Abstract
Background:
Insulin-like growth factor-1 (IGF-1) plays a key role in the pathogenesis of metabolic syndrome (MS), which is in turn associated with asthma. Whether IGF-1 contributes to asthma causation or asthma severity is largely unknown.
Objective :
To examine the relation between serum IGF-1 and asthma, asthma outcomes, and lung function in adults.
Methods:
Cross-sectional study of 297,590 adults (aged 40–69 years) who participated in the United Kingdom Biobank, had no diagnosis of diabetes, and were not on insulin. Multivariable logistic or linear regression was used to analyze serum IGF-1 and physician-diagnosed asthma, current wheeze, asthma hospitalizations, and lung function measures (FEV 1, FVC and FEV 1 /FVC).
Results:
Serum IGF-1 levels above the lowest quartile (Q1) were significantly associated with lower odds of asthma (adjusted odds ratio [aOR] for fourth quartile (Q4) vs. Q1=0.88, 95% confidence interval [CI]=0.85–0.91). Among participants with asthma, IGF-1 levels above Q1 were significantly associated with lower odds of current wheeze (aOR for Q4 vs. Q1=0.89, 95% CI=0.83–0.96), but not with asthma hospitalizations. Serum IGF-1 was significantly and positively associated with FEV 1 (b=20.9 mL, 95%CI=19.1–22.7) and FVC (b=25.6 mL, 95%CI=23.4–27.7), regardless of an asthma diagnosis; these associations were significant in men and women, with larger estimated effects in men.
Conclusion:
In a large study of British adults, higher serum IGF-1 levels were associated with lower odds of asthma and current wheeze, and higher FEV 1 and FVC. Our findings suggest potential beneficial effects of circulating IGF-1 on asthma and asthma outcomes in adults.
Keywords: insulin-like growth factor-1, asthma, wheeze, lung function, FEV1, UK Biobank
INTRODUCTION
Asthma affects approximately 339 million children and adults worldwide1. Total yearly asthma costs exceed $81 billion dollars in the U.S.2 and ~72 billion Euros in Europe3. In the United Kingdom (U.K.), 1.1 million children and 4.3 million adults currently receive treatment for asthma, and the disease leads to 93,000 hospitalizations per year and at least £1.1 billion medical care costs annually4, 5. While asthma mortality has been reduced in most age groups, a recent study reported a rise in deaths from asthma among the elderly in England and Wales6.
Insulin-like growth factors (IGFs) play an important role in metabolism and growth7, 8. Through bi-directional interactions with the immune system, IGFs can contribute to the pathogenesis of diseases such as cancer, cardiovascular disorders, and inflammatory lung diseases9–12. IGF-1 (somatomedin C) is a 70-aminoacid polypeptide with similar sequence and molecular structure to insulin. IGF-1 production primarily occurs in the liver and is induced by growth hormone (GH). IGF-1 can act as a hormone that mediates anabolic and linear growth, thus promoting cell growth and differentiation7, 13. IGF-1 also regulates the metabolism of glucose and lipids8.
Although IGF-1 deficiency has been linked to metabolic syndrome (MS), which has in turn been associated with asthma, few human studies have investigated the role of IGF-1 in asthma pathogenesis. IGF-1 may play a role in asthma by modulating airway inflammation, airway hyperresponsiveness, and airway smooth hyperplasia14. In murine models of asthma, IGF-1 is upregulated in lung tissue, and administration of IGF-1-neutralizing antibodies ameliorates airway resistance and inflammation15. In an in vivo study, IGF-1 promoted airway inflammation by upregulating IGF binding protein 3 (IGFBP-3), which can have profibrotic effects16. A recent case-control study reported that serum IGF-1 was higher in subjects with asthma than in control subjects, but those findings must be cautiously interpreted due to small sample size and potential bias by confounding17. In contrast to those results, serum IGF-1 was positively associated with lung function in two epidemiologic studies in adults18, 19.
Given inconsistent findings for a potential role of IGF-1 in asthma pathogenesis from animal and epidemiologic studies, we examined the relation between peripheral serum IGF-1 and asthma, current wheeze, asthma hospitalizations, and lung function in 297,590 non-diabetic British adults aged 40 to 69 years.
METHODS
Study design and study population
The UK Biobank is a national, prospective, population-based study established to identify determinants of complex diseases and improve the health outcomes of British adults. Approximately 9.2 million individuals aged 40–69 years who lived within 25 miles of twenty-two assessment centers in England, Wales, and Scotland were invited to enter the cohort, and ~500,000 (5.5%) of those 9.2 million people volunteered to participate in the baseline assessment20. Extensive data, including questionnaires, physical measures, and biological samples were collected at baseline, with longitudinal follow-up for a wide range of health-related outcomes20. The UK Biobank was approved by the UK National Health Service National Research Ethics Service (Ref 11/NW/0382) and informed consent was obtained from all participants. Details of the methods, protocols, and definitions used in the study can be found at the UK Biobank website (https://www.ukbiobank.ac.uk/).
The current study was conducted using the UK Biobank Resource under application #43252. The flowchart for selection of participants from the UK Biobank into the current analysis is shown in Figure 1. Of 502,506 participants recruited at baseline, 297,590 were not pregnant, had neither a diagnosis of diabetes nor reported use of insulin, and had complete information on asthma status and IGF-1 levels, and were thus included in the current analysis.
Figure 1-.
