Abstract
BACKGROUND
A disease caused by a novel coronavirus virus, named coronavirus disease 2019 (COVID-19), broke out in Wuhan, China in December 2019, and spread around the word. As of March 4, 2020, 93090 confirmed cases and 2984 deaths have been reported in more than 80 countries and territories. It has triggered global public health security. However, the features and prognosis of COVID-19 are incompletely understood.
CASE SUMMARY
We here report that the erythrocyte sedimentation rate (ESR) increased in a confirmed COVID patient. The high level of ESR sustained for a long time even after the patient recovered from COVID-19, while all results related to tumor, tuberculosis, rheumatic diseases, anemia, etc. cannot explain the abnormal elevation of ESR presented in this case.
CONCLUSION
Although the increased ESR cannot be explained by all existing evidence, it possibly links the abnormal pathologic change in some COVID-19 patients and negative prognosis, and provides the clue to dissect the mechanism of illness progressing in COVID-19 and its prognosis.
Keywords: COVID-19, Erythrocyte sedimentation rate, Prognosis, SARS-CoV-2, Joint damage, Case report
Core Tip: So far, there have been many reports on the coronavirus disease 2019 (COVID-19) around the world that present different epidemiological and clinical features. Here we report a case that had a sustaining high level of erythrocyte sedimentation rate in a patient recovering from COVID-19. The high level of erythrocyte sedimentation rate was not from the tumor, inflammation, tuberculosis, rheumatic diseases, or autoimmune diseases. Therefore, we suspected that COVID-19 possibly damaged the blood or immune system. The abnormal erythrocyte sedimentation rate would be a precursor causing the joint damage after COVID-19 infection, such as osteoarthritis in future. Our report provides the supplement to understand the features of COVID-19 and shows some clues helping understand the prognosis of severe acute respiratory syndrome coronavirus 2 infection.
INTRODUCTION
In December, 2019, a cluster of patients with pneumonia of unknown cause emerged in Wuhan, China, and later were confirmed with infection of a novel coronavirus virus [named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)][1-3]. Although most coronavirus disease 2019 (COVID-19) patients presented the initial signs and symptoms as fever or cough[4], extra-pulmonary symptoms have also been reported[5]. Recent research has shown that patient with no clinical signs could be infected by the SARS-CoV-2[6], while other studies have shown that COVID-19 can rapidly evolve into the acute respiratory distress syndrome and even multiple organ dysfunction[4,7]. Since most studies focused the changes of the respiratory system, the understanding of COVID-19 is still superficial, and the features of other organ involvement and prognosis in COVID-19 patients are unclear.
We herein describe an unexplained phenomenon that a high level of erythrocyte sedimentation rate (ESR) was present in a case of COVID-19, and aim to dissect the cause of abnormal ESR elevation in this case, in order to provide insight to understand the illness progression, and the long-term impact of COVID-19 on patients.
CASE PRESENTATION
Chief complaints
A 51-year-old male patient presented with high fever, dry cough, malaise, and pronounced tiredness for 8 d.
History of present illness
The patient, residing in Guizhou Province, China, returned from Wuhan, China on January 22, 2020, where there had been an outbreak of COVID-19. The patient was admitted to Guizhou Provincial People’s Hospital on January 29, 2020 with high fever, dry cough, malaise, and pronounced tiredness.
History of past illness
The patient had a 5-year history of hypertension but denied any history of arthritis, hyperthyroidism, tuberculosis, autoimmune disease, and other chronic diseases.
Personal and family history
The patient had no particular individual or family history.
Physical examination
On admission, the physical examination revealed a body temperature of 37.8 ℃ and no physical signs suggestive of lymphadenopathy, leukemia, and hyperthyroidism. Detailed systemic physical examination revealed no abnormalities in the cardiovascular or abdominal systems.
