Abstract
BACKGROUND
The pathogenesis of gastroesophageal reflux disease (GERD) is closely associated with the intestinal bacteria composition and their metabolites.
AIM
To investigate whether washed microbiota transplantation (WMT) improves symptoms of nonerosive reflux disease (NERD) with proton pump inhibitor (PPI) dependency.
METHODS
Patients with recurrent NERD and PPI dependency at the First Affiliated Hospital of Guangdong Pharmaceutical University from 2017 to 2018 were included and divided into a WMT or PPI group treated with PPI with/without WMT. The endpoint was NERD symptom frequency evaluated 1 mo after WMT using reflux disease questionnaire (RDQ) and GERD questionnaire (GERDQ) scores, remission time, PPI dose, and the examination of intestinal mucosal barrier function.
RESULTS
In the WMT (n = 15) and PPI (n = 12) groups, the total remission rate at 1 mo after treatment was 93.3% vs 41.7%. Compared with the PPI group, the WMT group showed better results in GERDQ (P = 0.004) and RDQ (P = 0.003) and in remission months (8 vs 2, P = 0.002). The PPI dose was reduced to some extent for 80% of patients in the WMT group and 33.3% in the PPI group. In 24 patients, intestinal mucosal barrier function was examined before treatment, and changes in the degree of damage were observed in 13 of these patients after treatment. Only one of the 15 patients had minor side effects, including a mushy stool two or three times a day, which resolved on their own after 1 wk.
CONCLUSION
This study is the first to demonstrate that WMT may be safe and effective for relieving NERD symptoms and reducing PPI dependency and recurrence.
Keywords: Nonerosive reflux disease, Washed microbiota transplantation, Proton pump inhibitor dependency, Intestinal bacteria, Lipopolysaccharide, Small intestinal bacterial overgrowth
Core Tip: In this study, we demonstrated for the first time that washed microbiota transplantation (WMT) is safe and effective for treating patients with nonerosive reflux disease (NERD) and proton pump inhibitor (PPI) dependency compared with PPI treatment. WMT significantly relieved the symptoms of NERD in patients, reduced PPI dependency, prolonged the duration of symptom remission, and reduced symptom recurrence.
INTRODUCTION
Gastroesophageal reflux disease (GERD) is divided into nonerosive reflux disease (NERD), reflux esophagitis, and Barrett's esophagus, and more than 70% of GERD cases are NERD[1]. The current treatment for GERD is proton pump inhibitors (PPIs)[2]. However, due to the chronic nature and recurrence of GERD, current PPI treatments for GERD do not provide satisfactory effects, especially in patients with NERD, who have a poorer response to PPIs than patients with reflux esophagitis[3-7]. In addition, many patients need to take PPIs for a long time[8], potentially leading to changes in their intestinal microbiota, such as increases in Enterococcus, Streptococcus, Staphylococcus, and potentially pathogenic Escherichia coli[6,9]. Escherichia coli is a Gram-negative bacterium, and a component of its cell wall, lipopolysaccharide, can cause lower esophageal sphincter (LES) relaxation and gastric empty-out delay and lead to the occurrence of GERD[6]. Many studies have also found that long-term use of PPIs can result in small intestinal bacterial overgrowth (SIBO)[9-11], which can cause chronic inflammation; immune reactions may give rise to reduced reactivity of esophageal smooth muscle[12]. At the same time, SIBO leads to the production of excess amounts of methane gas by the intestinal bacteria, which inhibits contractile activity, slows intestinal transit, and, consequently, affects gastric emptying and induces gastroesophageal reflux[13,14]. These studies suggest that the recurrence of symptoms and long-term use of PPI in patients with GERD (including NERD) may be related to changes in the intestinal microbiota and SIBO induction after previous PPI use.
In recent years, research on the application of fecal microbiota transplantation (FMT) in clinical diseases has developed rapidly. Good clinical effects of FMT have been observed for many diseases, including refractory Clostridium difficile infection[15], irritable bowel syndrome[16], inflammatory bowel disease[17], and constipation[18]. FMT reconstructs the balance of the intestinal flora in patients by using healthy donor feces, reducing the colonization of pathogenic bacteria, and leading to a treatment effect by regulating metabolism and immunity[19]. The latest study showed that the method of generating bacterial solutions through automatic purification systems is very popular among doctors and patients; therefore, FMT is also called washed microbiota transplantation (WMT)[20].
