Abstract
Human immunodeficiency virus type 1 (HIV) infection persists despite years of antiretroviral therapy (ART). To remove the stigma and burden of chronic infection, approaches to eradicate or cure HIV infection are desired. Attempts to augment ART with therapies that reverse viral latency, paired with immunotherapies to clear infection have advanced into the clinic, but the field is still in its infancy. We review foundational studies and highlight new insights in HIV cure research. Together with advances in ART delivery and HIV prevention strategies, future therapies that clear HIV infection may relieve society of the affliction of the HIV pandemic.
eTOC blurb Margolis
Margolis and colleagues review progress and prospects toward developing a true cure for HIV-1 infection, focusing on the biological underpinnings of the “kick and kill” approach to curing HIV-1 infection, which couples reactivation of latent, integrated provirus with immunotherapies to recognize and kill infected cells.
Human immunodeficiency virus type 1 (HIV), has led to nearly 50 million deaths and inflicted suffering across the globe. As this pandemic emerged, a remarkable response of clinicians, researchers, social activists, the pharmaceutical industry, and public authorities resulted in the development and implementation of potent antiretroviral therapy (ART), able to arrest disease, restore health, and reduce the spread of new infection. Developments in ART continue, with long-acting antivirals and engineered antibodies in advanced clinical trials that offer the promise of replacing daily pills for both treatment and prevention with only a few treatments per year (Gulick and Flexner, 2019). Sequential prime and boost vaccinations might accelerate the evolution of broadly neutralizing antibodies (bnAbs) that could reduce the incidence of new infection across the globe (Eisinger and Fauci, 2018), although recent efforts to replicate the success of RV144 (Kim et al., 2015) have recently failed with the early closure of HVTN 702.
Should these potential advances be effectively implemented across the globe, the impact of the HIV pandemic would be greatly reduced. However, millions will still be burdened by decades of chronic medical therapy and the stigma of HIV-1 infection, with the attendant burden on health systems worldwide. Therapy that could yield a cure, or short of viral eradication allow durable and stringent immunological control without the need for medication (“functional cure”), would provide a transformative tool for the millions living with HIV. The major barrier to HIV cure is a population of infected, long-lived cells containing persistent and latent viral genomes that cannot be detected or eliminated by host defenses. Previous decades of study uncovered several molecular mechanisms that establish and enforce post-integration latency of this retrovirus (last reviewed in this journal in 2013 (Ruelas and Greene, 2013). Ten years ago, a funding initiative was put forth by the National Institutes of Health entitled “Martin Delaney Collaboratory: Towards an HIV-1 Cure” which sought to bring together teams of researchers to focus on the daunting, multidisciplinary task of a developing an HIV cure. Since then numerous parallel efforts have been initiated across the world. The past decade of research has resulted in deeper understanding of the molecular and cellular mechanisms of HIV latency, novel assays developed to improve our ability to measure the latent reservoir, and studies in animal models of HIV latency. While other efforts have sought to develop cellular or gene therapies to control or clear infection, strategies to permanently silence viral genomes or induce apoptotic death in infected cells, or to induce a viral remission in the absence of viral eradication, this overview will more narrowly focus on efforts towards targeting and eliminating the persistent reservoirs of HIV infection, to develop curative therapy. Although pilot human trials seeking to reverse HIV latency and deplete the reservoir of persistent infection have begun, there is still more to learn and much to be done.
The Current State of HIV Cure Research
Among the countless infection events that occur within an untreated HIV-infected individual, a select few result in the integration of a fully intact, functional provirus that establishes stable infection with negligible viral gene expression. Most viral genomes that can be measured are defective due to errors in viral reverse transcription that result in small deletions or mutations, or through large deletions caused by the effects of the host APOBEC3 proteins. The rare surviving intact viral genomes persist within cellular reservoirs. By definition these latent proviruses can revert to the productive state in vivo, but during latency are unaffected by ART and unable to be detected by the host immune response. This state of proviral latency has been quantitated in peripheral blood and lymphoid tissues of HIV-infected subjects, and an array of molecular mechanisms that allow the establishment and maintenance of persistent, latent HIV infection have been described [Fig 1; (Ruelas and Greene, 2013) (Mbonye and Karn, 2014)]. Evidence that cellular factors are required to maintain quiescence implies that proviral latency is an unstable state of HIV infection, amenable to therapeutic attack.
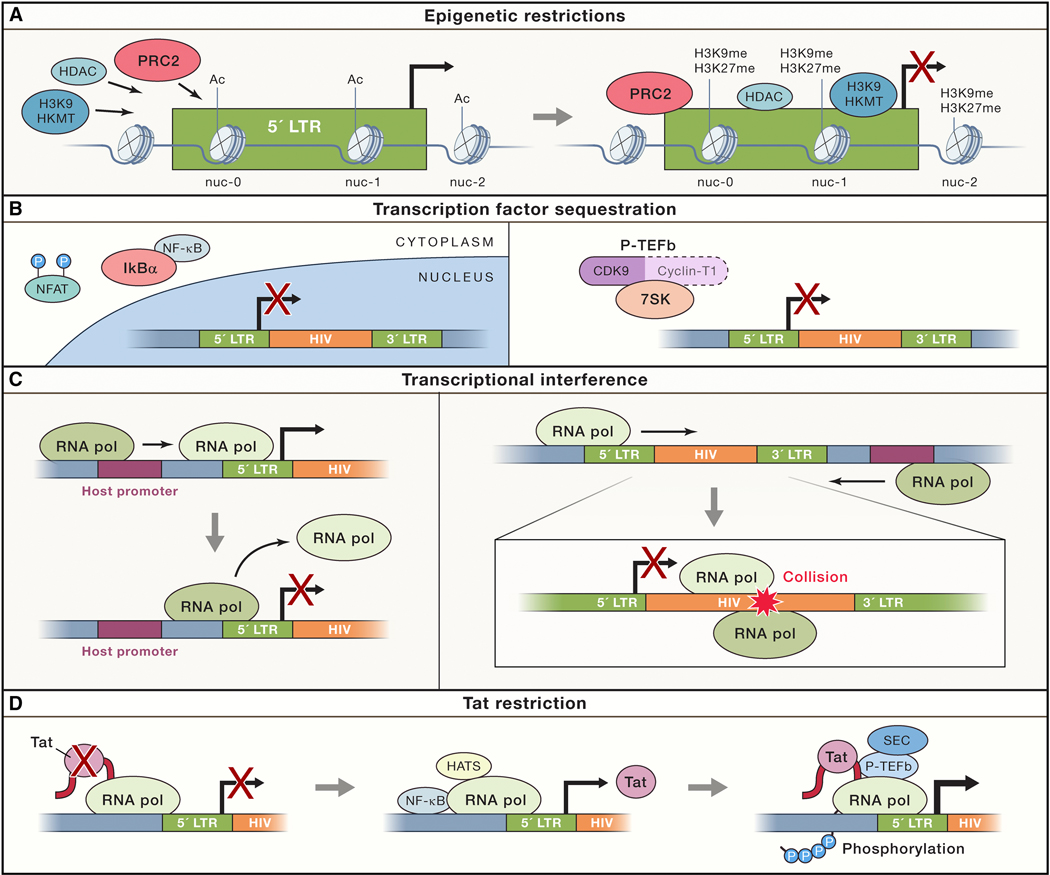
Figure 1.
Proviral silencing and latency is founded and enforced via multiple restrictions to expression. (A) Epigenetic modifiers, such as histone deacetylases (HDACs) and histone lysine methyltransferases (HKMTs), are recruited to HIV-1 LTR promoter, notably by the PRC2 complex. This results in histone modifications within chromatin at the HIV promoter that limit the ability of RNA polymerase to initiate transcription. (B) Sequestration of essential transcription factors like NFAT and NF-kB, and the pTEF-b cyclin complex, are sequestered in resting CD4+ T cells by cellular regulatory complexes (IkB and HEXIM/7skRNA, respectively). (C) Transcriptional interference can occur by promoter occlusion, when a host gene polymerase positioned upstream of the provirus reads through the HIV-1 LTR, causing displacement of necessary transcription factors. Alternatively convergent transcription abort viral expression when the proviral and the host gene RNA Pol II complexes are in opposite orientation, and collide. (D) In the absence of sufficient viral Tat transactivator, viral transcripts are paused. The switch to processive elongation requires the kinase activity of P-TEFb along with the recruitment of processivity factors that constitute the superelongation complex (SEC). Tat efficiently transactivates HIV transcription by recruiting P-TEFb and the SEC to the paused RNAP II complex at the TAR hairpin.
The most studied strategy to eliminate the persistent reservoir of infection seeks to pair therapies that induce the expression of latent HIV with continued ART to prevent infection of new cells, while augmenting the clearance of infected cells, now identifiable due to their production of viral antigen. HIV latency reversal agents (LRAs) have been developed to accomplish the first step of this process: induce the expression of virus within reservoirs. As viral latency is driven by host cellular programming, LRAs must be targeted to host processes, analogous to many agents designed for use in oncology. While LRAs tested to date are capable of inducing expression of RNA or viral protein from latent viral genomes in vitro and in vivo, none have yet convincingly depleted the viral reservoir or extended virologic remission after treatment interruption (Rasmussen and Søgaard, 2018). LRAs must be carefully selected to avoid unacceptable off-target toxicities. As such, identification of new approaches that potently and selectively induce HIV expression would represent a significant advance in progress toward an HIV cure. Ideally, LRAs might also potentiate clearance mechanisms to facilitate reduction of the reservoir, but at a minimum, they should not impede viral clearance (Clutton and Jones, 2018).
Emblematic of the early state of the field, few studies have paired LRAs with a viral clearance strategy [Table 1]. In the publication of one pilot study (Fidler et al., 2020), and the reports of two others employing broadly similar approaches (C. Gay, 2019) (Beatriz Mothe, 2017), depletion of latent, persistent infection was not seen. While latency reversal activity appears measurable as an increase of HIV RNA expression within circulating lymphocytes, it is unclear if this response will translate to sufficiently robust and durable HIV-1 protein expression to enable immune clearance of infected cells. Further, immune responses induced by the immunotherapies tested to date may have been insufficient to identify and clear such challenging targets. New tools and approaches are needed, and recent insights into the biology of HIV latency may offer a path towards more effective viral clearance strategies.
Table 1.
Research Toward a Cure Trials of January 30, 2020. Source: Treatment action group, https://www.treatmentactiongroup.org/cure/trials/
| Combination LRA and Immunotherapy Trials | Trial Registry Identifier(s) | Sponsor(s) | Phase | Estimated End Date |
|---|---|---|---|---|
| maraviroc, dolutegravir, dendritic cell vaccine, auranofin, nicotinamide | NCT02961829 (closed) | Federal University of São Paulo | Not listed | March 2020 |
| ROADMAP: romidepsin + 3BNC117 | NCT02850016 (closed to enrollment) | Rockefeller University | Phase IIa | December 2019 |
| TITAN: lefitolimod + 3BNC117 + 10–1074 (TLR9 agonist + broadly neutralizing antibodies) | NCT03837756 | University of Aarhus | Phase IIa | February 2021 |
| eCLEAR: romidepsin + 3BNC117 | NCT03041012 | Aarhus University Hospital | Phase II | April 2021 |
| Research In Viral Eradication of HIV Reservoirs (RIVER): ART, ChAdV63.HIVconsv & MVA.HIVconsv vaccines, vorinostat | NCT02336074 UK CPMS18010 (closed) | Imperial College London | Phase II | November 2022 |
| iHIVARNA, MVA vector HIV vaccine, 10–1074, romidepsin, HIVACAR01 (personalized HIV RNA vaccine) | NCT03619278 (not yet open) | David Garcia Cinca | Phase I/IIa | July 2020 |
| N-803 (recombinant human super agonist interleukin- 15 complex), VRC07–523LS, PGT121, haploidentical NK cells (haNK) | NCT04144335 (not yet open for enrollment) | University of Minnesota | Phase Ib | December 2020 |
| Chidamide + CAR-T or TCR-T cell therapy | NCT03980691 | Guangzhou 8th People’s Hospital | Phase I | December 2021 |
| VRC07–523LS + vorinostat | NCT03803605 | University of North Carolina, Chapel Hill | Phase I | July 2022 |
| vorinostat + HXTC: HIV 1 antigen expanded specific T cell therapy | NCT03212989 | University of North Carolina, Chapel Hill | Phase I | June 2021 |
| Vorinostat +/− tamoxifen in postmenopausal women | NCT03382834 (closed) | NIAID | Phase I | June 2023 |
| Vacc-4x + romidepsin | NCT02092116 | Bionor Immuno AS/Celgene | Phase I/II | 2019 |
| AGS-004 + vorinostat | NCT02707900 | University of North Carolina, Chapel Hill | Phase I | 2019 |
| MVA.HIVconsv + romidepsin | NCT02616874 | IrsiCaixa | Phase I | 2017 |
Multiple Mechanisms May Lead to Proviral Latency and Persistent HIV Infection
Initially, HIV infection was only understood to have a clinically latent phase prior to the appearance of AIDS. The development of RNA PCR then enabled the recognition that viremia persisted throughout HIV infection, but the state of post-integration viral latency was not clearly recognized until 1995 (Chun et al., 1995). Persistent and latent infection was thought to be established when productively infected, activated CD4+ T cells returned to the resting G0 state, where minimal transcription of viral genes was expected. Consistent with this notion, in resting CD4+ T-cells, various host transcription factors critical to driving HIV transcription including NF-kB, NFAT, and P-TEFb are sequestered, present at low levels, and/or lacking post translational modifications necessary for full activation. However, proviral latency has been shown to be driven by more than simply a deficiency of transcription factors in the environment of a resting cell.
