Abstract
Introduction:
Patients with hormone receptor-positive/HER2-negative (HR+/HER2−) metastatic breast cancer have benefitted from treatment with palbociclib, a cyclin-dependent kinase (CDK) 4/6 inhibitor capable of selectively targeting mechanisms of cell cycle progression that contribute to tumor cell proliferation. Palbociclib use in this setting demonstrates improved progression-free survival when given in combination with aromatase inhibitors or fulvestrant.
Areas covered:
The authors describe the current state of research surrounding palbociclib use in breast cancer, present evidence supporting a role for palbociclib in additional subtypes of metastatic breast cancer such as HER2-positive (HER2+) and triple-negative, report ongoing clinical trials aimed at expanding the scope of use for palbociclib, and discuss expected clinical results that will better inform decisions on including palbociclib as a part of breast cancer treatment strategies.
Expert opinion:
Preclinical and clinical studies have shown promising evidence for palbociclib use in metastatic HER2+ and androgen receptor-expressing triple-negative breast cancer but mixed results in the adjuvant/neoadjuvant setting, where differences may only be detectable in high-risk disease. Palbociclib combinations may constitute viable replacements for chemotherapy in the neoadjuvant setting as part of de-escalation strategies. Investigation into synergy of palbociclib with immunotherapies is also ongoing based on non-canonical effects of CDK4/6 inhibition on the tumor immune microenvironment.
1. Introduction
Palbociclib is an orally available cyclin-dependent kinase (CDK) 4/6-specific inhibitor. Along with two other approved drugs of this class, ribociclib and abemaciclib, the CDK4/6 inhibitors have transformed the treatment of metastatic hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2−) breast cancer. Palbociclib is indicated for use as first-line therapy for metastatic HR+/HER2− breast cancer with the non-steroidal aromatase inhibitor letrozole or as second-line therapy with the selective estrogen receptor degrader fulvestrant [1]. Additional clinical trials have commenced investigating palbociclib use in HER2-positive (HER2+) and triple-negative receptor subtypes of metastatic breast cancer, in HR+/HER2− metastatic breast cancer resistant to CDK4/6 inhibitors, as adjuvant or neoadjuvant intervention in non-metastatic breast cancer, and in ductal carcinoma in situ (DCIS). In this review, we give a background of the biology of CDK4/6 inhibitors, explore clinical developments of palbociclib in HER2+ and triple-negative breast cancers, discuss palbociclib use in the adjuvant and neoadjuvant settings, and examine the clinical data regarding palbociclib and endocrine therapy use as an alternative to cytotoxic chemotherapy. Finally, we provide our expert opinion on the current and future state of palbociclib use in breast cancer.
1.1. Cyclin D-CDK4/6 activity at the G1/S checkpoint
During the early G1 phase of the cell cycle, mitogenic signaling converges upon the induction of expression of D-type cyclins, which assemble with CDK4 or CDK6 to phosphorylate retinoblastoma protein (RB) and its related family members [2–9]. The role of RB is to repress activity of the E2F family of transcription factors that would otherwise drive transcription of cell cycle-progression genes [10]. Active cyclin D-CDK4/6 complexes phosphorylate and inactivate RB, which permits E2F-driven transcription of cyclin E and forms a feed-forward loop as cyclin E joins with CDK2 to further phosphorylate and inactivate RB [5]. Subsequently, remaining inhibitory pressure from RB is mitigated and the full suite of E2F-regulated genes is transcribed to produce the proteins necessary to carry out DNA synthesis and progress to later stages of the cell cycle [11,12]. Cyclin-CDK complexes are regulated by endogenous inhibitory proteins and post-translational modification [13]. INK4 family proteins such as the prototypical p16INK4A directly bind CDK4 to inhibit cyclin D-CDK4 activity [14]. Cyclin D-CDK4 is also regulated by cyclin H-CDK7, which critically activates the complex by phosphorylation of CDK4 at the T172 residue [15]. p21CIP1 and p27KIP1 regulate cyclin E-CDK2 complexes, and sequestration of these inhibitors by cyclin D-CDK4/6 enables further phosphorylation of RB and RB-like family members at the G1/S checkpoint [14] (Figure 1).
Figure 1:
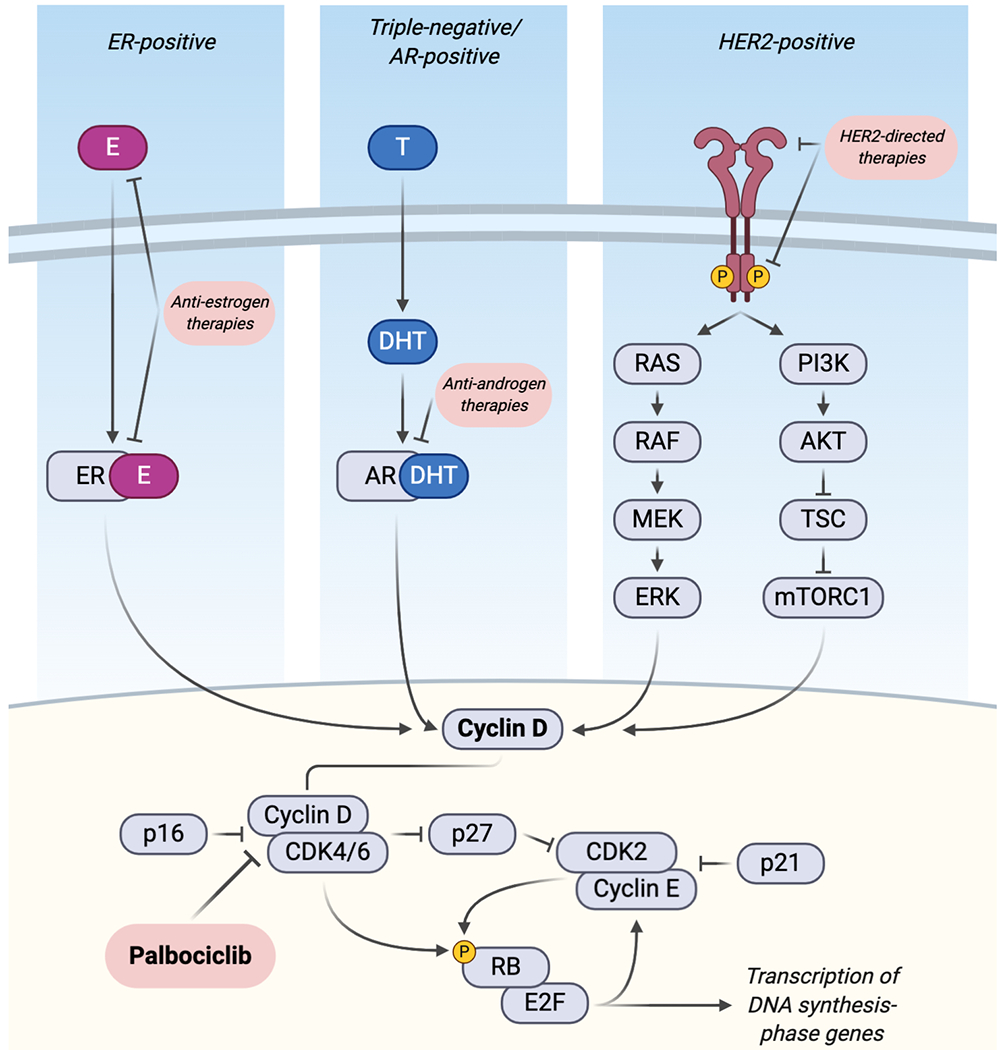
Mitogenic signal transduction pathways converge on cyclin D-CDK4/6 activity across multiple breast cancer subtypes and can be inhibited by targeted therapies and palbociclib. Left panel: estrogen receptor (ER) interaction with estradiol (E) in ER-positive breast cancers. Middle panel: testosterone (T) and dihydroxytestosterone (DHT) activity with the androgen receptor (AR) in AR-positive triple negative breast cancer. Right panel: HER2-signaling stimulates the MAP kinase and PI3K pathways, which transduce extracellular growth factor signals. Downstream, endogenous cell cycle inhibitors in the p16, p21, and p27 protein families negatively regulate CDK activity. Palbociclib inhibits cyclin D-CDK4/6-mediated phosphorylation of the retinoblastoma protein (RB) to prevent E2F-driven transcription of genes that commit the cell to DNA replication and cell division. This figure was created using BioRender.
