Abstract
BACKGROUND:
We examined whether the National Comprehensive Cancer Network distress thermometer (DT), a patient-reported outcome measure, could be used to identify levels and causes of distress associated with racial/ethnic disparities in time to care among patients with breast cancer.
METHODS:
We identified women aged ≥18 years with stage O-IV breast cancer who were diagnosed in a single health system between January 2014 and July 2016. The baseline visit was defined as the first postdiagnosis, pretreatment clinical evaluation. Zero-inflated negative binomial (ZINB) regression (modeling non-zero DT scores and DT scores = 0) and logistic regression (modeling DT score ≥ 4, threshold for social services referral) were used to examine associations between baseline score (0 = none to 10 = extreme) and types of stressors (emotional, familial, practical, physical, spiritual) after adjustment for race/ethnicity and other characteristics. Linear regression with log transformation was used to identify predictors of time to evaluation and time to treatment.
RESULTS:
A total of 1029 women were included (median baseline DT score = 4). Emotional, physical, and practical stressors were associated with distress in both the ZINB and logistic models (all P < .05). Black patients (n = 258) were more likely to report no distress than Whites (n = 675; ZINB zero model odds ratio, 2.72; 95% CI, 1.68-4.40; P < .001) despite reporting a similar number of stressors (P = .07). Higher DT scores were associated with shorter time to evaluation and time to treatment while being Black and having physical or practical stressors were associated with delays in both (all P < .05).
CONCLUSIONS:
Patient-reported stressors predicted delays in time to care, but patient-reported levels of distress did not, with Black patients having delayed time to care despite reporting low levels of distress. We describe anticipatory, culturally responsive strategies for using patient-reported outcomes to address observed disparities.
Keywords: breast cancer, distress, health disparities, modifiable risk factors, patient-reported outcomes, race/ethnicity
INTRODUCTION
The National Comprehensive Cancer Network (NCCN) distress thermometer (DT) and problem list is a widely used instrument through which patients with cancer can report overall distress and identify psychosocial and logistical sources of distress. In our health system, it is completed by patients with breast cancer at most appointments, with the exception of nurse-only visits for radiation or systemic therapy1 DT scores ≥4 are used as a threshold for clinically significant distress in our system and others,2,3 and this threshold is intended to trigger clinician-initiated referrals to cancer support services—including psycho-oncology and financial counseling—depending on patient-identified stressors. Accordingly, assessment of distress at a patient’s initial postdiagnosis appointment offers an opportunity to identify and address potentially modifiable barriers to timely, guideline-concordant care.
We recently demonstrated that many patients who were newly diagnosed with breast cancer had clinically significant distress levels at initial consultation after diagnosis, but that over time, distress levels eventually declined to low levels for a majority of women.4 However, we noted significant racial/ethnic differences in levels of self-reported distress—measured on a scale of 0 (no distress) to 10 (severe distress)—at patients’ first postdiagnosis oncology appointments. Given known disparities in time to care5-8 and the racial and ethnic differences in self-reported distress we had also observed, we sought to identify patient-reported stressors associated with clinically significant distress in patients with newly diagnosed breast cancer and to determine whether these causes of distress were independently associated with racial/ethnic disparities in time to evaluation and time to treatment.9
MATERIALS AND METHODS
Patient and clinical data, including DT score at baseline visit, were collected from the electronic health records for all women aged ≥18 years with newly diagnosed clinical stage 0-IV breast cancer who were first seen in the Duke Health System between January 2014 and July 2016, a cohort that has been described previously.4 The baseline visit was defined as the first cancer-center evaluation after pathological diagnosis and before any treatment including surgery, chemotherapy, endocrine therapy, targeted therapy, or radiation.
Patient characteristics including median levels of distress were summarized by racial/ethnic group with n (%) for categorical variables and median (interquartile range [IQR]) for continuous variables. Fisher’s exact tests or chi-square tests were used to compare categorical variables, and a Wilcoxon rank-sum test was used to compare continuous variables as appropriate.
Using multivariate regression, we examined the association between individual patients’ DT scores at baseline visit (dependent variable) and the causes of distress they selected from the DT problem list, which includes 39 individual stressors grouped into 5 different categories: emotional (6 items), familial (4 items), practical (6 items), physical (22 items), and spiritual (1 item). In addition to logistic regression modeling DT score ≥4 (the threshold for referrals in our health system), zero-inflated negative binomial regression (ZINB, modeling both likelihood of non-zero score and DT score = 0)10 was also used to examine the relationship between DT score and individual stressors, because many patients (22%) had baseline scores of 0 and the data displayed overdispersion.