Flowchart for selection of the UK Biobank participants included in the current analysis
Outcomes
Asthma was defined by selection of “asthma” as an answer to the following question: “Has a doctor ever told you that you have had any of the following conditions: blood clot in the leg, blood clot in the lung, emphysema/chronic bronchitis, asthma, hay fever, allergic rhinitis or eczema?” Control subjects were participants who did not select “asthma” as an answer to the question above. Current wheeze was defined as a “Yes” answer to the following question: “In the last year, have you ever had wheeze or whistling in the chest?” In subjects with asthma, a hospitalization for asthma was defined as ever having had a hospitalization with an International Classification of Diseases Clinical Modification (ICD) code of main diagnosis compatible with asthma (ICD-9: 493.x or ICD-10: J45.x and J46.x), excluding hospitalizations with an ICD code for a main diagnosis consistent with chronic obstructive pulmonary disease (COPD, ICD-9: J43, J44, J47 or ICD-10: 490, 491, 492, 494, 496). Data on asthma hospitalizations were available before (range=0 to 27 years, median=6 years) and after (range=1 to 9 years, median=4 years) the participant’s IGF-1 measurement.
Spirometry was performed using a Vitalograph Pneumotrac 6800 spirometer (Vitalograph Ltd., Buckingham, England), following European Respiratory Society/American Thoracic Society criteria for acceptability and reproducibility21. Participants were excluded from spirometry testing if they were being treated for tuberculosis, or if they had a chest infection in the last month, history of a detached retina, a heart attack, eye surgery, collapsed lung or a pneumothorax, or surgery in the chest or abdomen in the previous 3 months. Participants with extreme values for FEV1 or FVC (< 1st or > 99th percentile) were excluded from the analysis of lung function measures.
Peripheral serum insulin-like growth factor-1
The UK Biobank collected blood samples during the baseline assessment visit22. Serum IGF-1 was measured at the UK Biobank central laboratory by a one-step sandwich chemiluminescent immunoassay, on a DiaSorin Liaison XL (Diasorin S.p.A, Borsa Italiana). Internal quality control and external quality assurance schemes were used to verify assay performance. Samples with results exceeding the reportable range of the assay were diluted and re-analyzed (automatic dilution)23. Serum glycated hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography on a Bio-Rad VARIANT II Turbo hemoglobin testing system. Serum high sensitivity C-reactive protein (CRP) was measured by immuno-turbidimetric method on Beckman Coulter AU5800 analyzer (Beckman Coulter, UK, Ltd).
Statistical analysis
Bivariate analyses were conducted using two-sided Wald chi-square tests or t-tests, as appropriate. Logistic regression was used for the multivariable analyses of serum IGF-1 (as quartiles) and asthma, current wheeze, and at least one asthma hospitalization (ever). Known or potential confounders of the relation between IGF-1 and asthma were included in the multivariable models. All models were adjusted for age, sex, race/ethnicity (Caucasian vs. other), annual household income (< £31,000 vs. ≥ £31,000 per year, near the median household income for the UK in 201924), body mass index (BMI), smoking status (never, former, or current), packyears of cigarette smoking, serum levels of HbA1c and CRP, and season and time of the day when the samples were collected (to account for daily and seasonal variation). MS was defined by the presence of at least 3 of the following criteria: fasting glucose level of 110 mg/dL or greater, waist circumference value greater than 102 cm in men or 88 cm in women, triglyceride level of 150 mg/dL or greater, high-density lipoproteins (HDL) level less than 50 mg/dL in men or 40 mg/dL in women, and either systolic blood pressure of 130 mmHg or greater or diastolic blood pressure of 85 or greater25. After the final models were built, we tested for an interaction between IGF-1 and sex, obesity, or MS. Linear regression was used for the multivariable analysis of IGF-1 and lung function measures (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], and FEV1/FVC), which was first conducted in all subjects, and then separately in subjects with and without asthma. Models for lung function measures were adjusted for an asthma diagnosis (in all participants), age, sex, race/ethnicity, annual household income, BMI, smoking status, pack-years of cigarette smoking, serum levels of HbA1c and CRP, and season and time of the day when the samples were collected. Models for FEV1 and FVC were additionally adjusted for height and height squared.
Because 142,170 (32%) of the eligible participants were excluded from the analysis due to missing data for covariates (Figure 1), a multiple imputation procedure was used to include those participants in a sensitivity analysis. A fully conditional specification (FCS) method was used to create five imputed datasets, and regression analyses were then repeatedly performed for each of these five datasets. Final results were generated by combining five sets of regression coefficients using Rubin’s combining rules26. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and two-sided P-values <0.05 were considered significant.
RESULTS
Table 1 shows the main characteristics of the study participants by asthma status. Of the 297,590 participants, 34,023 (11.4%) reported having physician-diagnosed asthma (hereinafter referred to as “asthma”). Compared to control subjects (n=263,567), participants with asthma were younger and more likely to be female, non-Caucasian, and non-current smokers; and to have higher BMI and higher serum HbA1c and CRP levels, but lower serum IGF-1 and lower lung function measures (FEV1, FVC and FEV1/FVC). Participants with asthma were also more likely to have MS. Serum IGF-1 decreased with age in both women and men, regardless of asthma status (Figure 2).
Table 1.