Epigenetic controls:
Shortly after the histone code hypothesis was put forward (Jenuwein and Allis, 2001) repressive epigenetic modifications at the chromatin of the HIV promoter (long terminal repeat, LTR) was appreciated to play a key role in establishing and enforcing HIV latency. At the level of chromatin, histone acetylation is largely absent due to recruitment of histone deacetylases (HDACs) to the LTR (Turner and Margolis, 2017).
The proviral promoter is bound by three nucleosomes - Nuc-0, Nuc-1 and Nuc-2 - which flank two DNase hypersensitivity regions. Extensive work in cell model systems have shown that HIV latency can be enforced by marking of the nucleosomes at the LTR with either of the major repressive posttranslational modifications: methylation at histone 3 lysine 27 (H3K27me) or at histone 3 lysine 9 (H3K9me), or by the recently identified mono-methyl mark of histone 4 lysine 20 (reviewed in (Turner and Margolis, 2017)). Whether these histone methyl marks can co-exist on the same nucleosomes in vivo, or to what extent chromatin restriction is dependent on or affected by the viral integration site is currently unknown. However, interestingly recent work suggests both H3K27me and H3K9me, and the epigenetic factors which write and read these marks, are critical for the establishment and maintenance of HIV latency in primary cells (Nguyen et al., 2017). The removal of repressive histone methylation is far less understood; however, a role for the H3K9 demethylase LSD1 and the H3K27 demethylase UTX have been suggested (Boehm and Ott, 2017).
While the switch between activating histone acetylation and repressive deacetylation has been understood as a key epigenetic driver in HIV latency, less is known about other histone marks. A histone modification more recently described, histone crotonylation, is a chromatin mark recently linked to de-repression of HIV transcription (Jiang et al., 2018). While work on histone crotonylation is an emerging area of research, this could suggest that the use of crotonyl-CoA represents an alternative pathway to proviral activation when acetyl-CoA is limiting, and efficient histone acetylation can otherwise not be achieved.
Other chromatin remodelers have been linked to HIV latency. BAF (BRG1- or BRM -associated, ATP-dependent factor) can be selectively recruited to the HIV LTR by the short isoform of the BRomo- and extra-terminal Domain protein 4 (Conrad et al., 2017). The presence of the BAF complex can position the critical HIV nucleosome Nuc-1 at a less energetically favorable DNA sequence, aiding to the enforcement of transcriptional repression. Deepening understanding of the epigenetic regulation of HIV expression may yield additional targets for HIV latency reversal therapies.
Other limiting factors for viral transcription:
Although proviruses can be reactivated by epigenetic changes without cellular activation, a limitation of this approach is that epigenetic agents induce expression in minority of proviruses when compared to cellular activation signals. T-cell activation mediates a cascade of T cell receptor (TCR) signaling events that are generally expected to relieve all restrictions to proviral expression. Induction and binding of NF-kB and SP1, as well as other host transcription factors, to the LTR results in sufficient transcriptional initiation and elongation to produce the HIV activator protein Tat. Tat subsequently recognizes and binds a viral RNA structure, TAR (Tat-associated Region), found at the 5ʹ end of viral transcript. The Tat/TAR interaction initiates a positive feedback loop at the LTR via Tat-mediated recruitment of host cell factors P-TEFb, the super elongation complex, and SWI/SNF activating complex PBAF. These factors collaborate to drive sustained productive elongation from the viral promoter. Remodeling of the repressive chromatin environment at the LTR occurs during this process via recruitment of histone acetyltransferases CBP/p300 by NF-kB independent of Tat production, followed by Tat-mediated recruitment of additional acetyltransferases (Mbonye and Karn, 2014).
Perversely, the expectation that robust T cell activation would promptly activate expression of all latent proviral genomes was shattered by the demonstration of non-induced proviruses in 2013 (Ho et al., 2013). In a single round of stimulation in vitro, Ho et al. demonstrated that a minority sub-population of fully replication-competent proviral genomes still failed to be expressed, despite supraphysiologic cellular activation. Ho carefully showed that these non-induced proviruses lacked sequence defects or other identifiable factors (eg. DNA methylation) to explain their lack of response to cellular activation. However, additional rounds of stimulation in vitro did yield the expression of additional proviruses, with diminishing returns over subsequent rounds of induction. This finding highlighted the possible challenges of reactivating 100% of latent proviruses in vivo. The “failure to activate” of a portion of the proviral population was hypothesized to be due to an underlying stochastic behavior of this viral biologic system (Razooky et al., 2015). However, an alternate explanation is simply there are numerous factors contributing to inhibition or required for expression, and multiple rounds of signaling are required to hurdle all these barriers in 100% of a viral population. The practical question, as yet unanswered, is how long this will take. Bradley et al. (Bradley et al., 2018) identified diverse transcriptomic environments that were significantly associated proviral latency, but prominently included a transcriptional signature of naive and central memory T cells. In this model system, viral genomes that were stimulated to leave latency often returned to quiescence when stimulus was withdrawn. A deeper understanding of these cellular programs may lead to new and more effective approaches to reverse latency. Finally, it must be said that nearly all of the observations on mechanisms of HIV latency have bene made in in vitro model systems. It remains to be seen which of these mechanisms is the most relevant in vivo, and which can be safely modulated for therapeutic purposes.
The Latent Reservoir is Unexpectedly Dynamic
While LRAs act ultimately at the level of proviral transcription, they do so in the context of the host cell environment. Therefore, it is also important to understand the cells that play host to persistent HIV. While most HIV target cells exist within tissue, nearly all studies of HIV reservoirs are based on analyses of cells harvested from the peripheral circulation during suppressive ART. These studies reproducibly documented that all HIV-infected people on ART have an inducible viral reservoir that decays very slowly (Crooks et al., 2015; Siliciano et al., 2003), resides principally in resting central memory CD4+ T cells, and is largely comprised of defective DNA (Bruner et al., 2016; Ho et al., 2013). Further, reservoir formation cannot be prevented by even extremely early ART (Henrich et al., 2017; Luzuriaga et al., 2015). While these studies yielded insights into the nature of persistent HIV-1 reservoirs during suppressive ART, until recently little was definitively known about the dynamics of reservoir formation before and after ART, reservoir persistence, and the composition of the pool of cells encoding replication-competent, but quiescent HIV over time.
The most common assumption was the simplest one: starting soon after transmission, virus enters the latent reservoir --- predominantly central memory CD4+ T cells, and remains latent until the cell dies, or until some external stimulus induces viral expression from latency. If viral expression occurs, there is some likelihood that the cell will be cleared due to viral cytopathic or immune clearance effects. These events eliminating infected cells from the reservoir are countered by events increasing the size of the reservoir, namely activated infected cells returning to a resting state and re-entering the reservoir. These events must be in a slightly negative balance, as indicated by slow decline in the reservoir over time on ART uniformly seen in the presence of ART (Crooks et al., 2015; Siliciano et al., 2003).
However, a second major force shaping the population of the persistent, latent proviral reservoir is now known to be clonal proliferation, also termed clonal expansion. Proliferation of cells harboring HIV may be driven by the natural homeostatic proliferation of T cells, by adaptive antigen-induced T cell proliferation, or, less likely, by dysfunctional CD4 cell proliferation triggered by an HIV integration event in a critical genomic site. Whatever the cause(s), cell duplication has been demonstrated as a mechanism of sustaining the long lived, stable reservoir (Maldarelli et al., 2014) (Wagner et al., 2014). Consistent with this, residual viremia is often dominated by clonal sequences, and sequencing of proviral DNA also reveals clonal expansion of identical viral sequences (Tobin et al., 2005) (Bailey et al., 2006). While it appears that clonal expansion favors defective proviruses (Bruner et al., 2019), expansion of replication-competent clones (Simonetti et al., 2016) (Hosmane et al., 2017) (Bui et al., 2017) appears to be sufficient to maintain the longevity of the viral reservoir.
One aspect of this model was recently challenged by several studies showing that HIV does not enter the durable latent reservoir at a constant rate over time prior to the initiation of ART (Abrahams et al., 2019; Brodin et al., 2016). Both studies longitudinally examined HIV-1 RNA in the plasma of people prior to ART (N=10, Brodin et al; N=9, Abrahams, Joseph et al) and compared this pre-ART virion RNA to either viral DNA persisting after at least 2 years of ART (Brodin et al., 2016) or replication-competent virus recovered from rCD4+ T cells isolated from the blood after approximately 5 years of ART (Abrahams et al., 2019). In both studies, the majority of the persistent reservoir observed as either proviruses or inducible viruses, was most closely related to variants isolated from the plasma near the time of ART initiation. Further, based on detailed phylogenetic analyses, Abrahams, Joseph et al were able to show that approximately 80% of the replication competent reservoir appeared to originate from viruses circulating in the plasma in the year prior to ART, although there a minority of vial genomes found in the reservoir that are closely related to viruses circulating much earlier in untreated infection. Most recently, a third cohort demonstrated very similar findings (Pankau et al., 2020).
These results may be explained by a new view of latency, in which ART initiation fundamentally changes the rate at which HIV-infected cells enter latency, and the rate at which proviruses become reactivated and expressed, leaving the reservoir. Prior to ART, infected cells enter and exit at a relatively constant rate, with entry being governed by high rates of infection prior to ART. Exit from the reservoir in the pre-ART state is hypothesized to be driven by immune activation which favors viral expression and/or cell death. Under these conditions prior to ART, the frequency of latent infection (“size of the reservoir”) reaches homeostasis, but the specific viral genomes in the reservoir are continually replaced by viruses entering from the recently circulating pool [Fig. 2].
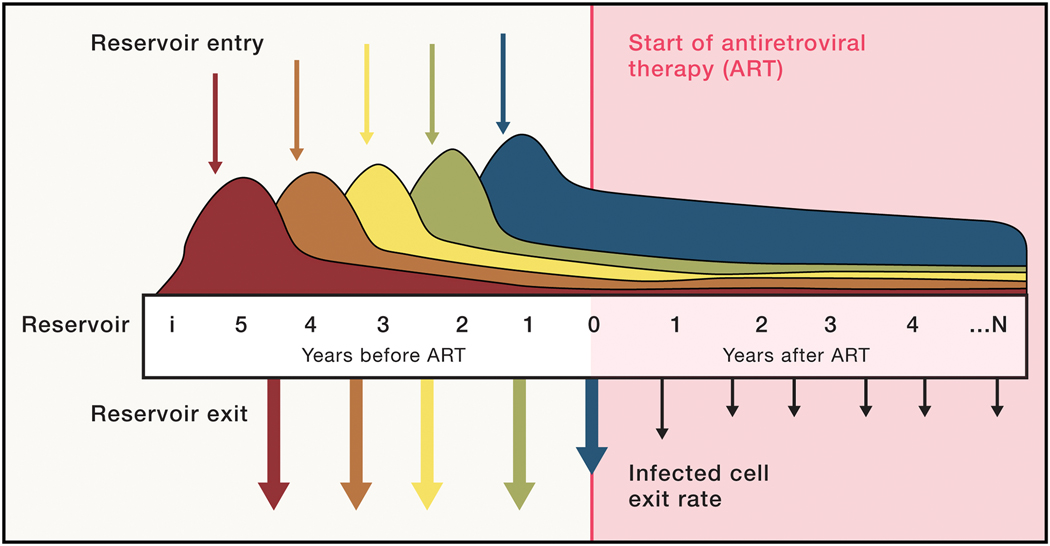
Figure 2.
A model of the dynamic, latent reservoir. Viruses enter the latent, persistent reservoir from the earliest days of infection, but most do not persist due to a short half-life of the host cells or immune activation or both. Early viruses are serially replaced by viruses that circulate later in infection, prior to ART initiation (dotted line). Upon ART initiation, viruses no longer enter the reservoir as replication is blocked. But the exit rate of infected cells is much decreased, perhaps due to the dampening of generalized immune activation and/or increases in CD4 cell half-life. The slower loss of persistently infected cells is thereafter nearly matched by homeostatic proliferation, resulting in the slow decay of latent viruses.
In this model, ART initiation alters this equation by blocking new infections, thereby terminating entry of new viral genomes into the reservoir. However, a new setpoint is reached as exit from the reservoir is slowed by an indirect effect of ART by at least one of several mechanisms: i) enhanced entry of integrated proviruses into latency due to changes in cell signaling milieu and cellular gene expression programs, ii) gradual enforcement of latency by epigenetic marks laid down over time, and iii) the extension of CD4+ T cell half-life (including HIV-infected CD4+ T cells; see Fig. 2) that accompanies viral suppression. The broad effects ART-mediated suppression of viremia on the immune system are likely to play a role in the establishment and behavior of the latent, persistent cellular reservoir. Ablation of viremia may extend the half-life of HIV-infected cells by restoring the ability to form long-lived memory cells (Abrahams et al., 2019; Goonetilleke et al., 2019). Alternatively, or additionally, the general dampening of immune activation and the reduction of circulating viral antigen that is mediated by ART may create an intracellular environment that allows epigenetic silencing of the proviral integrant and is less favorable to proviral expression. Together the reduction of entry and exit may stabilize the reservoir and allow it to form the long-lived reservoir that will persist during prolonged ART. Further, slowing exit from the reservoir skews long-lived reservoir composition towards those viruses that have most recently entered latency from the circulating pool. Nevertheless, it would be expected that a small number of older, earlier proviruses could be found to persist, as reported by Abrahams et al., 2019.
