ABSTRACT
Background
Microgreens are the young leafy greens of many vegetables, herbs, grains, and flowers with potential to promote human health and sustainably diversify the global food system. For successful further integration into the global food system and evaluation of their health impacts, it is critical to elucidate and optimize their nutritional quality.
Objectives
We aimed to comprehensively evaluate the metabolite and mineral contents of 6 microgreen species, and the influence of maturity on their contents.
Methods
Plant species evaluated were from the Brassicaceae (arugula, broccoli, and red cabbage), Amaranthaceae (red beet and red amaranth), and Fabaceae (pea) plant families. Nontargeted metabolomics and ionomics analyses were performed to examine the metabolites and minerals, respectively, in each microgreen species and its mature counterpart.
Results
Nontargeted metabolomics analysis detected 3321 compounds, 1263 of which were annotated and included nutrients and bioactive compounds. Ionomics analysis detected and quantified 26 minerals including macrominerals, trace minerals, ultratrace minerals, and other metals. Principal component analysis indicated that microgreens have distinct metabolite and mineral profiles compared with one another and with their mature counterparts. Several compounds were higher (P < 0.05; fold change ≥2) in microgreens compared with their mature counterparts, whereas some were not different or lower. In many cases, compounds that were higher in microgreens compared with the mature counterpart were also unique to that microgreen species.
Conclusions
These data provide evidence for the nutritional quality of microgreens, and can inform future research and development aimed at characterizing and optimizing microgreen nutritional quality and health impacts.
Keywords: bioactive compounds, food systems, functional foods, human health, microgreens, micronutrients, nutritional quality, phenolic compounds, phytochemicals, vitamins
The 6 microgreen species evaluated had an extensive and diverse range of metabolites and minerals, providing evidence for their nutritional quality and potential to promote human health.
Introduction
The United Nations projects the world population will reach 9.6 billion by 2050 (1). Population growth is leading to increased urbanization, and chronic disease prevalence associated with poor diet quality, malnutrition, and an aging population, among other factors (2–5). Human food and nutritional security are strongly dependent on sustainable crop production, food nutritional quality, and biodiversity, which in turn are reliant upon environmental health and adequate resources (5, 6). As the global climate changes, natural resources and biodiversity are declining (6, 7). This presents a challenge that requires evidence-based solutions to feed the growing global population, while meeting demands of changing environments and producing foods of high nutritional quality to support human health. Importantly, solutions should also be environmentally and economically sustainable.
Microgreens are an emerging horticultural food crop with potential to help address these challenges. They are the young and tender leafy greens of many vegetables, herbs, grains, and flowers from diverse plant families including Brassicaceae, Asteraceae, Apiaceae, Amaryllidaceae, Amaranthaceae, Cucurbitaceae, Fabaceae, and Lamiaceae (8–10). They contain the cotyledons (and hypocotyl if applicable), stems, and first true leaves and are harvested ∼10–20 d after seedling emergence (8). Research suggests they are of high nutritional quality, and can be sustainably produced and grown year-round in controlled environments with applications for urban agriculture, as well as in homes, restaurants, and schools (9, 11–13). Microgreens have gained popularity in culinary establishments as garnishes and are often referred to as “vegetable confetti” or “funfetti” due to their diverse colors, flavors, and textures. However, their potential for widespread application in agriculture, human health, and economic and environmental sustainability suggests that they can be viewed and utilized as a major vegetable food crop. We and others have demonstrated that microgreens are acceptable to consumers, but this is influenced by sensory perceptions, among other factors (14–16). We also showed that although consumers are likely to purchase them, their intent was impacted by knowledge regarding microgreens including nutrition, availability/access, cost, and shelf-life/freshness (15). Microgreens can be further integrated into the global food system to promote vegetable consumption, increase micronutrient and plant bioactive compound (or phytochemical) intake for the promotion of health, and to achieve specific health effects as functional foods. For this to occur, it is critical to elucidate and optimize their nutritional quality, confirm consumer acceptability, assess feasibility and tolerability of daily consumption, and evaluate impacts on human and population health, and economic and environmental sustainability.
Here, we report the metabolite and mineral contents of 6 microgreen species from 3 plant families (Brassicaceae: arugula, broccoli, and red cabbage; Amaranthaceae: red beet and red amaranth; and Fabaceae: pea). Metabolomics and ionomics were utilized to quantify differences in metabolites and minerals, respectively, among these microgreen species, and compared with their mature counterparts. Previous research evaluated micronutrient and bioactive compound contents (namely polyphenols and carotenoids) of various microgreen species. Some studies compared concentrations in microgreens with those in their mature counterparts, primarily through comparisons with concentrations provided in previous reports but with exceptions. To our knowledge, this study is the first comprehensive analysis of metabolites and minerals in these 6 microgreen species, with a direct comparison with their mature counterparts. Taken together, this research supports the high nutritional quality of microgreens including the extensive and diverse range of metabolites and minerals. This research also suggests the need for future work aimed at elucidating and optimizing their nutritional quality through agricultural practices, investigating the bioaccessibility and bioavailability of their nutrients and bioactive compounds, and exploring health effects.
Methods
Overview
This was a proof-of-concept study to test the hypothesis that microgreens have equivalent or higher metabolite (namely bioactive compounds) and mineral contents relative to their mature counterparts when grown in the same geographic location using conventional growing techniques. Botanically diverse species of commercial, human health, and consumer importance were investigated. The technical aspects of our experimental protocols are detailed in the following sections.
Microgreen materials and growing conditions
Microgreens were grown at the Colorado State University (CSU) Horticulture Center in Fort Collins, CO, USA, as previously described (15). The data from our ongoing microgreens research, including that of this present study, will inform future research aimed at evaluating the health impacts of microgreens. Therefore, we selected microgreen species based on previous research evaluating their consumer acceptance and sensory attributes, known nutritional characteristics, and potential for environmental sustainability and health impacts (12–14). In addition, consideration was given to researcher input, and/or their potential for use as functional foods and to influence human health. The microgreens belonged to the following families: 1) Brassicaceae—arugula [Eruca sativa (L.) Cav.], broccoli (Brassica oleracea L. Italica Group, organic), red cabbage (Brassica oleracea L. Capitata Group); 2) Amaranthaceae—bull's blood beet (Beta vulgaris L. Crassa Group) and red garnet amaranth (Amaranthus tricolor L., organic); and 3) Fabaceae—tendril pea (Pisum sativum, organic) (Figure 1). Seeds were purchased from a commercial provider (Johnny's Selected Seeds). Approximately 1.5-cm coir fiber (Botanicare COCOGRO) was used as the growing medium and layered in each standard 1020 black polystyrene germination tray (26.7 × 53 cm). Seeds were evenly sown at rates described in Table 1, and trays were covered with black polyethylene sheets for 24–48 h to increase the germination rate and maintain moisture. Seeds were irrigated twice each day with a hand pump spray and grown under light-emitting diode (LED) lamps (GreenPower LED production module; Philips). For each species, 8 replicates were grown and distributed in a randomized block design, where each tray represented 1 replication. Five of the most uniform trays were harvested 20 d after they were sown, except for peas, which were harvested after 10 d due to their faster growth rate. Each replicate was harvested with a sharp knife and its fresh weight was recorded. A total of 45 plant samples were immediately immersed in liquid nitrogen, lyophilized, and then stored at −80°C until processing.
FIGURE 1.

Images of (A) arugula, (B) broccoli, (C) bull's blood beet; (D) red cabbage, (E) red garnet amaranth, and (F) tendril pea microgreens. Reproduced with permission from reference 15.
TABLE 1.
Vegetable species grown as microgreens and their sowing rate for each standard 1020 tray (26.7 × 53 cm) with coir fiber medium.
| Species1 | Plant family | Average sowing rate per 1020 tray |
|---|---|---|
| Arugula [Eruca vesicaria (L.) Cav.] | Brassicaceae | 10 g |
| Bull's blood beet (Beta vulgaris L.) | Amaranthaceae | 23 g |
| Broccoli (Brassica oleraceae L. Italica Group), organic | Brassicaceae | 13 g |
| Red cabbage (Brassica oleracea L. Capitata Group) | Brassicaceae | 10.5 g |
| Red garnet amaranth (Amaranthus tricolor L.), organic | Amaranthaceae | 7.5 g |
| Tendril pea (Pisum sativum), organic | Fabaceae | 50 g |
Seeds purchased from Johnny's Selected Seeds.
Reproduced with permission from reference 15.
