Abstract
Background
Atrial fibrillation (AF) is one of the most common cardiac disorders affecting adults and is associated with significant morbidity and mortality. Efforts to manage AF through anti‐arrhythmics and rate control have been largely unsatisfactory. It has become clear that AF causes structural alterations in the atrial myocardium that propagate further AF, and that some of these alterations are the result of inflammation.
Methods
An in‐depth review of the available literature was undertaken using Google Scholar and keyword searches including [Atrial fibrillation] in combination with [inflammatory markers], [myocardial fibrosis], and [immunomodulators], limiting the search to English language articles. All articles were reviewed for relevance and collated by the author.
Results
Multiple markers of inflammation have been shown to be elevated in AF and to predict responses to treatments of AF including anti‐arrhythmics and cardioversion. The nidus of inflammation is not clear but seems to be related to the pulmonary veins.
Conclusions
The inflammatory cascade induces fibrotic changes in the myocardium, an arrhythmogenic process that stimulates further inflammation. Advances in treatment are focusing on biological agents and immunomodulators that inhibit the inflammatory cascade.
Keywords: atrial fibrillation, cardiac arrhythmia, inflammatory biomarkers
Our paper describes the most current understanding of the role of inflammation in the pathophysiology of atrial fibrillation. Current science has shown that atrial fibrillation causes inflammation and structural changes in the myocardium, and that inflammation and the changes in structure work together to reduce the threshold for atrial fibrillation. The mediators of this process are delineated in the paper along with recommendations for future research and treatment.
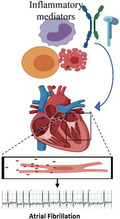
1. INTRODUCTION
Atrial fibrillation (AF) is the most common clinically relevant cardiac arrhythmia in adults. It is more common in the elderly, affecting about 1% of people under 65 years old but 5% of people over 65. 1 AF is associated with significant morbidity and mortality and has adverse effects on overall quality of life. Even patients with short‐term (≤7 days) AF have an increased risk of stroke. 2 Furthermore, given the aging of our society, the economic burden of AF is enormous.
Current management options are suboptimal. Outcomes of rhythm and rate control efforts have shown the limited efficacy and high side effects of both methods. 3 Therefore, there is a significant need for alternative, safe, and effective methods of treatment. This strong need has prompted a fundamental re‐evaluation of the pathophysiology of AF with the goal of finding new approaches to treatment.
Inflammatory processes are among the major focuses of current medical research and there are increasing evidences that inflammation may play a major role in cardiovascular diseases including myocardial disease, atherosclerotic disease, and strokes. 4 The role of inflammation in AF is becoming more clear, changing the paradigm from one of an electrical abnormality to a biochemical, structural disorder. Previously Wu et al 5 , 6 have compiled list of studies reporting role of inflammation in the perpetuation of AF. Guadino et al 7 linked an inflammatory marker (Interleukin‐6 [IL‐6]) polymorphism with postoperative AF while C‐reactive protein has been implicated in AF both post‐ 8 and non‐postoperative patients. 9 By means of multivariable proportional hazards model, Schnabel et al reported association of 12 inflammatory markers with AF incidence. 10 Therapies focused at reducing the inflammatory affliction are encouraging for AF treatment. Chokesuwattanaskul et al have compiled a list of studies linking NSAIDs with AF reduction. 11 The aim of this article is to describe the role of different inflammatory markers in AF.
2. WHAT IS THE PATHOPHYSIOLOGY OF AF?
Atrial fibrillation, once established, causes changes in the atrial myocardium which are known as “cardiac remodeling” (Figure 1). Electrical remodeling in AF promotes ongoing re‐entrant depolarizations that cause more AF. 12 The atrial refractory period shortens and atrial conductivity lengthens, while calcium accumulation in atrial myocytes further shortens the refractory period. Multiple small re‐entrant circuits develop. 13 It has also been reported that reactive oxygen species from mitochondria activate CAMKII, leading to the phosphorylation of another marker RYR2. This initiates AF, as observed in mouse model studies. Oxidative stress heightens AF and triggers the marker CAMKII to associate Ca2+ with calmodulin and stimulate it. 14 The adage “AF begets AF” applies.
FIGURE 1.
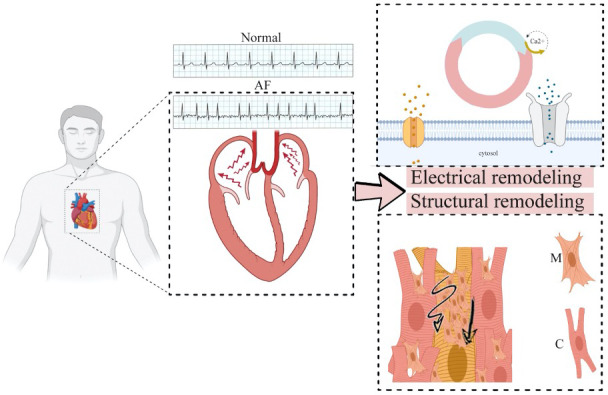
Major changes caused by atrial fibrillation (AF) include electrical and structural remodeling. Minute but irregular changes in electrocardiogram of AF patient can be seen in the figure. Ca2+ is the center of the electrical changes, with several more perturbations linked to it. Increased calcium leads to short refractory period (depicted by blue in the cycle), leading to transmission of re‐entrant waves, breaking normal sequence, and setting up more cycles. At tissue scale structural remodeling, conduction is slowed by the myofibroblast (represented by M) secreting collagen (shown in yellow), leading to fibrosis. Myofibroblast is involved in ionic remodeling and its interaction with cardiomyocyte (represented by C) also slackens conduction
There are structural remodeling changes as well, including left atrial dilatation and atrial fibrosis. The intercellular fibrosis impedes atrial conduction, affecting the biophysical and electrical properties of the atrial wall. 15 The loss of signal coordination and control also adds to the perpetuation of AF.
