Abstract
Introduction
Acute kidney injury (AKI) is one of the most common organ dysfunction in sepsis, and increases the risk of unfavourable outcomes. Renal replacement therapy (RRT) is the predominant treatment for sepsis-associated AKI (SAKI). However, to date, no prospective randomised study has adequately addressed whether initiating RRT earlier will attenuate renal injury and improve the outcome of sepsis. The objective of the trial is to compare the early strategy with delayed strategy on the outcomes in patients with SAKI in the intensive care unit (ICU).
Methods and analysis
This is a large-scale, multicentre, randomised controlled trial about SAKI. In total, 460 patients with sepsis and evidence of AKI stage 2 of Kidney Disease Improving Global Outcomes (KDIGO) will be recruited and equally randomised into the early group and the delay group in a ratio of 1:1. In the early group, continuous RRT (CRRT) will be started immediately after randomisation. In the delay group, CRRT will initiated if at least one of the following criteria was met: stage 3 of KDIGO, severe hyperkalaemia, pulmonary oedema, blood urea nitrogen level higher than 112 mg/dL after randomisation. The primary outcome is overall survival in a 90-day follow-up period (90-day all-cause mortality). Other end points include 28-day, 60-day and 1-year mortality, recovery rate of renal function by day 28 and day 90, ICU and hospital length of stay, the numbers of CRRT-free days, mechanical ventilation-free days and vasopressor-free days, the rate of complications potentially related to CRRT, CRRT-related cost, and concentrations of inflammatory mediators in serum.
Ethics and dissemination
The trial has been approved by the Clinical Research and Application Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University (2017–31-ks-01). Participants will be screened and enrolled from patients in the ICU with SAKI by clinicians, with no public advertisement for recruitment. Results will be disseminated in research journals and through conference presentations.
Trial registration
Keywords: acute renal failure, dialysis, infectious diseases, intensive & critical care
Strengths and limitations of this study.
The CRTSAKI Study is one of the very few large-scale, multicentre, prospective, two-arm randomised controlled trials designed to compare early with delayed initiation of continuous renal replacement therapy (CRRT) on the outcomes in patients with sepsis-associated acute kidney injury (SAKI) in the intensive care unit.
All participants will be enrolled at Kidney Disease Improving Global Outcomes stage 2 AKI and receive CRRT within 8 hours of randomisation in the early group.
Positive results will help clinicians choose the appropriate timing to initiate CRRT and improve outcomes of SAKI.
The risk of ‘unnecessary’ CRRT and the rate of complications potentially related to CRRT might increase in the early group.
A limitation of the study is its single-blind design, which would yield bias, although blind evaluation is adopted to minimise bias.
Introduction
As a life-threatening syndrome with organ dysfunction caused by infection, sepsis continues to be a major global concern because of its increasing incidence and high mortality.1 2 As far as we know, sepsis is a leading cause of acute kidney injury (AKI) in the intensive care unit (ICU). The presence of AKI significantely increases the mortality of sepsis and is an independent risk factor for the death of patients with sepsis. It is reported that the mortality of sepsis-associated AKI (SAKI) ranges from 30% to 60%.3–6
Undoubtedly, renal replacement therapy (RRT) is an important method for AKI. But the optimal timing of RRT initiation remains controversial. Several studies have tried to provide an answer to this dilemma. A meta-analysis by Karvellas et al7 in 2011 demonstrated that earlier initiation of RRT may have a significant improvement in 28-day mortality. Several meta-analyses in recent years8–10 suggested that while early initiation of RRT didn’t provide an advantage of improving survival, it was associated with a significant reduction in hospital length of stay (LOS). However, the strength of the conclusion was weakened by the heterogeneous definition of ‘early’ or ‘late’ in these studies. Two high-quality RCTs published in 2016 also reported contradictory results. The Early versus Late Initiation of Renal Replacement Therapy in Citical Ill Patients with Acute Kidney Injury (ELAIN) Study11 showed that early initiation of RRT significantly reduced 90-day mortality. Here ‘early RRT’ was initiated at stage 2 of Kidney Disease Improving Global Outcomes (KDIGO) classification, and ‘delayed RRT’ was initiated at stage 3 of KDIGO classification or if absolute indications for RRT were present. The Artificial Kidney Initiation in Kidney Injury (AKIKI) Study12 found no significant difference in 60-day mortality between an early and a delayed strategy for the initiation of RRT. In this study, all patients were required to have KDIGO stage 3 AKI. ‘Early’ meant initiating RRT within 6 hours after randomisation, and ‘delayed’ meant initiating RRT if the absolute indications for RRT were present or if oliguria or anuria lasted for more than 72 hours after randomisation. Another large RCT, Standard versus Accelerated Initiation of Renal Replacement Therapy in Acute Kidney Injury (STARRT-AKI), published in this year, that included 3019 patients with a twofold increase in serum creatinine (SCr), demonstrated that an accelerated renal-replacement strategy was not associated with a lower risk of death at 90 days than a standard strategy. In this study, the timing of RRT initiation in the accelerated-strategy group was comparable to that of the ELAIN Study, while the timing of RRT initiation in the standard strategy group was comparable to that of the AKIKI Study.13 In addition to differences in population, it is hard to exclude that the differences in outcomes of these studies are caused by differences in timing of RRT initiation.
