To the Editor,
We read with interest the report from London et al. regarding the efficacy of convalescent plasma in the treatment of severe COVID-19 in two patients with primary immunodeficiencies [1]. Herein, we highlight our experience of using remdesivir and convalescent plasma to treat persistent symptomatic COVID-19 in a patient with secondary immunodeficiency following long-term rituximab treatment.
Case Report
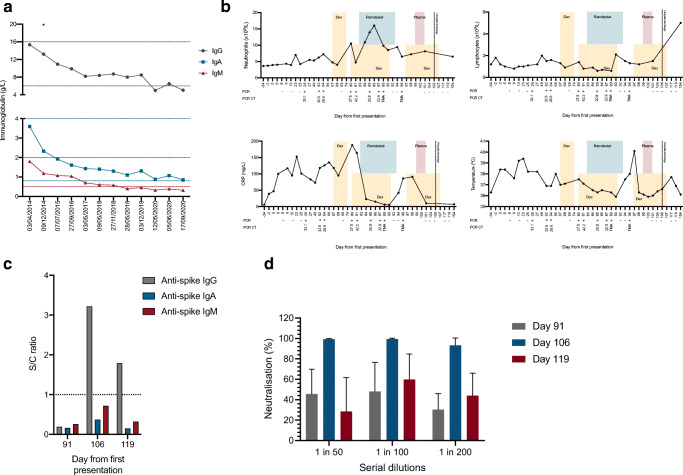
A 57-year-old lady presented to the emergency department on 16/4/2020 with shortness of breath, a 2-week history of dry cough, pyrexia (38.4 °C), and raised inflammatory markers. Chest radiography demonstrated bilateral and lower-zone peripheral air space opacities; however, she was not hypoxic. She had a 13-year history of anti-CCP seropositive rheumatoid arthritis, previously treated with glucocorticoids, disease-modifying anti-rheumatic drugs, etanercept, and, most recently, 6-monthly rituximab infusions commenced in 2014. Her last dose of rituximab was on 13/3/2020, approximately 3 weeks prior to the onset of symptoms. Serial immunoglobulin measurements are shown in Fig. 1a. The patient had previously suffered from intermittent lower respiratory tract infections with fully sensitive Streptococcus pneumoniae and non-typable Haemophilus influenzae previously grown in the sputum, despite protective IgG antibody concentrations in 8 out of 12 measured pneumococcal serotypes (Supplementary Table 1).
Fig. 1.
a Serial serum IgG (gray), IgA (blue), and IgM (red) measurements over time. Normal ranges are shown by dotted lines. Asterisk “*” represents date rituximab was initiated. b Changes in peripheral blood neutrophil count, lymphocyte count, C-reactive protein, and temperature over the time course of disease relative to pharmacological treatments received. Time course is relative to the first presentation on 16/4/2020. c Level of serum IgG, IgA, and IgM anti-SARS-CoV-2 trimeric spike glycoprotein antibodies were measured before and after treatment with convalescent plasma. Results are expressed as a signal to the cutoff calibrator ratio with the dotted line representing the cutoff for positivity. d In vitro SARS-CoV-2 neutralizing activity of serum from the patient before (day 91) and after (day 106 and day 119) the administration of convalescent plasma. Dex, dexamethasone; PCR, polymerase chain reaction; CT, cycle threshold; TMA, transcription-mediated amplification
A diagnosis of likely COVID-19 was made, but not proven by polymerase chain reaction (PCR) studies; the patient was discharged with broad-spectrum, oral antibiotics but re-represented 8 days later with persistent pyrexia (39.2 °C), cough, dyspnoea, and new diarrhea and vomiting. Inflammatory markers remained elevated and she was admitted for treatment with intravenous antibiotics and fluids. Three nasopharyngeal swabs taken within the first 3 days of admission returned negative results; however, radiological progression suggested ongoing viral pneumonia; SARS-CoV-2 RNA was first detected in a sputum sample on day 25 and subsequently found on multiple nasopharyngeal swabs between May and July 2020.
