Abstract
Cutaneous T cell lymphomas (CTCLs) are malignancies of skin-trafficking T cells. Patients with advanced CTCL manifest immune dysfunction that predisposes to infection and suppresses the anti-tumor immune response. Therapies that stimulate immunity have produced superior progression free survival compared to conventional chemotherapy, reinforcing the importance of addressing the immune deficient state in the care of CTCL patients. Recent research has better defined the pathogenesis of these immune deficits, explaining the mechanisms of disease progression and revealing potential therapeutic targets. The features of the malignant cell in mycosis fungoides (MF) and Sézary syndrome (SS) are now significantly better understood, including TH2 phenotype, Treg cytokine production, immune checkpoint molecule expression, chemokine receptors, and interactions with the microenvironment. The updated model of CTCL immunopathogenesis provides understanding into clinical progression and therapeutic response.
Keywords: cutaneous T cell lymphoma, CTCL, mycosis fungoides, Sezary syndrome, dermatologic oncology, immunotherapy, immune deficiency, immunopathogenesis, drug response
Introduction
The cutaneous T cell lymphomas (CTCLs) are a heterogenous group of T cell malignancies.1 The best studied and most common subtypes are mycosis fungoides (MF) and Sézary syndrome (SS), which together account for approximately 60% of cases.2 With advancing stage, patients with MF or SS typically face worsening immune dysfunction. The state of immune disorder in these patients impacts their risk of serious infections, the robustness of the anti-tumor immune response, and the efficacy of therapies that rely on immune stimulation.3,4
Therapies that stimulate immunity have long been a mainstay in CTCL management. Indeed, for advanced disease, immune targeted treatments have been associated with longer median progression free survival than traditional chemotherapy.5 While management of advanced stage CTCL is complex, the use of multimodality therapy is common, including some combination of interferons, extracorporeal photopheresis (ECP), monoclonal antibodies (mAbs), oral retinoids, histone deacetylase (HDAC) inhibitors, and total skin electron beam (TSEB) radiation.4 A newer generation of therapies, including immune checkpoint inhibitors, Toll-like receptor (TLR) agonists, and chimeric antigen receptor (CAR) T cells, are in development and demonstrate the enthusiasm around immune-stimulating strategies.
Recent research has more thoroughly characterized the cellular biology of immune suppression in CTCL. The nature of the malignant cell in MF and SS, as typically being mature CD4+ T cells with TH2 phenotype, has been known since the 1990s and explains defects in TH1 immunity, dendritic cell (DC) function, and cytokine production in CTCL patients.6 However, the genetic basis for the TH2 bias, etiology of tropism to the skin, pathogenesis of symptoms including pruritus, and tumorigenic influence of the microenvironment are now substantially better understood and provide intriguing targets for intervention.
In this review, we characterize the immune dysfunction in CTCL and describe the recent advances in our understanding of its pathogenesis. Particular attention is devoted to MF and SS, which are by far the best studied subtypes. Finally, part II considers emerging CTCL immunotherapies, which in the future may help to satisfy the significant remaining need for tolerable and effective treatments for advanced disease.
Immune dysfunction in CTCL
The influence of infection, the microbiome, and comorbidities
In MF and SS, the risk of infection is high, corresponds to disease stage, and in some estimates contributes more to mortality than the malignancy per se.3,7 In the early 1990s, a large retrospective cohort study found that staphylococcal, streptococcal, and herpetic skin infections, in decreasing order, are the most common in MF and SS.7 While many cutaneous infections are treated successfully without hospital admission, some patients develop bacteremia or pneumonia with a high risk of mortality.7 At a subclinical level, patients with MF and SS, especially the latter, also display a higher incidence of S. aureus colonization, particularly in lesional tissue.8 This finding is reminiscent of the increased S. aureus colonization in atopic dermatitis, which shares with MF and SS the impaired induction of anti-microbial peptides, including cathelicidins and β-defensins, in affected skin.9 Oral vitamin D administration can upregulate cathelicidin production in atopic dermatitis, but this pathway is relatively unexplored in CTCL.10,11 Rarer infectious complications, such as progressive multifocal leukoencephalopathy (PML), P. jirovecii pneumonia, and toxoplasmosis, have also been observed in CTCL, but do not dominate the picture to the same extent as bacterial and herpesvirus infections.7,12
One hypothesis is that microbial products may stimulate disease progression. In erythrodermic CTCL, there is a high incidence of colonization with S. aureus strains producing superantigenic staphylococcal enterotoxins (SEs) or toxic shock syndrome toxin (TSST-1).13 Both bacterial isolates from patients and recombinant SEs can activate STAT3 and induce expression of IL-17 in CTCL cell lines.14 SEs also trigger expression of the regulatory T cell (Treg) marker FOXP3 in SS cells, in a STAT5 dependent manner.15 The oncogenic microRNA miR-155 is also STAT5 dependent, raising the possibility that SEs may contribute to the high expression of this molecule in CTCL cells.16 Only a subset of T cell receptor (TCR) variable region β chains are responsive to specific SEs or TSST-1. For example, TSST-1 is specific to Vβ2, and SED targets Vβ1 and Vβ5.17 However, these sensitive TCRs are overrepresented in the expanded clones of CTCL in patients colonized with toxicogenic S. aureus strains.13 Even when the clonal TCR is unresponsive to SE, the toxins may activate benign cells which then stimulate neighboring malignant cells.14,18 Moreover, benign T cells are sensitive to cell death induced by S. aureus alpha toxin, whereas malignant cells are resistant.19 To our knowledge, two small studies have examined the use of antibiotics in CTCL, and both found that treatment led to clearance of S. aureus colonization and improvement in clinical symptoms.20,21 While antibiotics can have off-target effects on pathways relevant to CTCL, e.g. doxycycline’s inhibition of NF-kB22, the studies supporting exogenous, infectious drivers of CTCL are intriguing and promising.21
With certain comorbidities, CTCL seems to be more common and sometimes more aggressive. A population based survey suggested that CTCL may be more common in areas with higher prevalence of HIV, a finding in keeping with the known 15-fold higher incidence of T cell lymphomas in AIDS patients.23,24 The decreased complexity of the TCR repertoire in advanced CTCL is similar to that found in AIDS, illustrating the compromised growth of normal T cells in both conditions.6 In post-transplantation patients, CTCL may take a particularly aggressive course.25 Clearly, medications that inhibit T cell function, such as anti-TNF agents and cyclosporine, can drive sudden progression in undiagnosed CTCL.26,27 Dupilumab, an anti-IL4 receptor antibody, may also hasten CTCL progression through unknown mechanisms, perhaps by depleting benign tumor reactive lymphocytes.28 Collectively, these associations reinforce the role of T cell immune function in controlling disease progression.