Characteristics of study participants, by asthma status
| Characteristics | Asthma (n=34,023) | Controls (n=263,567) |
|---|---|---|
| Age at recruitment | 55.0 ± 8.2* | 55.9 ± 8.1 |
| Female sex | 19394 (57.0)* | 141031 (53.5) |
| Ethnicity | ||
| Caucasian | 32517 (95.6)* | 252582 (95.8) |
| Other | 1512 (4.4) | 11019 (4.2) |
| Annual household income < £30,000 | 16083 (47.3) | 124012 (47.1) |
| BMI (kg/m2) | 27.8 ± 5.1* | 27.1 ± 4.5 |
| Obesity (BMI > 30 kg/m2) | 9094 (26.9)* | 57050 (21.8) |
| Smoking | ||
| Never | 21981 (64.6)* | 170159 (64.6) |
| Former | 8983 (26.4) | 67130 (25.5) |
| Current | 3059 (9.0) | 26278 (10.0) |
| Pack-years of cigarette smoking | 8.1 ± 15.6 | 7.9 ± 15.1 |
| IGF-1 (nmol/L | 21.4 ± 5.8* | 21.6 ± 5.6 |
| HbA1c (mmol/mol) | 35.3 ± 4.6* | 35.1 ± 4.6 |
| CRP (mg/L) | 3.0 ± 4.9* | 2.5 ± 4.1 |
| Metabolic syndrome† | 8496 (29.2)* | 59055 (26.3) |
| FEV1 (ml)† | 2677 ± 725* | 2913 ± 724 |
| FVC (ml)† | 3682 ± 929* | 3837 ± 919 |
| FEV1/FVC (%)† | 72.5 ± 7.6* | 76.1 ± 5.9 |
| Wheeze in the last year | 21991 (65.5) | - |
| Asthma-related hospitalization, ever | 1086 (3.4) | - |
Abbreviations: BMI, body mass index; IGF-1, insulin-like growth factor 1; HbA1c, glycated hemoglobin A1c; CRP, C-reactive protein; FEV1, forced expiratory volume in one second; FVC, forced vital capacity
Results are shown as mean ± standard deviation (SD) for continuous variables, and as N (%) for binary variables.
Numbers (%) may vary due to missingness.
P <0.05 for comparison of participants with current asthma vs. controls, within each study cohort.
Figure 2-.
Serum levels of IGF-1 in women and men, by age group and asthma status
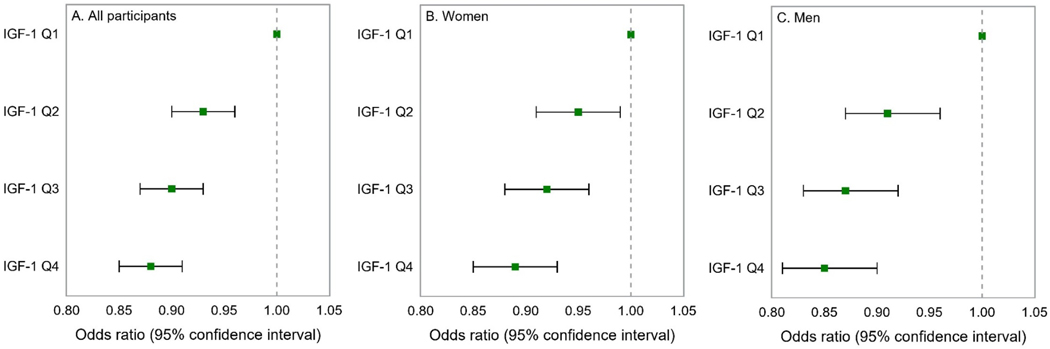
The results of the multivariable analysis of serum IGF-1 and asthma are shown in Figure 3. In this analysis, participants whose IGF-1 level was above the first (lowest) quartile (Q1) had 7% to 12% significantly lower odds of asthma than those with levels in the first quartile (e.g., adjusted odds ratio [aOR] for the fourth or highest quartile vs. the first quartile=0.88, 95% confidence interval [CI]=0.85–0.91). Because of significant modification of the estimated effect of IGF-1 on asthma by sex (P for interaction=0.01), we repeated the analysis after stratification by sex. In this analysis, women whose IGF-1 level was above the first quartile had 5%−11% lower odds of asthma than those whose levels were in the first quartile, and men whose IGF-1 level was above the first quartile had 9%−15% lower odds of asthma than those whose levels were in the first quartile. We found no significant modification of the estimated effect of IGF-1 on asthma by obesity (defined as BMI ≥30 kg/m2, P for interaction P=0.74).
Figure 3-.
Multivariable analysis of IGF-1 and asthma in all participants, and separately in women and men.
Footnote: Q=quartile. All models adjusted for sex (in all participants), age, ethnicity, annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season of the examination, the time of the day when the examination was performed, serum level of glycated hemoglobin A1c, and C-reactive protein.
To reduce the impact of potential misclassification of COPD as asthma, we repeated the multivariable analysis of asthma after stratification by smoking status (current smokers vs. never smokers and former smokers with <10 pack-years of cigarette smoking), obtaining similar results (Table 2). Similar results were obtained after excluding participants with a self-reported diagnosis of emphysema or chronic bronchitis (e-Table 1). In another sensitivity analysis, we repeated the multivariable analysis of serum IGF-1 and asthma after imputing data for missing covariates in all eligible participants (n=439,760). This analysis yielded similar results to those from the analysis that excluded participants with missing covariates (e-Table 1).
Table 2.
Multivariable analysis of IGF-1 and asthma by smoking status
| Current smokers (n=29,337) | Never smoker and former smokers with < 10 pack-year cigarette smoking (n=214,907) | |
|---|---|---|
| IGF-1 quartile (nmol/L) | Odds Ratio (95% Confidence Interval) | |
| Q1 (< 17.8) | 1.0 | 1.0 |
| Q2 (17.8– < 21.5) | 0.86 (0.78–0.95)* | 0.95 (0.91–0.98)* |
| Q3 (21.5– < 25.0) | 0.80 (0.72–0.89)* | 0.92 (0.88–0.95)* |
| Q4 (≥ 25.0) | 0.77 (0.69–0.86)*† | 0.89 (0.85–0.92)*† |
Abbreviation: IGF-1, insulin-growth like factor 1
All models adjusted for age, sex, ethnicity, annual household income, body mass index, packyears of cigarette smoking (in current smokers), the season of the examination, the time of the day when the examination was performed, serum level of glycated hemoglobin A1c, and C-reactive protein.
