Abstract
Background
Catheter ablation for paroxysmal supraventricular tachycardia (PSVT) is an established treatment, but the effect of deep sedation on PSVT inducibility remains unclear.
Aim
We sought to examine PSVT inducibility and outcomes of catheter ablation under deep sedation using adaptive servo ventilation (ASV).
Methods
We retrospectively evaluated consecutive patients who underwent catheter ablation for PSVT under deep sedation (Propofol + Dexmedetomidine) with use of ASV. Anesthetic depth was controlled with BIS™ monitoring, and phenylephrine was administered to prevent anesthesia‐induced hypotension. PSVT induction was attempted in all patients using extrastimuli at baseline, and after isoproterenol (ISP) infusion when necessary.
Results
PSVT was successfully induced in 145 of 147 patients, although ISP infusion was required in the majority (89%). The PSVT was atrioventricular nodal reentrant tachycardia (AVNRT) in 77 (53%), atrioventricular reciprocating tachycardia (AVRT) in 51 (35%), and atrial tachycardia (AT) in 17 (12%). A higher ISP dose was required for AT compared to other PSVT (AVNRT: 0.06 (IQR 0.03‐0.06) vs AVRT: 0.03 (0.02‐0.06) vs AT: 0.06 (0.03‐0.12) mg/h, P = .013). More than half (51%) of the patients developed hypotension requiring phenylephrine; these patients were older. Acute success was obtained in 99% (patients with AVNRT had endpoints with single echo on ISP in 46%). Long‐term success rate was 136 of 144 (94%) (AVNRT 96%, AVRT 92%, and AT 93%). There were no complications related to deep sedation.
Conclusions
Deep sedation with use of ASV is a feasible anesthesia strategy for catheter ablation of PSVT with good long‐term outcome. PSVT remains inducible if ISP is used.
Keywords: adaptive servo ventilation, catheter ablation, deep sedation, dexmedetomidine, paroxysmal supraventricular tachycardia
Deep sedation with use of ASV is a feasible anesthesia strategy for catheter ablation of PSVT with good long‐term outcome. PSVT remains inducible if ISP is used.
Abbreviations
- AF
atrial fibrillation
- AH jump
atrio‐Hisian jump
- AP
accessory pathway
- ASV
adaptive servo ventilation
- AT
atrial tachycardia
- AVNRT
atrioventricular nodal reentrant tachycardia
- AVRT
atrioventricular reciprocating tachycardia
- BIS
bispectral index
- BMI
body mass index
- DLP
dyslipidemia
- DM
diabetes mellitus
- EF
ejection fraction
- EPS
electrophysiological study
- HF
heart failure
- HR
heart rate
- HT
hypertension
- ISP
isoproterenol
- LAD
left atrial diameter
- PSVT
paroxysmal supraventricular tachycardia
- RF
radio frequency
- VA conduction
ventriculoatrial conduction
1. BACKGROUND
Catheter ablation is increasingly being utilized as first‐line therapy for treating paroxysmal supraventricular tachycardia (PSVT). While for atrial fibrillation (AF) ablation, deep sedation and general anesthesia are commonly used for the sake of patient comfort and improved catheter manipulation, 1 for PSVT ablation, minimal sedation is usually used. Effect of deep sedation on tachycardia inducibility during PSVT ablation is unknown, and optimal endpoint, long‐term outcomes are not well described.
Propofol is often used during deep sedation for its ease of anesthesia depth adjustment, but can lead to severe respiratory inhibition. 2 Dexmedetomidine is a safe anesthetic agent with minimal suppression of spontaneous breathing, 3 but its weakness is lack of immediate anesthesia depth control owing to its extended half‐life. Recently, combined dexmedetomidine and propofol use was reported to result in better anesthesia depth control with fewer sedation‐related adverse events during MRI. 4 Adaptive servo ventilation (ASV) is a respiratory support system which is often used in sleep apnea syndrome. It was recently applied to pulmonary vein isolation procedures, but its utility for PSVT ablation has not been studied. 5
We sought to evaluate inducibility, optimal endpoint, and long‐term outcomes in catheter ablation of PSVT under deep sedation using propofol and dexmedetomidine with respiratory support by ASV.
2. METHODS
2.1. Study population
We evaluated consecutive patients who underwent first‐time catheter ablation for PSVT from 2013 to 2018 under deep sedation (propofol + dexmedetomidine) with use of ASV. For accurate evaluation of PSVT inducibility, patients without documented ECG tracings of their tachycardia before the procedure, patients with PSVT coincidentally induced during AF ablation, and patients with prior AF ablation were excluded from this study. Patients were enrolled by reviewing electrophysiological study (EPS) summary and chart records. Intracardiac electrogram data during EPS/ablation were re‐analyzed offline. All patients signed a written informed consent for the ablation procedure. This study was approved by the Kameda Medical Center Review Board.