The mechanisms by which ART indirectly recalibrates exit from the reservoir are currently unknown, but when identified may inform strategies for reducing the size of the reservoir. While the specific mechanisms by which ART indirectly extends the half-life of latently infected cells must be confirmed, it may be possible use this new observation to design interventions deployed near the time of ART, and tip the balance away from latency enforcement, allowing the latent reservoir to shrink. For example, blockade of IL-7/IL7R signaling could prevent the transition of CD4+ T cell effectors to the memory cell pool, and their subsequent homeostatic maintenance. Such blockade for a relatively limited period of time (eg. several months) might block enforcement of latency, and thereby the formation of the majority of the long-lived reservoir (Abrahams et al., 2019; Goonetilleke et al., 2019). The development of strategies to counter these effects may require interventions deployed near the time of ART to tip the balance away from latency enforcement, allowing the latent reservoir to shrink or prevent HIV-infected cells from becoming long-lived.
The Diverse Cells of the Latent Reservoir
The first definitive studies that proved the existence of an inducible, replication-competent HIV reservoir were performed in memory CD4+ T cells lacking activation markers (Chun et al., 1997a; Chun et al., 1997b; Finzi et al., 1997; Wong et al., 1997) To date this cell population remains the most well-characterized reservoir of latent HIV. These long-lived cells have an estimated half-life of 3.7 years and represent a stable source of virus capable of reigniting infection in the absence of suppressive therapy. Within the CD4 T cell compartment, central memory T cells (TCM) are been most carefully studied longitudinally as a latent reservoir (Crooks et al., 2015; Siliciano et al., 2003). Other populations of T cells, such as naïve (TN), transitional, memory (TTM), effector memory (TEM), T memory stem cell (TSCM), gamma/delta T cells, and T regulatory T cells (Tregs) are all potential sources of persistent HIV infection (Buzon et al., 2014; Chomont et al., 2009; Soriano-Sarabia et al., 2014; Tran et al., 2008; Zerbato et al., 2019), although the frequency of replication-competent provirus has been less fully quantitated. While the stability of the reservoir in resting TCM cells has been carefully studied, the same cannot be said for individual T cell populations, a matter complicated by the natural differentiation process of T cells into various compartments (e.g central memory to effector memory), and the likelihood that a latent proviral genome may sometimes remain quiescent as an infected cell transitions in to the effector pool, or returns to the memory pool.
Studies aimed at measuring the stability of the latent reservoir in different T cell populations face the added hurdle of reconciling observations made in cells captured at a snapshot in time with the natural fate of that cell. While TEM generally have a shorter half-life compared to TCM (Farber et al., 2014), a higher frequency of integrated HIV DNA and inducible provirus in this compartment in people on ART has been reported (Hiener et al., 2017). TEM cells were also found to produce the highest quantities of HIV p24 capsid antigen following PMA/Ionomycin stimulation (Pardons et al., 2019). Interestingly, differentiating TCM into cells into a TEM phenotypes in vitro promotes more efficient reactivation of HIV by latency reversing agents (Kulpa et al., 2019). TEM also have higher levels of histone acetylation in their basal state (Pardons et al., 2019). However direct evidence that proviral genomes found in TEM cells are either expressed in vivo more often, or to higher levels, or without robust stimulation has not been fully elucidated. One model that fits these observations, but is as yet unproven, is that individual infected TEM cells are not long-lived, but are continuously replaced by persistent and proliferating TCM cells that are differentiating into TEM cells. [Fig 3].
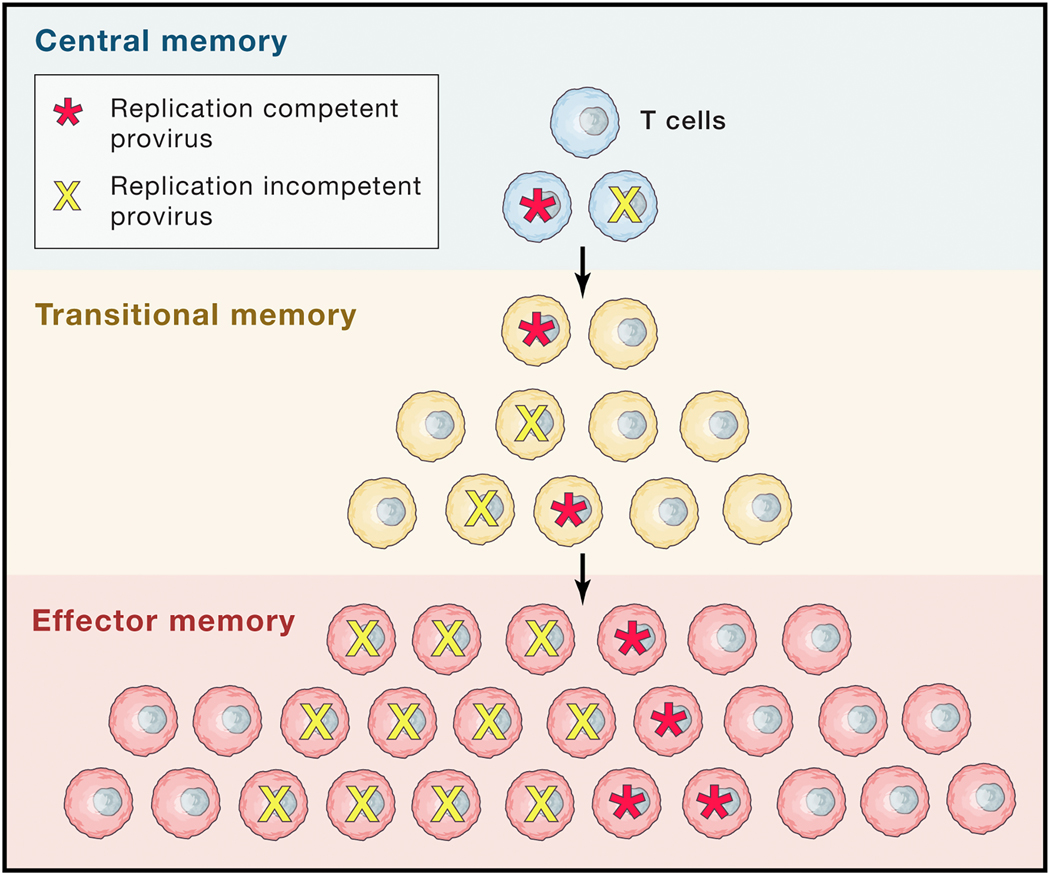
Figure 3.
Persistence and proliferation in latency. Both homeostatic and antigen-driven proliferation appears contribute to the persistence of proviral infection. One model that fits current observations holds that while more differentiated, activated, effector cells may have a shorter lifespan, they may be continually replaced by clonal proliferation of less differentiated memory cell populations.
Most of what is known about the different populations of cells contributing to the HIV reservoir has been determined using peripheral blood. Recently however, studies have been undertaken to better characterize the tissue reservoir [Fig. 4]. Memory CD4 T cells expressing the chemokine receptor CCR6 were reported to be a source of persistent HIV in gut tissues (Anderson et al., 2019; Gosselin et al., 2017) as are non-CD4 T cells (Yukl et al., 2013). Located within the B cell follicle of secondary lymphoid organs, T follicular helper cells (Tfh) are reported to be an HIV reservoir even in the context of anti-retroviral therapy (Banga et al., 2016; Pallikkuth et al., 2015). Given the limited access of immune effector cells into the B cell zone, clearing persistently infected Tfh cell may be a special challenge. Finally, in a recent study consistent with studies in animal models (Abreu et al., 2019; Honeycutt et al., 2017), Ganor et al used penile tissue from ART- treated individuals undergoing elective gender reassignment surgery to demonstrate the presence of replication competent HIV in urethral macrophages. This suggested that cells of the myeloid lineage are a potential source of persistent HIV infection despite ART. As investigators seek to reverse latency and clear persistent infection in the well-defined latent reservoir within TCM, attention must be given to the effects of such interventions on provirus in other populations. It is possible that efforts that yield depletion of the latently infected TCM pool may lead to depletion of depletion of infection in other, more differentiated cell types, or that these other reservoirs require alternate interventions.
Figure 4.
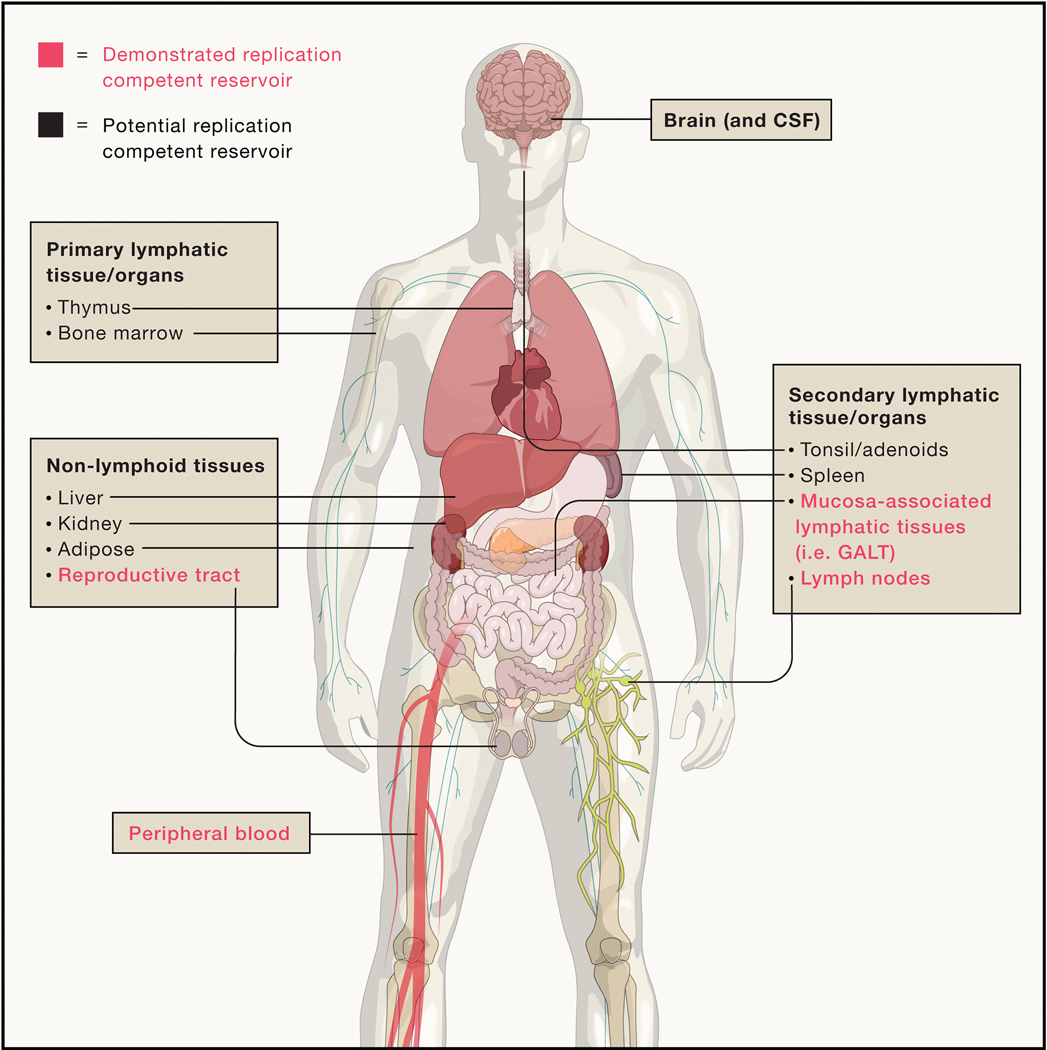
Potential and demonstrated sites of HIV reservoirs. Anatomic sites with demonstrated recovery of replication-competent virus in humans following years of suppressive ART are highlighted in bold. Potential replication-competent anatomic reservoir sites are in regular font. These sites represent tissues/organs where HIV nucleic acid has been detected either in humans or animal models but recovery of rebound-competent virus in humans after years of suppressive ART has not been demonstrated.
Targeting Privileged Sites:
There has long been concern about the ability to eliminate HIV from unique body compartments, especially the CNS and the genital tract (Wong and Yukl, 2016). The biology of the CNS has fueled suspicion that HIV may persist in this compartment during long-term ART and generate viral rebound after treatment interruption, but until recently there was little evidence to support this hypothesis. It has long been known that HIV-infected macrophages can be found in the CNS prior to ART (Joseph and Swanstrom, 2018) and given the putatively long half-life of macrophages and the fact that there is a low density of T cells in the CNS to eliminate HIV-infected cells, it has been hypothesized that HIV-infected macrophages persist in the CNS long after ART is initiated.
Consistent with this hypothesis, multiple studies have identified viral DNA and RNA in the CNS of HIV-infected humans and simian immunodeficiency virus (SIV)-infected macaques after extended ART (Estes et al., 2017; Lamers et al., 2016). Further, HIV continues to replicate in the CNS of a small percentage of otherwise well-suppressed people on ART (Joseph et al., 2019). Recently the persistence of replication competent virus in the CNS was confirmed by viral outgrowth assays, illustrating that SIV can be cultured from macrophages isolated from the CNS of ART-treated pigtailed macaques infected with a highly pathogenic SIV(Avalos et al., 2017), but the analogous studies have not been performed using human brain tissue. Together these findings indicate that replication competent viral reservoirs may persistent in the CNS during ART, but it remains unknown how often this happens, and the diversity of cells types comprising these reservoirs (e.g. macrophages, microglia, astrocytes or CD4+ T cells).