Mature plant material and growing conditions
The same 6 species of microgreens were grown to maturity in a greenhouse at the CSU Horticulture Center. Seeds were purchased from a commercial provider (Johnny's Selected Seeds). Seeds of broccoli, cabbage, and red beets were sown into 50-cell trays with misting benches set to irrigate for 10 s every 15 min. The potting medium used for the entire experiment was mixed in the following ratios (volume to volume): 3.8 cubic foot bale of Berger OM peat-based mix with perlite (approved in organic systems) amended with 3 cups of bonemeal (Down to Earth All Natural Fertilizers), 3 cups of blood meal (Down to Earth), and 28.426 gallons of worm castings. Arugula, peas, and red amaranth were seeded directly into #1 (“trade gallon”) containers. On the same date, broccoli, cabbage, and red beets transplants were potted into #1 containers. After transplanting and direct seeding into #1 containers, all crops were watered by hand daily until harvest at horticultural maturity for analyses, that is, the stage of maturity when plant foods will meet minimum quality standards and can be used by consumers postharvest and posthandling (17). Broccoli, red cabbage, and red beets were grown for 71 d after planting, and arugula, peas, and red amaranth were grown for 38 d after planting. Tissues harvested from mature plants included arugula full-sized leaves; pea shoots (leaves, stems, tendrils, and flowers); red beet leaves and root; red cabbage leaves, petioles, stem, and beginning head; broccoli leaves, petioles, stem, and beginning head; and red amaranth stems, petioles, and leaf tissue. A total of 45 plant samples were immediately immersed in liquid nitrogen, lyophilized, and then stored at −80°C until processing.
Metabolite extraction
In preparation for chemical analysis, plant samples were placed into a freeze dryer (Harvest Right) for 6 h of freezing and 12 h of drying at −67°C. The freeze-dried samples were stored at −80°C until analysis. For analysis, dry samples were homogenized using a standard home coffee grinder and then sifted through a fine-mesh sieve. Both the coffee grinder and sieve were sanitized with 70% methanol (MeOH) between each sample. A total of 100 mg (±0.5 mg) was transferred into a new 2-mL glass vial and kept on ice. Metabolite extraction was performed by adding 1 mL of methyl-tert-butyl-ether (MTBE) solution to obtain a 2:3 MeOH:MTBE solution (75% by volume MeOH and 100% by volume MTBE) and vortexing at 4°C for 60 min followed by centrifugation for 15 min at 3500 × g at 4°C. Next, 350 µL of cold water (LC-MS grade) was added and samples were centrifuged again for 25 min at 4°C at 3500 × g. The organic layer (top) was transferred to a clean 2-mL glass vial. An additional 500 µL of cold MTBE was added to the remaining aqueous layer and the samples were vortexed at 4°C for 10 min followed by centrifugation for 15 min at 3500 × g at 4°C. The organic layer (top) was added to the tube containing the previous organic layer. This process was repeated 1 additional time (a total of 3 organic layers were combined) and stored at −80°C until analysis. Next, the 400 µL of the organic layer extract was evaporated under a stream of nitrogen gas and resuspended in 200 μL 1:1 toluene/methanol (v/v) and vortexed for 5 s to mix. A 100-µL aliquot was put in a clean glass vial and stored at −80°C until analysis. Additionally, 20 µL of each sample was combined into a pooled quality control (QC) sample.
To the final remaining aqueous layer, 500 µL of 50% MeOH in water was added followed by vortexing at 4°C for 10 min and centrifugation for 15 min at 3500 × g at 4°C. The aqueous sample was transferred to a fresh 2-mL glass vial and then the process was repeated 1 additional time (total of 2 aqueous layers were combined). Five hundred microliters of the aqueous layer extract was transferred to a clean 2-mL vial and evaporated under a stream of nitrogen gas. The samples were resuspended with 500 µL of 3.5:3.5:3 MeOH/acetonitrile/water, vortexed at 4°C for 60 min, and then centrifuged for 10 min at 3500 × g at 4°C. The aqueous layer sample was then transferred to a fresh 2-mL glass vial and the dry down and resuspension protocol was repeated. A 100-µL aliquot was put in a clean glass vial and stored at −80°C until analysis. Additionally, 20 µL of each sample was combined into a pooled QC sample.
Metabolite detection using ultra-high-performance liquid chromatography-mass spectrometry (UHPLC-MS)
Samples were analyzed in randomized order with a pooled QC injection after every 6 samples. To ensure optimal metabolome coverage, a stacked injection approach was used (18). A total of 1 µL of the organic layer extract was preloaded onto a Waters Acquity UPLC system (Waters Corp.) equipped with a Waters Acquity UPLC CSH Phenyl Hexyl column (1.7 µM, 1.0 × 100 mm) with a constant flow of 99.9% solvent A (2 mM ammonium hydroxide, 0.1% formic acid) and 0.1% solvent B (acetonitrile, 0.1% formic acid) at 200 µL/min. This isocratic method was allowed to run for 30 s before the injection of 2 µL of aqueous layer extract. Separation was performed using a gradient that began at 99.9% A, was held at 99.9% A for 1 min, ramped to 98% B over 12 min, held at 98% B for 3 min, and then returned to starting conditions over 0.05 min and allowed to re-equilibrate for 3.95 min. Flow rate was constant at 200 µL/min. The column and samples were held at 65°C and 6°C, respectively. The column eluent was infused into the mass spectrometer (Xevo G2 Q-TOF; Waters Corp.) equipped with an electrospray source operating in positive-ion mode, scanning 50–2000 m/z at 0.2 s/scan, alternating between MS (6 V collision energy) and MSE mode (15–30 V ramp). Calibration was performed using sodium iodide with 1 ppm mass accuracy. The capillary voltage was held at 2200 V, source temp at 150°C, and nitrogen desolvation temperature at 350°C with a flow rate of 800 L/h.
Metabolomics data analysis and statistics
MS files were converted to .cdf format and processed by XCMS (19) in R (20). Samples were normalized to total ion current. UHPLC-MS data were deconvoluted into spectral clusters using RAMClustR (21). Metabolites were identified by matching mass spectra and retention indices and/or experimental or predicted retention times with in-house and external databases (22) including National Institute of Standards and Technology (http://nist.gov), 1SToP spectral and retention databases of Lipid Maps (23), and Human Metabolome database (24) using RAMSearch (22). Spectra were interpreted using the findMAIN function of the interpretMSSpectrum (25) package and MSFinder (26, 27), and validated annotations based on manual interpretation of the computationally determined annotations based on each metabolite's accurate mass information and fragmentation patterns. Annotation confidence for all metabolites is reported as level 2 based on guidelines of the Metabolomics Standards Initiative (28). Univariate statistics were performed using GraphPad Prism (Version 8.2.1; GraphPad Software). Comparison of microgreens and their mature counterparts was performed using a Student t test with false discovery rate (FDR) correction based on a 2-stage linear set-up procedure of Benjamini, Krieger, and Yekutieli (Q = 1%). Comparison across microgreen species was performed using a 2-factor ANOVA corrected for multiple comparisons using the Tukey test. Principal component analysis (PCA) was conducted on Pareto-scaled data using SIMCA software (Version 15.0.2.5959; Sartorius Stedim Data Analytics, Goettingen, Germany).
Microwave digestion
Dry homogenized microgreen samples were randomized into batches of 15. A total of 300 mg (±0.5 mg) of dry homogenized microgreen samples were weighed into 75-mL Teflon microwave vessels. Digestion was performed by adding 8 mL of redistilled concentrated nitric acid (HNO3) spiked with internal standard, indium (In, 562.5 ppb) followed by 2 mL of 30% hydrogen peroxide (Ultrex, J.T.Baker; Avantor). Each vessel was left to react for 10 min prior to sealing to allow any prereactions to occur safely before being capped. Vessels were sealed with a vessel rupture disk and pressure seal and placed in a programmable Titan MPS microwave digestion system (PerkinElmer) and samples were digested following the program in Supplemental Table 1. The Titan microwave digestion system can hold 16 samples and thus a blank sample was run with each batch. Upon completion of the digestion, all samples were diluted with MilliQ water (18.2 MΩ) to a final volume of 15 mL. Samples were vortexed and subsequently 1 mL of sample was diluted to a final volume of 15 mL with MilliQ water. This resulted in a dilution factor of 28.13× with a sample matrix consisting of 20 ppb of indium and 2.5% HNO3. Additionally, 750 µL of each sample was combined into a pooled QC sample.
