Abstract
Eradication of wild poliovirus (WPV) types 1 and 3, prevention and cessation of circulating vaccine-derived polioviruses, and achievement and maintenance of a world free of paralytic polio cases requires active risk management by focusing on population immunity and coordinated cessation of oral poliovirus vaccine (OPV). We suggest the need for a complementary and different conceptual approach to achieve eradication compared to the current case-based approach using surveillance for acute flaccid paralysis (AFP) to identify symptomatic poliovirus infections. Specifically, we describe a modeling approach to characterize overall population immunity to poliovirus transmission. The approach deals with the realities that exposure to live polioviruses (e.g., WPV, OPV) and/or vaccination with inactivated poliovirus vaccine provides protection from paralytic polio (i.e., disease), but does not eliminate the potential for re-infection or asymptomatic participation in poliovirus transmission, which may increase with time due to waning immunity. The AFP surveillance system provides evidence of symptomatic poliovirus infections detected, which indicate immunity gaps after outbreaks occur, and this system represents an appropriate focus for controlling disease outbreaks. We describe a conceptual dynamic model to characterize population immunity to poliovirus transmission that helps identify risks created by immunity gaps before outbreaks occur, which provides an opportunity for national and global policy makers to manage the risk of poliovirus and prevent outbreaks before they occur. We suggest that dynamically modeling risk represents an essential tool as the number of cases approaches zero.
Introduction
Since its launch in 1988, the Global Polio Eradication Initiative (GPEI) achieved significant progress toward the goal of eradicating all wild polioviruses (WPVs), including the successful eradication of WPV type 2 (WPV2) in 1999. However, efforts to eradicate the other two serotypes, WPV1 and WPV3, continue in a few endemic and re-infected countries, and the threats of importation of these WPVs and outbreaks of circulating vaccine-derived poliovirus (cVDPV) associated with the use of the live, attenuated oral poliovirus vaccine (OPV) represent ongoing concerns.(1) Ultimately, cessation of the use of OPV will need to occur to end all cases of paralytic poliomyelitis.(2–4) Clinical poliomyelitis cases of acute flaccid paralysis (AFP) detected by the Global Polio Laboratory Network(5) serves as the foundation for the eradication effort, because they reveal the circulation of live polioviruses (LPVs). However, as the GPEI approaches the situation of increasingly fewer cases, it must focus on managing the risks of outbreaks prior to their occurrence. Thus, while AFP surveillance provides important insights about immunity gaps among children after outbreaks occur and plays a key role in controlling disease, it does not provide a tool for prevention. Managing poliovirus risks represents a complex undertaking, in part because three serotypes exist (i.e., managers must deal with diseases caused by 3 different serotypes instead of one viral serotype in the case of smallpox)(6, 7) and infections typically lead to paralytic clinical symptoms in a relatively small fraction of individuals, with on average 1:200 susceptible individuals developing paralytic poliomyelitis in the pre-vaccine era(8) and reported type-specific paralysis-to-infection ratios ranging from 1:200 or more for WPV1 to 1:1000 or less for WPV2 and WPV3.(9, 10) Dynamic modeling facilitates visualization of infections and their transmission within a population, which we cannot directly observe from a surveillance system that detects paralytic cases (i.e., disease after it occurs) but does not detect asymptomatic infections.(11) Specifically, modeling population immunity to manage the risks of outbreaks can identify immunity gaps before outbreaks occur by looking beyond the subset of the population captured by AFP surveillance to consider all individuals in the population and their potential participation in poliovirus transmission. Policy makers increasingly recognize the importance of managing population immunity in the context of measles control and elimination, specifically with respect to determining the amount of time that can pass between supplemental immunization activities (SIAs) without risking outbreaks(12–14) and we suggest the need for a risk and population immunity management focus for polio.
This paper provides background context related to the individual and population immunity to polioviruses and the population immunity requirements for national elimination. We highlight the need to combine both measurements and models to adequately use insights about extrapolation of individual immunity to characterize population immunity. Population immunity represents the critical concept for health leaders interested in disease eradication or control, and in the methods section we present a conceptual model of population immunity. We develop a model informed by an expert review process(15, 16) and discuss its use to help monitor population immunity in different settings. Important uncertainties remain about the role of partially immune older individuals in poliovirus transmission and the cost, feasibility, and availability of some of the decision options. We discuss the potential impact of these uncertainties on population immunity and management strategies. While polio may seem like a relatively low priority in polio-free countries, maintaining the eradication of WPVs requires active risk management, which implies the need to continue to vaccinate despite no observed cases. Managing the risks requires understanding how individual immunity integrates to overall population immunity and how population immunity changes with time.
Context
Individual immunity
Polioviruses, human enteroviruses of the Picornaviridae family, comprise a single-stranded, positive-sense RNA genome and a protein capsid of three serotypes (types 1, 2, and 3). Individual protection from polioviruses is mediated by the immune system, which includes a collection of cells, tissues, and molecules that respond to invading pathogens in order to prevent infections and eliminate infections that occur within the host.(17) In healthy individuals, the immune system mounts both an innate (or natural or native) immune response, which rapidly provides initial protection from pathogens by stopping entry and destroying microbes that get past barriers, and an adaptive (or specific or acquired) immune response, which uses a slower, more complex process to target the specific pathogen. The role of innate immune response in the process of infection remains complex. Host susceptibility depends on many factors, including general health (which depends on both genetic and environmental conditions), the presence of other pathogens, and the nature of exposure to the pathogen. The adaptive response provides a particularly important defense, because many human pathogens developed strategies to resist or evade innate responses. Once triggered, the adaptive immune response retains a memory of a prior infection. This memory enables the immune system to respond more quickly when re-infected by the same (or in some cases a similar) pathogen (i.e., an anamnestic response).
Adaptive immunity changes over time in response to exposures. While innate immunity mechanisms recognize structures shared by classes of microbes, adaptive immunity mechanisms involve lymphocytes that express receptors to detect antigens (i.e., different substances produced by, or constituents of pathogens). Generally, adaptive immunity includes two mechanisms: cell-mediated immunity, which involves actions by T-lymphocytes (T-cells), macrophages, natural killer cells, and cytokines to present antigens to the immune system and attack pathogens that live and divide inside infected cells, and humoral immunity, which involves the production of antibodies (also called immunoglobulins) by B lymphocytes (B-cells) that selectively target specific antigens.(17) Continued excretion of polioviruses by patients with severe B lymphocyte-related immune deficiencies provides evidence of the important role that B lymphocytes and antibodies play in preventing and controlling poliovirus infections.(18) The antibodies excreted by B lymphocytes into the circulation neutralize polioviruses present in the blood (i.e., they provide humoral immunity) and those excreted into mucosal fluids neutralize polioviruses at mucosal surfaces (i.e., they increase mucosal immunity), and both types of antibodies reduce the extent of replication and infection by preventing the virus particles from gaining access to host cells and tissues.(19) For polioviruses, laboratory assays exist to detect the presence of antibodies in the blood(20, 21) and confirm prior exposure to live or inactivated viruses (i.e., active humoral immunity) and serological studies typically report the detection of antibodies in terms of titers. While even low titers likely indicate protection from disease, interpretation of titers with respect protection from asymptomatic infection depends on the exposure history, with a negative correlation between titers and probability of intestinal infection for individuals with prior live poliovirus infection, but possibly no such correlation for individuals with only inactivated poliovirus exposure history.(15) Even for a population with only IPV-derived immunity, serologic data provide useful information, because higher titers probably correlate with a reduction in oropharyngeal infection and infectiousness.(15, 16) Transfer of antibodies can also occur between individuals, most notably from mothers to their newborns via the placenta and colostrum, and to a much lesser extent, if any, breast milk during later infancy (i.e., passive immunity), which protects the otherwise naïve individual until the transferred antibodies get exhausted.(22) For polioviruses, maternal antibodies provide some protection for infants, but some countries recommend giving a birth dose of OPV.(8, 22)
In general, adaptive immune responses typically proceed in five sequential phases: antigen recognition, activation of lymphocytes, antigen elimination, immune response modulation, and memory.(17) The most important aspects of adaptive immunity include specificity for distinct antigens and memory to prior antigenic exposure. The first exposure to a pathogen leads to a primary immune response, in which naïve lymphocytes learn to locate and recognize the antigen(s) and mount a response. This complex process typically takes a fair amount of time, which means that significant infection may occur prior to an effective immune response. However, once the adaptive immune response occurs, memory B lymphocytes (i.e., long-lived B-cells induced during primary response) facilitate a secondary (i.e., anamnestic) immune response to subsequent exposures to the same pathogen, which leads to more rapid production of antibodies.(15, 23) However, relatively little evidence exists related to the length of time that memory lymphocytes persist in immune individuals. In an environment in which individuals periodically encounter circulating live viruses, the presentation of antigens to the immune system may effectively remind the memory lymphocytes about the pathogen. However, in the absence of exposure to pathogens, waning of immunity may occur. For polioviruses, the nature of waning remains relatively poorly characterized, although once infected with a live poliovirus (LPV, i.e., WPV, OPV, cVDPV, or OPV-related poliovirus) or effectively vaccinated, it appears that individuals benefit from lifelong protection from homotypic disease (i.e., paralytic polio). However, vaccinated and previously-infected individuals, as well as infants with maternal antibodies, can become asymptomatically infected or re-infected when exposed to a LPV. Consequently, they may potentially participate in poliovirus transmission, even though they will do so covertly, and the extent to which the waning of immunity affects the ability to participate in poliovirus transmission remains highly uncertain.(16)
By stimulating the adaptive immune system using a noninfectious or less infectious version of a pathogen, vaccination offers a highly effective method to protect individuals from paralytic poliomyelitis and reduce their ability to become infected and participate in transmission. For polio, countries currently use two kinds of licensed vaccines to stimulate adaptive immunity: inactivated poliovirus vaccine (IPV) and attenuated, live OPV. The capsid proteins of the three poliovirus serotypes differ with regard to antigenicity, which means that protection against one type does not protect against the other two, and thus individuals require vaccination for all three serotypes (i.e., at least one effective dose against each serotype).
