Abstract
Growth differentiation factor-15 (GDF-15) is cytokine involved in the regulation of multiple systems. Because it has regularly been shown to be increased in cardiovascular disease (CVD) and diabetes, it has been suggested that GDF-15 could be used as a biomarker for these diseases and their severity. However, several studies have demonstrated that GDF-15 has a protective role in regulation of inflammation, endothelial cell function, insulin sensitivity, weight gain, and is cardioprotective in myocardial infarction (MI). While GDF-15 has been implicated in the pathophysiology of many conditions including cancer, this review focuses on the potential functions of GDF-15 and signaling pathways implicated in its role regulating metabolism, insulin sensitivity, and the cardiovascular system.
Keywords: GDF-15, diabetes, cardiovascular disease
Graphical Abstract:
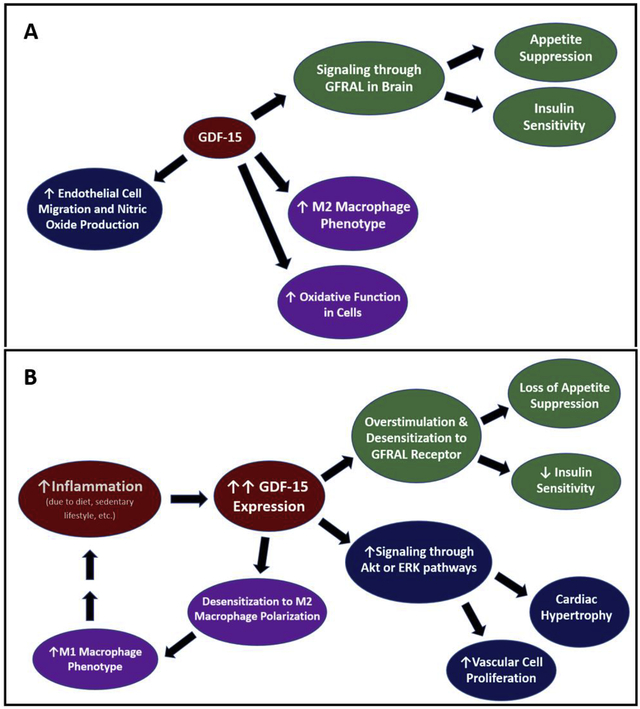
GDF-15 signaling in normal and pathogenic conditions. GDF-15 has been implicated to signal in many pathways, leading to metabolic changes such as insulin sensitivity and increased oxidative functions, as well as additional effects such as cell growth and proliferation (A). In chronic disease, inflammation can lead to dramatic increases in GDF-15 expression. It has been postulated that desensitization of the receptors can occur, causing a decrease in the GDF-15 impact on appetite suppression and insulin sensitivity, thereby contributing to diabetes and obesity. Desensitization may also contribute to the increase in M1 macrophages, promoting further inflammation and causing a vicious cycle (B).
Introduction
Growth differentiation factor-15 (GDF-15) was discovered in 1997 as a distant member of the transforming growth factor β (TGF-β) superfamily. This cytokine was identified by a number of research groups, which each identified the protein under differing conditions. Therefore, additional names for GDF-15 include: macrophage inhibitory cytokine-1 (MIC-1), placental bone morphogenic protein (PLAB), placental transforming growth factor beta (PTGFB), or NSAID activated gene-1 (NAG-1) [1–4]. Lastly, Bottner and colleagues [5] designated the name growth differentiation factor-15, due to the cytokine’s widespread expression and activity [5], which is the name that will be used throughout this review.
Normal Physiological Function
The signaling and effect of GDF-15 is largely dependent on the tissue and body condition, as will be discussed below. As a member of the TGF-β superfamily, and its identification as macrophage inhibitory peptide, it is no surprise that GDF-15 is involved in inflammation. It has been shown that GDF-15 is significantly elevated in serum from critically ill and/ or septic patients compared to healthy, regardless of the source of the patient’s ailment[6]. GDF-15 is also extremely important for the acute immune response. In a study examining the role of GDF-15 in either bacterial or viral infections, mice given a GDF-15 blocking antibody died in a significantly shorter time period compared to those who had active GDF-15 signaling. The mortality in these animals was believed to be due to excess inflammation [7]. Therefore, GDF-15 appears to be protective in infection, preventing the tissues from experiencing damage from inflammation.
GDF-15 has also been described as a myomitokine, which is cytokine that originates from mitochondria in skeletal muscle [8]. The mitochondria is an essential organelle to maintain both local and systemic homeostasis. Studies have shown that expression of proteins involved in oxidative metabolism are decreased in type 2 diabetes mellitus (T2DM) patients [9, 10]. Additionally, Petersen et al [11] demonstrated that elderly patients have decreased mitochondrial oxidative phosphorylation activity, which contributes to insulin resistance in old age [11]. When the mitochondria encounters proteotoxic stress, the adaptive response known as the unfolded protein response (UPR) may be activated in an attempt to reestablish normal function and oxidative phosphorylation [12, 13]. Increased GDF-15 mRNA and protein expression is associated with UPR induction in mice, and these mice are protected from diet-induced obesity and maintain increased sensitivity to insulin. In conjunction with this study, cultured myoblasts and hepatocytes were treated with recombinant GDF-15. Treated cells were found to have increased respiration and fatty acid oxidation in a dose dependent manner [8]. These findings suggest that GDF-15 may be important in maintaining cellular respiration, particularly under conditions of stress.
Another highly studied physiological function of GDF-15 is its ability to cause anorexia and cachexia, or weakness. During an experiment in which GDF-15 was overexpressed in mice, the animals were kept on a normal chow diet for 14 weeks. Animals with increased GDF-15 expression had significant decreases in body weight and this was associated with a decrease in food consumption. Additionally, mice with increased GDF-15 exhibited improved glucose handling, with a reduction in blood glucose measured. These trends were also observed when the mice were put on a high fat diet [14]. These studies demonstrated that GDF-15 might be a regulator of body weight and metabolism, with effects on glucose handling. A summary of GDF-15 signaling is depicted in the Graphical Abstract.
Receptors
Due to the anorexic effects, and therapeutic potential in treating obesity, studies sought to find a specific receptor for GDF-15. Yang et al [15] postulated that the receptor for growth differentiation factor-15 would be located in the brain [15]. This conclusion was based on other findings that an intact brainstem was required for the anorexic phenotype if GDF-15 was given peripherally [16]. Four groups identified the glial-derived neurotrophic factor (GDNF) family receptor α-like (GFRAL) as the receptor for GDF-15, with the co-receptor Ret. The researchers demonstrated the effects of GDF-15 binding of GFRAL by creating GFRAL knockout animals. The animals lacking GFRAL receptor did not have the anti-obesity protection offered by GDF-15 binding [15, 17–19]. GRFAL is an orphan receptor of the GDNF-α receptor family. Because GDNF is an alternative cytokine superfamily, it has been speculated that GDF-15 is not an appropriate description for the protein [20]. Because the anorexic effects of GDF-15 are mediated via GFRAL signaling, therapies which target GFRAL activation, thereby mimicking the binding of GDF-15, might offer a solution to the concerns of the obesity epidemic seen worldwide.
There has been a great deal of controversy over the ability of GDF-15 to bind TGF-β receptors (TGF-βR). Despite this, there have been several studies that have demonstrated GDF-15 binding to TGF-β receptors. Artz and colleagues [21] identified ALK-5/TGF-βRII as a heterodimer receptor pair for GDF-15 binding using both in vitro and in vivo methods [21]. Similarly, Zhang et al [22] found that GDF-15 was able to signal through both TGF-βRI and TGF-βRII [22]. Others have also shown GDF-15 signaling through different receptors, including an epithelial growth factor receptor ErbB2 [23, 24]. Though the GDF-15-specific receptor GFRAL is relegated to the brain, GDF-15 appears to signal throughout the body. Thus, signaling of growth differentiation factor-15 is not limited to the binding of GFRAL.