Ionomics (mineral) detection using inductively coupled plasma MS (ICP-MS)
Elemental concentrations of aluminum, arsenic, boron, barium, beryllium, calcium, cadmium, copper, chromium, copper, iron, potassium, lithium, magnesium, manganese, molybdenum, sodium, nickel, phosphorus, lead, sulfur, selenium, strontium, vanadium, tungsten, and zinc were measured using a NexION 350D mass spectrometer (PerkinElmer) connected to a perfluoroalkoxy alkane-screw thread (Elemental Scientific) nebulizer and a Peltier-controlled (PC3x, Elemental Scientific) quartz cyclonic spray chamber (Elemental Scientific) set at 4°C. Lithium, beryllium, boron, sodium, phosphorus, sulfur, magnesium, potassium, calcium, tungsten, arsenic, and lead were measured in standard mode. Cadmium, selenium, and arsenic were measured in dynamic reaction mode (DRC) mode using oxygen as the reactive gas. Aluminum, vanadium, chromium, manganese, iron, cobalt, nickel, copper, zinc, strontium, molybdenum, and barium were measured in DRC mode using ammonia as the reactive gas. Samples were measured in randomized order with analysis of the pooled QC after every 9 samples and introduced using a prepFAST SC-2 (Elemental Scientific) autosampler. Before analysis the nebulizer gas flow and quadrupole ion deflector were optimized for maximum indium signal intensity. A daily performance check was run to ensure instrument performance by reducing the formation of oxides and doubly charged species by obtaining a ratio of celenium oxide to celenium (CeO:Ce) of <0.025 and a Ce++:Ce of <0.030. A 7-point calibration curve was prepared by serial dilution of commercially available single-element standard stock solutions (Inorganic Ventures). These were matrix matched to the samples, 2.5% HNO3 and 20 ppb indium. For correction of instrument drift, internal standard solution consisting of lithium, rhodium, and iridium was added to each sample via the autosampler.
Ionomics data analysis and statistics
Data were processed using Microsoft Excel (Microsoft Corp.). Each element was subjected to internal standard corrections and subsequently drift corrected (29). Corrections were chosen based on minimizing the CV for the QC samples. After drift correction, samples were corrected for the dilution factor. Limits of detection and limits of quantification (LOQ) were calculated as 3 times or 10 times the SD of the blank divided by the slope of the calibration curve, respectively (30, 31). Final concentrations are given in micrograms per gram of freeze-dried plant material. Measured calculations below the LOQ were assigned to LOQ/2 (32). Statistical analysis was performed in GraphPad Prism. Analysis consisted of a pair-wise t test using the FDR method of Benjamini, Krieger, and Yekutieli (microgreen compared with mature) or a 2-factor ANOVA using the identified FDR method of Benjamini, Krieger, and Yukutieli to correct for multiple comparisons at an FDR of 0.05 was conducted on each element across microgreen samples. PCA was conducted on unit variance scaled data using SIMCA®software (Version 15.0.2.5959; Sartorius Stedim Data Analytics, Goettingen, Germany).
Results
Metabolites and minerals in microgreens and mature counterparts
Nontargeted metabolomics analysis using UHPLC-MS detected 3321 metabolites in microgreens and their mature counterparts (Supplemental Table 2), and of these, 38% (1263/3321) were annotated. Within the MS data, a total of 21 nutrients within 4 major nutrient classes (i.e., protein/amino acids, lipids/fats, carbohydrates, and vitamins) were detected including essential (e.g., isoleucine, lysine) and nonessential amino acids (e.g., glycine, glutamine), lipids (e.g., fatty acids such as long-chain fatty acids), carbohydrates (e.g., saccharides such as oligosaccharides), and vitamins such as vitamin E (α-tocopherol), vitamin B-2 (riboflavin), vitamin B-5 (pantothenic acid), and vitamin K-1 (phylloquinone). Most of the remaining metabolites can be classified as bioactive compounds, that is, known secondary plant metabolites or “constituents in foods or dietary supplements, other than those needed to meet basic human nutritional needs, which are responsible for changes in health status” (33, 34). The bioactive compounds included several types of alkaloids (e.g., indoles, phenolic amines), betacyanins (e.g., betanin, amaranthin), organosulphurs (e.g., glucosinolates, isothiocyanates), phenolics (e.g., coumarins, flavonoids), and terpenes (e.g., carotenoids).
Ionomics analysis using inductively coupled plasma MS detected 26 elements (Supplemental Table 3). Of the elements detected, 6 were macrominerals (i.e., calcium, potassium, magnesium, phosphorus, sodium, and sulfur), 7 were trace minerals (i.e., chromium, copper, iron, manganese, molybdenum, selenium, and zinc), 4 were ultratrace minerals (i.e., arsenic, boron, nickel, and vanadium), and 9 were other metals (i.e., aluminum, barium, beryllium, cadmium, cobalt, lithium, lead, strontium, and tungsten).
Effects of maturity on metabolite classes and known bioactives
Metabolite profiles of microgreens and their mature counterparts were visualized using PCA (Figure 2; 12 significant components; PC1 and PC2 accounted for 41% of the variation). A PCA scores plot showed separation between all microgreens and their mature counterparts. Specifically, red beet, red amaranth, and pea microgreens were separated from their mature counterparts along PC1 (23.5% of the variation). Arugula, broccoli, and red cabbage microgreens were separated from their mature counterparts along PC2 (17.5% of the variation), but there was some commonality with their mature counterparts. Overall, the mature counterparts clustered more closely to each other than to their respective microgreens.
FIGURE 2.
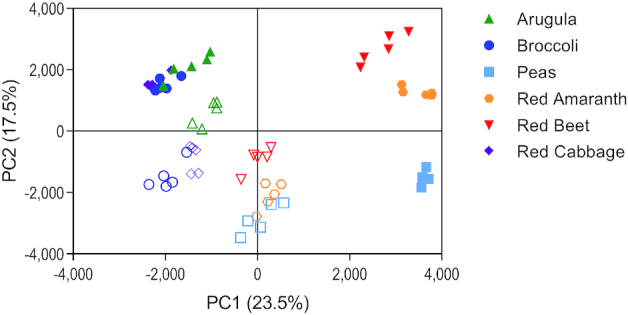
Principal component analysis of metabolites detected from 6 microgreen species and their mature counterparts. Solid shapes represent microgreens and open shapes represent the mature counterpart for each species. PC, principal component.
Further evaluation was focused on metabolites that were significantly different (P < 0.05) and ≥2-fold higher in microgreens than their mature counterpart (Supplemental Table 4). Broccoli microgreens contained 95 metabolites meeting these criteria, red cabbage microgreens contained 110, arugula microgreens contained 87, red beet microgreens contained 80, pea microgreens contained 93, and red amaranth microgreens contained 101. Classification of these metabolites by chemical superclass (Figure 3) demonstrates variable composition among species, but phenolics and lipids make up the majority of the compounds.
FIGURE 3.
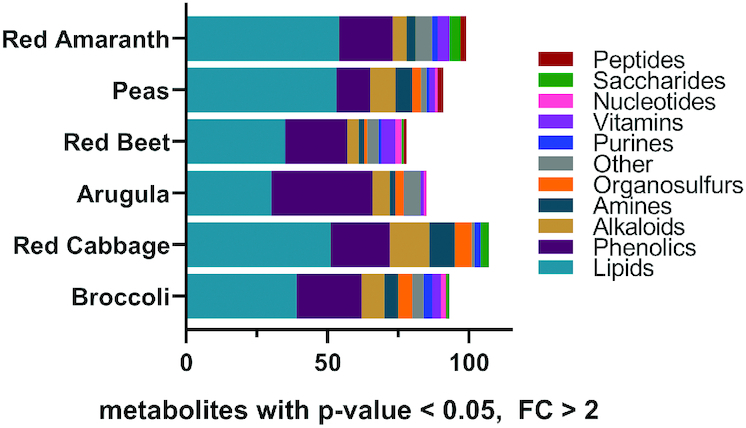
Bar charts representing the total number of metabolites that differ between mature tissues and microgreens, within each species. Color is used to indicate a designated chemical class for each metabolite. Only metabolites that were significantly different (Student t test P value < 0.05 and fold change >2) between microgreens and their mature counterparts are included. FC, fold change.
A total of 365 metabolites were significantly different and ≥2-fold higher in microgreens compared with mature counterparts, and were found to group according to plant family (Supplemental Table 5). Only 8 metabolites were common across all plant families (Supplemental Figure 1A), whereas Amaranthaceae and Brassicaceae shared 40 metabolites, Amaranthaceae and Fabaceae shared 23 metabolites, and Brassicaceae and Fabaceae shared 8 metabolites. Within the Brassicaceae plant family (Supplemental Figure 1B), 19 metabolites were common among all members, whereas arugula and broccoli shared 10 metabolites, arugula and red cabbage shared 10 metabolites, and broccoli and red cabbage shared 37 metabolites. Within the Amaranthaceae plant family (Supplemental Figure 1C), 19 metabolites were common between red beet and red amaranth.
Six microgreen species differed in metabolite profiles
Metabolomics analysis and PCA visualization revealed that each of the microgreen species contained a distinct metabolite profile that included variation in bioactive compounds (Figure 4; 6 significant components; PC1 and PC2 accounted for 61.1% of the variation). Further, the PCA scores plot showed grouping by plant family along PC1, where the Brassicaceae family of microgreens clustered together and were separate from the other families. Those in the Amaranthaceae family showed some grouping, but to a lesser degree than that of the Brassicaceae family. The variation in the microgreen metabolome was further validated by ANOVA, which revealed 454 of 1263 detected and annotated metabolites varied in ≥ 1 of the species (35.9%; FDR adjusted P < 0.05; Supplemental Table 6).