With respect to vaccination, the amount and timing of the OPV “dose” probably play important roles in determining whether exposure to the pathogen leads to an infection and immune response (i.e., whether it “takes” or not).(24–26) Given the importance of ensuring the effective “take” of vaccine doses (i.e., doses that surpass the capability of the innate immune response and replicate to cause infection), the World Health Organization requires a minimum median cell culture infective dose (CCID50) of 106.0 for type 1 OPV, 105.0 for type 2 OPV, and 105.8 for type 3 OPV in trivalent OPV (tOPV) formulations.(8) As a live virus, OPV infects vaccine recipients and the attenuated strains can lead to vaccine-associated paralytic polio (VAPP) in rare instances (i.e., OPV may cause paralysis in susceptibles approximately 1 per million first doses(27, 28) which is orders of magnitude lower than the paralysis rates of WPV). Vaccine recipients infected with OPV excrete the virus in their feces and sometimes in oropharyngeal secretions, and these excreted viruses can transmit and infect others, leading to secondary spread (i.e., they induce infection/inadvertent vaccine protection in susceptible individuals or boost immunity in others).(29–31) Close contacts appear more likely to become infected, although the infections can spread to community contacts as well.(27, 32, 33) Secondary spread of OPV offers the advantage of extending the impact of the immunization (i.e., it provides protective immunity benefits beyond the direct vaccine recipients), but it also means that fully susceptible non-vaccine recipients rarely may develop VAPP through contact with a vaccine recipient.(27) Secondary spread also means that in populations with relatively large numbers of susceptibles, the excreted viruses can continue to spread and genetically revert back toward WPVs, potentially to the point of becoming cVDPVs that cause outbreaks.(34)
In contrast, IPV does not lead to infection, VAPP,(27) or VDPVs. Instead, IPV stimulates immunity by directly presenting poliovirus-specific antigens to the adaptive immune system to stimulate the production of antibodies. Doses of IPV must also surpass the capability of the innate immune response to be effective, and currently the World Health Organization requires IPV vaccine formulations produced from WPV seed strains to contain at least 40, 8, and 32 D-antigen units of serotypes 1, 2 and 3, respectively.(8) Only intact poliovirus particles express D-antigen, and consequently D-antigen serves as an indicator of effective particle concentrations. Due to interference of maternal antibodies with the immune response, children may not benefit from a first dose of IPV until after 6 weeks of age.(35) Evidence from OPV challenge studies shows that individuals with only IPV-induced immunity acquire much less enteric mucosal immunity than individuals with a history of LPV infections.(15, 16) Consequently, they experience a higher probability of infection if exposed and excrete more poliovirus in feces if infected, although IPV and LPV may provide similar humoral immunity protection from oropharyngeal infection and excretion.(15, 16)
Although we mainly focus on immunocompetent individuals, the immune systems of individuals with common variable immunodeficiency (CVID) syndrome and other B lymphocyte deficiencies may not effectively mount a defense against a LPV infection, such that these individuals may excrete poliovirus for prolonged periods of time if infected.(36–38) Although very rare, immunodeficiency-associated vaccine-derived polioviruses (iVDPVs) occur, and current research efforts target the development of antivirals and other tools that might help clear the infections.(39) Due to risk of paralytic disease, many countries use CVID and other immunodeficiencies as an exclusion criterion for receiving OPV,(1) and with the now-known risk of creating a potential iVDPV, we expect that CVID might become a more universal exclusion criterion for OPV vaccination. Although some countries exclude HIV-exposed individuals from OPV vaccination, current evidence does not suggest that HIV infection represents a risk factor for prolonged excretion or the creation of iVPDVs (i.e., HIV primarily affects T-cells, not B-cells).(37, 40)
Population immunity
From an individual perspective, vaccination provides personal protection against paralytic disease, but from a population perspective the aggregate effect of individual immunity affects the ability of poliovirus infections to transmit throughout the entire population. Population immunity, as we define it, reflects the overall level of protection from poliovirus transmission within a population, including all of the individuals in the population. Population immunity to poliovirus transmission depends on the ability of individuals to prevent or limit infections if exposed and thus prevent or reduce poliovirus excretion that might cause infections in others. This mainly depends on systemic and local mucosal immunity from prior exposure to a LPV or vaccination with IPV, but it may also include poorly-characterized, non-specific innate immunity effects.(23, 26, 41) Evidence suggests that poliovirus infections typically involve the gut, leading to excretion of substantial amounts of poliovirus in the feces, but studies also report recovering virus from the throat, which suggests infection of the oropharynx.(26, 42, 43) Fecal excretion may result in transmission by the fecal-oral route via contaminated materials and hands, while oropharyngeal excretion may lead to transmission by the oropharyngeal route via aerosol droplets and possibly contaminated materials and hands.(42) The amount of excretion required for effective transmission and the relative importance of fecal-oral and oropharyngeal excretion in transmission remain uncertain and most likely depend on the setting.(16) Given that antibody levels change over time as a result of poliovirus infections, vaccination, and waning, individual excretion following poliovirus exposure also depends on the number and timing of prior infections and vaccinations. Moreover, immunological responses of individuals may differ depending on the route, dose, and strain of the infection. Thus, characterization of population immunity to poliovirus transmission requires an understanding of: (1) the extent to which different immunity states reduce both fecal and oropharyngeal excretion, (2) the impact of waning on protection from excretion over time, (3) the effect of different reductions of both fecal and oropharyngeal excretion on the ability to infect others, and (4) the relative importance of fecal-oral and oropharyngeal transmission. Population immunity to poliovirus transmission as defined above refers to the ability of a population to prevent sustained poliovirus transmission, and thus involves the collective immunity to excretion of all members of the population and the conditions leading to effective transmission of polioviruses. Sufficiently high population immunity provides a barrier against infection and inhibits sustained transmission,(44) and, on a population level it therefore protects even susceptible individuals from exposure to circulating poliovirus. We note that fully susceptible individuals remain even in populations that attain very high levels of vaccine coverage, because vaccine is contraindicated for some individuals (e.g., children too young to receive the vaccine, some immunocompromised individuals), gaps in health care systems miss some people (e.g., migrants, poor, and underserved), and some healthy individuals may put themselves and others at risk by refusing vaccination. If substantial heterogeneity exists with respect to population immunity, then a virus introduced in a pocket of susceptibles could still circulate and cause local outbreaks.(45)
The complexity of individual immunity, with multiple types of exposures (i.e., WPV, cVDPV, OPV vaccination, IPV vaccination, secondary OPV), different classifications of immunity (e.g., cell-mediated, humoral, innate, adaptive, mucosal, intestinal, oropharyngeal), and differential waning of the types of immunity, implies that modeling of population immunity involves more than just tracking the fraction of individuals immune to disease and assuming that these individuals can never again participate in transmission.(11) In this context, we emphasize that our definition of population immunity to poliovirus transmission contrasts sharply with the epidemiological-based definition of population immunity to disease used by others(46–48) (e.g., Jenkins et al. (2008) define “vaccine-induced population immunity” as the “fraction of children younger than 5 years of age who were protected by direct vaccination”(47, p. 1669)). By characterizing population immunity only with respect to protection from disease (and primarily in children under 5 years old captured by the AFP surveillance system), epidemiological inferences remain limited to understanding immunity as it relates to the incidence of disease in young children if they get exposed to WPVs or cVDPVs. Moreover, the definition does not account for the indirect effects of vaccination on the immunity of unvaccinated members of the population. This epidemiological definition of population immunity based only on vaccination history does not adequately capture the risk of poliovirus transmission and disease for several reasons. First, it does not account for the potential participation in poliovirus transmission of individuals protected from disease but not from asymptomatic infection. Second, it does not account for the possibility that individual immunity to poliovirus transmission may wane, particularly for older children and adults, and thus it does not represent all age groups potentially participating in poliovirus transmission. Third, it does not account for the effect of LPV infections from circulating viruses, including secondary OPV infections, on population immunity.
Sstatistical epidemiological analysis of risk factors based on using retrospective data to characterize population immunity performs relatively poorly with respect to predicting the dynamic risks of outbreaks and the extent of potential exposure to WPVs or cVDPVs.(48) Such retrospective models fail to consider that exposure to polioviruses changes over time and depends dynamically on population immunity to poliovirus transmission. Effectively characterizing the risks of poliovirus transmission and disease must account for the dynamic process of poliovirus transmission and tools for managing population immunity to poliovirus transmission must address the dynamic nature of transmission. While the nature of waning of immunity and the potential transmission between age groups remain poorly understood, these factors play an important role in the dynamics of poliovirus transmission that models must address. WPV introductions into populations not exposed to WPVs for many years typically lead to outbreaks with high incidence of cases among adolescents and adults, which suggests active exposure to WPVs across all age groups.(49–51) We note that the importance of waning of immunity may increase as we transition to increasing proportions of the population with only vaccine-induced protection (i.e., in the absence of periodic infections that historically occurred with recurring exposure to endemic WPVs) and/or shift to IPV, which does not boost immunity in unvaccinated individuals.(45) The epidemiological results provide important insights, but we suggest that achieving eradication requires understanding the potential for both symptomatic and asymptomatic poliovirus transmission within the entire population in the absence of cases, such that national and global managers can take appropriate risk management efforts to ensure the prevention of any future cases by ending the circulation of all indigenous WPVs and cVDPVs and limiting the ability of imported viruses to sustain transmission. Thus, the focus on immunity to disease among AFP cases as a signal for the identification of immunity gaps will provide incomplete information for an eradication effort, and this will become most apparent as the effort approaches the observation of zero cases.
