Abstract
Background:
Successful anticoagulation is critical for stroke prevention in adults with atrial fibrillation (AF). Anticoagulation satisfaction is a key indicator of treatment success. While physical, cognitive, and psychosocial limitations are common in elderly AF patients, their associations with anticoagulation satisfaction are unknown.
Objective:
Examine whether anticoagulation satisfaction differs among AF patients with and without physical, cognitive, and psychosocial conditions.
Methods:
The study comprised AF patients greater than or equal to 65 years old who were prescribed an oral anticoagulant (warfarin 57%; direct oral anticoagulant [DOAC] 43%). Frailty, cognitive function, social support, depressive symptoms, vision, hearing, and anxiety were assessed using validated measures. Anticoagulation satisfaction was measured using the anticlot treatment scale.
Results:
Participants (n = 1037, 50% female) were on average 76 years old. The following conditions were prevalent: frailty (14%), cognitive impairment (42%), social isolation (13%), vision impairment (35%), hearing impairment (36%), depression (29%), and anxiety (24%). Average anticlot treatment burden scale was 55 out of 60 (lower burden scales indicating higher perceived burden). Patients with high perceived burden were older, more likely to be female, and receive warfarin. After adjusting for confounders, visual impairment (adjusted odds ratio [95% confidence interval]: 1.7 [1.2-2.4]), depressive symptoms (2.4 [1.6-3.7]), and anxiety (1.8 [1.2-2.7]) were significantly associated with high perceived burden. Different conditions were associated with high perceived burden in warfarin vs DOAC users.
Conclusion:
Physical, cognitive, and psychosocial limitations are prevalent and associated with high perceived anticoagulation burden among elderly AF adults. These conditions merit consideration in anticoagulation prescribing.
Keywords: anticoagulation satisfaction, anxiety, atrial fibrillation, depression, vision
1 |. INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmia in the elderly and markedly increases stroke risk.1 Successful anticoagulation is central for stroke prevention in patients with AF.2 However, anticoagulation management is complex and frequently associated with treatment burden and bleeding.3,4 Unfortunately, approximately one-half of patients with AF have poor anticoagulation control which contributes to an increased risk of stroke and all-cause mortality.5,6
Anticoagulation satisfaction, which consists of patient-reported feelings of the limitations and burden of anticoagulation treatment, is an important but commonly ignored aspect in assessing treatment benefit. Patients being treated with warfarin who are more, vs those who are less, satisfied with anticoagulation have better treatment adherence and international normalized ratio (INR) time in the therapeutic range.7
Physical, cognitive, and psychosocial conditions have strong prognostic value in AF. Depression and anxiety are independently associated with ischemic stroke and intracranial bleeding in patients with AF.8 Also, impaired cognition is a risk factor for warfarin instability.9,10 However, whether these conditions are associated with anticoagulation satisfaction in patients with AF is unclear. Furthermore, in previous studies patients were typically treated with warfarin, and not with the more contemporary direct oral anticoagulants (DOACs).
Using data from the Systematic Assessment of Geriatric Elements in Atrial Fibrillation (SAGE-AF) study, we examined the association between six conditions commonly seen in geriatrics, including frailty, cognitive impairment, lack of social support, depressive symptoms, vision and hearing impairment, and anxiety in relation to anticoagulation satisfaction as characterized by the anticlot treatment scale (ACTS).11
This analysis provides data on anticoagulation satisfaction and will facilitate anticoagulation decision making in elderly patients with AF.
2 |. METHODS
2.1 |. Study population
The SAGE-AF study is an ongoing, prospective study of adults 65 years and older with AF who received oral anticoagulation. Participants completed a comprehensive baseline geriatric assessment, a structured interview, and a medical record review in the context of their routine medical care.
The eligibility criteria for SAGE-AF include: (a) be scheduled for an ambulatory care visit at one of four Central Massachusetts practices (University of Massachusetts Memorial Health Care internal medicine, cardiology, or electrophysiology, Heart Rhythm Associates of Central Massachusetts), one practice in Eastern Massachusetts (Boston University cardiology), or one of two practices in Central Georgia (Family Health Center and Georgia Arrhythmia Consultants); (b) have AF (if the arrhythmia was present on an electrocardiogram or Holter monitor or if it was noted in any clinic note or hospital record); and (c) have a CHA2DS2VASC12 risk score greater than or equal to 2. Participants are not eligible for enrollment if they have an absolute contraindication to oral anticoagulation, if they have an indication for oral anticoagulation other than AF (eg, mechanical heart valve), if they are unable to provide signed informed consent, if they do not speak English, if they have a planned invasive high bleeding risk procedure, if they are pregnant, if they are prisoners, or if they are unwilling or unable to participate in planned 1- and 2-year follow-up visits.