2.2. Anesthesia and electrophysiological study
Antiarrhythmic drugs were discontinued more than 5 half‐lives before the ablation procedure. Personnel for each electrophysiology (EP) study consisted of fully trained electrophysiologists, EP fellows, and EP nurses, all of whom were trained in airway management and administration of anesthetic drugs. Patients were continuously monitored by pulse oximetry. The ASV was set after bolus administration of fentanyl citrate 0.05 mg and propofol 40 mg, with oxygen supplementation, usually 4 L/min, targeting peripheral oxygen saturation >95%. A full face mask was used for the ASV (Mirage Quattro™; ResMed Ltd). The normal settings used during treatment were expiratory positive airway pressure, 7 cm H2O; inspiratory positive airway pressure, minimum 6 cm H2O and maximum 11 cm H2O. Oropharyngeal airway tubes were inserted if patients had anesthesia‐induced glossoptosis. End‐tidal CO2 was routinely used to monitor ventilation. Electrocardiograms were recorded throughout the procedure and arterial blood pressure was measured invasively using Arterial catheter mini kit™ (20ga x 6 inch Argon Medical). Sedation was administered by a trained EP nurse under supervision of the operating physician. After an initial bolus administration of fentanyl citrate and propofol, continuous administration of propofol and dexmedetomidine was started. Dexmedetomidine was administered with initial high dose of 6 μg/kg/h over 10 minutes, followed by a maintenance dose of 0.6 μg/kg/h. Anesthetic depth was controlled with BIS™ monitoring (A‐3000 BIS XP Platform™; Aspect Medical System, Natick, MA, USA) which was adjusted around 40 to achieve Ramsay sedation scale of 6. Local anesthesia was administered after the initiation of intravenous anesthesia, and the catheter sheaths were inserted from the right femoral vein and right subclavian vein. According to the pain and consciousness of the patients, additional fentanyl citrate was administered and propofol was titrated with attention to hypotension and respiratory depression. Electrode catheters were placed at the right ventricular apex, His region and high right atrium (RA) via the right femoral vein, and another was placed at the coronary sinus via the right subclavian vein. Phenylephrine was administered to prevent anesthesia‐induced hypotension, and systolic blood pressure was kept to >80 mmHg. After measuring effective refractory period and Wenckebach rate in both atrioventricular (AV) conduction and ventriculoatrial (VA) conduction, PSVT induction was attempted using extrastimuli up to double extrastimuli and/or burst pacing from atrium/ventricle at baseline, including in patients with manifest WPW syndrome. If PSVT was not inducible at baseline, then isoproterenol (ISP) was administered targeting a heart rate (HR) increase of 10%, and PSVT induction was attempted again. If PSVT was still not inducible, the dose of ISP was increased incrementally and induction attempted at the new dose. This process was repeated until sustained PSVT was induced. PSVT was categorized into 3 groups: atrioventricular node reentrant tachycardia (AVNRT), atrioventricular reciprocating tachycardia (AVRT), and atrial tachycardia (AT), as defined by others. 6 , 7 , 8 , 9 We diagnosed the mechanism of AT as re‐entry when any of the following criteria were fulfilled: (i) AT could be reproducibly initiated and terminated with programmed stimulation; (ii) fulfillment of the criteria for manifest and concealed entrainment; (iii) reentry circuit shown by 3D activation mapping, and differentiated from other AT mechanisms (abnormal automaticity and triggered activity). 10 EPS and ablation were performed using an electroanatomical mapping system (Ensite™).
2.3. Ablation strategy and success
Fentanyl citrate 0.05 mg was routinely administered before start of radiofrequency (RF) application, and was also administered when necessary for pain control according to the patient's body weight. Propofol administration was also adjusted to control pain and to maintain adequate BIS score.
Slow pathway ablation was performed for slow/fast AVNRT using non‐irrigated RF catheter typically started at 20 W, and increased to a maximum 30W with target temperature of maximum 55°C. For slow/slow and fast/slow AVNRT, slow pathway ablation was performed first, and then additional ablation of the earliest atrial VA conduction site via slow pathway was performed if tachycardia was still inducible. During slow pathway ablation, RF application was continued until appearance of junctional beats, and repeat induction was performed permitting one single echo under ISP. If 2 or more echo beats or tachycardia induction remained, RF application was repeated after discontinuing ISP infusion, and tachycardia induction was performed again after resumption of ISP infusion.
As for AVRT, accessory pathway ablation was performed after the induction of tachycardia. A supravalvular approach was used for left‐side accessory pathways (AP) via transseptal approach first, and then subsequently a subvalvular approach via transaortic approach was tried for difficult cases. After elimination of AP, non‐inducibility of the tachycardia was confirmed.
In patients with AT, the earliest activation site indicated by electroanatomical mapping was targeted for ablation. As for perinodal ATP‐sensitive AT of which the mechanism is considered to be macro‐reentry, ablation was tried at the reentrant site outside of Koch's triangle first, and then the earliest activation site was targeted. 11 , 12 Either of non‐irrigated or irrigated catheters were used in AVRT and AT ablation by physician choice, with maximum power up to 35 W.
Ablation was deferred if the target site was in close proximity (≤5 mm) to the His region.
After successful ablation of PSVT, reinduction of tachycardia was attempted using the same amount of ISP that had been necessary for induction before ablation. After the procedure, antiarrhythmic drugs for PSVT were discontinued excluding beta blockers when they were being prescribed for hypertension.
2.4. Follow‐up
Patients were evaluated in the outpatient setting at 3‐6 weeks after ablation and subsequently at 3‐ to 6‐month intervals if possible. Holter monitoring (24‐hour duration) was performed if they had palpitations or symptoms which suggested recurrence of tachycardia. After several follow‐up visits to our hospital, patients were sent back to referring cardiologists/physicians if they had no symptoms. Patients were referred back to our hospital if the same symptoms recurred or when recurrence of the tachycardia was suspected.
2.5. Statistical analysis
All values are expressed as median with interquartile range. Comparisons of continuous variables were analyzed with one‐way ANOVA. Categorical variables expressed as numbers and percentages between different groups were compared with Pearson's chi‐squared test, and two group comparisons were performed with Fisher's exact test. HR change was validated with Student's t test. Statistical significance was defined as P < .05. JMP® 14.3 (SAS Institute Inc, Cary, NC, USA) was used for analysis.
3. RESULTS
3.1. Population and PSVT category
Of 153 patients who underwent first‐time EPS/ABL for PSVT from July 2013 to September 2018, 6 patients were excluded (no documented tachycardia tracing before the procedure in 2, ASV not used in 3, and data missing in 1), leading to inclusion of 147 patients in this study (76 women (52%), age 62 (45‐70)). Of these, 145 patients (99%) had successful tachycardia induction. The remaining 2 patients only had induction of single‐ or two‐echo beats by programmed stimulation, even with ISP. In one of those two patients, tachycardia induction was also attempted after discontinuation of deep sedation, but was unsuccessful. Of the 145 patients who had successful tachycardia induction, catheter ablation was deferred in two patients because of the risk for cardiac conduction system injury; one had junctional tachycardia, AT originated close to the sinus node in the other. In total, 144 patients underwent catheter ablation, with the inclusion of one of the two patients in whom we failed to induce sustained PSVT (Figure 1). This particular patient had induction of two‐echo beats, had no accessory pathways, had documented short RP’ tachycardia on the preprocedure 12‐lead ECG, and was diagnosed as having slow/fast AVNRT.