FIGURE 4.
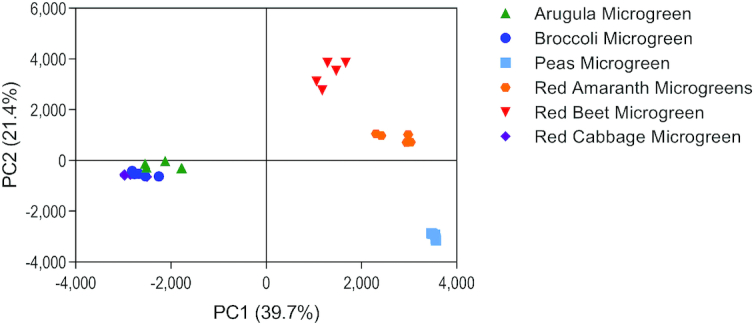
Principal component analysis of metabolites detected in 6 microgreen species. PC, principal component.
The uniqueness of the metabolite profiles for each species was further investigated by a modified z-score analysis. In this analysis, each metabolite is assigned a “z” that indicates if it is excessively higher in 1 species relative to the rest of the species combined, with a confidence threshold of z >1.96 associated with a significant P value. Metabolite z-values are reported in Supplemental Table 1 for each microgreen species. Overall, 334 of the 1263 metabolites were uniquely high in the microgreens, where arugula microgreens had 52 unique metabolites, broccoli microgreens had 16, red cabbage microgreens had 29, red beet microgreens had 45, red amaranth microgreens had 83, and pea microgreens had 109. Several interesting trends were observed in the uniqueness data. For instance, arugula microgreens were uniquely high in organosulfur compounds including isothiocyanates, sulfoxides, and glucosinolates, as well as vitamin K-1, polyphenolic compounds such as prenylated xanthones, and flavonols including rutin and quercetin glucosides. Red amaranth microgreens were uniquely high in amaranthin (betacyanin/betalain), phenolic acids such as coumaric acid esters and compounds involved in the formation of phenolic compounds such as cinnamic acid esters, polyphenolic compounds such as lignan glycosides, and oligosaccharides. Red beet microgreens were uniquely high in betanin (betacyanin/betalain), polyphenolic compounds such as apigenin (flavone), and salvianolic acid D. Red cabbage microgreens were uniquely high in numerous polyphenolic compounds such as flavonols including kaempferol and quercetin, flavones including isovitexin, as well as other compounds such as niazinin A (phenolic glycoside), sinapine (alkaloidal amine involved in flavonoid biosynthesis), the 2′-dehydroplectaniaxanthin (xanthophyll/carotenoid), and a quinic acid derivative. Pea microgreens were uniquely high in α-tocopherol (bioavailable form of vitamin E), polyphenolic compounds (flavonols) including kaempferol and quercetin, and flavonoid-7-O-glycosides. The findmainPlots.pdf file (SupplementalMaterial) contains mass spectra for each metabolite as constructed by RAMclust and interpreted by the MS Interpret R package.
Effects of maturity on mineral profiles
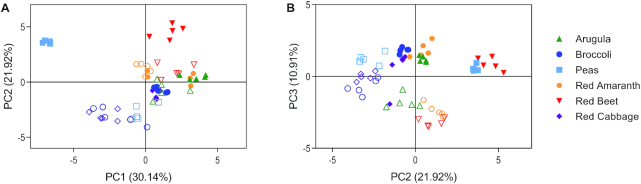
Mineral profiles of microgreens and mature counterparts were visualized using PCA (Figure 5; 9 significant components; PC1, PC2, and PC3 account for 63% of the variation). The PCA loadings plot showed separation between pea microgreens and all other samples along PC1 (30.14% of the variation). Separation of all microgreens and mature counterparts was observed along PC2 (21.92% of the variation).
FIGURE 5.
Principal component analysis of minerals detected in 6 microgreen species and their mature counterparts: (A) PC1 vs. PC2; (B) PC2 vs. PC3. Solid shapes represent microgreens and open shapes represent the mature counterpart for each species. PC, principal component.
Minerals were compared between microgreens and their mature counterparts through determination of minerals significantly different (P < 0.01) and ≥2-fold higher (microgreens compared with mature counterpart; Supplemental Table 7). Of nutritional relevance, selenium and copper were higher in arugula and broccoli microgreens than mature counterparts. Indeed, copper was 13.7-fold higher in broccoli microgreens than mature broccoli. Pea microgreens were significantly higher in both selenium and molybdenum, with fold changes of 16.8 and 12.2, respectively. Red amaranth microgreens had 2.2-fold higher concentrations of copper than the mature counterpart. Red beet microgreens were particularly rich in selenium compared to the mature counterpart (10.2-fold higher) and were also higher in chromium (3.1-fold) and copper (2.5-fold). Red cabbage microgreens were higher in phosphorus (2.1-fold), iron (2.4-fold), copper (9.1-fold), and zinc (3.8-fold) than the mature counterpart. It is important to note that some minerals were found in significantly lower quantities in microgreens compared with mature counterparts. Arugula microgreens were lower in sulfur, potassium, and molybdenum than mature arugula. Red amaranth microgreens were lower in cadmium, phosphorus, sulfur, and molybdenum than mature red amaranth. Surprisingly, pea and red beet microgreens were lower in numerous minerals such as magnesium, calcium, manganese, and copper, than mature counterparts. Of note, broccoli and red cabbage microgreens were not significantly lower in any minerals than their mature counterpart.
In terms of heavy metals that could be harmful to human health, arugula and broccoli microgreens had 3.5- and 2.7-fold higher arsenic concentrations, respectively, than their mature counterpart. Broccoli, red amaranth, red beet, and red cabbage microgreens were found to have 9.0-, 6.9-, 2.0-, and 11.4-fold higher lead concentrations than mature counterparts. Arugula and broccoli had 3.8- and 2.7-fold higher concentrations of strontium, respectively, than mature counterparts, whereas barium concentrations were higher in arugula (4.8-fold), broccoli (6.0-fold), red amaranth (6.8-fold), red beet (6.1-fold), and red cabbage (7.4-fold) microgreens compared with the mature counterparts. Importantly, none of the metals in any of the samples were detected at quantities sufficient to pose a threat to human health.
Six microgreen species differed in mineral profiles
Mineral profiles of microgreens were visualized using PCA (Figure 6; 5 significant components; PC1 and PC2 account for 70.23% of the variation). The PCA loadings plot showed grouping of arugula, red amaranth, red cabbage, and broccoli microgreens, whereas red beet and pea microgreens were distinct from one another and all other microgreens.
FIGURE 6.
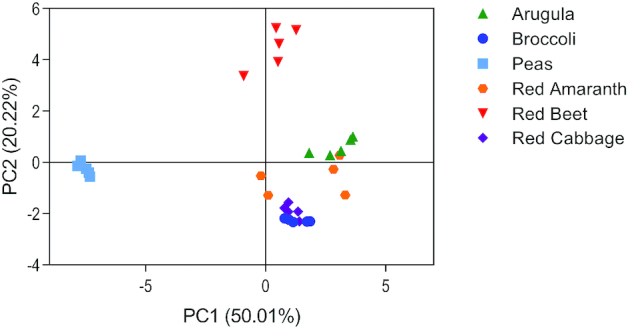
Principal component analysis of minerals detected in 6 microgreen species. PC, principal component.