Laboratory examinations
Laboratory examinations after admission showed mildly abnormal routine blood parameters and other inflammation parameters such as C-reactive protein (CRP), procalcitonin, interleukin-6 (IL-6) and ESR, adn normal liver and renal function parameters (Table 1). Arterial blood gas analysis was as follows: pH, 7.418; oxygen partial pressure, 71 (oxygen Index 173); partial pressure of carbon dioxide, 42 mmHg on room air. The nucleic acid assay of SARS-CoV-2 of throat swab was tested on January 29 and 30, 2020, and the results showed positive nucleic acid assay of SARS-CoV-2 twice.
Table 1.
Main results of routine blood test, inflammation parameters, and liver and renal function
|
Item/result
|
WBC (× 109/L)
|
NEUT (%)
|
LYM (× 109/L)
|
PCT (ng/mL)
|
IL6 (pg/mL)
|
ESR (mm/h)
|
CRP (mg/L)
|
CREA (umol/L)
|
UREA (mmol/L)
|
TBIL (umol/L)
|
ALT (U/L)
|
AST (U/L)
|
| Result | 3.57 | 53.5 | 1.29 | 0.06 | 35.65 | 22 | 43.58 | 103 | 4.6 | 17.3 | 9.73 | 38.45 |
| Reference range | 4-10 | 45-77 | 0.8-4 | < 0.5 | 0-7 | 0-15 | 0-8 | 40-106 | 2.9-8.2 | 5.1-20 | 5-40 | 8-40 |
WBC: White blood cells; NEUT: Neutrophil granulocytes; LYM: Lymphocytotoxicity; PCT: Procalcitonin; IL-6: interleukin-6; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; CREA: Creatinine; TBIL: Total bilirubin; ALT: Alanine transaminase; AST: Aspartate aminotransferase.
Imaging examinations
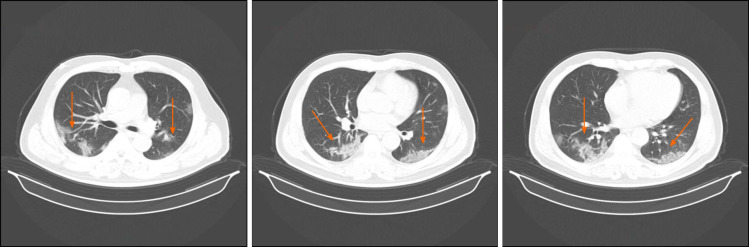
Chest computed tomography (CT) on admission showed the feature of multiple ground glass opacifications in both lower lobes (Figure 1).
Figure 1.
Chest computed tomography images obtained on January 31, 2020. After admission, the high-resolution computed tomography scan of chest on January 31, 2020 showed ground-glass shadowing in both the left and right lower lobes (orange arrows).
FINAL DIAGNOSIS
Based on the positive nucleic acid assay of SARS-CoV-2, symptoms, laboratory data, and CT results, COVID-19 was confirmed on January 31, 2020 according to the diagnostic criteria for COVID-19.
TREATMENT
After admission, main treatments included moxifloxacin, oseltamivir, γ-interferon, and Lianhua antipyretic granules. On February 2, 2020, the patient still had the symptoms of intermittent fever and dry cough, therefore, the anti-viral drugs, lopinavir and ritonavir tablets and methylprednisolone were given.
OUTCOME AND FOLLOW-UP
Up to February 14, 2020, the patient’s symptoms of malaise and pronounced tiredness were significantly improved, but he still had dry cough and intermittent fever. For centralized management of COVID-19 patients, he was transferred to Jiang Jun Shan Hospital (the designated hospital for COVID-19 patients in Guizhou Province, China). Laboratory data after transferring indicated that the count of leukocytes and lymphocytes decreased, while IL-6, CRP, and ESR increased, and liver function parameters were mildly abnormal (Table 2). Arterial blood gas analysis was as follows: pH, 7.41; oxygen partial pressure, 78 (oxygen index 270); partial pressure of carbon dioxide, 42 mmHg on room air (Table 3). Chest CT showed the streaky or coarse reticular pattern opacities, and lesions in both lower lobes were improved compared to those scanned before (Figure 2). Treatments with moxifloxacin and oseltamivir were replaced with the anti-virus drug Arbido. On February 21, 2020, the symptoms of dry cough and fever significantly resolved. Chest CT showed that the lesions in both lower lobes significantly improved (Figure 3), and the assay of blood sample indicated that all parameters of inflammation were normal except ESR (Table 4) tested on February 25, 2020. Also, both the nucleic acid assay of throat swab of SARS-CoV-2 tested on February 22 and 24, 2020 were negative, indicating that this patient had recovered from COVID-19. With the improvement of the patient’s condition, we stopped the use of all anti-viral drugs on February 24, 2020. The patient’s situation was stable from February 24, 2020 to March 1, 2020, and he was discharged and entered the period of medical observation from March 1, 2020.