Collagen‐based tissue fibrosis is important as a reparative process after tissue damage or cell death. Normal atrial myocytes are stacked end‐to‐end, connected by specialized intercellular proteins called connexins. The connexins work in the gap junctions, forming low‐resistance communications channels between cells. 15 In the myocardium, apoptotic or necrotic cells are replaced by collagen following infarction or exposure to toxins, so‐called reparative fibrosis, replacing individual cells within a stack of myocytes. This results in a separation of cells from end‐to‐end, interfering with intercellular communication through endplates. Reactive fibrosis occurs in response to inflammation, tachycardia, and altered clearing of collagen. This is most commonly seen in hypertension and diabetes mellitus, through effects of the renin‐angiotensin aldosterone system, beta‐adrenergic system, excessive reactive oxygen species, and metabolic disturbances caused by hyperglycemia. 16 , 17 Collagen fibers are deposited between strands of myocyte stacks, increasing the volume of intercellular matrix and altering communication between bundles of myocytes. 15 While reactive fibrosis begins early, eventually individual myocytes become necrotic and are replaced by reparative fibrosis. 18 Since reparative fibrosis directly impacts on connexins, it is felt to be a more significant problem in chronic atrial fibrillation.
At a molecular level, fibrosis is mediated by several signaling pathways including angiotensin II, transforming growth factor (TGF)‐beta 1, platelet‐derived growth factor (PDGF), and connective tissue growth factor. TGF‐β1 works in concert with a transmembrane protein called CD44 that stimulates cell's STAT3 pathway, increasing expression of hyaluronic acid (HA) and collagen. The angiotensin II receptor AT1 stimulates the CD44/HA/STAT3 interaction, promoting fibrosis. 19 Other mediators have been described in the fibrosis of AF, including oxidative stress marker GDF‐15 linked with bleeding, stroke and death in AF. 20 Oxidative stress in diabetes and obesity aggravates structural remodeling and hence, AF. 21
Osteopontin, an extracellular bridging protein, was recently shown to stimulate atrial fibrosis by promoting the proliferation of fibroblasts, production of collagen I, and secretion of fibronectin. 22 Atrial natriuretic protein, its receptor, and its clearance receptor may also play a role in atrial wall fibrosis contributing to AF. 23 Spronk et al focused on the effects of atrial fibrillation and a hypercoagulable state. 24 The findings of their experiments confirmed that, while AF induced a hypercoagulable state, the hypercoagulable state during AF induced profibrotic and proinflammatory responses in atrial fibroblasts. They proposed that the inhibition of coagulation may not only help prevent strokes but may also help prevent the fibrosis seen in AF.
The location of the initial trigger of AF is generally not known, but the pulmonary veins have been implicated in recent research. 25 Inflammation in the pulmonary vein may act as the nidus for AF, and continued inflammation likely mediates the remodeling process as described above. Studies have identified structural changes within the myocardium that begins without a few hours of onset of AF 26 , consistent with the difficulty of successful restoration of sinus rhythm by cardioversion after 24 hours. This reinforces the concept of structural rather than simply electrical changes.
3. IS THERE A CONNECTION BETWEEN INFLAMMATION AND AF?
There is increasing evidence to link inflammation to AF as in several other cardiovascular diseases. 27 AF is commonly seen as a complication of pericarditis, myocarditis, and endocarditis. The earliest reference to the idea that AF and inflammation were connected was posed by Bruins after the observation that AF occurred on the second or third day after coronary artery bypass surgery at about the same time the C‐reactive protein (CRP) levels peaked. 28 The following year, a large percentage of patients with intermittent AF of unknown etiology were noted to have antibodies against cardiac myofibril components. 29 These two studies supported the idea that AF was an inflammatory condition.
4. WHAT IS THE LABORATORY EVIDENCE OF INFLAMMATION IN AF?
After these two landmark studies were published, investigators started to examine the possible role of variety of inflammatory markers in AF. Although in most of these analyses, it was not clear whether there was a cause‐and‐effect relationship between AF and inflammation, but there has been strong evidence of an association between these conditions (Figure 2). Laboratory studies have revealed basis of higher cardiac inflammation and AF in aged females. 30 Inflammation association with AF in rat studies has been summarized in Table 1. Postoperative link of inflammation and AF has been established in canine model. 31 Hamanaka et al further reported in a single center study, that the bleeding risk is elevated in patients with nonvalvular AF. 32 Choi et al reported association between augmented chance of AF in patients with inflammatory bowel disease. 33 The two markers of inflammation most often mentioned in relation to AF are high‐sensitivity CRP (hsCRP) and IL‐6. Other reported markers include tumor necrosis factor‐α (TNF‐α), interleukin‐2 (IL‐2), and interleukin‐8 (IL‐8).
FIGURE 2.
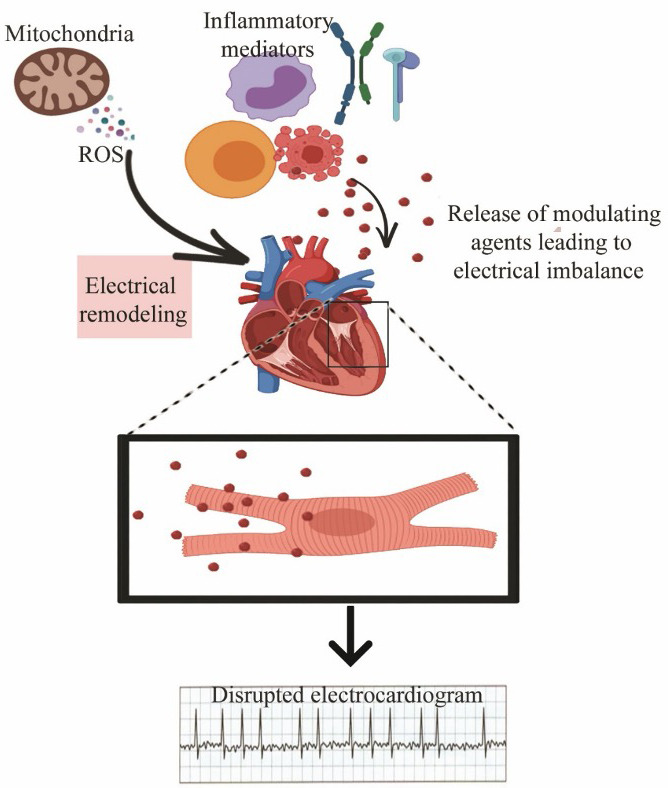
Mitochondrial‐derived reactive oxygen species and inflammatory mediators such as interleukins, cytokines (monocytes shown in yellow, macrophages shown in red, lymphocytes in purple) etc trigger atrial fibrillation
TABLE 1.