GDF-15 Downstream Signaling
Because GDF-15 appears to interact with multiple receptors in many tissues, it is difficult to narrow down a specific signaling pathway for the cytokine. GFRAL, the GDF-15 receptor isolated to the brain, is present on neurons containing the neurotransmitter cholecystokinin (CCK). Binding of GDF-15 to GFRAL results in release of the neurotransmitter and eliciting the appetite suppression response, as shown in Figure 1A. Interestingly, cancer drugs, such as cisplatin, have also been shown to activate these receptors, providing a direct relationship between chemotherapy and loss of appetite in cancer patients [23]. Li et al [25] demonstrated that endothelial cells exposed to high glucose used p53 to upregulate GDF-15 expression. GDF-15 then led to activation of the PI3K/Akt/eNOS pathway while downregulating the NF-κB/ JNK pathway. The group concluded that these were potential examples of protective effects of GDF-15 in diabetes [25]. As mentioned briefly above, GDF-15 signaling through ALK receptors has been speculated. ALK- 4/5/7 have all been identified as potential candidates due to GDF-15 activation of Smad2 and Smad3 downstream of these receptors, in addition to activation of Akt and ERK1/2 in cardiomyocytes [26]. Artz et al [21] further showed signaling through ALK-5 by GDF-15, showing a loss of signaling when a specific inhibitor to the receptor was applied [21], though specific binding studies have not been demonstrated. Activation of Akt and ERK1/2 were also upregulated upon GDF-15 binding to Erb2, an association that was confirmed by immunoprecipitation (Figure 1B) [27]. Whitson et al [28] identified yet another pathway, demonstrating a direct association of GDF-15 with connective tissue growth factor CCN2, confirmed via pulldown assays. The interaction of GDF-15 with CCN2 inhibits angiogenesis [28]. GDF-15 has been shown to be upregulated in several cancers, and it is possible that this prevention of blood vessel formation is the body’s attempt to fight tumor formation. Numerous studies have been performed identifying various signaling pathways of GDF-15, and it seems evident that GDF-15 follows many signaling patterns of TGF-β, but it may also have communication independent of the superfamily. Given the extensive potential of downstream activation, it seems reasonable that GDF-15 may have specific interactions in particular tissues and physiological environments, though future study on GDF-15 actions is still required.
Figure 1. Receptors binding GDF-15:
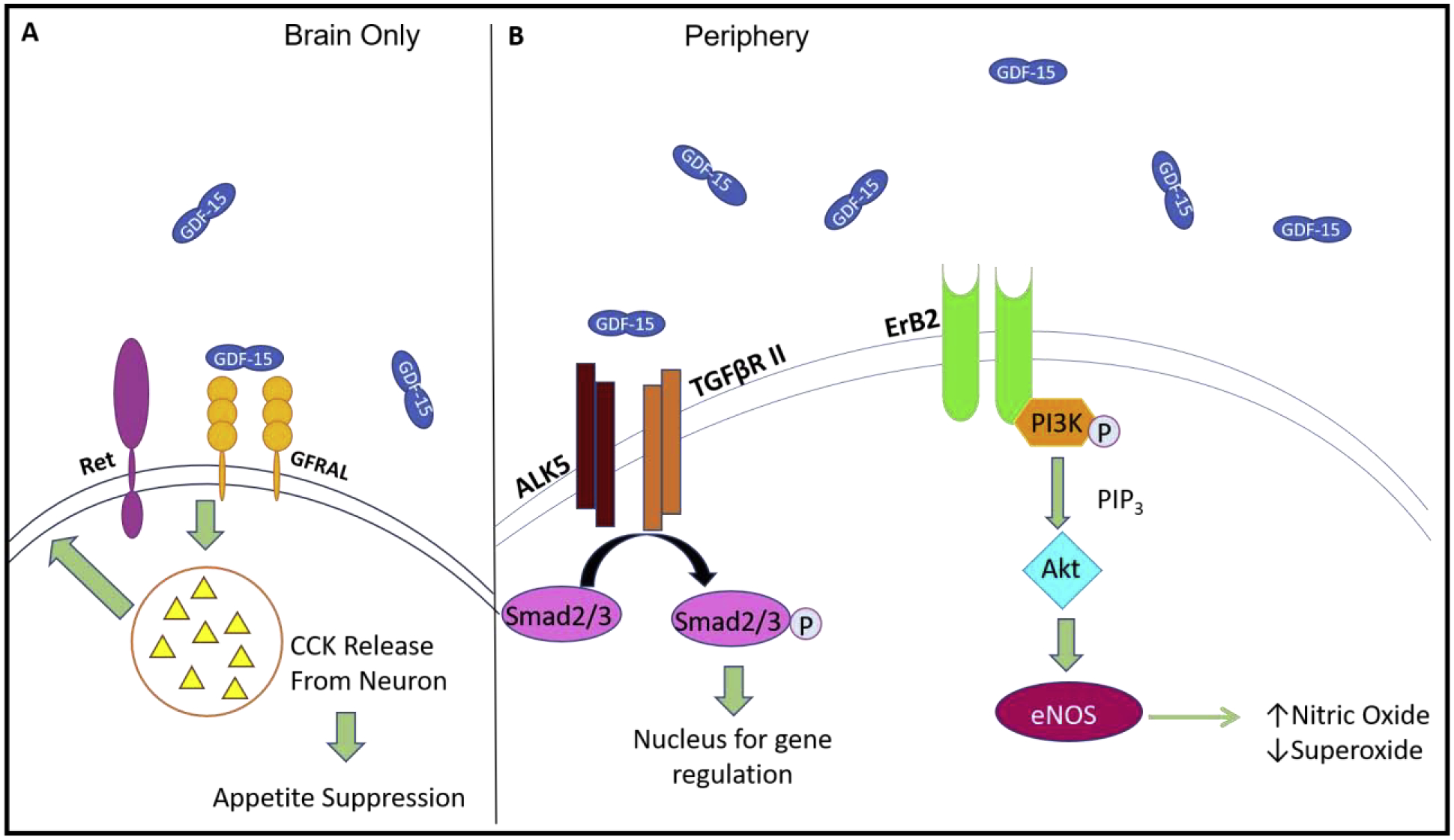
In the brain, GDF-15 binds GFRAL and interacts with the co-receptor Ret. Binding of GDF-15 to the receptor in the brain ultimately leads to release of neurotransmitter CCK, which works to suppress appetite (A). Throughout the rest of the body, GDF-15 is able to bind several other receptors including TGF-βRs and ALKs. Binding of these receptor combinations leads to activation of Smad2/3, ultimately leading to changes in gene expression (ie decrease in NF-κB expression). GDF-15 also binds endothelial receptor Erb2. Activation of Erb2 leads to activation of the PI3K and Akt pathways, ultimately resulting in upregulation of eNOS (B).
GDF-15 and Body Growth Regulation during Development
In the neonatal state, newborn babies are evaluated in numerous categories, one of which is their weight and size. This is because it has been well established that babies born premature or small for gestational age (SGA) are at an increased risk to develop metabolic disorders later in life [29]. Diaz et al[30] examined a cohort of infants which were either SGA or appropriate for gestational age (AGA). Parameters such as weight, length, GDF-15 and fasting glucose were recorded at various intervals until 24 months age. They found that SGA infants had significantly lower levels of circulating GDF-15 compared to AGA infants at birth. Though these levels dropped in both groups over time, the decrease was greater in the SGA group. The authors postulated that this decreased level of GDF-15 could be responsible for the “catch up” seen in the size and weight of SGA infants, and could lead to priming for obesity and metabolic disease later in life (Figure 2A) [30].