Table 2.
Main results of routine blood tests, inflammation parameters, and liver and renal function
|
Item/result
|
WBC (× 109/L)
|
NEUT (%)
|
LYM (× 109/L)
|
PCT (ng/mL)
|
IL6 (pg/mL)
|
ESR (mm/h)
|
CRP (mg/L)
|
CREA (umol/L)
|
UREA (mmol/L)
|
TBIL (umol/L)
|
ALT (U/L)
|
AST (U/L)
|
| Results | 2.27 | 54.8 | 0.73 | 0.1 | 14.2 | 102 | 16.69 | 89 | 3.1 | 10.2 | 46 | 43 |
| Reference range | 4-10 | 45-77 | 0.8-4 | < 0.5 | 0-7 | 0-15 | 0-8 | 40-106 | 2.9-8.2 | 5.1-20 | 5-40 | 8-40 |
WBC: White blood cells; NEUT: Neutrophil granulocytes; LYM: Lymphocytotoxicity; PCT: Procalcitonin; IL-6: interleukin-6; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; CREA: Creatinine; TBIL: Total bilirubin; ALT: Alanine transaminase; AST: Aspartate aminotransferase.
Table 3.
Results of examination related to erythrocyte sedimentation rate
|
Item
|
Result
|
Reference range
|
| FT3 (ρmol/L) | 5.52 | 3.28-6.47 |
| FT4 (ρmol/L) | 11.04 | 7.64-16.03 |
| TSH (µIU/mL) | 1.473 | 0.34-5.6 |
| RF (U/mL) | 10 | 0-20 |
| ANCA | Negative | Negative |
| Hb (g/L) | 134 | 110-150 |
| AFP (ng/mL) | 3.35 | 0-10.9 |
| CA125 (U/mL) | 5 | 0-35 |
| CA19-9 (U/mL) | 20 | 0-37 |
| CEA (ng/mL) | 1.3 | 0-5 |
| Mp-IgM | Negative | Negative |
| TB-IgG | Negative | Negative |
| AFB | Negative | Negative |
| T-SPOT.TB | Negative | Negative |
| HBsAg (IU/mL) | 0 | 0-0.05 |
| HIV | Negative | Negative |
TSH: Thyroid stimulating hormone; RF: Rheumatic diseases; ANCA: Autoimmune diseases; Hb: Hemoglobin; AFP: Alpha fetoprotein; CEA: Carcinoma embryonic antigen; HIV: Human immunodeficiency virus.
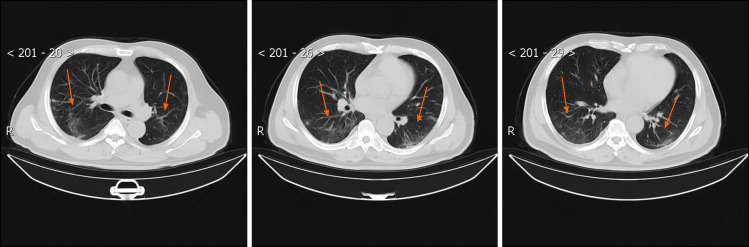
Figure 2.
Chest computed tomography images obtained on February 15, 2020. A high-resolution computed tomography scan of the chest was performed on February 15, 2020, which showed that the ground-glass shadowing in both the left and right lower lobes was improved, and there were streaky or coarse reticular pattern opacities (orange arrows).