The importance of cellular immunity
An in-situ host immune response is thought to control progression among many with patch stage MF, perhaps accounting for the indolent course of those with limited clinical disease. On histopathology, the presence of a reactive CD8+ cytotoxic T cell infiltrate is common and its intensity is associated with a more favorable prognosis.6,29 In peripheral blood, circulating CD8+ T cells display increased activation markers and possess lytic capacity against autologous malignant cells.30,31 The CD4+ T cells in early stage MF lesions tend to display a TH1 phenotype, suggesting that the cytokine milieu is supportive of cell-mediated cytotoxicity.32 These TH1 cells could represent either reactive benign helper T cells or malignant cells which have a malleable phenotype and have not yet acquired a dominant TH2 bias.3 At least one study has suggested that the visible inflammation in MF lesions is due in large part to the reactive immune response.33 Importantly, the cellular immune response to CTCL depends at least in part on MHC-dependent recognition of tumor antigens, indicating that these malignancies manifest enough acquired mutations that a healthy immune system would recognize them.30,34,35
However, the cellular immune response is profoundly depressed in progressive MF and SS. As the disease burden grows, there is a reduction in CD8+ infiltrate in skin lesions and a shift of cytokine production toward a TH2 profile.29,36,37 The TH2 cytokines secreted by malignant cells suppress TH1 immunity and enforce a global TH2 bias in benign helper T cells.38,39 With reduced TH1 function, the host is substantially deprived of cellular immunity and has impaired immunological memory to MHC-presented antigens.40 Patients may possess reactive T cells that are capable of recognizing tumor cells yet are incapacitated by the TH1/TH2 imbalance.41 In keeping with this, CD8+ lymphocytes from patients with SS demonstrate markers of exhaustion, cytokine unresponsiveness, and attenuated cytotoxicity.42,43 Stimulating TH1 function by supplementing IL-2, IL-12, and IFN-γ restores the lytic capacity of effector T cells.31 The cytokine IL-2 has also been shown to activate natural killer (NK) cells to lyse Sézary cell lines..44 Apart from suppressing TH1 function, the TH2 phenotype of MF and SS cells helps to explain common findings including eosinophilia and increased levels of immunoglobulins IgE and IgA.36 In addition to their TH2 phenotype, the malignant cells in SS generally also express varying levels of the immune checkpoint molecules PD-1, TIGIT, and CTLA4.45–47 Similarly, T cells from those with HTLV-1 associated adult T cell leukemias (ATLL) have demonstrated biomarkers of Tregs, expressing high levels of FOXP3, and other T cell lymphomas may have yet different immunosuppressive phenotypes.48–50
There are additional defects in DCs and polymorphonuclear granulocytes (PMNs). In SS, there is a stage-dependent decline in the number of circulating DCs in the blood and in the IL-12 production of each cell.51 In response to challenge with influenza virus or TLR9 agonists, peripheral blood mononuclear cells from SS patients produce significantly less IFN-α.51,52 PMNs from SS patients have reduced phagocytic activity and intracellular killing against K. pneumonia.53 These defects in antigen presenting cells (APCs) and in innate immunity also favor the development of infections in these patients.
A reduction in the burden of malignant T cells in SS through the use of immunotherapy can lead to the restoration of more normal immune function. The normal T cell repertoire that is lost in advanced disease tends to be restored after elimination of the malignant clone.54,55 Treatments including ECP, TSEB, interferons, and TLR agonists have been shown to restore some TH1/TH2 balance, likely in part by debulking malignant cells.56–58 Restoration of dendritic and NK cell populations is also typical.4 Importantly, there is a reasonable concern that traditional chemotherapy regimens, being immunotoxic, may delay immune reconstitution relative to treatments that stimulate immunity.4
Cellular mechanisms of immune evasion and progression
The definition of the malignant cell
The tumor cells in MF and SS are noted morphologically as ‘atypical’ lymphocytes with convoluted, cerebriform nuclei.4 While these qualitative features are useful, more objective measures have been developed for quantitating malignant cells. Flow cytometry evaluation of peripheral blood can reveal the characteristic deletion of markers including CD7 and CD26, the latter being much more frequent.59–62 The expansion of clonal T cell populations can be detected by polymerase chain reaction (PCR), antibodies specific for Vβ TCR families, or high throughput sequencing (HTS) of the complementarity determining region 3 (CDR3) of the TCRB gene complex.63–65 At the transcript level, tumor cells may be distinguished by the expression of TH2 cytokine mRNA.41 TCR sequencing has also better defined the histogenesis of CTCL, because malignant cells were found to have two rearranged copies of Vγ, a marker of ‘mature’ or post-thymic T cells.66
Studies have identified distinct molecular and genetic signatures in MF and SS, which suggest the two may be distinct entities rather than different stages of the same disease. Immunophenotyping analysis has suggested that Sézary cells and MF cells may have central memory and effector memory phenotypes, respectively.67 A genomic study found highly recurrent chromosomal abnormalities in MF that were not common in SS and vice versa.68 Therefore, despite many shared features, such as a predominant CD4+CLA+CD26− phenotype with TH2 cytokine elaboration, MF and SS may have distinct molecular signatures that may prove important for future targeted therapies.