P <0.01
P for trend <0.05
e-Table 1.
Sensitivity analysis on IGF-1 and asthma
| All participants | Women | Men | |
|---|---|---|---|
| IGF-1 quartile (nmol/L) | Odds Ratio (95% Confidence Interval) | ||
| Excluding participants with a diagnosis of emphysema or chronic bronchitis | n=293,114 | n=158,226 | n=134,888 |
| Q1 (< 17.6) | 1.0 | 1.0 | 1.0 |
| Q2 (17.6– < 21.3) | 0.93 (0.90–0.97)† | 0.95 (0.91–0.99)* | 0.92 (0.87–0.96)† |
| Q3 (21.3– < 24.9) | 0.90 (0.87–0.93)† | 0.91 (0.87–0.95)† | 0.87 (0.83–0.92)† |
| Q4 (≥ 24.9) | 0.87 (0.84–0.90)†‡ | 0.87 (0.85–0.93)†‡ | 0.85 (0.81–0.90)†‡ |
| Multiple imputation | n=439,760 | n=242,249 | n=197,511 |
| Q1 (< 17.6) | 1.0 | 1.0 | 1.0 |
| Q2 (17.6– < 21.3) | 0.92 (0.90–0.95)† | 0.92 (0.89–0.96)† | 0.92 (0.88–0.96)† |
| Q3 (21.3– < 24.9) | 0.89 (0.87–0.92)† | 0.91 (0.88–0.94)† | 0.88 (0.84–0.92)† |
| Q4 (≥ 24.9) | 0.88 (0.85–0.90)†‡ | 0.89 (0.86–0.92)†‡ | 0.86 (0.83–0.90)†‡ |
All models adjusted for age, sex (in all participants), ethnicity, annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season of the examination, the time of the day when the examination was performed, serum level of glycated hemoglobin A1c, and C-reactive protein.
P <0.05
P <0.05
P for trend <0.05
Table 3 shows the results of the multivariable analysis of serum IGF-1 and current wheeze or at least one asthma-related hospitalization. In this analysis, participants with asthma whose IGF-1 level was above the first quartile had 11%−12% lower odds of current wheeze (aOR for the fourth vs. the first quartile=0.89, 95% confidence interval [CI]=0.83–0.96). This association was significant in both women and men. Serum IGF-1 was not associated with having ever had an asthma hospitalization (in the whole cohort or in each sex). Furthermore, there was no significant association between IGF-1 and subsequent asthma hospitalizations (i.e. those occurring after IGF-1 was measured) or asthma hospitalizations occurring either one year prior to or after the IGF-1 measurement (e-Table 2).
Table 3.
Multivariable analysis of quartiles of serum IGF-1 level and asthma outcomes in participants with asthma (n=34,023)
| All participants | Women | Men | |
|---|---|---|---|
| IGF-1 quartile (Q), nmol/L | Odds Ratio (95% Confidence Interval) | ||
| Wheeze in the last year | n=21,991 | n=12,477 | n=9,514 |
| Q1 (< 17.4) | 1.0 | 1.0 | 1.0 |
| Q2 (17.4– < 21.2) | 0.88 (0.83–0.95)† | 0.90 (0.82–0.97)* | 0.87 (0.78–0.97)* |
| Q3 (21.2– < 24.8) | 0.88 (0.83–0.95)† | 0.89 (0.82–0.98)* | 0.87 (0.78–0.97)* |
| Q4 (≥ 24.8) | 0.89 (0.83–0.96)†‡ | 0.92 (0.84–1.00) | 0.87 (0.78–0.97)*‡ |
| At least one asthma-related hospitalization | n=1,086 | n=713 | n=373 |
| Q1 (< 17.4) | 1.0 | 1.0 | 1.0 |
| Q2 (17.4– < 21.2) | 0.98 (0.82–1.16) | 0.95 (0.77–1.17) | 1.04 (0.75–1.44) |
| Q3 (21.2– < 24.8) | 1.18 (0.99–1.40) | 1.17 (0.95–1.44) | 1.23 (0.90–1.68) |
| Q4 (≥ 24.8) | 0.97 (0.81–1.17) | 0.87 (0.69–1.10) | 1.17 (0.85–1.62) |
Abbreviation: IGF-1, insulin-like growth factor 1
All models adjusted for sex (in all participants), age, ethnicity, annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season of the examination, the time of the day when the examination was performed, serum level of glycated hemoglobin A1c, and C-reactive protein.
P <0.5
P <0.01
P for trend <0.05
e-Table 2.
Multivariable analysis of IGF-1 and asthma hospitalization in participants with asthma (n=34,023)
| All participants | Women | Men | |
|---|---|---|---|
| IGF-1 quartile (nmol/L) | Odds Ratio (95% Confidence Interval) | ||
| ≥1 asthma hospitalization after IGF-1 measure | n=440 | n=286 | n=154 |
| Q1 (< 17.4) | 1.0 | 1.0 | 1.0 |
| Q2 (17.4– < 21.2) | 0.88 (0.68–1.15) | 0.87 (0.63–1.20) | 0.92 (0.57–1.48) |
| Q3 (21.2– < 24.8) | 1.04 (0.80–1.35) | 0.98 (0.71–1.35) | 1.16 (0.74–1.82) |
| Q4 (≥ 24.8) | 0.82 (0.61–1.09) | 0.82 (0.58–1.18) | 0.84 (0.51–1.37) |
| ≥1 asthma hospitalization 1 year prior to or after IGF-1 measure | n=147 | n=95 | n=154 |
| Q1 (< 17.4) | 1.0 | 1.0 | 1.0 |
| Q2 (17.4– < 21.2) | 0.99 (0.62–1.59) | 0.97 (0.56–1.71) | 1.03 (0.42–2.50) |
| Q3 (21.2– < 24.8) | 1.32 (0.84–2.07) | 1.14 (0.65–2.00) | 1.67 (0.75–3.76) |
| Q4 (≥ 24.8) | 1.02 (0.62–1.68) | 0.96 (0.52–1.78) | 1.17 (0.49–2.80) |
All models adjusted for age, sex (in all participants), ethnicity, annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season of the examination, the time of the day when the examination was performed, serum level of glycated hemoglobin A1c, and C-reactive protein.