Several lines of evidence demonstrate that the genital tract may also serve as a unique compartment. In parallel to what is known about CNS reservoirs, urethral macrophages isolated from penile tissue after at least 3 years of ART were recently shown to harbor replication competent, inducible HIV-1 proviruses and RNA (Ganor et al., 2019). In addition, HIV RNA can readily be recovered from the genital fluids in men and women in spite of successful suppression of HIV in blood. Detection of viral RNA in the genital tract may be due to viral replication in the presence of non-inhibitory drug concentrations in this compartment, a possibility supported by observed variation in the ability of drugs to accumulate in this compartment and the fact that protease residence mutations can be detected in the semen of some men on protease inhibitor-based regimens with undetectable plasma viral loads (Houzet et al., 2014). Alternatively, and more likely, cells harboring HIV may simply release HIV into the genital tract. Regardless of whether there is viral replication in the genital tract during ART, there is growing evidence that HIV-infected cells can persist in that compartment during ART.
The Evolving Approach to Inducing Latency Reversal
To rigorously demonstrate the existence of latent HIV infection, initial studies in the 1990’s were carried out in carefully purified resting CD4+ T cells. In this cell population reversible silencing of viral expression in the cells under study --- the true definition of latency --- could be clearly demonstrated. As the integrated, latent HIV provirus in T cells is responsive to activation of immune signaling pathways, latency in resting central memory CD4+ T cells was reversed using the T cell receptor agonists phytohemagglutinin (PHA) or anti-CD3 antibodies (Chun et al., 1997b; Finzi et al., 1997) (Wong et al., 1997). These observations led to the first proposals for strategic HIV eradication therapy (Hamer, 2004), and the first clinical trial seeking to clear HIV infection studied the effects of the anti-CD3 antibody OKT3 and interleukin 2 (IL-2) (Prins et al., 1999). However, the strategy of global T cell activation proved untenable, as this first clinical trial resulted in profound T cell activation and depletion, substantial increase in inflammatory cytokine production, accompanied by severe toxicities (van Praag et al., 2001). Attempts to purge the viral reservoir then fell by the wayside for a time, but the emergence of the role of epigenetic control of proviral persistence led to renewed effort to reverse viral latency without global T cell activation.
Epigenetic LRAs:
Seeking to avoid global cellular activation, the next LRAs to emerge targeted the chromatin restrictions that maintain HIV latency. The epigenetic LRAs fall into three groups: 1) histone deacetylase inhibitors (HDACi), 2) histone methyltransferase inhibitors (HMTi), and 3) bromo and extra-terminal domain inhibitors (BETi). Of the three groups, only HDACi are currently in clinical trials for specific evaluation as latency reversal agents. Current HDACi are pan-inhibitors and include, among others, Vorinostat (SAHA), Panobinostat, Belinostat, and Romidepsin, some of which are approved for treatment of T-cell lymphomas (Rasmussen and Sogaard, 2018). HDACi have been shown to induce HIV activation in vitro and induce detectable cell-associated HIV RNA in vivo, however HDACi as single agents have failed to measurably decrease the size of the viral reservoir. This is likely due to the failure to yet engage an effective viral clearance response, but the possibility remains that these agents are ineffective in vivo. It is important to note that these clinical trials have reported minimal safety concerns regarding HDACi in vivo.
Currently, there are no FDA approved HMTi or BETi trials directed at HIV infection, however pre-clinical small molecule HMTi including GSK-343 and EPZ-6482 targeted to EZH2, the HMT responsible for H3K27me, and UNC-0638, an inhibitor targeted to H3K9 HMT G9a, have been demonstrated to reactivate HIV in laboratory models of latent infection (Nguyen et al., 2017; Turner and Margolis, 2017). Similarly, BET inhibitors have also demonstrated similar latency reactivation activity in vitro (reviewed in (Boehm et al., 2013)), but interest in evaluating HMTi and BETi as LRAs will likely depend on tolerability observed in ongoing oncology trials. Should these epigenetic LRAs advance to the clinic, there may be an opportunity for testing in combination with HDACi, other classes of LRAs, and/or immune modulating therapies to identify synergistic relationships that may allow lower and/or less frequent dosing strategies to limit adverse events.
Signal Agonist LRAs:
Given the incomplete success of epigenetic inhibitors in mediating robust and universal latency reversal, efforts have continued to develop safer strategies to reverse latency through the use of cellular signaling pathways. Activation of protein kinase C (PKC) engages many of the signaling pathways induced by natural activation of the TCR. Small molecule PKC agonists (PKCa) such as the phorbol esters and diterpenes have been studied for HIV latency reversal and have been shown to readily induce T cell activation and HIV expression in the laboratory setting across a range of model systems (Jiang and Dandekar, 2015). However, progression of PKCa to preclinical and clinical studies has been limited due to the potential for dangerous side effects of broad and potent PKC activation.
Bryostatin-1 is a potent molecule studied in a large number of clinical trials for several indications including oncology and Alzheimer’s and has been studied for HIV latency reversal. In a humanized mouse model bryostatin-1 and an analog induced HIV expression, but at doses that are only slightly lower than lethal exposures (Marsden et al.). A cautious clinical trial of bryostatin-1 studied very low doses that did not lead to activation of the immune system but also did not induce HIV expression (Gutierrez et al.). Ingenol B and the stabilized derivative GSK’445A were reported to induce SIV expression in ART-suppressed, SIV-infected macaques, accompanied by strong cellular activation, cytokine production, and unacceptable clinical signs such as fever (Gama L, 2015) (Brehm J, 2017) (A. Okoye). Recently, Jiang, et al., found evidence of latency reversal in skin of patients treated with topical ingenol mebutate as part of an actinic keratosis regimen (Jiang et al.), demonstrating that in a setting where PKCa can be administered safely, HIV expression can be induced. Others are currently pursuing ingenols administered as part of a traditional Chinese herbal preparation, kansui tea, in primate studies and clinical trials (NCT02531295). While PKCa are genuine latency reversing agents with in vivo activity, the relatively small window between an efficacious and a toxic exposure makes clinical development unacceptable in an otherwise healthy population of ART-treated people living with HIV infection (PLWH).
In another approach to signal CD4 cells and disrupt HIV latency, similar to the earlier use of IL2, the administration of IL7 has been explored. It was found to have some LRA activity in vitro (Lehrman et al.; Wang et al.), but this effect was overshadowed in vivo by the parallel CD4 cell proliferation induced by the cytokine, thwarting its use in viral eradication strategies (Katlama et al.). More recently research in oncology has brought into clinical testing several engineered forms of IL15, including a heterodimeric form of the molecule (Thaysen-Andersen et al.) and a synthetic receptor superagonist (ALT-803) (Rhode et al.). There is significant evidence that the use of forms of IL15 may confer immunological benefits, perhaps augmenting the antiviral immune response and improving clearance of persistent infection (Garrido et al.; Watson et al.). However, there is less evidence that IL15 can, by itself, directly reverse HIV latency in vivo (Webb et al.). Nevertheless, due to its immunomodulatory properties, further study of this cytokine as part of a latency reversal and viral clearance strategy appears warranted, and selected studies in people with HIV are underway.
Toll-like receptor (TLR) agonists have been studied as an orthogonal approach with the potential for both HIV latency reversal activity, and as immunomodulators capable of augmenting an antiviral response. TLRs are pathogen-recognition receptors that sense molecular motifs conserved across microbial organisms and mediate signaling responses. (Macedo et al.). Both in vitro and in vivo, TLR agonists increase immune activation, induce innate antiviral responses, and can augment pre-existing adaptive ones. However, reminiscent of the experience with IL15, while there is in vitro evidence that selected TLR agonists can reverse viral latency, there has only limited in vivo evidence for LRA activity thus far (Vibholm et al.). However, due to the unique immunodulatory properties of TLR agonists, further study of this approach in combination latency reversal and viral clearance strategies is warranted, and selected studies in people with HIV are underway.
Most recently, efforts to directly trigger cell signaling pathways for the activation of HIV expression without the pleiotropic effects of PKCa have been renewed. Among the transcription factors activated by TCR ligation or PKCa, NF-kB has been described to play a critical role in inducing HIV expression (Williams et al.). Selective activation of NF-kB would thus induce HIV expression, but spare the activation of additional pathways, perhaps minimizing the side effects of a latency reversal regimen in vivo. Pache et al. reported a class of small molecules known as SMAC mimetics or IAP agonists that selectively activate the non-canonical NF-kB signaling pathway, and induced HIV expression in cell line model systems (Pache et al.). Sampey et al. (Sampey et al.) studied a range of IAPi originally discovered to promote tumor cell apoptosis and found that AZD5582 potently induces HIV expression in cell line models and in CD4+ T cells isolated from ART-suppressed HIV-infected study participants. An optimized dosing strategy for application in humanized mice and rhesus macaques was developed and demonstrated that AZD5582 activates the non-canonical NF-kB pathway in vivo at tolerated doses. In ART-suppressed HIV-infected humanized mice and SIV-infected rhesus macaques, AZD5582 robustly induced HIV or SIV expression as evidenced by periods of detectable plasma viremia and increased viral RNA in resting CD4+ T cells isolated from tissues (Nixon et al., 2020). Importantly, induction of both HIV or SIV in each model system occurred in the absence of substantial inflammatory cytokine induction, the absence of robust cellular activation, and was generally well tolerated. These findings demonstrate that IAPi can repeatedly induce HIV expression in regimens that are tolerated in the two leading animal models of HIV persistence. Further animal model studies are underway in combination with clearance agents, as well as progression toward clinical studies.
Augmenting Immune Responses to Clear Persistent Infection
The clearance of residual HIV infection that remains despite effective and prolonged suppression of viral replication by ART is a unique challenge for the immune system and for immunotherapeutics. In the current paradigms of therapies to disrupt latency and clear persistent infection, the targets for clearance are rare populations of cells, induced by LRAs to express HIV proteins in quantities that are likely to be limited. Immunologic “danger signals” to recruit effectors to the site of the pathogen may be absent or limiting. Further, these cell populations may be widely distributed across anatomical compartments, and the HIV-specific immune response may have waned in the absence of recent antigen exposure, and/or may be dysfunctional or depleted.
CD8 T cells:
CD8 T cells play a major role in control of HIV viremia, and early T cell responses exert significant immune pressure on HIV-1, reflected in the emergence of viral escape variants within weeks (Goonetilleke et al., 2009). The rate of virus escape was used to infer the level of CD4 T cell killing in mathematical models, suggesting primary HIV-1 specific CD8 T cells can kill up to 30% of virus-infected cells/day (Goonetilleke et al., 2009). However, in the vast majority of individuals CD8 T cell immunity is insufficient to durably control viremia. In turn, ongoing viremia contributes to the emergence of generalized CD8 T cell dysfunction including of loss of proliferative and cytolytic capacity.
Although ART largely restores CD8 T cell function, CD8 T cells of PLWH exhibit an immune-ageing phenotype (reviewed in (Warren et al., 2019)). HIV-1 specific CD8 T cells remain stably detectable in durably suppressed individuals, albeit at ~10-fold lower levels than untreated infected, is highly stable over time (Xu et al., 2019). While little to no selective evolution is observed in durably suppressed individuals, T cell escape variants generated in untreated infection remain archived in the HIV-1 replication competent reservoir (Deng et al., 2015; Meier et al., 1995), very likely compromising effective CD8 T cell immunity following treatment interruption. An additional hurdle, detailed above, is that latently infected cells are both rare and widely anatomically distributed. CD8 T cells typically migrate in response to chemotactic signals produced by foci of replication. In an ART-treated individual (i.e. no virus replication), the inflammatory signal produced by rare HIV infected cells may be insufficient to attract circulating T cells.
CD8 T cell immunotherapies are being designed to address the challenges of viral eradication. Current therapies can be broadly divided into traditional therapeutic vaccine approaches that seek to boost and/or redirect HIV-1 specific CD8 T cells or chimeric/biologic approaches that harness non-HIV-1 specific CD8 T cells to kill infected cells. Therapeutic vaccines seek to induce or boost T cells that target regions of HIV-1 that are highly conserved. The rationale here, which is based on extensive studies in natural infection including studies of elite controllers, is that virus escape in conserved HIV-1 regions is slower because escape imparts a fitness cost to the virus. Therapeutic HIV vaccines include live-attenuated viral vectors, DNA vaccines and both dendritic cell and adoptive T-cell therapy.
Multiple chimeric/biologic approaches are being tested including chimeric antigen receptors in which HIV TCRs are expressed in memory CD8 T cells subverting their antigen, and wherein CD3 T cells are re-directed to kill infected cells expressing HIV-1 proteins and/or presenting HIV-derived peptides. Harnessing the killing capacity of the nearest CD3+ cell is a very attractive approach to overcome the challenge of the broad distribution of persistently-infected cells. Cytokines such as IL-15 which have also been proposed for study as LRAs may also promote access of CD8 T cells to infected cells in B cell follicles in LN (Bronnimann et al., 2018).
Antibodies for viral clearance:
Humoral responses are broadly divided into two classes of antibodies - those that i) block infection by binding to the envelope of free viruses, called neutralizing antibodies (NAbs), or ii) recognize envelope during virus entry or on the surface of infected cells (non-NAbs). The importance of these antibodies against HIV-1 was demonstrated by passive protection studies in NHP and their ability to exert immune pressure and rapidly select virus escape variants. Protection studies suggested that neutralization and Fc-mediated NAbs effector functions cooperate in providing protection from SHIV infection (Moog et al., 2014). Non-NAbs could also limit the number of transmitted/founder isolates compared to the control (Santra et al., 2015). However, the use of the broad NAbs 3BNC117 and VRC01 for treatment of HIV-1 infection revealed the presence of pre-existing escape mutants (Caskey et al., 2015; Lynch et al., 2015), and such pre-existing resistance might complicate the use of NAbs to eradicate persistent infection.
