Abstract
Introduction
The use of sedation in intensive care units (ICUs) is necessary and ubiquitous. The impact of sedation strategy on outcome, particularly when delivered early after initiation of mechanical ventilation, is unknown. Evidence is increasing that volatile anaesthetic agents could be associated with better outcome. Their use in delirium prevention is unknown.
Methods and analysis
This study is an investigator-initiated, prospective, multicentre, two-arm, randomised, control, open-trial comparing inhaled sedation strategy versus intravenous sedation strategy in mechanically ventilated patients in ICU. Two hundred and fifty patients will be randomly assigned to the intravenous sedation group or inhaled sedation group, with a 1:1 ratio in two groups according to the sedation strategy. The primary outcome is the occurrence of delirium assessed using two times a day confusion assessment method for the ICU (CAM-ICU). Secondary outcomes include cognitive and functional outcomes at 3 and 12 months.
Ethics and dissemination
The study has been approved by the Regional Ethics Committee (CPP Ouest) and national authorities (ANSM). The results will be submitted for publication in peer-reviewed journals.
Trial registration number
Keywords: adult intensive & critical care, delirium & cognitive disorders, adult anaesthesia
Strengths and limitations of this study.
The INASED study is a multicentre, randomised, controlled and open-label trial, comparing two sedation strategies.
The primary outcome is the occurrence of delirium up until intensive care unit (ICU) discharge.
Neurocognitive evaluation will be performed for at least 3 months after ICU discharge, which will enable investigators to evaluate patients’ outcome on a strong indicator.
The main limitation of the study is that considering the characteristics of the two strategies under evaluation, a double-blind trial is not possible.
However, blinding of outcome data assessment is ensured as the cognitive function is evaluated by a research assistant that will not be aware of patient assignment group.
Introduction
Background and rationale
The use of sedative drugs in intensive care units (ICUs) is essential and ubiquitous. Sedatives are administered to critically ill patients to relieve anxiety, reduce the stress of mechanical ventilation and prevent agitation-related harm.1 However, sedative drugs and their active metabolites can accumulate, leading to prolonged deep sedation, respiratory depression, immune suppression and hypotension. Undersedation leads to agitation, hypercatabolism, self-harm and unplanned extubation.2 Oversedation may increase the duration of mechanical ventilation, exposing patient to ventilator acquired pneumonia.3 Yet, these drugs, used as part of sedation titration protocol or daily sedation stop protocol, have improved patient outcomes.4–6
However, all these drug regimen, by uncertain mechanisms, favour the occurrence of ICU delirium. ICU delirium and ICU delirium duration are independent factors associated with the duration of mechanical ventilation, ICU length of stay and 6 months mortality.7 8 It has been demonstrated that patients who survive admission to ICU but who have experienced delirium suffer moderate to severe cognitive impairment at 6 months and show persistent depression, anxiety and post-traumatic stress 1 year after hospitalisation leading to public health burden.9–12
Halogenated gases have been used for a long time in anaesthesia. Thanks to technical innovations, they can be used with ICU ventilators.13 Their dose adjustment is simple, they have no active metabolites and are cleared by breathing. Several studies on selected populations have shown the feasibility and the benefits of this use in ICU.13–16 Safety use for the staff in charge of the patient has been established.17 18
To the best of our knowledge, no study has yet prospectively examined the potential clinical effect of isoflurane sedation on delirium as the primary outcome in the ICU setting.
Objectives
We aim to conduct a prospective multicentre randomised controlled trial comparing two sedation strategies in ICU with the hypothesis that inhaled sedation strategy would decrease delirium occurrence.
Primary objective
Determine the impact on the delirium occurrence of an inhaled sedation strategy versus an intravenous sedation strategy in ICU mechanically ventilated patients.
Trial design
The inhaled sedation in ICU (INASED) study is an investigator-initiated, prospective, multicentre, randomised, open-label trial comparing inhaled versus intravenous sedation in ICU mechanically ventilated patients. Patients will be assigned to the intravenous sedation group or the inhaled sedation group, with a 1:1 ratio.
Methods: participants, interventions and outcomes
Study setting
The INASED study will take place in 10 ICUs in France.
Inclusion criteria
Patients eligible to be enrolled in this trial are adult ICU patients (>18 years) within 24 hours of intubation and who are expected to require mechanical ventilation for at least 24 hours; patient requiring immediate ongoing sedative medication for comfort, safety, and to facilitate the delivery of life support measures.
Exclusion criteria
Age less than 18 years; the patient who has been intubated for more than 24 hours in the ICU; admission for a cardiac arrest, a traumatic brain injury and/or a stroke; the patient who is unable to complete the neuropsychological test due to aphasia, deafness, blindness or dementia; contraindication to isoflurane (personal or familial history of malignant hyperthermia; liver failure with prothrombin <30%; acute or chronic neuromuscular disease); occurrence of a severe acute respiratory distress syndrom (ARDS) (P/F ratio <100), a partial pressure of CO2 (PaCO2) >50 mm Hg at the time of randomisation; death is deemed to be imminent or inevitable during the ICU admission; pregnancy or breastfeeding woman; patient under the guardianship or curatorship.