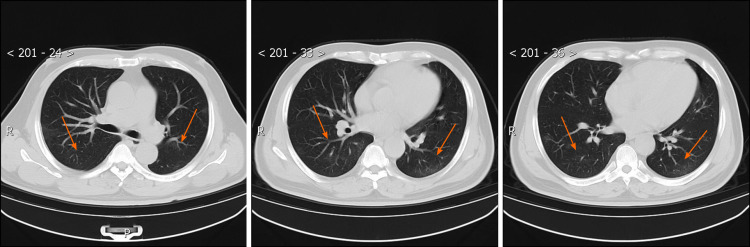
Figure 3.
Chest computed tomography images obtained on February 25, 2020. After the patient’s signs and symptoms resolved, a high-resolution computed tomography scan of the chest was performed on February 25, 2020, which showed that the ground-glass shadowing in both lower lobes, and the streaky or coarse reticular pattern opacities were further significantly improved (orange arrows).
Table 4.
Main results of routine blood tests, inflammation parameters, and liver and renal function
|
Item/result
|
WBC (× 109/L)
|
NEUT (%)
|
LYM (× 109/L)
|
PCT (ng/mL)
|
IL6 (pg/mL)
|
ESR (mm/h)
|
CRP (mg/L)
|
CREA (umol/L)
|
UREA (mmol/L)
|
TBIL (umol/L)
|
ALT (U/L)
|
AST (U/L)
|
| Result | 5 | 47.6 | 1.12 | < 0.05 | 13.97 | 120 | 8.3 | 99 | 7.1 | 11.4 | 28 | 24 |
| Reference range | 4-10 | 45-77 | 0.8-4 | < 0.5 | 0-7 | 0-15 | 0-8 | 40-106 | 2.9-8.2 | 5.1-20 | 5-40 | 8-40 |
WBC: White blood cells; NEUT: Neutrophil granulocytes; LYM: Lymphocytotoxicity; PCT: Procalcitonin; IL-6: interleukin-6; ESR: Erythrocyte sedimentation rate; CRP: C-reactive protein; CREA: Creatinine; TBIL: Total bilirubin; ALT: Alanine transaminase; AST: Aspartate aminotransferase.
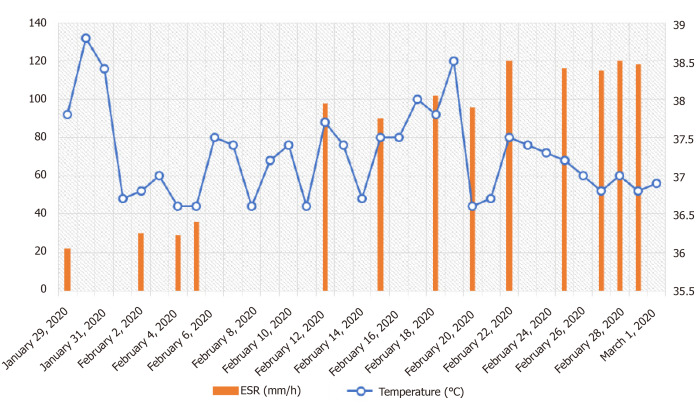
Strangely, the patient’s ESR gradually increased after admission, and reached 120 mm/h on February 22, 2020. To figure out the possible reasons of ESR increase in this case, we tested the relevant parameters of tumor, inflammation, tuberculosis, rheumatic diseases, autoimmune diseases, hyperthyroidism, and anemia, but there was no evidence indicating that the elevation of ESR resulted from these possible factors (Table 3). The ESR tested on February 28 and 29, 2020 still reached 120 mm/h and 118 mm/h, respectively (Figure 4). Therefore, we excluded the possibility that the elevation of ESR resulted from the negative effect of drugs. Moreover, the high level of ESR sustained more than 1 mo after the patient was discharged from hospital, and the ESR tested on April 18, 2020 and April 24, 2020 was 18 and 15 mm/h, respectively. However, the exact cause of sustained ESR elevation in this case is unclear.