P <0.5
P <0.01
P for trend <0.05
To examine whether the estimated effect of the IGF-1 on asthma or asthma outcomes is modified by MS, we conducted a separate analysis that included MS in the multivariate models. This analysis yielded similar results to those from the analysis that did not include MS in the models (Table 4). There were no significant interactions between IGF-1 level and MS on asthma (P=0.24), current wheeze (P=0.75), or asthma-related hospitalizations (P=0.72).
Table 4.
Multivariable analysis of IGF-1 and asthma and asthma outcomes adjusting for metabolic syndrome
| All participants (n=253,718) | Women (n=135, 541) | Men (n=118, 177) | |
|---|---|---|---|
| IGF-1 quartile (nmol/L) | Odds Ratio (95% Confidence Interval) | ||
| Asthma | n=29,146 | n=16,463 | n=12,683 |
| Q1 (< 17.8) | 1.0 | 1.0 | 1.0 |
| Q2 (17.8– < 21.5) | 0.94 (0.91–0.97)† | 0.95 (0.91–0.99)* | 0.92 (0.87–0.97)† |
| Q3 (21.5– < 25.0) | 0.90 (0.87–0.93)† | 0.92 (0.88–0.97)† | 0.87 (0.82–0.92)† |
| Q4 (≥ 25.0) | 0.88 (0.85–0.91)†‡ | 0.89 (0.85–0.94)†‡ | 0.85 (0.81–0.90)†‡ |
| Participants with asthma | |||
| Wheeze in the last year | n=18,824 | n=10,587 | n=8,237 |
| Q1 (< 17.4) | 1.0 | 1.0 | 1.0 |
| Q2 (17.4– < 21.2) | 0.87 (0.81–0.94)† | 0.87 (0.80–0.96)† | 0.87 (0.77–0.97)* |
| Q3 (21.2– < 24.8) | 0.89 (0.82–0.95)† | 0.90 (0.82–0.99)* | 0.86 (0.77–0.97)* |
| Q4 (≥ 24.8) | 0.87 (0.81–0.94)†‡ | 0.91 (0.83–1.01) | 0.83 (0.74–0.93)†‡ |
| At least one asthma -related hospitalization | n=896 | n=590 | n=306 |
| Q1 (< 17.4) | 1.0 | 1.0 | 1.0 |
| Q2 (17.4- < 21.2) | 0.99 (0.82–1.20) | 0.94 (0.75–1.19) | 1.12 (0.78–1.60) |
| Q3 (21.2- < 24.8) | 1.21 (0.99–1.46) | 1.14 (0.91–1.44) | 1.37 (0.96–1.92) |
| Q4 (≥ 24.8) | 1.01 (0.83–1.24) | 0.91 (0.71–1.17) | 1.23 (0.86–1.76) |
Abbreviation: IGF-1, insulin-like growth factor 1
All models adjusted for age, sex (in all participants), ethnicity, annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season of the examination, the time of the day when the examination was performed, serum level of glycated hemoglobin A1c, C-reactive protein, and metabolic syndrome. Analyses stratified by sex, given evidence of effect modification (P for interaction=0.01).
P <0.05
P <0.01
P for trend <0.05
Table 5 shows the results of the multivariable analysis of lung function measures in all participants and separately in subjects with and without asthma. Among all participants, each quartile increment in IGF-1 was significantly associated with an increment of 21–26 ml in FEV1 or FVC, with similar findings in subjects with or without asthma. There was a significant interaction between sex and IGF-1 on lung function measures (P for interaction <0.05 in all instances). In the analysis stratified by sex, each quartile increment in serum IGF-1 was significantly associated with 16–20 mL increased FEV1 or FVC in women, and 28–34 ml increased FEV1 or FVC in men. We obtained similar results in a secondary analysis of lung function in which Global Lung Function Initiative (GLI) z-scores were calculated for lung function measures (e-Table 3).
Table 5 –
Multivariable analysis of IGF-1 and lung function measures
| All participants (n=212,462) | Women (n=113,769) | Men (n=98,693) | |
|---|---|---|---|
| IGF-1 per quartile increment | β (95% Confidence Interval) | ||
| All Participants | |||
| FEV1 (ml) | 20.9 (19.1, 22.7)* | 15.9 (13.8, 17.9)* | 28.4 (25.3, 31.5)* |
| FVC (ml) | 25.6 (23.4, 27.7)* | 19.5 (17.0, 22.0)* | 34.0 (30.4, 37.6)* |
| FEV1/FVC (%) | 0.01 (−0.02, 0.03) | 0.001 (−0.03, 0.03) | 0.02 (−0.02, 0.06) |
| Participants without asthma | n=188,123 | n=100,031 | n=88,092 |
| FEV1 (ml) | 20.8 (18.9, 22.7)* | 15.9 (13.7, 18.1)* | 27.8 (24.6, 31.0)* |
| FVC (ml) | 25.5 (23.2, 27.7)* | 19.8 (17.1, 22.4)* | 33.2 (29.4, 37.0)* |
| FEV1/FVC (%) | 0.003 (−0.02, 0.03) | −0.01 (−0.03, 0.02) | 0.02 (−0.02, 0.05) |
| Participants with asthma | n=24,339 | n=13,738 | n=10,601 |
| FEV1 (ml) | 21.37 (15.5, 27.2)* | 15.4 (9.0, 21.8)* | 33.0 (22.5, 43.6)* |
| FVC (ml) | 26.3 (19.8, 32.7)* | 17.6 (10.3, 24.9)* | 40.2 (28.9, 51.6)* |
| FEV1/FVC (%) | 0.03 (−0.05, 0.12) | 0.04 (−0.06, 0.14) | 0.07 (−0.07, 0.21) |
Abbreviation: IGF-1, insulin-like growth factor 1; FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
All models adjusted for asthma status (in all participants), age, sex (in all participants), ethnicity, annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season and the time of the day when the examination was performed, and serum level of glycated hemoglobin A1c, and C-reactive protein. Models for FEV1 and FVC were additionally adjusted for height and height squared.
