Abstract
The angiotensin-converting enzyme 2 (ACE2) is the receptor for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is highly expressed in adipose tissue, possibly associated with progression to severe coronavirus disease 2019 (COVID-19) in obese subjects. We searched the Gene Expression Omnibus (GEO) and reanalyzed the GSE59034 containing microarray data from subcutaneous white adipose tissue (sWAT) biopsies from 16 women before and 2 years after RYGB, and 16 controls matched by sex, age, and BMI. After RYGB, there was a significant decrease in sWAT ACE2 gene expression (logFC=-0.4175, P=0.0015). Interestingly, after RYGB the sWAT ACE2 gene expression was significantly lower than in non-obese matched controls (LogFC=-0.32875, P=0.0014). Our data adds to the well-known benefits of RYGB, a potential protective mechanism against COVID-19.
Graphical Abstract
Keywords: Bariatric surgery, COVID-19, ACE-2, Obesity
Introduction
Obesity is an independent risk factor for severe forms of coronavirus disease 2019 (COVID-19). Large case series and meta-analyses have demonstrated an association between obesity and hospital admission, intensive care unit (ICU) admission, need for mechanical ventilation, and mortality due to COVID-19, even when adjusted for underlying conditions [1]. The pro-inflammatory pro-thrombotic state and the altered adaptive immune response in obesity might be partially implicated in those poorer outcomes [2]. However, the adipocytes highly express the angiotensin-converting enzyme 2 (ACE2), which is the receptor for the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. Since ACE2 gene expression in lungs is extremely low, the SARS-CoV-2 enters the host cells by directly binding to ACE2 in tissues where it is highly expressed, triggering a systemic response that will ultimately upregulate ACE2 gene expression in lungs [4]. As a consequence, the hypertrophied adipose tissue from obese patients might become infected by SARS-CoV-2 and allow spread to other organs, as well as serve as an important viral reservoir during COVID-19 infection [5]. This mechanism might represent a specific link between obesity and COVID-19.
Bariatric surgery, and specifically Roux-en-Y gastric bypass (RYGB), leads to a significant and sustained weight loss [6] and has been advocated as a possible strategy to reduce the risk of COVID-19 infection in patients with severe obesity. In addition to its effects on improving or resolving comorbidities and reducing cardiovascular risk, weight loss through RYGB might significantly decrease the volume of adipose tissue, reducing the availability of the receptor for SARS-CoV-2. Also, RYGB results in metabolic effects that are partially independent of weight loss [6] and could affect the renin angiotensin aldosterone system, leading to changes in ACE2 gene expression in adipose tissue. Our aim was to study ACE2 gene expression in subcutaneous white adipose tissue (sWAT) from patients with severe obesity, before and after RYGB, using publicly available gene expression data.
Methods
Gene expression data were explored using the publicly available Gene Expression Omnibus (GEO) DataSets (https://www.ncbi.nlm.nih.gov/geo/info/) [7]. The GEO is a public repository that archives and freely distribute comprehensive genomic data submitted by the scientific community, which can be searched and re-used after the index publication.
We used the search terms “adipose tissue” AND “bariatric surgery”, filtered for “Homo sapiens” species and “expression profiling by array”, to identify eligible datasets from studies performed in adult patients who had paired sWAT biopsies before and after bariatric surgery. To make adequate comparisons, only studies that included also matched non-obese individuals were considered. Pre-processed log2-transformed gene expression data were obtained from GEO using GEOquery R package v. 2.54.1. Data quality was analyzed by visual inspection of expression distributions with box plots. Additionally, ArrayQualityMetrics R package v. 3.42.0 was applied for quality control. Probes were mapped to HGNC Gene Symbols using the annotation file provided by platform’s manufacturer. Gene expression changes among pairs of groups were quantified as log-fold changes (logFC).
Statistical Analyses
All statistical analyses were conducted using the statistical software R. Differential expression was assessed using linear models provided by the limma R package v. 3.42.2, which are more stable and improve the statistical power of differential expression analysis, especially for small sample sizes.