Since our hospital launched WMT to treat diseases such as GERD, ulcerative colitis, irritable bowel syndrome, constipation, non-alcoholic fatty liver, and autism for two years and obtained good clinical results, more than 2500 times have been treated. This study mainly assessed the therapeutic effects of WMT in patients with PPI-dependent NERD.
MATERIALS AND METHODS
Subjects
Patients with a clear diagnosis of NERD and dependency on PPIs who were admitted to the Department of Gastroenterology of the First Affiliated Hospital of Guangdong Pharmaceutical University from January 1, 2017 to November 30, 2018 and had poor curative effects and recurrent symptoms after PPI use were included and divided into WMT and PPI groups. Patients who received WMT treatment provided informed consent.
Inclusion criteria
The age of the included patients was 18-85 years old. Consistent with the diagnosis of GERD, endoscopy excluded reflux esophagitis and Barrett's esophagus. The patients had a history of disease for more than 6 mo with PPI dependency, and no antibiotics were used 1 wk before treatment and during treatment. PPI dependency was defined that after standard PPI treatment, complete remission of GERD symptoms was not achieved or the symptoms recurred after the drug was stopped. The inclusion criteria of the WMT group were to meet both the above criteria and the following criteria: Patients had consented to WMT treatment, if they were not included in the PPI group.
Exclusion criteria
Patients were excluded if they had severe heart and lung disease, liver and kidney failure, malignant tumors, pregnancy, or other diseases that significantly affect quality of life, or they refused or failed to complete the follow-ups.
Treatment plan
The PPI group was treated with a previous treatment plan (mainly PPI treatment), while WMT treatment was added in the WMT group. The source of the bacterial suspension for WMT was mixed multidonor feces. All donors were healthy people aged 18 to 25 years, and they were required to undergo health examinations to exclude digestive tract diseases, tumors, infectious diseases, metabolic diseases, genetic diseases, and other related diseases and not to take antibiotics, as well as drugs that affect digestive tract dynamics and/or cause intestinal microecological disorders, for the last 3 mo. Two hundred milliliters of fresh fecal liquid was separated using an automatic purification system (GenFMTer; FMT Medical, Nanjing, China), and the prepared bacteria were injected into the patient's intestine via the middle or lower digestive tract within half an hour. There were two transplantation routes. One was the middle-gut route: Transendoscopic enteral tubing was placed in the jejunum under gastroscopy, and PPIs (such as lansoprazole 30 mg + normal saline 100 mL) were administered intravenously 1 h before injection of bacteria (to reduce the inactivation of bacteria when moving through the stomach). Metoclopramide hydrochloride (10 mg) was injected intramuscularly (to reduce adverse reactions such as vomiting or abdominal distension caused by irritation of the gastrointestinal tract by the bacterial fluid). The patients were placed in a sitting position when injecting the bacterial solution. The injection process was slow, requiring an injection time of at least 30 min for 200 mL of bacterial solution. After the injection, the patient was asked to remain sitting or standing for at least 2 h. The other route was the lower gut route: Transendoscopic enteral tubing was placed into the caecum via enteroscopy. When injecting the bacterial solution, the patient was in the right lateral position, and the time of injection was 30 min. After the injection was completed, the patient was instructed to rest in the right lateral position for at least 2 h. One course was administered once daily for 3 d. Four courses were administered, with one course per month given in the first month, second month, third month, and sixth month.
Outcome measurement
The main outcome measure was NERD symptom frequency evaluated 1 mo after WMT. The reflux disease questionnaire (RDQ) and GERD questionnaire (GERDQ) were administered to the patient before and 1 mo after treatment and reviewed. The RDQ integrates the severity and frequency of heartburn, chest pain, acid regurgitation, and regurgitation over the past 4 wk. The GERDQ measures the frequency of symptoms, such as reflux, heartburn, nausea, upper abdominal pain, sleep disturbance, additional antacids, and other symptoms during the past 7 d.
