Abstract
Introduction
Temporary ileostomy is a valuable aid in reducing the severity of complications related to rectal cancer surgery. However, it is still unclear what is the best timing of its closure in relation to the feasibility of an adjuvant treatment, especially considering patient-reported outcomes and health system costs. The aim of the study is to compare the results of an early versus late closure strategy in patients with indication to adjuvant chemotherapy after resection for rectal cancer.
Methods and analysis
This is a prospective multicentre randomised trial, sponsored by Rete Oncologica Piemonte e Valle d’Aosta (Oncology Network of Piedmont and Aosta Valley-Italy). Patients undergone to rectal cancer surgery with temporary ileostomy, aged >18 years, without evidence of anastomotic leak and with indication to adjuvant chemotherapy will be enrolled in 28 Network centres. An early closure strategy (between 30 and 40 days from rectal surgery) will be compared with a late one (after the end of adjuvant therapy). Primary endpoint will be the compliance to adjuvant chemotherapy with and without ileostomy. Complications associated with stoma closure as well as quality of life, costs and oncological outcomes will be assessed as secondary endpoints.
Ethics and dissemination
The trial will engage the Network professional teams in a common effort to improve the treatment of rectal cancer by ensuring the best results in relation to the most correct use of resources. It will take into consideration both the patients’ point of view (patient-reported outcome) and the health system perspective (costs analysis). The study has been approved by the Ethical Review Board of Città della Salute e della Scienza Hospital in Turin (Italy). The results of the study will be disseminated by the Network website, medical conferences and peer-reviewed scientific journals.
Trial registration number
Keywords: quality in health care, colorectal surgery, chemotherapy
Strengths and limitations of this study.
The study will involve all the referral centres for the treatment of colorectal cancer in the Northwestern area of Italy in a joint effort to improve the quality of rectal cancer care.
It will have a high external validity given the large multicentric territorial context and the pragmatic approach with large inclusion criteria.
The results will be analysed taking into account both the point of view of patients (patient-reported outcome analysis) and the costs for the health system.
The main weakness of the study is the relative rarity of the patient population that could slow the accrual rate.
Introduction
The temporary ileostomy is effective in reducing the severity of anastomotic complications in anterior resections for tumours of the rectum at risk of dehiscence and is therefore widely used, particularly after radiotherapy treatment.1 In patients with indication for adjuvant chemotherapy, current practice consists of closing the stoma after the end of treatment.
The prolonged presence of the stoma can however favour the onset of stoma-related complications, such as prolapse, parastomal hernia, mechanical ileus, high-flow dehydration and damage to kidney function. Ostomy-related complications may require unscheduled hospitalisations and result in increased costs.2 In addition, the presence of the stoma impacts on patients’ quality of life (QoL), causing alteration of the body image and imposing changes in the daily routine, lifestyle and sexual sphere.3 4 Therefore, early closure of the ileostomy has been proposed in patients without signs of postoperative fistula.
The early closure (within 1 month of surgery) of the temporary ileostomy resulted not inferior to late closure (over 12 weeks) in two randomised studies that evaluated postoperative complications as an outcome.5 6 Early closure saves days of life with ileostomy for the patient and costs related to ostomy care for the health system and could represent the most desirable and convenient choice.7 It was also associated with better long-term functional results in a secondary analysis of a randomised study.8 However, in patients with indication for adjuvant therapy, it is not known what the best timing is for closing the stoma (before the start, during or at the end of the treatment) in terms of therapy tolerability, QoL and overall costs.9
Both a start date delayed more than 8 weeks from surgery10 and a received dose <70% of that planned11 have been reported to reduce the effectiveness of adjuvant therapy in colorectal cancer patients. The presence of a stoma or the consequences of its early closure may interfere with an optimal delivery of chemotherapy. A recent multicentre retrospective study reported an increase in gastrointestinal toxicity in chemo-treated patients with stoma, with significant reduction in treatment compliance.12 On the other hand, early closure of the stoma could reveal an low anterior resection syndrome (LARS) before chemotherapy, with a potential negative impact on the tolerability of the treatment itself,13 or delay its initiation due to postoperative complications.14
This randomised study aims to identify the best timing for the stoma closure in relation to adjuvant therapy in terms of compliance to chemotherapy, complications, costs and QoL.