1.2. Targeting CDK4/6 in breast cancer
Cyclin-dependent kinases present ideal targets for anti-cancer therapy as several cancers are growth-dependent on their activity. Although they play an integral role in cell cycle progression, CDKs are remarkably dispensable in many normal cell types. Systematic knock out of CDK loci in mouse models revealed that the interphase CDKs (CDK2/4/6) are functionally redundant in most adult tissues, and their deficiency causes defects only in specialized cell types such as hematopoietic precursors [16]. However, cancer cells may be addicted to growth through CDK4/6. Work using the MMTV-Erbb2 mouse model of mammary carcinoma led to the discovery that intact CDK4 expression was required for tumorigenesis, though its ablation did not affect normal mammary gland development [17].
Given that cell cycle proteins serve a critical role in maintaining balance between proliferation and quiescence, it is unsurprising that aberrant cyclin D-CDK4/6 activity leads to dysregulated growth in a broad set of tumor types. In normal estrogen-responsive cells, active estrogen receptor (ER) can bind the CCND1 promoter to cause transcription of cyclin D and mitogenesis through the cyclin D-CDK4/6 axis. Cyclin D can also directly bind ER and induce ER activity [18–20]. This pathway is often hijacked in breast cancer by CCND1 amplification or cyclin D overexpression, aberrations that are associated with more aggressive disease, relapse, recurrence, and metastasis [21,22]. Palbociclib is a selective inhibitor of CDK4 and CDK6 developed to inhibit phosphorylation of RB by active CDK4/6 to induce G1 arrest in tumor cells [23] (Figure 1). An in vitro breast cancer cell line screen revealed that luminal-subtype cell lines are more likely to be sensitive to palbociclib than other subtypes, and that there is synergism when palbociclib is combined with tamoxifen [24]. RB pathway expression is typically intact in luminal-subtype breast cancers, which are characterized by estrogen and progesterone hormone receptor expression and HER2 non-amplification [25]. This has provided rationale for targeting HR+/HER2− breast cancer with CDK4/6 inhibition. Non-luminal tumors, such as those that are basal-like, are more likely to be resistant to palbociclib due to the increased frequency of lost RB protein expression in this subtype [25]. However, some basal-like breast cancer cell lines retain RB expression and are indeed sensitive to CDK4/6 inhibition in vitro, which lends to the complexity of predicting sensitivity or resistance to palbociclib or other CDK4/6 inhibitors [24–27].
1.3. Clinical development of palbociclib
Palbociclib is the first orally available CDK4/6-specific inhibitor approved by the FDA. Phase I trials demonstrated that the dose-limiting toxicities were neutropenia and thrombocytopenia and that treatment was tolerable in a schedule of three weeks of 125 mg daily followed by one week off [28,29]. Subsequently, the randomized, open-label phase II PALOMA-1/TRIO-18 clinical trial demonstrated that palbociclib in combination with letrozole was superior to letrozole alone in postmenopausal women with previously untreated HR+/HER2− metastatic breast cancer, leading to its accelerated approval by the FDA in 2015 [30]. A larger randomized phase III study, PALOMA-2, verified the results of the prior trial and indicated that median progression-free survival in the palbociclib plus letrozole group is increased to 24.8 months versus 14.5 months in the placebo plus letrozole group (hazard ratio 0.58, p<0.001), which was the primary study endpoint [31].
Palbociclib has also been approved for use in the second-line setting with fulvestrant based on the results of a second phase III study, PALOMA-3 [32]. Pre- and post-menopausal women with metastatic HR+/HER2− breast cancer were eligible to be enrolled in the study if their disease had progressed despite treatment with a prior line of endocrine therapy. Median progression-free survival was increased in the palbociclib plus fulvestrant group to 9.5 months versus 4.6 months in the placebo plus fulvestrant group (hazard ratio 0.46, p<0.0001) [33]. Overall survival (OS) trended higher in the palbociclib plus fulvestrant group (34.9 months versus 28.0 months; p<0.09) but did not reach a statistically significant difference, perhaps due to the study becoming less powered for OS analysis after 16% of patients in the placebo-fulvestrant group received treatment with a CDK4/6 inhibitor following disease progression [34].