Figure 2. GDF-15 in Growth and Development:
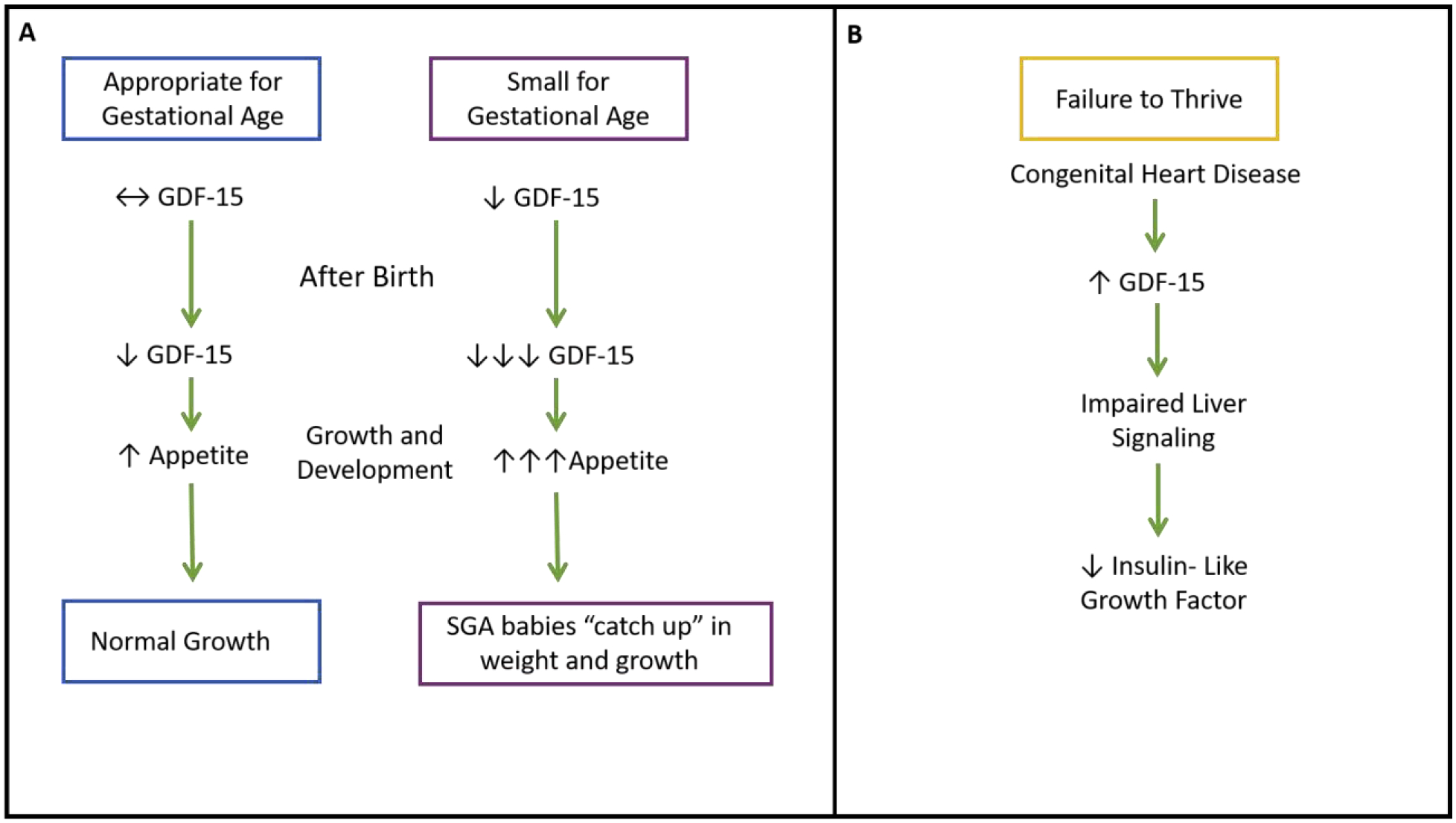
Babies born small for gestational age (SGA) have significantly less GDF-15. Like babies born at the appropriate weight, GDF-15 continues to drop during growth and development, but the drop is far greater in SGA infants. This is believed to contribute to SGA babies “catching up” in weight to the appropriate weight counterparts (A). Failure to thrive is a condition commonly caused by congenital heart defects. GDF-15 is increased here, leading to impaired liver signaling, and decreased insulin-like growth factor (B).
Another ailment common in children is known as failure to thrive (FTT). FTT refers to poor growth, typically measured by height and weight gain. Congenital heart disease has been established as one of the common causes of FTT. Using a mouse model of congenital heart disease-induced FTT, Wang et al established that GDF-15 is elevated in FTT and played a pivotal role in liver GH signaling. When GDF-15 was additionally knocked out from the mice, their liver signaling was restored, and IGF-1 significantly increased (Figure 2B) [31]. These studies suggest there is a delicate balance in the level of circulating GDF-15 that should be present during development. Elevated or diminished GDF-15 could have negative consequences during these important development years.
GDF-15, Obesity, and Diabetes
Linking GDF-15 to Appetite Through Cancer Studies
As mentioned above, GDF-15 has been linked to appetite suppression. This discovery first came about through studies investigating proteins that are elevated in cancer [32]. Expression of genes from 150 different cancers from 10 different anatomical regions were compared with 46 healthy tissues to look for patterns of gene upregulation for potential diagnostic purposes. 76 genes were found to be overexpressed in cancer tissue, several being known diagnostic markers such as kallikreins 6 and 10 in ovarian cancer. GDF-15 was upregulated in prostate, breast and colorectal carcinomas [32]. Additional studies showed that GDF-15 could be used as a marker for cancer progression and risk of mortality [33–35]. In late stage cancer, decreased appetite, weakness, and body wasting are common occurrences which may lead to death. Due to the significant increases in GDF-15 in cancer, and further increases in later stages, GDF-15 was studied for potential ability to effect appetite. In a study by Johnen et al [36], prostate cancer cells were transfected with either control vector or GDF-15 expressing vector. The cancer cells were then injected into nude mice which were monitored for six weeks. Mice which were overexpressing GDF-15 were shown to have decreased food consumption as well as a decrease in body weight compared to control animals [36].
Effects on Appetite and Weight
Similar to above, Macia et al [14] treated mice with control or GDF-15 overexpression vectors in the absence of cancer. Again, it was found that mice with increased GDF-15 expression had lower body weight, fat mass, and food intake compared to control. Additionally, the animals with increased GDF-15 were more sensitive to insulin compared to control animals. These same effects were observed even when the mice were given a high-fat diet [14]. This group continued their work to look at the effects of a germline knock out the expression of growth differentiation factor-15. Though there was no change in the lean mass of animals, knockout animals had significantly greater body weight and fat mass compared to controls. Interestingly, female knockout mice, but not males, had decreased food intake and had a decrease in metabolic activity compared to control counterparts, suggesting there are gender differences in the effects of GDF-15. The researchers also found that when GDF-15 was infused, body weight and food intake was reduced [37]. This demonstrated that the weight gain and diet changes were a direct result of impaired GDF-15 signaling.
Adipose Tissue Regulation
White adipose tissue is considered an endocrine organ due to its signaling in energy and metabolic homeostasis. Along with leptin and adiponectin, GDF-15 is also expressed in the adipose tissue and could be considered an adipokine. To examine determinants of GDF-15 expression and release from adipose, Ding and colleagues[38] exposed adipocytes to various conditions, including the satiety hormone leptin, hydrogen peroxide, and proinflammatory cytokine IL-1β. Exposure to hydrogen peroxide (reactive oxygen species) led to a significant increase in GDF-15 expression, while leptin and IL-1β stimuli caused significant decreases in GDF-15 expression. Interestingly, despite the ability of adipose to produce GDF-15, the group found that increasing body weight or fat mass was negatively correlated with growth differentiation factor-15 expression in adipocytes [38]. Because hyperleptinemia and leptin resistance are common in obesity [39], the increased circulating leptin could lead to decreased GDF-15 expression and release.
