Abstract
Background: Early identification of cognitive decline is critical for identifying individuals for inclusion in clinical trials and for eventual care planning.
Methods: A sample (ages 60–90 years) of consensus‐diagnosed, community‐dwelling Blacks (61 cognitively typical [HC], 28 amnestic mild cognitive impairment [aMCI], and 14 nonamnestic MCI [naMCI]) were recruited from the Michigan Alzheimer's Disease Research Center and the Wayne State University Institute of Gerontology. Participants received two resting state electroencephalograms (rsEEG, eyes closed) between which they engaged in a visual motion direction discrimination task. rsEEG %change current source densities across all frequency bands and regions of interest were calculated.
Results: EEG current density was not different across groups for pre‐task resting state. However, compared to HC, aMCI showed significantly greater declines at temporal and central cortical sites, while naMCI showed significant parietal declines.
Conclusion: This novel approach of post–pre/cognitive challenge rsEEG successfully discriminated older persons with MCI from those without was sensitive to cognitive decline.
Keywords: EEG markers, mild cognitive impairment, neuropsychology, resting‐state electroencephalography (rsEEG)
1. INTRODUCTION
Developing assessments that are sensitive to pre‐clinical cognitive decline and neural dysfunction, before frank Alzheimer's disease (AD) pathology, is critical for the study of neurodegenerative mechanisms and interventions to promote cognitive resiliency. The present study investigates if cognitive engagement changes resting state electroencephalogram (rsEEG) cortical activity and if the pattern of change may identify persons with mild cognitive impairment (MCI), a pre‐clinical stage to AD.
There is constant formation and dissolution of resting‐state patterns involved in functional and coherent network configurations around a more deterministic anatomical structure. 1 With respect to diagnosing MCI, the rsEEG research mainly focuses on abnormalities in the frequency and topographical features of rsEEG rhythms to unveil neural dysfunctions. 2 MCI patients show global ‘‘slowing’’ of the baseline EEG (i.e., EEG containing more low‐frequency power; for review see Babiloni et al. 2 and Vecchio et al. 3 ). Other studies 4 , 5 showed a reduced level of functional connectivity in patients with MCI. We also reported that older Blacks with MCI had reduced functional connectivity and brain topology as compared to cognitive typical adults. 6 These investigations highlight the use of rsEEG to detect differences in functional architecture between MCI and controls; however, greater sensitivity to detect pre‐clinical cognitive decline may be evident in functional changes after cognitive engagement. Lingering brain activity after a task is expected to interfere with subsequent task accuracy and speed, as suggested by dual‐task interference, 6 and cognitive deficits in MCI may in part be related to slowed disengagement and return to baseline function.
Based upon the reviewed literature, we hypothesized that MCI will show slower return to baseline compared to controls, that is, greater relative percent change in post‐cognitive engagement rsEEG spectral current source density (CSD). In line with the defining diagnostic feature of amnestic complaints, we expected to observe greater differences in percent change of rsEEG CSD between persons with amnestic MCI (aMCI) and controls in the temporal cortices. Deficits related to non‐amnestic MCI (naMCI) may be more variable, and we expected differences across regions outside of the temporal cortex. We test these hypotheses in a community sample of older Blacks.
HIGHLIGHTS
Resting state electroencephalographic (rsEEG) measures offer promise as biomarkers.
We compared rsEEG density immediately prior to and after a cognitive task.
Post–pre rsEEG% changes discriminate controls from amnestic and non‐amnestic mild cognitive impairment (MCI).
Within a Black community, this provided reliable MCI characterization.
RESEARCH IN CONTEXT
Systematic review: Early recognition of cognitive changes is critical in identifying those persons at risk for mild cognitive impairment (MCI) and dementia. Resting state electroencephalographic (rsEEG) measures offer promise as potential neurophysiological biomarkers for Alzheimer's disease (AD).