To overcome these limitations, engineered mAbs-based molecules that could either recognize multiple HIV-1 antigens or simultaneously redirect the cytotoxic cells to kill HIV-1 infected cells have been pursued (Ferrari et al., 2016). Bispecific T cell engagers (BiTEs) or dual affinity re-targeting (DART®) molecules are novel small molecules designed to recognize infected cells and recruit effector cells (Ferrari et al., 2016). These molecules can eliminate cells that have been infected in vitro, as well as reactivated latently infected CD4 cells obtained from ART-treated donors (Pegu et al., 2015; Sung et al., 2015b). DART® are currently being tested for safety (ClinicalTrials.gov NCT03570918) in first-in-human clinical trial.
Innate immune responses:
The first to be mobilized during acute HIV-1 infection, innate responses contribute to the initial control of virus replication (Stacey et al., 2009). Among the first cytokines to reach peak plasma levels are the interferons and interleukin-15 that help activate natural killer (NK) cells (Garrido et al., 2018) and favor Ab engagement to mediate antibody-dependent cellular cytotoxicity (ADCC) (Fisher et al., 2019). NK immunity can be targeted for HIV clearance strategies in several ways. The cytotoxic effects of NK cells can be harnessed using CAR technology similar to that being used for T cells (Zhen et al., 2015). Administration of HIV-specific antibodies targeting membrane-associated proteins will likely harness NK cells to mediate ADCC to kill HIV-1 infected cells (Pollara et al., 2013). Again, IL-15 which activates NK cells to augment killing of HIV-1 infected cells (Garrido et al., 2018), could be administered with the above strategies to both promote NK activity and increase effector cell access to lymph node follicles.
Special Translational Considerations
The Window of Vulnerability:
Although the understanding of viral latency and proviral persistence is still imperfect, clinical studies seeking to combine immunotherapies with latency reversing agents have begun. However, the leap to translational studies is challenged by the lack of validated metrics and assays that can guide the pathway for clinical development. Detailed virologic, immunologic, and pharmacodynamic assessment of interventions must be made in vivo, and such assessments are complicated by the fact that the ultimate efficacy of latency reversal is difficult to quantify without a parallel clearance intervention. Conversely, immunotherapies that might target the latent reservoir cannot be fully tested in the absence of effective latency reversal.
The first challenge is to define the metrics for effective HIV latency reversal. Typically “more is better,” but the most potent current LRAs tested in vitro induce cytokine storm and other clinically untenable toxicities As immunotherapies are likely to be needed to clear persistent infection, it is rational to define effective LRA activity as that which induces the presentation of viral protein or antigen in a latently infected cell at a sufficient quantity and for a sufficient length of time to allow immune-mediated clearance. Measurement of rare or low-level HIV-1 protein or peptide production following LRA exposure is currently challenging, although assays detecting antigen at the single cell level are emerging (Cabrera et al., 2015). A latency clearance assay has been presented (Sung et al., 2015a) seeking to define in an ex vivo assay the “window of vulnerability” that can be achieved by a selected intervention. However, in its current form this assay is demanding, has low throughput, and only semi-quantitative. Better assays for this purpose are needed, to guide selection of LRA regimens that are sufficient to allow clearance but avoid unacceptable toxicities.
Of Mice and Monkeys
In Vivo Models:
Historically, in vivo models have proven to be exceptionally informative in virtually all aspects of HIV research. This has also been the case for HIV cure research. In vivo models provide a complex substrate that may more closely resemble the human condition than in vitro studies, and may provide critical information regarding the safety of implementation in humans. Currently the two models most commonly used to study HIV cure approaches in vivo are NHP and humanized bone marrow/liver/thymus (or BLT) mice.
Non-human primates have been extensively used for HIV cure research. NHP can be infected with several different types of SIV. Infection results in relatively high initial viral loads and the rapid establishment of a long-lasting viral reservoir (Whitney et al., 2018). In addition, the types of experiments that can be conducted in NHP was expanded by the use of HIV/SIV chimeras that permit the evaluation of Ab interventions targeted to HIV (Haynes et al., 2019). Several different modalities of ART have been used to suppress viremia in NHP. Currently, the most commonly used combination includes DTG, TDF and FTC (Nixon et al., 2020). ART is administered daily as a subcutaneous injection that results in a rapid drop of peripheral blood viral RNA to below the level of detection of most commonly used viral load assays (30–60 copies per ml of blood). Viral suppression results in the establishment of latency and therapy interruption results in relatively rapid viral rebound. Virtually all current modalities of HIV cure approaches have been evaluated in vivo using NHP models. These include stem cell transplantation (SCT), immune modulation/vaccines, gene and cell therapy, as well as induction of latent SIV and destruction of infected cells (Borducchi et al., 2018; Del Prete et al., 2019; Hansen et al., 2011; Lim et al., 2018; Peterson et al., 2018). For the most part when similar experiments have also been conducted in humans the outcomes have correlated well between species. Examples of this include the effect of bone marrow transplantation and pre-conditioning, the persistence of transduced cells after transplant, the induction of plasma viremia and cellular activation after latency reversal agent administration.
Rodents are refractory to HIV infection and as such they have very limited utility for HIV cure research. However, the introduction of human cells and/or tissues renders these “humanized” mice susceptible to HIV infection. Mice can be humanized in several different ways including hematopoietic SCT (Garcia, 2016). SCT results in robust levels of human cells in peripheral blood and tissues including human T cells. Importantly, human T cells produced in these models are presumably educated in the mouse thymus and not in the context of human MHC molecules (i.e. HLA). This limitation was elegantly overcome by the development of humanized BLT mice in which mice are implanted with small pieces of human thymic and liver tissue that give rise to a bona fide human thymic organ where T cell precursors can develop into fully functional T cells educated in the context of HLA (Melkus et al., 2006). Because like NHP, humanized BLT mice can be infected by all physiological routes, infection can be efficiently suppressed by ART resulting in the establishment of HIV latency and analytical treatment interruption results in viral rebound (Denton et al., 2008; Denton et al., 2010; Denton et al., 2012; Sun et al., 2007; Wahl et al., 2015; Wahl et al., 2012). This and other humanized mouse models have been extensively used to investigate gene/cell therapy, immune modulation, HIV infected cell killing approaches, HIV silencing approaches and HIV induction by latency reversal agents (Badamchi-Zadeh et al., 2018; Cheng et al., 2017; Dash et al., 2019; Denton et al., 2014; Zhen et al., 2017). It is in this last area of investigation where the synergy and complementarity between the NHP and humanized mouse models have become most evident. Specifically, in two recent manuscripts two different HIV induction approaches were independently evaluated in both models (McBrien et al., 2020; McBrien et al., In Press; Nixon et al., 2020). The results obtained were essentially the same and nicely complemented each other. In the BLT mouse model, it was demonstrated that both latency reversal approaches were effective at inducing systemic production of HIV from resting human CD4+ T cells both in the periphery and tissues. In the NHP model, it was demonstrated that these latency reversal approaches can be serially administered resulting in reproducible induction of SIV in plasma and resting CD4+ T cells from lymph nodes. Both models also provided important information regarding the tolerability of the different LRAs.
It should be made clear that neither NHP nor BLT mice fully recapitulate the human condition. NHP provide a robust platform for the evaluation of in vivo efficacy and toxicity using significantly longer time frames for experimentation and providing larger samples for analysis. BLT mice provide a nimble platform for the rapid evaluation of in vivo efficacy and potential toxicity using human cells and relevant human viruses. However, both models complement each other in ways that result in robust information useful in determining the suitability of novel approaches to an HIV cure. Moving forward, a new generation of BLT humanized mice that incorporate autologous human non-hematopoietic cells are rapidly providing opportunities for the in vivo evaluation of novel approaches to HIV cure that require interventions that are specific for humans (Wahl et al., 2019).
Experimental Medicine Studies
Given the nascent state of HIV cure research, there are limited clinical studies of latency reversal, immunotherapeutic, or combination strategies (Table 1). In addition to the immunological and virological challenges of eradicating HIV, progression into clinical studies has launched discussions on the balance of risk versus benefit at a stage when studies involve little or no benefit to the participant within the context of largely translational research.
Due to concerns of ongoing viral replication despite ART, numerous studies of ART intensification were performed, without evidence of decrease in the HIV reservoir or in HIV expression (Rasmussen and Sogaard, 2018). Although still in dispute by a few investigators, overall studies strongly suggest that ongoing replication does not contribute to maintaining the HIV reservoir in patients suppressed on currently available ART (Bale and Kearney, 2019).
Latency reversal:
Several clinical studies have demonstrated modest induction of viral expression in response to LRAs, with HDACi remaining the most fully characterized. Although HDACi in clinical studies have been well-tolerated and resulted in reproducible increases in cell-associated viral RNA expression, (Archin et al., 2017; Archin et al., 2012; Sogaard et al., 2015), (Winckelmann et al., 2017), (Rasmussen et al., 2014), none have shown to significantly deplete the viral reservoir. The absence of a reduction in the viral reservoir with LRAs may be due to i) only partial or “ineffective” reactivation of the overall pool of latently infected cells, ii) inadequate LRA penetration into tissue compartments harboring latent HIV, or iii) ineffective clearance of reactivated cells. A variety of other biomolecules are under investigation as LRAs as described, but experience in clinical studies remains limited due to safety concerns related to potential off-target effects.
However, the TLR-9 agonist (MGN1703) was well-tolerated in PLWH on suppressive ART as a twice-weekly subcutaneous injection for 24 weeks (Vibholm et al., 2019). Despite observed increases in HIV-1 specific T cell responses and T cell activation with multiple doses of MGN1703, there were no difference in the time to viral rebound in participants who underwent ART interruption with or without continued MGN1703 dosing (Vibholm et al., 2019). N-803, an IL-15 superagonist, resulted in quantifiable low level viremia in 2 of 9 participants on suppressive ART, perhaps due to indirect mechanisms (Davis et al., 2018).
Vaccines:
Therapeutic vaccine have been proposed as a method for improving clearance of HIV-infected cells in PLWH suppressed on ART and the focus has been predominantly on vaccines that induce T cell responses. Although clinical studies of therapeutic HIV vaccines have shown an impact on HIV-specific T-cell immunity, overall response has been disappointing (reviewed in (Robinson, 2018)), and to date therapeutic vaccination has not resulted in sustained viral suppression following ATI (Mothe et al., 2017; Sneller et al., 2017).
Broadly Neutralizing and other Antibodies:
An immunotherapeutic strategy for HIV cure includes the passive transfer of NAbs. Several monoclonal NAbs have been shown to reduce viremia in untreated PLWH (Caskey et al., 2015; Caskey et al., 2017; Lynch et al., 2015). However, a randomized controlled trial of two infusions of the NAb VRC01 versus placebo in PLWH on suppressive ART showed no difference in cell-associated HIV RNA/DNA ratio, and no impact on low level viremia (Riddler et al., 2018). As mentioned above, bNAbs have been shown to delay the time to viral rebound in ART-suppressed participants following ATI (Bar et al., 2016; Scheid et al., 2016), but also to select for resistant viral variants. Monoclonal bNAbs clearly have an antiretroviral effect and a dual combination can maintain suppression without resistance emergence (Mendoza et al., 2018). Two participants in this study sustained suppression even after antibody concentration waned, suggesting an additional mechanism of activity beyond direct inhibition. Whether bNAbs with greater potency and breadth than VRC01 will help clear HIV infected cells is being studied.
Other monoclonal antibodies used for treatment of other conditions have also been explored, including a mAb against α4β7 integrin, which enables homing of T lymphocytes to the gut. Preclinical studies of anti-α4β7 mAb suggested a role in preventing SIV infection and preserving CD4+ T cells in gut associated lymphoid tissue (GALT) and peripheral blood, as well as limiting SIV infection in GALT. However, administration of anti-α4β7 mAb to suppressed PLWH did not impact time to viral rebound during ATI (Sneller et al., 2019).
Immune checkpoint inhibitors:
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy by reversing cancer-related immune dysfunction associated with specific malignancies. HIV-specific T cell exhaustion persists even on effective ART and may be a barrier to HIV cure. In addition, CD4+ T cells expressing immune checkpoint markers are enriched for latent HIV (Banga et al., 2016; Chomont et al., 2009; Fromentin et al., 2016). Therefore, ICIs could provide a strategy for reversing HIV-associated immune dysfunction and targeting cells with latent HIV-1. There has been one clinical study of ICIs in PLWH on ART without malignancy, in which a single low dose infusion of an anti-PD-L1 mAb (BMS-936559) appeared to enhance HIV-1 specific immune responses in 2 of 6 participants (Gay et al., 2017).
The role of immune checkpoint pathways in maintaining latency was suggested in a case report of a PLWH on ART, with a 20-fold increase in ca-HIV RNA in CD4+ T cells observed following multiple infusions of anti-CTLA-4 (ipilimumab) (Wightman et al., 2015). The same individual later received a single infusion of nivolumab for metastatic melanoma treatment, with a 24-fold significant increase in ca-HIV RNA post-infusion, both suggesting in vivo latency reversal following ICI administration. However, anti-HIV immune enhancement and LRA activity are not universally observed, and in otherwise healthy PLWH on effective ART the relatively frequent and sometimes severe autoimmune adverse events associated with ICIs may make this approach unacceptable.
