Abstract
Introduction
This study investigated alternative pre‐analytical handling of blood for neurofilament light (NfL) analysis where resources are limited.
Method
Plasma NfL was measured with single molecule array after alternative blood processing procedures: dried plasma spots (DPS), dried blood spots (DBS), and delayed 48‐hour centrifugation. These were compared to standardized plasma processing (reference standard [RS]). In a discovery cohort (n = 10) and a confirmatory cohort (n = 21), whole blood was obtained from individuals with unknown clinical etiology. In the confirmatory cohort, delayed centrifugation protocol was paired with either 37°C incubation or sample shaking to test the effect of these parameters.
Results
Delayed centrifugation (R2 = 0.991) and DPS (discovery cohort, R2 = 0.954; confirmatory cohort, DPS: R2 = 0.961) methods were strongly associated with the RS. Delayed centrifugation with higher temperatures (R2 = 0.995) and shaking (R2 = 0.975) did not affect this association. DPS (P < 0.001) returned concentrations considerably lower than the RS.
Discussion
DPS or delayed centrifugation are viable pre‐analytical procedures for the accurate quantification of plasma NfL.
Keywords: dried blood spots, dried plasma spots, neurofilament light, pre‐analytical handling
1. INTRODUCTION
There is increasing evidence supporting the value of blood biomarkers in research contexts, clinical evaluation, and pharmaceutical trials of neurological diseases. This is to a large extent owing to the rapid evolution of technological advances of immunoassay‐based platforms, such as single molecule array (Simoa). One breakthrough has been the femtomolar quantification of neurofilament light (NfL) in blood, 1 one of the main constituents of the axonal cytoskeleton. NfL has emerged as a valuable biomarker of general neuroaxonal injury, which is increased in most neurodegenerative diseases, 2 including Alzheimer's disease (AD), 3 as well in infectious, 1 neuroinflammatory, 4 and acute neurological conditions. 5 , 6 , 7 However, virtually all research on NfL has been performed in well‐characterized research cohorts or clinical settings with immediate access to advanced laboratory equipment such as ultra‐low temperature freezers and centrifuges, allowing for quick processing and storage of plasma or serum. Several studies have reported the very limited effects of freeze‐thaw cycles and plasma storage at room temperature (RT) on NfL stability, 8 , 9 , 10 but for NfL to be accessible also in remote and less privileged conditions, alternative blood sampling protocols need to be considered. To overcome this issue, quick and inexpensive methods not reliant upon strict cold‐chain transport should be developed. Furthermore, methods in which a blood sample could be conceivably obtained in patient/participant homes would increase clinical outreach and participant involvement in therapeutic trials. This would also be of great value to enable research studies being continued during the COVID‐19 pandemic. Methods with potential to meet these requirements include dried blood (DBS) 11 or plasma spots (DPS), 12 the latter being simplified recently in the form of Noviplex cards, with built‐in technology to separate soluble proteins from the cell fraction of whole blood. These methods have proven feasible to use for the measurement of NfL. 12 Alternatively, one could transport collecting tubes with whole blood and delay sample preparation to the arrival at an advanced laboratory. This would also simplify blood collection at point‐of‐care.
Blood‐based biomarkers hold great promise, and reaching distant populations would even further increase their value. Therefore, we aimed to evaluate alternative methods to simplify the pre‐analytical procedure of blood NfL measurements in 31 individuals from two cohorts.
2. METHODS
2.1. Samples
Fresh blood samples (leftover whole blood from clinical routine analyses) were collected at the Clinical Chemistry Laboratory, Sahlgrenska University Hospital, Mölndal, Sweden, as a part of routine evaluation. The discovery cohort used blood samples from n = 10 individuals, whereas n = 21 individuals were included in the confirmatory cohort. The study was conducted in accordance with the Ethics Committee at University of Gothenburg (EPN140811). Because all samples were provided anonymously by laboratory personnel and left over from clinical routine, no informed consent was necessary for this study to be conducted.