Methods and analysis
STOMAD is a multicentre open-label randomised phase III trial designed to evaluate the best timing of the closure of temporary ileostomy in patients operated on for rectal cancer and with indication for adjuvant chemotherapy.
Objectives
-
Primary objective
To compare the compliance with adjuvant therapy between early and late closure of temporary ileostomy.
The compliance with adjuvant chemotherapy in relation to the timing of ileostomy closure (before the start or after the end of treatment) will be assessed considering any therapeutic delay or dose reduction compared with the initially planned.
-
Secondary objectives
To compare patients with early and late closure of temporary ileostomy in terms of:
Surgical morbidity.
Chemotherapy toxicity.
Patient-reported QoL.
Costs.
Progression-free survival (PFS).
Overall survival (OS).
Target population and setting
Patients undergone to rectal resection (±neoadjuvant therapy) for cancer with protective ileostomy and candidates for adjuvant chemotherapy in the Centres for the treatment of colorectal neoplasms recognised by Rete Oncologica Piemonte e Valle d’Aosta.
The list of participating centres is reported in table 1.
Table 1.
List of participating centres
| Local PI | Local trial manager | Centre | Location |
| Paolo Millo | Elisa Ponte | AUSL Aosta–Surgical Unit–Parini Hospital | Aosta |
| Mario Morino | Massimiliano Mistrangelo | AOU Città Salute e Scienza–Academic Surgical Unit–Molinette Hospital | Torino |
| Paolo De Paolis | Mauro Santarelli | AOU Città Salute e Scienza–Surgical Unit–Molinette Hospital | Torino |
| Alessandro Ferrero | Paolo Massucco | AO Ordine Mauriziano–Surgical Unit–Umberto I Hospital (Coordineting centre) | Torino |
| Maurizio Degiuli | Rossella Reddavid | AOU S. Luigi Gonzaga–Academic Surgical Unit–S. Luigi Hospital | Orbassano (TO) |
| Roberto Saracco | Francesco Tomaselli | ASL Città Torino–Surgical Unit–Martini Hospital | Torino |
| Mauro Garino | Simone Birolo | ASL TO3–Surgical Unit–Infermi Hospital | Rivoli (TO) |
| Andrea Muratore | Marcello Calabrò | ASL TO3–Surgical Unit–Agnelli Hospital | Pinerolo (TO) |
| Nicoletta Pipitone | |||
| Lodovico Rosato | Luca Panier Suffat | ASL TO4–Surgical Unit–Civile Hospital | Ivrea (TO) |
| Eraldo Personnettaz | Monica Carrera | ASL TO4–Surgical Unit–Ciriè Hospital | Ciriè (TO) |
| Pietro Cumbo | Francesco Potente | ASL TO5–Surgical Unit–S. Croce Hospital | Moncalieri (TO) |
| Felice Borghi | Maria Carmela Giuffrida | AO S. Croce e Carle–Surgical Unit–S. Croce Hospital | Cuneo |
| Franco Bertolino | Marco Brunetti | ASL CN1–Surgical Unit–SS. Annunziata Hospital | Savigliano (CN) |
| Andrea Gattolin | Roberto Rimonda | ASL CN1–Surgical Unit–Regina Montis Regalis Hospital | Mondovì (CN) |
| Marco Calgaro | Vincenzo Adamo | ASL CN2–Surgical Unit–S. Lazzaro Hospital | Alba (CN) |
| Fabio Priora | Igor Monsellato | AO SS. Antonio e Biagio e Cesare Arrigo-Surgical Unit–SS. Antonio e Biagio Hospital | Alessandria |
| Domenico Piscioneri | |||
| Marco Amisano | Francesco Cravero | ASL AL–Surgical Unit–S. Spirito Hospital | Casale Monf.to (AL) |
| Alberto Serventi | Alberto Serventi | ASL AL–Surgical Unit–Mons. Galliano Hospital | Acqui Terme (AL) |
| Carmine Di Somma | Eliana Giaminardi | ASL AL–Surgical Unit–S. Giacomo Hospital | Novi Ligure (AL) |
| Vincenzo Sorisio | Luca Mazza | ASL AT–Surgical Unit–Cardinal Massaia Hospital | Asti |
| Sergio Gentilli | Paolo Bellora | AOU Maggiore Carità–Academic Surgical Unit–Maggiore Hospital | Novara |
| Raffaele Romito | Fabio Colli | AOU Maggiore Carità–Surgical Unit–Maggiore Hospital | Novara |
| Roberto Polastri | Roderto Perinotti | ASL BI–Surgical Unit–Infermi Hospital | Biella |