FIGURE 1.
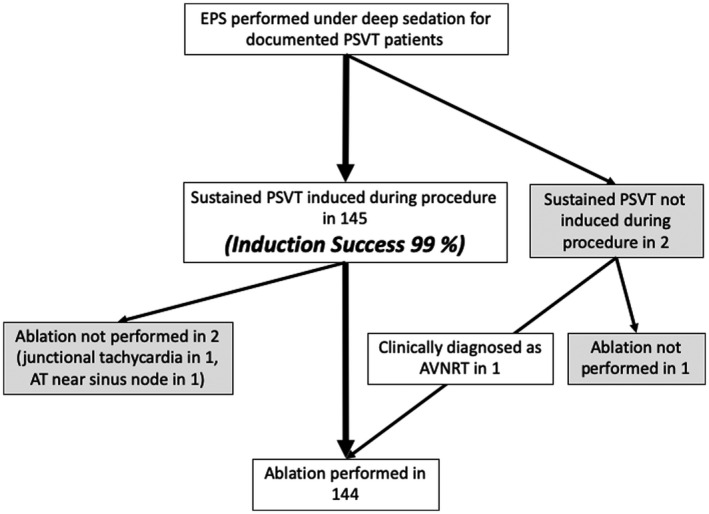
Patient selection for the study. EPS, electrophysiological study; PSVT, paroxysmal supraventricular tachycardia; AVNRT, Atrioventricular nodal reentrant tachycardia; ASV, adaptive servo ventilation; AT, atrial tachycardia
PSVT was diagnosed as AVNRT in 77 (54%), AVRT in 51 (35%), and AT in 17 (11%). AVNRT was diagnosed after using entrainment pacing from the ventricle (ventricular burst pacing and/or ventricular pacing simultaneous with His bundle activation). AT patients were more likely older, female, and had lower EF, higher incidence of diabetes mellitus, dyslipidemia, and heart failure (Table 1).
TABLE 1.
Clinical characteristics of the patients with successful PSVT induction
| All (N = 145) | AVNRT (N = 77) | AVRT (N = 51) | AT (N = 17) | P value | |
|---|---|---|---|---|---|
| Age (y.o.) | 61 (45‐70) | 62 (51‐73) | 57 (36‐69) | 65 (53‐71) | .0084 |
| Female | 75 (52%) | 45 (58%) | 19 (37%) | 11 (65%) | .033 |
| BMI (kg/m2) | 23.0 (20.6‐26.6) | 23.0 (20.2‐30.1) | 23.1 (21.1‐26.6) | 24.0 (22.2‐25.8) | .75 |
| EF (%) | 68 (64‐71) | 69 (66‐72) | 68 (63‐71) | 66 (58‐72) | .0074 |
| LAD (mm) | 36 (32‐40) | 35 (32‐40) | 36 (32‐40) | 35 (31‐38) | .66 |
| DM | 17 (12%) | 8 (10%) | 4 (8%) | 5 (29%) | .05 |
| HT | 31 (21%) | 18 (23%) | 10 (20%) | 3 (18%) | .85 |
| DLP | 27 (18%) | 13 (17%) | 7 (14%) | 7 (41%) | .04 |
| HF | 8 (6) | 1 (1%) | 3 (6%) | 4 (24%) | .0013 |
Abbreviations: BMI, body mass index; EF, ejection fraction; LAD, left atrial diameter; DM, diabetes mellitus; HT, hypertension; DLP, dyslipidemia; HF, heart failure.
Values less than 0.05 are written in bold.
3.2. PSVT induction
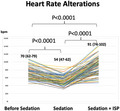
Of the 145 patients with inducible PSVT, only 16 (11%) had sustained PSVT induction at baseline, and the majority of patients (89%) required ISP infusion. Median HR of the 129 patients dropped from an initial 70 (62‐79) to 54 (47‐62) bpm after deep sedation introduction, but increased after ISP initiation. Median HR at the time of PSVT induction was 91 (74‐102) bpm, which was significantly higher than both before ISP initiation and baseline (P < .0001, each) (Figure 2) for the patients receiving ISP.
FIGURE 2.
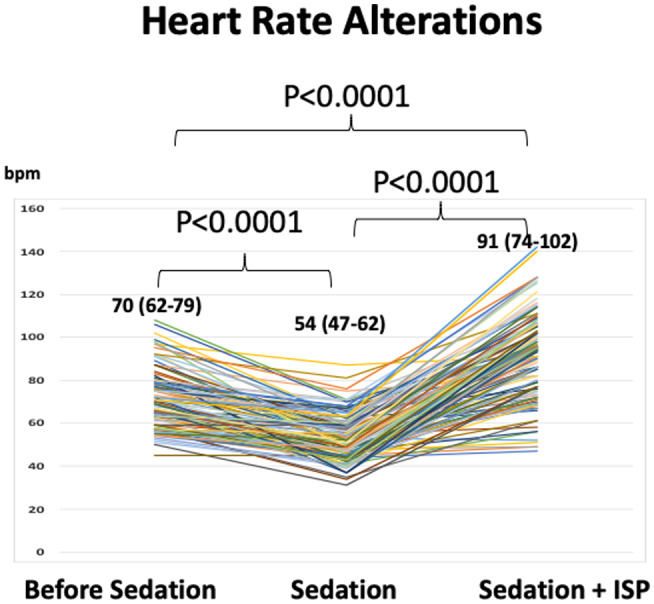
Heart rate (HR) changes owing to sedation and isoproterenol (ISP). After the initiation of sedation, average HR decreased from 70 to 54 bpm. Patients whose tachycardia could not be induced received ISP, and the average HR had increased to 91 bpm at the time of PSVT induction. Note that average HR at PSVT induction is higher than that before sedation
During EPS and catheter ablation, transient hypotension requiring phenylephrine infusion was observed in 74 patients (51%) (0.2 (0.1‐036) mg). Patients who needed phenylephrine administration were significantly older compared to those who did not (66 (57‐75) vs 55 (38‐67) y.o., P < .0001) (Table 2). Vital signs and the amount of ISP/phenylephrine administered are given in Table 3. A higher ISP dose was required for induction of AT compared to other types of PSVT (AVNRT: 0.06 (0.03‐0.06) vs AVRT: 0.03 (0.02‐0.06) vs AT: 0.06 (0.06‐0.12) mg/h, P = .013).