2.2. Blood collection and preparation
Blood was collected by venipuncture in EDTA tubes (Vacuette® tube 6 mL, #456243, Greiner Bio‐One GmbH, Kremsmuenster, Austria). As reference standard (RS), in both cohorts, blood was centrifuged within 2 hours of collection at 20°C, spun at 2000 g for 10 minutes and stored at –80°C pending biochemical analysis. In the discovery cohort, 65 μL of blood was spotted onto (1) Noviplex Duo Card, returning an equivalent of 14 μL of plasma (Noviplex DPS; https://www.shimadzu.co.uk/noviplex‐cards‐1); (2) protein saver cards (DBS) (#10534612; Whatman #903, GE Healthcare), two spots for each patient. Both Noviplex DPS and DBS cards were then wrapped in plastic foil and stored in zip‐lock bags at RT until further processing. Additionally, whole blood was aliquoted into new EDTA tubes, which were then kept at RT for 48 hours before being handled according to standard plasma protocol. In the confirmatory cohort, whole blood was again spotted onto Noviplex DPS cards but not DBS. Instead of storing samples on a lab bench at RT, the aim was to simulate transport in equatorial conditions. Thus, whole blood was stored either at 37°C for 48 hours, or shaken on a rocker for 3 hours at 300 RPM, and then standing on a lab bench for 45 hours before being handled according to standard plasma protocol.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the available scientific literature on PubMed for articles examining alternative sample handling methods for plasma measurements in neurological disease. One recent publication reports the use of dried blood and plasma spots (DBS/DPS, respectively) in amyotrophic lateral sclerosis patients and healthy controls. Several studies have reported the effects of freeze‐thaw cycles and leaving plasma at different temperatures. However, no studies report delayed centrifugation of whole blood (WB). Therefore, in this study, we compare DPS and DBS methods to delayed sample centrifugation as options to regular processing of WB.
Interpretation: Our findings suggest that DPS and delayed 48 hours room temperature sample centrifugation are feasible options, but with potentially different contexts of use.
Future directions: Further validation studies in larger cohorts with relevant diseases, method optimization, and testing other analytes will be needed if these biomarkers are to be incorporated into clinical practice or research studies.
2.3. Biochemical analysis
Prior to analysis, DBS cards were collected by cutting with a sterilized knife along the lines of the card and then incubated in 120 μL phosphate‐buffered saline at 37°C and 400 g for 1 hour. The elution and cards were then transferred to a 0.5 mL bottomless Eppendorf tube, slotted inside a 1.5 mL Eppendorf, which was spun at 20°C and 2000 g for 10 minutes. Noviplex DPS cards were prepared by eluting the protein‐containing disc using 100 μL of buffer used for the Simoa analysis (Sample Diluent, #502186 NF‐light, Quanterix). All samples were measured for NfL on the same analytical run, using a Simoa HD‐X analyzer (Quanterix) at the Neurochemistry Laboratory, Sahlgrenska University Hospital. All results were compensated for dilutions, except for DBS cards for which the dilution factor was not known. One Noviplex DPS sample in the discovery cohort fell below the limit of detection of the assay.
2.4. Statistical analysis
Associations between the RS and alternative sample processing methods were assessed using linear regression models. Wilcoxon signed rank tests were used to compare the absolute concentrations of alternative processing methods against the RS. All statistical analyses were performed in GraphPad Prism version 8.0.0 for Mac (GraphPad Software). Two‐sided significance was set at P < 0.05.
3. RESULTS
Strong associations were found for all three alternative sample handling protocols with the RS. Noviplex DPS cards (R2 = 0.954, P < 0.0001: Figure 1A) and DBS cards (R2 = 0.589, P = 0.009, Figure 1B) were slightly less associated than delayed centrifugation at RT, which displayed a near perfect association (R2 = 0.991, P < 0.0001: Figure 1C). These results were largely replicated in the confirmatory cohort (Noviplex DPS: R2 = 0.961, P < 0.0001: Figure 1D). It was also proven that shaking (R2 = 0.975, P < 0.0001: Figure 1E) of the samples or exposure to higher temperature (R2 = 0.995, P < 0.0001: Figure 1F) did not affect the promising results of delayed centrifugation from the discovery cohort.
FIGURE 1.