Figure 4.
Dynamic changes of the patient’s body temperature and erythrocyte sedimentation rate during hospitalization. After admission, the patient’s body temperature was monitored and the highest temperature in each day was recorded. The results are shown in this figure as a blue scatter curve. The erythrocyte sedimentation rate was examined at several indicated time points as indicated with red histograms.
DISCUSSION
As its high susceptibility, COVID-19 caused a serious public heath issue around the world[3,8]. Although the treatments and prevention for COVID-19 progressed, little is known about the prognosis and clinical impact of COVID-19[2,9].
In this virus-imported case, the fever was the typical clinical feature of COVID-19 even though several inflammation parameters such as the count of leukocyte and neutrophils were normal (Table 1). Consistent with common characteristics of viral infection, lymphopenia presented after COVID-19 and returned to normal after recovery from virus infection (Tables 1 and 4). With the illness progression, COVID-19 could be associated with bacterial infection, as the results showed that inflammation parameters such as the count of leukocyte and neutrophils, IL-6, and CRP increased. However, the procalcitonin results indicated that viral infection rather than bacterial infection was mainly involved in this case (Tables 1, 2, and 4). As the nucleic acid assay of virus turned negative, the patient’s physical sign of fever and dry cough significantly relieved, therefore, the signs and symptoms of the patient are correlated with the activity of SARS-CoV-2 infection.
Strangely, we found an unexplained laboratory data in this case that ESR began to significantly increase at about 2 wk after COVID-19. The high level of ESR sustained for a long time even though the sign of fever and dry cough resolved, the change of chest CT manifestations improved (Figure 3), and the test of nucleic acid assay of throat swab turned negative. Current results indicated the abnormal elevation of ESR in this case was not caused by the tumor, inflammation, tuberculosis, rheumatic diseases, hyperthyroidism, anemia, autoimmune diseases, or the side-effects of drugs (Table 3).
ESR is affected by the size, shape, and concentration of red blood cells and plasma characteristics[10]. The exact causes of the increased ESR in this case are not yet clear. We speculated that COVID-19 might trigger the change of the form of erythrocytes or plasma characteristics including the immune system via an unknown mechanism to increase the ESR. The sustained high level of ESR possibly brings a negative effect on COVID-19 patients’ prognosis, since high ESR could damage the joint and thus leads to joint diseases such as osteoarthritis[11,12]. Furthermore, it may be a precursor of hepatic and renal dysfunction[12-15]. Thus, COVID-19 may influence the long-term prognosis of patients; however, it is difficult to predict the long-term prognosis of COVID-19 patients on the basis of only ESR. More cases and evidence are needed to address this issue.
CONCLUSION
In summary, we have documented a patient with a sustained high level of ESR, even after he recovered from COVID-19. The increased ESR in this patient cannot be explained by the tumor, inflammation, tuberculosis, rheumatic diseases, hyperthyroidism, anemia, autoimmune diseases, or the side-effects of drugs. It is possibly associated with the patient’s prognosis, although it is difficult to predict the long-term prognosis of COVID-19 based on this single parameter.
ACKNOWLEDGEMENTS
We thank Dr. Ting-Ting Lou and Dr. Wen-Qi Zheng at Jiang Jun Shan Hospital for their assistance in the preparation and collection of clinical data.
Footnotes
Informed consent statement: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict-of-interest statement: We confirm that the manuscript has been read and approved by all named authors, and the order of authors listed in the manuscript has been approved by all of us. All authors declare that they have no competing interests to disclose.
CARE Checklist (2016) statement: We have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Peer-review started: November 7, 2020
First decision: December 13, 2020
Article in press: January 5, 2021
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H, Velikova TV S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
Contributor Information
Sheng-Lan Pu, Department of General Medicine, The First People's Hospital of Zunyi, Zunyi 563000, Guizhou Province, China; Department of General Medicine, The Third Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China.