Intervention
As in the VALTS trial, two sedation strategy will be compared: one with volatile agent (isoflurane), the other with intravenous sedative (propofol).19 Patients who are eligible for inclusion will be randomised and assigned to one of the two following groups (figure 1): (1) The patients assigned to control group will receive continuous infusion of intravenous propofol, (2) The patients assigned to interventional group will receive continuously inhaled isoflurane with use of AnaConDa (Sedana Medical, Uppsala, Sweden). Sedation and pain management in both arms will be guided using a standardised nurse-driven bedside protocol. Sedation in both arms will be titrated every hour to reach a Richmond Agitation Sedation Scale of (−2; 1) (or as clinically indicated) until extubation.20 Supplemental sedatives can be used, always at the minimum effective dose, to optimise sedation and achieve the level of sedation specified by the treating clinician at any time when allocated treatment alone is insufficient to provide patient comfort and safety, provide rescue sedation for immediate control of sudden breakthrough agitation at any time. Benzodiazepines will not be administered to any patient, unless deemed mandatory by the treating clinician for conditions such as convulsions, palliation, procedural anaesthesia, concomitant neuromuscular blockade or refractory agitation. Patients will be reviewed daily for assessment of withdrawing sedation to assist ventilator weaning (resolving the underlying pathology that led to mechanical ventilation; inspired fraction of oxygen (FiO2) <50%; PEEP 5–8 cm H2O; haemodynamic stability with mean arterial pressure >60 mm Hg, which may be assisted with stable doses of vasoactive drug support) and extubated according to predefined criteria. Pain scores will be monitored every 2 hours in both groups using the Behavorial Pain Scale (BPS), the Face Legs Activity Cry Consolability or the VICOMORE and/or numerical pain score. Pain treatment is based on the ABCDEF bundle.21 It uses the nurse-driven analgesia protocol of each ward involved in the study. It uses a pain assessment score (BPS, VICOMORE, face, leg, activity, cry, consolability scale (FLACC)), local or regional anaesthesia, non-opioid adjuncts (acetaminophen, NSAIDs, nefopam), opioids (per os opioids, bolus of sufentanyl followed by continuous infusion if necessary, continuous infusion of remifentanyl). We decided to avoid morphine use for analgesia-based sedation because of its long half-life of action and its accumulation.22 In both groups, light sedation is encouraged (Richmond agitation sedation scale (RASS) −2; 1). Whatever the treatment arm allocated, ABCDEF bundle will be used.21
Figure 1.
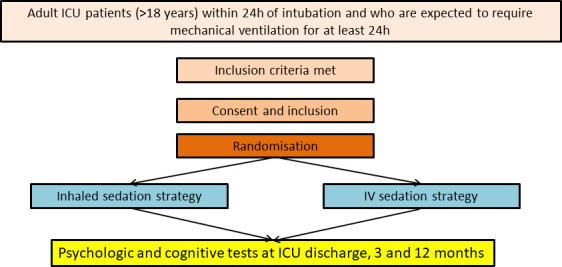
Intervention. Patients who are eligible for inclusion will be randomised and assigned to one of the two groups (inhaled or intravenous sedation). outcomes will be evaluated during ICU stay, at discharge and at 3 and 12 months. ICU, intensive care unit.
Control group: intravenous sedation
The patients assigned to the control group will receive continuously intravenous propofol. Sedation and pain management will be guided using an explicit bedside nurse driven sedation opioid analgesia algorithm.
Interventional group: inhaled sedation
Isoflurane will be infused into the AnaConDa device (Sedana Medical, Uppsala, Sweden), which is placed between the endotracheal tube and the ventilator breathing circuit. Isoflurane is placed in a standard syringe pump. The AnaConDa is placed in the breathing circuit between the Y-piece and the ET-tube. Liquid isoflurane is delivered from the syringe through the dedicated line into the AnaConDa where it is vaporised within the device. In order to limit the dead space, INASED study will only use 50 mL AnaConDa S filters. The gas monitor samples the gas from the AnaConDa port and displays the exhaled anaesthetic concentration in Fet% or MAC values (which indicates the concentration of the drug). Due to AnaConDa’s design, most of the exhaled anaesthetic agent is adsorbed and reflected to the patient on inspiration.23 Thus, AnaConDa recycles more than 90% of the expired volatile agent, which facilitates low infusion rates. The residual anaesthetic agent passes through the ventilator and exits through the exhaust where it is captured in the FlurAbsorb. The AnaConDa is changed every 24 hours. When patients are being prepared for extubation, isoflurane will be discontinued, and the AnaConDa device will be removed from the breathing circuit to facilitate rapid drug elimination. Gas scavenging is performed with a commercially available canister connected to the ventilator output. The canister contains 500 g of activated charcoal and removes isoflurane from the expired air up to a weight increase of 150 g, which provides 24 hours with the AnaConDa.