Between May and July 2020, she was re-admitted to the hospital on four occasions with similar symptoms, increasing oxygen requirements (peak oxygen requirements 4 L via nasal cannula to achieve peripheral oxygen saturation of 96%; desaturation to 82% on room air on exertion) and progressive radiological changes (Supplementary Figure 1). Blood cultures, urine cultures, sputum cultures, sputum mycobacterial PCR, and virology for cytomegalovirus and Epstein-Barr virus undertaken during these admissions did not identify another concurrent infection. During these admissions, she received three separate courses dexamethasone (6 mg once daily, orally; one 7-day course; two 10-day courses) and one course of remdesivir (200-mg loading dose followed by 100 mg once daily, 10 days, intravenously). Monotherapy with dexamethasone achieved little objective change in physiological and biochemical parameters of infection but treatment with intravenous remdesivir was associated with a dramatic reduction in C-reactive protein (Fig. 1b). Twenty-four hours after the cessation of remdesivir, a rebound increase in CRP and temperature was observed suggestive of a release of suppression of viral replication; SARS-CoV-2 RNA remained detectable following nasopharyngeal swabbing. Despite prolonged PCR positivity, anti-spike and anti-nucleocapsid SARS-CoV-2 antibodies were not detectable in the patient’s serum by mid-July 2020 secondary to ongoing, complete, peripheral B cell aplasia. On day 99, the patient received two units of convalescent plasma (275 mL/unit, units were centrally pre-screened by the NHS Blood Transfusion Service for a SARS-CoV-2 neutralizing antibody titer greater than 1:100). Convalescent plasma was well tolerated with rapid symptomatic improvement, reduction in inflammatory markers, and defervescence.
Following the administration of convalescent plasma, SARS-CoV-2 PCR was not detected on seven consecutive swabs taken between day 101 and day 119. IgG but not IgA or IgM antibodies directed against the spike protein remained detectable in the patients’ serum 3 weeks following administration of the plasma product (Fig. 1c). A serum sample taken 7 days after the administration of convalescent plasma showed > 95% neutralization of SARS-CoV-2 infectivity at a 1/200 dilution compared to pre-convalescent plasma serum (Fig. 1d). Neutralizing activity had been lost by day 119. A full description of the neutralizing assay is available in the supplementary methods. Resolution of plain-film radiological changes was observed by day 154 (Supplementary Figure 1).
Discussion
Adults with primary and secondary immunodeficiencies are at increased risk of morbidity and mortality from COVID-19 compared to the general population [2]. There are limited treatments for severe COVID-19; of the empirical treatments used in this study (dexamethasone, remdesivir, convalescent plasma), the only dexamethasone is associated with reduced 28-day mortality in the general population [3–5]. Whether the results from these large trials can be rationally generalized to patients with immune deficiencies is unknown.
Remdesivir is a ribonucleotide analog that inhibits the SARS-CoV-2 RNA-dependent RNA polymerase and arrests viral RNA synthesis by acting as a delayed chain terminator [6]. Concordant with previous case reports (Supplementary Table 2), we found the administration of remdesivir was associated with significant improvements in immunological and physiological biomarkers of SARS-CoV-2 infection. However, the rapid recrudescence of symptomatic COVID-19 following the cessation of remdesivir supports the hypothesis that remdesivir monotherapy is insufficient to support virological clearance and resolution of COVID-19 in patients with humoral immunodeficiency.
In our patient, and others treated with both remdesivir and convalescent plasma (summarized in Supplementary Table 2), complete virological clearance and prolonged symptomatic resolution were only achieved following the administration of convalescent plasma. The efficacy of convalescent plasma in the treatment of COVID-19 in patients has been reported in small case series of individuals with both congenital and acquired B cell aplasia [7–9]. Of note, two reports demonstrate that viral persistence can occur despite the generation of CD4 and CD8 T cell responses to viral antigens and that magnitude of ex vivo antiviral T cell responses is augmented following the administration of convalescent plasma [10, 11]. These data point to a non-redundant role for humoral immunity in immunological control of SARS-CoV-2, potentially via mechanisms such as antibody-dependent cellular cytotoxicity or opsonization, and suggest convalescent plasma is a rational therapeutic choice for individuals with humoral immune deficiencies, particularly when current immunoglobulin replacement products lack SARS-CoV-2-specific antibodies [12].
However, the administration of convalescent plasma to immunodeficient patients has been associated with the emergence of novel genomic variants within SARS-CoV-2 encoding spike proteins that demonstrate reduced susceptibility to neutralization in vitro [13]. Thus, combination treatments that simultaneously suppress viral RNA synthesis and facilitate viral clearance may be appropriate in immunocompromised individuals, to prevent the emergence of novel viral variants. A more comprehensive understanding of mechanisms through which convalescent plasma mediates its therapeutic effect and how these mechanisms are defective in primary and secondary immune deficiency may allow the selection of individuals who would benefit from such interventions.