Studies reporting AF linkage to inflammatory markers in laboratory rats
| Serial no. | Marker | Correlation | Reference |
|---|---|---|---|
| 1 | TNF, interleukin‐1β and interleukin‐6 | Negative | 34 |
| 2 | Interleukin‐10 | Positive | 34 |
| 3 | Natriuretic peptide A mutant | Positive | 35 |
| 4 | Collagen 1, Collagen 3, fibronectin, matrix metalloproteinases 2 and 9 | Positive | 36 |
| 5 | Interleukin‐17A, IL‐6, IL‐1β and TGF‐β1 | Positive | 37 |
| 6 | Col‐1, Col‐3, and α‐SMA | Negative | 37 |
| 7 | IL‐6 and TNF‐α | Positive | 38 |
| 8 | CC chemokine receptor 2+ ED‐1+, ED‐1+ macrophages Interleukin‐6, TNF‐α and nuclear p65 | Positive | 39 |
4.1. C‐reactive protein
Of the various inflammatory markers, hsCRP has proven to be the most predictable indicator of vascular inflammation. CRP is described as an acute‐phase reactant secreted by the liver in response to IL‐6 production by macrophages and T‐cells. Physiologically, it binds to dead or dying cells and activates the complement system. CRP is synthesized in response to circulating factors released by macrophages and adipocytes, explaining the low‐level inflammatory state that accompanies obesity. 40 Elevated hsCRP has consistently been associated with the risk of cardiovascular events including myocardial ischemia and infarction, sudden cardiac death, stroke, and peripheral vascular disease. 41 Patients with AF tend to have a higher level of hsCRP than people in normal sinus rhythm. 8 Patients in chronic atrial fibrillation have a higher level of hsCRP than people with paroxysmal AF. Finally, people with long‐standing AF tend to show higher levels of hsCRP and more atrial remodeling and dilatation than those with shorter duration AF. After cardioversion, patients with higher hsCRP are more likely to relapse into AF than those with lower serum levels. 42 Patients in normal sinus rhythm with high levels of hsCRP are more likely to develop AF. These studies emphasize the relationship between inflammation and AF.
The underlying cause of the relationship between hsCRP and AF is still unclear. CRP appears to bind to the membranes of myocardial cells, activating the complement cascade and triggering damage to the tissues. 43 Immunohistochemical studies have shown high levels of CRP in atheroma in coronary artery disease models. 44 hsCRP appears to fall in response to statin therapy. 45
4.2. Interleukin‐6
Interleukin‐6 is produced by macrophages, T‐cells, and endothelial cells and has both pro‐inflammatory and anti‐inflammatory functions. It is a cytokine that stimulates the production of CRP, fibrinogen, and serum amyloid‐A. Released after the interaction of immune cells and pathogens, it stimulates the production of several downstream cytokines and triggers fever. In muscle, it is released in response to exercise and downregulates inflammation through its control of tumor necrosis factor TNF‐α. 46
Although IL‐6 is considered cytoprotective in cardiac and muscle tissues 47 , studies of IL‐6 levels in AF have been mixed. The ARISTOTLE trial of 18 201 patients with AF showed higher mortality in patients with higher IL‐6 levels compared to patients with normal levels. 48 Another large cohort study, CRIC (Chronic Renal Insufficiency Cohort), found that plasma IL‐6 level is an independent and consistent predictor of AF in patients with chronic kidney disease. 49 Landiolol, a super‐short‐acting β‐blocker used in Europe and Japan during thoracic surgery to control heart rate, was associated with significantly lower postesophagectomy AF and lower IL‐6 levels. 50 IL‐6 was shown to rapidly induce electrical remodeling through its effect on intercellular connexins, decreasing cell‐to‐cell communication. 51 In a large randomized placebo‐controlled trial, the IL‐6 antagonist canakinumab approved for treatment of rheumatologic disorders was shown to reduce cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death) vs placebo in a dose‐dependent manner. 52 That study did not evaluate the impact of canakinumab on atrial fibrillation. Curiously, tocilizumab, a biologic immunosuppressant used in the treatment of rheumatoid disorders, selectively blocks IL‐6 receptors. It is being studied as an anti‐rejection agent in cardiac transplantation, 53 but it has not been used in the treatment or prevention of AF because of its potentially serious side effects and high cost. IL‐6 may have a role in increasing the prothrombotic state seen in patients with AF. 54
4.3. Tumor necrosis factor
Tumor necrosis factor (TNF), formerly called TNF‐α, is a cell‐signaling protein involved in the inflammatory cascade. Produced by macrophages, CD4+ lymphocytes, NK cells, neutrophils, mast cells, eosinophils, and neurons, TNF is an immune cell activator and a pyrogen, directly triggering fever in animals. It has been shown to cause CRP production by the liver and to stimulate systemic cytokine reactions to infection. 55
Similar to other inflammatory markers, TNF levels are higher in patients with AF. A study by Hai of 67 patients who were scheduled to have cardiac surgery (31 with rheumatic heart disease and AF, 36 with other heart disease and sinus rhythm) found higher levels of TNF in the AF group compared with the sinus rhythm group. 56 Multiple animal studies have shown reductions in atrial fibrillation with the use of TNF and TNF‐receptor blockers. A review of the literature by Ren et al showed beneficial effects of TNF blockade in preventing AF. 57
4.4. Interleukin‐2
Interleukin‐2 is another inflammatory cytokine that is produced by activated CD4+ and CD8+ T‐cells. It is involved in the inflammatory cascade and stimulates immune cells, often in response to microbial invaders. It functions through an interaction between the IL‐2 receptor and a cell's JAK/STAT signaling pathway. 58
Interleukin‐2 has been shown to correlate with the risk of developing AF after cardiac surgery. In one study by Haq, 33 patients undergoing coronary artery bypass graft (CABG) surgery were followed. Eleven developed AF within 1‐2 days postoperatively. Those with AF had a higher IL‐2 level than those without AF, and those who developed AF early (<24 hours) had a higher level of IL‐2 than those who developed AF later. 59 Rizos et al showed that patients undergoing cardioversion for AF were less often successful if they had higher IL‐2 levels. 60 Another study showed that IL‐2 level was an independent predictor of recurring AF at the 1‐year follow‐up postpulmonary vein catheter ablation for AF. 61 Based on our current knowledge, one of the mechanisms of action of lovastatin is inhibition of IL‐2 production with reduction in the level of systemic inflammation in patients with atherosclerosis 62 with potential therapeutic implications in patients with AF.