TABLE 2.
Clinical characteristics of the patients with or without phenylephrine administration
| Phenylephrine (+), N = 74 | Phenylephrine (−), N = 71 | P | |
|---|---|---|---|
| Age (y.o.) | 66 (57‐75) | 55 (38‐67) | <.0001 |
| Female | 41 (55%) | 35 (49%) | .51 |
| BMI (kg/m2) | 22.9 (21.0‐26.1) | 23.1 (20.3‐26.8) | .5 |
| EF (%) | 69 (65‐72) | 67 (63‐71) | .47 |
| LAD (mm) | 36 (33‐39) | 35 (32‐40) | .6 |
| DM | 9 (12%) | 8 (11%) | 1 |
| HT | 17 (23%) | 14 (19%) | .69 |
| DLP | 17 (23%) | 10 (14%) | .2 |
| HF | 5 (7%) | 3 (4%) | .72 |
| AVNRT | 42 (57%) | 35 (49%) | .57 |
| AVRT | 23 (31%) | 28 (39%) | |
| AT | 9 (12%) | 8 (11%) |
Abbreviations: BMI, body mass index; EF, ejection fraction; LAD, left atrial diameter; DM, diabetes mellitus; HT, hypertension; DLP, dyslipidemia; HF, heart failure.
Values less than 0.05 are written in bold.
TABLE 3.
Vital signs and ISP use during procedure
| All (N = 145) | AVNRT (N = 77) | AVRT (N = 51) | AT (N = 17) | P value | |
|---|---|---|---|---|---|
| Systolic BP at baseline (mmHg) | 141 (125‐170) | 151 (110‐177) | 140 (133‐175) | 143 (114‐158) | .81 |
| Diastolic BP at baseline (mmHg) | 70 (62‐93) | 78 (54‐95) | 77 (65‐93) | 65 (62‐82) | .76 |
| HR at baseline (bpm) | 70 (62‐79) | 71 (62‐80) | 67 (62‐76) | 75 (63‐84) | .34 |
| Phenylephrine use | 75 (51%) | 42 (54%) | 23 (45%) | 9 (53%) | .57 |
| Dose of phenylephrine (mg) | 0.04 (0‐0.2) | 0.05 (0‐0.2) | 0 (0‐0.2) | 0.1 (0‐0.2) | .74 |
| ISP use for induction | 129 (89%) | 71 (92%) | 43 (84%) | 15 (88%) | .38 |
| ISP dose for induction (mg/h) | 0.06 (0.03‐0.06) | 0.06 (0.03‐0.06) | 0.03 (0.02‐0.06) | 0.06 (0.06‐0.12) | .0013 |
Abbreviations: BP, blood pressure; HR, heart rate; ISP: isoproterenol.
Values less than 0.05 are written in bold.
Of the 78 patients with AVNRT who underwent catheter ablation, 11 patients (14%) did not show retrograde AV node conduction at the beginning of the procedure under deep sedation. It appeared only after ISP administration (Figure 3). Clinical characteristics of the patients who required ISP infusion for induction are shown in the Table S1.
FIGURE 3.
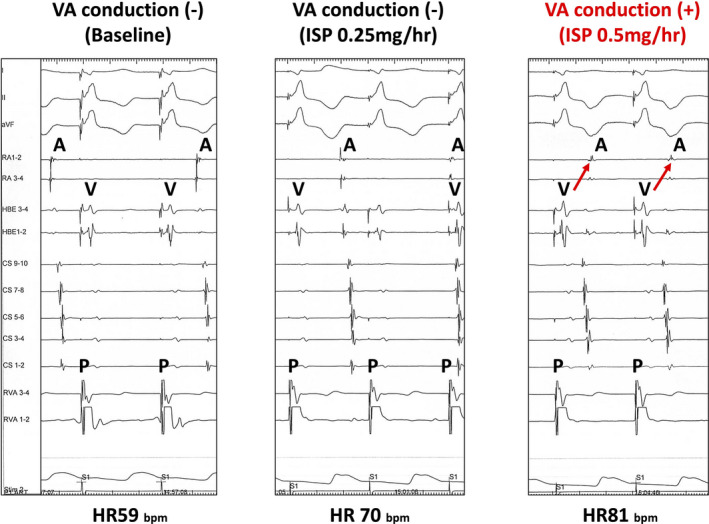
Representative case of a patient whose VA conduction appeared after titrated ISP administration. This patient (34 y.o. woman) had no VA conduction before ISP (left panel). Administration of ISP 0.25 mg/h increased the heart rate, but VA conduction did not appear (middle panel). After increase in ISP dose to 0.5 mg/h, VA conduction appeared (right panel) after which slow/fast atrioventricular nodal reentrant tachycardia became inducible. ISP, isoproterenol; AVNRT, atrioventricular nodal reentrant tachycardia; VA conduction, ventriculoatrial conduction
3.3. Ablation outcome
Total procedure time was 169 (136‐205) min (ASV time 167 (138‐205) min), and EPS time (from induction to diagnosis) was 113 (91‐138) min. Total dose of propofol and dexmedetomidine administered was 280 (200‐400) mg and 152 (124‐192) μg, respectively (Table 4). Patients with AT had longer procedural duration compared to those with AVNRT or AVRT (218 (141‐288) vs 157 (132‐191) vs 175 (146‐202) min, P = .0081). The same was true of ASV duration (P < .0001). A higher dose of ISP was used for the reinduction of tachycardia after ablation in patients with AT compared to those with AVNRT or AVRT.
TABLE 4.