Modeling and measuring population immunity to manage risks
Understanding poliovirus transmission and the risk and consequences of outbreaks requires modeling poliovirus transmission, which involves mainly asymptomatic infections, even though observed paralytic polio cases represent the primary focus of surveillance and public concern. Using a model-based definition of population immunity, we can focus on the integration of the overall protection from poliovirus transmission in a population as a function of time and view population immunity as a “stock.” The level of the stock depends on inflows and outflows, where inflows of susceptible individuals occur due to births, and deaths lead to outflows of individuals from all groups. For purposes of analogy, it may help to consider a familiar stock, like a bank account balance, for which deposits (inflows) and withdrawals (outflows) change the level of the balance (the value of the stock). While banks make it easy to observe individual account balances, observing individual immunity for a disease presents a challenge. Scaling up to the population level becomes even more challenging. For bank balances representing multiple individuals (e.g., corporations), accounting departments need to aggregate all of the inflows and outflows as a function of time to correctly assess and monitor the balance and manage the risks associated with cash flows. Population immunity for polio similarly represents an accounting challenge, but it becomes more complicated because individuals shift to relatively higher levels of protection as they experience infection with a LPV or get vaccinated with IPV and the process includes time delays (i.e., unlike bank deposits that can essentially increase the balance instantaneously, immunizations take some time to deliver and to provide protection, and decreasing population immunity also occurs gradually with births of susceptible children and decaying antibodies levels in immunes). Although challenging, we emphasize the importance of the accounting, because achieving and maintaining a high level of population immunity effectively prevents infections from transmitting, thus leading to national elimination and potential eradication of the disease, and to the prevention of outbreaks due to reintroductions.
By modeling population immunity to manage risks, we can help to visualize the impacts of changes in vaccine choices. For example, we recently demonstrated that population immunity continues to change as countries increasingly shift toward greater use of IPV, because IPV provides less mucosal immunity than OPV and does not induce or boost immunity secondarily in contacts of recipients, although it may represent a more widely accepted vaccine given that it does not cause VAPP.(45) Similarly, switching to monovalent or bivalent OPV formulations impacts population immunity by serotype (e.g., using mOPV1 instead of trivalent OPV (tOPV) provides a relative increase in population immunity for type 1, but results in relatively lower levels of population immunity for types 2 and 3).(52) Considering the complete population immunity profile, we can assess the average population immunity and compare this to theoretical threshold levels required to interrupt transmission, and we can identify and explore options to increase (and maintain) population immunity above the threshold to reduce the risk of outbreaks. We note that countries may differ significantly with respect to the thresholds, because conditions (e.g., sanitation, crowding, climate) in some countries may be more conducive to facilitating transmission than in others (e.g., countries with high population density areas and relatively poor sanitation will require relatively higher thresholds). For this reason alone, strategies that work effectively in some countries may prove insufficient in others. With WPVs continuing to circulate and posing a threat, countries must decide whether and when to conduct SIAs, and we suggest that a model of population immunity might help inform their decisions. Heterogeneity within countries may also represent an important consideration.
As with any model, the quality of the input data matters significantly with respect to performance. Although we cannot measure population immunity, serological studies offer an important opportunity to assess the levels of antibodies and to further refine population immunity estimates in the context of uncertain inputs. In addition, field studies that provide validation of estimates for key model inputs represent an essential activity. Specifically, given the critical role of vaccination coverage as an input to the model, independent measurements that provide validation of vaccine coverage estimates provide important information. Characterizing population immunity well also requires complete records of all SIAs conducted in different areas of a country.
Methods
Based on an expert review process on poliovirus immunity and transmission,(15, 16) we developed a conceptual model designed to monitor population immunity in different settings that captures the dynamics of different events such as birth, death, aging, vaccination (OPV or IPV), infection with a circulating LPV (i.e., WPV, VDPV, OPV, or Sabin-like poliovirus), recovery, and waning of immunity. The model generally follows how people in aggregate move between 8 discrete immunity states as a result of these different events:(15, 16)
“Maternally immune: Individuals born with maternal antibodies that wane rapidly with age (if not infected with LPV or successfully vaccinated with IPV)
Fully susceptible: Individuals never infected with LPV or successfully vaccinated with IPV and maternal antibodies effectively waned to 0
1 successful IPV dose: Individuals with 1 IPV dose that reached and stimulated the immune system and no history of LPV infection, including those “primed” and without measureable serum antibody, with all of these individuals assumed to benefit from protection from paralysis (as occurs for all immunity states except fully susceptible)
2 successful IPV doses: Individuals with 2 IPV doses that reached and stimulated the immune system and no history of LPV infection
≥ 3 successful IPV doses: Individuals with at least 3 IPV doses that reached and stimulated the immune system and no history of LPV infection
IPV and LPV: Individuals exposed to both LPV and IPV, in any order
1 LPV infection: Individuals with a history of a single LPV infection and no history of successful IPV vaccinations
≥ 2 LPV infections: Individuals with a history of multiple LPV infections and no history of successful IPV vaccinations” (16, p. TBD)
We emphasize that transitions between immunity states only occur following successful vaccine doses and actual infections (i.e., we require “take” of the vaccine rather than mere receipt of a vaccine and for an LPV we require actual replication of virus in the host rather mere ingestion without replication and/or an immune response). Waning of immunity represents a continuous process that we capture by considering multiple discrete waning stages and properties that evolve with time since entering a recent immunity state.(53)
The model assumes that individuals with any active or passive (i.e., maternal) immunity cannot contract paralytic poliomyelitis disease.
Three properties characterize the degree to which individuals in different immunity states can participate in poliovirus transmission in the model based on review and synthesis of the literature:(15, 16)
Relative susceptibility (Sr) characterizes the relative probability of becoming infected in a given immunity state compared to fully susceptibles in an identical setting.
Relative contribution to fecal-oral transmission if infected (RCFTI) characterizes the relative number of secondary infections generated via fecal-oral transmission by an infected individual in a given immunity state compared to an infected fully susceptible individual in an identical setting.
Relative contribution to oropharyngeal transmission if infected (RCOTI) characterizes the number of secondary infections generated via oropharyngeal transmission by an infected individual in a given immunity state compared to an infected fully susceptible individual in an identical setting.
Table 1 provides the values for these properties based on expert assessments and testing of the model against data on polio incidence in multiple different situations.(15, 16, 53) We can estimate Sr using the results from OPV challenge studies that included subjects in different immunity states and susceptible controls required for comparison, and these data informed the expert assessments.(15, 16, 53) RCFTI and RCOTI represent more complicated concepts for estimation. They depend on the average proportion excreting and the concentration of virus excreted in feces and from the oropharynx at different points in time by infected individuals in each immunity state, as well as the relationship between the concentration of excreted virus and infectiousness to others.(16) While infectiousness depends on numerous factors beyond the concentration of excreted virus, the experts provided an assessment of the average relationship controlling for all factors that might impact infectiousness. The expert assessments for the proportion excreting and the concentration of virus excreted in feces and from the oropharynx at different points in time and the infectiousness as a function of virus concentration lead to estimates for RCFTI and RCOTI for recent immunity states.(15, 16) For historic immunity states, limited data imply very large uncertainty with respect to RCFTI and RCOTI, and we determined how waning may affect these properties based on model calibration.(53) Finally, given that we characterize both fecal-oral and oropharyngeal transmission, the relative contribution of each transmission mode in a given setting affects the overall ability to participate in transmission. Thus, we also provide estimates for the proportion of transmission that occurs via the oropharyngeal route (PO) in different settings. Due to limitations in the existing data,(15) substantial uncertainty exists to inform these model inputs.(16)
Table 1:
Estimates for model inputs that characterize the level of immunity to transmission of type 1 WPV infection in each immunity state, based on an expert review process and model calibration.(15, 1,6, 53)
| Immunity state | Relative susceptibility (Sr) | Relative contribution to fecal-oral transmission by infected individuals (RCFTI) | Relative contribution to oropharyngeal transmission by infected individuals (RCOTI) |
|---|---|---|---|
| Fully susceptible | 1 (by definition) | 1 (by definition) | 1 (by definition) |
| Maternally immune | 0.78 | 0.84 | 0.61 |
| 1 successful IPV dose | |||
| 2 successful IPV doses | |||
| ≥ 3 successful IPV doses | |||
| 1 LPV infection | |||
| ≥ 2 LPV infections | |||
| IPV and LPVb |
Acronyms: IPV = inactivated poliovirus vaccine; LPV = live poliovirus; R0 = basic reproductive number; WPV = wild poliovirus
Notes:
Historic defined in the model as three (for type 3) or four (for types 1 and 2) years after entering the immunity state, with properties of 3 intermediate historic stages specified according to a logarithmic increase(53)
Given the small differences in assessments between the “≥ 2 LPV infections” and “IPV and LPV” immunity states, we assumed that the properties of “≥ 2 LPV infections” for “IPV and LPV”
We emphasize that our approach characterizes immunity to poliovirus transmission in relative terms compared to fully susceptibles. For example, if we know that an infected fully susceptible generates 5 new infections on average while infectious via fecal-oral transmission in a specific setting, then an RCFTI of 0.4 for a given immunity state means that an infected individual in that immunity state would generate 2 new infections on average. The absolute probabilities of infection and transmission depend on the virus transmissibility and the rate at which individuals contact infectious individuals in the specific population, for which the basic reproductive number (R0) provides an aggregate, average measure.