2. Expansion of use and future potential
2.1. Use in HER2-positive breast cancer
While palbociclib is not FDA-approved for use in HER2+ breast cancer, promising preclinical evidence exists of a role for CDK4/6 inhibition in this subtype. HER2+ breast cancers have been shown to depend on intracellular signaling via cyclin D-CDK4, which lies downstream from the activated HER2 receptor [35,36] (Figure 1). Synergy of CDK4/6 inhibition and anti-HER2 therapies was demonstrated in HER2+ breast cancer cell lines during the preclinical development of palbociclib [24]. An additional study using HER2+ cell lines found that sensitivity to lapatinib is augmented by palbociclib and that both drugs decrease DNA synthesis through disruption of E2F-target genes [37]. Unfortunately, a major shortcoming of targeted therapy in metastatic HER2+ breast cancer is the eventual development of therapeutic resistance. Evidence of a role for CDK4/6 inhibition in overcoming anti-HER2 therapy resistance was shown using another FDA-approved CDK4/6 inhibitor, abemaciclib. In a mouse model of inducible HER2-driven breast cancer, combined CDK4/6 inhibitor treatment and HER2 blockade acted synergistically on primary tumors growing in vivo, and intervention with abemaciclib at the outset of HER2 withdrawal significantly prolonged the time until tumor recurrence [38].
Clinical investigation of palbociclib in HER2+ breast cancer also includes intervention as adjuvant and neoadjuvant therapy in early breast cancer (Table 1). Only one ongoing clinical study has published interim results. The NA-PHER2 (NCT02530424) open-label phase II trial is investigating the combination of neoadjuvant trastuzumab, pertuzumab, palbociclib, and fulvestrant in women with non-metastatic invasive ER+/HER2+ breast cancer. Twenty-two patients were assessed at the interim analysis and their tumors were found to exhibit decreased expression of the proliferation marker Ki67 at the time of surgery compared to the pre-therapy baseline. The treatment regimen was deemed tolerable, as the most frequent grade 3 adverse events were neutropenia (29%) and diarrhea (14%) and no grade 4 events were recorded [39]. Other ongoing clinical trials involving palbociclib use in HER2+ breast cancer include PATRICIA II (NCT02448420), T-DM1 (NCT03530696), and PATINA/AFT-38 (NCT02947685).
Table 1:
Active clinical trials in metastatic breast cancer expanding the use of palbociclib to HER2-positive or triple-negative subtypes.
| Name/Identifier | Phase | Subtype | Setting | Interventions | Primary Outcome Measures |
|---|---|---|---|---|---|
|
NCT02530424 NA-PHER2 |
II | HER2+ | NA | Palbociclib, trastuzumab, pertuzumab and fulvestrant | Serial measures of Ki67 Serial measures of apoptosis |
|
NCT02907918 PALTAN |
II | HER2+ | NA | Palbociclib, letrozole, trastuzumab and goserelin if pre-menopausal | Rate of pCR |
|
NCT03644186 TOUCH |
II | HER2+ | NA | Palbociclib, letrozole, trastuzumab and pertuzumab vs. paclitaxel, trastuzumab and pertuzumab | Rate of pCR |
| NCT01976169 | I | HER2+ | M | Palbociclib and T-DM1 | Maximum tolerated dose Dose-limiting toxicities |
| NCT03054363 | I/II | HER2+ | M | Palbociclib, letrozole and tucatinib | Adverse events at 2.5
years Progression-free survival at 2.5 years |
| NCT03304080 | I/II | HER2+ | M | Palbociclib, anastrozole, trastuzumab and pertuzumab | Maximum tolerated dose Dose-limiting toxicities Clinical benefit rate |
| NCT03709082 | I/II | HER2+ | M | Palbociclib, letrozole and T-DM1 | Rate of overall response at 5 years |
|
NCT02448420 PATRICIA II |
II | HER2+ | M | Palbociclib and trastuzumab ± endocrine
therapy Palbociclib, trastuzumab, and endocrine therapy vs. trastuzumab, T-DM1, and chemotherapy |
PFS |
|
NCT03530696 T-DM1 |
II | HER2+ | M | T-DM1 ± palbociclib | PFS |
|
NCT02947685 PATINA |
III | HER2+ | M | Trastuzumab, pertuzumab and endocrine therapy ± palbociclib | PFS |
| NCT02605486 | I/II | TN (AR+) | M | Palbociclib and bicalutamide | Recommended phase II dose PFS |
Abbreviations: (AR+) Androgen receptor-positive; (HER2+) HER2-positive; (M) Metastatic; (NA) Neoadjuvant; (pCR) Pathological complete response; (PFS) Progression-free survival; (T-DM1) Trastuzumab emtansine; (TN) Triple-negative
2.2. Use in triple-negative breast cancer
Palbociclib use is also currently being investigated in the treatment of triple-negative breast cancer (TNBC), which is defined by the lack of expression of estrogen, progesterone, and HER2 receptors. TNBC is an aggressive subtype that is associated with a poor prognosis owing to several factors such as its increased tendency to metastasize and limited duration of response to chemotherapy in the metastatic setting [40–42]. TNBC is often resistant to CDK4/6 inhibition [24], possibly due to the increased proportion of tumors that lack functional RB compared to other breast cancer subtypes [25]. Functional RB is a requirement for sensitivity to CDK4/6 inhibitors, though it does not necessarily rule in sensitivity [43] (Figure 1).
A phase II study investigated palbociclib monotherapy for patients with metastatic breast cancer that had progressed on prior lines of therapy and included four patients with RB-positive TNBC; however, these four patients rapidly progressed on treatment [44]. Elsewhere, a phase I study demonstrated feasibility of alternating paclitaxel and palbociclib in patients with advanced breast cancer and included nine patients with TNBC. Of the nine, one patient’s disease was stable for over six months, though this response may have been from paclitaxel alone [45].
One promising area for use of palbociclib in TNBC is in the luminal androgen receptor (LAR) subtype, which expresses the androgen receptor (AR) and has been shown in cell line models to be sensitive both to bicalutamide, an AR antagonist, and to palbociclib [46,47]. A phase II clinical trial demonstrated that bicalutamide is safe and may be effective in patients with AR-positive (AR+) TNBC [40]. Subsequently, it was hypothesized that the addition of palbociclib will increase the efficacy of bicalutamide in patients with metastatic AR+ TNBC (Figure 1), and this is being investigated in an ongoing clinical trial (NCT02605486) [48] (Table 1).
2.3. Adjuvant and neoadjuvant use in HR+/HER2− breast cancer
Considerable interest has developed in expanding the use of palbociclib to earlier stages of HR+/HER2− breast cancer. Neoadjuvant treatment is used in cancer to improve surgical options, evaluate response to therapy, tailor individualized treatment approaches based on surgical tissue analyses, and obtain long-term disease-free survival [49]. Neoadjuvant palbociclib combined with endocrine therapies or compared to chemotherapy is being investigated in a number of clinical trials (Table 2).