When compared with non-septic AKI, patients with SAKI are generally sicker, with greater aberrations in haemodynamics and laboratory parameters, have a higher need for mechanical ventilation and vasoactive drug therapy, and have a longer duration of stay in both ICU and hospital.14 15 The pathophysiology of SAKI is complex and remains only partially understood. Animal studies revealed that at the early stage SAKI mainly involves functional changes and minimal structural kidney lesions.16 In the past, it was believed that renal hypoperfusion was the major contributor of SAKI. Recently, our understanding of this topic has improved. Inflammatory damage was thought to be the key pathophysiological process. Toll-like receptors recognise pathogen-associated molecular pattern and damage-associated molecular pattern, trigger inflammation and tissue injury, induce microvascular thrombus formation, vascular permeability and interstitial oedema, impair renal microcirculatory disturbance, cause renal venous congestion, and lower the glomerular filtration rate at last.17 18 It is reported that a correlation was observed between the concentrations of circulating inflammatory cytokines and mortality in patients with septic shock.19–21 In an observational study, Sood et al3 found that early reversible AKI was associated with improved survival in sepsis. Therefore, the hypothesis is that adequate removal of inflammatory mediators from the circulation, avoiding fluid overload to reduce renal venous congestion and interstitial oedema, may provide a potential therapy for this devastating condition. It is demonstrated that early continuous RRT (CRRT) can stabilise the internal environment and remove inflammatory mediators.22 Thus, it is believed that early strategy may control excessive inflammation as early as possible, thus reducing kidney injury and improving survival. A systematic review and meta-analysis in 2019 of five trials,23 that included 900 patients with SAKI, suggests that earlier initiation is not associated with improved survival. However, the finding should be interpreted with caution. In fact, three of them suggested that early initiation of CRRT could improve 28-day mortality, but the sample size was small.24–26 Among these five studies, one was a multicentre RCT, Initiation of Dialysis Early versus Delayed in the Intensive Care Unit (IDEAL-ICU), which included 488 patients from 29 mixed medical and surgical ICUs. RRT was initiated within 12 hours following the diagnosis of Risk, Injury, Failure, Loss and End-stage renal failure criteria (RIFLE) failure (RIFLE-F) AKI (equivalent to KDIGO stage 3) in the early group, and 48 hours after diagnosis of RIFLE-F AKI or if the absolute indications for RRT were present in the delayed group. No difference was seen between the two strategies. However, 41 patients (17 %) in the delayed group needed emergency RRT. Of note, mortality in this subgroup increased significantly (68 %).27
To the best of our knowledge, only one such large randomised controlled study focusing on the timing of RRT in patients with SAKI has been published.27 More studies are needed to clarify this issue. Moreover, considering the characteristics of SAKI, the ‘early strategy’ in the AKIKI Study and the IDEAL-ICU Study may still be too late. Therefore, we propose another multicentre RCT to investigate the impact of timing of CRRT initiation on mortality in patients with SAKI.
Methods and analysis
Study design, setting and patient population
The Continuous RRT Timing in Sepsis-associated AKI in ICU (CRTSAKI) Study is a prospective, open-label, two-arm, multicentre, randomised, controlled study. All patients admitted to the ICUs of participating centres will be considered as potential candidates for the study. Once the patient is diagnosed with SAKI stage 2, he/she should be screened for eligibility by physicians within 2 hours. When the patient fulfils the criterion of recruitment, written informed consent (online supplemental file 1) should be obtained from the patient or a responsible surrogate before randomisation.
bmjopen-2020-040718supp001.pdf (53.1KB, pdf)
The study will be conducted in 13 ICUs in Guangdong, China. Patient enrolment, intervention and follow-up are performed at the Second Affiliated Hospital of Guangzhou Medical University, Dongguan People’s Hospital, Guangdong Provincial People’s Hospital, Guangdong No.2 Provincial People’s Hospital, Guangzhou Red Cross Hospital, Huizhou Municipal Central Hospital, Guangzhou Panyu Central Hospital, the Sixth Affiliated Hospital of Guangzhou Medical University (Qingyuan People’s Hospital), Shenzhen People’s Hospital, Yue Bei People’s Hospital, People’s Hospital of Yangjiang, Zhongshan City People’s Hospital and Sun Yat-sen Memorial Hospital in China. The study is expected to last for 4 years. Recruitment of participants has started in August 2019.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination of our research. The results will be available to the public if necessary.