As mentioned above, adipose tissue has endocrine functions, which can contribute to glucose tolerance. Human and mouse adipose tissue contain an abundance of M1-like adipose tissue macrophages [40]. M1-like macrophages are considered pro-inflammatory, whereas M2-like macrophages are anti-inflammatory [41]. Obesity is associated with increased inflammation in adipose tissue [42]. Chronic adipose inflammation can be ameliorated by activation of the JAK-STAT6 pathway [43]. T-helper (Th)-2 cells secrete several cytokines in adipose tissue. Lee et al [44] showed that Th2 cytokines signal through the JAK-STAT6 pathway to induce GDF-15 expression. This is important because GDF-15 promotes the polarization of macrophages to the M2 phenotype in adipose [44]. Insulin resistance is associated with a decrease in M2 macrophages [45], and impaired oxidative metabolism favors the M1-like phenotype [40]. GDF-15 increases oxidative function in macrophages, and macrophage-specific GDF-15 knockout in mice led to significantly increased adipose tissue weight, blood glucose, and insulin resistance. Addition of recombinant GDF-15 was able to reverse the insulin resistance [40]. These studies suggest that GDF-15 is important to maintaining an increase in M2-like macrophage phenotype in adipose tissue, contributing to insulin sensitivity. A summary of these interactions can be seen in Figure 3.
Figure 3. GDF-15 in Diabetes:
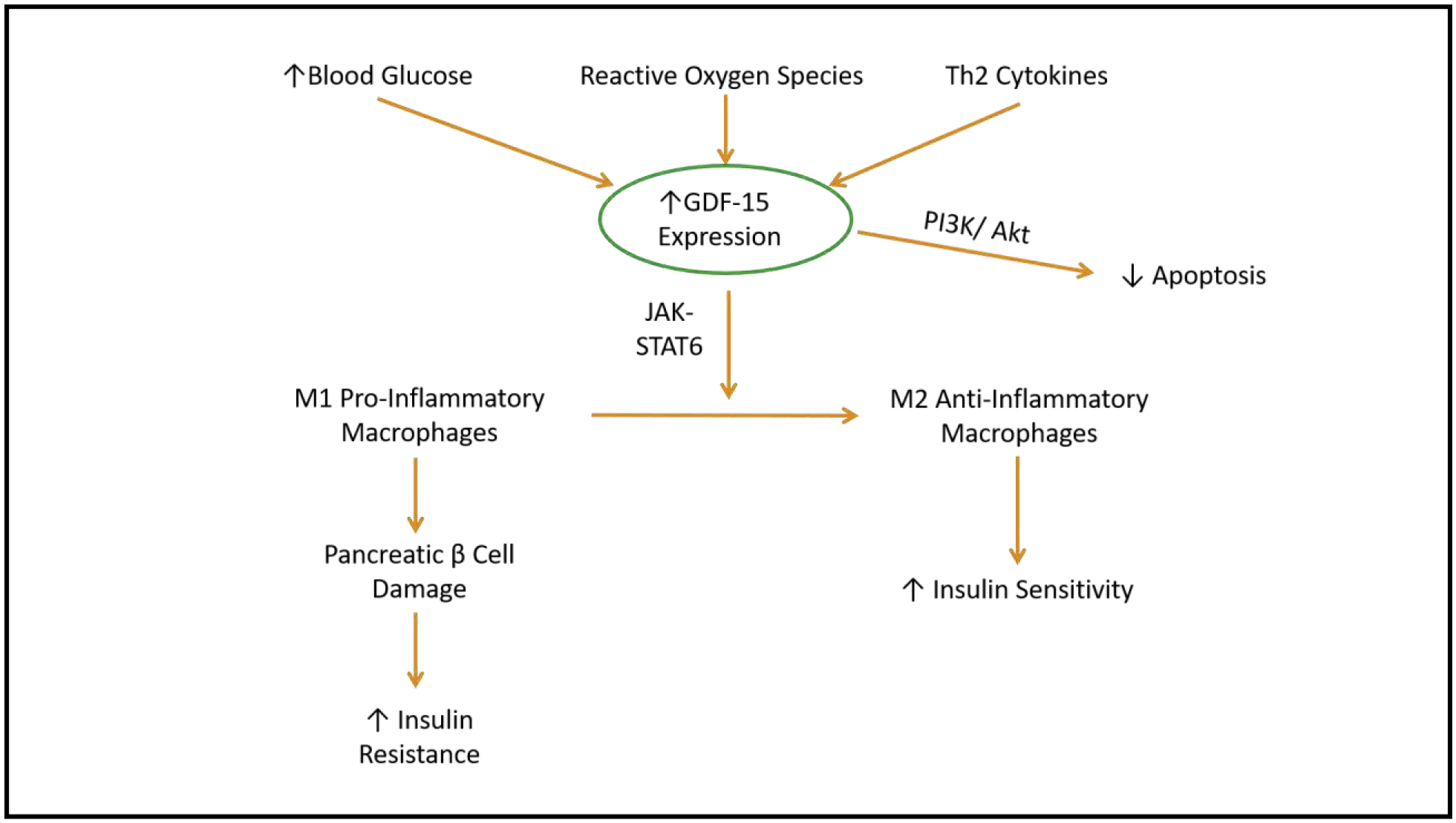
Several environments can lead to increased GDF-15 expression including increased blood glucose and reactive oxygen species, which are both common in pre-diabetes and type 2 diabetes mellitus. To combat these, GDF-15 activates the JAK/STAT6 pathway, promoting the shift in M1 pro-inflammatory macrophages to M2 anti-inflammatory macrophages. This shift helps to increase insulin sensitivity, while GDF-15 activation of PI3K/Akt pathway additionally acts to decrease apoptosis.
Patients with type 2 diabetes mellitus (T2DM) have been shown to have increased circulating levels of GDF-15. Despite the negative correlation discussed above, the circulating cytokine has been repeatedly shown to be positively correlated with body weight, fat mass, triglycerides, glucose, and HbA1c [46]. It is well established that the onset of diabetes is associated with inflammation. It has been shown that diabetics have an increase in resident macrophages associated with the islet cells of the pancreas [47]. Activated macrophages can secrete cytokines and chemokines which impair the function of pancreatic β cells, causing cellular dysfunction and decreased insulin sensitivity [48, 49]. Though GDF-15 could be detected prior to the onset of T2DM, some believe this is a response to the increased inflammation, thus acting as a response and not a cause. It is therefore not a sufficient independent predictor of type 2 diabetes [49]. However, it has been suggested to be a marker for those at risk for diabetes or obesity [26].
Because diabetes is a major risk factor for CVD, the effects of diabetes have been studied in order to better understand the development of CVD. Endothelial cells cultured in elevated glucose exhibit a significantly higher rate of apoptosis, but this is relieved through activation of PI3K/Akt pathways [50]. GDF-15 has been shown to be induced in endothelial cells exposed to high glucose, and is able to reduce apoptosis via induction of the PI3K/Akt pathway [25]. Therefore, increased GDF-15 appears to have a protective effect on the vasculature in maintaining endothelial cell health and function. Increased levels with severe disease appear to be the body’s attempt to mitigate further damage and potentially begin repairing. Because diabetes and obesity are both known to be conditions of increased inflammation, GDF-15 upregulation is to be expected to some degree. It is possible that the increase in GDF-15 due to the inflammation leads to a desensitization of receptor activity, accounting for lack of appetite suppression and increased insulin sensitivity in these conditions.
Weight Loss
Vila et al [51] performed a study in which they examined the association of GDF-15 with various factors before and after bariatric surgery in obese patients. Obese patients had significantly higher GDF-15 compared to non-obese, and GDF-15 was further increased in patients with diabetes. The level of GDF-15 was positively correlated with age, insulin levels, and the homeostasis model assessment (HOMA) insulin resistance index. One year following bariatric surgery, patients had a decrease in weight, fasting blood glucose, insulin levels, leptin, and improved HOMA insulin resistance index value. However, these post-surgery patients also had increased GDF-15. The researchers believed that GDF-15 and insulin resistance might be correlated despite the increase in GDF-15 after surgery because individuals with greater decreases in weight and improved insulin resistance had lesser increases in circulating GDF-15 [51]. Perhaps, like leptin and insulin, there is a point when the body becomes desensitized to the effects of GDF-15. Resistance to the effects of the cytokine would account for the disproportional results observed in patients post-bariatric surgery.