Table 2:
Active clinical trials expanding palbociclib use in the adjuvant or neoadjuvant settings of HR+/HER2− breast cancer.
| Name/Identifier | Phase | Setting | Interventions | Primary Outcome Measures |
|---|---|---|---|---|
| NCT04130152 | I | NA | Palbociclib, letrozole and goserelin | Rate of cell cycle arrest at 4 weeks post-surgery |
| NCT03774472 | I/II | NA | Palbociclib, letrozole and hydroxychloroquine | Incidence of adverse events at 30
days Change in breast tumor proliferation index (Ki67) Change in autophagy, senescence, and cell cycle markers |
|
NCT01723774 NeoPalAna |
II | NA | Palbociclib, anastrozole and goserelin | Rate of cell cycle arrest in PI3K-mutant tumors or endocrine-resistant tumors |
|
NCT02008734 POP (Completed) |
II | NA | Palbociclib vs. no intervention | Change in Ki67 at time of surgery |
|
NCT02296801 PALLET |
II | NA | Letrozole Letrozole then palbociclib and letrozole Palbociclib then palbociclib and letrozole Palbociclib and letrozole |
Change in Ki67 at 14 weeks Rate of cCR |
|
NCT02400567 NeoPal |
II | NA | Chemotherapy Palbociclib and letrozole |
Number of patients with no residual cancer burden |
|
NCT02592083 PREDIX LumA |
II | NA | Endocrine therapy ± palbociclib | Rate of clinical and radiological response after 16 weeks of preoperative treatment |
|
NCT02603679 PREDIX LumB |
II | NA | Paclitaxel Palbociclib and tamoxifen Palbociclib and aromatase inhibitor Palbociclib, aromatase inhibitor and goserelin |
Rate of radiological objective response rate after completion of 12 weeks of preoperative treatment |
|
NCT02624973 PETREMAC |
II | NA/A | Neoadjuvant palbociclib and endocrine therapy followed by adjuvant palbociclib | Evaluate predictive and prognostic value of mutations in 300 cancer-related genes |
|
NCT02764541 PELOPS |
II | NA | Tamoxifen Letrozole then tamoxifen Tamoxifen then palbociclib and tamoxifen Letrozole then palbociclib and tamoxifen |
Anti-proliferative response rate after 2
years Rate of pCR after 2 years |
|
NCT03065621 NeoRHEA |
II | NA | Palbociclib and endocrine therapy | Identify biomarkers of resistance |
| NCT03535506 | II | NA | Palbociclib vs. no intervention | Feasibility and comparison of histological findings between groups |
|
NCT03573648 ImmunoADAPT |
II | NA | Avelumab and tamoxifen ± palbociclib | Rate of cCR at 2 years |
|
NCT03628066 NSABP FB-13 |
II | NA | Palbociclib, letrozole, goserelin and Oncotype DX Breast Recurrence Score | Complete cell cycle arrest at 6 weeks |
|
NCT03819010 DxCARTES (Completed) |
II | NA | Palbociclib and letrozole | Difference in Oncotype DX Breast Recurrence Score® at the time of surgery between groups with high or low socres at the time of diagnosis |
|
NCT04075604 CheckMate 7A8 |
II | NA | Palbociclib, anastrozole and
nivolumab Palbociclib and anastrozole then palbociclib, anastrozole and nivolumab Palbociclib and anastrozole |
Dose-limiting toxicity Residual cancer burden rate at 20 weeks |
|
NCT03447132 SAFIA |
III | NA | Palbociclib and fulvestrant vs. placebo and fulvestrant | Rate of pCR |
| NCT03969121 | III | NA | Palbociclib and endocrine therapy vs. placebo and endocrine therapy | Change in Pre-operative Endocrine Prognostic
Index score Change in EndoPredict™ EPclin Score |
| NCT02040857 | II | A | Palbociclib and endocrine therapy | Treatment discontinuation rate |
|
NCT03609047 Appalaches |
II | A | Palbociclib Chemotherapy |
Distant recurrence-free survival rate at 5 years |
|
NCT04247633 HIPEx |
II | A | Palbociclib and endocrine therapy | 3-year event-free survival |
|
NCT01864746 PENELOPE-B |
III | A | Palbociclib vs. placebo | Invasive disease-free survival time |
|
NCT02513394 PALLAS |
III | A | Palbociclib and endocrine therapy vs. endocrine therapy alone | Invasive disease-free survival time |
|
NCT03820830 POLAR |
III | A | Palbociclib and endocrine therapy vs. endocrine therapy alone | Invasive disease-free survival time |
Abbreviations: (A) Adjuvant; (cCR) Clinical complete response; (HR+/HER2−) Hormone receptor-positive/HER2-negative; (NA) Neoadjuvant; (pCR) Pathological complete response
Common primary outcome measures in the ongoing neoadjuvant clinical trials of palbociclib include histological evaluation of tumor proliferation markers such as Ki67 or the determination of the rate of pathological complete response (pCR), or residual cancer burden (RCB) at the time of surgery. For example, NeoPAL is a phase II clinical trial that evaluated the efficacy and safety of 20 weeks of chemotherapy or letrozole-palbociclib as neoadjuvant therapy in patients with HER2-negative stage II-IIIA invasive breast carcinoma who were not candidates for breast conserving surgery. Although the rate of pCR was low for both experimental arms, both groups demonstrated equivalent decreases in Ki67 levels. Additionally, patients in the letrozole-palbociclib arm experienced fewer serious adverse events than those in the chemotherapy arm, which provides promising evidence for replacing chemotherapy with targeted therapies in the future [50]. The PALLET trial is a phase II study that evaluated 14 weeks of combination palbociclib plus letrozole versus letrozole alone as neoadjuvant therapy in postmenopausal women with HR+ breast cancer. While the clinical response was not different between the two groups, the combination of palbociclib and letrozole significantly enhanced suppression of Ki67 in primary tumors [51].