Inclusion criteria
Age between 18 years and 90 years
-
Patients admitted into ICU with sepsis (sepsis-3)1 compatible with the diagnosis of AKI at stage 2 of KDIGO classification
Sepsis-3: an increase in the Sequential Organ Failure Assessment (SOFA) Score of 2 points or more caused by a dysregulated host response to infection
More than twofold increase of SCr level compared with the baseline value and/or urine output (UO) <0.5 mL/kg/hour for 12 hours
Informed consent provided by the patient or person with decisional responsibility
Exclusion criteria
-
Presence of one of the emergent CRRT conditions before randomisation:
Hyperkalaemia >6.0 mmol/L or >5.5 mmol/L persisting despite medical treatment
Acute pulmonary oedema due to fluid overload responsible for severe hypoxaemia requiring oxygen flow rate >5 L/min to maintain a percutaneous oxygen saturation >95% or a fraction of inspired oxygen >50% in patients already on invasive or non-invasive mechanical ventilation and despite diuretic therapy
Blood urea nitrogen (BUN) >112 mg/dL (40 mmol/L)
Pre-existing severe chronic renal failure (estimated glomerular filtration rate <30 mL/min)
Previous RRT
Prior kidney transplant
AKI caused by permanent postrenal obstruction or surgical lesion of renal vessel
Pregnancy
Hepatorenal syndrome
AIDS
Patient for whom survival to 90 days is unlikely due to end-stage diseases
Patient is moribund with expected death within 24 hours
Patient included in another interventional clinical trial
Study definitions
Sepsis and septic shock
Sepsis is defined according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)1 as a life-threatening syndrome with organ dysfunction caused by infection. For clinical diagnosis, patients can be identified by an increase in the SOFA Score of 2 points or more caused by a dysregulated host response to infection. Septic shock, a subset of sepsis, can be identified by a vasopressor requirement to maintain a mean arterial pressure of 65 mm Hg or greater and serum lactate greater than 2 mmol/L despite resuscitation.
Acute kidney injury
AKI will be defined and classified according to the KDIGO classification (table 1). AKI is identified by at least one of the following:
Table 1.
KDIGO stage criteria for AKI
| Stage | SCr | UO |
| 1 | 1.5–1.9 times baseline* or ≥0.3 mg/dL (26.5 μmol/L) increase |
<0.5 mL/kg/hour for 6–12 hours |
| 2 | 2.0–2.9 times baseline | <0.5 mL/kg/hour for ≥12 hours |
| 3 | ≥3 times baseline or ≥4.0 mg/dL (353.6 μmol/L) increase or initiation of RRT or in patients <18 years a decrease in eGFR <35 mL/min/1.73 m2 | <0.3 mL/kg/hour for ≥24 hours or anuria ≥12 hours |
*If the patient presents with AKI without a reliable baseline SCr on record, baseline SCr can be estimated using the Modification of Diet in Renal Disease Study equation.33
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease Improving Global Outcomes; RRT, renal replacement therapy; SCr, serum creatinine; UO, urine output.
An increase in SCr by ≥0.3 mg/dL (26.5 μmol/L) within 48 hours
An increase in SCr to ≥1.5 times baseline within the previous 7 days
Urine volume ≤0.5 mL/kg/hour for 6 hours
Recovery of renal function
Recovery of renal function will be classified as complete recovery, partial recovery or no recovery.
Complete recovery of renal function will be defined as SCr elevated ≤0.5 mg/dL (44.2 μmol/L) compared with the baseline.
Partial recovery will be defined as SCr elevated >0.5 mg/dL (44.2 μmol/L) compared with the baseline, but not depending on dialysis.
No recovery of renal function will be defined as patients remaining dialysis-dependent at the time of death or study completion.