4.5. Interleukin‐8
Physiologically, IL‐8 recruits inflammatory cells to attack and phagocytize an antigen. Also known as the neutrophil chemotactic factor, it stimulates migration of neutrophils and granulocytes to a tissue, then stimulates phagocytosis once they arrive. IL‐8 increases angiogenesis and triggers local histamine release. 63
Interleukin‐8 levels rise in the serum of patients with AF. 64 In a cohort of 113 patients undergoing CABG surgery, patients with postoperative or sustained AF after surgery had significantly higher levels of IL‐8 than patients in sinus rhythm. 6 A similar study in 2014 found a correlation between inflammatory markers including IL‐8 and the development of AF after CABG surgery. 65 The origin of the IL‐8 in patients with AF is unclear, but work by Liuba et al suggests that it originates from the peripheral blood stream rather than locally. 66
4.6. White blood cell counts
Leukocytosis, while a non‐specific indicator of inflammation appears to predict AF after cardiac surgery. In a study of 272 patients who underwent lobectomy, pneumonectomy, or esophagectomy, a twofold increase in white blood cell count was associated with a threefold increase in the risk of AF. 67 A similar study by Lamm et al of 253 patients with normal left ventricular (LF) function undergoing elective cardiac surgery found that leukocytosis was associated with an increased risk of postoperative AF. 68 Preoperative leukocytosis in CABG predicted AF in a cohort of 66 patients in a study published in 2009. 69 Leukocytosis is seen as a general indicator of systemic inflammatory and oxidative state, signaling which patients may be at risk for the development of AF.
5. IMPLICATIONS FOR PRACTICE
Management of the AF requires treatment and prevention strategies (Figure 3). Prevention can be done via adopting healthy life style and reducing the risks while for treatment, several options are available. Given the number of inflammatory markers that are elevated in AF, it is reasonable to classify AF as an inflammatory state. There are still lingering arguments about cause and effect, with some experts arguing that AF causes inflammation instead of the converse. Clearly there is crosstalk as well as other variables such as other hormones and cytokines involved. There have been several classes of medications that have been shown to be helpful in the prevention of AF.
FIGURE 3.
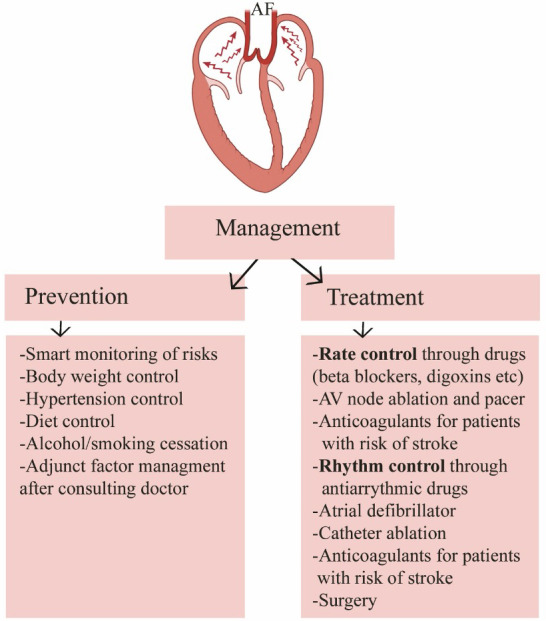
Overview of management of the atrial fibrillation (AF). Both prevention and treatment measures are necessary for successful therapy
5.1. Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is a key hormone in regulating blood pressure, vascular smooth muscle tone, renin production, and renal reabsorption of sodium in the distal tubule. Angiotensin II has been found to be pro‐inflammatory, and numerous studies have documented the effectiveness of both ACEi and ARBs in the primary and secondary prevention of AF. 70 Modulation of the renin‐angiotensin‐aldosterone system (RAAS) reduces the development of atrial fibrosis and cardiac remodeling, key findings in patients with AF. Animal studies have shown the effects of angiotensin II on remodeling, fibrosis, and repolarization in the atria as contributors to the development of AF. 71 Both ACEi and ARBs appear to be equally effective. 72
There have been several proposed mechanisms for the effectiveness of ACEi and ARBs in the prevention of AF, including a decrease in atrial stretch, lowered atrial pressures, prevention of angiotensin‐induced fibrosis, reduced sympathetic tone, and direct antiarrhythmic effects. 73 Furthermore, RAAS modulators likely reduce atrial wall inflammation, oxidative stress, and remodeling. 74
5.2. Statins
Hydroxymethyl glutaryl coenzyme A reductase (HMG CoA) inhibitors are potent lipid lowering agents with secondary anti‐inflammatory properties. These agents have also been shown to enhance the function of endothelial cells, increase nitric oxide, reduce thrombosis, stabilize plaques, and reduce oxidative stress. 75
More recently, statins' anti‐arrhythmic properties have been explored in relation to AF. In fact, they have been shown to be effective in primary and secondary prevention of AF. Siu et al studied 62 patients with chronic AF undergoing successful cardioversion, and showed significant reductions in recurrence of AF with the use of statins. 76 A study by Young‐Xiu evaluated a cohort of 499 patients with coronary artery disease and found that those taking statins were significantly less likely to develop AF. 77 Studies by Reilly et al suggested that statins are more effective early in the course of AF rather than later as a management strategy of chronic AF. 78 The combination of ACEi and statins was shown to be highly effective at reducing new‐onset AF in hypertensive patients. 79 Even in the presence of heart failure, statins reduce the prevalence of AF. 80
5.3. Steroids
Given the relationship between inflammation and AF, it would seem reasonable that steroids should help in the prevention or treatment of AF. In fact, studies have supported the use of glucocorticoids in primary and secondary prevention of AF. One study of 88 patients undergoing CABG given methylprednisolone postoperatively showed reductions in onset of AF. 81 Another study of 138 patients given pulse steroid therapy after catheter ablation found reduced rates of AF. 82 A higher, single dose of injected methylprednisolone worked better at preventing AF after catheter ablation than did a lower dose in a prospective study of 448 patients. 83 However, a more recent study of 60 patients undergoing ablation showed no impact on the rate of postprocedural AF despite a reductions in inflammatory markers. 84 Considering their overall unfavorable side effect profile, the role of steroids in the prevention or treatment of AF is unclear.