Theoretically, in the absence of exogenous introduction of infections, transmission can persist in a population as long as each infected individual can infect at least 1 contact. In an entirely susceptible population, if a new infection can generate R0 secondary infections, then transmission cannot persist if the actual proportion of the population susceptible to infection and transmission equals less than 1/R0 (e.g., if R0 equals 10, then with only 10% of the population susceptible to poliovirus transmission each new infection will only find 1 susceptible contact, on average). For poliovirus, everyone can potentially participate in transmission to some degree, regardless of prior immunity. Thus, the ability of transmission to persist depends on the effective susceptible proportion (ESP), defined as the sum of the proportion of individuals in each immunity state weighted by their relative contributions to each type of transmission:
where superscript IS indicates a given immunity state and pIS represents the proportion of individuals in a given immunity state. For simplicity, we ignore the small effect of mortality due to poliomyelitis or other causes on ESP in this conceptual model (i.e., a very small proportion of infected individuals dies during the infectious period because mortality occurs at a much lower rate than recovery from infection). The effective immune proportion (EIP) equals 1-ESP and represents a summary measure of population immunity as it relates to the ability of the population to sustain poliovirus transmission. Sustained transmission can occur if polioviruses can find enough effectively susceptible individuals to infect that they can continue to circulate, which occurs when ESP exceeds the threshold level ESP*. Equivalently, given the relationship between ESP and EIP, transmission can occur when the EIP falls below the threshold level (EIP*) such that:
We note that R0 may change over time due to seasonal oscillations and long term changes in poliovirus transmissibility,(53) which implies that the thresholds EIP* and ESP* may change with time. In a traditional mass-action model, ESP will approach ESP* at the endemic equilibrium.(54) To characterize population immunity using the EIP, we must track the numbers of individuals in each recent and historic immunity state. We combine the estimates of the numbers of people in each state with the relative susceptibility (Sr), relative contribution to fecal-oral transmission if infected (RCFTI), and relative contribution to oropharyngeal transmission if infected (RCOTI), using an assumed proportion of transmission that occurs via the oropharyngeal route (PO). We assume that Sr, RCFTI, and RCOTI reflect average values over all possible settings and individuals (except certain immunocompromised individuals, which the model should treat separately) that represent inherent properties of each immunity state. With respect to RCFTI and RCOTI, Table 1 shows important although uncertain differences between immunity states with recent LPV-induced immunity and IPV-induced or maternal immunity. To illustrate the conceptual model for population immunity to poliovirus transmission, we modeled a hypothetical low-income population with simplified conditions indicated by the model input values in Table 2 implemented in JAVA Eclipse™.
Table 2:
| Model input | Value | Notes |
|---|---|---|
| Proportion of transmissions via oropharyngeal route | 0.3 | Based on average of mean expert assessments for community and close contacts for type 1 (Table 1)(16) |
| Initial population distribution | Based on 1980 population in low-income countries(74) aged less than 5 years (5%) and more than 5 years (95%)(75) | |
| Death rate | 0.02 per person per year | Based on assumed life expectancy of 50 and applied to all three modeled age groups |
| Birth rate | 0.02 per person per year | Assumed equal to death rate to fix total population size |
| Basic reproductive number (R0) | 10 | High-medium estimate for low-income country based on prior work(11) (highly uncertain) |
| Effective routine OPV coverage | 50% | Fraction of newborns successfully vaccinated with OPV by 3 months of age |
| Effective cumulative OPV SIA coverage per year (ε) | 80% | Fraction of fully susceptibles aged less than 5 years successfully vaccinated by SIAs in a yearb |
| Number of SIA rounds for intense OPV with SIAs (nr) | 3 per year | Assume SIAs occur on days 0, 60, and 120 in each year during the “intense OPV with SIAs” mode |
| Step size for numerical integration | 1/2 day | Numerical integration according to Euler method |
Acronyms: OPV = oral poliovirus vaccine; R0 = basic reproductive number; SIAs = supplemental immunization activities
Notes:
For generic model inputs, see Duintjer Tebbens et al. (2012)(53)
Effective coverage in each round equals 1-(1-ε)1/nr. Individuals in other immunity states subject to same effective OPV SIA coverage, but with assumed lower susceptibility than fully susceptibles.
Results
Figure 1 shows our conceptual population immunity model that estimates the level of population immunity to transmission of infection as a function of time for a given population. As noted above, interrupting transmission or preventing a re-introduced virus from causing an outbreak requires that the effective immune proportion (EIP) exceeds EIP* = 1− 1/R0, where EIP provides a summary measure of population immunity and R0 provides a summary measure of the transmissibility of a poliovirus in a given population, independent of the level of population immunity.
Figure 1: Conceptual model to track population immunity to a given serotype.
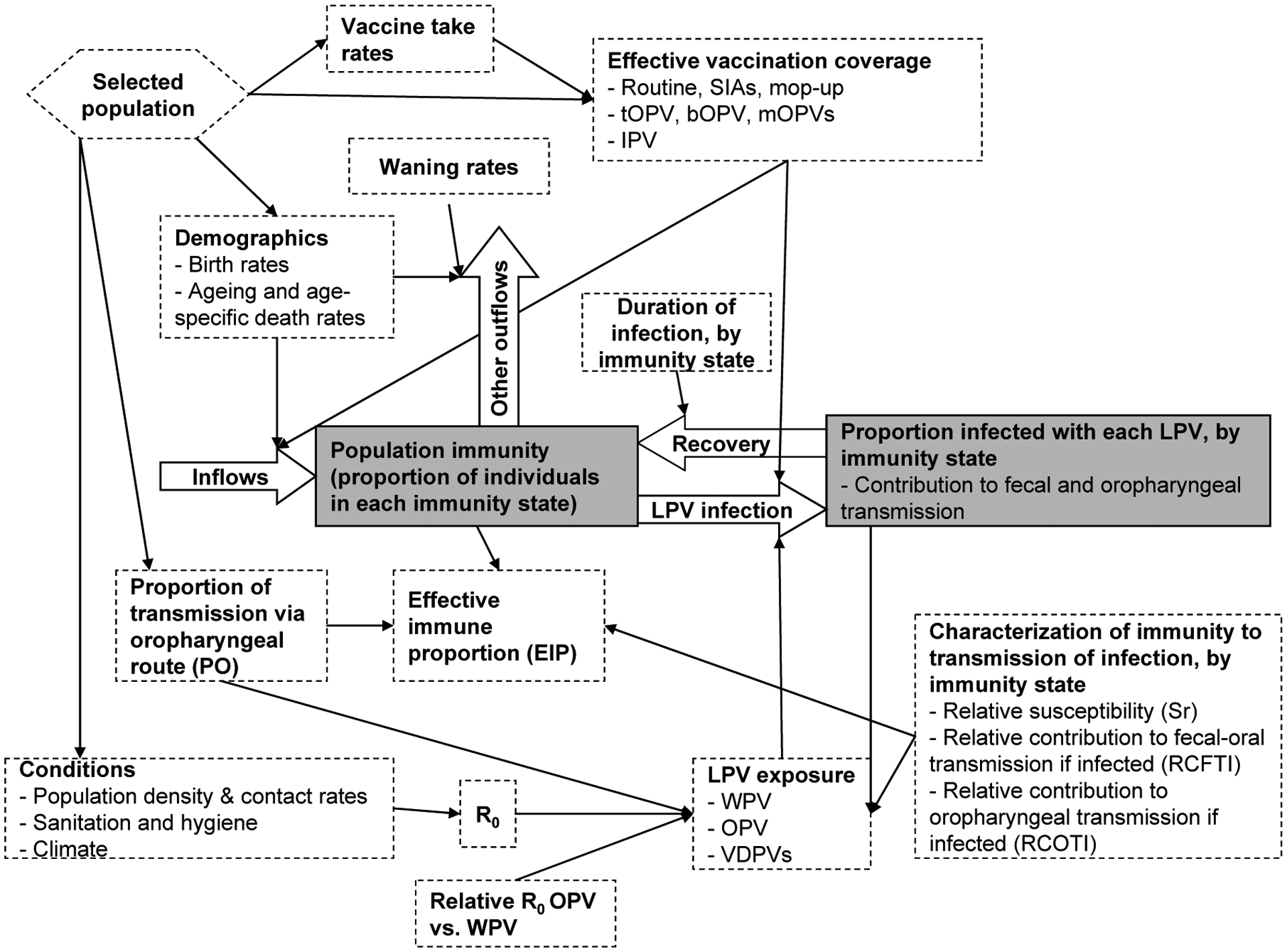
Acronyms: bOPV = bivalent OPV; IPV = inactivated poliovirus vaccine; mOPV = monovalent OPV; LPV = live poliovirus; OPV = oral poliovirus vaccine; R0 = basic reproductive number; tOPV = trivalent oral poliovirus vaccine; VDPVs = vaccine-derived polioviruses; SIAs = supplemental immunization activities; WPV = wild poliovirus
To monitor the number of individuals in each immunity state, the model must track the inflows and outflows from each immunity state (i.e., each stock). Figure 1 shows all the factors that influence the flows and represents population immunity as a single stock, although in reality it consists of a collection of multiple immunity states (i.e., stocks) with transition events dictating the flows between them. The factors influencing the flows include those related to births and deaths (largely independent of poliovirus transmission due to very low polio mortality in all countries), vaccination (with OPV or IPV), infection with a circulating LPV, and waning immunity. Flows related to vaccination come from a large number of possible vaccination strategies (e.g., routine immunization, supplemental immunization activities (SIAs) that administer vaccine to a large fraction of the population in a short period of time, and mop-up or response SIAs triggered by outbreaks).(1) The model must account for incomplete immunogenicity resulting from less than 100% vaccine take rates to correctly account only for successful IPV doses and actual LPV infections, because the appropriate assignment of the immunity states depends on vaccine take. Take rates vary in different populations and thus depend on the selected population.(1, 23, 55) LPV exposure depends dynamically on population immunity, because excretion of LPVs by individuals in each immunity state determines the rate of subsequent LPV exposure (i.e., the force of infection). Based on the assumption of homogeneous mixing typically used in dynamic transmission models,(54) the rate of LPV exposure determines both the proportion of individuals entering each immunity state after recovery from the infection and the proportion of individuals leaving each immunity state due to the LPV exposure. LPV exposure further depends on the assumptions about R0 in the given population, and in the case of OPV exposure it also depends on the assumed relative R0 for OPV vs. WPV. OPV represents an attenuated poliovirus with demonstrated lower neurovirulence (i.e., less likely to cause paralysis) and apparently lower transmissibility than WPVs.(56) However, the relative transmissibility remains highly uncertain, with lower transmissibility in a community compared to a close contact setting.(15, 16) In our model, we assume that OPV viruses evolve towards WPV-like neurovirulence and transmissibility as they replicate in individuals and circulate in a population, as characterized by a set of discrete reversion stages with different paralysis-to-infection ratios and relative R0s.(53) The absolute transmissibility of polioviruses varies widely between populations(11, 57, 58) and we previously used estimates of ranges for R0 that depend on World Bank income groups (e.g., 2–9 for high-income countries, 4–12 for upper middle-income countries, 6–14 in lower middle-income countries, and 8–16 in low-income countries).(11)