Out of the 6507 individuals screened, a total of 1244 participants have been enrolled in this longitudinal study and completed their baseline examination (Figure 1). Study protocols were approved by the University of Massachusetts Medical School, Boston University, and Mercer University Institutional Review Boards.
FIGURE 1.
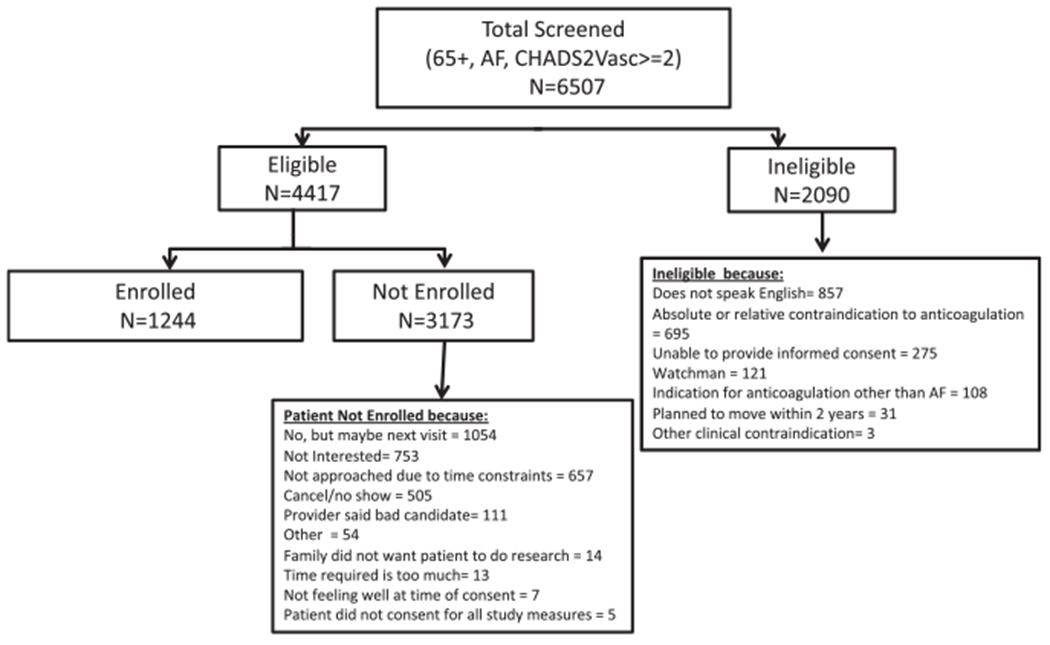
Enrollment flow chart. AF, atrial fibrillation
2.2 |. Data abstraction
Demographic, clinical, treatment, and laboratory characteristics were abstracted from hospital and clinic medical records by trained study staff. Information abstracted from the health record included participants’ age, sex, race, insurance type, comorbidities relevant to stroke and bleeding risk (eg, diabetes, hypertension, heart failure, anemia, chronic kidney disease), and cardiovascular treatments. Information about key laboratory findings, including serum creatinine, hemoglobin, and INR values (over the past 4 weeks), were also abstracted from the health record. CHA2DS2VASC and HAS-BLED13 scores were calculated. Alcohol use was ascertained from the medical record as well as from patient self-report during the baseline interview. Labile INR was defined as less than 60% of the 12 most recent INR values before baseline visit in the range of 2 to 3. Participants on a DOAC did not score on “L” in the HAS-BLED score.
2.3 |. Comprehensive geriatric and mood assessment
All SAGE-AF participants complete a six-component geriatric assessment using validated measures of frailty, cognitive function, social support, depressive symptoms, vision, and hearing. Frailty is assessed using the Cardiovascular Health Survey frailty scale.14 It has five components: weight loss/shrinking, exhaustion, low physical activity, slow gait speed, and weakness. Each component receives a point and the scale ranges from 0 to 5. A participant is considered to be frail if 3 or more criteria are present, prefrail (1-2), and not frail (0). Cognitive function is assessed by the Montreal Cognitive Assessment Battery.15 It is a 30-item screening tool validated to detect mild cognitive impairment. Higher scores indicate greater cognitive function, with a score less than 23 indicating cognitive impairment. We use a 5-item modified version of the Social Support Scale and the 6-item Social Network Scale to assess breadth and depth of social support available to participants.16 High scores indicate more social support with a score of less than 12 indicating social isolation. The Patient Health Questionnaire (PHQ-9) was used to assess for depressive symptoms17 with a score greater than or equal to 5 indicating high depressive symptoms. Vision and hearing were self-reported and categorized as binary (impaired or not). Anxiety was assessed using the Generalized Anxiety Disorder-7 scale18 with a score greater than or equal to 5 indicating anxiety symptoms.