Study intervention
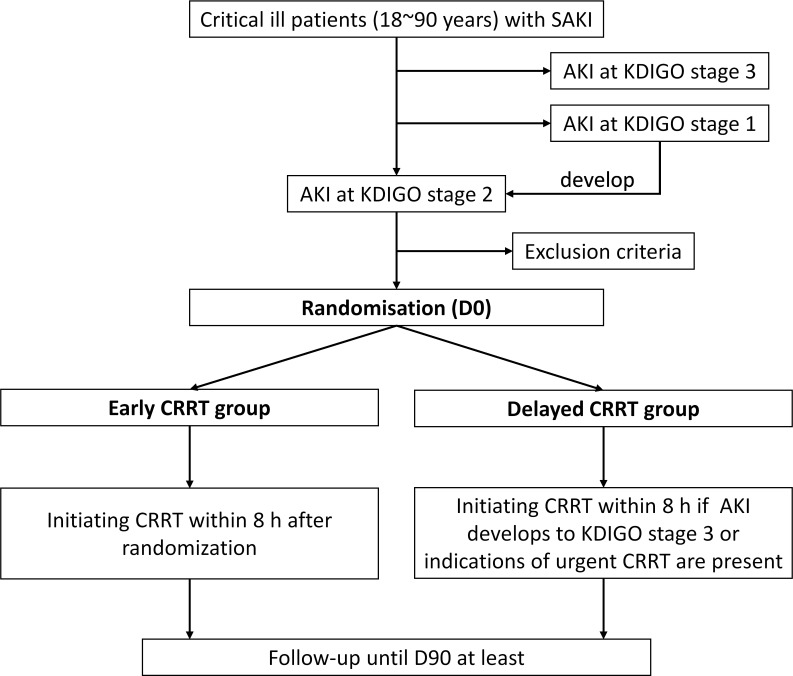
The study flow chart is detailed in figure 1.
Figure 1.
Flow chart of the trial. AKI, acute kidney injury; CRRT, continuous renal replacement therapy; D90, day 90; KDIGO, Kidney Disease Improving Global Outcomes; SAKI, sepsis-associated acute kidney injury.
Early CRRT group
Patients randomised to the early CRRT group will initiate CRRT as fast as possible. A maximum of 8 hours is allowed between randomisation and the actual initiation of CRRT. The initiation and cessation time, and the data of CRRT are recorded by nurses.
Delayed CRRT group
Patients randomised to the delayed CRRT group will be observed closely. SCr will be measured at least once/12 hours until renal function recovery or one of the following criteria is met:
AKI develops to stage 3 of KDIGO classification (≥3 times baseline SCr level or SCr concentration >4.0 mg/dL (353.6 μmol/L), and/or UO <0.3 mL/kg/hour for more than 24 hours or anuria (UO<100 mL) for more than 12 hours).
-
Presence of one of the emergent CRRT conditions after randomisation:
Hyperkalaemia >6.0 mmol/L.
Acute pulmonary oedema.
BUN >112 mg/dL (40 mmol/L).
CRRT will be initiated when one of the above criteria is met. A maximum of 8 hours is allowed between the appearance of indication and the actual initiation of CRRT. If the renal function of patients is recovery in the delayed group, initiation of CRRT can be avoided. The indication and time of initiation and cessation, and the data of CRRT are recorded by nurses.
Modality of CRRT
In order to keep uniformity of therapy between the early and delayed CRRT groups, identical settings of CRRT will be used in both groups. All patients undergoing CRRT will be treated using continuous veno-venous haemofiltration or continuous veno-venous haemodiafiltration. The rate of blood flow is recommended for 100–200 mL/min. The effluent flow will be prescribed based on the patient’s body weight at the time of randomisation and will be 20–25 mL/kg/hour. The choice of vascular access, the strategy of anticoagulation is left to the investigator’s discretion. Once CRRT is initiated, it is recommended to maintain treatment for at least 24 hours. The membranes should be replaced at least every 72 hours. The interval of CRRT interruption should notexceed 24 hours when the criteria for CRRT cessation or ‘intermittern’ are not met.
Cessation or alternation of CRRT
‘Intermittent’ CRRT
If UO ≥500 mL/24 hours without diuretics or ≥1000 mL/24 hours with diuretics, the interval of CRRT interruption is left to the investigator’s discretion.
Cessation of CRRT
CRRT will be discontinued if UO is ≥1000 mL/24 hours without diuretics or ≥2000 mL/24 hours with diuretics, and creatinine clearance is >20 mL/min.
Alternation of CRRT
If cessation criteria are not fulfilled until the patient leaves the ICU, CRRT can be changed to another RRT modality (ie, intermittent haemodialysis, peritoneal dialysis, prolonged intermittent RRT).
Additional treatments
Management of sepsis and septic shock will follow the international guidelines of the Surviving Sepsis Campaign 2016 and 2018.28 29 The patient’s primary physicians will determine the management of other comorbidities. The dose of all medications will be adjusted for renal function and CRRT.
Primary objective
The primary study end point is overall mortality measured from the date of randomisation (day 0) until death or day 90. For patients who were discharged alive from ICU, information on the primary end point will be acquired by a telephone call.