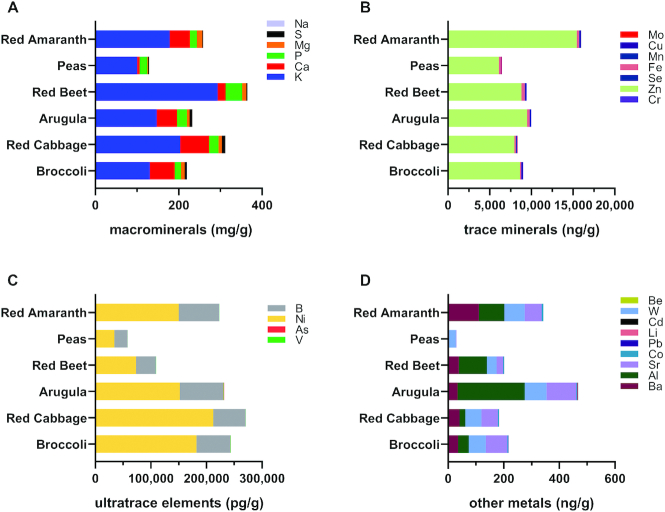
Proportions of each mineral were compared across microgreen species (Figure 7, Supplemental Tables 3 and 8) and several observations of nutritional relevance were noted with respect to macrominerals/major minerals. Calcium concentrations were higher in arugula [49.2 µg/g gram dry weight (gdw)], broccoli (60.0 µg/g gdw), and red cabbage (69.6 µg/g gdw) microgreens compared with red beet (19.0 µg/g gdw) and pea (5.59 µg/g gdw) microgreens, whereas red amaranth (48.3 µg/g gdw) microgreens were rich in calcium compared with red beet (19.0 µg/g gdw) and pea (5.59 µg/g gdw) microgreens. Potassium concentrations were higher in red beet microgreens (293.6 µg/g gdw) than in arugula (147.0 µg/g gdw), broccoli (130.6 µg/g gdw), and pea (100.6 µg/g gdw) microgreens, whereas red cabbage microgreens (203.4 µg/g gdw) were higher in potassium than pea microgreens (100.6 µg/g gdw). Arugula (6.4 µg/g gdw), broccoli (8.3 µg/g gdw), red amaranth (13.6 µg/g gdw), red beet (9.7 µg/g gdw), and red cabbage (8.2 µg/g gdw) microgreens were high in magnesium compared with pea microgreens (2.4 µg/g gdw), whereas red amaranth microgreens (13.6 µg/g gdw) also contained higher magnesium concentrations than arugula microgreens (6.4 µg/g gdw). Phosphorus concentrations were higher in red beet microgreens (39.8 µg/g gdw) than in broccoli (15.3 µg/g gdw), pea (18.2 µg/g gdw), and red amaranth (16.7 µg/g gdw) microgreens.
FIGURE 7.
Bar charts representing the proportions of minerals within each microgreen species. Color is used to indicate a specific mineral. Results of statistical analysis are not included in the figure. Macrominerals (A), trace minerals (B), ultratrace elements (C), and other metals (D). Al, aluminum; As, arsenic; B, boron; Ba, barium; Be, beryllium; Ca, calcium; Cd, cadmium; Co, cobalt; Cr, chromium; Cu, copper; Fe, iron; K, potassium; Li, lithium; Mg, magnesium; Mn, manganese; Mo, molybdenum; Na, sodium; Ni, nickel; P, phosphorus; Pb, lead; S, sulfur; Se, selenium; Sr, strontium; V, vanadium; W, tungsten; Zn, zinc.
With respect to trace minerals, numerous significant differences were observed among microgreens. Zinc made up the highest proportion of trace minerals in all microgreens, but the only significant difference noted was between red amaranth microgreens (15.4 µg/g gdw) and pea microgreens (6.1 µg/g gdw). Red beet microgreens (0.39 µg/g gdw) had more iron than broccoli microgreens (0.16 µg/g gdw) and red cabbage microgreens (0.20 µg/g gdw). Pea microgreens had higher selenium (0.0004 µg/g gdw) than all other microgreens. All microgreens had higher copper concentrations than pea microgreens (0.04 µg/g gdw). Arugula had higher chromium concentrations (0.004 µg/g gdw) than broccoli, pea, red amaranth, and red cabbage microgreens, whereas red amaranth and red beet microgreens had higher concentrations than broccoli and pea microgreens, and red amaranth and red beet had higher concentrations than red cabbage. Manganese was highest in red amaranth microgreens (0.13 µg/g gdw) and lowest in pea microgreens (0.02 µg/g gdw).
With respect to heavy metals harmful to human health, numerous significant differences were observed among microgreens for aluminum, arsenic, cadmium, and lead, among others. However, as mentioned above, none of the detected concentrations were above thresholds for risk to human health.
Discussion
This study comprehensively evaluated the metabolite and mineral contents of arugula, broccoli, red cabbage, red beet, red garnet amaranth, and pea microgreens, and the influence of maturity through application of nontargeted metabolomics and ionomics. To our knowledge, this is the first comprehensive analysis and comparison of metabolites and minerals in these 6 microgreen species and their mature counterparts. Overall, the findings of this study support that microgreens have distinct metabolite and mineral profiles compared with one another and with their mature counterparts. Microgreens were found to have an extensive and diverse range of metabolites (including vitamins and bioactive compounds) and minerals, providing further evidence for their high nutritional quality. We found that several, but not all, metabolites and minerals were higher in microgreens compared with the mature counterparts. In fact, certain compounds were not different or lower in microgreens compared with their respective mature counterparts. We also found that each microgreen species had numerous metabolites that were unique to that species.
Increased concentrations (P < 0.05 and ≥2-fold higher) of metabolites in microgreens compared with mature counterparts were largely driven by compounds classified within the lipid and phenolic superclasses, followed by those in the alkaloid, organosulfur, vitamin, and amine superclasses, among others. In some cases, higher concentrations of compounds in microgreen species compared with the mature counterpart were paralleled by the compound being unique (modified z-score >1.96) to that specific microgreen species. For instance, arugula microgreens were uniquely high in numerous indoles, glucosinolates, prenylated xanthones, and certain flavonoids relative to other microgreens, and these were present in higher quantities in arugula microgreens compared with mature arugula. Glucosinolates are known to have bitter and acrid flavors (35). Thus, these compounds are a likely contributor to our previous observation that consumers found arugula to be the least acceptable microgreen of the 6 studied (15), and as having the highest intensity for astringency, bitterness, and sourness. Nonetheless, arugula microgreens were still considered acceptable among consumers, and high variability was noted in that study suggesting that many individuals liked arugula microgreens. This is favorable considering the beneficial health effects of glucosinolates (36), as well as other compounds in arugula like polyphenols (37). Arugula was also found to be unique in containing vitamin K-1, but concentrations were not higher in the microgreens than mature arugula. Red cabbage microgreens were noted to be both unique in containing sinapine (hydrocinnamic acid/phenolic acid), and to contain higher concentrations of it than mature red cabbage. Sinapine has been reported to modulate circulating lipids, inflammation, and the gut microbiota in mice fed a high-fat diet (38), and to attenuate mitochondrial oxidative stress in cardiomyocytes (39), suggesting the potential of red cabbage microgreens to exert cardioprotective effects. Indeed, Huang et al. (40) demonstrated that red cabbage microgreens modulated circulating and liver lipids, and inflammatory cytokines in mice fed a high-fat diet. Here, we also found that red cabbage microgreens were higher than mature red cabbage in numerous health-promoting bioactive compounds including glucosinolates and their breakdown products (indoles and isothiocyanates), and various phenolics including flavonoid and nonflavonoid compounds. These compounds can protect against numerous chronic diseases including cardiovascular and metabolic disease and cancer, likely through diverse mechanisms including but not limited to their antioxidant and anti-inflammatory properties, ability to induce phase II detoxification enzymes, and/or modulation of the gut microbiome (36, 37, 41, 42). Pea microgreens were unique in containing kaempferol glycosides and trifolin, a kaempferol galactoside (both flavonols/flavonoid polyphenols), as well as numerous triterpene saponins, and also contained higher concentrations of these compounds than mature peas. Triterpene saponins have been shown to have numerous bioactivities, such as anti-inflammatory, immunomodulatory, anticarcinogenic, and cardiovascular-protective properties in preclinical models (43, 44). Saponins are often perceived as having bitter, astringent, and metallic sensory properties (45). Despite this, we previously observed that pea microgreens were rated favorably by consumers for acceptability and sensory perceptions often associated with adverse flavor (i.e., lower intensity for astringency, bitterness, etc.), although variability among individuals was noted (15). Here, peas were also found to be uniquely high in α-tocopherol (bioavailable vitamin E), compared with other microgreens, but concentrations were not higher in the pea microgreens than their mature counterparts. Vitamin E is an essential vitamin with several functions important to human health including its potent antioxidant and anti-inflammatory activities (46). Red beet microgreens also contained various triterpene saponin compounds that were both unique to the species and found in greater quantities in microgreens compared with their mature counterpart. We previously observed that red beet microgreens were perceived as being more bitter than pea microgreens, which also contain saponins, but were rated as acceptable to consumers (15). Red beet microgreens were also unique in betanin (betacyanin/betalain), and in phenolic compounds including apigenin (flavone/flavonoid polyphenol) and salvianolic acid D. They were also noted to contain higher concentrations of several flavonoid glycoside compounds than the mature red beets. Red amaranth microgreens were found to be both uniquely high in various triterpene saponin compounds and coumaric acid esters (phenolic acids) relative to other microgreens, and to contain higher quantities of these compounds compared with mature red amaranth. Red amaranth microgreens were also noted to be unique in other health-promoting compounds such as amaranthin (betacyanin/betalain), lignan glycosides (nonflavonoid polyphenols), and oligosaccharides. Additionally, they contained certain vitamins (pantothenic acid/vitamin B-5, tocotrienols/vitamin E, and vitamin A) and flavonoid-3-O-glycosides (flavonoid polyphenols) in higher concentrations than mature red amaranth. Broccoli microgreens were noted to contain N1,N10-diferuloylspermidine (hydroxycinnamic acid derivative/phenolamide), which was both unique to that species, and in higher quantities compared with mature broccoli. This compound has been previously demonstrated to be a potent antioxidant, and phenolamides in general have been shown to have bioactivity in vivo including antioxidant, anti-inflammatory, anticancer, and cardiovascular-protective effects, among others (47, 48). Broccoli was also uniquely high in a flavonoid-7-O-glycoside relative to other microgreens, and to contain higher concentrations of various flavonoid and nonflavonoid polyphenols, glucosinolates, tryptophanol (indole), sinapine, tocotrienols (vitamin E), linatine (vitamin B-6 antagonist/antinutrient), and ergosterols and derivatives (provitamin D-2) compared with mature broccoli.