Interpretation: Comparison of rsEEG cortical activity immediately prior to and after a challenging cognitive task may effectively identify persons with MCI, a pre‐clinical stage of AD. Compared to cognitively normal participants, persons with MCI showed significantly greater post–pre rsEEG % changes in specific cortical areas important for memory and problem solving.
Future directions: This novel approach of post–pre rsEEG comparisons after cognitive challenge may discriminate older Blacks at heightened risk for MCI from those with still preserved cognition. Approaches such as this that are portable and readily accepted may provide important information for community‐based recruitment in clinical trials or better target those persons needing more invasive testing or imaging.
2. METHODS
2.1. Subjects
We recruited 87 Black participants from the greater Detroit area (see Table 1 for demographics of the participants) who endorsed a change in memory or other cognitive functions over the past year. All participants underwent the National Alzheimer's Disease Coordinating Centers (NACC) Uniform Data Set (UDS) assessment battery, including standardized medical, neurological, and neuropsychological components. Consensus conference diagnosis followed NACC criteria (https://www.alz.washington.edu/WEB/researcher_home.html; UDS Form D1: Clinician Diagnosis), diagnosing 58 participants as normally functioning (HC), and 41 MCI. UDS further characterizes both aMCI (N = 28) and naMCI (N = 13) based on established criteria 7 and reflecting whether a MCI participant meets UDS criteria for an amnestic disorder in addition to whatever other cognitive domains were affected within the MCI diagnostic pattern. All participants provided written informed consent and procedures were approved by university institutional review boards.
TABLE 1.
Demographic information and computer anxiety scores of our participants
| Controls | aMCI | naMCI | |||||
|---|---|---|---|---|---|---|---|
| Demographic | M | SD | M | SD | M | SD | P |
| Sex | |||||||
| Females (N, %) | 53 (91%) | 23 (81%) | 12 (92%) | ||||
| Males (N, %) | 5 (9%) | 5 (19%) | 1 (8%) | ||||
| Age | 71.10 | 6.18 | 74.04 | 6.89 | 73.08 | 8.03 | .22 |
| Education | 15.26 | 2.34 | 14.22 | 2.36 | 14.85 | 2.64 | .18 |
| GDS | .81 | 1.19 | .65 | .78 | 1.11 | 1.54 | .59 |
Abbreviations: aMCI, amnestic mild cognitive impairment; GDS, Geriatric Depression Scale; naMCI, non‐amnestic mild cognitive impairment; SD, standard deviation.
2.2. EEG recordings
Brain vision (Brain Vision, Inc.) equipment was used to record scalp EEG activity with high‐density Acti Cap (64 active electrodes), modified according to the International 10‐20 System. The recording locations included the FCz electrode as an online reference and the AFz electrode at midline location as a ground. Filter settings for low and high pass were 70 Hz and 0.1 Hz, respectively. For these filters the cutoff frequencies were set at 3 dB down; the rolloff was 12 dB per octave at both sides. We maintained impedances below 10 kΩ for each channel and within a 5 kΩ range balance across all channels. The sampling rate was 500 Hz with 32 bit resolution.
We recorded baseline EEG twice: (1) at the beginning of the EEG session, and (2) at the end of EEG session, immediately after a participant performed motion direction discrimination task. After proper placing of the electrode cap with 64 electrodes and obtaining satisfactory impedances, the participant was seated behind the desk in a comfortable chair, adjusted for height, in a dimly lit room. Initially, we recorded rsEEG for at least 3 minutes with eyes closed, followed by 3 minutes with eyes open. Here we present spectral analyses obtained only with eye‐closed baseline EEG recordings before and after motion direction discrimination task to be as close as possible to the true baseline. 8 Barry et al. 9 showed that during the eyes‐open, as compared to eyes‐closed, mean activity in the delta, theta, alpha, and beta bands are reduced across sites.