Secondary and other objectives
The secondary end points include: (1) 28-day, 60-day and 1-year all-cause mortality; (2) Recovery rate of renal function by day 28 and day 90; (3) ICU and hospital LOS; (4) The percentage of receipt of CRRT at least once in the delayed group; (5) The number of days alive without CRRT, mechanical ventilation and vasopressor (the numbers of CRRT-free days, mechanical ventilation-free days and vasopressor-free days, between day 0 and up to day 90); (6) The SOFA Score at day 0, day 1, day 3, day 7, day 14 and day 28; (7) Impacts on other organ functions (heart, lung, liver); (8) The rate of complications potentially related to CRRT, including: (a) Major bleeding associated with anticoagulants (defined as fatal bleeding, and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in haemoglobin level of 20 g/L or more, or leading to transfusion of two or more units of whole blood or red cells),30 (b) Thrombosis of a large venous axis diagnosed by Doppler ultrasound, (c) Catheter-related bloodstream infection,31 (d) Thrombocytopenia (<100 × 109 platelets/mm3), (e) Hypothermia (defined as a core body temperature of less than 35℃ or need electric blanket to keep warm), (f) Haemodynamic instability due to CRRT and requiring the introduction or increase of vasopressor, (g) Pulmonary oedema during CRRT, (h) Hyperkalemia (defined as serum potassium concentration >6.5 mmol/L), (i) Hypokalaemia (defined as serum potassium concentration <3.0 mmol/L), (j) New onset of serious arrhythmia during CRRT (including atrial fibrillation, ventricular tachycardia, ventricular fibrillation and torsades de pointes).
Other end points include: (1) Cost analysis of CRRT; (2) Duration between randomisation to CRRT initiation; (3) Duration between appearance of at least one of the criteria that initiated CRRT in the delayed group and actual initiation.
Add-on study: (1) New biomarkers of AKI (such as angiotensinogen, neutrophil gelatinase associated lipocalin (NGAL)); (2) Concentrations of inflammatory mediators in serum will be analysed between two groups (such as interleukin (IL)−1, IL-6 and tumour necrosis factor-α (TNF-α)).
Sample size
The primary end point in this study is to compare the effect of early and delayed CRRT strategy on overall survival at day 90 in patients with SAKI. Our primary hypothesis is that the early CRRT strategy might beneficial to patients with SAKI. According to the IDEAL-ICU Study, the expected mortality of patients with SAKI in the delayed group may be estimated around 55%. Considering that the timing setting of CRRT in this study is similar to the ELAIN Study, a reduction in mortality of 15% in the early group (55% mortality in the delayed CRRT group vs 40% in the early CRRT group) can be expected. Differences between two groups will be detected with a power of 85% at a bilateral α risk of 0.05. Considering that in China, some patients will give up treatment and request discharge from hospital due to economic reasons or customs, the rate of non-evaluable cases is expected to be 15% for the worst. Hence, a total of 460 patients (230 per group) is required. Power calculations were performed using the Power Analysis & Sample Size (PASS) V.14.0 software.
Randomisation and blinding
Eligible patients are consecutively randomly assigned to either the early group or the delayed group in a 1:1 ratio. This allocation is achieved using a centralised, secure, computer-generated, web response system accessible from each study centre. The randomisation is balanced by blocks of variable and undisclosed sizes, and stratified according to centre. The block size is 6. The randomisation day is study day 0. Because this is an interventional study, blinding is not possible for physicians, nurses and patients. However, the analysis will be blinded to allocation of groups.
Data collection and follow-up
Each investigator from the 13 participating ICUs was trained for the protocol, the electronic random system and data collection in the electronic Case Record Form (eCRF) before trial initiation. The electronic random system and eCRF are developed by Guangzhou Baofeng Pharmaceutical Technology, managed and closed by the clinical research centre of the Second Affiliated Hospital of Guangzhou Medical University (China). It is a centralised, secure, web response system accessible from each study centre.
The flow chart of patient follow-up is shown in table 2. Potential exposure to nephrotoxic agents used nearly 1 week before inclusion (ie, contrast agent, aminoglycoside antibiotics, tacrolimus, amphotericin B, hydroxyethyl starch or vancomycin) and demographic data will be collected. Detailed data including reasons for ICU admission, cause of SAKI, Acute Physiology and Chronic Health Evaluation II Score, and dates of hospital and ICU admission will be recorded. Details of mechanical ventilation, vasopressor, CRRT, diuretics, UO, and fluid balance will be documented daily. SOFA scores will be evaluated at baseline, day 1, day 3, day 7, day 14, day 28. Blood will be collected at baseline, day 1, day 3, day 7 and day 14. Results of laboratory tests including white blood cell count and differentials in peripheral circulation, serum electrolyte levels, serum glucose level, urea and creatinine concentration, liver and myocardial enzyme concentrations, arterial blood gas analysis, procalcitonin and lactate will be recorded. Biomarkers of AKI (ie, angiotensinogen and NGAL) and inflammatory factors [ie, IL-1β, IL-6, TNF-α, C-reactive protein (CRP)] are planned to be tested at day 0, day 1, day 3 and day 7.