Staff education and training
This trial involves centres where the use of volatile sedation may be uncommon. Thus, education of medical, nursing and respiratory therapy staff regarding the use of volatile agents is supported by the development of a web-based teaching programme. Training sessions with a dedicated nurse include information regarding the use of the AnaConDa device, equipment setup and safety.
Masking protocol
It is not possible to blind local investigators to allocation as it is obvious which patients are receiving inhaled sedation: AnaConDa is connected to the endotracheal tube and requires the use of exhaled isoflurane monitor and a syringe driver. As the INASED study uses a nurse driven protocol, withdrawing of sedation is not initiated by the medical investigator but by the nurse in charge of the patient, based on this prespecified protocol. This is similar to what is used for spontaneous breathing trial (SBT), which are triggered daily by the nurse without medical consent if all the prespecified criteria are met.24 If SBT fails, patient is not extubated. If it succeeds, patient is extubated. Blinding of outcome data assessment is ensured as the cognitive function is evaluated by a research assistant that will not be aware of patient assignment group. Physicians treating the patients will be blinded for the final evolution of the neurocognitive assessment. However, the study remains an open-blinded study while physicians will be aware of the sedation group.
Equipment licensing and approvals
The AnaConDa device is licensed for use in Europe and isoflurane use in ICU is permitted (EC certificate CE 667826).
Duration of treatment
In both groups, patients will be treated for a minimal duration of 24 hours. Sedation continuation will be decided on an individual basis, according to the patient clinical status and will continue until no longer indicated up to 14 days maximum after enrolment. If sedation is deemed necessary beyond 14 days after enrolment, the choice of sedative regimen will be determined solely by the treating clinician.
Outcomes
Primary outcome
The primary outcome is the occurrence of delirium (yes/no) up until ICU discharge assessed using the confusion assessment method for the ICU (CAM-ICU). As delirium is fluctuating, CAM-ICU is to be evaluated twice a day, first time in the morning during first daily medical examination, second time in the evening at the beginning of the night shift. We decided not to evaluate delirium during the night in order to avoid sleep disorders within our patients and to follow recommendation of the ABCDEF bundle.21
Secondary outcomes
Secondary outcome variables include the following:
ICU outcomes:
Number of days with vasopressors or inotropic agents.
Number of days with sedation.
Cumulative dose and duration of anaesthetics drugs.
Maximum dose of vasopressors or inotropic agents.
Ventilation free days at 28 days following randomisation.
Proportion of RASS measurements in target range.
Incidence and duration of delirium (delirium free days at 28 days). Additionally, we consider a positive CAM-ICU assessment to be hyperactive delirium if the corresponding RASS is >0 and hypoactive delirium if the corresponding RASS is <0.
Number of days until RASS 0; −1 is reach.
Mortality at ICU discharge, at 28 days.
Length of ICU stay.
Requirement of physical restraints, of patients with unplanned extubation, unplanned catheter, urinary probe or gastric probe removal.
Self or heteroaggressive act.
Hospital outcome
Mortality at hospital discharge.
Length of hospital stay.
Readmission to ICU.
Discharge destination.
Posthospital outcomes.
Cost-effectiveness; institutional perspective and cost of lives saved (if positive).
Cognitive function and functional outcome will be evaluated at discharge, 3 and 12 months using two kinds of scores:
CANTAB test, combining six cognitive evaluations with an iPad during a 45–60 min medical consultation (those tests were also used in the Spice functional and neuro-psychological outcomes SPICEFANS substudy25).
Post-traumatic stress disorder Checklist Scale (PCLS), Hospital Anxiety and Depression scale (HADS), Short Form 36 (SF36) (medical outcome study SF 36), instrumental activities of daily living (IADL) performed by a clinical research associate.
Sample size
We determined that enrolment of 250 patients would provide a power of 80% to show a reduction by half (30% vs 15%) in the rate of delirium occurrence between the control group using intravenous sedation and the interventional group using inhaled sedation at a two-sided alpha level of 0.05, accounting for 3% lost to follow-up.
Recruitment
The initial duration of patient enrolment expected is 2 years, starting in July 2020. The 2020: approval by an independent Ethics Committee; 2020–2022: recruitment period; 2022: end of recruitment, monitoring of participating centres and queries to investigators; blind review to determine protocol violation, to define intention-to-treat and per-protocol analysis populations; new queries to investigators, cleaning and closure of the database; 2023: data analysis, writing of the manuscript and submission for publication.