Due to its role in metabolism, GDF-15 was examined in exercise. Healthy, male individuals were examined prior to and after 60 minutes of exercise. Immediately following exercise, there was a significant increase in circulating GDF-15, which increased further two hours after exercise. This post-exercise increase in GDF-15 is believed to explain exercise-induced appetite suppression experienced by many individuals [52]. Zhang et al [53] performed a study in which they examined the effects of exercise on stable adults with obesity. Individuals underwent a 12-week study consisting of 60 minutes of exercise five days per week. In obese individuals, exercise training led to significant increases in circulating GDF-15 immediately following exercise. Interestingly, a subset of the cohort, termed “responders,” also had elevated resting GDF-15 levels after the study’s completion. The concentration of growth differentiation factor 15 was correlated to loss of abdominal fat and fat-free mass [53]. These studies show that GDF-15 might play a part in the alteration of metabolism that leads to exercise-induced weight loss.
GDF-15 and Cardiovascular Disease
Cardiovascular disease (CVD), which includes hypertension, atherosclerosis, heart failure, coronary artery disease (CAD), myocardial infarction (MI), or stroke, is the leading cause of death worldwide [54]. Though not expressed under normal conditions, GDF-15 has been found to play a protective role in the heart in CVD, as described in Figure 4A [26, 55].
Figure 4. Cardiovascular Disease and GDF-15:
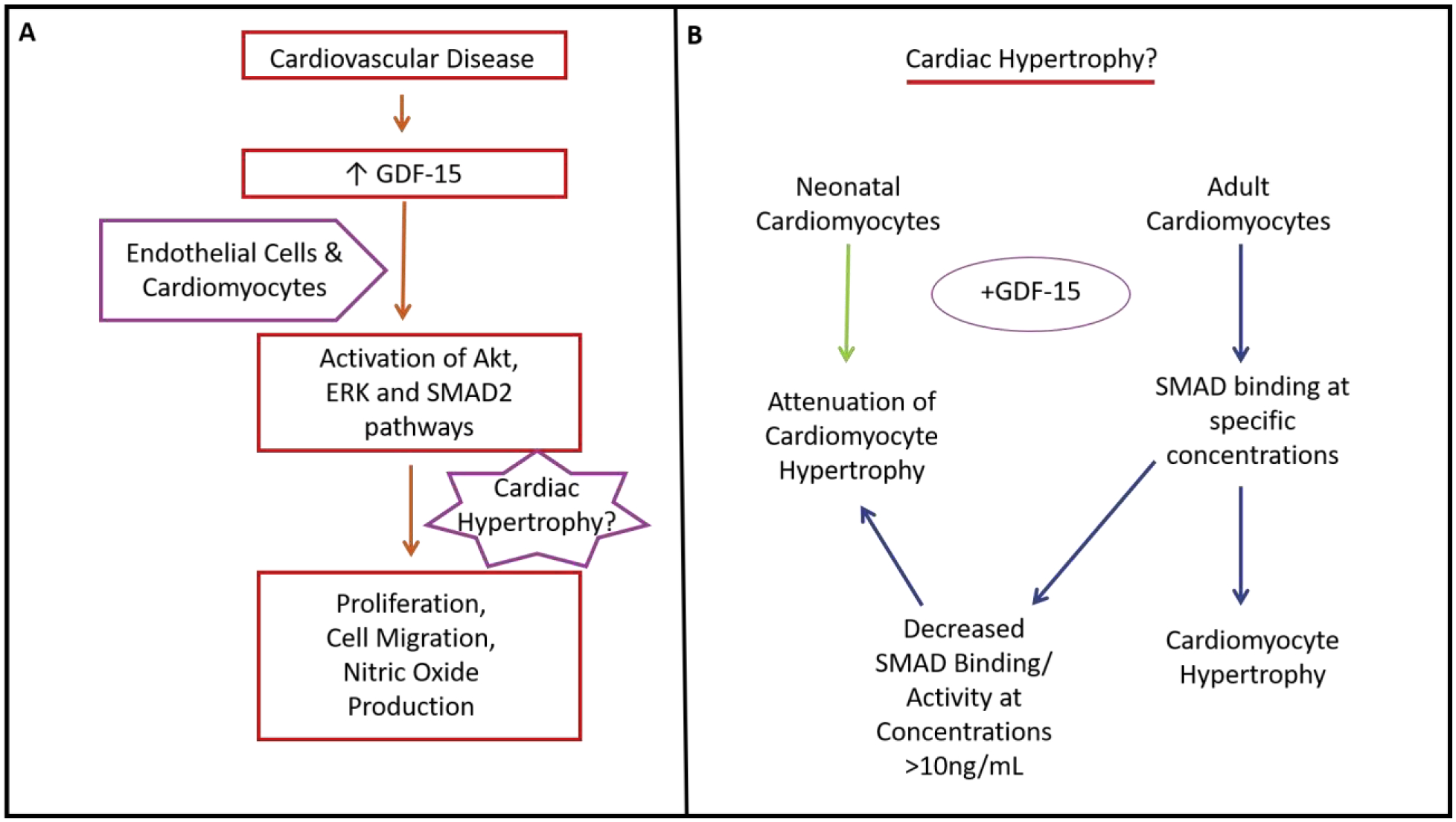
GDF-15 is increased in cardiovascular disease, leading to activation of Akt, ERK, and SMAD2 pathways in both endothelial cells and cardiomyocytes. These pathways promote cell migration, nitric oxide production, cell proliferation and possibly cardiac hypertrophy (A). Using cardiomyocytes from either neonates or adults, addition of GDF-15 can either cause attenuation of cardiomyocyte hypertrophy or promotion of cardiac hypertrophy by SMAD binding, respectively (B).
GDF-15 and Endothelial Dysfunction
One of the hallmark features of cardiovascular disease is the development of endothelial dysfunction. Ha et al [56] demonstrated that senescent endothelial cells express significantly more GDF-15 compared to non-senescent cells. Additionally, the group showed that GDF-15 had a paracrine effect on non-senescent cells to promote proliferation, migration, and nitric oxide production through activation of Akt, ERK, and SMAD2 pathways [56]. Peripheral artery disease (PAD) is caused by endothelial dysfunction and atherosclerosis. In patients with PAD, high serum GDF-15 levels are associated with increased risk of limb amputation or death due to limb ischemia [57].
GDF-15 in MI and CAD
There have been several studies which demonstrate a correlation between circulating GDF-15 and risk of recurring MI or death. Khan et al. used the Killip classification system to show that increased serum GDF-15 was associated with increased risk [58]. The Killip classification is a means of categorizing patients in various stages of CVD with risk of death (class 1 being lowest risk and class 4 being the highest risk) [59]. Additionally, the group found that analysis of N-terminal pro-B-type natriuretic peptide (NT-proBNP) in conjunction with GDF-15 gave a more accurate prediction of outcomes. Kaplan-Meier survival curves were used to demonstrate how these factors impacted the survival of the patients during the 7.75-year follow-up period. Patients in the lowest risk group, having below median levels of both GDF-15 and NT-proBNP, had approximately 90% survival. Intermediate risk groups, having above median levels of either GDF-15 or NT-proBNP, had approximately 50% survival during the follow up time period. The highest risk group, having above median levels of both GDP-15 and NT-proBNP, had less than 10% event-free survival during this time period[58]. Furthermore, a study by Schaub and colleagues [60] showed that patients suffering from acute MI had significantly higher GDF-15 than patients who were admitted to the hospital for other reasons[60]. Bonaca et al [61] conducted a study in which patients with acute coronary syndrome were examined, treated with statin, and evaluated for 2-year risk. After adjusting for factors such as age, sex, BMI, diabetes, and hypertension, increased GDF-15 was associated with nearly three times increased risk of death and more than three times increased of developing congestive heart failure [61].