P <0.01
e-Table 3 –
Multivariable analysis of IGF-1 and lung function measures (as z-scores)
| All participants (n=212,462) | Women (n=113,769) | Men (n=98,693) | |
|---|---|---|---|
| IGF-1 quartile | β (95% Confidence Interval) | ||
| All Participants | |||
| FEV1 z-score | 0.020 (0.016, 0.024)* | 0.021 (0.015, 0.026)* | 0.027 (0.021, 0.034)* |
| FVC z-score | 0.020 (0.017, 0.024)* | 0.022 (0.017, 0.027)* | 0.028 (0.022, 0.034)* |
| FEV1/FVC z-score | 0.002 (−0.001, 0.006) | −0.002 (−0.006, 0.002) | 0.001 (−0.004, 0.006) |
| Participants without asthma | n=188,123 | n=100,031 | n=88,092 |
| FEV1 z-score | 0.020 (0.016, 0.024)* | 0.020 (0.014, 0.026)* | 0.025 (0.018, 0.031)* |
| FVC z-score | 0.020 (0.016, 0.024)* | 0.022 (0.016, 0.027)* | 0.026 (0.020, 0.032)* |
| FEV1/FVC z-score | 0.003 (−0.004, 0.006) | −0.004 (−0.006, 0.002) | −0.001 (−0.006, 0.004) |
| Participants with asthma | n=24,339 | n=13,738 | n=10,601 |
| FEV1 z-score | 0.023 (0.010, 0.036)* | 0.025 (0.008, 0.042)* | 0.049 (0.028, 0.070)* |
| FVC z-score | 0.027 (0.015, 0.038)* | 0.025 (0.010, 0.040)* | 0.046 (0.028, 0.064)* |
| FEV1/FVC z-score | 0.001 (−0.010, 0.012) | 0.003 (−0.010, 0.017) | 0.017 (−0.001, 0.036) |
Abbreviation: IGF-1, insulin-growth like factor 1. FEV1, forced expiratory volume in one second; FVC, forced vital capacity.
All models adjusted for asthma status (in all participants), annual household income, body mass index, smoking status, pack-years of cigarette smoking, the season and the time of the day when the examination was performed, and serum level of glycated hemoglobin A1c, and C-reactive protein.
P <0.01
DISCUSSION
Among adult non-diabetic participants in the UK Biobank, a higher peripheral serum IGF-1 levels was significantly associated with lower odds of physician-diagnosed asthma and current wheeze, independently of BMI, HbA1c, and other potential confounders. Moreover, serum IGF-1 was significantly and positively associated with FEV1 and FVC in adults of both sexes, with larger effect estimates in men. To our knowledge, this is the first large epidemiological study of serum IGF-1, asthma, and asthma outcomes.
Our findings are consistent with some but not all prior studies of IGF-1 and lung function. In a population-based survey of 1,326 German healthy adults aged 20 to 79 years, IGF-1 was positively associated with FEV1 and FVC19. A similar association was found in women, but only in those 50 years and older19. In another study of 843 Welsh men aged 45 to 59 years, the IGF-1 to IGFBP-3 ratio was positively associated with FEV1/FVC. However, that study did not include women and no information was collected on asthma status18. Others have reported similar results in other pulmonary diseases, as serum IGF-1 was positively associated with percent-predicted FEV1 before and after the treatment of children with cystic fibrosis27. Our multivariable analyses reported positive associations between serum IGF-1 and FEV1 or FVC in both sexes, regardless of a diagnosis of asthma, with larger effect estimates in men. Our sex-specific results may be explained by larger airway diameter and lung volumes in men than in women, as well as differences in the levels of sex steroid hormones28.
In contrast to our finding of an inverse association between IGF-1 level and asthma or wheezing, a case-control study of 50 subjects reported a positive association between serum IGF-1 and asthma, and a negative correlation between IGF-1 and FEV1/FVC and asthma control17. However, that study was limited by small sample size and under-representation of males (3/27 cases and 4/23 controls), having IGF-1 levels well below the expected normal range, and lack of analyses unadjusted for potential confounders.
Despite the dearth of epidemiological and clinical studies of IGF-1 and asthma, there is substantial preclinical evidence of a potential role of IGF-1 in asthma pathobiology. In an ovo-albumin (OVA)-induced murine model of asthma, IGF-1 was upregulated in the airways, and administration of IGF-1 neutralizing antibodies improved airway resistance, airway inflammation, and airway wall thickening. An increase in IGFBP-3, an inhibitor of cell growth and promoter of apoptosis, was also observed after OVA challenge15. In vivo, IGFBP-3 has been shown to be induced by IGF-1, and IGFBP-3 level has been found to increase in airway epithelial cells of patients with asthma as compared to individuals without asthma16. In another murine model of asthma, IGF‑1 in the lungs was upregulated by interleukin (IL)-33, and such elevated IGF‑1 was predominantly derived from alveolar macrophages. Moreover, IGF-1 prevented the phagocytosis of apoptotic cells by alveolar epithelial cells and increased the release of inflammatory contents of apoptotic cells, leading to increased airway inflammation29, 30. Thus, findings from preclinical experimental studies suggest that increased IGF-1 or IGF-1 receptor signaling increase asthma risk, while results from the current study and other epidemiologic studies suggest that IGF1 is associated with lower risk for asthma.