The finding that microgreens contained numerous bioactive compounds that were significantly increased relative to mature counterparts is in line with previous reports. An initial study by Sun et al. (49) profiled 164 polyphenolic compounds in 5 Brassica microgreen species (mizuna, red cabbage, purple kohlrabi, red mustard, and purple mustard) and found that microgreens had more complex polyphenol profiles and more polyphenol varieties compared with their mature counterparts based on comparisons with previous reports. Huang et al. (40) determined polyphenol and glucosinolate contents in red cabbage microgreens and mature red cabbage and found that total concentrations of nonanthocyanin polyphenols were higher in the red cabbage microgreens than mature red cabbage, though total anthocyanin concentrations were lower in the microgreens than the mature counterpart. They also found that total glucosinolate concentrations were higher in red cabbage microgreens than mature red cabbage, which is also in line with our observation that numerous organosulfur compounds were significantly increased in red cabbage microgreens relative to mature red cabbage. Similarly, El-Nakhel et al. (50) directly compared bioactive compounds in green and red Salanova butterhead lettuce microgreens and found that polyphenols were more concentrated in the microgreens, and particularly in red pigmented lettuce microgreens, than mature lettuce. Conversely, de la Fuente et al. (51) found that broccoli, green curly kale, red mustard, and radish hydroponic microgreens contained similar amounts of total isothiocyanates and total polyphenols compared with their mature counterparts based on information from previous reports. Several of the studies above noted that the mature vegetables contained higher concentrations of carotenoids than the microgreens, which is in line with our observation that microgreens contained similar or lower concentrations of carotenoids compared with their mature counterparts. However, Xiao et al. (12) studied 25 microgreen species and found that several, but not all, contained higher concentrations of carotenoids than their mature counterparts based on information found in the USDA national nutrient database. These findings support that microgreens are concentrated sources of various health-promoting bioactive compounds, but that variation exists within the literature potentially due to factors such as plant genotype, methods of metabolite detection, and/or growing conditions. More research is needed to elucidate the bioactive compound types and quantities in various microgreen species, and the influence of the above-mentioned factors.
The finding that microgreens are concentrated sources of metabolites, namely bioactive compounds, and in many cases more concentrated than the mature counterpart, is of relevance to human nutrition and health and necessitates further investigation. Microgreen consumption could increase plant bioactive compound intake beyond usual intake to achieve specific functional health effects. An important area of consideration is the bioaccessibility (the quantity or fraction released from the food matrix in the gastrointestinal tract for absorption) and bioavailability (the fraction of ingested nutrient/compound that reaches systemic circulation and is utilized) of the compounds (52, 53). Ascorbic acid (vitamin C) and bioactive compounds including carotenoids, isothiocyanates, and polyphenols in Brassicaceae microgreens were shown to be bioaccessible through simulated gastrointestinal in vitro digestion, but this was significantly impacted by plant genotype (51). It is currently unknown whether microgreen bioactive compounds have the same bioaccessibility as their mature counterparts, which has implications for bioavailability and bioactivity of compounds in vivo. Additionally, previous research has demonstrated that modulation of microgreens’ growing conditions (e.g., LED lighting and nutrient solutions) can enhance the concentrations of various health-promoting compounds such as β-carotene (provitamin A), anthocyanins, and α-tocopherol (vitamin E) (54–56). The impact that these enhancements have on bioaccessibility, bioavailability, and thus bioactivity of compounds should be evaluated, as should the impacts on consumer acceptability and sensory perceptions of microgreens.
With respect to minerals, microgreens contained various minerals important to human health, with some minerals higher in microgreens than mature counterparts, including selenium, copper, iron, zinc, and molybdenum. We also found that the concentrations of some minerals were lower in microgreens than mature counterparts (e.g., magnesium, calcium, and manganese in pea and red beet microgreens). The higher concentration of minerals in microgreens in some cases represents their potential in helping increase mineral intake, particularly for minerals where deficiency is more common globally (e.g. iron, zinc, and selenium) (57). Paradiso et al. (58) evaluated the mineral contents of chicory, lettuce, and broccoli microgreens and compared them with those found in found in the USDA National Nutrient Database. Butterhead lettuce microgreens were higher in phosphorus, calcium, magnesium, and zinc than mature butterhead lettuce, whereas broccoli microgreens contained similar mineral compositions to mature broccoli but were higher in calcium. El-Nakhel et al. (50) found that green and red Salanova butterhead lettuce microgreens had higher concentrations of calcium, magnesium, sulfur, and sodium, whereas the mature lettuce was higher in nitrate, phosphorus, and potassium. It should be noted that factors contributing to the differences between our study and previous reports might include genotype, and growing and postharvest conditions. Indeed, both studies by Paradiso et al. and El-Nakhel et al. fertilized the microgreens with nutrient solutions, whereas we did not apply a supplemental fertilizer in our study. Our findings, as well as the above-mentioned reports, also shed light on opportunities for enhancing the nutritional quality of microgreens with respect to minerals in general, and in cases where minerals were lower in microgreens than their mature counterpart. Indeed, previous research has demonstrated that the mineral contents of various microgreens can be modulated through biofortification [e.g., selenium and iodide (59, 60)] and different light spectra [e.g., potassium, calcium, iron, and magnesium (61, 62)]. Bioaccessibility and bioavailability are also important for prevention of micronutrient deficiencies (52). Previous research using simulated gastrointestinal in vitro digestion (as a model of bioaccessibility), and mineral uptake in vitro (as a model of bioavailability), suggests that minerals (e.g., iron, potassium, magnesium, and zinc) in microgreens are bioaccessible and bioavailable, but with differences observed among plant species. Interestingly, differences in in vitro iron uptake between fenugreek microgreens and mature fenugreek were previously observed (63), where iron uptake was greater from microgreens and enhanced by ascorbic acid (vitamin C). These findings are promising with respect to bioaccessibility and bioavailability, but require confirmation and further investigation in vivo, particularly in humans. Additionally, bioaccessibility and bioavailability should be confirmed when enhancing the concentrations of minerals, as well as the consumer acceptance and sensory perceptions of the microgreens.
Overall, our findings support that the 6 microgreens studied contain a wide variety of nutrients and metabolites. We found that metabolite and mineral profiles of microgreens and their mature counterparts were more similar among themselves than each other (microgreens compared with mature counterparts). Further evaluation revealed microgreens are concentrated sources of some, but not all, vitamins, minerals, and bioactive compounds, known to exert beneficial impacts on human health. We found that in some instances, the concentrations of health-promoting nutrients and bioactive compounds were lower in microgreens relative to their mature counterpart. This is an important consideration for dietary recommendations and intervention studies involving microgreens, but also represents an area for improvement through agricultural practices such as modulation of lighting and supplemental fertilizer for biofortification. Our data, and the variety of species available, suggest microgreens have potential to increase food and agricultural biodiversity necessary for sustainable food and agricultural systems, and thus human health (6). They also suggest the potential of microgreens to increase dietary diversity to promote a nutritionally adequate diet and reduce disease risk (64). It is important to note that because growing conditions for microgreens have been shown to impact their nutritional and phytochemical quality, our findings should not be extrapolated to microgreens grown in different conditions. Additionally, specific red cabbage anthocyanins could not be annotated in these data, likely because the metabolites were only detected in positive MS mode, and although this enables broad detection of many compounds, a limitation is the inability to discriminate cyanidin-based flavonoids from noncolored isomers (65). Lastly, while we can evaluate relative differences of individual metabolites among samples, it is not appropriate to compare abundances of metabolites per se due to the inherent non-quantitative nature of mass spectrometry. Research of targeted metabolomics is necessary to compare absolute abundances of metabolites. Nonetheless, the z uniqueness scores are intended to provide insight into the metabolites most unique to each microgreens species relative to the other microgreens. Moving forward, research is warranted to better characterize nutritional and bioactive compound profiles in microgreens, and to understand potential impacts on human health including bioaccessibility, bioavailability, and bioactivity of the compounds. Research to improve the nutritional quality of microgreens should align with assessments and confirmation of consumer acceptability to promote translation of research to the global food system, including consumer markets.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge Dr Laura L Bellows for her contributions to funding acquisition and conceptualization of the research, and Natalie Yoder and Kimberley Freedman for their assistance with plant production.