Despite the correlation between GDF-15 and negative outcomes with MI, the cytokine could have a protective role. Kempf and colleagues [55] showed that cardiomyocytes subjected to ischemia/reperfusion (I/R) in vitro significantly increased their expression and excretion of GDF-15. A similar trend was observed when mice underwent permanent or transient coronary ligation. Cardiomyocytes in the infarcted area of the murine hearts were shown to have significantly greater expression of GDF-15 compared to sham or non-affected area. Further experiments showed that GDF-15 knockout mice had significantly greater infarct sizes compared to wildtype littermates[55]. Because TGF-β [62] and several other signaling factors [63] have been shown to be cardioprotective via activation of the ERK and PI3K/Akt pathways in I/R, GDF-15 was studied for similar effects. It was determined that GDF-15 acts through the PI3K/Akt pathway in I/R in order to mitigate the infarct size and damage [55].
In addition to patients suffering from MI, individuals with coronary artery disease have been shown to have significantly higher circulating GDF-15 compared to healthy counterparts [64]. Others have also shown that increased GDF-15 positively correlated with CAD and its severity by Gensini score [65] (the Gensini score is a measure of CAD severity, where a higher score indicates worsening disease [66]). Though it seems evident that growth differentiation factor-15 may be used as a biomarker for cardiovascular disease and severity, it remains unclear if there is any therapeutic potential in this pathway.
Hypertrophy and Heart Disease
Given its name, it may not come as a surprise that growth differentiation factor-15 has been implicated in hypertrophy. Numerous studies have found that GDF-15 is increased in cardiac hypertrophy. Kou and colleagues showed that patients with left ventricular hypertrophy had elevated GDF-15 compared to healthy individuals [67]. Similarly, Xue et al found that patients in China with hypertension and left ventricular hypertrophy had significantly greater levels of serum GDF-15 compared to individuals with hypertension without hypertrophy [68]. Patients with hypertrophic cardiomyopathy had increasing levels of GDF-15 with disease severity [69]. Additionally, Izumiya et al showed that patients with heart failure with preserved ejection fraction (HFpEF) had significantly greater serum GDF-15 compared to patients with heart failure with reduced ejection fraction (HFrEF). This is significant because left ventricular hypertrophy is characteristic of HFpEF due to the hypertension that is common in the condition [70]. Though these studies are interesting and point to an association between growth differentiation factor-15 and cardiac hypertrophy, they do not give a mechanism by which GDF-15 might be acting in these circumstances.
There have been only a few studies which examine the direct action of GDF-15 in hypertrophy, and the data from these have been conflicting. In a study examining the role of GDF-15 in isolated cardiomyocytes, GDF-15 was shown to attenuate hypertrophy [26]. Similarly, GDF-15 was shown to have protective effects against norepinephrine (NE)-induced hypertrophy in isolated cardiomyocytes [71]. However, it is important to note that both groups used cardiomyocytes from neonates, which may have differing signaling pathways compared to the terminally differentiated adult cardiomyocyte. Additionally, Xu et al demonstrated that NE treatment to cells lead to significant release of GDF-15 [71]. It is possible that when both NE and GDF-15 were added to the cultured cells, there was negative feedback signaling due to the substantial increase in GDF-15. The two groups also examined increased GDF-15 signaling in vivo. The transverse aortic constriction (TAC) procedure was used to induce pressure overloading in normal and transgenic mice. Though the group concluded that GDF-15 was protective against hypertrophy, they failed to adequately demonstrate GDF-15 overexpression in the hearts of their transgenic mice. Furthermore, GDF-15−/− mice exhibited similar phenotypes in cardiomyocyte size, skeletal muscle actin expression and B-type natriuretic peptide expression compared to mice with GDF-15 overexpression [26].
In contrast, Heger and colleagues [72] examined isolated adult cardiomyocytes exposed to GDF-15. They found that GDF-15 led to significant increase in cell size and rate of protein synthesis compared to control untreated cells. They predicted that this signaling was through proteins known as SMADs (small mothers against decapentaplegic), which is the primary signaling pathway of TGF-β superfamily members. When subjecting the cardiomyocytes to various concentrations of GDF-15, they found significantly increased binding activity of SMADs with 3ng/mL of GDF, but this activity was reduced when the concentration was increased to 10 ng/mL [72]. This study supports the idea that GDF-15 might be involved in cardiac hypertrophy in adults, and that when GDF-15 levels become too great, the hypertrophic effect might be lost (Figure 4B).
When a patient reaches end-stage heart failure, the standard treatment is to perform a heart transplant. It has been shown that GDF-15 protects transplanted hearts from rejection by inhibiting the pro-inflammatory nuclear factor- κB (NF-κB) [22]. As mentioned above, GDF-15 protects endothelial cells in the beginning stages of cardiovascular disease, as well as cardiomyocytes in the event of myocardial infarction. It is evident that GDF-15 is significantly elevated in cardiovascular disease. Though the role of GDF-15 in the development of cardiac hypertrophy remains unclear, growth differentiation factor-15 appears to be overall protective in the cardiovascular system.
Future Perspectives and Conclusions
Growth differentiation factor-15 is involved in many processes, from cellular respiration to appetite control, as well as several others not discussed in this review. It is clear that GDF-15 offers beneficial effects to manage metabolism and insulin sensitivity through its binding and activation of GFRAL. However, the data as to the mechanism in which GDF-15 acts in other environments remains controversial. While it appears that GDF-15 acts as a compensatory agent in diabetes and CVD, its role as a potential driver of these pathologies cannot be ruled out. Due to these unknowns, future studies need to be performed to examine the role of GDF-15 as a biomarker or therapeutic target for various ailments.
Highlights.
Growth differentiation factor 15 is cytokine that signals in many physiological pathways.
In healthy conditions, GDF-15 is able to decrease appetite, decrease inflammation, and increase sensitivity to insulin.
GDF-15 is upregulated in several conditions including obesity, diabetes, and inflammation.
GDF-15 receptors may develop resistance, accounting for the dramatic increase in circulating GDF-15 levels during disease.
Conflicting evidence suggests that GDF-15 participates in the structural remodeling of the cardiovascular system.
Acknowledgements
This work was supported in part by the U.S. National Institutes of Health (T32 HL098039 to ACE; R00 HL116769, R21 EB026518, and S10 OD023438 to AJT) and The Research Institute at Nationwide Children’s Hospital (to AJT).
Biographies
Author Biography

Adrian Eddy, PhD
Dr. Eddy completed her PhD in Physiology and Biophysics at the University of Mississippi Medical Center in Jackson, MS. Her dissertation focused on the pregnancy disorder preeclampsia, novel therapies, and potential sources of the pathogenic sFlt-1 protein. Upon completion of her PhD, Dr. Eddy joined the Trask Lab at Nationwide Children’s Hospital as a postdoctoral fellow.

Aaron J. Trask, PhD, FAHA, FCVS
Dr. Trask earned his PhD in Physiology and Pharmacology from Wake Forest University. Dr. Trask then joined Nationwide Children’s Hospital as a postdoctoral fellow in the Center for Cardiovascular Research before opening his own laboratory in the same center. His laboratory studies coronary microvascular disease in type 2 diabetes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None.
