritis: a Bayesian network meta-analysis of randomized controlled trials. Acupunct Med [Internet]. 2020 Jun [cited 2021 Jan 28];2020:964528420921193. Available from: https://journals.sagepub.com/doi/10.1177/0964528420921193 [DOI] [PubMed]; Shi X, Yu W, Zhang W, Wang T, Battulga O, Wang L. et al. A comparison of the effects of electroacupuncture versus transcutaneous electrical nerve stimulation for pain control in knee osteoarthritis: a Bayesian network meta-analysis of randomized controlled trials. Acupunct Med. 2020 Jun doi: 10.1177/0964528420921193. [Internet] [cited 2021 Jan 28];2020:964528420921193. Available from: https://journals.sagepub.com/doi/10.1177/0964528420921193. [DOI] [PubMed] [Google Scholar]
- [40].Kim BH, Moon YK, Kim MH, Nam HJ. Comparing the effects of manual acupuncture, electroacupuncture, and transcutaneous electrical nerve stimulation on chronic tinnitus: a randomized controlled trial. Integr Med Res [Internet]. 2020 Jun [cited 2021 Jan 28];9(2):100409 [about 5 p.]. Available from: https://www.sciencedirect.com/science/article/pii/S221342202030041X?via%3Dihub [DOI] [PMC free article] [PubMed]; Kim BH, Moon YK, Kim MH, Nam HJ.. Comparing the effects of manual acupuncture, electroacupuncture, and transcutaneous electrical nerve stimulation on chronic tinnitus: a randomized controlled trial. Integr Med Res. 2020 Jun doi: 10.1016/j.imr.2020.100409. [Internet] [cited 2021 Jan 28];9(2):100409 [about 5 p.]. Available from: https://www.sciencedirect.com/science/article/pii/S221342202030041X?via%3Dihub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].DeMayo MM, Young LJ, Hickie IB, Song YJ, Guastella AJ. Circuits for social learning: a unified model and application to autism spectrum disorder. Neurosci Biobehav Rev. 2019 Dec;107(107):388–98. [DOI] [PMC free article] [PubMed]; DeMayo MM, Young LJ, Hickie IB, Song YJ, Guastella AJ.. Circuits for social learning: a unified model and application to autism spectrum disorder. Neurosci Biobehav Rev. 2019 Dec;107(107):388–98. doi: 10.1016/j.neubiorev.2019.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li Z, Chen J, Cheng J, Huang S, Hu Y, Wu Y, et al. Acupuncture modulates the cerebello-thalamo-cortical circuit and cognitive brain regions in patients of Parkinson’s disease with tremor. Front Aging Neurosci [Internet]. 2018 Jul [cited 2021 Jan 28];10:206 [about 11 p.]. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00206/full [DOI] [PMC free article] [PubMed]; Li Z, Chen J, Cheng J, Huang S, Hu Y, Wu Y. et al. Acupuncture modulates the cerebello-thalamo-cortical circuit and cognitive brain regions in patients of Parkinson’s disease with tremor. Front Aging Neurosci. 2018 Jul doi: 10.3389/fnagi.2018.00206. [Internet] [cited 2021 Jan 28];10:206 [about 11 p.]. Available from: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00206/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang R, Jia MX, Zhang JS, Xu XJ, Shou XJ, Zhang XT, et al. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: a prospective single-blinded controlled study. Res Dev Disabil. 2012 Jul-Aug;33(4):1136–46. [DOI] [PubMed]; Zhang R, Jia MX, Zhang JS, Xu XJ, Shou XJ, Zhang XT. et al. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: a prospective single-blinded controlled study. Res Dev Disabil. 2012 Jul-Aug;33(4):1136–46. doi: 10.1016/j.ridd.2012.02.001. [DOI] [PubMed] [Google Scholar]
- [44].Wei D, Allsop S, Tye K, Piomelli D. Endocannabinoid signaling in the control of social behavior. Trends Neurosci. 2017 Jul;40(7):385–96. [DOI] [PMC free article] [PubMed]; Wei D, Allsop S, Tye K, Piomelli D.. Endocannabinoid signaling in the control of social behavior. Trends Neurosci. 2017 Jul;40(7):385–96. doi: 10.1016/j.tins.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun [Internet]. 2012 Sep [cited 2021 Jan 28];3:1080 [about 11 p.]. Available from: https://www.nature.com/articles/ncomms2045 [DOI] [PMC free article] [PubMed]; Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H. et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat Commun. 2012 Sep doi: 10.1038/ncomms2045. [Internet] [cited 2021 Jan 28];3:1080 [about 11 p.]. Available from: https://www.nature.com/articles/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kerr DM, Downey L, Conboy M, Finn DP, Roche M. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav Brain Res. 2013 Jul;249(249):124–32. [DOI] [PubMed]; Kerr DM, Downey L, Conboy M, Finn DP, Roche M.. Alterations in the endocannabinoid system in the rat valproic acid model of autism. Behav Brain Res. 2013 Jul;249(249):124–32. doi: 10.1016/j.bbr.2013.04.043. [DOI] [PubMed] [Google Scholar]