Methods: assignment of intervention, data collection, management and analysis
Allocation, data collection and monitoring will be carried out according to the same methods described in the HIGH-WEAN protocol.26
Allocation and sequence intervention
A computer-generated, centre stratified randomisation is performed in a 1:1 ratio, using a centralised web-based management system (Cleannfile). The strategy assigned to the patient (intravenous or inhaled sedation) will be initiated immediately after randomisation.
Data collection and management
Data will be collected on an electronic case report form (e-CRF) by a trained investigator or research assistant at each centre. A blank copy of the e-CRF can be printed from the e-CRF. This enables the investigator or research assistant to fill it out with the data of the included patients, which will be captured. Once data collection has been completed, the investigator or research assistant shall sign and date the copy. This document will constitute an integral part of the patient’s medical records; as such, it shall be retained permanently. Data recorded in the e-CRF that originate in source documents must be consistent with each other; if they are not, the differences have got to be justified and documented. Blinded and patient identifiable data are stored separately in secure databases. All patient identifiable data are stored by the coordinating centre. Site staff will be available to facilitate the monitoring visits and ensure that all required documentation is available for review. At time of inclusion, the following data will be collected:
Patient characteristics, severity scores (Acute Physiology and Chronic Health Evaluation score, SOFA score), haemodynamics, vasoactive drug support, ventilation mechanics, laboratory findings, clinical ICU complications, length of stay and mortality will be recorded. Delirium will be assessed twice daily using the CAM-ICU.27 All these parameters will be collected each day from day 1 to ICU discharge.
Cognitive function and functional outcome will be evaluated at discharge, 3 and 12 months using two kinds of scores:
CANTAB test, combining six cognitive evaluations with an iPad during a 45–60 min medical consultation (those tests were also used in the Spice functional and neuro-psychological outcomes SPICEFANS substudy.25
PCLS, HADS, SF36 (medical outcome study SF 36), IADL performed by a clinical research associate.
Statistical methods
All the analyses will be performed by an independent statistician, following a predefined statistical analysis plan. The analysis will be performed on an intention-to-treat basis, after a blind review of the data and final database lock. All the analyses will be conducted using SAS V.9.3 statistical software (SAS Institute). A two-tailed p value equal or less than 0.05 will be considered as statistically significant. All tests, except for the primary outcome, will be exploratory.
Descriptive analysis of patient groups at baseline
Wrongly included subjects as well as those lost to follow-up will be described. Deviations from the protocol will be described. The baseline characteristics of the study participants will be described according to their randomisation group.
Analysis pertaining to the main criteria of evaluation
The frequency of delirium occurrence will be compared between the two groups using a χ2 test or an exact Fisher;s exact test if required. The probability of delirium occurrence will then be modelled (secondary analysis) using a multivariate logistic regression.
Analysis pertaining to the secondary criteria of evaluation
Secondary criteria of evaluation will be compared between the two treatment groups by means of Student’s t-test (or the Mann-Whitney U test, if necessary) for continuous quantitative variables and by means of the χ2 test (or Fisher’s exact test) for qualitative variables. Linear models and logistics models will be used to compare the two groups in multivariate analyses.
Time-to-event analyses will involve the Kaplan-Meier method and the Cox proportional hazards model.
Predetermined subgroup analysis
Duration of delirium will be compared between the two groups among patients who suffered from delirium, using the Student’s t-test or the Mann-Whitney U test if required.
Data monitoring
An investigator at each centre will be responsible for daily patient screening, enrolling patients in the study, ensuring adherence to the protocol and completing the e-CRF. Research assistants will regularly monitor all the centres on site to check adherence to the protocol and the accuracy of the data recorded.
Ethics and dissemination
Consent or assent
The patient will be included after having provided a written informed consent to the investigator according to the decision of the central Ethics Committee. If the patient is not able to understand the information given, he/she can be included if the same procedure is completed with a next of kin. After the patient’s recovery, he/she will be asked if he/she agrees to continue the trial. Her/his consent will again be necessary for the continuation of the study.
Confidentiality
Data will be handled according to French law. All original records will be archived at trial sites for 15 years. The clean database file will be anonymised and kept for 15 years.
Declaration of interest
The study is promoted by the University Hospital of Brest. Sedana Medical funded the promoter for study monitoring and will provide sedation equipment and monitoring for all the participating centres, but will have no other involvement in the study, data analysis, the writing of the manuscript, or in the decision to submit the manuscript.
Access to data
All investigators will have access to the final data set. Participant-level data sets will be made accessible on a controlled access basis.
Dissemination policy
The protocol is reported according to the Standard Protocol Items: Recommendations for Interventional Trials guidelines. Findings will be published in peer-reviewed journals and presented at local, national and international meetings and conferences to publicise and explain the research to clinicians, commissioners and service users.