Since both WPV and OPV infections contribute to population immunity, the initial conditions for the model depend on past LPV exposures and vaccination histories. Thus, starting the model requires looking back in time to reconstruct population immunity with respect to each serotype with several different modes yielding different population immunity level characteristics. In contrast to major epidemiologic phases for general eradicable diseases,(59–61) we focus specifically on the following modes relevant to current poliovirus population immunity levels:
I: Endemic, ubiquitous WPV transmission, leading to a roughly endemic equilibrium
II: Routine OPV vaccination, leading to a significant reduction in WPV incidence associated with an increase in OPV-induced immunity (including impacts from secondary OPV exposure)
III: Intensified efforts to interrupt WPV transmission using SIAs and mop-up campaigns, leading to very high levels of OPV-induced immunity and very low WPV exposure
IV: WPV eliminated with sustained, but possibly decreased, vaccination intensity
Countries progress through these modes differently and not necessarily in a synchronous linear fashion, although that may represent the general trend in the context of an eradication effort. For example, three countries in Africa that previously eliminated polio recently re-established transmission of WPV for more than 12 months following importations (i.e., they reverted from mode IV to III).(62)
Figure 2 illustrates the four modes in a hypothetical low-income country population for the model with the inputs provided in Table 2. As long as substantial WPV exposure continues in a given population, the WPVs will eventually find pockets of susceptible people, keeping the population immunity near the threshold to sustain transmission regardless of the extent of vaccination. During the endemic equilibrium, this comes at a large cost in terms of paralytic incidence (mode I). With the beginning of noticeable routine OPV use (mode II), population immunity shifts from only WPV-derived to mostly OPV-derived among children, which substantially reduces the incidence of cases. During this mode, EPI continues to hover around the EIP* threshold, because WPV exposure eventually leads to immunity in the fraction of new birth cohorts left unimmunized with routine OPV. Once the intensity of OPV use increases (in the example due to frequent SIAs, although this could occur with a large, sustained increase in routine immunization coverage), population immunity can significantly exceed the EIP* threshold, so that the last indigenous WPV case and interruption of WPV transmission can occur following intensification (mode III). During mode III, immunity derives mainly from direct vaccination, but some older individuals not vaccinated during campaigns may also benefit from boosting of their individual immunity due to a high prevalence of OPV viruses. The effect of secondary OPV becomes smaller as population immunity increases, and thus we see the most important impact of SIAs on population immunity immediately after the addition of SIAs. In the absence of waning immunity, population immunity remains high with continued SIAs, but with waning immunity the level of population immunity may decrease as SIAs target only children less than five years of age and thus provide less boosting to older individuals.
Figure 2: Example of a hypothetical low-income country population as it goes through various modes affecting population immunity.
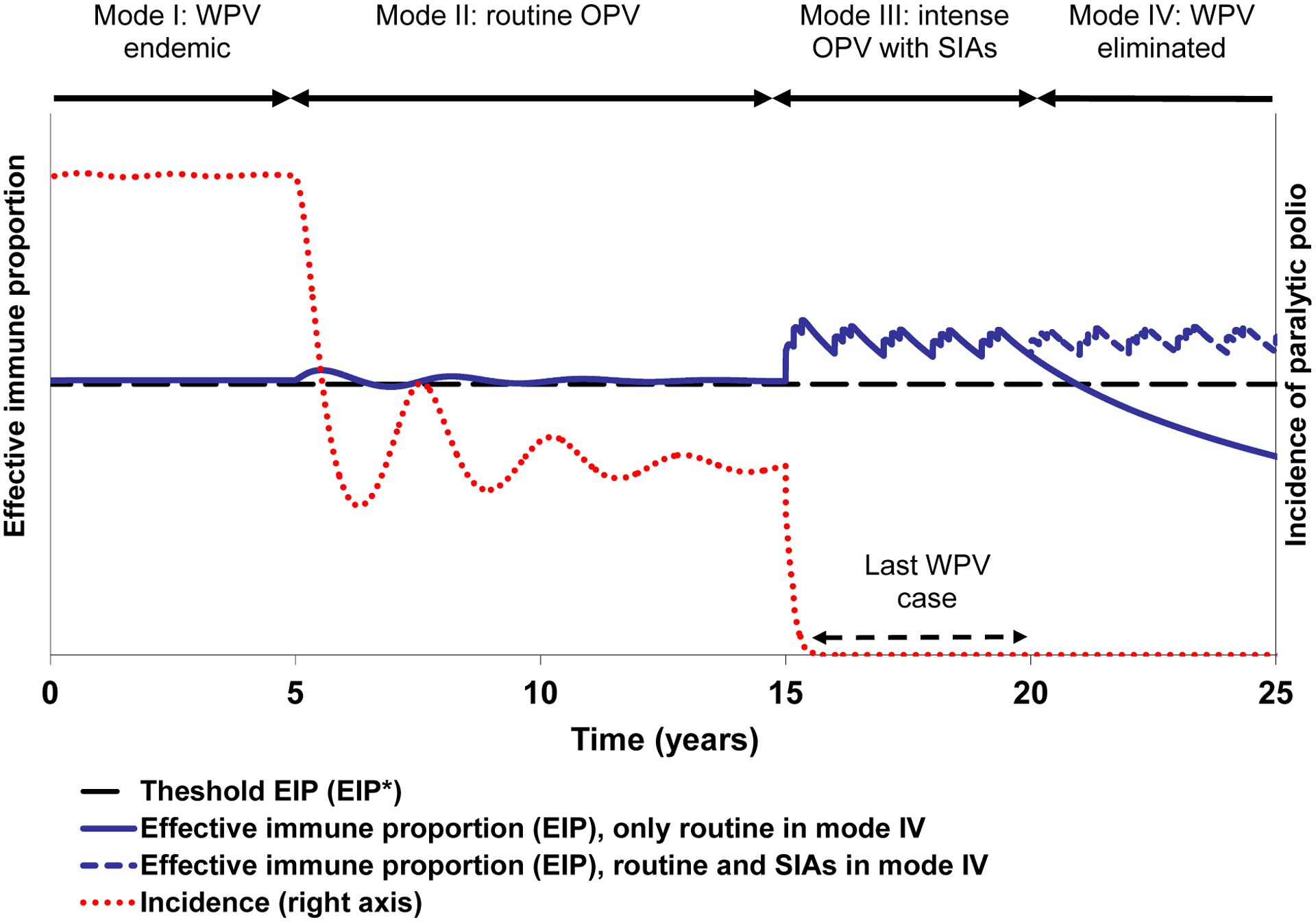
Acronyms: OPV = oral poliovirus vaccine; SIAs = supplemental immunization activities; WPV = wild poliovirus
We emphasize that an expert review process identified important uncertainties related to waning immunity (Table 1)(15, 16) and that modeling of population immunity in actual countries must use appropriate assumptions about waning and the role of boosting with secondary OPV. After WPV elimination (mode IV), population immunity may remain high with sustained high vaccination intensity, but, with little or no immunity derived from circulating WPV infections it may begin to decrease and drop below the threshold over time if the vaccination intensity decreases, as shown by the two scenarios. Experience suggests that unless vaccination intensity remains high after WPV interruption, the state of decreasing population immunity ultimately ends in either an outbreak from an imported WPV or emergence of a cVDPV. Thus, paradoxically, while the successful elimination of WPVs from most of the world demonstrates the achievement of unprecedented high levels of population immunity, the subsequent absence of substantial LPV exposure due to WPV elimination and decreasing vaccination in some places creates conditions of population immunity levels low enough to support ongoing transmission of polioviruses. Consequently, some populations experience explosive outbreaks following WPV introductions,(51, 63–65) and other populations experience the emergence of cVDPV outbreaks, which to date only occurred in places free of transmission of the corresponding WPV serotype.(66–68) This analysis demonstrates that to remain polio-free countries must maintain high population immunity levels until global WPV interruption, and they must do so in the absence of cases when the perception of the need for vaccination continues to decline.(2, 3, 69, 70)
Discussion
Our conceptual model of population immunity shows the factors needed to characterize outbreak risks and track population immunity, which extends far beyond tracking the vaccination status in children. The fact that population immunity can substantially decrease after WPV interruption underscores the critical importance of managing population immunity to achieve and maintain WPV interruption and prevent the emergence of cVDPV outbreaks. With most countries in the world currently free of all three types of WPVs, maintaining high levels of population immunity for polio represents a critical issue that nations should continue to prioritize due to the known risk of outbreaks following reintroduction of an LPV and continued exportation of WPV1 and WPV3 from endemic and re-infected countries. The outbreak in Tajikistan serves as a painful reminder that the absence of observed cases over a period of several years does not guarantee the maintenance of sufficiently high levels of population immunity to keep any future imported viruses from causing an outbreak.(63, 64) It also substantiates the need for a population immunity model applicable to countries with limited routine immunization coverage to help with risk assessment and to support expenditures on risk management and prevention. Sadly, recognition of the risk in Tajikistan prior to the outbreak led to a request of an approximately $750,000 for a preventative SIA, which the GPEI did not deem a priority due to financial pressures, and ultimately the world spent approximately $11 million to respond to the outbreak in Tajikistan and related outbreaks that occurred (Rebecca Martin and Rudi Tangermann, Personal communication, April 30, 2012). National, regional, and global immunization and health leaders will need to invest in efforts to effectively communicate the benefits of vaccines and sustain prevention activities as their efforts succeed in decreasing cases.(52) Achieving eradication requires moving entirely to prevention (i.e., achieving and sustaining high enough levels of population immunity in all areas long enough such that all circulating WPVs die out).