Table 2.
Flow chart of patient follow-up
| Screening | Day 0 inclusion | Study period | Death/D90 | |
| Baseline information | ||||
| Demographic data and history | √ | √ | ||
| Inclusion and exclusion criteria | √ | |||
| Written informed consent | √ | |||
| Vital signs | √ | |||
| APCHEII/SOFA | √ | √D1, 3, 7, 14, 28 | ||
| Efficacy observation | ||||
| Mechanical ventilation | √ | √ | ||
| Treatment with vesopressor | √ | √ | ||
| CRRT initiation and application | √ | √ | ||
| UO and diuretic application | √ | √ | ||
| Renal function recovery | √D28 | √ | ||
| UCG and ECG | √D1, D14 | |||
| Laboratory tests | √ | √ | √D1, 3, 7, 14 | |
| Biomarkers and inflammatory factors | √ | √D1, 3, 7 | ||
| Safety observation | ||||
| Complications of CRRT | √ | √ | ||
| Adverse event | √ | √ | ||
| Additional observation | ||||
| Total cost of CRRT | √ | |||
| ICU and hospital LOS | √ | |||
| Alive or dead status | √D28, 60 | √ | ||
APCHEII, Acute Physiology and Chronic Health Evaluation II; CRRT, continuous renal replacement therapy; D90, day 90; LOS, length of stay; SOFA, Sequential Organ Failure Assessment; UCG, ultrasonic cardiogram; UO, urine output.
During CRRT intervention, details of initiation, cessation, setting parameters and complications will be collected. Scr and UO will be monitored daily to ensure whether criteria for CRRT discontinuation is present. All recruited patients will be followed to determine adverse events, renal function recovery and mortality until death or at 28, 60, 90 days and 1 year after randomisation.
Statistical analysis
Data will be double checked by the clinical research team, and the database is managed and closed by the clinical research centre of the Second Affiliated Hospital of Guangzhou Medical University (China).
For each group, quantitative variables with normal distribution will be described as mean and SD. Quantitative variables with skewed distribution will be described as median (M) and IQR (25th centile to 75th centile). Qualitative variables will be described as frequency and percentage.
The primary end point (all-cause mortality at 90 days) will be performed according to the intent-to-treat principle. This data set includes all trial subjects enrolled into the trial and randomised. Additional sensitivity analyses will be performed according to the per-protocol principle. This data set includes all trial subjects who were treated according to protocol and reached a defined end point in the trial. The main comparison of all-cause 90 days mortality between two treatment groups will be performed using the χ2 test, with secondary analysis by the Kaplan-Meier method and log-rank test. Analyses were performed at a two-sided alpha level of 5%.
Safety will be analysed by the frequency of complications related to CRRT in both the treatment groups and by comparing rates using χ2 or Fisher’s exact tests, with an α risk set at 0.05.
Statistical analyses of the prespecified secondary end points will be performed with descriptive and inductive statistical methods. Categorical variables will be compared using the χ2 or Fisher’s exact tests, as appropriate. Continuous variables will be compared using Student’s t-test or the Mann-Whitney test, as appropriate.
The primary efficacy analysis provides confirmative evidence. Further analyses will be regarded explorative (hypothesis generating) and will be interpreted accordingly. All point estimates of parameters of interest will be supplemented by 95% CIs. Type I error enhancement due to multiple significance testing will be accounted for if applicable.
Statistical analyses will be performed according to the principles of the International Conference on Harmonisation (ICH)-guideline E9 ‘Statistical Principles for Clinical Trials’ using SAS V.9.4, or R software V.3.6.3 or later.
Ethics and dissemination
The study protocol has been approved by the Clinical Research and Application Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University (V.2.0, registration number: 2017–31-ks-01; date of approval: 29 October 2018). Participants will be screened and enrolled from patients in the ICU with SAKI by clinicians, with no public advertisement for recruitment. When the patient fulfils the criterion of recruitment, written informed consent (online supplemental file 1) should be obtained from the patient or a responsible surrogate before randomisation. All information from the participants will be kept private and will not be provided to any company or institution. Results will be disseminated in research journals and through conference presentations.