2.3. Experimental procedures
We performed computerized testing and EEG recordings in a community center at the University of Michigan (UM) Detroit Center. We evaluated several available spaces with a Gauss meter prior to EEG recording to find the area with the least external noise (preferably <.3 mG) to obtain acceptable EEG signal. Active electrodes also were used to additionally isolate external noise, to minimize cable movement artefacts, and to keep impedances at bellow 10kΩ. 10
2.4. EEG data analyses
Analyzer 2 (Brain Vision, Inc.) was used for pre‐processing of the baseline EEG data following the recommendations from the OHBM COBIDAS MEEG committee. 11 Off‐line was used for inspection to identify and remove segments of EEG contaminating either excessive noise, saturation, or lack of EEG activity. We segmented cleaned EEG data in consecutive epochs of 2 seconds and analyzed off‐line (1024 data points; 0.488 Hz resolution; Hanning window). An automatic computerized procedure using a rejection criterion of +/– 100 mV on any channel affected by artifacts (muscular, instrumental) was used to identify acceptable epochs. The artifact‐free segments were additionally detrended and baseline corrected before averaging. On average we obtained 90 (range, 64–115) 2‐second artifact‐free segments per subject to perform fast Fourier transform (FFT) analyses to evaluate power in major frequency bands: delta (2–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–30 Hz). Frequencies > 30 Hz were not included in power analyses due to contamination of pericranial musculature electrical activity of scalp‐recorded neuroelectric activity. 12 To reduce the number of recording sites for further analysis we designated a total of six regions of interest (ROIs; Figure 1): frontal—F (Fp1, Fp2, AF3, AF7, AF4, AF8, F7, F5, F3, F1, Fz, F2, F4, F6, F8, Fc5, FC3, FC1, FC2, FC4, FC6), left temporal—LT (FT9, FT7, T7, TP7, TP9) and right temporal—RT (FT10, FT8, T8, TP8, TP10), central—C (C5, C3, C1, Cz, C2, C4, C6, CP5, CP3, CP1, CPz, CP2, CP4, CP6), parietal—P (P7, P5, P3, P1, Pz, P2, P4, P6, P8, PO7, PO3, POz, PO4, PO8), and occipital—Occ (PO9, O1, Oz, O2, PO10). Before calculating absolute power for each ROI, EEG epochs were transformed into the reference‐free CSD distribution, which removes nearly all volume conduction effects. 13
FIGURE 1.
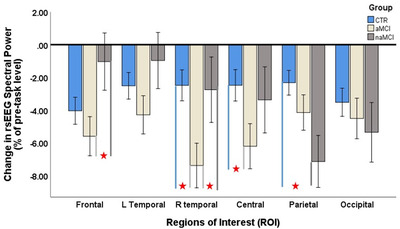
Overall effects of motion direction discrimination task on post as compared pre resting state electroencephalogram (rsEEG). Effect estimates are post versus pre % change in EEG current source density for six different cortical regions separate for controls (HC), amnestic mild cognitive impairment (aMCI), and nonamestic MCI (naMCI).  indicates statistically significant pairwise comparison
indicates statistically significant pairwise comparison
2.5. STATISTICAL PROCEDURES
SPSS for Windows (release 22.0.0) was used for data analyses. Initially, all the measures were evaluated for normality using a Shapiro–Wilk test. As expected, all the EEG spectral power measures were skewed and a log transformation was applied. The primary hypothesis was tested as a 6 (ROI) × 4 (Band) × 3 (Group) repeated‐measures general linear model that tested MCI‐related difference in spectral power. The analysis was made of measures taken prior to the task, as well as in percentage change ([(post–pre) / pre] x 100) in rsEEG after cognitive engagement. Age, years of education, and sex were included as covariates in the models. Significant omnibus effects were evaluated with paired comparisons between groups by region, adjusted for covariates.
3. RESULTS
3.1. Demographic data
Demographic information and computer anxiety scores of our participants are presented in Table 1. As expected, there was no difference between HC, aMCI, and naMCI subjects in terms of age and education, nor Geriatric Depression Scale (GDS).
3.2. Resting state EEG spectral power before cognitive engagement
Resting state EEG spectral power measurements before and after cognitive engagement are presented in Table 2 separately for normal controls, aMCI, and naMCI.