2.4 |. Anticoagulation satisfaction measurement
Anticoagulation satisfaction is measured by ACTS.11 The ACTS is a validated 15-item survey designed to assess patients’ satisfaction with their oral anticoagulation. It has been used in major clinical trials19,20 and registries.21,22 In contrast to some other assessments of anticoagulation satisfaction, it includes questions that pertain to both DOAC and warfarin users. It has two scales: a 12-item burden scale and a 3-item benefit scale. The burden item is coded from “Extremely” (coded 1) to “Not at all” (coded 5). The burden scale sums the individual burden items and ranges from 12 to 60. Higher scores represent less favorable perceptions of anticoagulation. There is no published consensus on what constitutes a clinically important difference on the ACTS scale. We defined high anticoagulation burden as being in the lowest quartile of the burden scale. We also examined the scale as a continuous variable. The benefit items are coded from “Not at all” (coded 1) to “Extremely” (coded 5). The benefit scale is the sum of the benefit items and ranges from 3 to 15, higher scores representing more favorable perceptions.
2.5 |. Data analysis
Differences in selected baseline characteristics between participants reporting high anticoagulation burden were compared to participants with lower burden by analysis of variance for continuous variables and the χ2 test for categorical variables. The associations between exposures and anticoagulation burden scale were examined by logistic regression and linear regression. Model 1 included six conditions (frailty, cognitive impairment, social isolation, visual impairment, hearing impairment, and depression), plus anxiety and presence of AF symptoms. Model 2 additionally adjusted for demographical variables including age, sex, education, income, insurance, and provider type. Lastly, model 3 additionally adjusted for comorbidities summarized as CHA2DS2VASC and HAS-BLED scores. The association between exposures and the benefit scale was examined separately by linear regression.
Because anticoagulation satisfaction is higher among DOAC users than warfarin users,3 an analysis was also performed examining the association between anticoagulation burden and each condition separately among DOAC and warfarin users. In addition, since the proportion of patients receiving DOACs is higher among participants enrolled from Georgia, analyses were stratified by site (Massachusetts vs Georgia). Statistical analyses were performed using SAS 9.4 (SAS Institute, Inc, Cary, NC).
3 |. RESULTS
SAGE-AF enrolled 1244 participants. Of these, 83% (N = 1037) were taking oral anticoagulants and are included in the analysis. Participants were on average 76 (±7) years old and approximately one half were women. Participants who reported high anticoagulation burden were younger, more likely to be female, and were more likely to have been previously diagnosed with anemia, chronic lung disease, and have an implantable cardiac device. Participants who perceived a higher burden from their anticoagulation were more often treated with warfarin (Table 1).
TABLE 1.
Characteristics of elderly adults with atrial fibrillation according to perceived anticoagulation burden
| Characteristics | High burdena (n = 254) | Low burden (n = 783) | P |
|---|---|---|---|
| Age | 74 (7) | 76 (7) | <.01 |
| Female | 145 (57) | 369 (47) | .01 |
| White | 210 (83) | 673 (86) | .2 |
| Married or living as married | 136 (54) | 441 (56) | .64 |
| Education | .36 | ||
| High school/some college | 147 (58) | 453 (58) | |
| College graduate | 43 (17) | 112 (14) | |
| Graduate degree or above | 60 (24) | 213 (27) | |
| Annual income (in dollars) | .06 | ||
| <10 000 | 16 (8) | 29 (4) | |
| 10 000-49 999 | 102 (50) | 301 (45) | |
| 50 000-99 999 | 54 (26) | 218 (33) | |
| ≥100 000-149 999 | 31 (15) | 119 (18) | |
| Insurance | .05 | ||
| Commercial/HMO/PPO | 39 (15) | 142 (18) | |
| Medicare | 191 (75) | 561 (72) | |
| Atrial fibrillation type | .99 | ||
| Paroxysmal | 142 (56) | 439 (56) | |
| Persistent | 70 (28) | 220 (28) | |
| Permanent | 17 (7) | 51 (7) | |
| CHADSVASC score | 4.6 (1.6) | 4.5 (1.6) | .24 |
| HAS-BLED score | 2.9 (1.0) | 2.9 (1.0) | .52 |
| Medical History | |||
| Heart failure | 114 (45) | 288 (37) | .02 |
| Myocardial infarction | 42 (17) | 165 (21) | .11 |
| Hypertension | 233 (92) | 708 (90) | .53 |
| Diabetes | 73 (29) | 228 (29) | .91 |
| Stroke | 25 (10) | 82 (10) | .77 |
| Alcohol use | 76 (30) | 248 (32) | .6 |
| Anemia | 95 (37) | 233 (30) | .02 |
| Asthma/chronic obstructive lung disease | 81 (32) | 182 (23) | .006 |
| Renal disease | 79 (31) | 225 (29) | .47 |
| Implantable cardiac device | 105 (41) | 249 (32) | .005 |
| Anticoagulant type | <.0001 | ||
| Warfarin | 180 (71) | 412 (53) | |
| Direct oral anticoagulant | 74 (29) | 371 (47) | |
Abbreviations: HMO, health maintenance organization; PPO, preferred provider organization.