| Silvio Testa | Clemente De Rosa | ASL VC–Surgical Unit–S. Andrea Hospital | Vercelli |
| Sandro Zonta | Francesco Battafarano | ASL VCO–Surgical Unit – S. Biagio Hospital | Domodossola (VB) |
| Renza Trapani | ASL VCO–Surgical Unit – Castelli Hospital | Verbania (VB) | |
| Dario Ribero | Alfredo Mellano | FPO–Colorectal Surgical Unit – IRCCS | Candiolo (TO) |
| Renzo Leli | Paola Bellomo | Surgical Unit–Humanitas Gradenigo Hospital | Torino |
| Carlo Bima | Enrico Gibin | Surgical Unit–Cottolengo Hospital | Torino |
PI, Principal Investigator.
Enrolment
Inclusion criteria
Patients undergone to radical intestinal resection (R0) for rectal neoplasia with protective ileostomy.
Age ≥18 years.
Absence of fistula (enema and/or endoscopy).
Indication to adjuvant chemotherapy.
Informed consent.
Exclusion criteria
ASA >3.
UICC stage IV.
ECOG Performance Status ≥2.
Severe and non-controlled systemic, oncological or infectious disease.
Before enrolment, the patient shall not show signs of ongoing complications. The integrity of the colorectal anastomosis will be confirmed with an enema and/or endoscopy according to local standards starting 15 days after surgery.
The presence of a discontinuation of the anastomotic rhyme in endoscopy or of a spreading of any entity of the contrast medium on the enema will represent an exclusion criterion. All patients will simultaneously perform an oncological evaluation to establish the indication for adjuvant therapy in the presence of the definitive histological examination. Patients without signs of anastomotic complications and with indication for adjuvant therapy will be enrolled for study by the local investigators. Enrolment and randomisation must take place within 21 days of the intervention.
The subjects who are eligible for the study, after the informed consent has been signed, will be stratified by previous neoadjuvant treatment (yes or no) and by the proposed adjuvant chemotherapy scheme (with or without platinum derivatives), and then randomised to one of the following arms:
Arm A (experimental): closure of the stoma between 30 and 40 days after surgery on the rectum, before starting adjuvant therapy.
Arm B (standard): closure of the stoma starting from 15 days and within 60 days from the end of the adjuvant therapy. The anastomosis instrumental evaluation will be repeated after the end of chemotherapy in this group.
Endpoint definition
-
Primary endpoint.
Proportion of patients with adequate compliance with adjuvant treatment.
Compliance with adjuvant therapy will be considered adequate if both of the following criteria are met: start of adjuvant therapy within the 70th day (≤10 weeks) after surgery on the rectum; and total cumulative dose delivered, compared with the theoretical planned, ≥70%.
Failure to adhere to at least 1 of the two criteria will correspond to a failure (inadequate compliance).
Patients with missing or non-performed assessment of compliance for any reason will also be considered unsuccessful adherence.
-
Secondary endpoints.
Morbidity. Incidence of complications related to the presence or to the closure of the ileostomy, during the hospitalisation or after discharge, using the Clavien-Dindo classification. Individual patient events, hospitalisations and reoperations will be recorded.
Chemotherapy toxicity. All adverse events according to CTCAE V.5.0 classification will be considered.
QoL. Patient-reported QoL will be measured at the baseline and at defined time points using validated questionnaires (EORTC C30 and CR29, EQ5D). Bowel function will be evaluated at 12 months from randomisation by means of the LARS score.