Other outcome measures included remission, relapse after remission, monosymptomatic remission, and biochemical coupling examination of intestinal barrier function. Remission was defined as RDQ and GERDQ scores reduced by 30% at 1 mo posttreatment. Relapse after remission was defined as: After WMT treatment, the patient's symptoms of NERD reached remission but then worsened to a level observed prior to WMT treatment; the RDQ and GERDQ scores increased by 30% from the previous period, and the duration of this increase exceeded 1 mo. Monosympto-matic remission was defined when the RDQ or GERDQ scores of heartburn, acid regurgitation, chest pain, regurgitation, and sleep disturbance declined posttreatment compared with pretreatment. Biochemical coupling examination of intestinal barrier function was performed by determining serum levels of diamine oxidase (DAO), D-lactic acid (DLA), and lipopolysaccharide[21] according to the test developed by the Institute of Biophysics, Chinese Academy of Sciences (Beijing, China), and the manufacturer’s protocol. DAO > 10 U/L indicated intestinal mucosal damage and increased intestinal permeability; DLA > 15 mg/L indicated abnormal intestinal permeability; and lipopolysaccharide > 20 U/L indicated intestinal bacterial translocation[22]. Thus, abnormal levels of any of these indicators reflect intestinal mechanical barrier dysfunction. PPI medication status, side effects of WMT treatment, and WMT course were also determined.
Statistical analysis
SPSS version 20.0 or GraphPad Prism version 5.0 was used for data analyses. The data are described as frequencies, percentages, medians, and interquartile ranges. Comparisons of lipopolysaccharide values and scores of RDQ and GERDQ were performed by applying the non-parametric Wilcoxon signed-rank test or Mann-Whitney test, and a two-tailed P value of < 0.05 was considered statistically significant.
Ethics statement
This retrospective study was approved by the Ethics Committee of Guangdong Pharmaceutical University (Approval No. Yilun Shen [2019] No. 93 01).
RESULTS
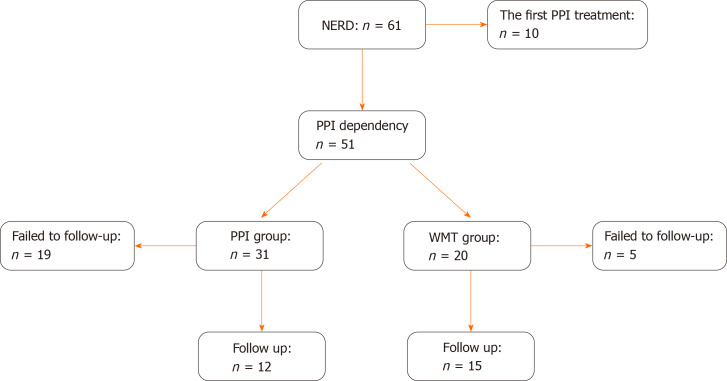
Of the 61 patients with NERD who were screened, 51 were eligible according to the inclusion criteria, and 27 completed the follow-ups (Figure 1). The WMT group included 15 patients, and the PPI group comprised 12 patients. There were no significant differences in the characteristics of the two study groups at enrolment (Table 1).
Figure 1.
Flow chart for the inclusion of patients. NERD: Nonerosive reflux disease; WMT: Washed microbiota transplantation; PPI: Proton pump inhibitor dependency.
Table 1.
Basic information of patients with nonerosive reflux disease
|
Item
|
PPI
|
WMT
|
P
value
|
| Female | 6 | 8 | |
| Age (median; IQR, yr) | 58.5 (53, 67.25) | 62 (55, 67) | 0.526 |
| Duration (median; IQR, mo) | 48 (15, 144) | 24 (6, 60) | 0.218 |
| BMI (median; IQR, kg/m2) | 21.63 (19.13, 24.38) | 24.24 (22.58, 25.43) | 0.079 |
WMT: Washed microbiota transplantation; PPI: Proton pump inhibitors; BMI: Body mass index; IQR: Interquartile range.
At 1 mo after treatment, the total remission rate in the WMT and PPI groups was 93.3% vs 41.7%. Compared with the PPI group, the WMT group showed better results in the GERDQ scores (7 vs 11, P = 0.004) and RDQ scores (8 vs 20.5, P = 0.003), as well as in the remission months [8 (3, 17) vs 2 (0, 4), P = 0.002] (Table 2); nine patients showed sustained remission for more than 6 mo in the WMT groups, while there were only two in the PPI group. Furthermore, the patients in the WMT group achieved better improvements in heartburn (9/10 vs 7/11), acid regurgitation (12/14 vs 7/11), chest pain (5/6 vs 1/5), regurgitation (9/12 vs 4/8), and sleep disturbance (9/11 vs 5/5) than the PPI group. However, 13.3% (2/15) of patients in the WMT group relapsed after remission (Table 3).