Xiang-Yan Zhang, Respiratory Institute, Guizhou Provincial People's Hospital, Guiyang 550000, Guizhou Province, China; NHC Key Laboratory of Pulmonary Immune related Disease, Guiyang 550000, Guizhou Province, China.
Dai-Shun Liu, Respiratory Department, The First People's Hospital of Zunyi, Zunyi 563000, Guizhou Province, China; Respiratory Department, The Third Affiliated Hospital of Zunyi Medical University, Zunyi 563000, Guizhou Province, China.
Ba-Ning Ye, Intensive Care Unit, Guizhou Provincial People's Hospital, Guiyang 550000, Guizhou Province, China.
Jian-Quan Li, NHC Key Laboratory of Pulmonary Immune related Disease, Guiyang 550000, Guizhou Province, China; Intensive Care Unit, Guizhou Provincial People's Hospital, Guiyang 550000, Guizhou Province, China. 401131098@qq.com.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Tang J, Wei F. Updated understanding of the outbreak of 2019 novel coronavirus (2019-nCoV) in Wuhan, China. J Med Virol. 2020;92:441–447. doi: 10.1002/jmv.25689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rello J, Tejada S, Userovici C, Arvaniti K, Pugin J, Waterer G. Coronavirus Disease 2019 (COVID-19): A critical care perspective beyond China. Anaesth Crit Care Pain Med. 2020;39:167–169. doi: 10.1016/j.accpm.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song Y, Liu P, Shi XL, Chu YL, Zhang J, Xia J, Gao XZ, Qu T, Wang MY. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 6.Ng OT, Marimuthu K, Chia PY, Koh V, Chiew CJ, De Wang L, Young BE, Chan M, Vasoo S, Ling LM, Lye DC, Kam KQ, Thoon KC, Kurupatham L, Said Z, Goh E, Low C, Lim SK, Raj P, Oh O, Koh VTJ, Poh C, Mak TM, Cui L, Cook AR, Lin RTP, Leo YS, Lee VJM. SARS-CoV-2 Infection among Travelers Returning from Wuhan, China. N Engl J Med. 2020;382:1476–1478. doi: 10.1056/NEJMc2003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020;92:548–551. doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsay ES, Lerman MA. How to use the erythrocyte sedimentation rate in paediatrics. Arch Dis Child Educ Pract Ed. 2015;100:30–36. doi: 10.1136/archdischild-2013-305349. [DOI] [PubMed] [Google Scholar]
- 11.Hanada M, Takahashi M, Furuhashi H, Koyama H, Matsuyama Y. Elevated erythrocyte sedimentation rate and high-sensitivity C-reactive protein in osteoarthritis of the knee: relationship with clinical findings and radiographic severity. Ann Clin Biochem. 2016;53:548–553. doi: 10.1177/0004563215610142. [DOI] [PubMed] [Google Scholar]
- 12.Atzeni F, Talotta R, Masala IF, Bongiovanni S, Boccassini L, Sarzi-Puttini P. Biomarkers in Rheumatoid Arthritis. Isr Med Assoc J. 2017;19:512–516. [PubMed] [Google Scholar]
- 13.Brouillard M, Reade R, Boulanger E, Cardon G, Dracon M, Dequiedt P, Pagniez D. Erythrocyte sedimentation rate, an underestimated tool in chronic renal failure. Nephrol Dial Transplant. 1996;11:2244–2247. doi: 10.1093/oxfordjournals.ndt.a027143. [DOI] [PubMed] [Google Scholar]
- 14.Hess CT. Monitoring laboratory values: transferrin, C-reactive protein, erythrocyte sedimentation rate, and liver function. Adv Skin Wound Care. 2009;22:96. doi: 10.1097/01.ASW.0000345287.28403.76. [DOI] [PubMed] [Google Scholar]
- 15.Stojan G, Fang H, Magder L, Petri M. Erythrocyte sedimentation rate is a predictor of renal and overall SLE disease activity. Lupus. 2013;22:827–834. doi: 10.1177/0961203313492578. [DOI] [PMC free article] [PubMed] [Google Scholar]