Costs. The costs related to hospitalisation, outpatient visits, ostomy care supplies and the management of complications and toxicity will be assessed.
PFS defined as the time elapsed between the randomisation date and the date of progression/death for any cause or the latest follow-up available.
OS defined as the time elapsed between the randomisation date and the date of death for any cause or the latest follow-up available
Patients will be followed for the duration of the study, regardless of the clinical course, and will conclude the active follow-up with a final evaluation 12 months after randomisation in both study arms. A longer follow-up, based only on routinely recorded data, will be conducted to assess long term OS. Enrolment is expected to start in September 2020.
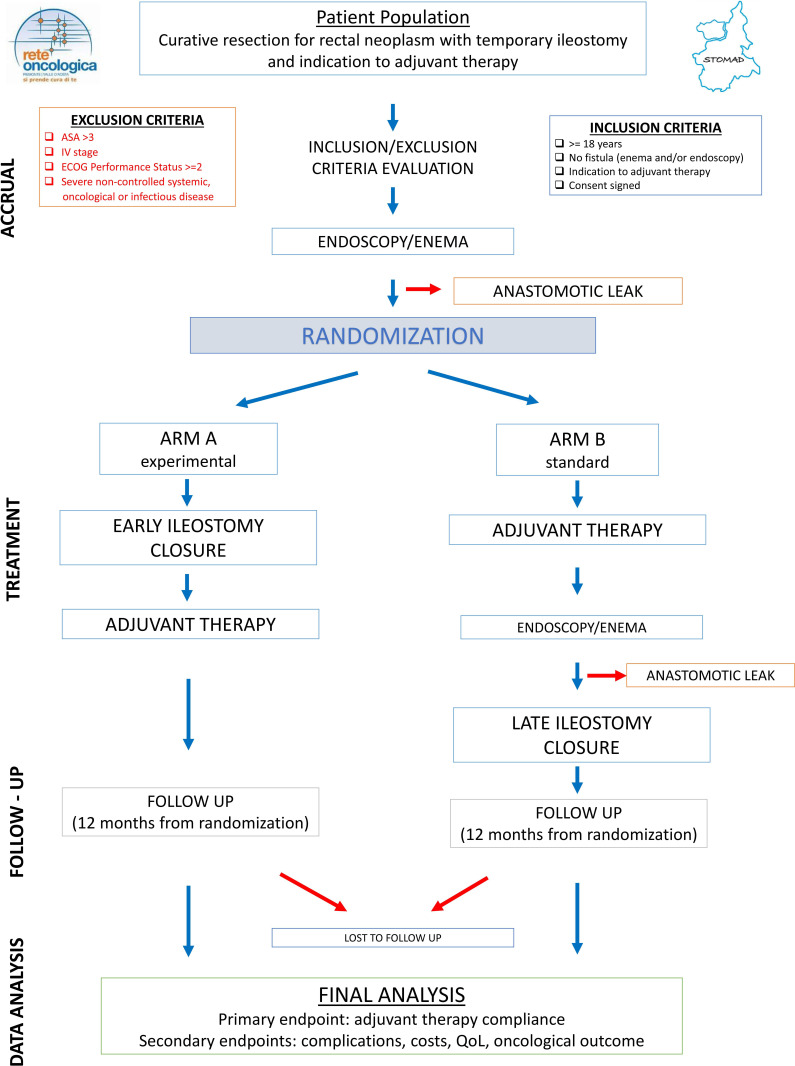
The study flow diagram is depicted in figure 1.
Figure 1.
Study flow diagram. QoL, quality of life.
Surgical technique and medical therapy
Hospitalisation will normally take place the day before or the morning of surgery. Antibiotic prophylaxis (usually short term with cefazoline within half an hour after skin incision) and thrombus embolic prophylaxis will be performed according to national guidelines.
The stoma closure will be performed manually or mechanically according to the surgeon’s judgement. The suture of the skin incision will be linear or purse string according to the local standards.
The postoperative management will be based on the ERAS strategy (early feeding and mobilisation). Discharge criteria will be passage of gas, adequate oral feeding and good pain control.