Patient and public involvement
Patients and public were not involved in the study.
Discussion
International guidelines on sedation and delirium in ICU have been developed and formulated by national and international Societies.1 Concerning sedation, four messages are important: using light sedation versus deep sedation, however, there is no consensus on the definition of light, moderate and deep sedation, using a daily sedative interruption protocol or a nurse-driven sedation protocol, using propofol or dexmedetomidine over benzodiazepines even if there is no difference between propofol and benzodiazepine use for delirium prevention and even if the pooled analysis of all evaluated studies in these guidelines did not show a significant benefit of dexmedetomidine compared with a benzodiazepine infusion for duration of mechanical ventilation extubation, ICU length of stay and the risk for delirium, monitor sedation.
Benzodiazepine use is to be avoided within the ICU.1 If propofol has a more favourable pharmacokinetics than benzodiazepine, its prolonged exposure can lead to hypotension, respiratory depression, hypertriglyceridaemia, pancreatitis and to the often lethal propofol infusion syndrome.28 29
Dexmedetomidine (alpha 2 adrenergic receptor agonist) seems to reduce the delirium duration, the coma duration and even mortality in septic patients.30 31 However, dexmedetomidine is often insufficient to deeply sedate.31 Since the publication of these guidelines, the SPICE study, a recent multicentre trial enrolling 4000 patients and comparing dexmedetomidine as the sole or primary sedative to usual sedation care (propofol, midazolam or other sedatives) failed to show a mortality reduction at day 90, showed that sedation targets were difficult to obtain with dexmedetomidine as the sole agent of sedation and that adverse effects were multiplied by 10.25
The NONSEDA study (comparing a no sedation group versus a light sedation group (RASS −2; −3)) enrolled 710 patients. Mortality at 90 days did not differ significantly between those assigned to a plan of no sedation and those assigned to a plan of light sedation. Fourteen per cent of screened patients declined to participate and about one third patient should have been sedated during the first 24 hours in the no sedation group.32
Delirium during sedation administration is frequent. Rapidly improving cognitive state concerns only a minority of delirium sedated patients (14%). Majority of delirium under sedation patient has a worse long-term prognosis.33 These results have been confirmed in a large study showing that delirium associated with sedation was the most common type of delirium in ICU, but also the most strongly associated with long-term cognitive impairment.34 Moreover, safety and efficacy of alternate sedation paradigms on delirium and long-term outcomes has been defined as one of the top trials to perform in the next years by a multinational, interprofessional board.35
Potential benefits of isoflurane use in ICU are the absence of accumulation or tachyphylaxis, the wide therapeutic range, the small interindividual variation, the rapidity of efficacy, the wake up speed and the analgesia effect The duration of use of isoflurane is long and range up to 96 hours in the study by Sackey et al,36 up to 348 hours in the study by L'Her et al,13 up to 323 hours in the study by Krannich et al.37 Despite these extended times, the duration of mechanical ventilation and lenghth of stay in the ICU are shorter in the study by Krannich et al, extubations were performed earlier in the study by Jerath et al, response to simple orders and the extubation are obtained earlier in the study by Sackey et al.36–38 Randomised clinical trials (RCTs) examining volatile anesthesics effects and safety aspects in ICU are currently recruiting (NCT01983800) or have been published demonstrating the safety and acceptability in ICUs with limited experience of using volatile anesthesics-based sedation.39 Inhaled sedation has shown decrease of epithelial injury and inflammation in ARDS.14 Those results should however be confirmed in an RCT (NCT04235608). Safety use for the staff in charge of the patient has been established.17 18 Recommendations for use have been issued.40 Inhaled volatile anaesthetics to conserve intravenous sedatives agents have proven to be effective during the COVID-19 pandemic (NCT04383730).41 42 In addition, their potential neuroprotective effect would make it an anaesthetic of choice in the prevention of ICU delirium.43 44 Schoen et al report that sevoflurane improved short-term postoperative cognitive ability in patients undergoing circulatory assisted heart surgery compared with propofol.45 Dabrowski et al have confirmed in patients undergoing bypass surgery that sevoflurane and isoflurane attenuate levels of MMP-9, GFAP, specific biochemical markers of brain injury.46
All of these results stress the importance of carrying out this study whose hypothesis is that inhaled sedation strategy would decrease delirium occurrence. The use of isoflurane preferentially over sevoflurane is justified by the absence of wake-up gain by the use of sevoflurane versus isoflurane in general anaesthesia, the absence of clear haemodynamic or pharmacodynamic differences between the molecules during their use in general anaesthesia and a more pronounced bronchodilator effect of isoflurane.47–49 Sevoflurane-induced diabetes insipidus is of concern in context of long-term sedation.50
The INASED study is the first randomised, controlled and open-label trial adequately powered to determine whether inhaled sedation strategy in ICU reduces delirium. Inclusion criteria are as broad as possible. This strategy maximises recruitment rates and improves the generalisation of results. All patients will be treated using the ABCDEF bundle which implies less variation in study quality, analgesic regimens, use of daily sedation breaks, reporting depth of sedation, type of sedative drug, and duration of use.21 It is not possible to blind local investigators to allocation treatment. However, withdrawing of sedation, SBT, extubation will follow a nurse-driven protocol. Blinding of outcome data assessment is ensured as the cognitive function is evaluated by a research assistant that will not be aware of patient assignment group.