TABLE 2.
Resting state eyes‐closed EEG CSD spectral powers for pre‐ and post‐cognitive engagement for controls (HC), amnestic MCI (aMCI), and nonamnestic MCI (naMCI)
| Group ROI | Controls | aMCI | No‐aMCI | |||
|---|---|---|---|---|---|---|
| Pre (M, SD) | Po (M, SD) | Pre (M, SD) | Po (M, SD) | Pre (M, SD) | Po (M, SD) | |
| Frontal | ||||||
| δ | 4.86 (0.63) | 4.60 (0.64) | 4.91 (0.66) | 4.55 (0.56) | 4.77 (0.57) | 4.71 (0.74 |
| θ | 4.48 (0.83) | 4.00 (0.77) | 4.71 (0.72) | 4.06 (0.60) | 4.32 (0.74) | 4.11 (0.77) |
| α | 4.13 (1.02) | 4.06 (1.04) | 4.18 (0.70) | 4.13 (0.68) | 4.16 (1.03) | 4.06 (0.60) |
| β | 4.77 (0.81) | 4.77 (0.76) | 4.79 (0.61) | 4.69 (0.65) | 4.85 (0.77) | 4.92 (0.75) |
| L temporal | ||||||
| δ | 5.04 (0.78) | 4.82 (0.76) | 5.10 (0.73) | 4.80 (0.58) | 5.08 (0.76) | 5.09 (0.85) |
| θ | 4.77 (0.92) | 4.41 (0.85) | 4.99 (0.74) | 4.50 (0.66) | 4.75 (0.70) | 4.56 (0.76) |
| α | 4.76 (1.02) | 4.76 (0.98) | 4.74 (0.74) | 4.72 (0.73) | 4.67 (0.91) | 4.66 (0.93) |
| β | 4.97 (0.87) | 5.02 (0.78) | 5.06 (0.62) | 4.98 (0.73) | 5.11 (0.92) | 5.15 (0.83) |
| R temporal | ||||||
| δ | 5.12 (0.70) | 4.95 (0.68) | 5.21 (0.72) | 4.79 (0.62) | 4.87 (0.75) | 4.67 (0.79) |
| θ | 4.83 (0.89) | 4.46 (0.82) | 5.05 (0.77) | 4.39 (0.68) | 4.46 (0.75) | 4.21 (0.79) |
| α | 4.71 (0.98) | 4.71 (0.97) | 4.76 (0.75) | 4.57 (0.84) | 4.47 (0.93) | 4.43 (0.95) |
| β | 5.03 (0.80) | 5.00 (0.75) | 5.04 (0.67) | 4.74 (0.87) | 4.93 (0.70) | 4.89 (0.59) |
| Central | ||||||
| δ | 4.66 (0.83) | 4.49 (0.83) | 4.76 (0.88) | 4.34 (0.75) | 4.61 (0.77) | 4.41 (0.70) |
| θ | 4.45 (0.98) | 4.11 (0.95) | 4.65 (0.85) | 4.21 (0.80) | 4.32 (0.93) | 4.02 (0.85) |
| α | 4.50 (1.07) | 4.49 (1.09) | 4.74 (0.93) | 4.60 (1.02) | 4.47 (1.27) | 4.37 (1.19) |
| β | 4.84 (0.90) | 4.83 (0.89) | 4.88 (0.68) | 4.65 (0.76) | 4.89 (0.91) | 4.74 (0.80) |
| Parietal | ||||||
| δ | 4.71 (0.74) | 4.57 (0.72) | 4.75 (0.75) | 4.45 (0.70) | 4.75 (0.65) | 4.40 (0.72) |
| θ | 4.60 (0.86) | 4.38 (0.85) | 4.67 (0.81) | 4.49 (0.86) | 4.60 (0.77) | 4.21 (0.88) |
| α | 5.14 (1.02) | 5.09 (1.05) | 5.20 (1.09) | 5.10 (1.15) | 4.96 (1.22) | 4.71 (1.36) |
| β | 5.06 (0.80) | 4.97 (0.81) | 5.04 (0.73) | 4.80 (0.93) | 5.17 (0.79) | 4.77 (0.79) |
| Occipital | ||||||
| δ | 4.74 (0.71) | 4.57 (0.75) | 4.70 (0.76) | 4.43 (0.66) | 4.80 (0.82) | 4.59 (0.86) |
| θ | 4.63 (0.77) | 4.39 (0.82) | 4.64 (0.78) | 4.43 (0.77) | 4.72 (0.81) | 4.39 (1.01) |
| α | 4.96 (0.92) | 4.82 (0.97) | 4.95 (0.95) | 4.80 (0.99 | 4.93 (1.19) | 4.71 (1.30) |
| β | 5.31 (0.83) | 5.12 (0.82) | 5.40 (0.76) | 5.10 (0.95 | 5.37 (0.79) | 5.11 (0.84) |
Abbreviations: ROI, region of interest; SD, standard deviation.