Discussion
Sepsis is the leading cause of AKI in the ICU, which often manifests as part of the multiple organ dysfunction syndrome. AKI is an independent contributor to mortality in sepsis. Early reversible AKI was associated with improved survival.3 It is reported that there is no acute tubular necrosis and only intracellular and metabolic modifications are observed in the early phases of SAKI.18 Therefore, it is supposed that an early strategy which can reduce kidney injury may benefit to improve survival.
Unfortunately, there are currently no pharmacological treatments available to SAKI therapy. CRRT is considered to be the most effective therapy to SAKI when the renal function becomes insufficient to maintain internal environmental stability. However, when to start CRRT remains a particularly challenging question during SAKI in critically ill patients. In 2018, the IDEAL-ICU Study showed no difference between the two strategies. However, 41 patients (17 %) in the delayed group needed emergency RRT and had higher mortality.27 On the one hand, Barbar et al32 thought it is unacceptable to expose 30% of patients to the potential risks of an extracorporeal support technique if they did not actually need it. On the other hand, others argued that although there may be a risk of ‘unnecessary’ RRT, there could be an even greater risk associated with not providing it. To the best of our knowledge, only one such large randomised controlled study focusing on timing of RRT in patients with SAKI has been published. Therefore, more studies are needed to clarify this issue.
Looking at the previous studies on the timing of RRT in AKI, the conclusions were different, and the definitions of ‘early’ or ‘late’ were different. In the ELAIN Study,11 ‘early RRT’ was initiated at stage 2 of the KDIGO classification. In the AKIKI Study,12 all patients were required to have KDIGO stage 3 AKI. ‘Early’ meant initiating RRT within 6 hours after randomisation. In the IDEAL-ICU Study, ‘early’ meant initiating RRT within 12 hours following the diagnosis of RIFLE-F AKI (equivalent to KDIGO stage 3). Considering that the patients with SAKI are generally sicker than the patients without SAKI, the ‘early strategy’ in the AKIKI Study and the IDEAL-ICU Study may still be too late. It might be preferable to initiate RRT at an earlier stage which was similar to that in the ELAIN Study. Therefore, we chose all patients with KDIGO stage 2 AKI. CRRT was initiated within 8 hours of randomisation in the early group, and 8 hours after development of AKI to stage 3 of KDIGO classification or presence of one of the emergent CRRT conditions after randomisation.
We hypothesise that initiating CRRT early enough may attenuate renal injury from systemic inflammation, acidaemia, uraemia and fluid overload in patients with sepsis. If this study confirms our hypothesis, it may help to improve mortality for patients with SAKI. Negative results will also encourage us to pay deeper attention to details underlining insightful information: is there a way to better predict which patients are likely to require RRT and which patients have a high likelihood of spontaneous recovery?
Supplementary Material
Footnotes
W-yC and L-hC contributed equally.
Contributors: X-mX, Z-hZ, W-yC and L-hC designed the trial. X-mX obtained funding for the trial. W-yC and L-hC drafted the manuscript. X-mX and Z-hZ provided critical revision of the manuscript. All authors discussed and helped to improve the protocol, and read and approved the final manuscript.
Funding: This study was funded by a Grant from Guangzhou Medical University (B195001009).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016;315:801. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock. JAMA 2016;315:775 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sood MM, Shafer LA, Ho J, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 2014;29:711–7. 10.1016/j.jcrc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 4.Bagshaw SM, George C, Bellomo R, et al. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008;12:R47 10.1186/cc6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopes JA, Jorge S, Resina C, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. Int J Infect Dis 2009;13:176–81. 10.1016/j.ijid.2008.05.1231 [DOI] [PubMed] [Google Scholar]
- 6.Jiang L, Zhu Y, Luo X, et al. Epidemiology of acute kidney injury in intensive care units in Beijing: the multi-center BAKIT study. BMC Nephrol 2019;20:468. 10.1186/s12882-019-1660-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karvellas CJ, Farhat MR, Sajjad I, et al. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care 2011;15:R72. 10.1186/cc10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasin L, Boraso S, Tiberio I. Early initiation of renal replacement therapy in critically ill patients: a meta-analysis of randomized clinical trials. BMC Anesthesiol 2019;19:62. 10.1186/s12871-019-0733-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Chen D, Tang X, et al. Timing of initiation of renal replacement therapy in acute kidney injury: an updated meta-analysis of randomized controlled trials. Ren Fail 2020;42:77–88. 10.1080/0886022X.2019.1705337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Jia L, Li R, et al. Early versus late initiation of renal replacement therapy for acute kidney injury in critically ill patients: a systematic review and meta-analysis. PLoS One 2019;14:e223493 10.1371/journal.pone.0223493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016;315:2190. 10.1001/jama.2016.5828 [DOI] [PubMed] [Google Scholar]