Given the current data and potential of isoflurane sedation to improve patient outcomes, INASED is a well designed, adequately powered RCT within a homogeneous population to truly understand the potential clinical effects of this sedation modality.
Supplementary Material
Footnotes
Contributors: PB and EL designed the study and wrote the manuscript together. EN provided substantial contributions to the conception and design of the study, wrote the statistical analysis plan and estimated the sample size. P-YE, SE, AWT, CG, GG, FR, OH and SJ contributed for drafting the work, revising it critically for important intellectual content and approved the final version of the manuscript. All authors gave their agreement to be accountable for all aspects of the work, and ensure the accuracy and integrity of any part of the work.
Funding: The study is funded by Sedana Medical which did not interfere with the design of the trial and have no other involvement in the study, data analysis, the writing of the manuscript, or in the decision to submit the manuscript. The study is promoted by the University Hospital of Brest.
Disclaimer: The firm Sedana provides therapy equipment and monitoring to all the participating centres but has no other involvement in the study.
Competing interests: PB reports financial support (travel expanse coverage to attend scientific meetings) from Sedana Medical. SE declares receiving consulting fees, unrestricted research grants and equipment research support from Aerogen, unrestricted research grant from Fisher & Paykel, unrestricted research grant form Hamilton medical, consulting fees from La Diffusion Technique Française. AWT reports financial support (payment for lectures and travel expanse coverage to attend scientific meetings) from Fisher & Paykel, Covidien, Maquet-Getinge and GE Healthcare. SJ reports receiving consulting fees from Drager, Medtronic, Baxter, Fresenius Medical and Fisher & Paykel. EL is cofounder and shareholder of Oxynov, a R and D Canadian company dedicated to automated oxygen administration. He is also a consultant for Sedana Medical, GE Healthcare and Smiths Medical.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Devlin JW, Skrobik Y, Gélinas C, et al. . Clinical practice guidelines for the prevention and management of pain, Agitation/Sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825–73. 9. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 2.Jackson DL, Proudfoot CW, Cann KF, et al. . The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care 2009;13:R204. 10.1186/cc8212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin JW The pharmacology of oversedation in mechanically ventilated adults. Curr Opin Crit Care 2008;14:403–7. 10.1097/MCC.0b013e32830280b3 [DOI] [PubMed] [Google Scholar]
- 4.De Jonghe B, Bastuji-Garin S, Fangio P, et al. . Sedation algorithm in critically ill patients without acute brain injury. Crit Care Med 2005;33:120–7. 10.1097/01.CCM.0000150268.04228.68 [DOI] [PubMed] [Google Scholar]
- 5.Arias-Rivera S, Sánchez-Sánchez MdelM, Santos-Díaz R, et al. . Effect of a nursing-implemented sedation protocol on weaning outcome. Crit Care Med 2008;36:2054–60. 10.1097/CCM.0b013e31817bfd60 [DOI] [PubMed] [Google Scholar]
- 6.Girard TD, Kress JP, Fuchs BD, et al. . Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet 2008;371:126–34. 10.1016/S0140-6736(08)60105-1 [DOI] [PubMed] [Google Scholar]
- 7.Pandharipande PP, Girard TD, Jackson JC, et al. . Long-Term cognitive impairment after critical illness. N Engl J Med 2013;369:1306–16. 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S, Cook D, Devlin JW, et al. . Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med 2015;43:557–66. 10.1097/CCM.0000000000000727 [DOI] [PubMed] [Google Scholar]
- 9.Griffiths J, Fortune G, Barber V, et al. . The prevalence of post traumatic stress disorder in survivors of ICU treatment: a systematic review. Intensive Care Med 2007;33:1506–18. 10.1007/s00134-007-0730-z [DOI] [PubMed] [Google Scholar]
- 10.Wade DM, Howell DC, Weinman JA, et al. . Investigating risk factors for psychological morbidity three months after intensive care: a prospective cohort study. Crit Care 2012;16:R192. 10.1186/cc11677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolters AE, Peelen LM, Welling MC, et al. . Long-Term mental health problems after delirium in the ICU. Crit Care Med 2016;44:1808–13. 10.1097/CCM.0000000000001861 [DOI] [PubMed] [Google Scholar]