Table 2.
Symptom scores and remission time before and after treatment in the two groups
|
Group
|
PPI (n = 12)
|
WMT (n = 15)
|
P
value1
|
| Pre-GERDQ (median; IQR) | 12 (9, 13.75) | 12 (10, 12) | 0.746 |
| Post-GERDQ (median; IQR) | 11 (8.25, 13.5) | 7 (7, 8) | 0.004 |
| Intra-group, P value2 | 0.477 | 0.003 | |
| Pre-RDQ (median, IQR) | 26 (18, 33) | 23 (16, 25) | 0.203 |
| Post-RDQ (median, IQR) | 20.5 (12.75, 26) | 8 (6, 12) | 0.003 |
| Intra-group, P value | 0.005 | 0.002 | |
| Remission time (median, IQR; mo) | 2 (0, 4) | 8 (3, 17) | 0.002 |
The RDQ and GERDQ scores between two groups were analyzed by non-parametric Mann-Whitney test.
The intra-group RDQ and GERDQ scores were analyzed by non-parametric Wilcoxon signed-rank test.
WMT: Washed microbiota transplantation; PPI: Proton pump inhibitors; RDQ: Reflux disease questionnaire; GERDQ: Gastroesophageal reflux disease questionnaire; IQR: Interquartile range.
Table 3.
Clinical responses to washed microbiota transplantation in patients with nonerosive reflux disease
|
Item
|
|
WMT, n = 15
|
| Remission1, n | 14 (93.3) | |
| Remission after the first course of WMT, n | 13 (86.7) | |
| Remission after the first course of FMT until the end of the study, n | 10 (66.7) | |
| No remission after the first course of WMT, n | 2 (13.3) | |
| Recurrence after remission, n | 2 (13.3) | |
| Side effects of WMT treatment2, n | No | 14 (93.3) |
| Yes | 1 (6.70) |
The scores of the reflux disease questionnaire or gastroesophageal reflux disease questionnaire at 1 mo post-washed microbiota transplantation (WMT) were reduced by 30%.
Only one of the 15 patients had minor side effects, with a mushy stool two or three times a day, which resolved on their own after 1 wk, but no abdominal pain, black stool, fever, or serious WMT-related side effects were observed.
WMT: Washed microbiota transplantation; FMT: Fecal microbiota transplantation.
The PPI dose was reduced to some extent in 80% (12/15) of the patients in the WMT group and 33.3% (4/12) in the PPI group. After receiving the WMT treatment, 72.7% (8/11) of the patients who continued using PPIs reduced their PPI doses, and all four patients with on-demand PPI use were also reduced. In addition, 33.3% (5/15) of the patients maintained symptomatic relief and stopped taking PPIs in the WMT group, while the percentage was 16.7% (2/12) in the PPI group (Table 4).
Table 4.
Use of proton pump inhibitors in nonerosive reflux disease patients
|
Items
|
PPI
|
WMT
|
|
| Continuous use before treatment (n) | No reduction after treatment | 3 | 4 |
| Reduction after treatment | 5 | 0 | |
| Withdrawal after treatment | 3 | 0 | |
| Usage as needed before treatment (n) | No reduction after treatment | 0 | 4 |
| Reduction after treatment | 2 | 2 | |
| Withdrawal after treatment | 2 | 2 | |
WMT: Washed microbiota transplantation; PPI: Proton pump inhibitors.
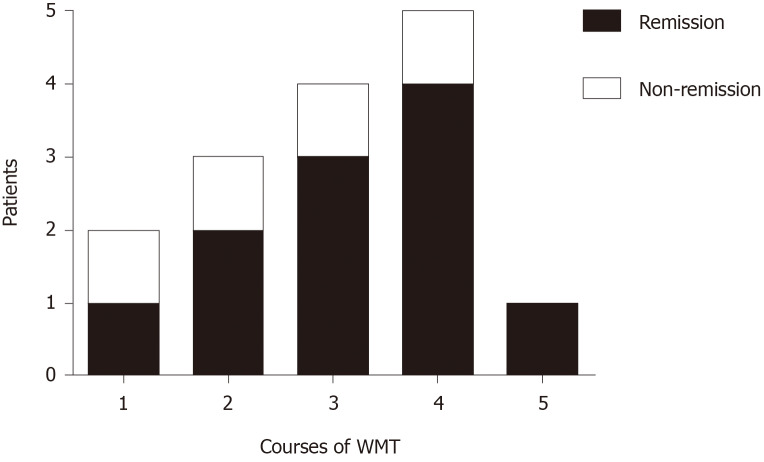
With regard to the relationship between courses of WMT and remission in patients with NERD symptoms (Figure 2), ten people who completed three or more WMT courses achieved an 80% symptom remission rate. With increasing courses of WMT treatment, the remission rate increased.