A benefit of neoadjuvant intervention is that it facilitates the discovery and evaluation of biomarkers that can be correlated with response to treatment, vulnerability to additional therapies, or survival benefit. The phase II NeoPalAna trial evaluated the effects of neoadjuvant palbociclib and anastrozole on tumor cell proliferation and found that palbociclib enhanced cell cycle control, measured by reduced Ki67, regardless of luminal subtype [52]. Subsequent analysis from this trial found that drug-induced suppression of thymidine kinase 1, measured in peripheral serum, correlated with decreased Ki67 levels in these patients [53]. The phase II preoperative-palbociclib (POP) trial examined the effects of 14 days of preoperative palbociclib monotherapy in patients with early breast cancer. Tissue analyses from this trial found that short-term palbociclib treatment decreased protein levels of Ki67 in tumors compared to the no-treatment group, and decreased cyclin E2 mRNA expression correlated with decreased Ki67 levels [54].
While adjuvant endocrine therapy reduces the risk of breast cancer recurrence and death [55], disease recurrence after surgery remains a major issue in the treatment of breast cancer [56]. Several clinical trials examining the addition of adjuvant palbociclib to standard endocrine therapy are underway, with the unifying hypothesis that adding palbociclib to the standard-of-care treatments will prolong invasive disease-free survival time (Table 2). The phase III PENELOPE-B trial (NCT01864746), which is a post-neoadjuvant placebo-controlled study comparing palbociclib plus endocrine therapy to endocrine therapy alone, seeks to define a role for adjuvant palbociclib in the treatment of patients with residual disease after having received neoadjuvant chemotherapy. The phase III POLAR study (NCT03820830), compares palbociclib and endocrine therapy to endocrine therapy alone for the treatment of locoregional recurrent breast cancer. The phase III PALLAS study (NCT02513394), was designed to evaluate the addition of two years of palbociclib to standard adjuvant endocrine therapy in prolonging invasive disease-free survival time for patients with stage II-III HR+/HER2− early-stage breast cancer who received definitive surgery; however, at the interim analysis this trial was deemed unlikely to show a significant improvement and adjuvant palbociclib treatment was discontinued [57].
2.4. Palbociclib-based regimens compared to chemotherapy
The prospect of combining CDK4/6 inhibitors with cytotoxic chemotherapy remains challenging. DNA synthesis-targeting or mitosis-targeting agents are mechanistically at odds with CDK4/6 inhibitors, which cause cell cycle stasis at the G1/S checkpoint and prevent entry into the DNA synthesis and mitosis phases. Alternatively, a combination of palbociclib and additional agents could be administered instead of chemotherapy. In women with HR+/HER2− early breast cancer randomized to receive neoadjuvant palbociclib plus letrozole or chemotherapy, the palbociclib-letrozole combination caused fewer adverse effects than chemotherapy, though achievement of pathological complete response was poor in both groups (3.8% in the palbociclib-letrozole group versus 5.9% in the chemotherapy group) [50]. In premenopausal women with HR+/HER2− metastatic breast cancer whose disease had progressed after tamoxifen therapy, treatment with palbociclib plus exemestane with ovarian suppression resulted in an increased median progression-free survival of 20.1 months in the palbociclib plus exemestane group versus 14.4 months in the capecitabine group (hazard ratio 0.659, p=0.0235) [58]. In the PEARL study, women whose metastatic disease had progressed on non-steroidal aromatase inhibitors were randomized to receive capecitabine or one of two treatment regimens: palbociclib plus exemestane (cohort 1) or palbociclib plus fulvestrant (cohort 2). Median progression-free survival was not significantly different between treatment groups in cohort 2 (n=305): 7.5 months in the palbociclib plus fulvestrant group versus 10 months in the capecitabine group (hazard ratio 1.09, p=0.537). The median progression free survival was also not different comparing endocrine therapy plus palbociclib with capecitabine in patients with pre-treatment ESR1 wild type ctDNA. However, stratification by luminal subtype revealed that palbociclib plus fulvestrant is inferior to capecitabine in patients with non-luminal tumors, with median progression-free survival of 2.7 months in the palbociclib plus fulvestrant group versus 13.7 months in the capecitabine group (hazard ratio 3.19, p=0.013) [59]. Overall, the decision to employ palbociclib plus endocrine therapy or chemotherapy in treatment of HR+/HER2− disease remains complex. A recent meta-analysis has shown that no chemotherapy regimen is superior to palbociclib plus letrozole for progression-free survival in the HR+/HER2− metastatic setting [60]; therefore, when weighing the use of chemotherapy or CDK4/6 inhibitor plus endocrine therapy it is crucial to consider the receptor and molecular subtypes of the tumor, the previous lines of therapy the patient has received, the patient’s tolerance for the toxicities of each therapy, and the financial impact of an additional targeted therapy.
3. Conclusion
The role of CDK4/6 inhibitors as first-line treatment for patients with metastatic HR+/HER2− breast cancer is firmly established. Palbociclib is under investigation in earlier clinical stages and other subtypes of breast cancer, and the combination of palbociclib with HER2-targeted therapies or with androgen receptor antagonists remains under evaluation. Combination palbociclib and endocrine therapy may provide an alternative to chemotherapy in early or advanced breast cancer, particularly in luminal subtype tumors, though strategies for appropriate patient selection must first be optimized. Investigation in the neoadjuvant and adjuvant settings is also ongoing. While a blanket-use scenario for palbociclib as a part of adjuvant therapy seems unlikely given the anticipated negative results of the PALLAS study, there may yet be subgroups that could benefit from treatment. Patient selection by less-than-optimal response to neoadjuvant therapy and correlative biomarker analyses of patient subgroups will inform future preclinical and clinical research alike.
4. Expert Opinion
Palbociclib is an effective, safe, and well-tolerated treatment for patients with HR+/HER2− metastatic breast cancer. Current clinical research is exploring the potential expansion of palbociclib use into new subtypes of breast cancer and earlier clinical stages, as well as in different sequences with existing approved therapies or in combination with experimental compounds. Anti-estrogen endocrine therapies for patients with metastatic HR+/HER2− breast cancer synergize with CDK4/6 inhibition owing to estrogen signaling biology (Figure 1). In HER2+ breast cancer, anti-HER2 therapy and CDK4/6 inhibition were shown to synergize in preclinical models, providing rationale for combining palbociclib with HER2-directed therapies such as anti-HER2 antibodies, receptor tyrosine kinase inhibitors and antibody-drug conjugates. In TNBC, combination treatment with enzalutamide provides hope for targeted therapy against the LAR subtype, which is AR+; however, cytotoxic chemotherapy and immune therapy rather than palbociclib remain the mainstay of treatment in metastatic TNBC. Interestingly, the novel intravenous CDK4/6 inhibitor trilaciclib is under evaluation in combination with cytotoxic chemotherapy for its potential effects in preventing chemotherapy-induced myelosuppression and its potential to alter the tumor-immune microenvironment, in addition to a potential impact on response rates and survival [61].