Results
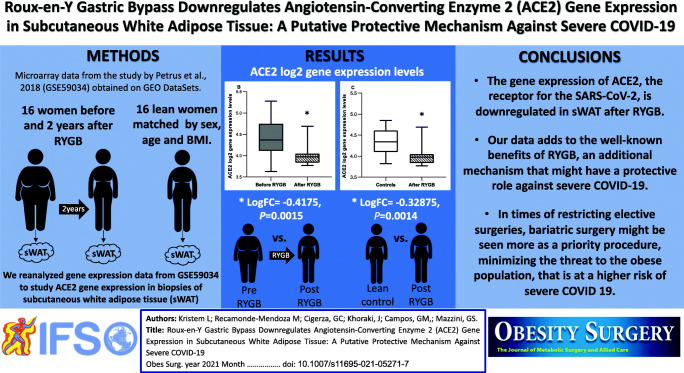
Our search revealed 21 studies in which sWAT biopsies were performed in bariatric surgery patients. Only one study had paired sWAT biopsies done before and after bariatric surgery, matched to non-obese individuals (GSE59034) [8], and was included in our analyses. In that study, Petrus et al [8] analyzed the transcriptional changes associated with changes in sWAT adipocyte volume and cellularity during weight gain (refer to the index publication for full methods). The study evaluated three cohorts of patients, but we included in our analysis only the two cohorts with microarray data available in GSE59034. The first cohort had 21 obese women, who underwent RYGB between 2006 and 2009 (NCT01785134), were re-examined after two years and had reduced their body mass index (BMI) to a non-obese level (< 30 kg/m2). In the second cohort, for each post-RYGB patient, a control subject who had never been obese was recruited, matched by sex, age, and current BMI. The sWAT biopsies were obtained from the para-umbilical region by needle aspiration under local anesthesia.
According to the index publication [8], the 21 patients in each cohort were women, mean age 47 years. Mean BMI significantly decreased from 41.1 kg/m2 before RYGB to 25.5 kg/m2 after RYGB (P<0.001), as well as the mean percentage of body fat (before RYGB: 52.8% vs. after RYGB: 32.9%, P<0.001). Of notice, there was a significant decrease on adipocyte volume after surgery (before RYGB: 957.3 pL vs. after RYGB: 369.4 pL, P<0.001), whereas the adipocyte cells count remained unaltered (before RYGB: 7.1×1010 vs. after RYGB: 7.6×1010, P=0.16). There were no differences in age, BMI, and percentage of body fat between the control subjects who had never been obese and patients after RYGB (P=0.6; P=0.07; P=0.3; respectively). However, despite similar BMI and percentage of body fat, mean adipocyte volume was significantly lower after RYGB (369.4 pL) relative to control subjects who had never been obese (485.1 pL, P=0.005), whereas adipocyte cells count was significantly higher after RYGB (7.6×1010) relative to the matched control subjects (5.7×1010, P=0.002).
ACE2 gene expression in sWAT
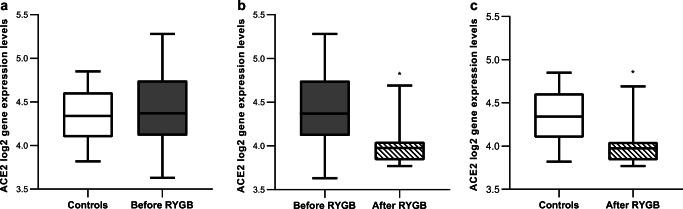
GSE59034 had microarray data available from 16 patients from each cohort, all paired (before and after RYGB) and matched (after surgery to controls). Quality assessment of preprocessed data deposited by authors indicated a good quality of arrays. Figure 1 shows the comparisons of sWAT ACE2 gene expression in log2 scale among groups. There was no difference in ACE2 gene expression in sWAT between the obese individuals before RYGB and control individuals who had never been obese (logFC=0.08875, 95% CI: -0.193 - 0.3705, P=0.53, Fig. 1a). After RYGB there was a significant decrease in ACE2 gene expression in sWAT (logFC=-0.4175, 95% CI: -0.6528–-0.1822, P=0.0015, Fig. 1b). Of notice, ACE2 gene expression in sWAT was significantly lower after RYGB compared to control subjects who had never been obese (LogFC=-0.32875, 95% CI: -0.512–-0.1454, P=0.0014, Fig. 1c), despite similar BMI and percentage of body fat.