Additional adaption of immunotherapeutic advances in cancer treatment to strategies for HIV cure, bolstered by the two cases of clinical cure following stem cell transplantation for cancer treatment (Gupta et al., 2019), includes adoptive T cell therapy, gene editing, bispecific antibodies and anti-HIV chimeric antigen receptor (CAR) T cells. While earlier studies of adoptive T cell therapy for HIV-1 established their safety (reviewed in (Lam and Bollard, 2013)), antiviral efficacy was transient, in part to a reliance on monoclonal T cells, extensive in vitro expansion resulting in a potentially exhausted phenotype, and infusion of T cells into viremic, untreated patients (Lam and Bollard, 2013). When applied in the setting of ART suppression, HIV-specific T cells persisted in blood and were capable of homing to rectal tissue, but virologic outcomes were not reported (Chapuis et al., 2011). Previous studies also examined infusing T cells specific for a single HLA-restricted epitope, either by clonal selection or introducing an artificial, high-affinity T cell receptor for the HLA-A2 Gag epitope SL9 (Varela-Rohena et al., 2008). Infused T cell clones showed little persistence and no clear long-term reduction in viral load. More recently, infusions of ex vivo polyclonal HIV-specific T cells targeting multiple HIV epitopes were safe and increased CD8+ T cell mediated antiviral activity in 2 of 6 participants, but did not increase the overall frequency of HIV-specific T cells, likely due to the low dose employed (Sung et al., 2018). Ex vivo site-specific modification of the CCR-5 gene in autologous CD4 cell using zinc-finger nuclease with re-infusion in PLWH on ART was safe and resulted in HIV RNA suppression in one of four participants who interrupted ART (Tebas et al., 2014). Studies employing gene-editing in stems cells for PLWH undergoing stem cell transplant for malignancy are ongoing.
Combination Studies:
As pilot studies of latency reversal and pilot studies of enhancement of HIV clearance mechanisms have now both demonstrated safety, the study of the combination of these two interventions may now proceed. Results from clinical studies combining these two strategies are limited. In a single arm trial of romidepsin in combination with an adjuvant vaccine, there was lack of HIV-1 reactivation in all participants following romidepsin administration and no effect on the time to viral rebound with an ATI (Leth et al., 2016). A randomized controlled trial combining latency reversal with an HIV vaccine enrolled participants treated within 4 weeks of diagnosis with primary HIV infection who received prime vaccination with ChAdV63.HIVconsv and MVA.HIVconsv boost 8 weeks later, followed by 10 doses of vorinostat every 3 days (Fidler et al., 2018; Fidler et al., 2020). No reduction in the viral reservoir was observed as measured by viral outgrowth between week 16 and 18 post-randomization, despite significantly higher HIV-specific CD4+ T cell responses and functional CD8+ T cell responses in the intervention vs ART only arm. In this population treated for a relatively short period of time after initial infection, the natural decay of persistent HIV DNA seen in early ART may have obscured a weak effect of the interventions. Initial reports of pilot studies employing similar approaches have also failed to achieve a measurable depletion of latent, persistent infection [(C. Gay, 2019) (Beatriz Mothe, 2017)].
In sum, several clinical studies of HIV cure have shown some ability to enhance HIV-specific immune responses without an associated impact on the viral reservoir or viral control in the absence of ART. More potent LRAs are needed to more effectively reactivate latent HIV. Whether any of the currently available immunotherapeutic strategies, in isolation or in combination, could then substantially deplete the pool of latently infected cells induced to express virus remains unanswered.
Towards an HIV cure in the 5th decade of AIDS
Eradication of infection in PLWH is still not achievable, at any scale. But now in the 5th decade of the AIDS pandemic, we mark more than 10 years of scientific effort refocused towards the goal of viral eradication. Increasingly, tools to approach this goal are emerging. As the understanding of the many mechanisms of viral persistence grows, novel approaches to disrupt and target latency are advancing toward clinical testing.
Advances in the use of HIV antibodies and HIV vaccine development may also be employed to enlist the body’s own defenses in recognition and clearance of infected cells. When these barriers are breached, we may discover additional cellular reservoirs of infection and mechanisms of persistence that require alternate or combinatorial therapeutic approaches. But the progress made so far in deciphering HIV pathogenesis and developing treatment strategies gives hope that eradication of established HIV infection is an attainable goal.
Acknowledgements:
We are grateful Luisa Ferrari for editorial support, and especially for the dedication of the PWLH who participate in research towards an HIV cure.
Funding statement: This study was supported by National Institutes of Health grant UM1-AI126619 to D.M.M., UL1TR002489 to the UNC TRaCS Institute, AI064518 to G.F., and AI50410 to the UNC Center for AIDS Research.
Footnotes
Declaration of Interests
JJ Eron reports grants and personal fees from Merck, and personal fees from Gilead outside the submitted work. DM Margolis reports personal fees from Merck and ViiV Healthcare, and holds common stock in Gilead. CL Gay reports research grants from Viiv and Gilead.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Okoye A, R.F., Brehm J, Maxwell A, Vaidya M, Pardons M, Tai V, Tang J, Smedly J, Axthelm M, Lifson J, Picker L, Trautmann L, Favre D, Chomont N. Activation of latent HIV and SIV RNA transcription in vitro and in vivo in ART suppressed SIV-infected rhesus macaques by the Ingenol-based protein kinase C agonist, GSK445A. In AIDS 2018. (Amsterdam, Netherlands). [Google Scholar]
- Abrahams MR, Joseph SB, Garrett N, Tyers L, Moeser M, Archin N, Council OD, Matten D, Zhou ST, Doolabh D, et al. (2019). The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci Transl Med 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu CM, Veenhuis RT, Avalos CR, Graham S, Parrilla DR, Ferreira EA, Queen SE, Shirk EN, Bullock BT, Li M, et al. (2019). Myeloid and CD4 T Cells Comprise the Latent Reservoir in Antiretroviral Therapy-Suppressed SIVmac251-Infected Macaques. mBio 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Khoury G, Fromentin R, Solomon A, Chomont N, Sinclair E, Milush JM, Hartogensis W, Bacchetti P, Roche M, et al. (2019). Human Immunodeficiency Virus (HIV)-Infected CCR6+ Rectal CD4+ T Cells and HIV Persistence On Antiretroviral Therapy. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, et al. (2017). Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest 127, 3126–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. (2012). Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos CR, Abreu CM, Queen SE, Li M, Price S, Shirk EN, Engle EL, Forsyth E, Bullock BT, Mac Gabhann F, et al. (2017). Brain Macrophages in Simian Immunodeficiency Virus-Infected, Antiretroviral-Suppressed Macaques: a Functional Latent Reservoir. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badamchi-Zadeh A, Tartaglia LJ, Abbink P, Bricault CA, Liu PT, Boyd M, Kirilova M, Mercado NB, Nanayakkara OS, Vrbanac VD, et al. (2018). Therapeutic Efficacy of Vectored PGT121 Gene Delivery in HIV-1-Infected Humanized Mice. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey JR, Sedaghat AR, Kieffer T, Brennan T, Lee PK, Wind-Rotolo M, Haggerty CM, Kamireddi AR, Liu Y, Lee J, et al. (2006). Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol 80, 6441–6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale MJ, and Kearney MF (2019). Review: HIV-1 phylogeny during suppressive antiretroviral therapy. Curr Opin HIV AIDS 14, 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, Corpataux JM, de Leval L, Pantaleo G, and Perreau M. (2016). PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med 22, 754–761. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, et al. (2016). Effect of HIV Antibody VRC01 on Viral Rebound after Treatment Interruption. N Engl J Med 375, 2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatriz Mothe JM, Christian Manzardo, Josep Coll, Puertas Maria C, Javier Martinez-Picado, Tomas Hanke, Bonaventura Clotet, Christian Brander(2017). VIRAL CONTROL INDUCED BY HIVCONSV VACCINES & ROMIDEPSIN IN EARLY TREATED INDIVIDUALS. In CONFERENCE ON RETROVIRUSES AND OPPORTUNISTIC INFECTIONS (Seattle WA USA). [Google Scholar]
- Boehm D, Conrad RJ, and Ott M. (2013). Bromodomain proteins in HIV infection. Viruses 5, 1571–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D, and Ott M. (2017). Host Methyltransferases and Demethylases: Potential New Epigenetic Targets for HIV Cure Strategies and Beyond. AIDS Research and Human Retroviruses 33, S-8-S-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borducchi EN, Liu J, Nkolola JP, Cadena AM, Yu WH, Fischinger S, Broge T, Abbink P, Mercado NB, Chandrashekar A, et al. (2018). Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature 563, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley T, Ferrari G, Haynes BF, Margolis DM, and Browne EP (2018). Single-Cell Analysis of Quiescent HIV Infection Reveals Host Transcriptional Profiles that Regulate Proviral Latency. Cell Rep 25, 107–117.e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- T.V. Brehm J, Irlbeck D, Ferris R, Archin N, Kanke M, Routy JP, Margolis DM, Tang J, Favre D (2017). DEVELOPMENT OF A PKC AGONIST DERIVED FROM INGENOL FOR HIV LATENCY DISRUPTION IN VIVO. In CONFERENCE ON RETROVIRUSES AND OPPORTUNISTIC INFECTIONS (Seattle, WA). [Google Scholar]
- Brodin J, Zanini F, Thebo L, Lanz C, Bratt G, Neher RA, and Albert J. (2016). Establishment and stability of the latent HIV-1 DNA reservoir. Elife 5, e18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronnimann MP, Skinner PJ, and Connick E. (2018). The B-Cell Follicle in HIV Infection: Barrier to a Cure. Front Immunol 9, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, Lai J, Strain MC, Lada SM, Hoh R, et al. (2016). Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med 22, 1043-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner KM, Wang Z, Simonetti FR, Bender AM, Kwon KJ, Sengupta S, Fray EJ, Beg SA, Antar AAR, Jenike KM, et al. (2019). A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature 566, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui JK, Sobolewski MD, Keele BF, Spindler J, Musick A, Wiegand A, Luke BT, Shao W, Hughes SH, Coffin JM, et al. (2017). Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog 13, e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon MJ, Sun H, Li C, Shaw A, Seiss K, Ouyang Z, Martin-Gayo E, Leng J, Henrich TJ, Li JZ, et al. (2014). HIV-1 persistence in CD4(+) T cells with stem cell-like properties. Nat Med 20, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay C, J.D.K. SD Warren Falcinelli J., Kirchherr JL, Sholtis K, Allard B, Stuelke E, Gamble A, Plachco A, Tcherapanova I, Eron JJ, Goonetilleke N, DeBenedette M, Nicolette CA, Archin NM, Margolis DM (2019). The impact of vorinostat and AGS-004, a dendritic cell-based immunotherapy, on persistent HIV-1 Infection In IAS 2019 (Mexico City, Mexico; ). [Google Scholar]
- Cabrera C, Chang L, Stone M, Busch M, and Wilson DH (2015). Rapid, Fully Automated Digital Immunoassay for p24 Protein with the Sensitivity of Nucleic Acid Amplification for Detecting Acute HIV Infection. Clin Chem 61, 1372–1380. [DOI] [PubMed] [Google Scholar]
- Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr., Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, et al. (2015). Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522, 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, Murrell B, Pfeifer N, Nogueira L, Oliveira TY, et al. (2017). Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 23, 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis AG, Casper C, Kuntz S, Zhu J, Tjernlund A, Diem K, Turtle CJ, Cigal ML, Velez R, Riddell S, et al. (2011). HIV-specific CD8+ T cells from HIV+ individuals receiving HAART can be expanded ex vivo to augment systemic and mucosal immunity in vivo. Blood 117, 5391–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Yu H, Li G, Li F, Ma J, Li J, Chi L, Zhang L, and Su L. (2017). Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, et al. (2009). HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 15, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. (1997a). Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387, 183–188. [DOI] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, and Siliciano RF (1995). In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med 1, 1284–1290. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, and Fauci AS (1997b). Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 94, 13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton GT, and Jones RB (2018). Diverse Impacts of HIV Latency-Reversing Agents on CD8+ T-Cell Function: Implications for HIV Cure. Front Immunol 9, 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad RJ, Fozouni P, Thomas S, Sy H, Zhang Q, Zhou M-M, and Ott M. (2017). The Short Isoform of BRD4 Promotes HIV-1 Latency by Engaging Repressive SWI/SNF Chromatin-Remodeling Complexes. Molecular Cell 67, 1001–1012.e1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks AM, Bateson R, Cope AB, Dahl NP, Griggs MK, Kuruc JD, Gay CL, Eron JJ, Margolis DM, Bosch RJ, et al. (2015). Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. Journal of Infectious Diseases 212, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Kaminski R, Bella R, Su H, Mathews S, Ahooyi TM, Chen C, Mancuso P, Sariyer R, Ferrante P, et al. (2019). Sequential LASER ART and CRISPR Treatments Eliminate HIV-1 in a Subset of Infected Humanized Mice. Nat Commun 10, 2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis Z, Anderson J, Thorkelson A, Wong HC, Karn J, Dobrowlski C, Miller JS, Cooley S, Douek DC, and Schacker TW (2018). Phase I study of ALT-803 (IL-15) superagonist) to clear latent HIV reservoirs. Paper presented at: Conference on Retroviruses and Opportunistic Infections (Boston, MA). [Google Scholar]