Ablation procedure
| All (N = 144) | AVNRT (N = 78) | AVRT (N = 51) | AT (N = 15) | P value | |
|---|---|---|---|---|---|
| RF application number | 8 (5‐13) | 8 (5‐14) | 8 (5‐12) | 8 (6‐16) | .76 |
| RF application time (sec) | 340 (217‐490) | 323 (200‐441) | 357 (226‐533) | 462 (305‐825) | .68 |
| ISP dose for reinduction (mg/h) | 0.06 (0‐0.06) | 0.06 (0.03‐0.06) | 0.03 (0‐0.06) | 0.06 (0.06‐0.18) | .0002 |
| Propofol dose (mg) | 280 (200‐400) | 280 (180‐360) | 280 (210‐460) | 300 (230‐540) | .11 |
| Dexmedetomidine dose (μg) | 152 (124‐192) | 140 (120‐184) | 156 (132‐212) | 160 (136‐244) | .063 |
| Fluoroscopy time (min) | 24 (17‐33) | 23 (15‐34) | 27 (20‐33) | 26 (18‐33) | 1 |
| Total procedure time (min) | 169 (136‐205) | 157 (132‐191) | 175 (146‐202) | 218 (141‐288) | .0081 |
| ASV time (min) | 167 (138‐205) | 152 (134‐192) | 148 (180‐203) | 257 (164‐296) | <.0001 |
| Time for EPS (min) | 113 (91‐138) | 104 (88‐130) | 121 (95‐139) | 133 (93‐197) | .029 |
| Systolic BP at the end of procedure | 119 (107‐131) | 121 (131‐106) | 119 (107‐135) | 114 (110‐123) | .75 |
| Diastolic BP at the end of procedure | 74 (64‐80) | 72 (62‐81) | 75 (64‐81) | 68 (65‐79) | .61 |
| HR at the end of procedure | 64 (58‐72) | 65 (60‐73) | 61 (56‐68) | 72 (60‐87) | .007 |
| Complication | 4 (3%) | 2 (3%) | 1 (2%) | 1 (7%) | .61 |
Abbreviations: RF, radiofrequency; ISP, isoproterenol; BP, blood pressure; HR, heart rate.
Values less than 0.05 are written in bold.
The 78 patients with AVNRT who received catheter ablation were classified into 3 subtypes: slow/fast AVNRT in 59, fast/slow AVNRT in 12, and slow/slow AVNRT in 7 (Table 5). Of the same 78 patients, 77 (99%) had successful ablation with different endpoints as follows; no atrio‐Hisian (AH) jump up in 27 (35%), AH jump without echo in 14 (18%), and single echo on ISP in 36 (46%). Of 51 patients with AVRT, 21 had manifest conduction; the remaining 30 had concealed or intermittent AP conduction. Of the same 51 patients, AP was left sided in 46 and right sided in 5. Successful AP ablation was achieved in all patients, tachycardia was not inducible after ablation. All 15 patients with AT had successful ablation. Distribution of the AT foci was as follows: perinodal ATP‐sensitive AT in 5, crista terminalis in 1, RA posterior in 1, coronary sinus ostium in 2, and one each in near tricuspid valve, RA lateral wall, left inferior pulmonary vein, non‐coronary cusp, right coronary cusp, and right atrial appendage. Of these, 6 ATs (5 perinodal ATP‐sensitive AT and RA posterior wall) were thought to have a reentrant mechanism, and the remaining 11 ATs, other mechanisms (abnormal automaticity or triggered activity). Of all patients, a total of 4 (3%) had complications unrelated to deep sedation or use of ASV, and there were no patients who needed mechanical ventilation as a result of respiratory arrest. Two patients had cardiac tamponade, one had pneumothorax, and one had subclavian artery puncture. All of them were discharged without aftereffects.
TABLE 5.
EPS results and ablation endpoints in each subset of AVNRT
| ALL AVNRT (N = 78) | Slow/fast (N = 59) | Fast/slow (N = 12) | Slow/slow (N = 7) | P value | |
|---|---|---|---|---|---|
| EPS | |||||
| HR at baseline (bpm) | 71 (62‐80) | 72 (65‐96) | 66 (57‐88) | 70 (57‐80) | .27 |
| HR under sedation (bpm) | 55(47‐61) | 55 (47‐63) | 50 (42‐63) | 57 (49‐61) | .33 |
| VA conduction at baseline | 66 (84%) | 44 (75%) | 10 (83%) | 6 (86%) | .68 |
| VA conduction WB rate at baseline (msec) | 430 (375‐500) | 375 (348‐500) | 403 (365‐500) | 500 (406‐575) | .28 |
| AV conduction WB rate at baseline (msec) | 430 (375‐500) | 430 (375‐500) | 375 (375‐465) | 500 (430‐500) | .26 |
| Jump up at baseline | 52 (67%) | 41 (69%) | 7 (58%) | 7 (100%) | .15 |
| One echo beat at baseline | 25 (32%) | 22 (37%) | 2 (17%) | 1 (14%) | .22 |
| SVT induction at baseline (including short run) | 15 (19%) | 11 (19%) | 2 (17%) | 2 (29%) | .53 |
| HR at induction (bpm) | 95 (82‐106) | 94 (82‐102) | 92 (82‐108) | 102 (76‐115) | .38 |
| Ablation endpoint | |||||
| No AH jump up | 27 (35%) | 22 (37%) | 4 (33%) | 1 (14%) | .84 |
| Single echo beat | 36 (46%) | 26 (44%) | 5 (42%) | 5 (71%) | |
| AH jump up only (no echo) | 14 (18%) | 10 (17%) | 3 (25%) | 1 (14%) | |
| Failure (SVT inducible) | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | |
3.4. Acute and long‐term success
Of the 144 patients who underwent catheter ablation, acute success was achieved in 143 patients (99%) (AVNRT 99%, AVRT 100%, and AT 100%). During 68 (32‐147) days of follow‐up, 6 patients (4%) had tachycardia recurrence (3 AVNRT, 1 AT, 2 AVRT). There were also 2 patients with recurrence of delta wave on the surface ECG. The AT that recurred in one patient originated in the left inferior pulmonary vein with mechanism of abnormal automaticity/triggered activity, and it was successfully treated in a repeat procedure. The long‐term success rate was 136 of 144 (94%) (AVNRT 96%, AVRT 92%, and AT 93%) (Figure 4).