5.4. Fish oils
The effectiveness of fish oil in the prevention or treatment of AF is controversial. Dietary intake of polyunsaturated fatty acids (PUFAs) improve cardiovascular outcomes, partly through their hypolipidemic effects and partly because of their antioxidant properties. 85 In fact, the n‐3 fatty acids are often used in the treatment of inflammatory diseases such as rheumatoid arthritis and Crohn's disease. A diet rich in n‐3 fatty acids correlates with reductions in inflammatory markers. The effect on AF is less clear. 86
In a prospective, randomized, placebo‐controlled study of 1516 patients undergoing cardiac surgery, PUFA did not prove to be protective against the development of AF. 86 Similarly, another prospective, randomized, placebo‐controlled study of 337 patients with AF found no positive effects on the inflammatory markers and recurrence of AF with the use of fish oils. 87 A large meta‐analysis of fish oil for prevention of AF in postoperative patients found no effect. 88 Our conclusions is that fish oil supplementation is unlikely to add benefit in the prevention or treatment of AF.
5.5. Vitamin C
Ascorbic acid (vitamin C) is an inexpensive nutritional supplement and is a potent antioxidant. This effect could potentially reduce the inflammation in patients at risk for AF. Vitamin C reduces atrial electrical remodeling, thereby reducing the incidence of AF, possibly through its effects of scavenging reactive oxygen species and reducing overall inflammation. 89 Studies have shown a modest effect of vitamin C supplementation. In one study of 44 patients undergoing electrical cardioversion, vitamin C was associated with a decrease in AF recurrence. 90 A recent meta‐analysis by Hemilä et al showed a benefit of vitamin C supplements for the prevention of AF, but only in countries outside of the United States. 91 Another recent meta‐analysis found that peri‐operative vitamin C reduced the onset of postoperative AF. 92
6. CONCLUSIONS
In summary, there are extensive and expanding evidence showing a connection between inflammation and the development and propagation of AF. These relationships do not adequately define exactly why they are related and further in vitro and in vivo studies can shed further light on the pathophysiology of this connection. Furthermore, several medications have shown protentional beneficial effects in the prevention and reduction of AF. Studies with larger patient population, in particular randomized clinical trials, are needed to better investigate their roles. Newer anti‐inflammatory medications including monoclonal antibodies which demonstrated significant benefit in decreasing events related to atherosclerotic coronary disease could also be the next generation of medications in the AF field and need to be investigated.
CONFLICT OF INTEREST
None.
Nso N, Bookani KR, Metzl M, Radparvar F. Role of inflammation in atrial fibrillation: A comprehensive review of current knowledge. J Arrhythmia.2021;37:1–10. 10.1002/joa3.12473
REFERENCES
- 1. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med. 1982;306(17):1018–22. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–8. [DOI] [PubMed] [Google Scholar]
- 3. Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck K‐H, Vardas P, et al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166(3):442–8. [DOI] [PubMed] [Google Scholar]
- 4. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96(9):939–49. [DOI] [PubMed] [Google Scholar]
- 5. Wu NA, Xu B, Xiang Y, Wu L, Zhang Y, Ma X, et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: a meta‐analysis. Int J Cardiol. 2013;169(1):62–72. [DOI] [PubMed] [Google Scholar]
- 6. Wu ZK, Laurikka J, Vikman S, Nieminen R, Moilanen E, Tarkka MR. High postoperative interleukin‐8 levels related to atrial fibrillation in patients undergoing coronary artery bypass surgery. World J Surg. 2008;32(12):2643–9. [DOI] [PubMed] [Google Scholar]
- 7. Gaudino M, Andreotti F, Zamparelli R, Di Castelnuovo A, Nasso G, Burzotta F, et al. The ‐174G/C interleukin‐6 polymorphism influences postoperative interleukin‐6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(suppl 1):II195–9. [DOI] [PubMed] [Google Scholar]
- 8. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–91. [DOI] [PubMed] [Google Scholar]
- 9. Dernellis J, Panaretou M. C‐reactive protein and paroxysmal atrial fibrillation: evidence of the implication of an inflammatory process in paroxysmal atrial fibrillation. Acta Cardiol. 2001;56(6):375–80. [DOI] [PubMed] [Google Scholar]
- 10. Schnabel RB, Larson MG, Yamamoto JF, Kathiresan S, Rong J, Levy D, et al. Relation of multiple inflammatory biomarkers to incident atrial fibrillation. Am J Cardiol. 2009;104(1):92–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chokesuwattanaskul R, Thongprayoon C, Bathini T, Torres‐Ortiz A, O'Corragain OA, Watthanasuntorn K, et al. Efficacy and safety of anticoagulation for atrial fibrillation in patients with cirrhosis: a systematic review and meta‐analysis. Dig Liver Dis. 2019;51(4):489–95. [DOI] [PubMed] [Google Scholar]
- 12. Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation. Time course and mechanisms.Circulation. 1996;94(11):2968–74. [DOI] [PubMed] [Google Scholar]
- 13. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91(1):265–325. [DOI] [PubMed] [Google Scholar]
- 14. Shan J, Xie W, Betzenhauser M, Reiken S, Chen B‐X, Wronska A, et al. Calcium leak through ryanodine receptors leads to atrial fibrillation in 3 mouse models of catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2012;111(6):708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51(8):802–9. [DOI] [PubMed] [Google Scholar]