Common mutations and dysregulations
A resistance to apoptosis is a fundamental feature of malignant cells, and several pathways combine to induce this resistance in CTCL. Typically, benign lymphocytes rely on Fas-mediated pathways to undergo regulated cell death after repeated TCR stimulation.69 This process, also referred to as activation induced cell death (AICD), is important to maintain homeostasis during the clonal expansion of activated lymphocytes.70 The activation of T cells leads to the expression of Fas ligand (FasL), which complexes with the Fas receptor and leads to T cell apoptosis.70 However, both CTCL cell lines and patient derived tumor cells have exhibited delayed expression of FasL following activation.71 Moreover, studies have identified inactivating splice variants and other mutations in the Fas gene as well as reduced TCR-proximal signaling that downregulates AICD.72–74 The decreased TCR signaling in CTCL cells appears to be due to a profound suppression of phospholipase C, gamma 1 (PLCG1).74 The FasL that CTCL cells do express may be sufficient to induce apoptosis in neighboring benign lymphocytes, and this ‘bystander cytotoxicity’ may be a mechanism by which CTCL cells evade the host immune response.71 Additional pathways implicated in apoptosis resistance include constitutive NF-kB signaling, PAK1 and STAT3 overexpression, mTOR signaling, and IL-7, 9, and 15 interactions.75–80
The pathogenesis of the TH2 phenotype of MF and SS cells is now significantly better understood. In benign helper T cells, cytokine and TCR-mediated pathways induce TH differentiation. The principal transcription factors that determine TH1 and TH2 phenotype, respectively, are T-bet and GATA3.81 Most TH determining pathways converge on these transcription factors. For example, the TH2 polarizing cytokines IL-4 and IL-13 signal through cytokine receptor associated JAKs to activate STAT6, which enhances the transcription of GATA3.81 Meanwhile, signaling through the TCR promotes GATA3 expression by PI3K-mTOR pathways and by downregulating RUNX1.81,82 The activation of STAT5 and STAT3 also appear necessary for TH2 differentiation.83,84 In MF and SS, the acquisition of the TH2 phenotype is associated with a loss of the TH1 polarizing STAT4 and a gain of STAT6.85 These changes appear to be due in part to aberrant histone acetylation and expression of the oncogenic miR-155 microRNA.85 Notably, blocking STAT3 in tumor cell lines abrogates the expression of IL-5 and IL-13 and encourages apoptosis.39,77 In both early and advanced stages of disease, constitutive STAT3 and STAT5 activity is observed. In early stages this may be due to cytokine signaling from the tumor microenvironment, while in advanced stages these transcription factors may become cytokine-independent and driven by constitutively active JAK signalling.86
The application of whole exome sequencing to patient derived SS cells has identified genes with common mutations and copy number alterations. Based on mutation frequency alone, TP53, PLCG1, CCR4, FAS, and TNFRSF1B emerge as likely driver genes87,88, all of which have been implicated in prior studies of CTCL pathogenesis.72,74 Mutations were also observed in well-known tumor suppressor, signaling, and epigenetic regulating proteins including RB1, CDKN1B, MAPK1, BRAF, TET2, and CREBBP.88 Overall, these genetic studies help elucidate the derangements behind tumor proliferation, resistance to apoptosis, and immune surveillance.
Surface factors
The cellular ‘surfaceome’ has acquired a new importance in the age of immunotherapy. With the advent of therapeutic mAbs, affinity-directed toxins, and (CAR) T cells, we are witnessing how surface proteomics can direct tumor eradication.
In CTCL, we now have a much higher resolution picture of cell surface proteomics. The immune checkpoint receptors are a particularly well-characterized group. In SS, both benign and malignant CD4+ T cells exhibit a stage-dependent increase in PD-1 expression, and the blockade of the PD-1 axis can restore TH1 cytokine production.45 With elimination of the malignant clone, PD-1 expression can normalize.45 Mechanistically, PD-1/PD-L1 complexes inhibit reactive immune T cells and promote induction of FOXP3+ Tregs and TH2 cells.89,90 A study of MF skin biopsies found that tumor cells strongly express the ligand PD-L1, while a separate report examining leukemic CTCL found lower PD-L1 expression.90,91 The anti PD-L1 antibodies atezolizumab and avelumab are currently being studied for mature T cell malignancies including CTCL (NCT03905135 and NCT03357224). The immune checkpoint receptors TIGIT and CTLA-4 are also overexpressed on Sézary cells, the latter likely due to proteasome dysfunction and GATA3 upregulation.46,47,92 The receptor CD47, which inhibits macrophage-mediated phagocytosis of tumor cells, is also elevated on Sézary cells through the influence of TH2 cytokines, and its expression is correlated with worse overall survival.93
To date, the screening of CTCL lines for tumor-specific plasma membrane proteins has produced only a limited number of candidates for immunotherapeutic targeting.94 Examples include a set of cancer/germline antigens that include cTAGE-1, MAGE-A9, and NY-ESO-1.95,96 However, the search for tumor-associated proteins has been more fruitful. These proteins are upregulated relative to benign cells, and some provide promising targets as well as insight into clinical manifestations. The chemokine receptors CCR4 and CCR7 facilitate T cell homing into the skin and lymph nodes, respectively.97 The former is upregulated in both CTCL cells and Tregs, making it an attractive dual target.98 CCR7 is overexpressed mainly on leukemic CTCL cells.67 The chemokine receptor CXCR4 also plays a role in skin trafficking, and the absence of CD26, which normally degrades the ligand of CXCR4, may also facilitate skin tropism.99 Other associated biomarkers of Sézary cells include the sialomucin core protein CD164, Fc receptor-like protein 3 (FCRL3), syndecan-4 (SD-4), and vimentin.100–102 The latter two are present on all activated T cells and may simply reflect the constitutively activated phenotype of Sézary cells. Tumor-associated antigens can be effective targets, with acceptable on-target off-tumor toxicity, as demonstrated by good results with anti-CCR4 and anti-CD52 mAbs. The discovery of other tumor-associated antigens is a promising direction of research.
The influence of the microenvironment
The interactions of cytokines, chemokines, and stroma contribute to CTCL tumorigenesis, suggesting potential therapeutic targets. The influences of TH2 and Treg cytokines are summarized in Fig 1..103,104 The cytokine IL-31 has a dose-dependent relationship with the clinical manifestation of pruritus.105,106 A set of chemokines including CCL17, CCL22, CCL27, and the stromal cell-derived factor 1 (SDF1) are implicated in directional migration into the skin.107 Importantly, in their normal function, these chemokines also exert pro-survival influences on their target cells, likely including PI3K/Akt signaling.107 In in vitro studies, the cytokines IL-2, IL-4, IL-7, IL-13, and IL-15 have all been implicated as lymphoma growth factors108–110.