FIGURE 4.
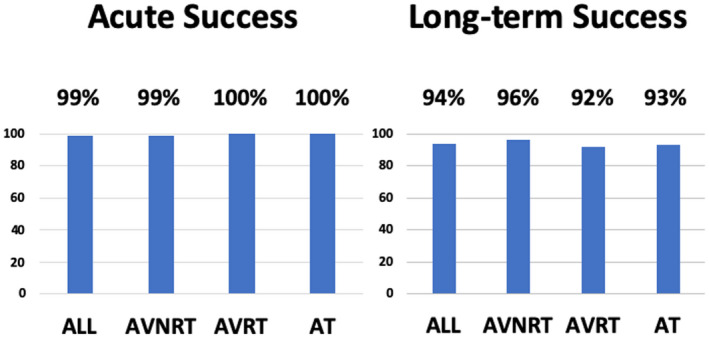
Acute and long‐term success
4. DISCUSSION
4.1. Major findings
Our major findings were as follows:
Deep sedation with use of ASV is a feasible anesthesia strategy for catheter ablation of PSVT regardless of its subtype (AVNRT, AVRT, and AT).
Inducibility of the PSVT remains preserved with the use of ISP. Required dose of ISP was higher in patients with the AT subtype.
In patients with AVNRT, “single echo beat by extra stimulation under ISP” is a feasible ablation endpoint even under deep sedation as it is for conventional minimal sedation.
4.2. Need of deep sedation for PSVT ablation
Currently, PSVT ablation is often performed under local anesthesia and minimal sedation with an oxygen mask or nasal cannula. 13 This conventional method is safe and inducibility for tachycardia is preserved. However, patients do experience pain during the groin puncture and RF application, and also palpitations during pacing and induced tachycardia, throughout a procedure that can last several hours. Indeed, EPS procedures produce anxiety, pain, and discomfort in more than 50% of patients. 13 If tachycardia inducibility is preserved and results in good outcomes, deep sedation should be given as an option to patients for PSVT ablation as it often is for AF ablation in recent years. Furthermore, we believe that steady anesthesia in the catheter laboratory has the advantage of preventing medication error. Our results expand the options for anesthesia strategy during PSVT ablation beyond minimal sedation, and we expect it to lead to greater patient satisfaction. 2
4.3. Deep sedation with ASV
Deep sedation with ASV lies between minimal sedation with an oxygen mask and deep sedation with laryngeal mask or general anesthesia requiring muscle relaxants. ASV assists spontaneous respiration using positive‐pressure ventilation, and is safer in preventing both hypoxia and oral complications such as dental injury related to intubation. However, even under the assistance of ASV, excessive deep sedation may cause hypoxia with glossoptosis, and BIS monitoring is required to adjust anesthesia depth for safety. The current study demonstrated the safety and utility of deep sedation with ASV and BIS monitoring in catheter ablation for PSVT.
That said, half of the patients needed vasopressor administration to counter hypotension, especially in older patients. Use of ASV can be one of the reasons for hypotension by reducing venous return, and high dose of ISP may also lead to the hypotension in those patients. Deep sedation should preferably be performed under direct blood pressure monitoring as well as oxygen monitoring. Our target for BIS sedation level was 40 which corresponds to a Ramsay sedation score of 6. 14 Interestingly, it is reported that administration of ISP itself often alters BIS level. 15 Careful BIS‐level monitoring is needed before and after ISP administration because ISP is frequently needed for tachycardia induction in this anesthesia strategy.
4.4. Induction during deep sedation
A majority of the patients (89%) needed ISP infusion for PSVT induction. Inhibition of sympathetic nerve activity by deep sedation makes it difficult to induce PSVT, which is reflected in the reduced HR (average 54 bpm) after the sedation. At dosages of ISP required for PSVT induction, HR increased to 91 bpm. The HR at which PSVT became inducible was faster than both baseline and after sedation. As a rule of thumb, we begin to attempt induction when HR has increased by 10% over baseline value in our laboratory. The HR at which PSVT was induced was about 20 bpm over presedation value on average.
Requirement for ISP infusion in inducing AVNRT under minimal sedation is not well researched, but by some estimates it is about 30%‐50% of cases. 16 , 17 Since 92% of AVNRT patients needed ISP under deep sedation in this study, it is likely that ISP amount required for AVNRT induction differs according to anesthesia depth. It took extra time to induce tachycardia, but our total procedure time was not different from past reports of PSVT ablation, 18 suggesting the possibility that the duration of RFCA itself is shortened under deep sedation.
While we have demonstrated the preserved inducibility (99%) of PSVT including AT under deep sedation, contrary results have been reported in pediatric patients with ectopic AT; in 4 of 7 pediatric patients with ectopic AT, the tachycardia terminated after propofol infusion and could not be induced by ISP. 19 Theoretically, if the mechanism of AT is abnormal automaticity or triggered activity, its inducibility can be decreased by deep sedation that reduces sympathetic nerve activity. However, based on the preserved inducibility of AT we found in the current study, it may be that this potential negative inducibility can be countered by increasing ISP, which is possible under deep sedation because discomfort and palpitation would not be an issue. In our study, the required dose of ISP was higher in patients with AT than for other PSVT types. Further study focusing on inducibility of AT according to its different mechanisms (reentry, abnormal automaticity, and triggered activity) is needed.
Most of the patients in this study (133 of 145 (92%)) had reentrant tachycardias as a mechanism of PSVT, such as AVNRT (77) and AVRT (51), including 5 patients with perinodal ATP‐sensitive AT of which the mechanism is supposed to be reentry. 12 , 20 Based on our results, it appears that PSVT with reentrant mechanism also have preserved inducibility under deep sedation.