The authors responsibilities were as follows—SAJ, JEP, ALH, SEN, MEU, MB, MTF, HJT, and TLW: designed research; HI: contributed to research design; HI, JMC, HMO, and KAM: conducted research; JEP, ALH, JMC, and HMO: analyzed data; SAJ, JEP, ALH, and JMC: interpreted results; SAJ: wrote paper with contributions from JEP, ALH, SEN, MEU, and JMC; SAJ: had primary responsibility for final content; and all authors: commented on and edited subsequent drafts, and read and approved the final manuscript.
Notes
Supported by the Colorado Agricultural Experiment Station, National Institute of Food and Agriculture, US Department of Agriculture, Hatch under 1016130 (SAJ, JEP, ALH, SEN, MEU, MB, MTF, HJT, TLW).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–8, Supplemental Figure 1 and Supplemental Material are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: CSU, Colorado State University; DRC, dynamic reaction mode; FDR, false discovery rate; gdw, gram dry weight; LED, light-emitting diode; LOQ, limits of quantification; MeOH, methanol; MTBE, methyl-tert-butyl-ether; PCA, principal component analysis; QC, quality control; Q-TOF, quadrupole time-of-flight; UHPLC-MS, ultra-high-performance liquid chromatography-mass spectrometry.
Contributor Information
Sarah A Johnson, Email: Sarah.Johnson@colostate.edu, Department of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO, USA.
Jessica E Prenni, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA; Analytical Resources Core: Bioanalysis and Omics, Colorado State University, Fort Collins, CO, USA.
Adam L Heuberger, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA; Department of Soil and Crop Sciences, Colorado State University, Fort Collins, CO, USA.
Hanan Isweiri, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA; Department of Biology, Faculty of Education, University of Benghazi, Benghazi, Libya.
Jacqueline M Chaparro, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA; Analytical Resources Core: Bioanalysis and Omics, Colorado State University, Fort Collins, CO, USA.
Steven E Newman, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA.
Mark E Uchanski, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA.
Heather M Omerigic, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA.
Kiri A Michell, Department of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO, USA.
Marisa Bunning, Department of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO, USA.
Michelle T Foster, Department of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO, USA.
Henry J Thompson, Department of Horticulture and Landscape Architecture, Colorado State University, Fort Collins, CO, USA.
Tiffany L Weir, Department of Food Science and Human Nutrition, Colorado State University, Fort Collins, CO, USA.
References
- 1. United Nations World population prospects 2019: highlights. New York: UN; 2019. [Google Scholar]
- 2. Urbanization and health. Bull World Health Organ. 2010;88:245–6. [Google Scholar]
- 3. Shlisky J, Bloom DE, Beaudreault AR, Tucker KL, Keller HH, Freund-Levi Y, Fielding RA, Cheng FW, Jensen GL, Wu Det al. Nutritional considerations for healthy aging and reduction in age-related chronic disease. Adv Nutr. 2017;8(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabaté J, Harwatt H, Soret S. Environmental nutrition: a new frontier for public health. Am J Public Health. 2016;106(5):815–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. D'Odorico P, Davis KF, Rosa L, Carr JA, Chiarelli D, Dell'Angelo J, Gephart J, MacDonald GK, Seekell DA, Suweis S. The global food-energy-water nexus. Rev Geophys. 2018;56(3):456–531. [Google Scholar]
- 6. Pilling D, Bélanger J, Hoffmann I. Declining biodiversity for food and agriculture needs urgent global action. Nat Food. 2020;1(3):144–7. [Google Scholar]
- 7. Dwivedi SL, Lammerts van Bueren ET, Ceccarelli S, Grando S, Upadhyaya HD, Ortiz R. Diversifying food systems in the pursuit of sustainable food production and healthy diets. Trends Plant Sci. 2017;22(10):842–56. [DOI] [PubMed] [Google Scholar]
- 8. Choe U, Yu LL, Wang TTY. The science behind microgreens as an exciting new food for the 21st century. J Agric Food Chem. 2018;66(44):11519–30. [DOI] [PubMed] [Google Scholar]
- 9. Mir SA, Shah MA, Mir MM. Microgreens: production, shelf life, and bioactive components. Crit Rev Food Sci Nutr. 2017;57(12):2730–6. [DOI] [PubMed] [Google Scholar]
- 10. Kyriacou MC, Rouphael Y, Di Gioia F, Kyratzis A, Serio F, Renna M, De Pascale S, Santamaria P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci Technol. 2016;57:103–15. [Google Scholar]
- 11. Xiao Z, Codling EE, Luo Y, Nou X, Lester GE, Wang Q. Microgreens of Brassicaceae: mineral composition and content of 30 varieties. J Food Compos Anal. 2016;49:87–93. [Google Scholar]
- 12. Xiao Z, Lester GE, Luo Y, Wang Q. Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J Agric Food Chem. 2012;60(31):7644–51. [DOI] [PubMed] [Google Scholar]
- 13. Weber CF Broccoli microgreens: a mineral-rich crop that can diversify food systems. Front Nutr. 2017;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao Z, Lester GE, Park E, Saftner RA, Luo Y, Wang Q. Evaluation and correlation of sensory attributes and chemical compositions of emerging fresh produce: microgreens. Postharvest Biol Technol. 2015;110:140–8. [Google Scholar]
- 15. Michell KA, Isweiri H, Newman SE, Bunning M, Bellows LL, Dinges MM, Grabos LE, Rao S, Foster MT, Heuberger ALet al. Microgreens: consumer sensory perception and acceptance of an emerging functional food crop. J Food Sci. 2020;85(4):926–35. [DOI] [PubMed] [Google Scholar]
- 16. Caracciolo F, El-Nakhel C, Raimondo M, Kyriacou MC, Cembalo L, De Pascale S, Rouphael Y. Sensory attributes and consumer acceptability of 12 microgreens species. Agronomy. 2020;10(7):1043. [Google Scholar]
- 17. Swiader J, Ware G. Producing vegetable crops. 5th ed Danville (IL): Interstate Publishers, Inc; 2002. [Google Scholar]
- 18. Broeckling CD, Prenni JE. Stacked injections of biphasic extractions for improved metabolomic coverage and sample throughput. Anal Chem. 2018;90(2):1147–53. [DOI] [PubMed] [Google Scholar]
- 19. Smith CA, Want EJ, O'Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78(3):779–87. [DOI] [PubMed] [Google Scholar]
- 20. R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2015. [Google Scholar]
- 21. Broeckling CD, Afsar FA, Neumann S, Ben-Hur A, Prenni JE. RAMClust: a novel feature clustering method enables spectral-matching-based annotation for metabolomics data. Anal Chem. 2014;86(14):6812–7. [DOI] [PubMed] [Google Scholar]
- 22. Broeckling CD, Ganna A, Layer M, Brown K, Sutton B, Ingelsson E, Peers G, Prenni JE. Enabling efficient and confident annotation of LC−MS metabolomics data through MS1 spectrum and time prediction. Anal Chem. 2016;88(18):9226–34. [DOI] [PubMed] [Google Scholar]
- 23. Fahy E, Sud M, Cotter D, Subramaniam S. LIPID MAPS online tools for lipid research. Nucleic Acids Res. 2007;35(Suppl 2):W606–W12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong Eet al. HMDB 3.0—the Human Metabolome Database in 2013. Nucleic Acids Res. 2012;41(D1):D801–D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jaeger C, Méret M, Schmitt CA, Lisec JiMS. Compound annotation in liquid chromatography/high-resolution mass spectrometry based metabolomics: robust adduct ion determination as a prerequisite to structure prediction in electrospray ionization mass spectra. Rapid Commun Mass Spectrom. 2017;31(15):1261–6. [DOI] [PubMed] [Google Scholar]
- 26. Lai Z, Tsugawa H, Wohlgemuth G, Mehta S, Mueller M, Zheng Y, Ogiwara A, Meissen J, Showalter M, Takeuchi Ket al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat Methods. 2018;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsugawa H, Kind T, Nakabayashi R, Yukihira D, Tanaka W, Cajka T, Saito K, Fiehn O, Arita M. Hydrogen rearrangement rules: computational MS/MS fragmentation and structure elucidation using MS-FINDER software. Anal Chem. 2016;88(16):7946–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sumner L, Amberg A, Barrett D, Beale M, Beger R, Daykin C, Fan TM, Fiehn O, Goodacre R, Griffin Jet al. Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3(3):211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haugen J-E, Tomic O, Kvaal K. A calibration method for handling the temporal drift of solid state gas-sensors. Anal Chim Acta. 2000;407(1–2):23–39. [Google Scholar]