- Del Prete GQ, Alvord WG, Li Y, Deleage C, Nag M, Oswald K, Thomas JA, Pyle C, Bosche WJ, Coalter V, et al. (2019). TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K, Pertea M, Rongvaux A, Wang L, Durand CM, Ghiaur G, Lai J, McHugh HL, Hao H, Zhang H, et al. (2015). Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517, 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Estes JD, Sun Z, Othieno FA, Wei BL, Wege AK, Powell DA, Payne D, Haase AT, and Garcia JV (2008). Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med 5, e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Krisko JF, Powell DA, Mathias M, Kwak YT, Martinez-Torres F, Zou W, Payne DA, Estes JD, and Garcia JV (2010). Systemic administration of antiretrovirals prior to exposure prevents rectal and intravenous HIV-1 transmission in humanized BLT mice. PLoS One 5, e8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, et al. (2014). Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog 10, e1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, et al. (2012). Generation of HIV latency in humanized BLT mice. J Virol 86, 630–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger RW, and Fauci AS (2018). Ending the HIV/AIDS Pandemic(1). Emerg Infect Dis 24, 413–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, Deeks SG, Luciw PA, Chipman JG, Beilman GJ, et al. (2017). Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med 23, 1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber DL, Yudanin NA, and Restifo NP (2014). Human memory T cells: generation, compartmentalization and homeostasis. Nature Reviews Immunology 14, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G, Haynes BF, Koenig S, Nordstrom JL, Margolis DM, and Tomaras GD (2016). Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection. In Nat Rev Drug Discov, pp. 823–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler S, Stohr W, Pace M, Dorrell L, Lever A, Pett S, Kinloch S, Fox J, Clarke A, Nelson M, et al. (2018). A randomised controlled trial comparing the impact of antiretroviral therapy (ART) with a ‘kick-and-kill’ approach to ART alone on HIV reservoirs in individuals with primary HIV infection (PHI); RIVER trial. Paper presented at: International AIDS Conference (Amsterdam, Netherlands). [Google Scholar]
- Fidler S, Stohr W, Pace M, Dorrell L, Lever A, Pett S, Kinloch-de Loes S, Fox J, Clarke A, Nelson M, et al. (2020). Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. (1997). Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300. [DOI] [PubMed] [Google Scholar]
- Fisher L, Zinter M, Stanfield-Oakley S, Carpp LN, Edwards RW, Denny T, Moodie Z, Laher F, Bekker L-G, McElrath MJ, et al. (2019). Vaccine-Induced Antibodies Mediate Higher Antibody-Dependent Cellular Cytotoxicity After Interleukin-15 Pretreatment of Natural Killer Effector Cells In Front Immunol (Frontiers; ), pp. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromentin R, Bakeman W, Lawani MB, Khoury G, Hartogensis W, DaFonseca S, Killian M, Epling L, Hoh R, Sinclair E, et al. (2016). CD4+ T Cells Expressing PD-1, TIGIT and LAG-3 Contribute to HIV Persistence during ART. PLoS Pathog 12, e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P.S. Gama L, Shirk E, Queen SE, Li M, Bullock B, Wietgrefe S, Pianowski L, Zink MC, Clements J (2015). Latency Reversing Agents Activate Latent Reservoirs in the Brain of SIV-Infected Macaques In CONFERENCE ON RETROVIRUSES AND OPPORTUNISTIC INFECTIONS (Boston, Massachusetts; ). [Google Scholar]
- Ganor Y, Real F, Sennepin A, Dutertre CA, Prevedel L, Xu L, Tudor D, Charmeteau B, Couedel-Courteille A, Marion S, et al. (2019). HIV-1 reservoirs in urethral macrophages of patients under suppressive antiretroviral therapy. Nat Microbiol 4, 633–644. [DOI] [PubMed] [Google Scholar]
- Garcia JV (2016). In vivo platforms for analysis of HIV persistence and eradication. J Clin Invest 126, 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Abad-Fernandez M, Tuyishime M, Pollara JJ, Ferrari G, Soriano-Sarabia N, and Margolis DM (2018). Interleukin-15-Stimulated Natural Killer Cells Clear HIV-1-Infected Cells following Latency Reversal Ex Vivo. J Virol 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CL, Bosch RJ, Ritz J, Hataye JM, Aga E, Tressler RL, Mason SW, Hwang CK, Grasela DM, Ray N, et al. (2017). Clinical Trial of the Anti-PD-L1 Antibody BMS-936559 in HIV-1 Infected Participants on Suppressive Antiretroviral Therapy. J Infect Dis 215, 1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke N, Clutton G, Swanstrom R, and Joseph SB (2019). Blocking Formation of the Stable HIV Reservoir: A New Perspective for HIV-1 Cure. Front Immunol 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, et al. (2009). The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. The Journal of experimental medicine 206, 1253–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin A, Wiche Salinas TR, Planas D, Wacleche VS, Zhang Y, Fromentin R, Chomont N, Cohen EA, Shacklett B, Mehraj V, et al. (2017). HIV persists in CCR6+CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 31, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick RM, and Flexner C. (2019). Long-Acting HIV Drugs for Treatment and Prevention. Annual review of medicine 70, 137–150. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Abduljawad S, McCoy L, Mok HP, Peppa D, Lee H, Nastouli E, Lambert J, Pace M, Frater J, et al. (2019). Sustained HIV-1 remission following homozygous CCR5 delta32 allogenic HSCT. Paper presented at: Conference on Retroviruses and Opportunistic Infections (Seattle, WA). [Google Scholar]
- Gutierrez C, Serrano-Villar S, Madrid-Elena N, Perez-Elias MJ, Martin ME, Barbas C, Ruiperez J, Munoz E, Munoz-Fernandez MA, Castor T, et al. (2016). Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. AIDS 30, 1385–1392. [DOI] [PubMed] [Google Scholar]
- Hamer DH (2004). Can HIV be Cured? Mechanisms of HIV persistence and strategies to combat it. Curr HIV Res 2, 99–111. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes BF, Burton DR, and Mascola JR (2019). Multiple roles for HIV broadly neutralizing antibodies. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich TJ, Hatano H, Bacon O, Hogan LE, Rutishauser R, Hill A, Kearney MF, Anderson EM, Buchbinder SP, Cohen SE, et al. (2017). HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: An observational study. PLos Med 14, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiener B, Horsburgh BA, Eden JS, Barton K, Schlub TE, Lee E, von Stockenstrom S, Odevall L, Milush JM, Liegler T, et al. (2017). Identification of Genetically Intact HIV-1 Proviruses in Specific CD4(+) T Cells from Effectively Treated Participants. Cell Rep 21, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y-C, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DIS, Lai J, Blankson JN, Siliciano JD, and Siliciano RF (2013). Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeycutt JB, Thayer WO, Baker CE, Ribeiro RM, Lada SM, Cao Y, Cleary RA, Hudgens MG, Richman DD, and Garcia JV (2017). HIV persistence in tissue macrophages of humanized myeloid-only mice during antiretroviral therapy. Nat Med 23, 638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane NN, Kwon KJ, Bruner KM, Capoferri AA, Beg S, Rosenbloom DI, Keele BF, Ho YC, Siliciano JD, and Siliciano RF (2017). Proliferation of latently infected CD4(+) T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J Exp Med 214, 959–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Matusali G, and Dejucq-Rainsford N. (2014). Origins of HIV-infected leukocytes and virions in semen. J Infect Dis 210 Suppl 3, S622–630. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, and Allis CD (2001). Translating the histone code. Science 293, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Jiang G, and Dandekar S. (2015). Targeting NF-kappaB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res Hum Retroviruses 31, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Maverakis E, Cheng MY, Elsheikh MM, Deleage C, Mendez-Lagares G, Shimoda M, Yukl SA, Hartigan-O’Connor DJ, Thompson GR 3rd, et al. (2019). Disruption of latent HIV in vivo during the clearance of actinic keratosis by ingenol mebutate. JCI Insight 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Nguyen D, Archin NM, Yukl SA, Méndez-Lagares G, Tang Y, Elsheikh MM, Thompson GR III, Hartigan-O’Connor DJ, Margolis DM, et al. (2018). HIV latency is reversed by ACSS2-driven histone crotonylation. The Journal of Clinical Investigation 128, 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, and Swanstrom R. (2018). The evolution of HIV-1 entry phenotypes as a guide to changing target cells. J Leukoc Biol 103, 421–431. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Trunfio M, Kincer LP, Calcagno A, and Price RW (2019). What can characterization of cerebrospinal fluid escape populations teach us about viral reservoirs in the central nervous system? Aids 33 Suppl 2, S171–s179. [DOI] [PubMed] [Google Scholar]
- Katlama C, Lambert-Niclot S, Assoumou L, Papagno L, Lecardonnel F, Zoorob R, Tambussi G, Clotet B, Youle M, Achenbach CJ, et al. (2016). Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS 30, 221–230. [DOI] [PubMed] [Google Scholar]
- Kim JH, Excler JL, and Michael NL (2015). Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annual review of medicine 66, 423–437. [DOI] [PubMed] [Google Scholar]
- Kulpa DA, Talla A, Brehm JH, Ribeiro SP, Yuan S, Bebin-Blackwell AG, Miller M, Barnard R, Deeks SG, Hazuda D, et al. (2019). Differentiation into an Effector Memory Phenotype Potentiates HIV-1 Latency Reversal in CD4(+) T Cells. J Virol 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S, and Bollard C. (2013). T-cell therapies for HIV. Immunotherapy 5, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers SL, Rose R, Maidji E, Agsalda-Garcia M, Nolan DJ, Fogel GB, Salemi M, Garcia DL, Bracci P, Yong W, et al. (2016). HIV DNA Is Frequently Present within Pathologic Tissues Evaluated at Autopsy from Combined Antiretroviral Therapy-Treated Patients with Undetectable Viral Loads. J Virol 90, 8968–8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman G, Ylisastigui L, Bosch RJ, and Margolis DM (2004). Interleukin-7 induces HIV type 1 outgrowth from peripheral resting CD4+ T cells. J Acquir Immune Defic Syndr 36, 1103–1104. [DOI] [PubMed] [Google Scholar]
- Leth S, Schleimann MH, Nissen SK, Hojen JF, Olesen R, Graversen ME, Jorgensen S, Kjaer AS, Denton PW, Mork A, et al. (2016). Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. The lancet HIV 3, e463–472. [DOI] [PubMed] [Google Scholar]
- Lim SY, Osuna CE, Hraber PT, Hesselgesser J, Gerold JM, Barnes TL, Sanisetty S, Seaman MS, Lewis MG, Geleziunas R, et al. (2018). TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, Mellors JW, Rosenbloom D, and Persaud D. (2015). Viremic Relapse after HIV-1 Remission in a Perinatally Infected Child. N Engl J Med 372, 786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, et al. (2015). Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7, 319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo AB, Novis CL, and Bosque A. (2019). Targeting Cellular and Tissue HIV Reservoirs With Toll-Like Receptor Agonists. Front Immunol 10, 2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, et al. (2014). Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden MD, Loy BA, Wu X, Ramirez CM, Schrier AJ, Murray D, Shimizu A, Ryckbosch SM, Near KE, Chun TW, et al. (2017). In vivo activation of latent HIV with a synthetic bryostatin analog effects both latent cell “kick” and “kill” in strategy for virus eradication. PLoS Pathog 13, e1006575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbonye U, and Karn J. (2014). Transcriptional control of HIV latency: Cellular signaling pathways, epigenetics, happenstance and the hope for a cure. Virology 454–455, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien JB, Mavigner M, Franchitti L, Smith SA, White E, Tharp GK, Walum H, Busman-Suhay K, Aguilera-Sandoval CR, Thayer WO, et al. (2020). Robust and persistent reactivation of SIV and HIV by N-803 and depletion of CD8(+) cells. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBrien JB, Mavigner M, Franchitti L, Smith SA, White E, Tharp GK, Walum H, Busman-Suhay K, Aguilera-Sandoval CR, Thayer WO, et al. (In Press). Robust and persistent SIV and HIV reactivation by N-803 and CD8 depletion. Nature. [Google Scholar]
- Meier UC, Klenerman P, Griffin P, James W, Koppe B, Larder B, McMichael A, and Phillips R. (1995). Cytotoxic T lymphocyte lysis inhibited by viable HIV mutants. Science 270, 1360–1362. [DOI] [PubMed] [Google Scholar]
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, Wege AK, Haase AT, and Garcia JV (2006). Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12, 1316–1322. [DOI] [PubMed] [Google Scholar]
- Mendoza P, Gruell H, Nogueira L, Pai JA, Butler AL, Millard K, Lehmann C, Suarez I, Oliveira TY, Lorenzi JCC, et al. (2018). Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature 561, 479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog C, Dereuddre-Bosquet N, Teillaud J-L, Biedma ME, Holl V, Van Ham G, Heyndrickx L, Van Dorsselaer A, Katinger D, Vcelar B, et al. (2014). Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques In Mucosal Immunol (Nature Publishing Group; ), pp. 46–56. [DOI] [PubMed] [Google Scholar]
- Mothe B, Molto J, Manzardo C, Coll J, Puertas MC, Martinez-Pcado J, Hanke T, Clotet B, and Brander C. (2017). Viral control induced by HIVCONSV vaccins and romidepsin in early treated individuals. In Conference on Retroviruses and Opportunistic Infections (Boston, MA). [Google Scholar]