High burden was defined as being the bottom quartile of the reversed anticlot treatment burden scale. Data were presented as n (%), mean (standard deviation).
Overall, the ACTS burden score has a mean of 55 with a standard deviation of 6 and is left-skewed (median, 57 and mode, 60; range, 12-60) (Figure 2). The ACTS burden scores were 56 (±5) and 54 (±6) among individuals taking DOAC and warfarin, respectively. The average burden scales of participants with high and low anticoagulation burden were 46 (±6) and 58 (±2), respectively.
FIGURE 2.
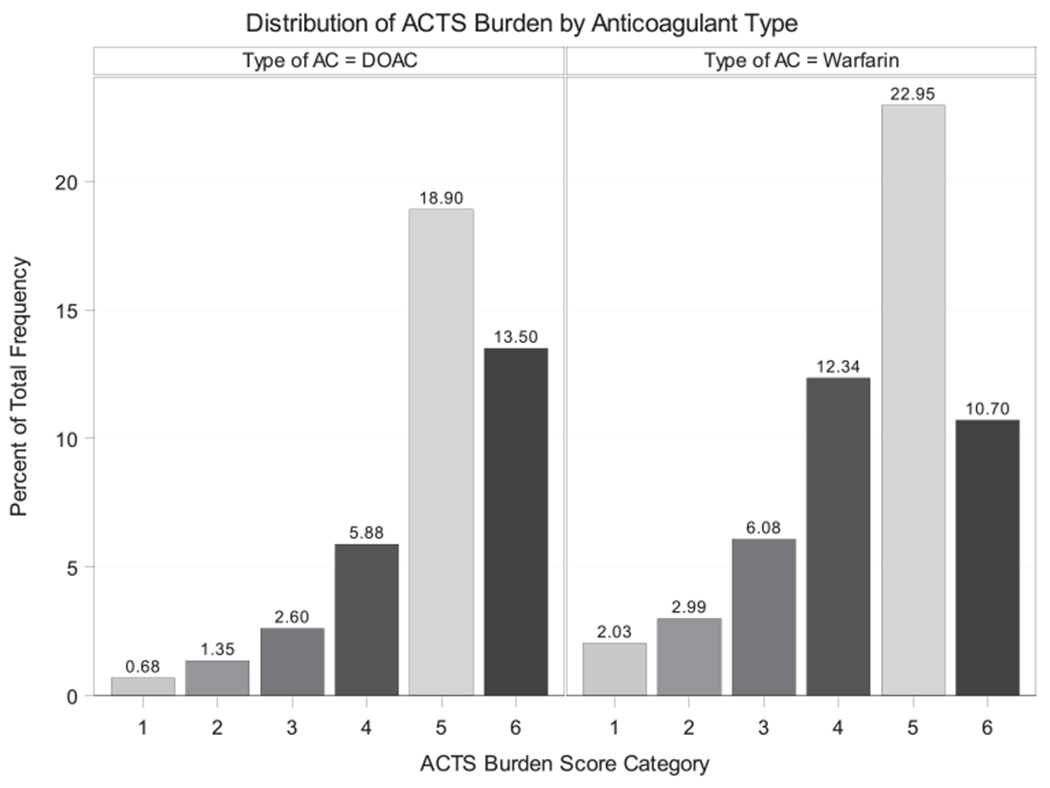
Histogram of ACTS burden scores (score category: 1: <40, 2: 40-44, 3: 45-49,4: 50-54, 5: 55-59, 6: 60). AC, anticoagulant; ACTS, the anticlot treatment scale; DOAC, direct oral anticoagulant
The prevalence of physical, cognitive, and psychosocial impairment was high among participants with AF, ranging from 14% to 42%. Visual impairment, depression, and anxiety were significantly associated with high anticoagulation burden, both in univariate and multivariate analyses. Frailty was significantly associated with high anticoagulation burden only in univariate analysis. Social isolation was significantly associated with high anticoagulation burden in univariate analysis and model 1 (Table 2). When the burden scale was examined as a continuous variable, the results were not substantially changed (Table S1).