We found that 11 of 79 patients with AVNRT (14%) had no retrograde AV node conduction under deep sedation until ISP administration. Deep sedation can hide retrograde AV node conduction as a result of its direct inhibitory effect on the AV node and indirect effect via the sympathetic nerve. Careful consideration is needed in identifying presence or absence of retrograde AV node conduction under deep sedation.
Of two patients who failed to have sustained PSVT induced in this study, we attempted induction after terminating deep sedation in one, but induction remained unsuccessful, suggesting deep sedation was not the cause of our inability to induce tachycardia in this patient.
We used a combination of sedation drugs in this study, dexmedetomidine, which suppresses spontaneous breathing less, and propofol, which has fast anesthesia depth control. Although dexmedetomidine has negative cardiovascular effects such as bradycardia as well as hypotension, its clinical feasibility for PSVT ablation has been previously demonstrated. 21 , 22 , 23 Propofol is supposed to have less negative influence on the cardiac conduction system, and can be a favorable anesthetic agent in PSVT ablation. 24 , 25 In our study, the combination of dexmedetomidine and propofol enabled us to achieve both preserved inducibility and a safe procedure without complications related to deep sedation. 19 Utility of propofol in catheter ablation for AVNRT has been described in the past, 24 , 26 but we evaluated the inducibility and the outcome not only for AVNRT but also for other types of PSVT, and under deep sedation with ASV instead of minimal sedation.
4.5. Ablation success
Both acute and long‐term success rates were comparable to studies using other anesthetic modalities. 27
In the patients with AVNRT, “single echo beat by extra stimulation under ISP” is a feasible ablation endpoint under deep sedation as well as conventional minimal sedation. Also, in the patients with AVRT or AT, disappearance of the accessory pathway and the elimination of tachycardia with confirmed non‐inducibility by pacing stimulation under ISP is a compatible ablation endpoint in deep sedation. It may be that deep sedation is associated with satisfactory long‐term outcome owing to the increased ease of catheter manipulation; the same concept has been reported in AF ablation. 5 To prove this, however, prospective evaluation comparing outcomes to minimal sedation is needed.
5. LIMITATIONS
This is a retrospective, single‐center, observational study. Additionally, not all patients were followed at our institution after undergoing the ablation procedure and, in those patients, we had to rely on the information gathered from the referring healthcare providers for arrhythmia outcomes. ISP dose for inducing PSVT and ablation endpoint in AVNRT were left to the physicians. Cardiac function was preserved in our population (average EF 68%), so we were not able to ascertain whether this anesthesia strategy requiring vasotropic support and high dose of isoproterenol could also be applied to patients with severe LV dysfunction.
6. CONCLUSION
Deep sedation with use of ASV is a feasible anesthesia strategy for catheter ablation of PSVT with good long‐term outcome. PSVT inducibility remains preserved when ISP is used. The required ISP dose differs based on PSVT type. In patients with AVNRT, “single echo beat by programmed stimulation under ISP” is a feasible ablation endpoint even under deep sedation, as it is for conventional minimal sedation.
DISCLOSURE
The authors declare no conflict of interest for this article.
The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. Committee of Kameda Medical Center, Approval No. 18‐156 (Approval date 15 January 2019).
Supporting information
Table S1
Hayashi T, Mizukami A, Kuroda S, et al. Outcomes of deep sedation for catheter ablation of paroxysmal supraventricular tachycardia, with adaptive servo ventilation. J Arrhythmia.2021;37:33–42. 10.1002/joa3.12476
REFERENCES
- 1. Hutchinson MD, Garcia FC, Mandel JE, Elkassabany N, Zado ES, Riley MP, et al. Efforts to enhance catheter stability improve atrial fibrillation ablation outcome. Heart Rhythm. 2013;10:347–53. [DOI] [PubMed] [Google Scholar]
- 2. Münkler P, Attanasio P, Parwani AS, Huemer M, Boldt L‐H, Haverkamp W, et al. High patient satisfaction with deep sedation for catheter ablation of cardiac arrhythmia: high patient satisfaction with deep sedation. Pacing Clin. Electrophysiol.. 2017;40:585–90. [DOI] [PubMed] [Google Scholar]
- 3. Takigawa M, Takahashi A, Kuwahara T, Okubo K, Nakashima E, Watari Y, et al. Airway support using a pediatric intubation tube in adult patients with atrial fibrillation: a simple and unique method to prevent heart movement during catheter ablation under continuous deep sedation. J Arrhythm. 2017;33:262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boriosi JP, Eickhoff JC, Klein KB, Hollman GA. A retrospective comparison of propofol alone to propofol in combination with dexmedetomidine for pediatric 3T MRI sedation. Pediatr Anesth. 2017;27:52–9. [DOI] [PubMed] [Google Scholar]
- 5. Murakami T, Yamaji H, Numa K, Kawamura H, Murakami M, Higashiya S, et al. Adaptive‐servo ventilation combined with deep sedation is an effective strategy during pulmonary vein isolation. EP Europace. 2013;15:951–6. [DOI] [PubMed] [Google Scholar]
- 6. Maruyama M, Kobayashi Y, Miyauchi Y, Ino T, Atarashi H, Katoh T, et al. The VA relationship after differential atrial overdrive pacing: a novel tool for the diagnosis of atrial tachycardia in the electrophysiologic laboratory. J Cardiovasc Electrophysiol. 2007;18:1127–33. [DOI] [PubMed] [Google Scholar]