Figure 1.
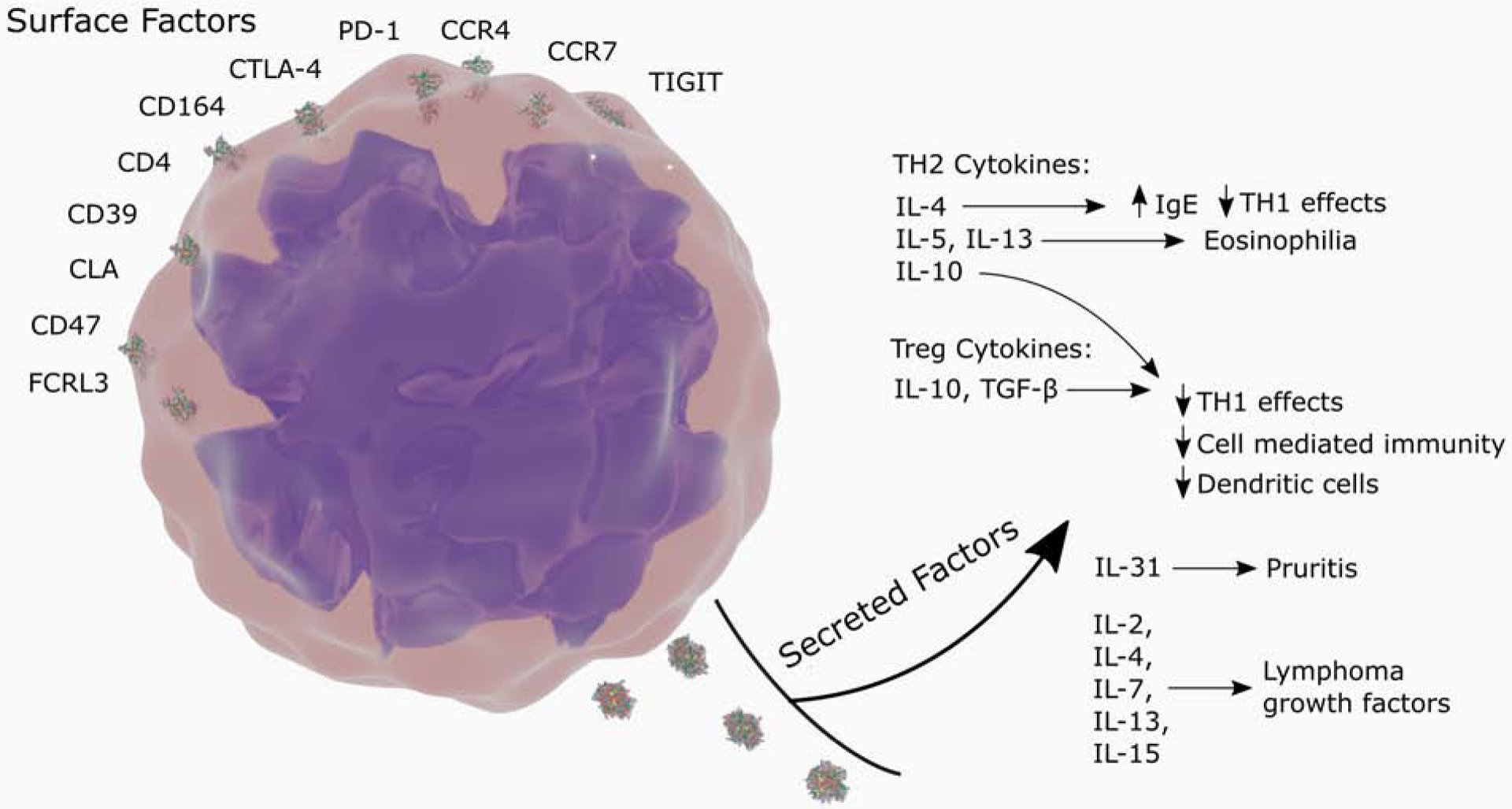
“Sézary syndrome. Malignant cells dysregulate the host immune response through surface and secreted factors.”
The influence of dysregulated or immature APCs can explain certain aspects of both tumor progression and therapeutic response. Direct contact with immature DCs promotes CTCL cell proliferation and Treg cytokine production in an MHC class 2-dependent manner.103 The presence of immature DCs in culture allows for the prolonged proliferation of CTCL cells, an effect which is inhibited by blocking CD40 or the clonotypic TCR.111 Conversely, CTCL cells do not proliferate when encountering mature DCs.112 The TH1 cytokine IFN-γ stimulates maturation of dendritic cells.113 While DCs can phagocytose both viable and apoptotic CTCL cells, only the latter induce DC maturation markers.112 The observation that apoptotic CTCL cells can induce APC maturation may explain why therapies like TSEB and ECP, which can induce massive apoptosis of malignant cells, are effective additions to many multimodality regimens.
Conclusion
Recent discoveries have advanced our understanding of the pathophysiology of CTCL, particularly MF and SS. The immune deficient state commonly observed in these patients may lead to severe infections or inadequate anti-tumor immunity. Therapies that stimulate immunity have been associated with longer progression free survival than traditional cytotoxic chemotherapy. The application of new technologies, including high throughput TCR sequencing and genomic analysis, has led to breakthroughs in diagnosis and in understanding tumor proliferation, escape from apoptosis, and dependency on the microenvironment. In the next part, we review in more depth the current and emerging immunotherapies for CTCL.
Funding sources:
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number TL1TR001880 (JSD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: MW and AHR are inventors on a pending patent for the use of resiquimod for cutaneous T cell lymphoma.