TABLE 2.
Prevalence and odds ratios of physical, cognitive, and psychosocial impairments in relation to high anticoagulation burden among elderly adults with atrial fibrillation receiving an anticoagulant
| Impairment and its prevalence | Odds ratio (95% confidence interval) | ||||
|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3 | ||
| Frailty | 145 (14%) | 2.0 (1.3-3.1) | 0.9 (0.5-1.5) | 1.3 (0.7-2.4) | 1.4 (0.7-2.5) |
| Cognitive impairment | 435 (42%) | 1.0 (0.7-1.3) | 0.8 (0.6-1.1) | 0.8 (0.5-1.2) | 0.8 (0.5-1.2) |
| Social isolation | 133 (13%) | 1.5 (1.0-2.2) | 1.6 (1.1-2.5) | 1.5 (0.9-2.4) | 1.5 (1.0-2.4) |
| Visual impairment | 361 (35%) | 2.1 (1.5-2.7) | 1.6 (1.1-2.1) | 1.6 (1.1-2.3) | 1.7 (1.2-2.4) |
| Hearing impairment | 376 (36%) | 1.2 (0.9-1.6) | 1.1 (0.8-1.5) | 1.1 (0.8-1.6) | 1.1 (0.8-1.6) |
| Depression | 296 (29%) | 3.7 (2.8-5.0) | 2.5 (1.7-3.7) | 2.4 (1.5-3.6) | 2.4 (1.6-3.7) |
| Anxiety | 245 (24%) | 3.5 (2.6-4.8) | 2.1 (1.5-3.1) | 1.8 (1.2-2.7) | 1.8 (1.2-2.7) |
Note: Model 1: all geriatric and mood impairments and symptoms of atrial fibrillation; Model 2: variables controlled for in model 1 and age, sex, education, income, insurance, and provider type; Model 3: variables controlled for in model 2 and CHADSVASC and HAS-BLED.
When stratified by anticoagulant type, visual impairment and depression were significantly associated with high anticoagulation burden in warfarin users, whereas social isolation and anxiety were significantly associated with high anticoagulation burden in participants receiving DOAC (Table 3). Among warfarin users, the burden scales with and without visual impairment were 52 (±7) and 55 (±6); the burden scales with and without depression were 51 (±7) and 55 (±5). Among DOAC users, the burden scales with and without social isolation were 54 (±7) and 56 (±5); the burden scales with and without anxiety were 53 (±6) and 57 (±4). The associations did not show significant heterogeneity according to the study site (Table S2).
TABLE 3.
Odds ratios of physical, cognitive, and psychosocial impairments for high anticoagulation burden by anticoagulant types
| Odds ratio (95% confidence interval) |
|||||
|---|---|---|---|---|---|
| Unadjusted | Impairment | Model 1 | Model 2 | Model 3 | |
| Warfarin (N = 592) | Frailty | 1.6 (0.9-2.8) | 0.6 (0.3-1.3) | 1.1 (0.5-2.4 | 1.1 (0.5-2.5) |
| Cognitive impairment | 0.9 (0.6-1.2) | 0.7 (0.5-1.1) | 0.7 (0.4-1.1) | 0.7 (0.4-1.1) | |
| Social isolation | 1.1 (0.7-2.0) | 1.3 (0.7-2.4) | 1.4 (0.7-2.7) | 1.4 (0.7-2.8) | |
| Visual impairment | 2.3 (1.6-3.3) | 1.7 (1.1-2.5) | 1.9 (1.2-3.0) | 1.9 (1.2-3.1) | |
| Hearing impairment | 1.2 (0.8-1.7) | 1.0 (0.7-1.5) | 1.2 (0.7-1.9) | 1.1 (0.7-1.8) | |
| Depression | 4.2 (2.9-6.2) | 3.3 (2.1-5.4) | 3.3 (1.9-5.7) | 3.3 (1.9-5.8) | |
| Anxiety | 3.4 (2.3-5.1) | 1.9 (1.2-3.1) | 1.4 (0.8-2.5) | 1.4 (0.8-2.5) | |
| Direct oral anticoagulant (N = 445) | Frailty | 3.0 (1.5-6.1) | 1.4(0.6-3.5) | 1.9 (0.7-5.3) | 2.1(0.7-5.9) |
| Cognitive impairment | 1.2 (0.7-2.0) | 0.9 (0.5-1.7) | 1.0 (0.5-2.0) | 1.0 (0.5-2.1) | |
| Social isolation | 2.7 (1.5-4.8) | 2.8 (1.5-5.4) | 2.3 (1.1-4.9) | 2.4 (1.1-5.0) | |
| Visual impairment | 1.9 (1.1-3.1) | 1.4 (0.8-2.5) | 1.4 (0.8-2.7) | 1.5 (0.8-2.8) | |
| Hearing impairment | 1.2 (0.7-1.9) | 1.0 (0.6-1.8) | 1.2 (0.6-2.3) | 1.2 (0.6-2.3) | |
| Depression | 3.2 (1.9-5.4) | 1.5 (0.8-3.0) | 1.3 (0.6-2.8) | 1.3 (0.6-2.8) | |
| Anxiety | 4.4 (2.6-7.4) | 3.2 (1.7-6.0) | 3.6 (1.8-7.4) | 3.7 (1.8-7.6) | |
Note: Model 1: geriatric and mood impairment and symptoms of atrial fibrillation; Model 2: model 1 and age, sex, education, income, insurance, and provider type; Model 3: model 2 and CHADSVASC and HAS-BLED.