Both regression models were adjusted for age (continuous), American Joint Commission on Cancer seventh edition clinical stage (0-IV), insurance status (Medicaid, Medicare, private, unknown, and other [including TriCare, self-pay]), marital status (married/partnered, divorced, singled, widowed, unknown), and self-reported race combined with self-reported ethnicity, which together were parsed into 3 categories: 1) non-Hispanic Black, 2) non-Hispanic White, and 3) other (including Asian/Pacific Islander [PI], Hispanic, Native American, multiracial, and race not reported or unknown), given the small sample sizes for Asian/PI and Hispanic patients. We also performed sensitivity analyses in which race/ethnicity was disaggregated into 5 categories: 1) Asian/PI, 2) non-Hispanic Black, 3) Hispanic, 4) non-Hispanic White, and 5) other (including Native American, multiracial, and race not reported or unknown), to ensure that important distinctions between Asian/PI and Hispanic patients were not elided. Interactions between race/ethnicity and having a stressor in any of the 5 domains were tested and are reported if significant.
Linear regression was used to identify covariates including DT score and race/ethnicity that could be predictive of time to evaluation and time to treatment after adjusting for age, clinical stage, insurance, marital status, and race/ethnicity, plus treatment sequence (neoadjuvant [preoperative chemotherapy, endocrine therapy, and/or targeted therapy] vs surgery first) for the time to treatment linear regression model only. Log transformations were used on both time to evaluation and time to treatment to ensure that the assumption of linearity was fulfilled. Time-to-evaluation was defined as time from date of pathologic diagnosis to first appointment with a medical, radiation, or surgical oncologist. Time-to-treatment was defined as time from date of pathologic diagnosis to first oncologic treatment. As with the logistic and ZINB regression models, we conducted our primary analyses with race/ethnicity parsed into 3 categories (Black, White, other) and conducted sensitivity analyses in which race/ethnicity was disaggregated into 5 categories (Asian/PI, Black, Hispanic, White, other). Interactions between race/ethnicity and DT score and between race/ethnicity and having a stressor in any of the 5 domains were tested, and significant interactions are reported.
We report incidence rate ratios and odds ratios (ORs) for the ZINB models, ORs for the logistic model, and exponentiated estimates (EEs) for the log-linear models with 95% CIs. A significance level of P < .05 was used for all analyses, which were performed in SAS version 9.4 (SAS Institute, Cary, North Carolina). Our study was approved by the institutional review board at Duke University (protocol Pro00083052).
RESULTS
Patient and Treatment Characteristics
A total of 1029 women were included (Fig. 1, Table 1).4 The median age at diagnosis was 58 years and ranged from 43 to 60 years, with the youngest group being Hispanic women (n = 23) and the oldest group being non-Hispanic Black women (n = 258). A majority of patients were non-Hispanic White (65.6%, n = 675), married/partnered (59.2%, n = 609), and had early-stage (0-I) disease (59.8%, n = 615). Among non-Hispanic Black patients, the most common form of insurance was Medicare (51.9%, n = 134); private insurance was more common for other groups except Hispanic patients, who had the lowest rates of any private and/or federal insurance (all P < .001).
Figure 1.
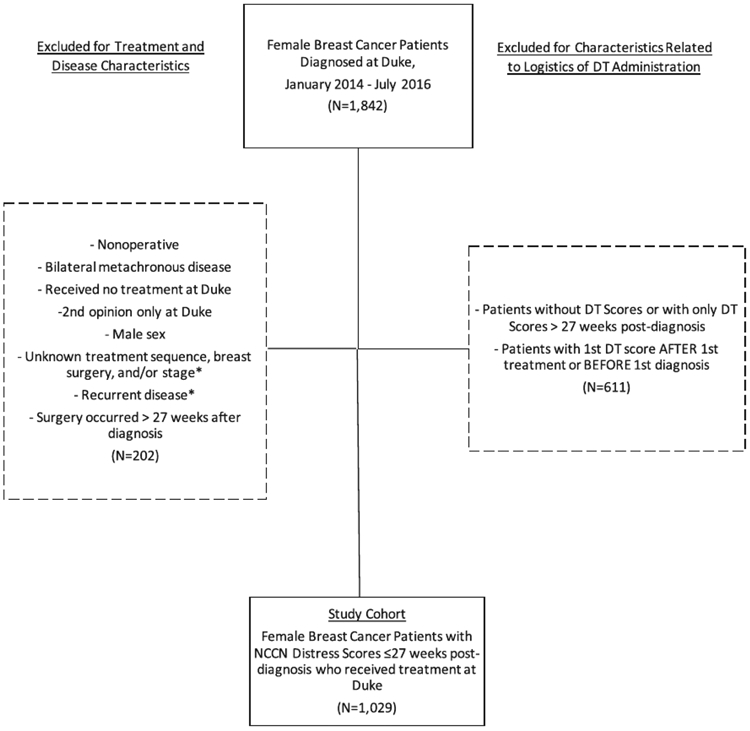
Flow diagram of patients newly diagnosed with breast cancer between January 2014 and July 2016. DT, distress thermometer; NCCN, National Comprehensive Cancer Network.