- 12.Vasilevskis EE, Chandrasekhar R, Holtze CH, et al. . The cost of ICU delirium and coma in the intensive care unit patient. Med Care 2018;56:890–7. 10.1097/MLR.0000000000000975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.L'her E, Dy L, Pili R, et al. . Feasibility and potential cost/benefit of routine isoflurane sedation using an anesthetic-conserving device: a prospective observational study. Respir Care 2008;53:1295–303. [PubMed] [Google Scholar]
- 14.Jabaudon M, Boucher P, Imhoff E, et al. . Sevoflurane for sedation in acute respiratory distress syndrome. A randomized controlled pilot study. Am J Respir Crit Care Med 2017;195:792–800. 10.1164/rccm.201604-0686OC [DOI] [PubMed] [Google Scholar]
- 15.Mesnil M, Capdevila X, Bringuier S, et al. . Long-Term sedation in intensive care unit: a randomized comparison between inhaled sevoflurane and intravenous propofol or midazolam. Intensive Care Med 2011;37:933–41. 10.1007/s00134-011-2187-3 [DOI] [PubMed] [Google Scholar]
- 16.Jerath A, Panckhurst J, Parotto M, et al. . Safety and efficacy of volatile anesthetic agents compared with standard intravenous Midazolam/Propofol sedation in ventilated critical care patients: a meta-analysis and systematic review of prospective trials. Anesth Analg 2017;124:1190–9. 10.1213/ANE.0000000000001634 [DOI] [PubMed] [Google Scholar]
- 17.Sackey PV, Martling C-R, Nise G, et al. . Ambient isoflurane pollution and isoflurane consumption during intensive care unit sedation with the anesthetic conserving device. Crit Care Med 2005;33:585–90. 10.1097/01.CCM.0000156294.92415.E2 [DOI] [PubMed] [Google Scholar]
- 18.Herzog-Niescery J, Vogelsang H, Gude P, et al. . The impact of the anesthetic conserving device on occupational exposure to isoflurane among intensive care healthcare professionals. Minerva Anestesiol 2018;84:25–32. 10.23736/S0375-9393.17.11770-0 [DOI] [PubMed] [Google Scholar]
- 19.Jerath A, Ferguson ND, Steel A, et al. . The use of volatile anesthetic agents for long-term critical care sedation (VALTS): study protocol for a pilot randomized controlled trial. Trials 2015;16:560. 10.1186/s13063-015-1083-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sessler CN, Gosnell MS, Grap MJ, et al. . The Richmond Agitation-Sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–44. 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 21.Pun BT, Balas MC, Barnes-Daly MA, et al. . Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med 2019;47:3–14. 10.1097/CCM.0000000000003482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Anesthesiol Clin 2011;29:567–85. 10.1016/j.anclin.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Kermad A, Speltz J, Daume P, et al. . Reflection efficiencies of AnaConDa-S and AnaConDa-100 for isoflurane under dry laboratory and simulated clinical conditions: a bench study using a test lung. Expert Rev Med Devices 2020:1–7. 10.1080/17434440.2021.1865151 [DOI] [PubMed] [Google Scholar]
- 24.Tonnelier J-M, Prat G, Le Gal G, et al. . Impact of a nurses' protocol-directed weaning procedure on outcomes in patients undergoing mechanical ventilation for longer than 48 hours: a prospective cohort study with a matched historical control group. Crit Care 2005;9:R83–9. 10.1186/cc3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shehabi Y, Howe BD, Bellomo R, et al. . Early sedation with dexmedetomidine in critically ill patients. N Engl J Med 2019;380:2506–17. 10.1056/NEJMoa1904710 [DOI] [PubMed] [Google Scholar]
- 26.Thille AW, Muller G, Gacouin A, et al. . High-Flow nasal cannula oxygen therapy alone or with non-invasive ventilation during the weaning period after extubation in ICU: the prospective randomised controlled HIGH-WEAN protocol. BMJ Open 2018;8:e023772. 10.1136/bmjopen-2018-023772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ely EW, Inouye SK, Bernard GR, et al. . Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 2001;286:2703–10. 10.1001/jama.286.21.2703 [DOI] [PubMed] [Google Scholar]
- 28.Marik PE Propofol: therapeutic indications and side-effects. Curr Pharm Des 2004;10:3639–49. 10.2174/1381612043382846 [DOI] [PubMed] [Google Scholar]
- 29.Hemphill S, McMenamin L, Bellamy MC, et al. . Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth 2019;122:448–59. 10.1016/j.bja.2018.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandharipande PP, Pun BT, Herr DL, et al. . Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644–53. 10.1001/jama.298.22.2644 [DOI] [PubMed] [Google Scholar]
- 31.Ruokonen E, Parviainen I, Jakob SM, et al. . Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med 2009;35:282–90. 10.1007/s00134-008-1296-0 [DOI] [PubMed] [Google Scholar]