Adjuvant chemotherapy will be administered, in terms of indications, drugs and schedules, according to the national guidelines (AIOM) and according to the consensus documents of the Colorectal Study Group of the Oncology Network (http://www.reteoncologica.it/area-operatori/gruppi-per-patologie/raccomandazioni-di-rete).
The ideal temporal target for the start of chemotherapy will be within 8 weeks of surgery on the rectum; the maximum time within 10 weeks. Randomised patients starting after this term are still followed up and evaluated until the end of follow-up for the evaluation of the other endpoints.
Endpoints assessment and follow-up
Morbidity
Complications related to stoma closure surgery, which occurred both during and after hospitalisation, will be recorded according to the Clavien-Dindo classification. Their overall weight per patient will be calculated through the CCI. Management problems and complications from the stoma presence will also be recorded. All reinterventions and hospitalisations during the study period will be captured.
Adjuvant therapy
Chemotherapy toxicity will be evaluated according to the CTC-EORTC. Prophylaxis and treatment of side effects and dose reductions will be applied according to international standards (NCI-CTCAE criteria). Starting date of the treatment, dose reductions and therapeutic scheme variations, suspensions or interruptions will be recorded. In relation to the total dose and the total number of programmed cycles, the percentage of completeness of the adjuvant therapy will be calculated. Grade and type of adjuvant therapy toxicity will be recorded for each patient.
Quality of life
QoL will be measured using the EORTC C30, CR29 and EQ5D validated questionnaires. The questionnaires will be administered, in both arms, on enrolment (baseline), at the beginning of the fourth cycle of adjuvant therapy and at 12 months after the intervention on rectal cancer.
The LARS score will be used to evaluate intestinal and sphincter function at 12 months after the rectal intervention in both arms (in the control group if ostomy closed since at least 2 months).
Costs
The costs will be estimated considering the days of hospitalisation related to the closure of the stoma, the treatment of complications or toxicity, the outpatient visits during the study period and the amount of supplies for stoma care. Regional averages costs will be used as the basic cost unit.
Statistical considerations
Sample size
The sample size was calculated in relation to the main objective. The null hypothesis (proportion of patients with adequate compliance to the adjuvant treatment in patients with closure of the stoma after treatment) was inferred from data of randomised trials of adjuvant chemotherapy after rectal cancer resection15 16 and studies on chemotherapy toxicity directly related to the presence of an ostomy,12 and was set at 0.70. The alternative hypothesis is an increase in the proportion up to 0.85, with an absolute increase of 0.15. This increase is considered clinically relevant. With a two-tailed alpha error of 0.05 and a power of 0.80, the sample size required is at least 242 patients (121 for each treatment arm). Taking into account a maximum drop-out rate of approximately 10%, the total number of patients enrolled and randomised will be 270. The estimated study duration is 36 months.
Randomisation
The randomisation list, stratified by neoadjuvant therapy (yes/no) and by type of planned adjuvant chemotherapy (fluoropyrimidine±platinum derivatives) will be generated by the Clinical Epidemiology Unit of Città della Salute e della Scienza University Hospital in Turin, using a block procedure of variable length in random order, completely concealed to clinicians. The 1:1 randomisation will be done online. After entering the inclusion/exclusion criteria and the stratification variables into the database, the patient will be randomised and registered in arm A or B. The computerised randomisation system will be accessible continuously.