The benefit scale was examined as a continuous outcome. Average benefit scale was 11 (±4) with a range from 3 to 15. Social isolation and visual impairment were significantly associated with lower perceived anticoagulation benefit in unadjusted and adjusted models. Specifically, the average unadjusted benefit scale in participants with and without social isolation were 8.6 (8.0-9.3) and 11.1 (10.9-11.4) (P for difference <.01). The average perceived anticoagulation benefit in participants with as compared to without visual impairment were 10.3 (9.9-10.7) and 11.1 (10.8-11.4) (P for difference <.01) (Table S3).
4 |. DISCUSSION
In this contemporary cohort of elderly adults with AF prescribed an oral anticoagulant, visual impairments, depressive symptoms, and anxiety symptoms were independently associated with higher burden from anticoagulation. Furthermore, the conditions associated with anticoagulation burden differed by anticoagulant type. Specifically, visual impairment and depression were associated with higher burden in warfarin users, whereas social isolation and anxiety were associated with higher burden among patients treated with a DOAC. With respect to perceived anticoagulation benefits, social isolation and visual impairment were associated with lower perceived anticoagulation benefits.
The links between physical, cognitive, and psychosocial conditions with anticoagulation outcomes and satisfaction in the contemporary era of stroke prevention for AF are not well established. Prior studies have demonstrated that cognitive impairment is associated with twofold higher risk of thromboembolic events, and less effective anticoagulation,9 presumably as a result of poor anticoagulation adherence.23 More recently, a community-based case-control study found depression to be independently associated with a 2.2-fold greater odds of having a supratherapeutic INR among warfarin-treated patients.10 Anxiety is also independently associated with 50% higher risk of stroke and intracranial hemorrhage in AF patients initiating warfarin. This suggests in aggregate that conditions common in geriatric patients influence anticoagulation adherence as well as effectiveness.8
Anticoagulation satisfaction is an important patient-reported outcome because it is strongly associated with anticoagulation adherence and clinical outcomes, including bleeding and stroke.24–26 The previous literature on anticoagulation satisfaction in AF has generally focused on comparing satisfaction among warfarin-treated patients to those treated with DOACs in whom the burden from anticoagulation treatment is generally lower.3,27 Patient perceptions of the burdens and benefits of anticoagulation are important in the context of AF management, since treatment is generally life-long, often requires behavior change (ie, change in diet), and is associated with potential harm. In addition to their associations with clinical outcomes, assessment of patient beliefs and preferences surrounding anticoagulation is a critical component of anticoagulation shared decision making, a process endorsed for providers and patients by the 2016 American College of Cardiology AF Quality Guidelines.28 In parallel with increasing recognition of its importance, treatment satisfaction has become increasingly integrated into health care research.29
We observed that visual impairment, depression, and anxiety were independently associated with higher self-reported burden of anticoagulation among elderly participants with AF. Our findings suggest that assessment of mood and vision in the context of the clinical care of patients with AF might help health care providers identify patients for whom anticoagulation is burdensome. It could facilitate more personalized and informed decisions about the risks and benefits of anticoagulation and identify patients who may need closer surveillance. Furthermore, clinical assessments that integrate these components of a geriatric examination have the potential to facilitate more efficient allocation of health care resources (ie, a visiting nurse to conduct an INR) and improve patient safety by targeting AF patients at higher risk for additional support.