Figure 2.
Relationship between courses of washed microbiota transplantation and remission of nonerosive reflux disease. WMT: Washed microbiota transplantation.
Twenty-four patients had the function of the intestinal mucosal barrier examined before treatment, which showed that over half of the patients (13/24) had different degrees of intestinal mucosal barrier function damage, such as the change of epithelial permeability (8/24), the damage of intestinal epithelial cells (4/24), intestinal mucosal ischaemia (4/24), and intestinal bacterial translocation (2/24). However, in the WMT group, eight samples were analyzed, and the function of the intestinal mucosal barrier observed as being worse in the post-treatment than pre-treatment, two patients before treatment with intestinal mucosal damage were positive to negative, while six patients with intestinal mucosal barrier damage were negative to positive. Wilcoxon signed-rank test showed that the numeric values of lipopolysaccharide, diamine oxidase, and D-lactate had no significant difference between before and after WMT treatment (Table 5).
Table 5.
Results of examining the function of the intestinal mucosal barrier in the washed microbiota transplantation group
|
Item
|
Lipopolysaccharide
|
Diamine oxidase
|
D-lactate
|
| Pre-treatment (median, IQR) | 7.5 (3.6, 10.4) | 4.1 (3.2, 6.5) | 8.9 (7.6, 11.8) |
| Post-treatment (median, IQR) | 10.1 (1.0, 23.6) | 7.7 (2.3, 13.54) | 16.3 (7.7, 18.3) |
| P value1 | 0.401 | 0.263 | 0.208 |
The results were analyzed by nonparametric Wilcoxon signed-rank test.
IQR: Interquartile range.
Only one of the 15 patients had minor side effects, including a mushy stool two or three times a day, which resolved on their own after 1 wk, but no abdominal pain, black stool, fever, or serious WMT-related side effects were observed.
DISCUSSION
PPIs are currently the first-line treatment for GERD. NERD, which accounts for most GERD cases, ruins patients' quality of life and is more difficult to treat than reflux esophagitis[23]. However, not all patients with NERD can achieve good treatment effects because PPIs are less effective in patients with NERD than in those with reflux esophagitis, and its chronic and recurrent nature means that some patients need to take PPIs for a long time. In addition, potential side effects of long-term use of PPIs are gradually being revealed, including SIBO, lack of micronutrients, and dementia[9,11,24]. A study with population-based follow-up for 10 years in Hong Kong, China in 2018 showed that long-term PPI use could increase the risk of gastric cancer[25]. Therefore, new challenges are arising for NERD treatment, and the results of our study show that WMT can solve this problem. This study confirmed that WMT had a significant clinical effect on NERD in patients with PPI dependency compared to PPI alone. It can reduce the PPI dose and the NERD recurrence rate. Our results showed that WMT, combined with PPI treatment, resulted in remission in 93.3% (14/15) of the patients, and 80% (12/15) of the patients reduced the PPI dose, which was far better than that of the PPI group (33.3%). Compared with the PPI group, the WMT group showed better results in the GERDQ scores and RDQ scores as well as in the remission months, which had obvious statistical significance.