- 32.Olsen HT, Nedergaard HK, Strøm T, et al. . Nonsedation or light sedation in critically ill, mechanically ventilated patients. N Engl J Med 2020;382:1103–11. 10.1056/NEJMoa1906759 [DOI] [PubMed] [Google Scholar]
- 33.Patel SB, Poston JT, Pohlman A, et al. . Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 2014;189:658–65. 10.1164/rccm.201310-1815OC [DOI] [PubMed] [Google Scholar]
- 34.Girard TD, Thompson JL, Pandharipande PP, et al. . Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med 2018;6:213–22. 10.1016/S2213-2600(18)30062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandharipande PP, Ely EW, Arora RC, et al. . The intensive care delirium research agenda: a multinational, interprofessional perspective. Intensive Care Med 2017;43:1329–39. 10.1007/s00134-017-4860-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sackey PV, Martling C-R, Carlswärd C, et al. . Short- and long-term follow-up of intensive care unit patients after sedation with isoflurane and midazolam--a pilot study. Crit Care Med 2008;36:801–6. 10.1097/CCM.0B013E3181652FEE [DOI] [PubMed] [Google Scholar]
- 37.Krannich A, Leithner C, Engels M, et al. . Isoflurane sedation on the ICU in cardiac arrest patients treated with targeted temperature management: an observational Propensity-Matched study. Crit Care Med 2017;45:e384–90. 10.1097/CCM.0000000000002185 [DOI] [PubMed] [Google Scholar]
- 38.Jerath A, Beattie SW, Chandy T, et al. . Volatile-based short-term sedation in cardiac surgical patients: a prospective randomized controlled trial. Crit Care Med 2015;43:1062–9. 10.1097/CCM.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 39. Jerath A, Wong K, Wasowicz M, et al. . Use of inhaled volatile anesthetics for longer term critical care sedation: a pilot randomized controlled trial. Crit Care Explor 2020;2:e0281 10.1097/CCE.0000000000000281 [DOI] [Google Scholar]
- 40.Herzog-Niescery J, Seipp H-M, Weber TP, et al. . Inhaled anesthetic agent sedation in the ICU and trace gas concentrations: a review. J Clin Monit Comput 2018;32:667–75. 10.1007/s10877-017-0055-6 [DOI] [PubMed] [Google Scholar]
- 41.Jerath A, Ferguson ND, Cuthbertson B. Inhalational volatile-based sedation for COVID-19 pneumonia and ARDS. Intensive Care Med 2020;46:1563–6. 10.1007/s00134-020-06154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrière N, Bodenes L, Bailly P, et al. . Shortage of anesthetics: think of inhaled sedation! J Crit Care 2020:30686–9. 10.1016/j.jcrc.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen F, Long Z, Yin J, et al. . Isoflurane post-treatment improves outcome after an embolic stroke in rabbits. PLoS One 2015;10:e0143931. 10.1371/journal.pone.0143931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y-Z, Li T-T, Cao H-L, et al. . Recent advances in the neuroprotective effects of medical gases. Med Gas Res 2019;9:80–7. 10.4103/2045-9912.260649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoen J, Husemann L, Tiemeyer C, et al. . Cognitive function after sevoflurane- vs propofol-based anaesthesia for on-pump cardiac surgery: a randomized controlled trial. Br J Anaesth 2011;106:840–50. 10.1093/bja/aer091 [DOI] [PubMed] [Google Scholar]
- 46.Dabrowski W, Rzecki Z, Czajkowski M, et al. . Volatile anesthetics reduce biochemical markers of brain injury and brain magnesium disorders in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2012;26:395–402. 10.1053/j.jvca.2011.10.014 [DOI] [PubMed] [Google Scholar]
- 47.Nyktari V, Papaioannou A, Volakakis N, et al. . Respiratory resistance during anaesthesia with isoflurane, sevoflurane, and desflurane: a randomized clinical trial. Br J Anaesth 2011;107:454–61. 10.1093/bja/aer155 [DOI] [PubMed] [Google Scholar]
- 48.Freiermuth D, Mets B, Bolliger D, et al. . Sevoflurane and Isoflurane-Pharmacokinetics, hemodynamic stability, and cardioprotective effects during cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2016;30:1494–501. 10.1053/j.jvca.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 49.Zorrilla-Vaca A, Núñez-Patiño RA, Torres V, et al. . The impact of volatile anesthetic choice on postoperative outcomes of cardiac surgery: a meta-analysis. Biomed Res Int 2017;2017:1–12. 10.1155/2017/7073401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.L'Heudé M, Poignant S, Elaroussi D, et al. . Nephrogenic diabetes insipidus associated with prolonged sedation with sevoflurane in the intensive care unit. Br J Anaesth 2019;122:e73–5. 10.1016/j.bja.2019.02.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