The apparent paradoxical findings for IGF-1 and asthma could be partly explained by the fact that preclinical models have measured IGF-1 in the lung, while epidemiologic studies have assessed peripheral (circulating) serum IGF-1 levels, which could plausibly have different effects on immune responses and airway inflammation. Moreover, a bidirectional feedback between IGF-1 and T helper (Th) 2 cytokines may contribute to the conflicting findings of experimental and epidemiologic studies. An in vitro study reported that IL-17F induces expression of IGF-1 in bronchial epithelial cells, while both IL-4 and IL-13 enhanced IL-17F-induced IGF-1 expression, suggesting that an IL-17F/IGF-1 axis may contribute to pulmonary allergic responses31. On the other hand, a negative feedback between proinflammatory cytokines and IGF-1 has been suggested12. Tumor necrosis factor (TNF)-α and IL-1β can decrease IGF-1 sensitivity by enhancing IGFBP production and impeding IGF-1 binding to its receptor IGF-1R through an insulin receptor substrate (IRS) – Akt pathway. In contrast, IGF-1 can suppress proinflammatory cytokine signaling by increasing IL-10 secretion or directly via c-Jun N terminal kinase (JNK) and NF-κB pathways12.
Proinflammatory cytokines implicated in IGF-1 resistance may further explain potential beneficial effects of IGF-1 on respiratory health from epidemiologic studies, which were not considered in preclinical IGF-1 models. Lower IGF-1 levels have been associated with characteristics of MS, including higher waist-to-hip ratio, triglycerides levels, and systolic and diastolic blood pressure, as well as impaired insulin sensitivity in non-diabetic individuals32. MS was associated with a 34% increase in asthma in a study of 4,060 elderly Koreans (≥65 years), and this association was substantially mediated by insulin resistance and systemic inflammation33. In a study of adolescents with and without asthma, we previously reported that insulin resistance is negatively associated with FEV1 and FVC, and that MS is associated with lower FEV1/FVC34. The absence of effect modification of MS in our analysis also highlights the importance of metabolic signaling pathways in lung health independent of MS or overt diabetes. Collectively, these findings support further investigation of how the endocrine system modulates key immune responses in asthma.
Our study has several limitations. First, we cannot examine temporal relationships in this cross-sectional study. Second, IGFBP-3 can modulate IGF-1 bioavailability or function, and it was not measured in the UK Biobank. Circulating IGF-1 is bound to one of seven IGF binding proteins, of which IGFBP-3 is the most abundant7, and the IGF-1/IGFBP-3 ratio may represent a better index for metabolic risk35. Third, the fasting time of UK Biobank participants varied (median =3 hours). Therefore, MS assessment was not ideal in our study, as fasting glucose levels should be measured after at least 8 hours of fasting. However, we excluded subjects with diabetes or taking insulin, and adjusted for HbA1c in our models to account for undiagnosed diabetes. Fourth, a “healthy volunteer” selection bias has been suggested for the UK Biobank, and thus our findings may not be generalizable to the British population at large. Compared with the general population, UK Biobank participants were less likely to be obese or to smoke, and had fewer self-reported health conditions36. Lastly, we lack data on several potential confounders, including allergic sensitization, environmental exposure to endocrine disruptors, physical activity, and use of medications such as metformin or corticosteroids. However, we excluded participants who were diagnosed with diabetes or who used insulin, and thus the number of participants on metformin (if any) would be small. Moreover, we obtained similar results in a sensitivity analysis excluding participants who reported current use of asthma medications (data not shown).
In summary, higher serum IGF-1 levels are associated with lower odds of physician-diagnosed asthma and current wheeze in middle-aged and older British adults in the UK Biobank. In this population, higher IGF-1 levels are also associated with higher FEV1 and FVC, with a slightly larger estimated effects in men. Given opposite results in prior preclinical studies, our findings warrant further assessment in longitudinal studies including young and old adults.