- 16. Chang SH, Yeh YH, Lee JL, Hsu YJ, Kuo CT, Chen WJ. Transforming growth factor‐beta‐mediated CD44/STAT3 signaling contributes to the development of atrial fibrosis and fibrillation. Basic Res Cardiol. 2017;112(5):58. [DOI] [PubMed] [Google Scholar]
- 17. Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biernacka A, Frangogiannis NG. Aging and cardiac fibrosis. Aging Dis. 2011;2(2):158–73. [PMC free article] [PubMed] [Google Scholar]
- 19. Bai F, Pang X‐F, Zhang L‐H, Wang N‐P, McKallip RJ, Garner RE, et al. Angiotensin II AT1 receptor alters ACE2 activity, eNOS expression and CD44‐hyaluronan interaction in rats with hypertension and myocardial fibrosis. Life Sci. 2016;153:141–52. [DOI] [PubMed] [Google Scholar]
- 20. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130(21):1847–58. [DOI] [PubMed] [Google Scholar]
- 21. Karam BS, Chavez‐Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. 2017;16(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin R, Wu S, Zhu D, Qin M, Liu X. Osteopontin induces atrial fibrosis by activating Akt/GSK‐3beta/beta‐catenin pathway and suppressing autophagy. Life Sci. 2020;245:117328. [DOI] [PubMed] [Google Scholar]
- 23. Rahmutula D, Zhang H, Wilson EE, Olgin JE. Absence of natriuretic peptide clearance receptor attenuates TGF‐beta1‐induced selective atrial fibrosis and atrial fibrillation. Cardiovasc Res. 2019;115(2):357–72. [DOI] [PubMed] [Google Scholar]
- 24. Spronk HMH, De Jong AM, Verheule S, De Boer HC, Maass AH, Lau DH, et al. Hypercoagulability causes atrial fibrosis and promotes atrial fibrillation. Eur Heart J. 2017;38(1):38–50. [DOI] [PubMed] [Google Scholar]
- 25. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–66. [DOI] [PubMed] [Google Scholar]
- 26. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54(2):230–46. [DOI] [PubMed] [Google Scholar]
- 27. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–70. [DOI] [PubMed] [Google Scholar]
- 28. Bruins P, Velthuis HT, Yazdanbakhsh AP, Jansen PGM, van Hardevelt FWJ, de Beaumont EMFH, et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C‐reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96(10):3542–8. [DOI] [PubMed] [Google Scholar]
- 29. Maixent JM, Paganelli F, Scaglione J, Levy S. Antibodies against myosin in sera of patients with idiopathic paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 1998;9(6):612–7. [DOI] [PubMed] [Google Scholar]
- 30. Manwani B, Inam ME, Morales D, Gelinas B, Lee J, McCullough L. Increased cardiac inflammation and atrial fibrillation inducibility in aged females: the niche for cardio‐embolic strokes? Stroke. 2020;51:AWP263. [Google Scholar]
- 31. Ishii Y, Schuessler RB, Gaynor SL, Yamada K, Fu AS, Boineau JP, et al. Inflammation of atrium after cardiac surgery is associated with inhomogeneity of atrial conduction and atrial fibrillation. Circulation. 2005;111(22):2881–8. [DOI] [PubMed] [Google Scholar]
- 32. Hamanaka Y, Sotomi Y, Hirata A, Kobayashi T, Ichibori Y, Makino N, et al. Persistent systemic inflammation is associated with bleeding risk in atrial fibrillation patients. Circulation. 2020;84(3):411–8. [DOI] [PubMed] [Google Scholar]
- 33. Choi Y‐J, Choi E‐K, Han K‐D, Park J, Moon I, Lee E, et al. Increased risk of atrial fibrillation in patients with inflammatory bowel disease: a nationwide population‐based study. World J Gastroenterol. 2019;25(22):2788–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu M, Li W, Wang H, Yin L, Ye B, Tang Y, et al. CTRP9 ameliorates atrial inflammation, fibrosis, and vulnerability to atrial fibrillation in post‐myocardial infarction rats. J Am Heart Assoc. 2020;8(21):e013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng C, Liu H, Tan C, Tong D, Zhao Y, Liu X, et al. Mutation in NPPA causes atrial fibrillation by activating inflammation and cardiac fibrosis in a knock‐in rat model. FASEB J. 2019;33(8):8878–91. [DOI] [PubMed] [Google Scholar]
- 36. Hiram R, Naud P, Xiong F, Al‐U'datt D, Algalarrondo V, Sirois MG, et al. Right atrial mechanisms of atrial fibrillation in a rat model of right heart disease. J Am Coll Cardiol. 2019;74(10):1332–47. [DOI] [PubMed] [Google Scholar]
- 37. Fu X‐X, Zhao N, Dong Q, Du L‐L, Chen X‐J, Wu Q‐F, et al. Interleukin‐17A contributes to the development of post‐operative atrial fibrillation by regulating inflammation and fibrosis in rats with sterile pericarditis. Int J Mol Med. 2015;36(1):83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dai H, Wang X, Yin S, Zhang Y, Han YU, Yang N, et al. Atrial fibrillation promotion in a rat model of rheumatoid arthritis. J Am Heart Assoc. 2017;6(12):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho E, Kim M, Ko YS, Lee HY, Song M, Kim MG, et al. Role of inflammation in the pathogenesis of cardiorenal syndrome in a rat myocardial infarction model. Nephrol Dial Transplant. 2013;28(11):2766–78. [DOI] [PubMed] [Google Scholar]
- 40. Du Clos TW. Function of C‐reactive protein. Ann Med. 2000;32(4):274–8. [DOI] [PubMed] [Google Scholar]
- 41. Ridker PM, Hennekens CH, Buring JE, Rifai N. C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–43. [DOI] [PubMed] [Google Scholar]
- 42. Liu T, Li G, Li L, Korantzopoulos P. Association between C‐reactive protein and recurrence of atrial fibrillation after successful electrical cardioversion: a meta‐analysis. J Am Coll Cardiol. 2007;49(15):1642–8. [DOI] [PubMed] [Google Scholar]
- 43. Lagrand WK, Niessen HWM, Wolbink G‐J, Jaspars LH, Visser CA, Verheugt FWA, et al. C‐reactive protein colocalizes with complement in human hearts during acute myocardial infarction. Circulation. 1997;95(1):97–103. [DOI] [PubMed] [Google Scholar]