- 12.Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016;375:122–33. 10.1056/NEJMoa1603017 [DOI] [PubMed] [Google Scholar]
- 13.STARRT-AKI Investigators, Canadian Critical Care Trials Group, Australian and New Zealand Intensive Care Society Clinical Trials Group, et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 2020;383:240–51. 10.1056/NEJMoa2000741 [DOI] [PubMed] [Google Scholar]
- 14.Bagshaw SM, George C, Bellomo R, et al. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008;12:R47. 10.1186/cc6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2007;2:431–9. 10.2215/CJN.03681106 [DOI] [PubMed] [Google Scholar]
- 16.Calzavacca P, Evans RG, Bailey M, et al. Cortical and medullary tissue perfusion and oxygenation in experimental septic acute kidney injury. Crit Care Med 2015;43:e431–9. 10.1097/CCM.0000000000001198 [DOI] [PubMed] [Google Scholar]
- 17.Shum H-P, Yan W-W, Chan TM. Recent knowledge on the pathophysiology of septic acute kidney injury: a narrative review. J Crit Care 2016;31:82–9. 10.1016/j.jcrc.2015.09.017 [DOI] [PubMed] [Google Scholar]
- 18.Dellepiane S, Marengo M, Cantaluppi V. Detrimental cross-talk between sepsis and acute kidney injury: new pathogenic mechanisms, early biomarkers and targeted therapies. Crit Care 2016;20:61. 10.1186/s13054-016-1219-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kellum JA, Kong L, Fink MP, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the genetic and inflammatory markers of sepsis (GenIMS) study. Arch Intern Med 2007;167:1655–63. 10.1001/archinte.167.15.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogos CA, Drosou E, Bassaris HP, et al. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 2000;181:176–80. 10.1086/315214 [DOI] [PubMed] [Google Scholar]
- 21.Peng Z, Pai P, Han-Min W, et al. Evaluation of the effects of pulse high-volume hemofiltration in patients with severe sepsis: a preliminary study. Int J Artif Organs 2010;33:505–11. 10.1177/039139881003300801 [DOI] [PubMed] [Google Scholar]
- 22.Davenport A Can modification of renal replacement therapy improve the outcome of patients with systemic inflammatory response syndrome? Blood Purif 2006;24:317–8. 10.1159/000091850 [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Li H, Zhang D. Timing of continuous renal replacement therapy in patients with septic AKI: a systematic review and meta-analysis. Medicine 2019;98:e16800. 10.1097/MD.0000000000016800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek SD, Yu H, Shin S, et al. Early continuous renal replacement therapy in septic acute kidney injury could be defined by its initiation within 24 hours of vasopressor infusion. J Crit Care 2017;39:108–14. 10.1016/j.jcrc.2016.12.014 [DOI] [PubMed] [Google Scholar]
- 25.Oh HJ, Kim MH, Ahn JY, et al. Can early initiation of continuous renal replacement therapy improve patient survival with septic acute kidney injury when enrolled in early goal-directed therapy? J Crit Care 2016;35:51–6. 10.1016/j.jcrc.2016.04.032 [DOI] [PubMed] [Google Scholar]
- 26.Chon GR, Chang JW, Huh JW, et al. A comparison of the time from sepsis to inception of continuous renal replacement therapy versus rifle criteria in patients with septic acute kidney injury. Shock 2012;38:30–6. 10.1097/SHK.0b013e31825adcda [DOI] [PubMed] [Google Scholar]
- 27.Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018;379:1431–42. 10.1056/NEJMoa1803213 [DOI] [PubMed] [Google Scholar]
- 28.Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign. Crit Care Med 2017;45:486–552. 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 29.Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med 2018;46:997–1000. 10.1097/CCM.0000000000003119 [DOI] [PubMed] [Google Scholar]
- 30.Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 31.Chaves F, Garnacho-Montero J, Del Pozo JL, et al. Diagnosis and treatment of catheter-related bloodstream infection: clinical guidelines of the Spanish Society of infectious diseases and clinical microbiology and (SEIMC) and the Spanish Society of Spanish Society of intensive and critical care medicine and coronary units (SEMICYUC). Med Intensiva 2018;42:5–36. 10.1016/j.medin.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 32.Barbar SD, Dargent A, Quenot J-P. Initiation of renal replacement therapy in patients with septic acute kidney injury: right timing or right patient? Ann Transl Med 2019;7:598. 10.21037/atm.2019.09.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khwaja A KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 2012;120:c179–84. 10.1159/000339789 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040718supp001.pdf (53.1KB, pdf)