Analysis
The principal analyses will be carried out on all subjects randomised according to the assigned treatment arm (intention to treat principle). The demographic and baseline characteristics will be described for the whole study population and for each treatment arm. Discrete variables will be summarised by frequencies and percentages. The continuous variables will be summarised with the use of standard measures of central tendency and dispersion (mean and SD or median deviation and IQR). The analysis of the primary endpoint will be based on the comparison of treatment compliance between the two arms by means of a stratified χ2 test. As a sensitivity analysis, further potential confounders detected at the baseline will be included in a logistic regression model. OS and PFS, calculated from the randomisation date, will be assessed with the Kaplan-Meier method and the differences in survival (overall and disease free) will be tested using the stratified Log-rank test. The 95% CI will be calculated for all the study endpoints. HR, adjusted for the stratification criteria and the main prognostic factors, will be estimated using the Cox model. Planned subgroup analyses will be carried out for the two stratification factors of the randomisation (neoadjuvant treatment and adjuvant chemotherapy) and by age (divided into three classes according to the tertiles) using interaction terms between treatment arm and the subgroup variable in the regression models. Multiplicity adjustments for secondary outcomes and subgroup analyses will not be performed because these results will be considered exploratory and no claims will be made on them. The incidence of individual adverse events during hospitalisation will be compared using the χ2 test or the Fisher’s exact test, as appropriate. The comparison on the QoL will be evaluated by comparing the average score between the two groups with the Student’s t-test (or with a non-parametric test and quartile regression, if necessary) and with generalised linear mixed model to take into account the repeated measurements over time on the same subjects. Per-protocol analyses will be performed for exploratory purposes.
Data collection
The data will be collected in each participating centre by filling in an electronic CRF. A local study manager will be identified for each participating centre. The completeness and congruity of the data will be checked periodically by a central study monitor and overviewed the study’s Steering Committee. The central monitor and Steering Committee will refer to the local managers for any request for clarification.
Ethics and dissemination
The Rete Oncologica Piemonte e Valle d’Aosta is a multidisciplinary organisation that includes specialists involved in the treatment of cancer disease in the north-western territory of Italy. The aim of the Oncology Network is to reduce the variability of treatments, guarantee uniform access to and improve the quality of cancer care. To this end, the Network issues recommendations, drafted through a peer-review process by its members, and defines the criteria for the designation as referral centres for cancer specific procedures.
STOMAD is a non-profit study conducted within the Network centres and is part of the research branch aimed at improving the healthcare delivery system. It is proposed to investigate which is the best adjuvant treatment delivery strategy in relation to the presence of the stoma for patients operated on for rectal cancer, taking into consideration both the patient’s point of view (patients reported outcome) and the health system perspective (costs analysis).
The study will be conducted according to the principles of the Helsinki Declaration and the ICH Guideline for Good Clinical Practice. It will be approved by the reference Ethics Committee of each participating centre. Each enrolled patient must express a written consent (the consent form in original language is provided as online supplemental material). Consent can be revoked at any time. Patients data will be collected on an existing online platform created by the clinical epidemiology unit of the main centre in the region, which will also be responsible for all statistical analyses. The data collected for the study will be processed in accordance with current national legislation (personal data protection code). The trial steering committee may request the premature termination of the study in case of adverse events with severity and frequency significantly higher than expected or if the primary end point in the experimental group is significantly worse than the control group before the end of the study. For these evaluations, the steering committee will not use predefined statistical criteria (statistical stopping rules) but will base the decision on a careful quantitative and qualitative evaluation of the events that will be discussed in scheduled meetings.
bmjopen-2020-044692supp001.pdf (83.4KB, pdf)
This trial will engage the Network professional teams in a common effort to improve the treatment of rectal cancer by ensuring the best results in relation to the most correct use of resources (value-based healthcare). Other positive effects could be the strengthening of collaboration relationships between the Network centres and the definition of a common platform for future Network research.
The results of this study will be presented at national and international meetings and reported in the Network website. A manuscript with the final results will be submitted for publication in a peer-reviewed journal.
Supplementary Material
Acknowledgments
A special acknowledgment goes to the Director of Rete Oncologica Piemonte e Valle d’Aosta, Dr O. Bertetto, for his support to this multicentre protocol and to Drs F. Saccona and M. Abdallah for the elaboration of the online platform.