TABLE 1.
Demographic and Clinical Characteristics, Newly Diagnosed Breast Cancer Patients With Baseline Visit Between January 2014 and July 2016 (n = 1029)
| Total (N = 1029) | Asian/PI (n = 28) | Non-Hispanic Black (n = 258) |
Hispanic (n = 23) | Non-Hispanic White (n = 675) |
Other (n = 45) | P | |
|---|---|---|---|---|---|---|---|
| Age, y, median (IQR) | 58 (48-67) | 51 (45-60) | 60 (50-67) | 43 (34-48) | 59 (48-67) | 52 (42-61) | <.001 |
| Days to first evaluation, median (IQR) | 19 (13-28) | 15 (12.5-25) | 21 (14-30) | 23 (17-43) | 17 (12-26) | 21 (14-35) | <.001 |
| Days to first treatment, median (IQR) | 47 (35-70) | 51 (33.5-115.5) | 47 (36-72) | 71 (47-180) | 45 (34-64) | 67(40-142) | <.001 |
| DT score, median (IQR) | 4 (1-7) | 5 (0-7) | 3 (0-6) | 5 (1-9) | 4 (1-7) | 4 (2-6) | .01 |
| DT score group | |||||||
| 0-3 | 481 (46.7) | 11 (39.3) | 143 (55.4) | 10 (43.5) | 299 (44.3) | 18 (40) | .03 |
| 4-10 | 548 (53.3) | 17 (60.7) | 115 (44.6) | 13 (56.5) | 376 (55.7) | 27 (60) | |
| Total no. of problem list items | |||||||
| Median (IQR) | 3 (1-7) | 3.5 (0.5-6) | 4 (1-7) | 5 (1-9) | 3 (1-6) | 5 (2-8) | .07 |
| ≥1 emotional stressor | 650 (63.2) | 19 (67.9) | 151 (58.5) | 17 (73.9) | 432 (64) | 31 (68.9) | .33 |
| ≥1 family stressor | 177 (17.2) | 2 (7.1) | 53 (20.5) | 5 (21.7) | 105 (15.6) | 12 (26.7) | .08 |
| ≥1 physical stressor | 574 (55.8) | 10 (35.7) | 160 (62) | 12 (52.2) | 363 (53.8) | 29 (64.4) | .02 |
| ≥1 spiritual stressors | 18 (1.7) | 3 (10.7) | 5 (1.9) | 0 (0) | 9 (1.3) | 1 (2.2) | .006 |
| ≥1 practical stressor | 358 (34.8) | 8 (28.6) | 95 (36.8) | 11 (47.8) | 222 (32.9) | 22 (48.9) | .10 |
| Treatment sequence | |||||||
| Neoadjuvant | 163 (15.8) | 3 (10.7) | 72 (27.9) | 3 (13) | 80 (11.9) | 5 (11.1) | <.001 |
| Surgery first | 866 (84.2) | 25 (89.3) | 186 (72.1) | 20 (87) | 595 (88.1) | 40 (88.9) | |
| Surgery type | |||||||
| Lumpectomy | 620 (60.3) | 18 (64.3) | 170 (65.9) | 10 (43.5) | 391 (57.9) | 31 (68.9) | .005 |
| Mastectomy without reconstruction | 291 (28.3) | 5 (17.9) | 74 (28.7) | 11 (47.8) | 190 (28.1) | 11 (24.4) | |
| Mastectomy with reconstruction | 118 (11.5) | 5 (17.9) | 14 (5.4) | 2 (8.7) | 94 (13.9) | 3 (6.7) | |