Literature Cited
- 1.Lawton LN, Bonaldo MF, Jelenc PC, Qiu L, Baumes SA, Marcelino RA, de Jesus GM, Wellington S, Knowles JA, Warburton D et al. : Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene 1997, 203(1):17–26. [DOI] [PubMed] [Google Scholar]
- 2.Hromas R, Hufford M, Sutton J, Xu D, Li Y, Lu L: PLAB, a novel placental bone morphogenetic protein. Biochimica et biophysica acta 1997, 1354(1):40–44. [DOI] [PubMed] [Google Scholar]
- 3.Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K et al. : MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proceedings of the National Academy of Sciences of the United States of America 1997, 94(21):11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek SJ, Kim KS, Nixon JB, Wilson LC, Eling TE: Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Molecular pharmacology 2001, 59(4):901–908. [PubMed] [Google Scholar]
- 5.Böttner M, Suter-Crazzolara C, Schober A, Unsicker K: Expression of a novel member of the TGF-beta superfamily, growth/differentiation factor-15/macrophage-inhibiting cytokine-1 (GDF-15/MIC-1) in adult rat tissues. Cell and tissue research 1999, 297(1):103–110. [DOI] [PubMed] [Google Scholar]
- 6.Buendgens L, Yagmur E, Bruensing J, Herbers U, Baeck C, Trautwein C, Koch A, Tacke F: Growth Differentiation Factor-15 Is a Predictor of Mortality in Critically Ill Patients with Sepsis. Disease markers 2017, 2017:5271203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luan HH, Wang A, Hilliard BK, Carvalho F, Rosen CE, Ahasic AM, Herzog EL, Kang I, Pisani MA, Yu S et al. : GDF15 Is an Inflammation-Induced Central Mediator of Tissue Tolerance. Cell 2019, 178(5):1231–1244.e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi HS, Kang SG, Choi MJ, Lee SE, Jung SB et al. : Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J Cell Biol 2017, 216(1):149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R et al. : Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America 2003, 100(14):8466–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FG, Goodpaster BH, Ruderman NB, Kelley DE: Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. American journal of physiology Endocrinology and metabolism 2010, 298(1):E49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI: Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003, 300(5622):1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng J, Bussière F, Hekimi S: Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Developmental cell 2001, 1(5):633–644. [DOI] [PubMed] [Google Scholar]
- 13.Owusu-Ansah E, Song W, Perrimon N: Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 2013, 155(3):699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macia L, Tsai VW, Nguyen AD, Johnen H, Kuffner T, Shi YC, Lin S, Herzog H, Brown DA, Breit SN et al. : Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One 2012, 7(4):e34868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjær SB, Wu X, Huang T, Hultman K, Paulsen SJ et al. : GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med 2017, 23(10):1158–1166. [DOI] [PubMed] [Google Scholar]
- 16.Tsai VW, Manandhar R, Jørgensen SB, Lee-Ng KK, Zhang HP, Marquis CP, Jiang L, Husaini Y, Lin S, Sainsbury A et al. : The anorectic actions of the TGFβ cytokine MIC-1/GDF15 require an intact brainstem area postrema and nucleus of the solitary tract. PLoS One 2014, 9(6):e100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK et al. : The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med 2017, 23(10):1215–1219. [DOI] [PubMed] [Google Scholar]
- 18.Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD et al. : GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med 2017, 23(10):1150–1157. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JY, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D et al. : Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550(7675):255–259. [DOI] [PubMed] [Google Scholar]
- 20.Breit SN, Tsai VW, Brown DA: Targeting Obesity and Cachexia: Identification of the GFRAL Receptor-MIC-1/GDF15 Pathway. Trends in molecular medicine 2017, 23(12):1065–1067. [DOI] [PubMed] [Google Scholar]
- 21.Artz A, Butz S, Vestweber D: GDF-15 inhibits integrin activation and mouse neutrophil recruitment through the ALK-5/TGF-βRII heterodimer. Blood 2016, 128(4):529–541. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Zhang G, Liu Y, Chen R, Zhao D, McAlister V, Mele T, Liu K, Zheng X: GDF15 Regulates Malat-1 Circular RNA and Inactivates NFκB Signaling Leading to Immune Tolerogenic DCs for Preventing Alloimmune Rejection in Heart Transplantation. Frontiers in immunology 2018, 9:2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worth AA, Shoop R, Tye K, Feetham CH, D’Agostino G, Dodd GT, Reimann F, Gribble FM, Beebe EC, Dunbar JD et al. : The cytokine GDF15 signals through a population of brainstem cholecystokinin neurons to mediate anorectic signalling. eLife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KK, Lee JJ, Yang Y, You KH, Lee JH: Macrophage inhibitory cytokine-1 activates AKT and ERK-1/2 via the transactivation of ErbB2 in human breast and gastric cancer cells. Carcinogenesis 2008, 29(4):704–712. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Yang L, Qin W, Zhang G, Yuan J, Wang F: Adaptive induction of growth differentiation factor 15 attenuates endothelial cell apoptosis in response to high glucose stimulus. PLoS One 2013, 8(6):e65549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD: GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res 2006, 98(3):342–350. [DOI] [PubMed] [Google Scholar]
- 27.Li S, Ma YM, Zheng PS, Zhang P: GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. J Exp Clin Cancer Res 2018, 37(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitson RJ, Lucia MS, Lambert JR: Growth differentiation factor-15 (GDF-15) suppresses in vitro angiogenesis through a novel interaction with connective tissue growth factor (CCN2). Journal of cellular biochemistry 2013, 114(6):1424–1433. [DOI] [PubMed] [Google Scholar]
- 29.Mericq V, Martinez-Aguayo A, Uauy R, Iñiguez G, Van der Steen M, Hokken-Koelega A: Long-term metabolic risk among children born premature or small for gestational age. Nature reviews Endocrinology 2017, 13(1):50–62. [DOI] [PubMed] [Google Scholar]
- 30.Díaz M, Campderrós L, Guimaraes MP, López-Bermejo A, de Zegher F, Villarroya F, Ibáñez L: Circulating growth-and-differentiation factor-15 in early life: relation to prenatal and postnatal growth and adiposity measurements. Pediatric research 2020, 87(5):897–902. [DOI] [PubMed] [Google Scholar]
- 31.Wang T, Liu J, McDonald C, Lupino K, Zhai X, Wilkins BJ, Hakonarson H, Pei L: GDF15 is a heart-derived hormone that regulates body growth. EMBO molecular medicine 2017, 9(8):1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ et al. : Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proceedings of the National Academy of Sciences of the United States of America 2003, 100(6):3410–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown DA, Hance KW, Rogers CJ, Sansbury LB, Albert PS, Murphy G, Laiyemo AO, Wang Z, Cross AJ, Schatzkin A et al. : Serum macrophage inhibitory cytokine-1 (MIC-1/GDF15): a potential screening tool for the prevention of colon cancer? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2012, 21(2):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koopmann J, Buckhaults P, Brown DA, Zahurak ML, Sato N, Fukushima N, Sokoll LJ, Chan DW, Yeo CJ, Hruban RH et al. : Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clinical cancer research : an official journal of the American Association for Cancer Research 2004, 10(7):2386–2392. [DOI] [PubMed] [Google Scholar]
- 35.Brown DA, Stephan C, Ward RL, Law M, Hunter M, Bauskin AR, Amin J, Jung K, Diamandis EP, Hampton GM et al. : Measurement of serum levels of macrophage inhibitory cytokine 1 combined with prostate-specific antigen improves prostate cancer diagnosis. Clinical cancer research : an official journal of the American Association for Cancer Research 2006, 12(1):89–96. [DOI] [PubMed] [Google Scholar]
- 36.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S et al. : Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med 2007, 13(11):1333–1340. [DOI] [PubMed] [Google Scholar]
- 37.Tsai VW, Macia L, Johnen H, Kuffner T, Manadhar R, Jørgensen SB, Lee-Ng KK, Zhang HP, Wu L, Marquis CP et al. : TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One 2013, 8(2):e55174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Q, Mracek T, Gonzalez-Muniesa P, Kos K, Wilding J, Trayhurn P, Bing C: Identification of macrophage inhibitory cytokine-1 in adipose tissue and its secretion as an adipokine by human adipocytes. Endocrinology 2009, 150(4):1688–1696. [DOI] [PubMed] [Google Scholar]