It is widely believed that GERD usually occurs through the following four mechanisms[26]: Transient LES relaxation, low LES pressure, swallowing-associated LES relaxation, and straining during periods with low LES pressure. Recently, new findings related to the pathogenesis of GERD showed that lipopolysaccharide, a component of Gram-negative cell walls, is involved in the mechanism of GERD. Research has shown that the state of esophageal diseases (oesophagitis, Barrett's esophagus, etc.) is mainly dominated by type II Gram-negative bacteria. We know that lipopolysaccharide is the main structure of the outer membrane in Gram-negative bacteria; it can activate Toll-like receptor 4 and the downstream nuclear factor kB pathway to induce an inflammatory response and upregulate the expression of inducible nitrous oxide synthase, which relaxes the LES, and COX-2, which delays gastric emptying[27]. In this retrospective study, 24 patients had the function of the intestinal mucosal barrier examined before treatment, which showed that over half of the patients (13/24) had different degrees of intestinal mucosal barrier function damage, which may be involved in the pathogenesis of GERD. We also analyzed the values of lipopolysaccharide, diamine oxidase, and D-lactate before and after WMT treatment for 8 patients. The results showed that the medians were increased after treatment compared with those before treatment, but there was no significant difference. This seems to be contrary to evidence from a previous study. However, this may be due to the small number of samples, and the test was not performed at the same time. This result gives us good inspiration to focus on researching this aspect in the future.
The results of this trial indicate that as the number of courses of WMT treatment increases, the remission rate increases. The response rate of patients who completed more than four courses of WMT reached 83.3%, but the results may have some limitations due to the small sample size in this study. Although WMT is increasingly used in clinical treatment, there is no uniform treatment course or dosage. According to previous reports, multiple fresh fecal transplants can improve clinical efficacy[28]. We recommend that WMT for GERD be administered in four courses at 1, 2, 3, and 6 mo (1 course is a continuous fecal treatment for 3 d, with 200 mL of fecal separated solution injected into the jejunum or caecum via the middle or lower digestive tract per day). The four courses of treatment recommended in this study are feasible, and it seems that increasing the treatment course can increase the rate of remission. The poor WMT responses observed in this study may be related to the existence of organic lesions that cause GERD, such as hiatal hernia, in some patients, and some may be related to failure to complete the recommended courses.
Because this is a retrospective study, its limitations are that the accuracy and completion of the relevant data and questionnaires could not be improved during follow-up. Memory bias may have affected the questionnaires. The analysis of related test items had a small sample size, which affects the analysis of the experimental results.
CONCLUSION
In conclusion, this study for the first time demonstrated that WMT is safe and effective in treating patients with NERD and PPI dependency. Compared to the PPI group, it can significantly relieve the symptoms of NERD patients, reduce PPI dependency, prolong the duration of remission in symptoms, and reduce recurrence. It can also increase the diversity and evenness of the bacterial community. The next step is to expand the sample size and perform further studies to confirm the role of WMT in the treatment of NERD and elucidate its mechanism.
ARTICLE HIGHLIGHTS
Research background
The pathogenesis of gastroesophageal reflux disease (GERD) is closely associated with the intestinal bacteria composition and their metabolites.
Research motivation
At present, the treatment of GERD has not achieved satisfactory clinical results.
Research objectives
To investigate whether washed microbiota transplantation (WMT) improves symptoms of nonerosive reflux disease (NERD) with proton pump inhibitor (PPI) dependency.
Research methods
Patients with recurrent NERD and PPI dependency at the First Affiliated Hospital of Guangdong Pharmaceutical University from 2017 to 2018 were included and divided into a WMT or PPI group treated with PPI with/without WMT. The endpoint was NERD symptom frequency evaluated 1 mo after WMT using reflux disease questionnaire (RDQ) and GERD questionnaire (GERDQ) scores, remission time, PPI dose, and the examination of intestinal mucosal barrier function.
Research results
In the WMT (n = 15) and PPI (n = 12) groups, the total remission rate at 1 mo after treatment was 93.3% vs 41.7%. Compared with the PPI group, the WMT group showed better results in GERDQ (P = 0.004) and RDQ (P = 0.003) and in remission months (8 vs 2, P = 0.002). The PPI dose was reduced to some extent for 80% of patients in the WMT group and 33.3% in the PPI group. In 24 patients, intestinal mucosal barrier function was examined before treatment, and changes in the degree of damage were observed in 13 of these patients after treatment. Only one of the 15 patients had minor side effects, including a mushy stool two or three times a day, which resolved on their own after 1 wk.
Research conclusions
This study is the first to demonstrate that WMT may be safe and effective for relieving NERD symptoms and reducing PPI dependency and recurrence.
Research perspectives
WMT could be a new treatment for NERD.
ACKNOWLEDGEMENTS
We acknowledge Professor Fa-Ming Zhang and his team at the Second Affiliated Hospital of Nanjing Medical University of China for technical guidance on washed microbiota transplantation. We also acknowledge the patients and donors for their participation in this study.