- 44. Reynolds GD, Vance RP. C‐reactive protein immunohistochemical localization in normal and atherosclerotic human aortas. Arch Pathol Lab Med. 1987;111(3):265–9. [PubMed] [Google Scholar]
- 45. Albert MA, Danielson E, Rifai N, Ridker PM, Investigators P. Effect of statin therapy on C‐reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286(1):64–70. [DOI] [PubMed] [Google Scholar]
- 46. Gabay C. Interleukin‐6 and chronic inflammation. Arthritis Res Ther. 2006;8 suppl 2(2):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wollert KC, Drexler H. The role of interleukin‐6 in the failing heart. Heart Fail Rev. 2001;6(2):95–103. [DOI] [PubMed] [Google Scholar]
- 48. Aulin J, Hijazi Z, Andersson U, Alexander JH, Gersh B, Granger CB, et al. P3626Serial IL‐6 levels and risk of death in anticoagulated patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2017;38(suppl 1):1.28110302 [Google Scholar]
- 49. Amdur RL, Mukherjee M, Go A, Barrows IR, Ramezani A, Shoji J, et al. Interleukin‐6 is a risk factor for atrial fibrillation in chronic kidney disease: findings from the CRIC study. PLoS ONE. 2016;11(2):e0148189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Horikoshi Y, Goyagi T, Kudo R, Kodama S, Horiguchi T, Nishikawa T. The suppressive effects of landiolol administration on the occurrence of postoperative atrial fibrillation and tachycardia, and plasma IL‐6 elevation in patients undergoing esophageal surgery: a randomized controlled clinical trial. J Clin Anesth. 2017;38:111–6. [DOI] [PubMed] [Google Scholar]
- 51. Lazzerini PE, Laghi‐Pasini F, Acampa M, Srivastava U, Bertolozzi I, Giabbani B, et al. Systemic inflammation rapidly induces reversible atrial electrical remodeling: the role of interleukin‐6‐mediated changes in connexin expression. J Am Heart Assoc. 2019;8(16):e011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 53. January S, Pottebaum A, Raymer D, Lavine K. Tocilizumab for antibody‐mediated rejection in the setting of cardiac allograft vasculopathy. J Heart Lung Transpl. 2019;38(4):S38–9. [Google Scholar]
- 54. Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin‐6 and C‐reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43(11):2075–82. [DOI] [PubMed] [Google Scholar]
- 55. Chouaib S, Branellec D, Buurman WA. More insights into the complex physiology of TNF. Immunol Today. 1991;12(5):141–2. [DOI] [PubMed] [Google Scholar]
- 56. Hai D, Xue Y‐M, Zhan X‐Z, Liao H‐T, Guo H‐M, Wu S‐L. Role of tumor necrosis factor‐alpha in the pathogenesis of atrial fibrillation. Chin Med J. 2011;124(13):1976–82. [PubMed] [Google Scholar]
- 57. Ren M, Li X, Hao L, Zhong J. Role of tumor necrosis factor alpha in the pathogenesis of atrial fibrillation: a novel potential therapeutic target? Ann Med. 2015;47(4):316–24. [DOI] [PubMed] [Google Scholar]
- 58. Beadling C, Guschin D, Witthuhn BA, Ziemiecki A, Ihle JN, Kerr IM, et al. Activation of JAK kinases and STAT proteins by interleukin‐2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994;13(23):5605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hak Ł, Myśliwska J, Wickiewicz J, Szyndler K, Siebert J, Rogowski J. Interleukin‐2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J Interferon Cytokine Res. 2009;29(6):327–32. [DOI] [PubMed] [Google Scholar]
- 60. Rizos I, Tsiodras S, Rigopoulos AG, Dragomanovits S, Kalogeropoulos AS, Papathanasiou S, et al. Interleukin‐2 serum levels variations in recent onset atrial fibrillation are related with cardioversion outcome. Cytokine. 2007;40(3):157–64. [DOI] [PubMed] [Google Scholar]
- 61. Cabrera‐Bueno F, Medina‐Palomo C, Ruiz‐Salas A, Flores A, Rodríguez‐Losada N, Barrera A, et al. Serum levels of interleukin‐2 predict the recurrence of atrial fibrillation after pulmonary vein ablation. Cytokine. 2015;73(1):74–8. [DOI] [PubMed] [Google Scholar]
- 62. Cheng S‐M, Lai J‐H, Yang S‐P, Tsao T‐P, Ho L‐J, Liou J‐T, et al. Modulation of human T cells signaling transduction by lovastatin. Int J Cardiol. 2010;140(1):24–33. [DOI] [PubMed] [Google Scholar]
- 63. Koch A, Polverini P, Kunkel S, Harlow L, DiPietro L, Elner V, et al. Interleukin‐8 as a macrophage‐derived mediator of angiogenesis. Science. 1992;258(5089):1798–801. [DOI] [PubMed] [Google Scholar]
- 64. Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7(4):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Anatolevna RO, Veniaminovich FO, Mikhaylovich KS. Predictors of new‐onset atrial fibrillation in elderly patients with coronary artery disease after coronary artery bypass graft. J Geriatr Cardiol. 2016;13(5):444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liuba I, Ahlmroth H, Jonasson L, Englund A, Jonsson A, Safstrom K, et al. Source of inflammatory markers in patients with atrial fibrillation. Europace. 2008;10(7):848–53. [DOI] [PubMed] [Google Scholar]
- 67. Amar D, Goenka A, Zhang H, Park B, Thaler HT. Leukocytosis and increased risk of atrial fibrillation after general thoracic surgery. Ann Thorac Surg. 2006;82(3):1057–61. [DOI] [PubMed] [Google Scholar]
- 68. Lamm G, Auer J, Weber T, Berent R, Ng C, Eber B. Postoperative white blood cell count predicts atrial fbirllation after cardiac surgery. J Cardiothorac Vasc Anesth. 2006;20(1):51–6. [DOI] [PubMed] [Google Scholar]
- 69. Fontes ML, Amar D, Kulak A, Koval K, Zhang H, Shi W, et al. Increased preoperative white blood cell count predicts postoperative atrial fibrillation after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2009;23(4):484–7. [DOI] [PubMed] [Google Scholar]