Resting state—eyes closed—EEG spectral powers were entered into mixed 6 × 4 × 3 mixed analysis of covariance with ROI (6 levels—F, LT, RT, C, P, Occ), Band (4 levels—delta, theta, alpha, beta) as within‐subjects variables and Group (controls, aMCI, naMCI) as between‐subjects variable. Sex, age, and education were included as covariates. Analyses did not reveal any group differences (F [2,93] = .24, P = .787), or between‐group × ROI interaction (Wilks’ λ [10,178] = .62, p = .792), Group × Band interaction (Wilks’ λ [6,182] = .96, P = .451), or Group × ROI x Band interaction (Wilks’ λ [30,158] = 1.20, P = .238) in rsEEG before cognitive engagement.
3.3. Change in resting state EEG spectral power after cognitive engagement
However, significant group differences were evident in the percent change in rsEEG after cognitive engagement. The results of the multivariate analysis showed the main effect of group (F [2,92]) = 3.45, P = .036) and significant interaction effect group × ROI (Wilks’ λ [10,176] = 2.45, P = .009) to indicate groups differed overall and the magnitude of the difference varied across cortical regions. There was no effect of band (Wilks’ λ [3,90] = 1.64, P = .186) or an interaction with group (Wilks’ λ [6,180] = .41, P = .871) or region (Wilks’ λ [15,78] = .96, P = .503), therefore further post hoc analysis evaluated between‐group differences by region in the average signal across bands. Post hoc analyses demonstrated differences between MCI and HC across ROIs (see Table 3 and Figure 1). Patients with aMCI showed significantly greater decrease of rsEEG in right temporal and the central ROIs as compared to HC (t = 2.82, and t = 2.20, respectively). Patients with naMCI showed significantly greater decrease of rsEEG in the parietal region compared to HC (t = 2.64). There was also statistically significant decrease of rsEEG in the frontal and right temporal ROIs in aMCI compared to naMCI (t = 2.05, and t = 2.03, respectively).
TABLE 3.
Pre versus post task % change of resting state EEG spectral power for controls, aMCI, and naMCI and the between‐group comparisons
| Group means | Between‐group comparisons | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | aMCI | naMCI | HC vs. aMCI | HC vs. naMCI | aMCI vs. naMCI | |||||||
| ROI | M | SD | M | SD | M | SD | t‐test | d | t‐test | d | t‐test | d |
| Frontal | −4.05 | 6.10 | −5.60 | 7.14 | −1.04 | 5.40 | 1.05 | .23 | −1.64 | .46 | ‐2.05 | .72 |
| L temporal | −2.52 | 5.34 | −4.30 | 7.37 | −0.97 | 5.31 | 1.22 | .28 | −0.88 | .28 | −1.45 | .52 |
| R temporal | −2.49 | 7.49 | −7.39 | 7.66 | −2.76 | 4.37 | 2.82 | .65 | 0.12 | .04 | ‐2.03 | .74 |
| Central | −2.49 | 7.39 | −6.22 | 7.26 | −3.38 | 6.68 | 2.20 | .51 | 0.40 | .13 | −1.19 | .41 |
| Parietal | −2.33 | 5.16 | −4.16 | 5.13 | −7.15 | 8.80 | 1.54 | .36 | 2.64 | .67 | 1.38 | .38 |
| Occipital | −3.52 | 6.73 | −4.27 | 6.05 | −5.36 | 6.87 | 0.66 | .12 | 0.88 | .27 | 0.40 | .17 |
Note: Group means and between‐group comparisons are reported, including an estimate of effect size (Cohen's d). Significant between‐group differences (P < 0.05) are in bold.