Ultimately, eradicating live polioviruses and ending all cases of paralytic polio will require also stopping all circulation of all LPVs, which requires cessation of OPV.(2–4) The complete eradication of WPVs continues to represent the best strategy to reduce the risk of importation, although this will not reduce the risk of live poliovirus outbreaks to zero.(71) Countries will continue to face a large number of choices to maintain high levels of population immunity, including whether to use IPV or OPV for routine immunization and whether to conduct periodic SIAs(1) and these collectively translate to complex global risk management choices.(52) Endemic countries or countries experiencing frequent WPV introductions face more complicated choices, because they must determine the number of SIAs to conduct with mOPVs, bOPVs, and/or tOPV to preempt or respond to serotype-specific threats. Recent experience with efforts that focused on using mOPV1 to reduce the burden of WPV1 between 2006–9 in India led to increased susceptibility to type 3 and outbreaks of WPV3.(72) Similarly, the shift to the nearly exclusive use of mOPV1 and mOPV3 in SIAs instead of tOPV between 2006–9 in Nigeria, which did not achieve high levels of routine tOPV coverage, increased susceptibility to type 2 and led to the largest cVDPV type 2 outbreak to date.(68) We suggest that understanding population immunity emerges as the key to risk management and outbreak prevention and to the identification and implementation of optimal programmatic and immunization policies. Practical considerations, such as costs, supply constraints, combination vaccines, and harmonization with other childhood vaccines all remain important factors that influence national decisions,(1) but we believe that shifting to a prevention approach that relies on population immunity management instead of chasing outbreaks detected by AFP surveillance will help to achieve WPV eradication and successfully stop the use of OPV. We hope that in the future, monitoring the national stock of population immunity for polioviruses will become a tool used to inform national and regional vaccination decisions, and that this might serve as a model for other vaccine-preventable diseases.
Our review and conceptual model for population immunity reveal several important sources of uncertainty for which we suggest the need for additional research.(16) First, serological studies with wide age ranges offer an important opportunity to assess the level of antibodies in members of populations and these data could significantly improve characterization of the speed and nature of waning. They may also provide an additional check on the performance of immunization efforts, because along with independent monitoring, they may provide insights about achieved vaccination coverage. Notably, since population immunity integrates over time, serological studies can also help to identify or document immunity gaps that may exist due to lapses in immunization that may imply risks for older children and adults who missed vaccination opportunities as children and who represent vulnerable and identifiable risk groups. For example, the outbreak that occurred in Congo in 2010 included a relatively large proportion of young adult males who missed immunization as children.(51) Longitudinal and/or repeated seroprevalence and OPV challenge studies could provide critical evidence to better characterize the impact of waning on population immunity to poliovirus transmission.(15, 16) Little understanding of maternal immunity exists, and no studies currently measure levels of maternal immunity in infants born to mothers with only IPV protection (i.e., never exposed to an LPV) in countries that use only IPV for vaccination.
Second, with numerous countries considering a switch from OPV to IPV, ongoing research and development efforts continue to focus on developing more cost-effective IPV options. Although we focused on the concept that countries need to manage their programs effectively with the goal of achieving and maintaining sufficient national levels of population immunity to prevent the transmission of LPV infections, we emphasize that many countries may also want to integrate these considerations with cost-effectiveness modeling to simultaneously consider the economic impact of their many options to reach this objective and this should represent a priority for additional research.
Third, with respect to conducting SIAs, we emphasize that the quality of the SIAs represents an important choice, which we capture explicitly in our population immunity model by requiring the specification of the percent of coverage within the target population. Recent research in Pakistan demonstrated important differences between reported and actual field performance,(73) which suggests the need for additional operational research related to the conduct of SIAs and characterization and use of realistic targets in population immunity models like the one we describe here.
Fourth, real differences will continue to exist between countries, within countries, and in the way individuals interact with others. Consequently, national and regional health leaders will need to understand the implications of heterogeneity with respect to managing their population immunity and risks. In this regard, we expect that countries may need to specifically target preventive vaccination activities for specific high-risk subpopulations to the extent possible to address immunity gaps before outbreaks occur. Reactive vaccination of specific subpopulations after outbreaks occur alone does not offer a long-term strategy for prevention because it does not maintain population immunity at a level that will prevent sustained transmission. However, reaching the unvaccinated represents a significant challenge and can imply relatively large costs.
Fifth, resource mobilization will continue to determine the ability to meet the immunization needs identified by regional and national modeling. The requirement to maintain high population immunity everywhere until global WPV eradication and OPV cessation suggests the need to create the expectation that polio eradication will require substantial resources until complete. In moving to a prevention approach, efforts should focus on creating incentives for prevention and not on rewarding failures. Unfortunately, financial pressures tend to shift resources toward fire fighting (e.g., paying $11 million for outbreak response associated with Tajikistan) instead of prevention (e.g., paying $750,000 for SIAs in Tajikistan), even though the human and economic cost of outbreaks appears to far exceed the costs of avoiding them. We hope that population immunity modeling will play an essential role in helping national, regional, and global health policy makers value the cases and costs saved by prevention, and that this will help to support the achievement and maintenance of global polio eradication as cost-effectively as possible.
Acknowledgment
Drs. Thompson and Duintjer Tebbens thank the Bill and Melinda Gates Foundation for providing a contract to Kid Risk, Inc. to support their work on this paper under Work Order 4533-17492. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Bill and Melinda Gates Foundation or the US Centers for Disease Control and Prevention.