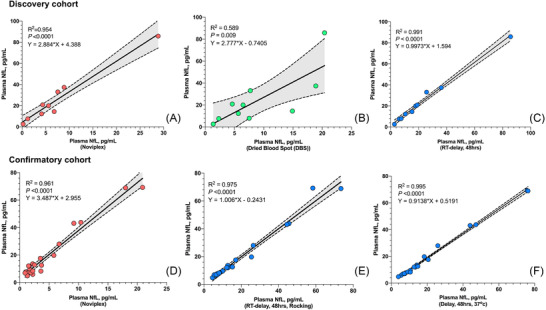
Correlation between RS NfL and alternative sample handling. This figure shows the correlation between NfL concentrations using the RS and (A) Noviplex DPS cards, (B) DBS cards, and (C) plasma stored at RT for 48 hours before analysis in the discovery cohort, as well as with (D) Noviplex DPS cards, (E) rocking for 3 hours at RT and then RT for 45 hours, and (F) 37°C incubation for 48 hours in the confirmatory cohort. Abbreviations: DBS, dried blood spots; DPS, dried plasma spots; NfL, neurofilament light; RS, reference standard; RT, room temperature. R2, equations and P values are derived from linear regression models
In both cohorts, Noviplex DPS and DBS cards returned significantly lower concentrations of NfL compared to the RS (DPS: Figure 2A, discovery cohort, P < 0.01; Figure 2D confirmatory cohort, P < 0.0001; DBS: P < 0.05, Figure 2). The delayed centrifugation at RT as well as incubation at 37°C gave slightly higher concentrations of NfL than the RS (P < 0.05, Figure 2C, 2F). No differences were observed for shaking in the confirmatory cohort (Figure 2E).
FIGURE 2.
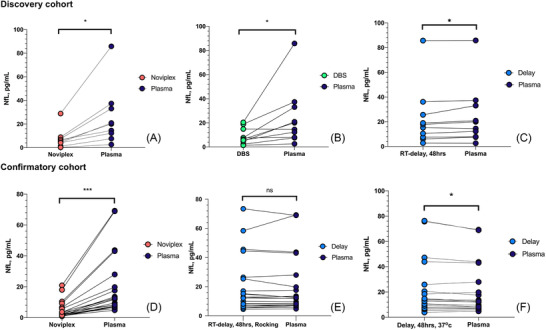
Comparison between NfL concentrations with the RS and alternative sample handling. Comparison of NfL concentrations using the RS versus (A) Noviplex DPS cards, (B) DBS cards, and (C) plasma stored at RT for 48 hours before analysis in the discovery cohort, as well as with (D) Noviplex DPS cards, (E) rocking for 3 hours at RT and then RT for 45 hours, and (F) 37°C incubation for 48 hours in the confirmatory cohort. Abbreviations: DBS, dried blood spots; DPS, dried plasma spots; NfL, neurofilament light; RS, reference standard; RT, room temperature. * = P < 0.05, ** = P < 0.01, *** = P < 0.001
4. DISCUSSION
In this study, we provide evidence that NfL concentrations remain stable after delaying centrifugation of whole blood. We also corroborate previous results that Noviplex DPS results strongly correlate with RS plasma concentrations, albeit at lower concentrations. 12
First, delayed centrifugation of whole blood while exposing it to shaking did not affect NfL concentrations, while they only slightly increased when whole blood was stored at 37°C and at RT in the discovery cohort. This highlights the stability of plasma NfL, and enables use of the same age‐related cut‐offs that have been suggested for commonly processed plasma in clinical routine may be used. 10
The concentrations found in samples processed as Noviplex DPS were substantially lower, and similar to those reported by Lombardi et al. 12 in patients with amyotrophic lateral sclerosis (ALS) and controls, but demonstrated strong correlations with standard procedures. Therefore, adjusted cut‐off values would have to be used for a DPS collection method. However, DPS offers significant advantages and remains a promising simplified plasma processing alternative. One context would be in epidemiological studies in developing countries, where relative associations between NfL and other factors could still be investigated on a population level. Further, a DPS method conceivably could be used as a “home testing” tool to reach vulnerable populations with difficulties visiting neurology or memory clinics, particularly during the COVID‐19 pandemic, as well as for regular monitoring in therapeutic trials. A limitation of the Noviplex DPS cards used in this study is the current cost, which is estimated at $30 per card.