- Nguyen K, Das B, Dobrowolski C, and Karn J. (2017). Multiple Histone Lysine Methyltransferases Are Required for the Establishment and Maintenance of HIV-1 Latency. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon CC, Mavigner M, Sampey GC, Brooks AD, Spagnuolo RA, Irlbeck DM, Mattingly C, Ho PT, Schoof N, Cammon CG, et al. (2020). Systemic HIV and SIV latency reversal via non-canonical NF-kappaB signalling in vivo. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pache L, Dutra Miriam S., Spivak Adam M., Marlett John M., Murry Jeffrey P., Hwang Y, Maestre Ana M., Manganaro L, Vamos M, Teriete, et al. (2015). BIRC2/cIAP1 Is a Negative Regulator of HIV-1 Transcription and Can Be Targeted by Smac Mimetics to Promote Reversal of Viral Latency. Cell Host & Microbe 18, 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallikkuth S, Sharkey M, Babic DZ, Gupta S, Stone GW, Fischl MA, Stevenson M, and Pahwa S. (2015). Peripheral T Follicular Helper Cells Are the Major HIV Reservoir within Central Memory CD4 T Cells in Peripheral Blood from Chronically HIV-Infected Individuals on Combination Antiretroviral Therapy. J Virol 90, 2718–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankau MD, Reeves DB, Harkins E, Ronen K, Jaoko W, Mandaliya K, Graham SM, McClelland RS, Matsen Iv FA, Schiffer JT, et al. (2020). Dynamics of HIV DNA reservoir seeding in a cohort of superinfected Kenyan women. PLOS Pathogens 16, e1008286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardons M, Fromentin R, Pagliuzza A, Routy JP, and Chomont N. (2019). Latency-Reversing Agents Induce Differential Responses in Distinct Memory CD4 T Cell Subsets in Individuals on Antiretroviral Therapy. Cell Rep 29, 2783–2795.e2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, et al. (2015). Activation and lysis of human CD4 cells latently infected with HIV-1 In Nat Comms (Nature Publishing Group; ), pp. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CW, Wang J, Deleage C, Reddy S, Kaur J, Polacino P, Reik A, Huang ML, Jerome KR, Hu SL, et al. (2018). Differential impact of transplantation on peripheral and tissue-associated viral reservoirs: Implications for HIV gene therapy. PLoS Pathog 14, e1006956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollara J, Bonsignori M, Moody MA, Pazgier M, Haynes BF, and Ferrari G. (2013). Epitope Specificity of Human Immunodeficiency Virus-1 Antibody Dependent Cellular Cytotoxicity [ADCC] Responses. In Curr HIV Res, pp. 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, ten Berge IJ, Yong SL, Fox CH, Roos MT, et al. (1999). Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. Aids 13, 2405–2410. [DOI] [PubMed] [Google Scholar]
- Rasmussen TA, and Sogaard OS (2018). Clinical Interventions in HIV Cure Research. Adv Exp Med Biol 1075, 285–318. [DOI] [PubMed] [Google Scholar]
- Rasmussen TA, and Søgaard OS (2018). Clinical Interventions in HIV Cure Research In HIV Vaccines and Cure : The Path Towards Finding an Effective Cure and Vaccine, Zhang L, and Lewin SR, eds. (Singapore: Springer Singapore; ), pp. 285–318. [Google Scholar]
- Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, et al. (2014). Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. The lancet HIV 1, e13–21. [DOI] [PubMed] [Google Scholar]
- Razooky BS, Pai A, Aull K, Rouzine IM, and Weinberger LS (2015). A hardwired HIV latency program. Cell 160, 990–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, Liu B, Zhu X, Wen J, You L, et al. (2016). Comparison of the Superagonist Complex, ALT-803, to IL15 as Cancer Immunotherapeutics in Animal Models. Cancer Immunol Res 4, 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddler SA, Zheng L, Durand CM, Ritz J, Koup RA, Ledgerwood J, Bailer RT, Koletar SL, Eron JJ, Keefer MC, et al. (2018). Randomized Clinical Trial to Assess the Impact of the Broadly Neutralizing HIV-1 Monoclonal Antibody VRC01 on HIV-1 Persistence in Individuals on Effective ART. Open Forum Infect Dis 5, ofy242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson HL (2018). HIV/AIDS Vaccines: 2018. Clin Pharmacol Ther 104, 1062–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelas DS, and Greene WC (2013). An integrated overview of HIV-1 latency. Cell 155, 519–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampey GC, Irlbeck DM, Browne EP, Kanke M, McAllister AB, Ferris RG, Brehm JH, Favre D, Routy J-P, Jones CD, et al. (2018). The SMAC Mimetic AZD5582 is a Potent HIV Latency Reversing Agent. bioRxiv. [Google Scholar]
- Santra S, Tomaras GD, Warrier R, Nicely NI, Liao H-X, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, et al. (2015). Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques In PLoS Pathog (Public Library of Science; ), pp. e1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu CL, Lorenzi JC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, et al. (2016). HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535, 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, and Siliciano RF (2003). Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4(+) T cells. Nat Med 9, 727–728. [DOI] [PubMed] [Google Scholar]
- Simonetti FR, Sobolewski MD, Fyne E, Shao W, Spindler J, Hattori J, Anderson EM, Watters SA, Hill S, Wu X, et al. (2016). Clonally expanded CD4+ T cells can produce infectious HIV-1 in vivo. Proc Natl Acad Sci U S A 113, 1883–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneller MC, Clarridge KE, Seamon C, Shi V, Zorawski MD, Justement JS, Blazkova J, Huiting ED, Proschan MA, Mora JR, et al. (2019). An open-label phase 1 clinical trial of the anti-alpha4beta7 monoclonal antibody vedolizumab in HIV-infected individuals. Sci Transl Med 11. [DOI] [PubMed] [Google Scholar]
- Sneller MC, Justement JS, Gittens KR, Petrone ME, Clarridge KE, Proschan MA, Kwan R, Shi V, Blazkova J, Refsland EW, et al. (2017). A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, Kjaer AS, Schleimann MH, Denton PW, Hey-Cunningham WJ, et al. (2015). The Depsipeptide Romidepsin Reverses HIV-1 Latency In Vivo. PLoS Pathog 11, e1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Sarabia N, Bateson RE, Dahl NP, Crooks AM, Kuruc JD, Margolis DM, and Archin NM (2014). The quantitation of replication-competent HIV-1 in populations of resting CD4+ T cells. J Virol 24, 01900–01914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey AR, Norris PJ, Qin L, Haygreen EA, Taylor E, Heitman J, Lebedeva M, DeCamp A, Li D, Grove D, et al. (2009). Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections In J Virol (American Society for Microbiology; ), pp. 3719–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Denton PW, Estes JD, Othieno FA, Wei BL, Wege AK, Melkus MW, Padgett-Thomas A, Zupancic M, Haase AT, et al. (2007). Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med 204, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JA, Lam S, Garrido C, Archin N, Rooney CM, Bollard CM, and Margolis DM (2015a). Expanded cytotoxic T-cell lymphocytes target the latent HIV reservoir. J Infect Dis 212, 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JA, Patel S, Clohosey ML, Roesch L, Tripic T, Kuruc JD, Archin N, Hanley PJ, Cruz CR, Goonetilleke N, et al. (2018). HIV-Specific, Ex Vivo Expanded T Cell Therapy: Feasibility, Safety, and Efficacy in ART-Suppressed HIV-Infected Individuals. Mol Ther 26, 2496–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JAM, Pickeral J, Liu L, Stanfield-Oakley SA, Lam C-YK, Garrido C, Pollara J, Labranche C, Bonsignori M, Moody MA, et al. (2015b). Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells In J Clin Invest (American Society for Clinical Investigation; ), pp. 4077–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, Spratt SK, Surosky RT, Giedlin MA, Nichol G, et al. (2014). Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med 370, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaysen-Andersen M, Chertova E, Bergamaschi C, Moh ES, Chertov O, Roser J, Sowder R, Bear J, Lifson J, Packer NH, et al. (2016). Recombinant human heterodimeric IL-15 complex displays extensive and reproducible N- and O-linked glycosylation. Glycoconj J 33, 417–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin NH, Learn GH, Holte SE, Wang Y, Melvin AJ, McKernan JL, Pawluk DM, Mohan KM, Lewis PF, Mullins JI, et al. (2005). Evidence that low-level viremias during effective highly active antiretroviral therapy result from two processes: expression of archival virus and replication of virus. J Virol 79, 9625–9634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TA, de Goer de Herve MG, Hendel-Chavez H, Dembele B, Le Nevot E, Abbed K, Pallier C, Goujard C, Gasnault J, Delfraissy JF, et al. (2008). Resting regulatory CD4 T cells: a site of HIV persistence in patients on long-term effective antiretroviral therapy. PLoS One 3, 0003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A-MW, and Margolis DM (2017). Chromatin Regulation and the Histone Code in HIV Latency. Yale J Biol Med 90, 229–243. [PMC free article] [PubMed] [Google Scholar]
- van Praag RM, Prins JM, Roos MT, Schellekens PT, Ten Berge IJ, Yong SL, Schuitemaker H, Eerenberg AJ, Jurriaans S, de Wolf F, et al. (2001). OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol 21, 218–226. [DOI] [PubMed] [Google Scholar]
- Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, et al. (2008). Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nature medicine 14, 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibholm LK, Konrad CV, Schleimann MH, Frattari G, Winckelmann A, Klastrup V, Jensen NM, Jensen SS, Schmidt M, Wittig B, et al. (2019). Effects of 24-week Toll-like receptor 9 agonist treatment in HIV type 1+ individuals. AIDS 33, 1315–1325. [DOI] [PubMed] [Google Scholar]
- Wagner TA, McLaughlin S, Garg K, Cheung CYK, Larsen BB, Styrchak S, Huang HC, Edlefsen PT, Mullins JI, and Frenkel LM (2014). Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 345, 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A, Baker C, Spagnuolo RA, Stamper LW, Fouda GG, Permar SR, Hinde K, Kuhn L, Bode L, Aldrovandi GM, et al. (2015). Breast Milk of HIV-Positive Mothers Has Potent and Species-Specific In Vivo HIV-Inhibitory Activity. J Virol 89, 10868–10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A, De C, Abad Fernandez M, Lenarcic EM, Xu Y, Cockrell AS, Cleary RA, Johnson CE, Schramm NJ, Rank LM, et al. (2019). Precision mouse models with expanded tropism for human pathogens. Nat Biotechnol 37, 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A, Swanson MD, Nochi T, Olesen R, Denton PW, Chateau M, and Garcia JV (2012). Human breast milk and antiretrovirals dramatically reduce oral HIV-1 transmission in BLT humanized mice. PLoS Pathog 8, e1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FX, Xu Y, Sullivan J, Souder E, Argyris EG, Acheampong EA, Fisher J, Sierra M, Thomson MM, Najera R, et al. (2005). IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest 115, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JA, Clutton G, and Goonetilleke N. (2019). Harnessing CD8(+) T Cells Under HIV Antiretroviral Therapy. Front Immunol 10, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DC, Moysi E, Valentin A, Bergamaschi C, Devasundaram S, Fortis SP, Bear J, Chertova E, Bess J Jr., Sowder R, et al. (2018). Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog 14, e1006902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, Stanton JJ, Legasse AW, Hobbs T, Martin LD, et al. (2018). The human IL-15 superagonist ALT-803 directs SIV-specific CD8(+) T cells into B-cell follicles. Blood Adv 2, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JB, Lim SY, Osuna CE, Kublin JL, Chen E, Yoon G, Liu PT, Abbink P, Borducci EN, Hill A, et al. (2018). Prevention of SIVmac251 reservoir seeding in rhesus monkeys by early antiretroviral therapy. Nat Commun 9, 5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman F, Solomon A, Kumar SS, Urriola N, Gallagher K, Hiener B, Palmer S, McNeil C, Garsia R, and Lewin SR (2015). Effect of ipilimumab on the HIV reservoir in an HIV-infected individual with metastatic melanoma. AIDS 29, 504–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Chen LF, Kwon H, Fenard D, Bisgrove D, Verdin E, and Greene WC (2004). Prostratin antagonizes HIV latency by activating NF-kappaB. J Biol Chem 279, 42008–42017. [DOI] [PubMed] [Google Scholar]
- Winckelmann A, Barton K, Hiener B, Schlub TE, Shao W, Rasmussen TA, Ostergaard L, Sogaard OS, Tolstrup M, and Palmer S. (2017). Romidepsin-induced HIV-1 viremia during effective antiretroviral therapy contains identical viral sequences with few deleterious mutations. AIDS 31, 771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, and Richman DD (1997). Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291–1295. [DOI] [PubMed] [Google Scholar]
- Wong JK, and Yukl SA (2016). Tissue reservoirs of HIV. Curr Opin HIV AIDS 11, 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Trumble IM, Warren JA, Clutton G, Abad-Fernandez M, Kirchnerr J, Adimora AA, Deeks SG, Margolis DM, Kuruc JD, et al. (2019). HIV-Specific T Cell Responses Are Highly Stable on Antiretroviral Therapy. Mol Ther Methods Clin Dev 15, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukl SA, Shergill AK, Ho T, Killian M, Girling V, Epling L, Li P, Wong LK, Crouch P, Deeks SG, et al. (2013). The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis 208, 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbato JM, McMahon DK, Sobolewski MD, Mellors JW, and Sluis-Cremer N. (2019). Naive CD4+ T Cells Harbor a Large Inducible Reservoir of Latent, Replication-competent Human Immunodeficiency Virus Type 1. Clin Infect Dis 69, 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A, Kamata M, Rezek V, Rick J, Levin B, Kasparian S, Chen IS, Yang OO, Zack JA, and Kitchen SG (2015). HIV-specific Immunity Derived From Chimeric Antigen Receptor-engineered Stem Cells. In Mol Ther, pp. 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen A, Rezek V, Youn C, Lam B, Chang N, Rick J, Carrillo M, Martin H, Kasparian S, Syed P, et al. (2017). Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J Clin Invest 127, 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]