References
- 1.Willemze R, Cerroni L, Kempf W, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood, The Journal of the American Society of Hematology. 2019;133(16):1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trautinger F, Eder J, Assaf C, et al. European organization for research and treatment of cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome–Update 2017. Eur J Cancer. 2017;77:57–74. [DOI] [PubMed] [Google Scholar]
- 3.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins. 2013;5(8):1402–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen EA, Rook AH, Zic J, et al. Sézary syndrome: Immunopathogenesis, literature review of therapeutic options, and recommendations for therapy by the united states cutaneous lymphoma consortium (USCLC). J Am Acad Dermatol. 2011;64(2):352–404. [DOI] [PubMed] [Google Scholar]
- 5.Hughes CFM, Khot A, McCormack C, et al. Lack of durable disease control with chemotherapy for mycosis fungoides and sézary syndrome: A comparative study of systemic therapy. Blood. 2015;125(1):71–81. [DOI] [PubMed] [Google Scholar]
- 6.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115(4):798–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Axelrod PI, Lorber B, Vonderheid EC. Infections complicating mycosis fungoides and sezary syndrome. JAMA. 1992;267(10):1354–1358. [PubMed] [Google Scholar]
- 8.Talpur R, Bassett R, Duvic M. Prevalence and treatment of staphylococcus aureus colonization in patients with mycosis fungoides and sézary syndrome. Br J Dermatol. 2008;159(1):105–112. [DOI] [PubMed] [Google Scholar]
- 9.Wolk K, Mitsui H, Witte K, et al. Deficient cutaneous antibacterial competence in cutaneous T-cell lymphomas: Role of Th2-mediated biased Th17 function. Clinical Cancer Research. 2014;20(21):5507–5516. [DOI] [PubMed] [Google Scholar]
- 10.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1, 25-dihydroxyvitamin D3. The FASEB journal. 2005;19(9):1067–1077. [DOI] [PubMed] [Google Scholar]
- 11.Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122(4):829–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Richardson SK, Melhem ER, Rook AH, Kim EJ. Progressive multifocal leukoencephalopathy from JC virus in a patient with advanced mycosis fungoides. J Am Acad Dermatol. 2007;57(5):893–895. [DOI] [PubMed] [Google Scholar]
- 13.Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive staphylococcus aureus, and oligoclonal T-cell receptor VÎ2 gene expansion. Blood, The Journal of the American Society of Hematology. 1997;89(1):32–40. [PubMed] [Google Scholar]
- 14.Willerslev-Olsen A, Krejsgaard T, Lindahl LM, et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood, The Journal of the American Society of Hematology. 2016;127(10):1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willerslev-Olsen A, Buus TB, Nastasi C, et al. Staphylococcus aureus enterotoxins induce FOXP3 in neoplastic T cells in sézary syndrome. Blood cancer journal. 2020;10(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp K, Ralfkiaer U, Mette Gjerdrum L, et al. STAT5-mediated expression of oncogenic miR-155 in cutaneous T-cell lymphoma. Cell cycle. 2013;12(12):1939–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas D, Dauwalder O, Brun V, et al. Staphylococcus aureus superantigens elicit redundant and extensive human VÎ2 patterns. Infect Immun. 2009;77(5):2043–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woetmann A, Lovato P, Eriksen KW, et al. Nonmalignant T cells stimulate growth of T-cell lymphoma cells in the presence of bacterial toxins. Blood. 2007;109(8):3325–3332. doi: 10.1182/blood-2006-04-017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blümel E, Munir Ahmad S, Nastasi C, et al. Staphylococcus aureus alpha-toxin inhibits CD8 T cell-mediated killing of cancer cells in cutaneous T-cell lymphoma. OncoImmunology. 2020;9(1):1751561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tokura Y, Yagi H, Ohshima A, et al. Cutaneous colonization with staphylococci influences the disease activity of sezary syndrome: A potential role for bacterial superantigens. Br J Dermatol. 1995;133(1):6–12. [DOI] [PubMed] [Google Scholar]
- 21.Lindahl LM, Willerslev-Olsen A, Gjerdrum LM, et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood. 2019;134(13):1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander-Savino CV, Hayden MS, Richardson C, Zhao J, Poligone B. Doxycycline is an NF-ΰB inhibitor that induces apoptotic cell death in malignant T-cells. Oncotarget. 2016;7(46):75954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arzoo KK, Bu X, Espina BM, Seneviratne L, Nathwani B, Levine AM. T-cell lymphoma in HIV-infected patients. JAIDS J Acquired Immune Defic Syndromes. 2004;36(5):1020–1027. [DOI] [PubMed] [Google Scholar]
- 24.Mirvish ED, Pomerantz RG, Geskin LJ. Infectious agents in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;64(2):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravat FE, Spittle MF, Russell-Jones R. Primary cutaneous T-cell lymphoma occurring after organ transplantation. J Am Acad Dermatol. 2006;54(4):668–675. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Escala ME, Posligua AL, Wickless H, et al. Progression of undiagnosed cutaneous lymphoma after anti–tumor necrosis factor-alpha therapy. J Am Acad Dermatol. 2018;78(6):1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zackheim HS, Koo J, LeBoit PE, et al. Psoriasiform mycosis fungoides with fatal outcome after treatment with cyclosporine. J Am Acad Dermatol. 2002;47(1):155–157. [DOI] [PubMed] [Google Scholar]
- 28.Espinosa ML, Nguyen MT, Aguirre AS, et al. Progression of cutaneous T-cell lymphoma after dupilumab: Case review of 7 patients. J Am Acad Dermatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeer MH, van Doorn R, Dukers D, Bekkenk MW, Meijer CJ, Willemze R. CD8 T cells in cutaneous T-cell lymphoma: Expression of cytotoxic proteins, fas ligand, and killing inhibitory receptors and their relationship with clinical behavior. Journal of clinical oncology. 2001;19(23):4322–4329. [DOI] [PubMed] [Google Scholar]
- 30.Asadullah K, Friedrich M, Döcke W, Jahn S, Volk H, Sterry W. Enhanced expression of T-cell activation and natural killer cell antigens indicates systemic anti-tumor response in early primary cutaneous T-cell lymphoma. J Invest Dermatol. 1997;108(5):743–747. [DOI] [PubMed] [Google Scholar]