- 70. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, et al. Angiotensin II receptor blockade reduces new‐onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45(5):712–9. [DOI] [PubMed] [Google Scholar]
- 71. Jansen H, Mackasey M, Moghtadaei M, Belke D, Egom E, Tuomi J, et al. Distinct patterns of atrial electrical and structural remodeling in angiotensin II mediated atrial fibrillatio. J Mol Cell Cardiol. 2018;124:12–25. [DOI] [PubMed] [Google Scholar]
- 72. Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin‐Angiotensin system inhibition a meta‐analysis. J Am Coll Cardiol. 2010;55(21):2299–307. [DOI] [PubMed] [Google Scholar]
- 73. Healey JS, Baranchuk A, Crystal E, Morillo CA, Garfinkle M, Yusuf S, et al. Prevention of atrial fibrillation with angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: a meta‐analysis. J Am Coll Cardiol. 2005;45(11):1832–9. [DOI] [PubMed] [Google Scholar]
- 74. Schneider MP, Hua TA, Böhm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by renin‐angiotensin system inhibition: a meta‐analysis. J Am Coll Cardiol. 2010;55(21):2299–307. [DOI] [PubMed] [Google Scholar]
- 75. Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97(12):1129–35. [DOI] [PubMed] [Google Scholar]
- 76. Siu CW, Lau CP, Tse HF. Prevention of atrial fibrillation recurrence by statin therapy in patients with lone atrial fibrillation after successful cardioversion. Am J Cardiol. 2003;92(11):1343–5. [DOI] [PubMed] [Google Scholar]
- 77. Young‐Xu Y, Jabbour S, Goldberg R, Blatt CM, Graboys T, Bilchik B, et al. Usefulness of statin drugs in protecting against atrial fibrillation in patients with coronary artery disease. Am J Cardiol. 2003;92(12):1379–83. [DOI] [PubMed] [Google Scholar]
- 78. Reilly SN, Jayaram R, Nahar K, Antoniades C, Verheule S, Channon KM, et al. Atrial sources of reactive oxygen species vary with the duration and substrate of atrial fibrillation: implications for the antiarrhythmic effect of statins. Circulation. 2011;124(10):1107–17. [DOI] [PubMed] [Google Scholar]
- 79. Horio T, Iwashima Y, Yoshihara F, Akiyama M, Okutsu M, Kawano Y. Combination therapy with renin‐angiotensin system inhibitors and statins is associated with reduced incidence of new‐onset atrial fibrillation in hypertensive patients. Eur Heart J. 2018;39(suppl 1):ehy566.6169. [Google Scholar]
- 80. Hanna IR, Heeke B, Bush H, Brosius L, King‐Hageman D, Dudley SC, et al. Lipid‐lowering drug use is associated with reduced prevalence of atrial fibrillation in patients with left ventricular systolic dysfunction. Heart Rhythm. 2006;3(8):881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Prasongsukarn K, Abel JG, Jamieson WRE, Cheung A, Russell JA, Walley KR, et al. The effects of steroids on the occurrence of postoperative atrial fibrillation after coronary artery bypass grafting surgery: a prospective randomized trial. J Thorac Cardiovasc Surg. 2005;130(1):93–8. [DOI] [PubMed] [Google Scholar]
- 82. Kim YR, Nam G‐B, Han S, Kim S‐H, Kim K‐H, Lee S, et al. Effect of short‐term steroid therapy on early recurrence during the blanking period after catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8(6):1366–72. [DOI] [PubMed] [Google Scholar]
- 83. Kim D‐R, Won H, Uhm J‐S, Kim J‐Y, Sung J‐H, Pak H‐N, et al. Comparison of two different doses of single bolus steroid injection to prevent atrial fibrillation recurrence after radiofrequency catheter ablation. Yonsei Med J. 2015;56(2):324–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Iskandar S, Reddy M, Afzal MR, Rajasingh J, Atoui M, Lavu M, et al. Use of oral steroid and its effects on atrial fibrillation recurrence and inflammatory cytokines post ablation – the Steroid AF Study. J Atr Fibrillation. 2017;9(5):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kinsella JE, Lokesh B, Stone RA. Dietary n‐3 polyunsaturated fatty acids and amelioration of cardiovascular disease: possible mechanisms. Am J Clin Nutr. 1990;52(1):1–28. [DOI] [PubMed] [Google Scholar]
- 86. Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, et al. Fish oil and postoperative atrial fibrillation: the Omega‐3 Fatty Acids for Prevention of Post‐operative Atrial Fibrillation (OPERA) randomized trial. JAMA. 2012;308(19):2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. J Am Coll Cardiol. 2014;64(14):1441–8. [DOI] [PubMed] [Google Scholar]
- 88. Mozaffarian D, Wu JHY, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, et al. Fish oil and post‐operative atrial fibrillation: a meta‐analysis of randomized controlled trials. J Am Coll Cardiol. 2013;61(21):2194–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Carnes CA, Chung MK, Nakayama T, Nakayama H, Baliga RS, Piao S, et al. Ascorbate attenuates atrial pacing‐induced peroxynitrite formation and electrical remodeling and decreases the incidence of postoperative atrial fibrillation. Circ Res. 2001;89(6):e32–8. [DOI] [PubMed] [Google Scholar]
- 90. Korantzopoulos P, Kolettis TM, Kountouris E, Dimitroula V, Karanikis P, Pappa E, et al. Oral vitamin C administration reduces early recurrence rates after electrical cardioversion of persistent atrial fibrillation and attenuates associated inflammation. Int J Cardiol. 2005;102(2):321–6. [DOI] [PubMed] [Google Scholar]
- 91. Hemila H, Suonsyrja T. Vitamin C for preventing atrial fibrillation in high risk patients: a systematic review and meta‐analysis. BMC Cardiovasc Disord. 2017;17(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Polymeropoulos E, Bagos P, Papadimitriou M, Rizos I, Patsouris E, Tauoumpoulis I. Vitamin C for the prevention of postoperative atrial fibrillation after cardiac surgery: a meta‐analysis. Adv Pharm Bull. 2016;6(2):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