Footnotes
Twitter: @renza.trapani@aslvco.it
Contributors: PM proposed the conception and design of the study protocol. AF, MMin, SP, GC, CG, MCG, DM, IM, MKP, RP, PR, CM and FS contributed to the initial conception, design, and the drafting of the protocol and represent the trial Steering Committee. EP, MMis, MS, FT, RR, SB, MC, NP, LPS, MC, FP, MB, RR, VA, DP, FCr, AS, EGi, LM, PBellora, FCo, CDR, FB, RT, AM, EGi and PBelloro evaluated and approved the protocol and will be responsible for local patient accrual and data registration. All authors have approved the final submitted manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Montedori A, Cirocchi R, Farinella E, et al. . Covering ileo- or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev 2010;55 10.1002/14651858.CD006878.pub2 [DOI] [PubMed] [Google Scholar]
- 2.Malik T, Lee MJ, Harikrishnan AB. The incidence of stoma related morbidity - a systematic review of randomised controlled trials. Ann R Coll Surg Engl 2018;100:501–8. 10.1308/rcsann.2018.0126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown H, Randle J. Living with a stoma: a review of the literature. J Clin Nurs 2005;14:74–81. 10.1111/j.1365-2702.2004.00945.x [DOI] [PubMed] [Google Scholar]
- 4.Herrle F, Sandra-Petrescu F, Weiss C, et al. . Quality of life and timing of stoma closure in patients with rectal cancer undergoing low anterior resection with diverting stoma: a multicenter longitudinal observational study. Dis Colon Rectum 2016;59:281–90. 10.1097/DCR.0000000000000545 [DOI] [PubMed] [Google Scholar]
- 5.Alves A, Panis Y, Lelong B, et al. . Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. Br J Surg 2008;95:693–8. 10.1002/bjs.6212 [DOI] [PubMed] [Google Scholar]
- 6.Danielsen AK, Park J, Jansen JE, et al. . Early closure of a temporary ileostomy in patients with rectal cancer: a multicenter randomized controlled trial. Ann Surg 2017;265:284–90. 10.1097/SLA.0000000000001829 [DOI] [PubMed] [Google Scholar]
- 7.Park J, Angenete E, Bock D, et al. . Cost analysis in a randomized trial of early closure of a temporary ileostomy after rectal resection for cancer (easy trial). Surg Endosc 2020;34:69–76. 10.1007/s00464-019-06732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keane C, Park J, Öberg S, et al. . Functional outcomes from a randomized trial of early closure of temporary ileostomy after rectal excision for cancer. Br J Surg 2019;106:645–52. 10.1002/bjs.11092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tulchinsky H, Shacham-Shmueli E, Klausner JM, et al. . Should a loop ileostomy closure in rectal cancer patients be done during or after adjuvant chemotherapy? J Surg Oncol 2014;109:266–9. 10.1002/jso.23493 [DOI] [PubMed] [Google Scholar]
- 10.Biagi JJ, Raphael MJ, Mackillop WJ, et al. . Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335–42. 10.1001/jama.2011.749 [DOI] [PubMed] [Google Scholar]
- 11.Aspinall SL, Good CB, Zhao X, et al. . Adjuvant chemotherapy for stage III colon cancer: relative dose intensity and survival among Veterans. BMC Cancer 2015;15:62. 10.1186/s12885-015-1038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson JP, Wells CI, Vather R, et al. . Effect of diversion ileostomy on the occurrence and consequences of chemotherapy-induced diarrhea. Dis Colon Rectum 2016;59:194–200. 10.1097/DCR.0000000000000531 [DOI] [PubMed] [Google Scholar]
- 13.Siassi M, Hohenberger W, Lösel F, et al. . Quality of life and patient’s expectations after closure of a temporary stoma. Int J Colorectal Dis 2008;23:1207–12. 10.1007/s00384-008-0549-2 [DOI] [PubMed] [Google Scholar]
- 14.Chow A, Tilney HS, Paraskeva P, et al. . The morbidity surrounding reversal of defunctioning ileostomies: a systematic review of 48 studies including 6,107 cases. Int J Colorectal Dis 2009;24:711–23. 10.1007/s00384-009-0660-z [DOI] [PubMed] [Google Scholar]
- 15.Hofheinz R-D, Wenz F, Post S, et al. . Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579–88. 10.1016/S1470-2045(12)70116-X [DOI] [PubMed] [Google Scholar]
- 16.Glynne-Jones R, Counsell N, Quirke P, et al. . Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 2014;25:1356–62. 10.1093/annonc/mdu147 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-044692supp001.pdf (83.4KB, pdf)