Abbreviations:
- ACIP
Advisory Committee on Immunization Practices
- bOPV
bivalent OPV for types 1 and 3
- cVDPV
circulating vaccine-derived poliovirus (cVDPV1, cVDPV2, and cVDPV3 indicating circulating vaccine-derived poliovirus types 1, 2, and 3, respectively)
- CVID
Common variable immunodeficiency
- EIP
effective immune proportion
- EIP*
threshold level of EIP below which a population can sustain transmission
- ESP
effective susceptible proportion
- ESP*
threshold level of ESP above which a population can sustain transmission
- GPEI
Global Polio Eradication Initiative
- IPV
inactivated poliovirus vaccine (trivalent)
- iVDPV
immunodeficiency-associated vaccine-derived poliovirus
- LPV
live poliovirus (WPV, VDPV, OPV, or OPV-related poliovirus)
- mOPV
monovalent OPV (mOPV1, mOPV2, and mOPV3 indicating monovalent types 1, 2, and 3, respectively)
- OPV
oral poliovirus vaccine (generally the trivalent formulation)
- OPV
related poliovirus – partially reverted OPV virus with one or more attenuating mutations still intact
- PO
proportion of transmissions via oropharyngeal route
- R0
basic reproductive number
- RCFTI
relative contribution to fecal-oral transmission if infected
- RCOTI
relative contribution to oropharyngeal transmission if infected
- SIAs
supplemental immunization activities
- Sr
relative susceptibility
- tOPV
trivalent OPV
- VAPP
vaccine-associated paralytic polio
- VDPV
vaccine-derived poliovirus
- WPV
wild poliovirus (WPV1, WPV2, and WPV3 indicating wild poliovirus types 1, 2, and 3, respectively)
References
- 1.Thompson KM, Pallansch MA, Duintjer Tebbens RJ, Wassilak SG, Kim J-H, Cochi SL. Pre-eradication vaccine policy options for poliovirus infection and disease control. Risk Analysis: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Assembly. Poliomyelitis: Mechanism for management of potential risks to eradication (resolution 61.1). Geneva: World Health Organization; 2008. http://apps.who.int/gb/ebwha/pdf_files/WHA61-REC1/A61_Rec1-part2-en.pdf [Google Scholar]
- 3.Thompson KM, Duintjer Tebbens RJ, Pallansch MA, Kew OM, Sutter RW, Aylward RB, Watkins M, Gary HE Jr., Alexander JP Jr., Jafari H, Cochi SL. The risks, costs, and benefits of possible future global policies for managing polioviruses. American Journal of Public Health 2008;98(7):1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson KM, Duintjer Tebbens RJ. The case for cooperation in managing and maintaining the end of poliomyelitis: Stockpile needs and coordinated OPV cessation. The Medscape Journal of Medicine 2008;10(8):190. [PMC free article] [PubMed] [Google Scholar]
- 5.de Gourville EM, Sangrujee N, Duintjer Tebbens RJ, Pallansch MA, Thompson KM. Global surveillance and the value of information: The case of the global polio laboratory network. Risk Analysis 2006;26(6):1557–1569 [DOI] [PubMed] [Google Scholar]
- 6.Thompson KM, Duintjer Tebbens RJ. Eradication versus control for poliomyelitis: An economic analysis. Lancet 2007;369(9570):1363–1371 [DOI] [PubMed] [Google Scholar]
- 7.Thompson KM, Duintjer Tebbens RJ. Using system dynamics to develop policies that matter: Global management of poliomyelitis and beyond. System Dynamics Review 2008;24(4):433–449 [Google Scholar]
- 8.World Health Organization. Polio vaccines and polio immunization in the pre-eradication era: WHO position paper. Weekly Epidemiological Record 2010;85:213–228 [PubMed] [Google Scholar]
- 9.Bernier RH. Some observations on poliomyelitis lameness surveys. Reviews of Infectious Diseases 1984;6 Suppl 2:S371–S375 [DOI] [PubMed] [Google Scholar]
- 10.Nathanson N, Kew OM. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. American Journal of Epidemiology 2010;172(11):1213–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Sutter RW, Thompson KM. A dynamic model of poliomyelitis outbreaks: Learning from the past to help inform the future. American Journal of Epidemiology 2005;162(4):358–372 [DOI] [PubMed] [Google Scholar]
- 12.Simons E, Mort M, Dabbagh A, Strebel P, Wolfson L. Strategic planning for measles control: Using data to inform optimal vaccination strategies. Journal of Infectious Diseases 2011;204 Suppl 1:S28–S34 [DOI] [PubMed] [Google Scholar]
- 13.Gay NJ. Modeling measles, mumps, and rubella: Implications for the design of vaccination programs. Infection Control and Hospital Epidemiology 1998;19(8):570–573 [DOI] [PubMed] [Google Scholar]
- 14.Bauch CT, Szusz E, Garrison LP. Scheduling of measles vaccination in low-income countries: Projections of a dynamic model. Vaccine 2009;27(31):4090–4098 [DOI] [PubMed] [Google Scholar]
- 15.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Expert review on poliovirus immunity and transmission. Risk Analysis:In Press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, Halsey NA, Hovi T, Minor PD, Modlin JF, Patriarca PA, Sutter RW, Wright PF, Wassilak SGF, Cochi SL, Kim J-H, Thompson KM. Review and assessment of poliovirus immunity and transmission: Synthesis of knowledge gaps and identification of research needs. Risk Analysis: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbas AK, Lichtman AH. Basic immunology: Functions and disorders of the immune system. 2nd ed. Philadelphia, PA: Saunders; 2006. [Google Scholar]
- 18.Modlin JF. Coxsackieviruses, echoviruses, and newer enteroviruses In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, douglas, and bennett’s principles and practice of infectious diseases. Philadelphia, PA: Churchill-Livingstone; 2000. p. 1904–1919. [Google Scholar]
- 19.Ogra PL. Mucosal immune response to poliovirus vaccines in childhood. Reviews of Infectious Diseases 1984;6 Suppl 2:S361–S368 [DOI] [PubMed] [Google Scholar]
- 20.Expanded Programme on Immunization. Report of a WHO informal consultation on polio neutralizing antibody assays, Nashville, 5–6 December 1991. Geneva: World Health Organization; 1991. Report No.: WHO/EPI/RD/91.3 Rev 1. [Google Scholar]
- 21.Sutter RW, Pallansch MA, Sawyer LA, Cochi SL, Hadler SC. Defining surrogate serologic tests with respect to predicting protecive vaccine efficacy: Poliovirus vaccination In: William JC, Goldenthal KL, Burns DL, Lewis BPJ, editors. Combined vaccines and simultaneous administration. Current issues and perspectives. New York: New York Academy of Sciences; 1995. p. 289–299. [DOI] [PubMed] [Google Scholar]
- 22.Halsey N, Galazka A. The efficacy of DPT and oral poliomyelitis immunization schedules initiated from birth to 12 weeks of age. Bulletin of the World Health Organization 1985;63(6):1151–69 [PMC free article] [PubMed] [Google Scholar]
- 23.Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine-live In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Fifth ed: Saunders Elsevier; 2008. p. 631–686. [Google Scholar]
- 24.Pujol JM, Eisenberg JE, Haas CN, Koopman JS. The effect of ongoing exposure dynamics in dose response relationships. PLoS Computational Biology 2009;5(6):e1000399.doi: 10.1371/journal.pcbi.1000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henry JL, Jaikaran ES, Mason PJ, Barnes JM, Beale AJ. A study of polio vaccination in infancy: Excretion following challenge with live virus by children given killed or living poliovaccine. Journal of Hygiene Cambridge 1966;64:105–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onorato IM, Modlin JF, McBean MA, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. Journal of Infectious Diseases 1991;163:1–6 [DOI] [PubMed] [Google Scholar]
- 27.Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA, Sutter RW. Vaccine policy changes and epidemiology of poliomyelitis in the United States. Journal of the American Medical Association 2004;292(14):1696–1701 [DOI] [PubMed] [Google Scholar]
- 28.Strebel PM, Sutter RW, Cochi SL, Biellik RJ, Brink EW, Kew OM, Pallansch MA, Orenstein WA, Hinman AR. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clinical Infectious Diseases 1992;14:568–79 [DOI] [PubMed] [Google Scholar]
- 29.Chen RT, Hausinger S, Dajani AS, Hanfling M, Baughman AL, Pallansch MA, Patriarca PA. Seroprevalence of antibody against poliovirus in inner-city preschool children. Journal of the American Medical Association 1996;275(21):1639–1645 [PubMed] [Google Scholar]
- 30.Más Lago P, Bravo JR, Andrus JK, Comellas MM, Galindo MA, de Quadros CA, Bell E. Lesson from Cuba: Mass campaign administration of trivalent oral poliovirus vaccine and seroprevalence of poliovirus neutralizing antibodies. Bulletin of the World Health Organization 1994;72(2):221–5 [PMC free article] [PubMed] [Google Scholar]
- 31.WHO Collaborative Study Group on Oral and Inactivated Poliovirus Vaccines. Combined immunization of infants with oral and inactivated poliovirus vaccines: Results of a randomized trial in the Gambia, Oman, and Thailand. WHO collaborative study group on oral and inactivated poliovirus vaccines. Bulletin of the World Health Organization 1996;74(3):253–268 [PMC free article] [PubMed] [Google Scholar]
- 32.Benyesh-Melnick M, Melnick JL, Rawls WE, Wimberly I, Barrera Ora J, Ben-Porath E, Rennick V. Studies of the immunogenicity, communicability and genetic stability of oral poliovaccine administered during the winter. American Journal of Epidemiology 1967;86(1):112–136 [DOI] [PubMed] [Google Scholar]
- 33.Gelfand HM, Potash L, Leblanc DR, Fox JP. Intrafamilial and interfamilial spread of living vaccine strains of polioviruses. Journal of the American Medical Association 1959;170(17):2039–2048 [DOI] [PubMed] [Google Scholar]
- 34.Kew OM, Sutter RW, de Gourville E, Dowdle WR, Pallansch MA. Vaccine-derived polioviruses and the endgame strategy for global polio eradication. Annual Review of Microbiology 2005;59:587–635 [DOI] [PubMed] [Google Scholar]