A significant portion of patients will not benefit from the addition of palbociclib in first-line treatment of metastatic HR+/HER2− breast cancer but will endure its side effects, frequent follow-up requirements and elevated costs. Should all patients receive this CDK4/6 inhibitor in the first line? The answer may be provided by the SONIA study (NCT03425838), which is designed to evaluate if first-line treatment with a CDK4/6 inhibitor and endocrine therapy is superior to first-line treatment with endocrine therapy alone, followed by a CDK4/6 inhibitor in the second line. Also, a palbociclib monotherapy arm was not evaluated in the main phase II/III trials leading to its approval for patients with metastatic HR+/HER2− breast cancer. Palbociclib monotherapy as treatment of metastatic HR+/HER2− breast cancer was tested in the TREnd trial, which compared palbociclib alone to palbociclib given with the same endocrine therapy that was received prior to disease progression, with both arms being active in terms of clinical benefit ratio (54-60%), and a non-statistically significant progression free survival of 10.8 months in the combination group, compared to 6.5 months in the palbociclib group (Hazard Ratio 0.69; 95% CI: 0.4-1.1, exploratory p=0.12) [62]. In an exploratory analysis, a progression-free survival advantage in TREnd was identified in patients receiving combination palbociclib and endocrine therapy who had a history of receiving prior endocrine therapy for 6 months of greater (hazard ratio 0.53, p=0.02) [62]. Such a finding adds to the potential role for palbociclib in reversing acquired endocrine therapy resistance, a hypothesis which has also been discussed in a recent case series of 5 women with HR+/HER2− metastatic breast cancer who received palbociclib plus fulvestrant as second- or third-line therapy after disease progression on prior endocrine therapy [63].
In the adjuvant setting, palbociclib added to endocrine therapy in the PALLAS trial was deemed unlikely at interim analysis to show a significant improvement versus adjuvant endocrine therapy alone. A few weeks later, the interim results of the phase III MonarchE (NCT03155997) were reported, indicating that abemaciclib used in combination with adjuvant endocrine therapy had met the primary endpoint of increased invasive disease-free survival [64,65]. The differences in the selected population for these studies may hold the explanation for the discrepant results. PALLAS enrolled patients with stage II-III disease, while MonarchE selected a higher risk population based on tumor size, number of positive lymph nodes in the axilla and higher Ki67 at baseline. The full reports of these two studies are eagerly awaited. Additionally, the post-neoadjuvant phase III PENELOPE-B trial (NCT01864746) is also selecting a high-risk population for adjuvant palbociclib by requiring the presence of residual disease after neoadjuvant chemotherapy and surgery. Unfortunately, patient selection for therapy with CDK4/6 inhibitors cannot be based on surrogate biomarkers of benefit since none beyond ER+ has been validated to-date.
Finally, considerable interest has been generated in understanding the non-canonical effects of CDK4/6 inhibitors. The full mechanistic understanding of CDK4/6 inhibition in cancer is just beginning, as active cyclin D-CDK4/6 may phosphorylate over 300 targets in addition to RB [66]. Among other effects, CDK4/6 inhibitor therapies can alter immune activity within the tumor microenvironment, which creates an opportunity for these drugs to be combined with immune-modulatory therapies [67,68]. Based on promising preclinical evidence (reviewed in [69]), palbociclib is being tested clinically in combination with endocrine therapy and several PD-1 and PD-L1 immune checkpoint inhibitors (Table 3). Ultimately, palbociclib use in breast cancer is firmly established in the metastatic HR+/HER2− setting, and the large number of ongoing clinical trials involving palbociclib use in other types and stages of breast cancer will provide impactful guidance on the expansion of its use.
Table 3:
Active clinical trials exploring palbociclib use with checkpoint inhibitors.
| Name/Identifier | Phase | Setting | Interventions | Primary Outcome Measures |
|---|---|---|---|---|
|
NCT03573648 ImmunoADAPT |
II | NA | Palbociclib, tamoxifen and
avelumab Tamoxifen and avelumab only |
Rate of cCR at 2 years Safety and tolerability |
|
NCT04075604 CheckMate7A8 |
II | NA | Palbociclib, anastrozole and
nivolumab Palbociclib and anastrozole then palbociclib, anastrozole, and nivolumab Palbociclib and anastrozole |
Residual cancer burden rate Dose limiting toxicity |
| NCT02778685 | II | M | Letrozole, palbociclib and pembrolizumab | Response rate and duration of response |
|
NCT03147287 PACE |
II | M | Fulvestrant Palbociclib and fulvestrant Palbociclib, fulvestrant and avelumab |
PFS at 2 years Overall response rate Safety and tolerability |
Abbreviations: (cCR) Clinical complete response; (M) Metastatic; (NA) Neoadjuvant; (PFS) Progression-free survival
Drug Summary Box.
| Drug name | Palbociclib |
| Phase | Launched |
| Indication | Breast Cancer |
| Pharmacology description | Cyclin-dependent kinase 4
inhibitor Cyclin-dependent kinase 6 inhibitor |
| Route of administration | Oral |
| Chemical structure |  |
| Pivotal trial(s) | PALOMA-1/TRIO-18 [NCT00721409]30 PALOMA-2 [NCT01740427]31 PALOMA-3 [NCT01942135]32 |
Pharmaprojects - copyright to Citeline Drug Intelligence (an Informa business). Readers are referred to Informa-Pipeline (http://informa-pipeline.citeline.com) and Citeline (http://informa.citeline.com).
Acknowledgments
Declaration of Interest:
GT Gallanis was supported by a National Institutes of Health (NIH)/National Cancer Institute (NCI) grant T32 CA009686 while AT Riegel was supported by NIH/NCI grants RO1 CA205632, R21 C226542 and T32CA009686. PR Pohlmann meanwhile has received research funding via their institution from Genentech/Roche, Pfizer Inc, Seattle Genetics and Cascadian Therapeutics. She has also served on the speaker’s bureau of Genentech/Roche. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Contributor Information
Gregory T. Gallanis, Department of Oncology, Georgetown University, 3970 Reservoir Rd NW Building NRB Room E307, Washington, DC 20057.
Ramon I. Pericas, Department of Oncology, Georgetown University, 3970 Reservoir Rd NW Building NRB Room E307, Washington, DC 20057.