- 39.Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF: Leptin resistance in obesity: An epigenetic landscape. Life sciences 2015, 140:57–63. [DOI] [PubMed] [Google Scholar]
- 40.Jung SB, Choi MJ, Ryu D, Yi HS, Lee SE, Chang JY, Chung HK, Kim YK, Kang SG, Lee JH et al. : Reduced oxidative capacity in macrophages results in systemic insulin resistance. Nature communications 2018, 9(1):1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porcheray F, Viaud S, Rimaniol AC, Léone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G: Macrophage activation switching: an asset for the resolution of inflammation. Clinical and experimental immunology 2005, 142(3):481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berg AH, Lin Y, Lisanti MP, Scherer PE: Adipocyte differentiation induces dynamic changes in NF-kappaB expression and activity. American journal of physiology Endocrinology and metabolism 2004, 287(6):E1178–1188. [DOI] [PubMed] [Google Scholar]
- 43.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, Mukundan L, Wu D, Locksley RM, Chawla A: IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America 2010, 107(52):22617–22622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SE, Kang SG, Choi MJ, Jung SB, Ryu MJ, Chung HK, Chang JY, Kim YK, Lee JH, Kim KS et al. : Growth Differentiation Factor 15 Mediates Systemic Glucose Regulatory Action of T-Helper Type 2 Cytokines. Diabetes 2017, 66(11):2774–2788. [DOI] [PubMed] [Google Scholar]
- 45.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A: Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell metabolism 2006, 4(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dostalova I, Roubicek T, Bartlova M, Mraz M, Lacinova Z, Haluzikova D, Kavalkova P, Matoulek M, Kasalicky M, Haluzik M: Increased serum concentrations of macrophage inhibitory cytokine-1 in patients with obesity and type 2 diabetes mellitus: the influence of very low calorie diet. European journal of endocrinology 2009, 161(3):397–404. [DOI] [PubMed] [Google Scholar]
- 47.Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G et al. : Increased number of islet-associated macrophages in type 2 diabetes. Diabetes 2007, 56(9):2356–2370. [DOI] [PubMed] [Google Scholar]
- 48.Kolb H, Mandrup-Poulsen T: An immune origin of type 2 diabetes? Diabetologia 2005, 48(6):1038–1050. [DOI] [PubMed] [Google Scholar]
- 49.Carstensen M, Herder C, Brunner EJ, Strassburger K, Tabak AG, Roden M, Witte DR: Macrophage inhibitory cytokine-1 is increased in individuals before type 2 diabetes diagnosis but is not an independent predictor of type 2 diabetes: the Whitehall II study. European journal of endocrinology 2010, 162(5):913–917. [DOI] [PubMed] [Google Scholar]
- 50.Ho FM, Lin WW, Chen BC, Chao CM, Yang CR, Lin LY, Lai CC, Liu SH, Liau CS: High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cellular signalling 2006, 18(3):391–399. [DOI] [PubMed] [Google Scholar]
- 51.Vila G, Riedl M, Anderwald C, Resl M, Handisurya A, Clodi M, Prager G, Ludvik B, Krebs M, Luger A: The relationship between insulin resistance and the cardiovascular biomarker growth differentiation factor-15 in obese patients. Clinical chemistry 2011, 57(2):309–316. [DOI] [PubMed] [Google Scholar]
- 52.Kleinert M, Clemmensen C, Sjøberg KA, Carl CS, Jeppesen JF, Wojtaszewski JFP, Kiens B, Richter EA: Exercise increases circulating GDF15 in humans. Molecular metabolism 2018, 9:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Fealy CE, Kirwan JP: Exercise training promotes a GDF15-associated reduction in fat mass in older adults with obesity. American journal of physiology Endocrinology and metabolism 2019, 316(5):E829–e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Organization WH: World health statistics 2015: World Health Organization; 2015.
- 55.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD et al. : The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res 2006, 98(3):351–360. [DOI] [PubMed] [Google Scholar]
- 56.Ha G, De Torres F, Arouche N, Benzoubir N, Ferratge S, Hatem E, Anginot A, Uzan G: GDF15 secreted by senescent endothelial cells improves vascular progenitor cell functions. PLoS One 2019, 14(5):e0216602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Haan JJ, Haitjema S, den Ruijter HM, Pasterkamp G, de Borst GJ, Teraa M, Verhaar MC, Gremmels H, de Jager SCA: Growth Differentiation Factor 15 Is Associated With Major Amputation and Mortality in Patients With Peripheral Artery Disease. Journal of the American Heart Association 2017, 6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, Davies JE, Ng LL: Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. European heart journal 2009, 30(9):1057–1065. [DOI] [PubMed] [Google Scholar]
- 59.Werns SW, Bates ER: The enduring value of Killip classification. American heart journal 1999, 137(2):213–215. [DOI] [PubMed] [Google Scholar]
- 60.Schaub N, Reichlin T, Twerenbold R, Reiter M, Steuer S, Bassetti S, Stelzig C, Wolf C, Winkler K, Haaf P et al. : Growth differentiation factor-15 in the early diagnosis and risk stratification of patients with acute chest pain. Clinical chemistry 2012, 58(2):441–449. [DOI] [PubMed] [Google Scholar]
- 61.Bonaca MP, Morrow DA, Braunwald E, Cannon CP, Jiang S, Breher S, Sabatine MS, Kempf T, Wallentin L, Wollert KC: Growth differentiation factor-15 and risk of recurrent events in patients stabilized after acute coronary syndrome: observations from PROVE IT-TIMI 22. Arterioscler Thromb Vasc Biol 2011, 31(1):203–210. [DOI] [PubMed] [Google Scholar]
- 62.Lefer AM, Tsao P, Aoki N, Palladino MA Jr., : Mediation of cardioprotection by transforming growth factor-beta. Science 1990, 249(4964):61–64. [DOI] [PubMed] [Google Scholar]
- 63.Hausenloy DJ, Yellon DM: New directions for protecting the heart against ischaemia-reperfusion injury: targeting the Reperfusion Injury Salvage Kinase (RISK)-pathway. Cardiovasc Res 2004, 61(3):448–460. [DOI] [PubMed] [Google Scholar]
- 64.Wang X, Chen LL, Zhang Q: Increased Serum Level of Growth Differentiation Factor 15 (GDF-15) is Associated with Coronary Artery Disease. Cardiovascular therapeutics 2016, 34(3):138–143. [DOI] [PubMed] [Google Scholar]
- 65.Liu H, Lyu YN, Li D, Cui Y, Dai W, Li Y: Association of circulating growth differentiation factor-15, Krüppel-like factor 4 and growth arrest-specific 6 with coronary artery disease. Clin Chim Acta 2019, 495:630–636. [DOI] [PubMed] [Google Scholar]
- 66.Rampidis GP, Benetos G, Benz DC, Giannopoulos AA, Buechel RR: A guide for Gensini Score calculation. Atherosclerosis 2019, 287:181–183. [DOI] [PubMed] [Google Scholar]
- 67.Kou H, Jin X, Gao D, Ma R, Dong X, Wei J, Wang X: Association between growth differentiation factor 15 and left ventricular hypertrophy in hypertensive patients and healthy adults. Clinical and experimental hypertension (New York, NY : 1993) 2018, 40(1):8–15. [DOI] [PubMed] [Google Scholar]
- 68.Xue H, Fu Z, Chen Y, Xing Y, Liu J, Zhu H, Zhou X: The association of growth differentiation factor-15 with left ventricular hypertrophy in hypertensive patients. PLoS One 2012, 7(10):e46534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montoro-García S, Hernández-Romero D, Jover E, García-Honrubia A, Vilchez JA, Casas T, Martínez P, Climent V, Caballero L, Valdés M et al. : Growth differentiation factor-15, a novel biomarker related with disease severity in patients with hypertrophic cardiomyopathy. European journal of internal medicine 2012, 23(2):169–174. [DOI] [PubMed] [Google Scholar]
- 70.Izumiya Y, Hanatani S, Kimura Y, Takashio S, Yamamoto E, Kusaka H, Tokitsu T, Rokutanda T, Araki S, Tsujita K et al. : Growth differentiation factor-15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. The Canadian journal of cardiology 2014, 30(3):338–344. [DOI] [PubMed] [Google Scholar]
- 71.Xu XY, Nie Y, Wang FF, Bai Y, Lv ZZ, Zhang YY, Li ZJ, Gao W: Growth differentiation factor (GDF)-15 blocks norepinephrine-induced myocardial hypertrophy via a novel pathway involving inhibition of epidermal growth factor receptor transactivation. J Biol Chem 2014, 289(14):10084–10094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heger J, Schiegnitz E, von Waldthausen D, Anwar MM, Piper HM, Euler G: Growth differentiation factor 15 acts anti-apoptotic and pro-hypertrophic in adult cardiomyocytes. Journal of cellular physiology 2010, 224(1):120–126. [DOI] [PubMed] [Google Scholar]