DBS sampling was first introduced in 1961 as a method to detect phenylketonuria as a part of the newborn screening program, 11 and has since been used in various conditions, such as in the determination of HbA1c concentration 13 and human immunodeficiency virus viral load. 14 Here, we show that concentrations of NfL in DBS cards correlate with the RS, similar to what was found in a prior study. 12 However, the complex matrix of the elute, similar to hemolyzed blood, likely contains proteases, membrane components, and hemoglobin, which could explain the results being inferior to Noviplex DPS and delayed centrifugation methods.
Strengths of this study include the availability of whole blood that could be prepared instantly after collection, as well as the comparison of several putative pre‐analytical methods in a controlled environment. In a clinical or research scenario, it is desired to measure several analytes implicated in neurological disease, such as tau phosphorylated at amino acid 181 15 , 16 , 17 or 217, 18 , 19 total tau, 20 glial fibrillary acidic protein, 21 or amyloid beta 1‐40 and 1‐42 22 using these pre‐analytical procedures. One limitation is the small sample size, with unknown pathology, but the RS NfL concentrations span the clinically relevant range, which speaks for the generalizability of the results. Additionally, it should be noted that this is a study focused on the pre‐analytics of a well‐characterized biomarker, and that the different sample handling conditions were compared head‐to‐head, providing a proof‐of‐concept for alternative sample handling methods in blood NfL measurements.
To conclude, these findings have several potential implications. Due to the excellent concordance between delayed centrifugation of whole blood and the RS, it could be a viable alternative in remote settings, such as distant health care centers or for home collection, while essentially adhering to the cut‐offs currently in use. Additionally, DPS sampling could be used in large population‐based studies in which accessibility is the main priority.
CONFLICTS OF INTEREST
Henrik Zetterberg has served on scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (all unrelated to the work presented in this paper). Kaj Blennow has served as a consultant, on advisory boards, or on data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Nicholas J. Ashton and Joel Simrén report no disclosures.
AUTHOR CONTRIBUTIONS
Joel Simren, Nicholas J. Ashton, Kaj Blennow, and Henrik Zetterberg conceptualized the research; Joel Simren and Henrik Zetterberg collected the blood samples; Joel Simren and Nicholas J. Ashton performed plasma NfL measurements, data quality control, and data compilation; Joel Simren, Nicholas J. Ashton, Kaj Blennow, and Henrik Zetterberg contributed to data analysis; Joel Simren, Nicholas J. Ashton, Kaj Blennow, and Henrik Zetterberg wrote the original manuscript draft, reviewed, edited, and approved the final manuscript for submission.
ACKNOWLEDGMENTS
Nicholas J. Ashton is supported by the Swedish Alzheimer Foundation (Alzheimerfonden; #AF‐931009), the Swedish Brain Foundation (Hjärnfonden), the Agneta Prytz‐Folkes & Gösta Folkes Foundation, and the Swedish Dementia Foundation (Demensförbundet). Kaj Blennow is supported by the Swedish Research Council (#2017‐00915); the Alzheimer Drug Discovery Foundation (ADDF), USA (#RDAPB‐201809‐2016615); the Swedish Alzheimer Foundation (#AF‐742881); Hjärnfonden, Sweden (#FO2017‐0243); the Swedish state under the agreement between the Swedish government and the County Councils; the ALF‐agreement (#ALFGBG‐715986); and European Union Joint Program for Neurodegenerative Disorders (JPND2019‐466‐236). Henrik Zetterberg is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2018‐02532); the European Research Council (#681712); Swedish State Support for Clinical Research (#ALFGBG‐720931); the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809‐2016862); the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska‐Curie grant agreement No 860197 (MIRIADE); and the UK Dementia Research Institute at UCL.