Footnotes
Institutional review board statement: This retrospective study was approved by the Ethics Committee of Guangdong Pharmaceutical University (Approval No. Yilun Shen[2019] No. 93 01).
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
Manuscript source: Unsolicited manuscript
Peer-review started: November 29, 2020
First decision: December 8, 2020
Article in press: January 12, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maslennikov R S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
Contributor Information
Ya-Mei Zheng, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Xian-Yun Chen, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Jie-Yi Cai, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Yu Yuan, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Wen-Rui Xie, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Jia-Ting Xu, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Harry Hua-Xiang Xia, Department of Science and Education, The First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou 510030, Guangdong Province, China.
Min Zhang, Department of Epidemiology and Health Statistics, Guangdong Pharmaceutical University, Guangzhou 510220, Guangdong Province, China.
Xing-Xiang He, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China.
Li-Hao Wu, Department of Gastroenterology, The First Affiliated Hospital of Guangdong Pharmaceutical University, Research Center for Engineering Techniques of Microbiota-Targeted Therapies of Guangdong Province, Guangzhou 510030, Guangdong Province, China. wulihao888@126.com.
Data sharing statement
No additional data are available.
References
- 1.Savarino E, de Bortoli N, De Cassan C, Della Coletta M, Bartolo O, Furnari M, Ottonello A, Marabotto E, Bodini G, Savarino V. The natural history of gastro-esophageal reflux disease: a comprehensive review. Dis Esophagus. 2017;30:1–9. doi: 10.1111/dote.12511. [DOI] [PubMed] [Google Scholar]
- 2.Hunt R, Armstrong D, Katelaris P, Afihene M, Bane A, Bhatia S, Chen MH, Choi MG, Melo AC, Fock KM, Ford A, Hongo M, Khan A, Lazebnik L, Lindberg G, Lizarzabal M, Myint T, Moraes-Filho JP, Salis G, Lin JT, Vaidya R, Abdo A, LeMair A Review Team: World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J Clin Gastroenterol. 2017;51:467–478. doi: 10.1097/MCG.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita Y, Ashida K, Hongo M Japan Rabeprazole Study Group for NERD. Randomised clinical trial: a multicentre, double-blind, placebo-controlled study on the efficacy and safety of rabeprazole 5 mg or 10 mg once daily in patients with non-erosive reflux disease. Aliment Pharmacol Ther. 2011;33:213–224. doi: 10.1111/j.1365-2036.2010.04508.x. [DOI] [PubMed] [Google Scholar]
- 4.Labenz J, Labenz G, Stephan D, Willeke F LOPA-Studiengruppe. [Insufficient symptom control under long-term treatment with PPI in GERD - fact or fiction? MMW Fortschr Med. 2016;158 Suppl 4:7–11. doi: 10.1007/s15006-016-8303-0. [DOI] [PubMed] [Google Scholar]
- 5.Jovov B, Que J, Tobey NA, Djukic Z, Hogan BL, Orlando RC. Role of E-cadherin in the pathogenesis of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1039–1047. doi: 10.1038/ajg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan Z, Alastal Y, Khan MA, Khan MS, Khalil B, Shrestha S, Kamal F, Nawras A, Howden CW. On-Demand Therapy with Proton Pump Inhibitors for Maintenance Treatment of Nonerosive Reflux Disease or Mild Erosive Esophagitis: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2018;2018:6417526. doi: 10.1155/2018/6417526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadlapati R, DeLay K. Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease. Med Clin North Am. 2019;103:15–27. doi: 10.1016/j.mcna.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson MA, Goodrich JK, Maxan ME, Freedberg DE, Abrams JA, Poole AC, Sutter JL, Welter D, Ley RE, Bell JT, Spector TD, Steves CJ. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 11.Naito Y, Kashiwagi K, Takagi T, Andoh A, Inoue R. Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion. 2018;97:195–204. doi: 10.1159/000481813. [DOI] [PubMed] [Google Scholar]