There is no consensus as to what constitutes a clinically important difference on the ACTS scale. However, many have suggested that a difference of 0.5 standard deviation or more constitute a minimally important clinical difference on patient-reported outcomes.30 In our study, most of the between-group differences were close to, or larger than, 0.5 standard deviations (0.5×6=3) for the ACTS burden scale and (0.5×4=2) for the ACTS benefit scale. Also, we know from prior work that DOACs are associated with higher satisfaction than warfarin. The average difference in the burden scales between DOACs and warfarin in this study was two. Since most of the differences we observed were bigger than two, we believe that our findings are clinically relevant.
The prescription of DOACs and proportion of anticoagulated patients receiving DOACs has increased dramatically since their introduction in 2010.31 However, more than half of AF patients on anticoagulation remain treated with warfarin according to recent national registry data.32 Our findings from wide-spectrum outpatient practices suggest that distinct conditions are associated with perceived burden from anticoagulation among warfarin, as compared with DOAC, users. The association between visual impairment and higher burden among warfarin users (but not patients on DOAC) may result from the fact that warfarin requires frequent dose adjustments using tablets of different colors and shapes. In contrast, social isolation and anxiety were associated with higher perceived burden from anticoagulation among DOAC but not warfarin-treated patients. It could be from the mitigating effect that in-person specialized anticoagulation care provided to anxious and isolated warfarin, but not DOAC, users. Our results suggest that a comprehensive geriatric assessment, including mood, vision, and social support, might better inform the type of anticoagulation selected for elderly adults with AF.
Our study has several strengths. The study population is geographically diverse. It includes elderly AF patients with a high degree of comorbidity. Also, validated and publicly available instruments were used for the comprehensive assessment of conditions common in geriatrics, including cognition, frailty, social isolation, and mood. Limitations should also be mentioned. The cross-sectional design precludes conclusions of any temporal and causal relationship. Second, we did not collect information about how long patients had received anticoagulants or whether they had switched agents before enrollment. We acknowledge that the perception of burden and benefit may be affected by prior switching or based on the duration of anticoagulant exposure. Third, we only examined patients receiving anticoagulants so individuals with extreme anticoagulation dissatisfaction which led to discontinuation were not included. Fourth, vision and hearing impairment were self-reported and thus less objective. However, this emulated the real-world practice because cardiology and internal medicine clinics are not usually equipped to have objective sensory assessment. Fifth, we did not have data on adherence to anticoagulants, which limits the exploration whether increased anticoagulation burden in elderly AF patients could lead to worse hard outcomes. Also, because our population has a heavy burden of physical, cognitive, and psychosocial illness, it may differ from the population in which the questionnaires used were validated. This may affect the performance of the questionnaires. Lastly, this is a risk factor analysis. It is hypothesis-generating and the findings can be from chance.
5 |. CONCLUSION
Physical, cognitive, and psychosocial conditions are common among elderly adults with AF receiving anticoagulation. Vision impairment, depressive symptoms, and anxiety are associated with high anticoagulation burden. The patterns of association differed by the type of anticoagulant used. Our findings suggest that vision, depression, and anxiety merit consideration in anticoagulation prescribing.
Supplementary Material
Acknowledgments
FUNDING INFORMATION
This study was supported by grant R01HL126911 from the National Heart, Lung, and Blood Institute. DDM’s time was also supported by grants R01HL137734, R01HL137794, R01HL13660, and R01HL141434, also from the National Heart, Lung and Blood Institute.
Disclosures: David D. McManus has received research grant support from Apple Computer, Bristol-Myers Squibb, Boeringher-Ingelheim, Pfizer, Samsung, Philips Healthcare, and Biotronik; has received consultancy fees from Bristol-Myers Squibb, Pfizer, Flexcon, and Boston Biomedical Associates; and has inventor equity in Mobile Sense Technologies, Inc (CT).