- 31.Seo N, Tokura Y, Matsumoto K, Furukawa F, Takigawa M. Tumour-specific cytotoxic T lymphocyte activity in Th2-type sézary syndrome: Its enhancement by interferon-gamma (IFN-γ) and IL-12 and fluctuations in association with disease activity. Clin Exp Immunol. 1998;112(3):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saed G, Fivenson DP, Naidu Y, Nickoloff BJ. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas sezary syndrome express a Th2-type profile. J Invest Dermatol. 1994;103(1):29–33. [DOI] [PubMed] [Google Scholar]
- 33.Vieyra-Garcia P, Crouch JD, O’Malley JT, et al. Benign T cells drive clinical skin inflammation in cutaneous T cell lymphoma. JCI insight. 2019;4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger CL, Wang N, Christensen I, Longley J, Heald P, Edelson RL. The immune response to class I-associated tumor-specific cutaneous T-cell lymphoma antigens. J Invest Dermatol. 1996;107(3):392–397. [DOI] [PubMed] [Google Scholar]
- 35.Winter D, Fiebiger E, Meraner P, et al. Definition of TCR epitopes for CTL-mediated attack of cutaneous T cell lymphoma. The Journal of Immunology. 2003;171(5):2714–2724. [DOI] [PubMed] [Google Scholar]
- 36.Vowels BR, Cassin M, Vonderheid EC, Rook AH. Aberrant cytokine production by sezary syndrome patients: Cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992;99(1):90–94. [DOI] [PubMed] [Google Scholar]
- 37.Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103(5):669–673. [DOI] [PubMed] [Google Scholar]
- 38.Guenova E, Watanabe R, Teague JE, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clinical cancer research. 2013;19(14):3755–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nielsen M, Nissen MH, Gerwien J, et al. Spontaneous interleukin-5 production in cutaneous T-cell lymphoma lines is mediated by constitutively activated Stat3. Blood, The Journal of the American Society of Hematology. 2002;99(3):973–977. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura T, Nakui M, Sato M, et al. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(1):S52–S61. [DOI] [PubMed] [Google Scholar]
- 41.Echchakir H, Bagot M, Dorothée G, et al. Cutaneous T cell lymphoma reactive CD4 cytotoxic T lymphocyte clones display a Th1 cytokine profile and use a fas-independent pathway for specific tumor cell lysis. J Invest Dermatol. 2000;115(1):74–80. [DOI] [PubMed] [Google Scholar]
- 42.Torrealba MP, Manfrere KC, Miyashiro DR, et al. Chronic activation profile of circulating CD8 T cells in sezary syndrome. Oncotarget. 2018;9(3):3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Querfeld C, Leung S, Myskowski PL, et al. Primary T cells from cutaneous T-cell lymphoma skin explants display an exhausted immune checkpoint profile. Cancer immunology research. 2018:canimm. 0270.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouaziz J, Ortonne N, Giustiniani J, et al. Circulating natural killer lymphocytes are potential cytotoxic effectors against autologous malignant cells in sezary syndrome patients. J Invest Dermatol. 2005;125(6):1273–1278. [DOI] [PubMed] [Google Scholar]
- 45.Samimi S, Benoit B, Evans K, et al. Increased programmed death-1 expression on CD4 T cells in cutaneous T-cell lymphoma: Implications for immune suppression. Arch Dermatol. 2010;146(12):1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jariwala N, Benoit B, Kossenkov AV, et al. TIGIT and helios are highly expressed on CD4 T cells in sezary syndrome patients. J Invest Dermatol. 2017;137(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong HK, Wilson AJ, Gibson HM, et al. Increased expression of CTLA-4 in malignant T cells from patients with mycosis fungoides cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126(1):212–219. [DOI] [PubMed] [Google Scholar]
- 48.Tendler CL, Burton JD, Jaffe J, et al. Abnormal cytokine expression in Sezary and adult T-cell leukemia cells correlates with the functional diversity between these T-cell malignancies. Cancer Res. 1994;54(16):4430–4435. [PubMed] [Google Scholar]
- 49.Ureshino H, Shindo T, Nishikawa H, et al. Effector regulatory T cells reflect the equilibrium between antitumor immunity and autoimmunity in adult T-cell leukemia. Cancer immunology research. 2016;4(8):644–649. [DOI] [PubMed] [Google Scholar]
- 50.Shimazu Y, Shimazu Y, Hishizawa M, et al. Hypomethylation of the Treg-specific demethylated region in FOXP3 is a hallmark of the regulatory T-cell subtype in adult T-cell leukemia. Cancer immunology research. 2016;4(2):136–145. [DOI] [PubMed] [Google Scholar]
- 51.Wysocka M, Zaki MH, French LE, et al. Sezary syndrome patients demonstrate a defect in dendritic cell populations: Effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood, The Journal of the American Society of Hematology. 2002;100(9):3287–3294. [DOI] [PubMed] [Google Scholar]
- 52.Wysocka M, Benoit BM, Newton S, Azzoni L, Montaner LJ, Rook AH. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104(13):4142–4149. [DOI] [PubMed] [Google Scholar]
- 53.Fierro MT, Cuffini AM, Novelli M, et al. Functional and phenotypical alterations of polymorphonuclear cells in sézary syndrome patients. European Journal of Dermatology. 2012;21(6):921–929. [DOI] [PubMed] [Google Scholar]
- 54.Yawalkar N, Ferenczi K, Jones DA, et al. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102(12):4059–4066. [DOI] [PubMed] [Google Scholar]
- 55.Yamanaka K, Fuhlbrigge RC, Mizutani H, Kupper TS. Restoration of peripheral blood T cell repertoire complexity during remission in advanced cutaneous T cell lymphoma. Arch Dermatol Res. 2010;302(6):453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo EK, Cassin M, Lessin SR, Rook AH. Complete molecular remission during biologic response modifier therapy for sezary syndrome is associated with enhanced helper T type 1 cytokine production and natural killer cell activity. J Am Acad Dermatol. 2001;45(2):208–216. [DOI] [PubMed] [Google Scholar]