- 12.Tugtepe H, Tugay M, Bozkurt S, Yildiz F, Utkan T, Yegen BC, Dagli TE. Esophageal smooth muscle reactivity is impaired in chronic reflux esophagitis by both receptor- and nonreceptor-mediated mechanisms. J Pediatr Surg. 2007;42:641–646. doi: 10.1016/j.jpedsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Suri J, Kataria R, Malik Z, Parkman HP, Schey R. Elevated methane levels in small intestinal bacterial overgrowth suggests delayed small bowel and colonic transit. Medicine (Baltimore) 2018;97:e10554. doi: 10.1097/MD.0000000000010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pimentel M, Lin HC, Enayati P, van den Burg B, Lee HR, Chen JH, Park S, Kong Y, Conklin J. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1089–G1095. doi: 10.1152/ajpgi.00574.2004. [DOI] [PubMed] [Google Scholar]
- 15.Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, Moore D, Colville A, Bhala N, Iqbal TH, Settle C, Kontkowski G, Hart AL, Hawkey PM, Williams HR, Goldenberg SD. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J Hosp Infect. 2018;100 Suppl 1:S1–S31. doi: 10.1016/j.jhin.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 16.Wen W, Zhang H, Shen J, Wei L, Shen S. Fecal microbiota transplantation for patients with irritable bowel syndrome: A meta-analysis protocol. Medicine (Baltimore) 2018;97:e12661. doi: 10.1097/MD.0000000000012661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ianiro G, Bibbò S, Scaldaferri F, Gasbarrini A, Cammarota G. Fecal microbiota transplantation in inflammatory bowel disease: beyond the excitement. Medicine (Baltimore) 2014;93:e97. doi: 10.1097/MD.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding C, Fan W, Gu L, Tian H, Ge X, Gong J, Nie Y, Li N. Outcomes and prognostic factors of fecal microbiota transplantation in patients with slow transit constipation: results from a prospective study with long-term follow-up. Gastroenterol Rep (Oxf) 2018;6:101–107. doi: 10.1093/gastro/gox036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang F, Cui B, He X, Nie Y, Wu K, Fan D FMT-standardization Study Group. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9:462–473. doi: 10.1007/s13238-018-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, Chen Y, Yin H, Wang H, Marcella C, Cui B, Cheng L, Ji G, Zhang F. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11:251–266. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li HC, Fan XJ, Chen YF, Tu JM, Pan LY, Chen T, Yin PH, Peng W, Feng DX. Early prediction of intestinal mucosal barrier function impairment by elevated serum procalcitonin in rats with severe acute pancreatitis. Pancreatology. 2016;16:211–217. doi: 10.1016/j.pan.2015.12.177. [DOI] [PubMed] [Google Scholar]
- 22.Shen S, Zhao J, Dai Y, Chen F, Zhang Z, Yu J, Wang K. Methamphetamine-induced alterations in intestinal mucosal barrier function occur via the microRNA-181c/ TNF-α/tight junction axis. Toxicol Lett. 2020;321:73–82. doi: 10.1016/j.toxlet.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Savarino E, Marabotto E, Bodini G, Pellegatta G, Coppo C, Giambruno E, Brunacci M, Zentilin P, Savarino V. Epidemiology and natural history of gastroesophageal reflux disease. Minerva Gastroenterol Dietol. 2017;63:175–183. doi: 10.23736/S1121-421X.17.02383-2. [DOI] [PubMed] [Google Scholar]
- 24.Vaezi MF, Katzka D, Zerbib F. Extraesophageal Symptoms and Diseases Attributed to GERD: Where is the Pendulum Swinging Now? Clin Gastroenterol Hepatol. 2018;16:1018–1029. doi: 10.1016/j.cgh.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Cheung KS, Chan EW, Wong AYS, Chen L, Wong ICK, Leung WK. Long-term proton pump inhibitors and risk of gastric cancer development after treatment for Helicobacter pylori: a population-based study. Gut. 2018;67:28–35. doi: 10.1136/gutjnl-2017-314605. [DOI] [PubMed] [Google Scholar]
- 26.Tack J, Pandolfino JE. Pathophysiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018;154:277–288. doi: 10.1053/j.gastro.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Yang L, Francois F, Pei Z. Molecular pathways: pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 2012;18:2138–2144. doi: 10.1158/1078-0432.CCR-11-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Z, Li P, Zhu J, Cui B, Xu L, Xiang J, Zhang T, Long C, Huang G, Ji G, Nie Y, Wu K, Fan D, Zhang F. Multiple fresh fecal microbiota transplants induces and maintains clinical remission in Crohn's disease complicated with inflammatory mass. Sci Rep. 2017;7:4753. doi: 10.1038/s41598-017-04984-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.