Acknowledgments
Funding Sources: This study was conducted using the UK Biobank Resource under Application Number 43252. Dr. Han’s contribution was supported by grant MD011764 from the U.S. National Institutes of Health (NIH). Dr. Celedón’s contribution was supported by grants HL117191, HL119952, and MD011764 from the U.S. NIH. Dr. Yan’s contribution was supported by grant HL138098 from the U.S. NIH. Dr. Forno’s contribution was supported by grant HL149693 from the U.S. NIH. The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Abbreviations:
- AM:
alveolar macrophages
- BMI:
body mass index
- CI:
confidence interval
- CRP:
C-reactive protein
- FEV1:
forced expiratory volume in 1 second
- FVC:
forced vital capacity
- GH:
growth hormone
- HbA1c:
glycated hemoglobin A1c
- ICD code:
International Classification of Diseases Clinical Modification code
- IGF-1:
insulin-like growth factor-1
- IGFBP-3:
insulin-like growth factor binding protein 3
- OR:
odds ratio
- MS:
metabolic syndrome
- UK Biobank:
United Kingdom Biobank
Footnotes
Conflicts of interest: Dr. Celedón has received research materials from Merck and GSK (inhaled steroids), and Pharmavite (vitamin D and placebo capsules), to provide medications free of cost to participants in NIH-funded studies, unrelated to the current work. The other authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Global Asthma Network. The Global Asthma Report 2018. Auckland, New Zealand2018. [Google Scholar]
- 2.Nurmagambetov T, Kuwahara R, Garbe P. The Economic Burden of Asthma in the United States, 2008–2013. Ann Am Thorac Soc. 2018;15:348–356. [DOI] [PubMed] [Google Scholar]
- 3.Gibson GJ, Loddenkemper R, Lundback B, Sibille Y. The economic burden of lung disease.. The European Lung White Book: Respiratory Health and Disease in Europe: European Respiratory Society; 2013:16–27. [Google Scholar]
- 4.Mukherjee M, Stoddart A, Gupta RP, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asthma UK. Asthma facts and statistics. Vol 2020. London, England2020. [Google Scholar]
- 6.Shaw DE, Gaynor CM, Fogarty AW. Changes in asthma mortality in England and Wales since 2001. Thorax. 2019;74:1174–1175. [DOI] [PubMed] [Google Scholar]
- 7.Laron Z. Insulin-like growth factor 1 (IGF-1): a growth hormone. Mol Pathol. 2001;54:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med. 2016;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. 2000;86:125–130. [DOI] [PubMed] [Google Scholar]
- 10.Simpson A, Petnga W, Macaulay VM, Weyer-Czernilofsky U, Bogenrieder T. Insulin-Like Growth Factor (IGF) Pathway Targeting in Cancer: Role of the IGF Axis and Opportunities for Future Combination Studies. Target Oncol. 2017;12:571–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z, Li W, Guo Q, Wang Y, Ma L, Zhang X. Insulin-Like Growth Factor-1 Signaling in Lung Development and Inflammatory Lung Diseases. Biomed Res Int. 2018;2018:6057589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor JC, McCusker RH, Strle K, Johnson RW, Dantzer R, Kelley KW. Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol. 2008;252:91–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakar S, Rosen CJ, Beamer WG, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H, Kim SR, Oh Y, Cho SH, Schleimer RP, Lee YC. Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am J Respir Cell Mol Biol. 2014;50:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita N, Tashimo H, Ishida H, et al. Role of insulin-like growth factor-I in allergen-induced airway inflammation and remodeling. Cell Immunol. 2005;235:85–91. [DOI] [PubMed] [Google Scholar]
- 16.Veraldi KL, Gibson BT, Yasuoka H, et al. Role of insulin-like growth factor binding protein-3 in allergic airway remodeling. Am J Respir Crit Care Med. 2009;180:611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acat M, Toru Erbay U, Sahin S, Arik O, Ayada C. High serum levels of IGF-I and IGFBP3 may increase comorbidity risk for asthmatic patients. Bratisl Lek Listy. 2017;118:691–694. [DOI] [PubMed] [Google Scholar]
- 18.Green CJ, Holly JM, Bolton CE, et al. Role of IGF-I, IGF-II and IGFBP-3 in lung function of males: the Caerphilly Prospective Study. Int J Mol Epidemiol Genet. 2014;5:112–119. [PMC free article] [PubMed] [Google Scholar]
- 19.Glaser S, Friedrich N, Ewert R, et al. Association between serum insulin-like growth factor (IGF) I and IGF binding protein-3 and lung function. J Clin Endocrinol Metab. 2009;94:2452–2458. [DOI] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. [DOI] [PubMed] [Google Scholar]
- 22.Elliott P, Peakman TC, Biobank UK. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–244. [DOI] [PubMed] [Google Scholar]
- 23.Biobank UK. UK Biobank Biomarker Project - Companion Document to Accompany Serum Biomarker Data2019. [Google Scholar]
- 24.Office for National Statistics. Average household income, UK: financial year ending 2019. London, England2020. [Google Scholar]
- 25.Grundy SM, Brewer HB Jr., Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. [DOI] [PubMed] [Google Scholar]
- 26.Rubin DB. Multiple imputation for nonresponse in surveys. New York, U.S: John Wiley & Sons; 1987. [Google Scholar]
- 27.Gifford AH, Nymon AB, Ashare A. Serum insulin-like growth factor-1 (IGF-1) during CF pulmonary exacerbation: trends and biomarker correlations. Pediatr Pulmonol. 2014;49:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LoMauro A, Aliverti A. Sex differences in respiratory function. Breathe (Sheff). 2018;14:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Mu M, Wang H, et al. Upregulated IGF1 in the lungs of asthmatic mice originates from alveolar macrophages. Mol Med Rep. 2019;19:1266–1271. [DOI] [PubMed] [Google Scholar]
- 30.Mu M, Wu F, He J, et al. Insulinlike growth factor 1 inhibits phagocytosis of alveolar epithelial cells in asthmatic mice. Mol Med Rep. 2019;20:2381–2388. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi M, Fujita J, Kokubu F, et al. Induction of insulin-like growth factor-I by interleukin-17F in bronchial epithelial cells. Clin Exp Allergy. 2010;40:1036–1043. [DOI] [PubMed] [Google Scholar]
- 32.Sesti G, Sciacqua A, Cardellini M, et al. Plasma concentration of IGF-I is independently associated with insulin sensitivity in subjects with different degrees of glucose tolerance. Diabetes Care. 2005;28:120–125. [DOI] [PubMed] [Google Scholar]
- 33.Park S, Choi NK, Kim S, Lee CH. The relationship between metabolic syndrome and asthma in the elderly. Sci Rep. 2018;8:9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–311 e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sierra-Johnson J, Romero-Corral A, Somers VK, et al. IGF-I/IGFBP-3 ratio: a mechanistic insight into the metabolic syndrome. Clin Sci (Lond). 2009;116:507–512. [DOI] [PubMed] [Google Scholar]
- 36.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]