Abbreviations: aMCI, amnestic mild cognitive impairment; EEG, electroencephalogram; HC, healthy control; naMCI, nonamnestic mild cognitive impairment; ROI, region of interest; SD, standard deviation.
4. DISCUSSION
Our results provide exploratory evidence for changes in cortical modulation as indexed by changes in rsEEG after cognitive engagement in HC, aMCI, and naMCI older Blacks. Results showed significant decrease in the power of rsEEG after a short motion direction discrimination task, as compared to rsEEG before the perceptual task. After the cognitive task, older adults with aMCI compared to HC displayed greater decreases in rsEEG in right temporal and central cortical regions, while naMCI showed a similar pattern only in parietal cortical region. Older adults with aMCI compared to naMCI showed greater change in rsEEG measured over the frontal and right temporal regions.
At present, we can only speculate as to the origin of the greater decrease in rsEEG at post‐task in MCI compared to HC. To the extent that the task engaged the cerebral cortex, greater decreases in rsEEG after the task may indicate MCI patients are slower to return to a baseline, or default, state. The observed pattern may be consistent with the hypothesis that cognitive deficits in MCI are in part attributed to lingering functional activation, which diminishes cognitive efficiency and increases the likelihood for error. The differentiation of brain regions is consistent with typical clinical features of MCI: it is plausible that variability in post‐task activation within the temporal cortices would contribute to declarative memory deficits in aMCI. The differentiation of the naMIC from HC regarding significant decrease of rsEEG over the parietal region is quite perplexing. The heterogeneous patterns of brain atrophy and brain activity in naMCI compared to aMCI and controls has been well established and further supported by our findings: the regional pre–post changes of rsEEG for naMCI are not as uniform as for as aMCI and controls: naMCI showed the peak of % change at parietal ROI while the minimal change was over the frontal temporal sites. To the best of our speculation we assume that in naMCI slowed return rsEEG to baseline at parietal ROI is due to impaired cortical activity resulted from parietal cortical atrophy, white matter lesions, and increased AD neuropathology at this region. The sources of the variability in post‐task rsEEG will require further study. For example, slowed return to baseline state may be due to persistent activation of task‐based functional networks, slowed re‐activation of resting state networks, or possibly new cognitive engagement related to self‐appraisal of past task performance.
Somewhat surprisingly, the results did not show significant difference among the three groups for pre‐task rsEEG. This finding is contrary to several reports of MCI patients displaying decreased EEG spectral power compared to HC, primarily in lower frequency bands (for review see Babiloni et al. 1 ). However, pre‐clinical functional deficit and AD‐related neuropathology may be more evident after the challenge of a task, and the recovery to baseline rsEEG appears to be a sensitive index to differentiate MCI from HC. This initial report warrants additional study with a larger, independent sample to determine whether changes in rsEEG CSD in specific cortical regions after cognitive engagement may be a sensitive biomarker to identify and classify persons with aMCI and naMCI. After further validation, this approach may be useful for clinical screening and community‐based research.