Supplementary Information
(PDF 4517 kb)
(DOCX 22 kb)
Acknowledgments
The authors would like to acknowledge the work of the University of Birmingham Clinical Immunology Service for facilitating laboratory studies, Dr. Andrew Bosworth (University Hospitals Birmingham NHS Foundation Trust), for providing and interpreting the RT-PCR CT values, and Nur Zinna for her assistance in analyzing data from neutralization experiments.
Author Contributions
E.M., A.M.S., A.B., S.G., A.G.R., D.D., and S.M. provided clinical care for the patient. A.M.S., A.G.R., S.E.F., H.H., and Z.S. performed and interpreted laboratory studies. E.M. and A.M.S. wrote the first draft of the manuscript. A.M.S. revised the manuscript. All authors reviewed and commented on the revised manuscript prior to submission.
Funding
The authors are grateful for funding from the Global Challenges Research Fund and The Institute for Global Innovation (IGI) of the University of Birmingham, and the UK Medical Research Council (Grant Reference Number MC_PC_17183).
Availability of Data and Materials
All available data is presented within the figures of this manuscript.
Declarations
Ethical Approval
The patient was consented into the following research studies: National Institute for Health Research Rare Disease Bioresource (13/EE/0325) approved by the East of England – Cambridge South Research Ethics Committee and the Analysis of the immunological response to coronavirus infection (20/NW/0240) approved by the North West - Preston Research Ethics Committee.
Consent to Participate
The patient provided written informed consent prior to enrolment in these studies.
Consent to Publish
The patient provided written informed consent prior to publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Emily McKemey and Adrian M. Shields contributed equally to this work.
Change history
3/31/2021
A Correction to this paper has been published: 10.1007/s10875-021-01029-z
References
- 1.London J, et al. Severe COVID-19 in patients with B cell alymphocytosis and response to convalescent plasma therapy. J Clin Immunol. 2021;41(2):356–61. [DOI] [PMC free article] [PubMed]
- 2.Shields AM et al. COVID-19 in patients with primary and secondary immunodeficiency: The United Kingdom experience. J Allergy Clin Immunol. 2020;S0091–6749(20)32406-4. [DOI] [PMC free article] [PubMed]
- 3.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group et al. Dexamethasone in hospitalized patients with covid-19 - Preliminary report. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed]
- 5.Simonovich VA et al. A randomized trial of convalescent plasma in covid-19 severe pneumonia. N Engl J Med 2021;384:619–29. [DOI] [PMC free article] [PubMed]
- 6.Gordon CJ, Tchesnokov EP, Woolner E, Perry JK, Feng JY, Porter DP, Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J Biol Chem. 2020;295(20):6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyts I et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J Allergy Clin Immunol. 2021;147(2):520–31. [DOI] [PMC free article] [PubMed]
- 8.Hueso T, Pouderoux C, Péré H, Beaumont AL, Raillon LA, Ader F, Chatenoud L, Eshagh D, Szwebel TA, Martinot M, Camou F, Crickx E, Michel M, Mahevas M, Boutboul D, Azoulay E, Joseph A, Hermine O, Rouzaud C, Faguer S, Petua P, Pommeret F, Clerc S, Planquette B, Merabet F, London J, Zeller V, Ghez D, Veyer D, Ouedrani A, Gallian P, Pacanowski J, Mékinian A, Garnier M, Pirenne F, Tiberghien P, Lacombe K. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin H, et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(10):3594–3596.e3. doi: 10.1016/j.jaip.2020.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckland MS, et al. Treatment of COVID-19 with remdesivir in the absence of humoral immunity: a case report. Nat Commun. 2020;11(1):6385. doi: 10.1038/s41467-020-19761-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Damme KFA, et al. Case report: convalescent plasma, a targeted therapy for patients with CVID and severe COVID-19. Front Immunol. 2020;11:596761. doi: 10.3389/fimmu.2020.596761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwaiger J, Karbiener M, Aberham C, Farcet MR, Kreil TR. No SARS-CoV-2 neutralization by intravenous Immunoglobulins produced from plasma collected before the 2020 pandemic. J Infect Dis. 2020;222(12):1960–1964. doi: 10.1093/infdis/jiaa593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemp SA, et al. Neutralising antibodies in spike mediated SARS-CoV-2 adaptation. medRxiv, 2020: p. 2020.12.05.20241927.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 4517 kb)
(DOCX 22 kb)
Data Availability Statement
All available data is presented within the figures of this manuscript.