- [47].Zamberletti E, Gabaglio M, Parolaro D. The endocannabinoid system and autism spectrum disorders: insights from animal models. Int J Mol Sci [Internet]. 2017 Sep [cited 2021 Jan 28];18(9):1916 [about 14 p.]. Available from: https://www.mdpi.com/1422-0067/18/9/1916 [DOI] [PMC free article] [PubMed]; Zamberletti E, Gabaglio M, Parolaro D.. The endocannabinoid system and autism spectrum disorders: insights from animal models. Int J Mol Sci. 2017 Sep doi: 10.3390/ijms18091916. [Internet] [cited 2021 Jan 28];18(9):1916 [about 14 p.]. Available from: https://www.mdpi.com/1422-0067/18/9/1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fusar-Poli L, Cavone V, Tinacci S, Concas I, Petralia A, Signorelli MS, et al. Cannabinoids for people with ASD: a systematic review of published and ongoing studies. Brain Sci [Internet]. 2020 Aug [cited 2021 Jan 28]; 10(9):572 [about 18 p.]. Available from: https://www.mdpi.com/2076-3425/10/9/572 [DOI] [PMC free article] [PubMed]; Fusar-Poli L, Cavone V, Tinacci S, Concas I, Petralia A, Signorelli MS. et al. Cannabinoids for people with ASD: a systematic review of published and ongoing studies. Brain Sci. 2020 Aug doi: 10.3390/brainsci10090572. [Internet] [cited 2021 Jan 28]; 10(9):572 [about 18 p.]. Available from: https://www.mdpi.com/2076-3425/10/9/572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wei D, Lee D, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH, et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci USA. 2015 Nov;112(45):14084–9. [DOI] [PMC free article] [PubMed]; Wei D, Lee D, Cox CD, Karsten CA, Peñagarikano O, Geschwind DH. et al. Endocannabinoid signaling mediates oxytocin-driven social reward. Proc Natl Acad Sci USA. 2015 Nov;112(45):14084–9. doi: 10.1073/pnas.1509795112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chen L, Zhang J, Li F, Qiu Y, Wang L, Li YH, et al. Endogenous anandamide and cannabinoid receptor-2 contribute to electroacupuncture analgesia in rats. J Pain. 2009 Jul;10(7):732–9. [DOI] [PubMed]; Chen L, Zhang J, Li F, Qiu Y, Wang L, Li YH. et al. Endogenous anandamide and cannabinoid receptor-2 contribute to electroacupuncture analgesia in rats. J Pain. 2009 Jul;10(7):732–9. doi: 10.1016/j.jpain.2008.12.012. [DOI] [PubMed] [Google Scholar]
- [51].Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, et al. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009 Jun;40(6):2157–64. [DOI] [PubMed]; Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J. et al. Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke. 2009 Jun;40(6):2157–64. doi: 10.1161/STROKEAHA.108.541490. [DOI] [PubMed] [Google Scholar]
- [52].Yang CJ, Tan HP, Du YJ. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience. 2014 May;267(267):1–10. [DOI] [PubMed]; Yang CJ, Tan HP, Du YJ.. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience. 2014 May;267(267):1–10. doi: 10.1016/j.neuroscience.2014.02.021. [DOI] [PubMed] [Google Scholar]
- [53].Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord [Internet]. 2012 Jul [cited 2021 Jan 28];4:19 [about 43 p.]. Available from: https://jneurodevdisorders.biomedcentral.com/articles/10.1186/1866-1955-4-19 [DOI] [PMC free article] [PubMed]; Dichter GS, Damiano CA, Allen JA.. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord. 2012 Jul doi: 10.1186/1866-1955-4-19. [Internet] [cited 2021 Jan 28];4:19 [about 43 p.]. Available from: https://jneurodevdisorders.biomedcentral.com/articles/10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010 Jun;16(3):e92–123. [DOI] [PMC free article] [PubMed]; Baskerville TA, Douglas AJ.. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010 Jun;16(3):e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW, et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature. 2018 Aug;560(7720):589–94. [DOI] [PMC free article] [PubMed]; Walsh JJ, Christoffel DJ, Heifets BD, Ben-Dor GA, Selimbeyoglu A, Hung LW. et al. 5-HT release in nucleus accumbens rescues social deficits in mouse autism model. Nature. 2018 Aug;560(7720):589–94. doi: 10.1038/s41586-018-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Cohen RM. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997; 350(9078):638. [DOI] [PubMed]; Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Cohen RM.. Low medial prefrontal dopaminergic activity in autistic children. Lancet. 1997;350(9078):638. doi: 10.1016/s0140-6736(05)63326-0. [DOI] [PubMed] [Google Scholar]
- [57].Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Res. 2010 Apr;3(2):53–67. [DOI] [PMC free article] [PubMed]; Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY.. Reward processing in autism. Autism Res. 2010 Apr;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017 Sep;357(6358):1406–11. [DOI] [PMC free article] [PubMed]; Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ. et al. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017 Sep;357(6358):1406–11. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yoshimoto K, Fukuda F, Hori M, Kato B, Kato H, Hattori H, et al. Acupuncture stimulates the release of serotonin, but not dopamine, in the rat nucleus accumbens. Tohoku J Exp Med. 2006 Apr;208(4):321–6. [DOI] [PubMed]; Yoshimoto K, Fukuda F, Hori M, Kato B, Kato H, Hattori H. et al. Acupuncture stimulates the release of serotonin, but not dopamine, in the rat nucleus accumbens. Tohoku J Exp Med. 2006 Apr;208(4):321–6. doi: 10.1620/tjem.208.321. [DOI] [PubMed] [Google Scholar]
- [60].Liang Y, Wang CX, Fang JQ, Shao XM, Ying XM. Effect of pre-electroacupuncture at “Zusanli” (ST 36) on DA and 5-HT contents and their ratio in hypothalamus and striatum in exercise rats. Zhongguo Zhenjiu. 2012 Jan;2011(31):1101–5. [PubMed]; Liang Y, Wang CX, Fang JQ, Shao XM, Ying XM.. Effect of pre-electroacupuncture at “Zusanli” (ST 36) on DA and 5-HT contents and their ratio in hypothalamus and striatum in exercise rats. Zhongguo Zhenjiu. 2012 Jan;2011(31):1101–5. [PubMed] [Google Scholar]