- 35.Plotkin SA, Vidor E. Poliovirus vaccine -- inactivated In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. Fifth ed: Saunders Elsevier; 2008. p. 605–630. [Google Scholar]
- 36.Fiore L, Plebani A, Buttinelli G, Fiore S, Donati V, Marturano J, Soresina A, Martire B, Azzari C, Nigro G, Cardinale F, Trizzino A, Pignata C, Alvisi P, Anastasio E, Bossi G, Ugazio AG. Search for poliovirus long-term excretors among patients affected by agammaglobulinemia. Clinical Immunology 2004;111(1):98–102 [DOI] [PubMed] [Google Scholar]
- 37.Halsey NA, Pinto J, Espinosa-Rosales F, Faure-Fontenla M, da Silva EE, Kahn AA, ADB W, Minor PD, Dunn G, Asturia E, Hussain H, Pallansch MA, Kew OM, Winkelstein J, Sutter RW, the Polio Project Team. Search for polio virus carriers among people with primary immune deficiency diseases in the United States, Mexico, Brazil and the United Kingdom. Bulletin of the World Health Organization 2004;82(1):3–8 [PMC free article] [PubMed] [Google Scholar]
- 38.Khetsuriani N, Prevots DR, Quick L, Elder ME, Pallansch MA, Kew OM, Sutter RW. Persistence of vaccine-derived polioviruses among immunodeficient persons with vaccine-associated paralytic poliomyelitis. Journal of Infectious Diseases 2003;188:1845–1852 [DOI] [PubMed] [Google Scholar]
- 39.National Research Council. Exploring the role of antiviral drugs in the eradication of polio: Workshop report. Washington, D.C.: National Academy Press; 2006. [Google Scholar]
- 40.Hennessey KA, Lago H, Diomande F, Akoua-Koffi C, Cáceres VM, Pallansch MA, Kew OM, Nolan M, Zuber PL. Poliovirus vaccine shedding among persons with HIV in Abidjan, Cote d’Ivoire. Journal of Infectious Diseases 2005;192(12):2124–2128 [DOI] [PubMed] [Google Scholar]
- 41.The Cuba IPV Study Collaborative Group. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba. New England Journal of Medicine 2007;356(15):1536–1544 [DOI] [PubMed] [Google Scholar]
- 42.Melnick JL. Poliovirus and other enteroviruses In: Evans AS, Kaslow RA, editors. Viral infections of humans: Epidemiology and control. 4th ed. New York: Plenum Medical; 1997. p. 583–663. [Google Scholar]
- 43.Glezen WP, Lamb GA, Belden EA, Chin TD. Quantitative relationship of preexisting homotypic antibodies to the excretion of attenuated poliovirus type 1. American Journal of Epidemiology 1966;83(2):224–237 [DOI] [PubMed] [Google Scholar]
- 44.Fine PEM. Herd immunity: History, theory, practice. Epidemiologic Reviews 1993;15(2):265–302 [DOI] [PubMed] [Google Scholar]
- 45.Thompson KM, Wallace GS, Duintjer Tebbens RJ, Smith PH, Barskey AE, Pallansch MA, Gallagher KM, Alexander JP, Armstrong GL, Cochi SL, Wassilak SG. Trends in the risk of U.S. Polio outbreaks and poliovirus vaccine availability for response. Public Health Reports 2012;127(1):23–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grassly NC, Wenger J, Durrani S, Bahl S, Deshpande JM, Sutter RW, Heymann DL, Aylward RB. Protective efficacy of a monovalent oral type 1 poliovirus vaccine: A case-control study. Lancet 2007;369(9570):1356–1362 [DOI] [PubMed] [Google Scholar]
- 47.Jenkins HE, Aylward RB, Gasasira A, Donnelly CA, Abanida EA, Koleosho-Adelekan T, Grassly NC. Effectiveness of immunization against paralytic poliomyelitis in Nigeria. New England Journal of Medicine 2008;359(16):1666–1674 [DOI] [PubMed] [Google Scholar]
- 48.O’Reilly KM, Chauvin C, Aylward RB, Maher C, Okiror S, Wolff C, Nshmirimana D, Donnelly CA, Grassly NC. A statistical model of the international spread of wild poliovirus in Africa used to predict and prevent outbreaks. PLoS Medicine 2011;8(10):e1001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prevots DR, Ciofi degli Atti ML, Sallabanda A, Diamanti E, Aylward RB, Kakariqqi E, Fiore L, Ylli A, van der Avoort HG, Sutter RW, Tozzi AE, Panei P, Schinaia N, Genovese D, Oblapenko G, Greco D, Wassilak SG. Outbreak of paralytic poliomyelitis in Albania, 1996: High attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clinical Infectious Diseases 1998;26(2):419–25 [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. Outbreak news. Poliomyelitis, Namibia: Weekly Epidemiological Record; 2006;81(24):238. [PubMed] [Google Scholar]
- 51.World Health Organization. Outbreak news - outbreak of poliomyelitis, republic of the congo, September 2010-February 2011. Weekly Epidemiological Record 2011;86(15):141–14221476332 [Google Scholar]
- 52.Thompson KM, Duintjer Tebbens RJ. Current polio global eradication and control policy options: Perspectives from modeling and prerequisites for OPV cessation. Expert Reviews of Vaccines 2012;11(4):449–459 [DOI] [PubMed] [Google Scholar]
- 53.Duintjer Tebbens RJ, Pallansch MA, Kalkowska DA, Wassilak SG, Cochi SL, Thompson KM. Characterizing poliovirus transmission and evolution: Insights from modeling experiences with wild and vaccine-related polioviruses. Risk Analysis 2012: In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson RM, May RM. Infectious diseases of humans: Dynamics and control. New York: Oxford University Press; 1991. [Google Scholar]
- 55.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: Review. Reviews of Infectious Diseases 1991;13:926–39 [DOI] [PubMed] [Google Scholar]
- 56.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: Implications for the global poliomyelitis eradication initiative. American Journal of Epidemiology 1999;150(10):1001–1021 [DOI] [PubMed] [Google Scholar]
- 57.Fine PEM, Carneiro IAM. Transmissibility and persistence of oral poliovirus vaccine viruses: Implications for the global poliomyelitis eradication initiative. London: Infectious Disease Epidemiology Unit, Department of Infectious and Tropical Diseases, London School of Hygiene; 1998. May. [Google Scholar]
- 58.Fine PEM, Clarkson JA. Individual versus public priorities in the determination of optimal vaccination policies. American Journal of Epidemiology 1986;124(6):1012–1020 [DOI] [PubMed] [Google Scholar]
- 59.Thompson KM, Duintjer Tebbens RJ. Economic evaluation of the benefits and costs of disease elimination and eradication initiatives In: Cochi SL, Dowdle WR, editors. Disease eradication in the 21st century: Implications for global health. Cambridge, MA: MIT Press; 2011. p. 115–130. [Google Scholar]
- 60.Thompson KM, Duintjer Tebbens RJ. Retrospective cost-effectiveness analyses for polio vaccination in the United States. Risk Analysis 2006;26(6):1423–1440 [DOI] [PubMed] [Google Scholar]
- 61.Duintjer Tebbens RJ, Thompson KM. Priority shifting and the dynamics of managing eradicable infectious disease. Management Science 2009;55(4):650–663 [Google Scholar]
- 62.World Health Organization. Global Polio Eradication Initiative -- wild poliovirus list 2000–2010.2010: http://www.polioeradication.org/Dataandmonitoring/Poliothisweek/Wildpolioviruslist.aspx, accessed November 12 2010
- 63.MacDonald N, Hébert PC. Polio outbreak in Tajikistan is cause for alarm. Canadian Medical Association Journal 2010;182(10):1013DOI: 10.1503/cmaj.100831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization Regional Office for Europe. WHO epidemiological brief: Tajikistan polio outbreak and regional response 2010: http://www.euro.who.int/__data/assets/pdf_file/0019/118342/EPI_TJK_Issue3.pdf, accessed July 27 2010
- 65.Moscrop A Yemen embarks on its seventh round of polio immunisations. British Medical Journal 2005;331(7529):1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Estivariz CF, Watkins M, Handoko D, Rusipah R, Deshpande J, Rana BJ, Irawan E, Widhiastuti D, Pallansch MA, Thapa A, Imari S. A large vaccine-derived poliovirus outbreak on Madura island--indonesia, 2005. Journal of Infectious Diseases 2008;197(3):347–54. 10.1086/525049 [DOI] [PubMed] [Google Scholar]
- 67.Kew O, Morris-Glasgow V, Landaverde M, Burns C, Shaw J, Garib Z, Andre J, Blackman E, Freeman CJ, Jorba J, Sutter R, Tambini G, Venczel L, Pedreira C, Laender F, Shimizu H, Yoneyama T, Miyamura T, van Der Avoort H, Oberste MS, Kilpatrick D, Cochi S, Pallansch M, de Quadros C. Outbreak of poliomyelitis in Hispaniola associated with circulating type 1 vaccine-derived poliovirus. Science 2002;296(5566):356–359 [DOI] [PubMed] [Google Scholar]
- 68.Jenkins HE, Aylward RB, Gasasira A, Donnelly CA, Mwanza M, Corander J, Garnier S, Chauvin C, Abanida E, Pate MA, Adu F, Baba M, Grassly NC. Implications of a circulating vaccine-derived poliovirus in Nigeria. New England Journal of Medicine 2010;326(25):2360–2369 [DOI] [PubMed] [Google Scholar]
- 69.Aylward RB, Sutter RW, Heymann DL. OPV cessation--the final step to a “Polio-free” World. Science 2005;310(5748):625–626 [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization. Polio eradication initiative. Cessation of routine oral polio vaccine (OPV) use after global polio eradication Framework for national policy makers in OPV-using countries. Geneva, Switzerland; 2005. Report No.: WHO/POL/05.02. [Google Scholar]
- 71.Duintjer Tebbens RJ, Pallansch MA, Kew OM, Cáceres VM, Jafari H, Cochi SL, Aylward RB, Thompson KM. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Analysis 2006;26(6):1471–1505 [DOI] [PubMed] [Google Scholar]
- 72.Grassly NC, Jafari H, Bahl S, Durrani S, Wenger J, Sutter RW, Aylward RB. Asymptomatic wild-type poliovirus infection among children with previous oral poliovirus vaccination. Journal of Infectious Diseases 2010;10:1535–1543 [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication --- Afghanistan and Pakistan, 2008. Morbidity and Mortality Weekly Report 2009;58(8):198–201 [PubMed] [Google Scholar]
- 74.World Bank. Country classification.2009: http://go.worldbank.org/K2CKM78CC0, accessed June 13 2009
- 75.UN Population Division. World population prospects population database: The 2008 revision population database.2010: http://esa.un.org/unpp/,accessed January 4 2010