Anna T. Riegel, Department of Oncology, 3970 Reservoir Rd NW Building NRB Room E307, Washington, DC 20057.
Paula R. Pohlmann, Lombardi Comprehensive Cancer Center, Georgetown University, 3800 Reservoir Rd NW, 2nd Floor, Washington, DC 20007.
References
- [1].Palbociclib (Ibrance) package insert [Internet]. [cited 2020 Jun 1]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212436lbl.pdf.
- [2].Baldin V, Lukas J, Marcote MJ, et al. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. [DOI] [PubMed] [Google Scholar]
- [3].Matsushime H, Roussel MF, Ashmun RA, et al. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. [DOI] [PubMed] [Google Scholar]
- [4].Kato J, Matsushime H, Hiebert SW, et al. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. [DOI] [PubMed] [Google Scholar]
- [5].Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cobrinik D, Whyte P, Peeper DS, et al. Cell cycle-specific association of E2F with the p130 E1A-binding protein. Genes Dev. 1993;7:2392–2404. [DOI] [PubMed] [Google Scholar]
- [7].Zhu L, van den Heuvel S, Helin K, et al. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993;7:1111–1125. [DOI] [PubMed] [Google Scholar]
- [8].Asghar U, Witkiewicz AK, Turner NC, et al. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016;6:353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. [DOI] [PubMed] [Google Scholar]
- [11].Harbour JW, Luo RX, Dei Santi A, et al. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. [DOI] [PubMed] [Google Scholar]
- [12].Yao G, Lee TJ, Mori S, et al. A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol. 2008;10:476–482. [DOI] [PubMed] [Google Scholar]
- [13].Massagué J G1 cell-cycle control and cancer. Nature. 2004;432:298–306. [DOI] [PubMed] [Google Scholar]
- [14].Lowe SW, Sherr CJ. Tumor suppression by Ink4a-Arf: progress and puzzles. Curr Opin Genet Dev. 2003;13:77–83. [DOI] [PubMed] [Google Scholar]
- [15].Paternot S, Bockstaele L, Bisteau X, et al. Rb inactivation in cell cycle and cancer: The puzzle of highly regulated activating phosphorylation of CDK4 versus constitutively active CDK-activating kinase. Cell Cycle. 2010;9:689–699. [DOI] [PubMed] [Google Scholar]
- [16].Malumbres M, Sotillo R, Santamaría D, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell. 2004;118:493–504. [DOI] [PubMed] [Google Scholar]; * This important work using murine knock-out models led to the discovery that cyclin-dependent kinases have redundant functions in mammalian cells and that mice lacking various combinations of CDKs can develop with only limited impairments.
- [17].Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. [DOI] [PubMed] [Google Scholar]; * This work led to the discovery that breast cancer can become addicted to growth through the cyclin D-CDK4 axis, paving the way for development of CDK4/6 inhibition as a therapeutic strategy in cancer.
- [18].Liu M-M, Albanese C, Anderson CM, et al. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J Biol Chem. 2002;277:24353–24360. [DOI] [PubMed] [Google Scholar]
- [19].Neuman E, Ladha MH, Lin N, et al. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zwijsen RML, Wientjens E, Klompmaker R, et al. CDK-Independent Activation of Estrogen Receptor by Cyclin D1. Cell. 1997;88:405–415. [DOI] [PubMed] [Google Scholar]
- [21].Gillett C, Fantl V, Smith R. Amplification and Overexpression of Cyclin D1 in Breast Cancer Detected by Immunohistochemical Staining. 1994;8. [PubMed] [Google Scholar]
- [22].Kenny FS, Hui R, Musgrove EA, et al. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 1999;5:2069–2076. [PubMed] [Google Scholar]
- [23].Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- [24].Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res BCR. 2009;11:R77. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This seminal work provided key preclinical evidence that led to the investigation of palbociclib use in clinical trials.
- [25].Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Raspé E, Coulonval K, Pita JM, et al. CDK4 phosphorylation status and a linked gene expression profile predict sensitivity to palbociclib. EMBO Mol Med. 2017;9:1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This key investigation into the role of T172 phosphorylation of CDK4 has led to development of a gene expression signature that predicts sensitivity to palbociclib across multiple breast cancer subtypes.
- [27].Álvarez-Fernández M, Malumbres M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell. 2020;37:514–529. [DOI] [PubMed] [Google Scholar]
- [28].Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:568–576. [DOI] [PubMed] [Google Scholar]
- [29].Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer. 2011;104:1862–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 2015;16:25–35. [DOI] [PubMed] [Google Scholar]; ** This phase II clinical trial led to the accelerated approval of palbociclib by the FDA as the first-in-class CDK4/6-specific inhibitor.
- [31].Finn RS, Martin M, Rugo HS, et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- [32].Turner NC, Ro J, André F, et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209–219. [DOI] [PubMed] [Google Scholar]
- [33].Loibl S, Turner NC, Ro J, et al. Palbociclib Combined with Fulvestrant in Premenopausal Women with Advanced Breast Cancer and Prior Progression on Endocrine Therapy: PALOMA-3 Results. The Oncologist 2017;22:1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Turner NC, Slamon DJ, Ro J, et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379:1926–1936. [DOI] [PubMed] [Google Scholar]
- [35].Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. [DOI] [PubMed] [Google Scholar]
- [36].Choi YJ, Li X, Hydbring P, et al. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22:438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nikolai BC, Lanz RB, York B, et al. HER2 Signaling Drives DNA Anabolism and Proliferation through SRC-3 Phosphorylation and E2F1-Regulated Genes. Cancer Res. 2016;76:1463–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goel S, Wang Q, Watt AC, et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell. 2016;29:255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This preclinical investigation of abemaciclib in murine models of HER2+ breast cancer has provided key evidence and rationale for investigating CDK4/6 inhibitors in the HER2+ setting.
- [39].Gianni L, Bisagni G, Colleoni M, et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol 2018;19:249–256. [DOI] [PubMed] [Google Scholar]
- [40].Gucalp A, Tolaney S, Isakoff SJ, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2013;19:5505–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. [DOI] [PubMed] [Google Scholar]
- [42].Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res Off J Am Assoc Cancer Res. 2007;13:4429–4434. [DOI] [PubMed] [Google Scholar]
- [43].Gong X, Litchfield LM, Webster Y, et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell. 2017;32:761–776. e6. [DOI] [PubMed] [Google Scholar]
- [44].DeMichele A, Clark AS, Tan KS, et al. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res Off J Am Assoc Cancer Res. 2015;21:995–1001. [DOI] [PubMed] [Google Scholar]
- [45].Clark AS, McAndrew NP, Troxel A, et al. Combination Paclitaxel and Palbociclib: Results of a Phase I Trial in Advanced Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2019;25:2072–2079. [DOI] [PubMed] [Google Scholar]
- [46].Lehmann BD, Bauer JA, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Asghar US, Barr AR, Cutts R, et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:5561–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]; * References 46 and 47: these investigations have led to the exploration of CDK4/6 inhibitor use in the androgen receptor-expressing subtype of triple-negative breast cancer.