- 7. Michaud GF, Tada H, Chough S, Baker R, Wasmer K, Sticherling C, et al. Differentiation of atypical atrioventricular node re‐entrant tachycardia from orthodromic reciprocating tachycardia using a septal accessory pathway by the response to ventricular pacing. J Am Coll Cardiol. 2001;38:1163–7. [DOI] [PubMed] [Google Scholar]
- 8. Padanilam BJ, Manfredi JA, Steinberg LA, Olson JA, Fogel RI, Prystowsky EN. Differentiating junctional tachycardia and atrioventricular node re‐entry tachycardia based on response to atrial extrastimulus pacing. J Am Coll Cardiol. 2008;52:1711–7. [DOI] [PubMed] [Google Scholar]
- 9. Hirao K, Otomo K, Wang X, Beckman KJ, McClelland JH, Widman L, et al. A new method for differentiating retrograde conduction over an accessory AV pathway from conduction over the AV node. Circulation. 1996;94:1027–35. [DOI] [PubMed] [Google Scholar]
- 10. Chen SA, Chiang CE, Yang CJ, Cheng CC, Wu TJ, Wang SP, et al. Sustained atrial tachycardia in adult patients. Electrophysiological characteristics, pharmacological response, possible mechanisms, and effects of radiofrequency ablation. Circulation. 1994;90:1262–78. [DOI] [PubMed] [Google Scholar]
- 11. Yamabe H, Okumura K, Morihisa K, Koyama J, Kanazawa H, Hoshiyama T, et al. Demonstration of anatomical reentrant tachycardia circuit in verapamil‐sensitive atrial tachycardia originating from the vicinity of the atrioventricular node. Heart Rhythm. 2012;9:1475–83. [DOI] [PubMed] [Google Scholar]
- 12. Yamabe H, Kanazawa H, Ito M, Kaneko S, Kanemaru Y, Kiyama T, et al. Slow potential at the entrance of the slow conduction zone in the reentry circuit of a verapamil‐sensitive atrial tachycardia originating from the atrioventricular annulus. J Am Heart Assoc. 2018;7:e009223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lau W, Kovoor P, Ross DL. Cardiac electrophysiologic effects of midazolam combined with fentanyl. Am J Cardiol. 1993;72:177–82. [DOI] [PubMed] [Google Scholar]
- 14. Mondello E, Panasiti R, Siliotti R, Floridia D, David A, Trimarchi G. BIS and Ramsay score in critically ill patient: what future? Minerva Anestesiol. 2002;68:7. [PubMed] [Google Scholar]
- 15. O'Neill DK, Aizer A, Linton P, Bloom M, Rose E, Chinitz L. Isoproterenol infusion increases level of consciousness during catheter ablation of atrial fibrillation. J Interv Card Electrophysiol. 2012;34:137–42. [DOI] [PubMed] [Google Scholar]
- 16. Wells P, Dubuc M, Klein GJ, Dan D, Roux J‐F, Lockwood E, et al. Intracardiac ablation for atrioventricular nodal reentry tachycardia using a 6 mm distal electrode cryoablation catheter: prospective, multicenter, North American study (ICY‐AVNRT STUDY). J Cardiovasc Electrophysiol. 2018;29:167–76. [DOI] [PubMed] [Google Scholar]
- 17. Kimman GP, Bogaard MD, Van Hemel NM, van Dessel PFHM, Jessurun ER, Boersma LVA, et al. Ten year follow‐up after radiofrequency catheter ablation for atrioventricular nodal reentrant tachycardia in the early days forever cured, or a source for new arrhythmias? Pacing Clin Electrophysiol. 2005;28:1302–9. [DOI] [PubMed] [Google Scholar]
- 18. Patel PJ, Segar R, Patel JK, Padanilam BJ, Prystowsky EN. Arrhythmia induction using isoproterenol or epinephrine during electrophysiology study for supraventricular tachycardia. J Cardiovasc Electrophysiol. 2018;29:1635–40. [DOI] [PubMed] [Google Scholar]
- 19. Lai L‐P, Huang G‐H, Stephenuang SK. Usefulness of intravenous propofol anesthesia for radiofrequency catheter ablation in patients with tachyarrhythmias: infeasibility for pediatric patients with ectopic atrial tachycardia. Pacing Clin Electrophysiol. 1999;22:1358–64. [DOI] [PubMed] [Google Scholar]
- 20. Iesaka Y, Takahashi A, Goya M, Soejima Y, Okamoto Y, Fujiwara H, et al. Adenosine‐sensitive atrial reentrant tachycardia originating from the atrioventricular nodal transitional area. J Cardiovasc Electrophysiol. 1997;8:854–64. [DOI] [PubMed] [Google Scholar]
- 21. Vladinov G, Fermin L, Longini R, Ramos Y, Maratea E. Choosing the anesthetic and sedative drugs for supraventricular tachycardia ablations: a focused review. Pacing Clin Electrophysiol. 2018;41:1555–63. [DOI] [PubMed] [Google Scholar]
- 22. Tirotta CF, Nguyen T, Fishberger S, Velis E, Olen M, Lam L, et al. Dexmedetomidine use in patients undergoing electrophysiological study for supraventricular tachyarrhythmias. Pediatr Anesth. 2017;27:45–51. [DOI] [PubMed] [Google Scholar]
- 23. Slupe AM, Minnier J, Raitt MH, Zarraga IGE, MacMurdy KS, Jessel PM. Dexmedetomidine sedation for paroxysmal supraventricular tachycardia ablation is not associated with alteration of arrhythmia inducibility. Anest Analg. 2018;129:1529–35. [DOI] [PubMed] [Google Scholar]
- 24. Warpechowski P, Lima GG, Medeiros CM, Santos ATL, Kruse M, Migloransa MH, et al. Randomized study of propofol effect on electrophysiological properties of the atrioventricular node in patients with nodal reentrant tachycardia. Pacing Clin Electrophysiol. 2006;29:1375–82. [DOI] [PubMed] [Google Scholar]
- 25. Niksch A, Liberman L, Clapcich A, Schwarzenberger JC, Silver ES, Pass RH. Effects of remifentanil anesthesia on cardiac electrophysiologic properties in children undergoing catheter ablation of supraventricular tachycardia. Pediatr Cardiol. 2010;31:1079–82. [DOI] [PubMed] [Google Scholar]
- 26. Fazelifar A, Eskandari A, Hashemi M, Alavi M, Totounchi M, Forghanian A, et al. Deep sedation in patients undergoing atrioventricular nodal reentry tachycardia ablation. Res Cardiovasc Med. 2013;2:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santangeli P, Proietti R, Di Biase L, Bai R, Natale A. Cryoablation versus radiofrequency ablation of atrioventricular nodal reentrant tachycardia. J Interv Card Electrophysiol. 2014;39:111–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