- 57.Manfrere KC, Torrealba MP, Miyashiro DR, et al. Toll-like receptor agonists partially restore the production of pro-inflammatory cytokines and type I interferon in sézary syndrome. Oncotarget. 2016;7(46):74592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Renzo M, Rubegni P, De Aloe G, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous t†cell lymphoma. Immunology. 1997;92(1):99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harmon CB, Witzig TE, Katzmann JA, Pittelkow MR. Detection of circulating T cells with CD4 CD7â^’ immunophenotype in patients with benign and malignant lymphoproliferative dermatoses. J Am Acad Dermatol. 1996;35(3):404–410. [DOI] [PubMed] [Google Scholar]
- 60.Wood GS, Hong SR, Sasaki DT, et al. Leu-8/CD7 antigen expression by CD3 T cells: Comparative analysis of skin and blood in mycosis fungoides/sezary syndrome relative to normal blood values. J Am Acad Dermatol. 1990;22(4):602–607. [DOI] [PubMed] [Google Scholar]
- 61.Jones D, Dang NH, Duvic M, Washington LT, Huh YO. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115(6):885–892. [DOI] [PubMed] [Google Scholar]
- 62.Bernengo MG, Novelli M, Quaglino P, et al. The relevance of the CD4 CD26–subset in the identification of circulating Sezary cells. Br J Dermatol. 2001;144(1):125–135. [DOI] [PubMed] [Google Scholar]
- 63.Wood GS, Tung RM, Heaffner AC, et al. Detection of clonal T-cell receptor Î3 gene rearrangements in early mycosis fungoides/Sezary syndrome by polymerase chain reaction and denaturing gradient gel electrophoresis (PCR/DGGE). J Invest Dermatol. 1994;103(1):34–41. [DOI] [PubMed] [Google Scholar]
- 64.Feng B, Jorgensen JL, Jones D, et al. Flow cytometric detection of peripheral blood involvement by mycosis fungoides and Sezary syndrome using T-cell receptor VÎ2 chain antibodies and its application in blood staging. Modern Pathology. 2010;23(2):284–295. [DOI] [PubMed] [Google Scholar]
- 65.Weng W, Armstrong R, Arai S, Desmarais C, Hoppe R, Kim YH. Minimal residual disease monitoring with high-throughput sequencing of T cell receptors in cutaneous T cell lymphoma. Science Translational Medicine. 2013;5(214):214ra171. [DOI] [PubMed] [Google Scholar]
- 66.Kirsch IR, Watanabe R, O’Malley JT, et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Science translational medicine. 2015;7(308):308ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell JJ, Clark RA, Watanabe R, Kupper TS. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: A biologic rationale for their distinct clinical behaviors. Blood, The Journal of the American Society of Hematology. 2010;116(5):767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Doorn R, van Kester MS, Dijkman R, et al. Oncogenomic analysis of mycosis fungoides reveals major differences with Sezary syndrome. Blood, The Journal of the American Society of Hematology. 2009;113(1):127–136. [DOI] [PubMed] [Google Scholar]
- 69.Meech SJ, Edelson R, Walsh P, Norris DA, Duke RC. Reversible resistance to apoptosis in cutaneous T cell lymphoma. Ann N Y Acad Sci. 2001;941(1):46–58. [DOI] [PubMed] [Google Scholar]
- 70.Green DR, Droin N, Pinkoski M. Activation induced cell death in T cells. Immunol Rev. 2003;193(1):70–81. [DOI] [PubMed] [Google Scholar]
- 71.Ni X, Hazarika P, Zhang C, Talpur R, Duvic M. Fas ligand expression by neoplastic T lymphocytes mediates elimination of CD8 cytotoxic T lymphocytes in mycosis fungoides: A potential mechanism of tumor immune escape? Clinical cancer research. 2001;7(9):2682–2692. [PubMed] [Google Scholar]
- 72.van Doorn R, Dijkman R, Vermeer MH, Starink TM, Willemze R, Tensen CP. A novel splice variant of the Fas gene in patients with cutaneous T-cell lymphoma. Cancer Res. 2002;62(19):5389–5392. [PubMed] [Google Scholar]
- 73.Dereure O, Levi E, Kadin ME, Vonderheid EC. Infrequent Fas mutations but no Bax or p53 mutations in early mycosis fungoides: A possible mechanism for the accumulation of malignant T lymphocytes in the skin. J Invest Dermatol. 2002;118(6):949–956. [DOI] [PubMed] [Google Scholar]
- 74.Klemke C, Brenner D, Weiss E, et al. Lack of T-cell receptor-induced signaling is crucial for CD95 ligand up-regulation and protects cutaneous T-cell lymphoma cells from activation-induced cell death. Cancer Research. 2009;69(10):4175–4183. [DOI] [PubMed] [Google Scholar]
- 75.Izban KF, Ergin M, Qin J, et al. Constitutive expression of NF-κB is a characteristic feature of mycosis fungoides: Implications for apoptosis resistance and pathogenesis. Hum Pathol. 2000;31(12):1482–1490. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, Gu X, Li W, Zhang Q, Zhang C. PAK1 overexpression promotes cell proliferation in cutaneous T cell lymphoma via suppression of PUMA and p21. J Dermatol Sci. 2018;90(1):60–67. [DOI] [PubMed] [Google Scholar]
- 77.Sommer VH, Clemmensen OJ, Nielsen O, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. evidence for an antiapoptotic function of STAT3. Leukemia. 2004;18(7):12881295. [DOI] [PubMed] [Google Scholar]
- 78.Marzec M, Liu X, Wysocka M, Rook AH, Odum N, Wasik MA. Simultaneous inhibition of mTOR-containing complex 1 (mTORC1) and MNK induces apoptosis of cutaneous T-cell lymphoma (CTCL) cells. PloS one. 2011;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Döbbeling U, Dummer R, Laine E, Potoczna N, Qin J, Burg G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood, The Journal of the American Society of Hematology. 1998;92(1):252–258. [PubMed] [Google Scholar]
- 80.Kumar S, Dhamija B, Marathe S, et al. The Th9 axis reduces the oxidative stress and promotes the survival of malignant T cells in cutaneous T-cell lymphoma patients. Molecular Cancer Research. 2020;18(4):657–668. [DOI] [PubMed] [Google Scholar]