Fig. 1.
Angiotensin-converting enzyme 2 (ACE2) log2 gene expression levels in subcutaneous white adipose tissue (sWAT) across groups (boxes represent median and quartiles, whiskers represent min. and max.). a log-fold change (logFC)=0.08875, P=0.53. b logFC=-0.4175, P=0.0015. c LogFC=-0.32875, P=0.0014. *P<0.05 for LogFC
Discussion
Our secondary analyses of the GEO dataset GSE59034 is the first report to demonstrate that RYGB downregulates the gene expression of ACE2, the receptor for SARS-CoV-2, in sWAT. Interestingly, sWAT ACE2 gene expression after RYGB was also significantly lower than in control individuals who had never been obese, despite similar BMI and percentage of body fat. RYGB also led to a significant weight and fat mass loss and, 2 years after surgery, average adipocyte volume was significantly lower relative to non-obese subjects, matched by age and BMI. Our findings also corroborate previous published data reporting similar levels of ACE2 gene expression in sWAT from obese and non-obese patients [9]. The higher availability of virus receptors in obese subjects is probably secondary to a greater volume of adipose tissue, and not due to an upregulation of sWAT ACE2 gene expression in obesity. Consequently, it is important to notice that RYGB provides for a significant reduction in adipose tissue volume, and also for downregulation in sWAT ACE2 gene expression, probably leading to additional protection during COVID-19 infection. Bariatric surgery positively impacts most of the comorbidities associated with poor COVID-19 outcomes in patients with morbid obesity (6), and has now come to attention as a possible protector factor. Accordingly, a retrospective cohort study [10] showed that previous bariatric surgery was independently associated with a reduced risk of mechanical ventilation and death in obese patients with COVID-19.
Our study has methodological limitations inherent in working with publicly available databases. We could not have access to clinical and demographic information such as metabolic or constitutional parameters for each of the 16 individuals in both cohorts that had microarray data in GSE59034. However, the fact that the patients in the dataset were paired (before to after surgery) and matched (after surgery to non-obese controls) keeps the accuracy of our analyses. This study has an exploratory nature, and the mechanistic hypothesis raised here should be a matter of deeper investigations.
Given these limitations, we conclude that RYGB downregulates ACE2 gene expression in sWAT, which might have an additional protective role against severe presentations of COVID-19 infection. Our data adds to the well-known benefits of RYGB, a potential protective mechanism against severe COVID-19, by downregulating the gene expression of the receptor for SARS-CoV-2 in sWAT. In times of restricting elective surgeries, bariatric surgery might be seen more as a priority procedure, minimizing the threat to a population at risk.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study formal consent is not required.
Informed Consent
Informed consent does not apply.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popkin B, Du S, Green W, Beck M, Algaith T, Herbst C, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. doi: 10.1111/obr.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sattar N, McInnes I, McMurray J. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. doi: 10.1161/CIRCULATIONAHA.120.047659. [DOI] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garvin M, Alvarez C, Miller J, Prates E, Walker A, Amos B, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;9:e59177. doi: 10.7554/eLife.59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchis-Gomar F, Lavie C, Mehra M, Henry B, Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arterburn D, Telem D, Kushner R, Courcoulas A. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324(9):879–887. doi: 10.1001/jama.2020.12567. [DOI] [PubMed] [Google Scholar]
- 7.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41(Database issue):D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrus P, Mejhert N, Corrales P, Lecoutre S, Li Q, Maldonado E, et al. Transforming growth factor-β3 regulates adipocyte number in subcutaneous white adipose tissue. Cell Rep. 2018;25(3):551–560.e5. doi: 10.1016/j.celrep.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 9.Jia X, Yin C, Lu S, Chen Y, Liu Q, Bai J, Lu Y. Two things about COVID-19 might need attention. Preprints. 2020:2020020315. 10.20944/preprints202002.0315.v1.
- 10.Iannelli A, Bouam S, Schneck A, Frey S, Gugenheim J, Alifano M. The impact of previous history of bariatric surgery on outcome of Covid-19: a nationwide medico-administrative French study. Obes Surg. 2020;18:1–9. doi: 10.1007/s11695-020-05120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]