- 30. Broccardo CJ, Schauer KL, Kohrt WM, Schwartz RS, Murphy JP, Prenni JE. Multiplexed analysis of steroid hormones in human serum using novel microflow tile technology and LC-MS/MS. J Chromatogr B. 2013;934:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shrivastava A, Gupta V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci. 2011;2(1):21–5. [Google Scholar]
- 32. Becker K, Kaus S, Krause C, Lepom P, Schulz C, Seiwert M, Seifert B. German Environmental Survey 1998 (GerES III): environmental pollutants in blood of the German population. Int J Hyg Environ Health. 2002;205(4):297–308. [DOI] [PubMed] [Google Scholar]
- 33. Crozier A, Clifford MN, Ashihara H. Plant secondary metabolites: occurrence, structure and role in the human diet. Hoboken (NJ): John Wiley & Sons; 2008. [Google Scholar]
- 34. Weaver CM Bioactive foods and ingredients for health. Adv Nutr. 2014;5(3):306S–11S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drewnowski A, Gomez-Carneros C. Bitter taste, phytonutrients, and the consumer: a review. Am J Clin Nutr. 2000;72(6):1424–35. [DOI] [PubMed] [Google Scholar]
- 36. Prieto M, López CJ, Simal-Gandara J. Glucosinolates: molecular structure, breakdown, genetic, bioavailability, properties and healthy and adverse effects. Adv Food Nutr Res. 2019;90:305–50. [DOI] [PubMed] [Google Scholar]
- 37. Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, Novellino E, Santini A. Polyphenols: a concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33(9):2221–43. [DOI] [PubMed] [Google Scholar]
- 38. Li Y, Li J, Su Q, Liu Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. 2019;10(6):3637–49. [DOI] [PubMed] [Google Scholar]
- 39. Boulghobra D, Grillet PE, Laguerre M, Tenon M, Fauconnier J, Fanca-Berthon P, Reboul C, Cazorla O. Sinapine, but not sinapic acid, counteracts mitochondrial oxidative stress in cardiomyocytes. Redox Biol. 2020;34:101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang H, Jiang X, Xiao Z, Yu L, Pham Q, Sun J, Chen P, Yokoyama W, Yu LL, Luo YSet al. Red cabbage microgreens lower circulating low-density lipoprotein (LDL), liver cholesterol, and inflammatory cytokines in mice fed a high-fat diet. J Agric Food Chem. 2016;64(48):9161–71. [DOI] [PubMed] [Google Scholar]
- 41. Raiola A, Errico A, Petruk G, Monti DM, Barone A, Rigano MM. Bioactive compounds in Brassicaceae vegetables with a role in the prevention of chronic diseases. Molecules. 2017;23(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Johnson SA, Litwin NS, Seals DR. Age-related vascular dysfunction: what registered dietitian nutritionists need to know. J Acad Nutr Diet. 2019;119(11):1790–6. [DOI] [PubMed] [Google Scholar]
- 43. Mroczek A Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem Rev. 2015;14(4):577–605. [Google Scholar]
- 44. Di Gioia F, Petropoulos SA. Phytoestrogens, phytosteroids and saponins in vegetables: biosynthesis, functions, health effects and practical applications. Adv Food Nutr Res. 2019;90:351–421. [DOI] [PubMed] [Google Scholar]
- 45. Roland WS, Pouvreau L, Curran J, van de Velde F, de Kok PM. Flavor aspects of pulse ingredients. Cereal Chemistry. 2017;94(1):58–65. [Google Scholar]
- 46. Weber P, Birringer M, Blumberg JB, Eggersdorfer M, Frank J. Vitamin E in human health. Hoboken (NJ): Springer; 2019. [Google Scholar]
- 47. Abe K, Matsuura H, Ukai M, Shimura H, Koshino H, Suzuki T. N1,N14-diferuloylspermine as an antioxidative phytochemical contained in leaves of Cardamine fauriei. Biosci Biotechnol Biochem. 2017;81(10):1855–60. [DOI] [PubMed] [Google Scholar]
- 48. Wang W, Snooks HD, Sang S. The chemistry and health benefits of dietary phenolamides. J Agric Food Chem. 2020;68(23):6248–67. [DOI] [PubMed] [Google Scholar]
- 49. Sun J, Xiao Z, Lin LZ, Lester GE, Wang Q, Harnly JM, Chen P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS(n.). J Agric Food Chem. 2013;61(46):10960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. El-Nakhel C, Pannico A, Graziani G, Kyriacou MC, Giordano M, Ritieni A, De Pascale S, Rouphael Y. Variation in macronutrient content, phytochemical constitution and in vitro antioxidant capacity of green and red butterhead lettuce dictated by different developmental stages of harvest maturity. Antioxidants (Basel). 2020;9(4):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. de la Fuente B, Lopez-Garcia G, Manez V, Alegria A, Barbera R, Cilla A. Evaluation of the bioaccessibility of antioxidant bioactive compounds and minerals of four genotypes of Brassicaceae microgreens. Foods. 2019;8(7):250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fernandez-Garcia E, Carvajal-Lerida I, Perez-Galvez A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr Res. 2009;29(11):751–60. [DOI] [PubMed] [Google Scholar]
- 53. Neilson AP, Goodrich KM, Ferruzzi MG. Bioavailability and metabolism of bioactive compounds from foods. In: Coulston AM, Boushey CJ, Ferruzzi MG, Delahanty LM, editors. Nutrition in the prevention and treatment of disease. Elsevier; 2017. pp. 301–19. [Google Scholar]
- 54. Palmitessa OD, Renna M, Crupi P, Lovece A, Corbo F, Santamaria P. Yield and quality characteristics of Brassica microgreens as affected by the NH4:NO3 molar ratio and strength of the nutrient solution. Foods. 2020;9(5):677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brazaityte A, Sakalauskiene S, Samuoliene G, Jankauskiene J, Virsile A, Novickovas A, Sirtautas R, Miliauskiene J, Vastakaite V, Dabasinskas Let al. The effects of LED illumination spectra and intensity on carotenoid content in Brassicaceae microgreens. Food Chem. 2015;173:600–6. [DOI] [PubMed] [Google Scholar]
- 56. Samuoliene G, Virsile A, Brazaityte A, Jankauskiene J, Sakalauskiene S, Vastakaite V, Novickovas A, Viskeliene A, Sasnauskas A, Duchovskis P. Blue light dosage affects carotenoids and tocopherols in microgreens. Food Chem. 2017;228:50–6. [DOI] [PubMed] [Google Scholar]
- 57. Bailey RL, West Jr KP, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66(Suppl. 2):22–33. [DOI] [PubMed] [Google Scholar]
- 58. Paradiso VM, Castellino M, Renna M, Gattullo CE, Calasso M, Terzano R, Allegretta I, Leoni B, Caponio F, Santamaria P. Nutritional characterization and shelf-life of packaged microgreens. Food Funct. 2018;9(11):5629–40. [DOI] [PubMed] [Google Scholar]
- 59. Germ M, Stibilj V, Sircelj H, Jerse A, Kroflic A, Golob A, Marsic NK. Biofortification of common buckwheat microgreens and seeds with different forms of selenium and iodine. J Sci Food Agric. 2019;99(9):4353–62. [DOI] [PubMed] [Google Scholar]
- 60. Puccinelli M, Malorgio F, Rosellini I, Pezzarossa B. Production of selenium-biofortified microgreens from selenium-enriched seeds of basil. J Sci Food Agric. 2019;99(12):5601–5. [DOI] [PubMed] [Google Scholar]
- 61. Kyriacou MC, El-Nakhel C, Pannico A, Graziani G, Soteriou GA, Giordano M, Zarrelli A, Ritieni A, De Pascale S, Rouphael Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front Plant Sci. 2019;10:1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Samuoliene G, Brazaityte A, Virsile A, Miliauskiene J, Vastakaite-Kairiene V, Duchovskis P. Nutrient levels in Brassicaceae microgreens increase under tailored light-emitting diode spectra. Front Plant Sci. 2019;10:1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khoja KK, Buckley A, Aslam MF, Sharp PA, Latunde-Dada GO. In vitro bioaccessibility and bioavailability of iron from mature and microgreen fenugreek, rocket and broccoli. Nutrients. 2020;12(4):1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. de Oliveira Otto MC, Anderson CA, Dearborn JL, Ferranti EP, Mozaffarian D, Rao G, Wylie-Rosett J, Lichtenstein AH. Dietary diversity: implications for obesity prevention in adult populations: a science advisory from the. American Heart Association. Circulation. 2018;138(11):e160–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun J, Lin LZ, Chen P. Study of the mass spectrometric behaviors of anthocyanins in negative ionization mode and its applications for characterization of anthocyanins and non-anthocyanin polyphenols. Rapid Commun Mass Spectrom. 2012;26(9):1123–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.