- 81.Wan YY. GATA3: A master of many trades in immune regulation. Trends Immunol. 2014;35(6):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Komine O, Hayashi K, Natsume W, et al. The Runx1 transcription factor inhibits the differentiation of naive CD4 T cells into the Th2 lineage by repressing GATA3 expression. J Exp Med. 2003;198(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu J Transcriptional regulation of Th2 cell differentiation. Immunol Cell Biol. 2010;88(3):244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stritesky GL, Muthukrishnan R, Sehra S, et al. The transcription factor STAT3 is required for T helper 2 cell development. Immunity. 2011;34(1):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Litvinov IV, Cordeiro B, Fredholm S, et al. Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell cycle. 2014;13(18):2975–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Netchiporouk E, Litvinov IV, Moreau L, Gilbert M, Sasseville D, Duvic M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle. 2014;13(21):3331–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Ni X, Covington KR, et al. Genomic profiling of Sezary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47(12):1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.da Silva Almeida, Ana Carolina, Abate F, Khiabanian H, et al. The mutational landscape of cutaneous T cell lymphoma and Sezary syndrome. Nat Genet. 2015;47(12):1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood, The Journal of the American Society of Hematology. 2009;114(10):2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saulite I, Ignatova D, Chang Y, et al. Blockade of programmed cell death protein 1 (PD-1) in sézary syndrome reduces Th2 phenotype of non-tumoral T lymphocytes but may enhance tumor proliferation. OncoImmunology. 2020;9(1):1738797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kantekure K, Yang Y, Raghunath P, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma (CTCL)/mycosis fungoides (MF). Am J Dermatopathol. 2012;34(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gibson HM, Mishra A, Chan DV, Hake TS, Porcu P, Wong HK. Impaired proteasome function activates GATA3 in T cells and upregulates CTLA-4: Relevance for sezary syndrome. J Invest Dermatol. 2013;133(1):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson LD, Banerjee S, Kruglov O, et al. Targeting CD47 in Sezary syndrome with SIRPαFc. Blood advances. 2019;3(7):1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee M, Kistler C, Hartmann TB, et al. Immuno-screening of a cutaneous T-cell lymphoma library for plasma membrane proteins. Cancer Immunology, Immunotherapy. 2007;56(6):783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.EichmÃ1/4ller S, Usener D, Thiel D, Schadendorf D. Tumor-specific antigens in cutaneous t-cell lymphoma: Expression and sero-reactivity. International journal of cancer. 2003;104(4):482–487. [DOI] [PubMed] [Google Scholar]
- 96.Koch J, Dübel S, Usener D, Schadendorf D, Eichmüller S. cTAGE: A cutaneous T cell lymphoma associated antigen family with tumor-specific splicing. J Invest Dermatol. 2003;121(1):198–206. [DOI] [PubMed] [Google Scholar]
- 97.Gonzalez BR, Zain J, Rosen ST, Querfeld C. Tumor microenvironment in mycosis fungoides and Sezary syndrome. Curr Opin Oncol. 2016;28(1):88–96. [DOI] [PubMed] [Google Scholar]
- 98.Chang D, Sui J, Geng S, et al. Humanization of an anti-CCR4 antibody that kills cutaneous T-cell lymphoma cells and abrogates suppression by T-regulatory cells. Molecular cancer therapeutics. 2012;11(11):2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Narducci MG, Scala E, Bresin A, et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107(3):1108–1115. [DOI] [PubMed] [Google Scholar]
- 100.Wysocka M, Kossenkov AV, Benoit BM, et al. CD164 and FCRL3 are highly expressed on CD4 CD26- T cells in Sezary syndrome patients. J Invest Dermatol. 2014;134(1):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chung J, Shiue LH, Duvic M, Pandya A, Cruz PD Jr, Ariizumi K. Sezary syndrome cells overexpress syndecan-4 bearing distinct heparan sulfate moieties that suppress T-cell activation by binding DC-HIL and trapping TGF-β on the cell surface. Blood, The Journal of the American Society of Hematology. 2011;117(12):3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huet D, Bagot M, Loyaux D, et al. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. The Journal of Immunology. 2006;176(1):652–659. [DOI] [PubMed] [Google Scholar]
- 103.Berger CL, Tigelaar R, Cohen J, et al. Cutaneous T-cell lymphoma: Malignant proliferation of T-regulatory cells. Blood. 2005;105(4):1640–1647. [DOI] [PubMed] [Google Scholar]
- 104.Walsh PT, Benoit BM, Wysocka M, Dalton NM, Turka LA, Rook AH. A role for regulatory T cells in cutaneous T-cell lymphoma; induction of a CD4^ CD25^ Foxp3^ T cell phenotype associated with HTLV-I infection. J Invest Dermatol. 2006;126:690–692. [DOI] [PubMed] [Google Scholar]
- 105.Singer EM, Shin DB, Nattkemper LA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133(12):2783. [DOI] [PubMed] [Google Scholar]
- 106.Cedeno-Laurent F, Singer EM, Wysocka M, et al. Improved pruritus correlates with lower levels of IL-31 in CTCL patients under different therapeutic modalities. Clinical Immunology. 2015;158(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee C, Hwang ST. Pathophysiology of chemokines and chemokine receptors in dermatological science: A focus on psoriasis and cutaneous T-cell lymphoma. Dermatologica Sinica. 2012;30(4):128–135. [Google Scholar]
- 108.Marzec M, Liu X, Kasprzycka M, et al. IL-2–and IL-15-induced activation of the rapamycin-sensitive mTORC1 pathway in malignant CD4 T lymphocytes. Blood, The Journal of the American Society of Hematology. 2008;111(4):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamanaka K, Clark R, Rich B, et al. Skin-derived interleukin-7 contributes to the proliferation of lymphocytes in cutaneous T-cell lymphoma. Blood. 2006;107(6):2440–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geskin LJ, Viragova S, Stolz DB, Fuschiotti P. Interleukin-13 is overexpressed in cutaneous T-cell lymphoma cells and regulates their proliferation. Blood, The Journal of the American Society of Hematology. 2015;125(18):2798–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berger CL, Hanlon D, Kanada D, et al. The growth of cutaneous T-cell lymphoma is stimulated by immature dendritic cells. Blood, The Journal of the American Society of Hematology. 2002;99(8):2929–2939. [PubMed] [Google Scholar]
- 112.Thumann P, Lüftl M, Moc I, et al. Interaction of cutaneous lymphoma cells with reactive T cells and dendritic cells: Implications for dendritic cell-based immunotherapy. Br J Dermatol. 2003;149(6):1128–1142. [DOI] [PubMed] [Google Scholar]
- 113.He T, Tang C, Xu S, Moyana T, Xiang J. Interferon gamma stimulates cellular maturation of dendritic cell line DC2. 4 leading to induction of efficient cytotoxic T cell responses and antitumor immunity. Cell Mol Immunol. 2007;4(2):105–111. [PubMed] [Google Scholar]