Simren J, Ashton NJ, Blennow K, Zetterberg H. Blood neurofilament light in remote settings: Alternative protocols to support sample collection in challenging pre‐analytical conditions. Alzheimer's Dement. 2021;13:e12145 10.1002/dad2.12145
REFERENCES
- 1. Gisslen M, Price RW, Andreasson U, et al. Plasma concentration of the Neurofilament Light Protein (NFL) is a biomarker of CNS injury in HIV infection: a cross‐sectional study. EBioMedicine. 2016;3:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashton NJ, Janelidze S, Al Ahmad K, Leuzy A, Van der Ende EL, Karikari TK, Diagnostic value of plasma neurofilament light: A multicentre validation study. PREPRINT (Version 1) available at Research Square [+ https://doi.org/1021203/rs3rs-63386/v1+]. August 31, 2020.
- 3. Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2019;76(7):791‐799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Williams T, Zetterberg H, Chataway J. Neurofilaments in progressive multiple sclerosis: a systematic review. J Neurol. 2020. 10.1007/s00415-020-09917-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shahim P, Zetterberg H, Tegner Y, Blennow K. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology. 2017;88(19):1788‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gattringer T, Pinter D, Enzinger C, et al. Serum neurofilament light is sensitive to active cerebral small vessel disease. Neurology. 2017;89(20):2108‐2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wihersaari L, Ashton NJ, Reinikainen M, et al. Neurofilament light as an outcome predictor after cardiac arrest: a post hoc analysis of the COMACARE trial. Intensive Care Med. 202147 1:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keshavan A, Heslegrave A, Zetterberg H, Schott JM. Stability of blood‐based biomarkers of Alzheimer's disease over multiple freeze‐thaw cycles. Alzheimers Dement (Amst). 2018;10:448‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hviid CVB, Knudsen CS, Parkner T. Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand J Clin Lab Invest. 2020;80(4):291‐295. [DOI] [PubMed] [Google Scholar]
- 10. Lewczuk P, Ermann N, Andreasson U, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guthrie R. Blood screening for phenylketonuria. JAMA. 1961;178(8):863‐863. [Google Scholar]
- 12. Lombardi V, Carassiti D, Giovannoni G, Lu CH, Adiutori R, Malaspina A. The potential of neurofilaments analysis using dry‐blood and plasma spots. Sci Rep. 2020;10(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mastronardi CA, Whittle B, Tunningley R, Neeman T, Paz‐Filho G. The use of dried blood spot sampling for the measurement of HbA1c: a cross‐sectional study. BMC Clin Pathol. 2015;15:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Andreotti M, Pirillo M, Guidotti G, et al. Correlation between HIV‐1 viral load quantification in plasma, dried blood spots, and dried plasma spots using the Roche COBAS Taqman assay. J Clin Virol. 2010;47(1):4‐7. [DOI] [PubMed] [Google Scholar]
- 15. Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho‐tau181 in the Alzheimer’s Disease Neuroimaging Initiative. Molecular Psychiatry. 2020; 10.1038/s41380-020-00923-z. [DOI] [PubMed] [Google Scholar]
- 16. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19(5):422‐433. [DOI] [PubMed] [Google Scholar]
- 17. Lantero Rodriguez J, Karikari TK, Suarez‐Calvet M, et al. Plasma p‐tau181 accurately predicts Alzheimer's disease pathology at least 8 years prior to post‐mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020;140(3):267‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmqvist S, Janelidze S, Quiroz YT, et al. Discriminative accuracy of plasma phospho‐tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;. 324(8):772‐781.324 8:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattsson‐Carlgren N, Janelidze S, Palmqvist S, et al. Longitudinal plasma p‐tau217 is increased in early stages of Alzheimer's disease. Brain. 2020;. 143:3234‐3241.143 11:3234–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moseby‐Knappe M, Mattsson N, Nielsen N, et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76(1):64‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katsanos AH, Makris K, Stefani D, et al. Plasma glial fibrillary acidic protein in the differential diagnosis of intracerebral hemorrhage. Stroke. 2017;48(9):2586‐2588. [DOI] [PubMed] [Google Scholar]
- 22. Verberk IMW, Thijssen E, Koelewijn J, et al. Combination of plasma amyloid beta(1‐42/1‐40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020;12(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]