- [48].Gucalp A, Edelweiss M, Patil S, et al. Abstract P3–11-04: Phase I/II trial of palbociclib in combination with bicalutamide for the treatment of androgen receptor (AR)+ metastatic breast cancer (MBC). Poster Sess Abstr [Internet]. American Association for Cancer Research; 2018. [cited 2020 Jun 4]. p. P3-11-04–P3-11-04. Available from: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.SABCS17-P3-11-04. [Google Scholar]
- [49].Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations From an International Expert Panel on the Use of Neoadjuvant (Primary) Systemic Treatment of Operable Breast Cancer: An Update. J Clin Oncol. 2006;24:1940–1949. [DOI] [PubMed] [Google Scholar]
- [50].Cottu P, D’Hondt V, Dureau S, et al. Letrozole and palbociclib versus chemotherapy as neoadjuvant therapy of high-risk luminal breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:2334–2340. [DOI] [PubMed] [Google Scholar]
- [51].Johnston S, Puhalla S, Wheatley D, et al. Randomized Phase II Study Evaluating Palbociclib in Addition to Letrozole as Neoadjuvant Therapy in Estrogen Receptor-Positive Early Breast Cancer: PALLET Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37:178–189. [DOI] [PubMed] [Google Scholar]
- [52].Ma CX, Gao F, Luo J, et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23:4055–4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bagegni N, Thomas S, Liu N, et al. Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib. Breast Cancer Res. 2017;19:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Arnedos M, Bayar MA, Cheaib B, et al. Modulation of Rb phosphorylation and antiproliferative response to palbociclib: the preoperative-palbociclib (POP) randomized clinical trial. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:1755–1762. [DOI] [PubMed] [Google Scholar]
- [55].Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet Lond Engl 2011;378:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Pan H, Gray R, Braybrooke J, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med. 2017;377:1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pfizer Provides Update on Phase 3 PALLAS Trial of Ibrance® (palbociclib) Plus Endocrine Therapy in HR+, HER2− Early Breast Cancer [Internet]. 2020. [cited 2020 Jun 10]. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-provides-update-phase-3-pallas-trial-ibrancer.
- [58].Park YH, Kim T-Y, Kim GM, et al. Palbociclib plus exemestane with gonadotropin-releasing hormone agonist versus capecitabine in premenopausal women with hormone receptor-positive, HER2-negative metastatic breast cancer (KCSG-BR15–10): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2019;20:1750–1759. [DOI] [PubMed] [Google Scholar]
- [59].Martín M, Zielinski C, Ruíz-Borrego M, et al. Abstract GS2–07: Results from PEARL study (GEICAM/2013–02_CECOG/BC.1.3.006): A phase 3 trial of Palbociclib (PAL) in combination with endocrine therapy (ET) versus Capecitabine (CAPE) in hormonal receptor (HR)-positive/human epidermal growth factor receptor (HER) 2-negative metastatic breast cancer (MBC) patients (pts) whose disease progressed on aromatase inhibitors (AIs). Gen Sess Abstr [Internet]. American Association for Cancer Research; 2020. [cited 2020 Sep 23]. p. GS2-07–GS2-07. Available from: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.SABCS19-GS2-07. [Google Scholar]
- [60].Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20:1360–1369. [DOI] [PubMed] [Google Scholar]
- [61].Tan AR, Wright GS, Thummala AR, et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol 2019;20:1587–1601. [DOI] [PubMed] [Google Scholar]
- [62].Malorni L, Curigliano G, Minisini AM, et al. Palbociclib as single agent or in combination with the endocrine therapy received before disease progression for estrogen receptor-positive, HER2-negative metastatic breast cancer: TREnd trial. Ann Oncol Off J Eur Soc Med Oncol. 2018;29:1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Schettini F, De Santo I, Rea C, et al. Palbociclib added to ongoing endocrine therapy for hormone receptor positive HER2 negative metastatic breast cancer: A case report series. Mol Clin Oncol [Internet]. 2020. [cited 2020 Sep 23]; Available from: http://www.spandidos-publications.com/10.3892/mco.2020.2016. [DOI] [PMC free article] [PubMed]
- [64].Rastogi P, Toi M, Harbeck N, et al. Abstract OT3-05-05: MonarchE: A randomized, open-label, phase 3 study of abemaciclib combined with standard adjuvant endocrine therapy versus standard adjuvant endocrine therapy alone in patients with high risk, node positive, early stage, HR+, HER2− breast cancer. Ongoing Clin Trials [Internet]. American Association for Cancer Research; 2018. [cited 2020 Jul 20]. p. OT3-05-05–OT3-05-05. Available from: http://cancerres.aacrjournals.org/lookup/doi/10.1158/1538-7445.SABCS17-OT3-05-05. [Google Scholar]
- [65].Verzenio® (abemaciclib) Significantly Reduced the Risk of Cancer Returning in People with High Risk HR+, HER2− Early Breast Cancer [Internet]. 2020. [cited 2020 Jun 30]. Available from: https://investor.lilly.com/news-releases/news-release-details/verzenior-abemaciclib-significantly-reduced-risk-cancer.
- [66].Anders L, Ke N, Hydbring P, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–634. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This key work has led to the discovery that cyclin D-CDK4/6 complexes phosphorylate hundreds of additional substrates in addition to retinoblastoma protein and has drastically increased the scope of investigation in CDK4/6 biology.
- [67].Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature. 2017;548:471–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bonelli M, La Monica S, Fumarola C, et al. Multiple effects of CDK4/6 inhibition in cancer: From cell cycle arrest to immunomodulation. Biochem Pharmacol. 2019;170:113676. [DOI] [PubMed] [Google Scholar]
- [69].Ameratunga M, Kipps E, Okines AFC, et al. To Cycle or Fight—CDK4/6 Inhibitors at the Crossroads of Anticancer Immunity. Clin Cancer Res. 2019;25:21–28. [DOI] [PubMed] [Google Scholar]