National Heart, Lung, and Blood Institute, Grant/Award Numbers: R01HL126911, R01HL13660, R01HL137734, R01HL137794, R01HL141434
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart study. Circulation. 1998;98:946–952. [DOI] [PubMed] [Google Scholar]
- 2.Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Euro Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 3.Coleman CI, Coleman SM, Vanderpoel J, et al. Patient satisfaction with warfarin- and non-warfarin-containing thromboprophylaxis regimens for atrial fibrillation. J Investig Med. 2013;61:878–881. [DOI] [PubMed] [Google Scholar]
- 4.Hylek EM, Go AS, Chang Y, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003;349:1019–1026. [DOI] [PubMed] [Google Scholar]
- 5.Haas S, Ten Cate H, Accetta G, et al. Quality of vitamin K antagonist control and 1-year outcomes in patients with atrial fibrillation: a global perspective from the GARFIELD-AF registry. PLOS One. 2016;11:e0164076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan EW, Lau WCY, Siu CW, et al. Effect of suboptimal anticoagulation treatment with antiplatelet therapy and warfarin on clinical outcomes in patients with nonvalvular atrial fibrillation: A population-wide cohort study. Heart Rhythm. 2016;13: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Kong MC, Lee LH, Ng HJ, Ko Y. Knowledge, satisfaction, and concerns regarding warfarin therapy and their association with warfarin adherence and anticoagulation control. Thromb Res. 2014;133:550–554. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner C, Fan D, Fang MC, et al. Anxiety, depression, and adverse clinical outcomes in patients with atrial fibrillation starting warfarin: cardiovascular research network WAVE study. J Am Heart Assoc. 2018;7:007814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flaker GC, Pogue J, Yusuf S, et al. Cognitive function and anticoagulation control in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2010;3:277–283. [DOI] [PubMed] [Google Scholar]
- 10.Diug B, Evans S, Lowthian J, et al. The unrecognized psychosocial factors contributing to bleeding risk in warfarin therapy. Stroke. 2011;42:2866–2871. [DOI] [PubMed] [Google Scholar]
- 11.Cano SJ, Lamping DL, Bamber L, Smith S. The anti-clot treatment scale (ACTS) in clinical trials: cross-cultural validation in venous thromboembolism patients. Health Qual Life Outcomes. 2012; 10:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 13.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. Chest. 2010;138: 1093–1100. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 15.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 53, 2005:695–699. [DOI] [PubMed] [Google Scholar]
- 16.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. [DOI] [PubMed] [Google Scholar]
- 17.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer RL, Kroenke K, Williams JBW, Loöwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 19.Bamber L, Wang MY, Prins MH, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosis. Thromb Haemost. 2013;110:732–741. [DOI] [PubMed] [Google Scholar]
- 20.Prins MH, Bamber L, Cano SJ, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of pulmonary embolism; results from the EINSTEIN PE trial. Thromb Res. 2015;135:281–288. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar AK, Mueller I, Bassand J-P, et al. International longitudinal registry of patients with atrial fibrillation at risk of stroke: global Anticoagulant Registry in the FIELD (GARFIELD). Am Heart J. 2012;163:13–19.e1. [DOI] [PubMed] [Google Scholar]
- 22.Piccini JP, Fraulo ES, Ansell JE, et al. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT-AF. Am Heart J. 2011;162:606–612.e1. [DOI] [PubMed] [Google Scholar]
- 23.Jankowska-Polańska B, Katarzyna L, Lidia A, Joanna J, Dudek K, Izabella U. Cognitive function and adherence to anticoagulation treatment in patients with atrial fibrillation. J Geriatr Cardiol. 2016; 13:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koretsune Y, Kumagai K, Uchiyama S, et al. Patient-reported treatment satisfaction with rivaroxaban in Japanese non-valvular atrial fibrillation patients: an observational study. Curr Med Res Opin. 2018;34:2157–2164. [DOI] [PubMed] [Google Scholar]
- 25.Dias-Barbosa C, Balp M-M, Kulich K, Germain N, Rofail D. A literature review to explore the link between treatment satisfaction and adherence, compliance, and persistence. Patient Prefer Adherence. 2012;6:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. [DOI] [PubMed] [Google Scholar]
- 27.Coleman CI, Haas S, Turpie AGG, et al. Impact of switching from a vitamin K antagonist to rivaroxaban on satisfaction with anticoagulation therapy: The XANTUS-ACTS substudy. Clin Cardiol. 2016;39:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidenreich PA, Solis P, Estes NAM, et al. 2016 ACC/AHA clinical performance and quality measures for adults with atrial fibrillation or atrial flutter. J Am Coll Cardiol. 2016;68:525–568. [DOI] [PubMed] [Google Scholar]
- 29.Anker SD, Agewall S, Borggrefe M, et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35:2001–2009. [DOI] [PubMed] [Google Scholar]
- 30.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo Clinic Proc. 2002;77:371–383. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Alexander GC, Nazarian S, Segal JB, Wu AW. Trends and variation in oral anticoagulant choice in patients with atrial fibrillation, 2010–2017. Pharmacotherapy. 2018;38:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzec LN, Wang J, Shah ND, et al. Influence of direct oral anticoagulants on rates of oral anticoagulation for atrial fibrillation. J Am Coll Cardiol. 2017;69:2475–2484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