In sum, the results of the present study demonstrate that repeated short rsEEG recording could be an effective EEG marker of cognitive deficit in older adults. Our present study indicates that the task‐related alterations in rsEEG can discriminate those older Blacks with MCI from those with age‐typical cognition. Furthermore, this study also attests to the feasibility of combined rsEEG as a sensitive recording technique for probing cognitive‐induced changes in brain activity. Future research using this technique in conjunction with systematic manipulation of specific cognitive engagement dose and modality parameters will no doubt lead to an increased understanding of the complex rsEEG dynamics. Future studies combining both behavioral and rsEEG outcome measures aiming to probe the effects of cognitive engagement on cortical network activity will no doubt aid scientific understanding as well as clinical relevance to use pre–post task EEG modulation approach.
CONFLICTS OF INTEREST
The authors declare no conflict of interest with respect to this research, authorship, and/or the publication of this article.
ACKNOWLEDGMENTS
This research was in part supported by grants from NIA/NIH, 1R21AG046637‐01A1, 1R01AG054484‐01A1; Alzheimer's Association Award HAT‐07‐60437 grant; and a grant from the Slovenian Research Agency, P3‐0366/2451 to Voyko Kavcic; and by partial support from NIH/NIA grant P30AG053760 to the Michigan Alzheimer's Disease Research Center (MADC).
Kavcic V, Daugherty AM, Giordani B. Post‐task modulation of resting state EEG differentiates MCI patients from controls. Alzheimer's Dement. 2021;13:1–6. 10.1002/dad2.12153
REFERENCES
- 1. Babiloni C, Del Percio C, Pascarelli MT, et al. Abnormalities of functional cortical source connectivity of resting‐state electroencephalographic alpha rhythms are similar in patients with mild cognitive impairment due to Alzheimer's and Lewy body diseases. Neurobiol Aging. 2019;77:112‐127. [DOI] [PubMed] [Google Scholar]
- 2. Babiloni C, Blinowska K, Bonanni L, et al. What electrophysiology tells us about Alzheimer's disease: a window into the synchronization and connectivity of brain neurons. Neurobiol Aging. 2020;85:58‐73. [DOI] [PubMed] [Google Scholar]
- 3. Vecchio F, Babiloni C, Lizio R, et al. Resting state cortical EEG rhythms in Alzheimer's disease: toward EEG markers for clinical applications: a review Suppl Clin Neurophysiol. 2013:223‐236. [DOI] [PubMed] [Google Scholar]
- 4. Tóth B, File B, Boha R, et al. EEG network connectivity changes in mild cognitive impairment—preliminary results. Int J Psychophysiol. 2014;92(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 5. Požar R, Giordani B, Kavcic V. Effective differentiation of mild cognitive impairment by functional brain graph analysis and computerized testing. PLoS One. 2020;15(3):e0230099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toosizadeh N, Ehsani H, Wendel C, Zamrini E, O'Connor K, Mohler J. screening older adults for amnestic mild cognitive impairment and early‐stage Alzheimer's disease using upper‐extremity dual‐tasking. Sci Rep. 2019;9(1):1‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160‐1163. [DOI] [PubMed] [Google Scholar]
- 8. Duncan NW, Northoff G. Overview of potential procedural and participant‐related confounds for neuroimaging of the resting state. J Psychiatry Neurosci. 2013;38(2):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barry RJ, Clarke AR, Johnstone SJ, Magee CA, Rushby JA. EEG differences between eyes‐closed and eyes‐open resting conditions. Clin Neurophysiol. 2007;118(12):2765‐2773. [DOI] [PubMed] [Google Scholar]
- 10. Kappenman ES, Luck SJ. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 2010;47(5):888‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pernet C, Garrido MI, Gramfort A, et al. Issues and recommendations from the OHBM COBIDAS MEEG committee for reproducible EEG and MEG research. Nat Neurosci. 2020:1‐11. [DOI] [PubMed] [Google Scholar]
- 12. Whitham EM, Pope KJ, Fitzgibbon SP, et al. Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin Neurophysiol. 2007;118(8):1877‐1888. [DOI] [PubMed] [Google Scholar]
- 13. Kayser J, Tenke CE. Issues and considerations for using the scalp surface Laplacian in EEG/ERP research: a tutorial review. Int J Psychophysiol. 2015;97(3):189‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]