| Clinical disease stage | |||||||
| 0 | 151 (14.7) | 4 (14.3) | 39 (15.1) | 3 (13) | 97 (14.4) | 8 (17.8) | .054 |
| 1 | 464 (45.1) | 12 (42.9) | 95 (36.8) | 6 (26.1) | 332 (49.2) | 19 (42.2) | |
| 2 | 323 (31.4) | 10 (35.7) | 91 (35.3) | 10 (43.5) | 197 (29.2) | 15 (33.3) | |
| 3 | 78 (7.6) | 1 (3.6) | 30 (11.6) | 4 (17.4) | 40 (5.9) | 3 (6.7) | |
| 4 | 13 (1.3) | 1 (3.6) | 3 (1.2) | 0 (0) | 9 (1.3) | 0 (0) | |
| Insurance | |||||||
| Private | 475 (46.2) | 19 (67.9) | 90 (34.9) | 8 (34.8) | 329 (48.7) | 29 (64.4) | <.001 |
| Medicaid | 13 (1.3) | 1 (3.6) | 5 (1.9) | 2 (8.7) | 4 (0.6) | 1 (2.2) | |
| Medicare | 449 (43.6) | 5 (17.9) | 134 (51.9) | 2 (8.7) | 299 (44.3) | 9 (20) | |
| Other | 26 (2.5) | 1 (3.6) | 8 (3.1) | 6 (26.1) | 9 (1.3) | 2 (4.4) | |
| Unknown | 66 (6.4) | 2 (7.1) | 21 (8.1) | 5 (21.7) | 34 (5) | 4 (8.9) | |
| Marital status | |||||||
| Married/Partnered | 609 (59.2) | 22 (78.6) | 98 (38) | 11 (47.8) | 449 (66.5) | 29 (64.4) | <.001 |
| Divorced | 124 (12.1) | 1 (3.6) | 50 (19.4) | 4 (17.4) | 66 (9.8) | 3 (6.7) | |
| Single | 164 (15.9) | 2 (7.1) | 67 (26) | 7 (30.4) | 81 (12) | 7 (15.6) | |
| Unknown | 49 (4.8) | 0 (0) | 12 (4.7) | 1 (4.3) | 30 (4.4) | 6 (13.3) | |
| Widowed | 83 (8.1) | 3 (10.7) | 31 (12) | 0 (0) | 49 (7.3) | 0 (0) | |
Abbreviations: DT, distress thermometer; IQR, interquartile range; PI, Pacific Islander; y, year.
Data are presented as n (%) unless indicated otherwise.
Black patients had the highest proportion of women receiving neoadjuvant treatment (27.9% vs ~11% among the rest of the cohort; P < .001). Lumpectomy was the most common surgical procedure across all groups except among Hispanic women, nearly half of whom (47.8%) underwent mastectomy without reconstruction (P= .005).
Patient-Reported Levels and Causes of Distress
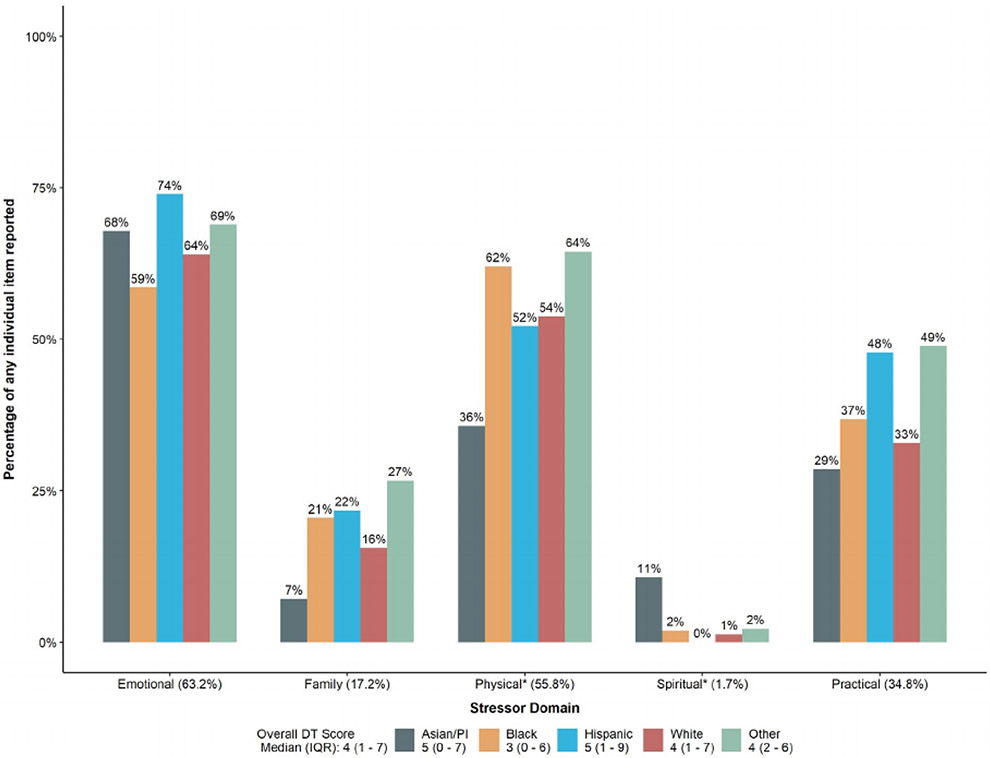
The median DT score for the entire cohort was 4 (IQR, 1-7) (Fig. 2, Table 1), the threshold for clinician-initiated referral to support services in our health system. Black patients had the lowest median DT score (3 [IQR, 0-6]) and were the only racial/ethnic group to have a median DT score below the referral-triggering threshold of 4, whereas Asian/PI patients (n = 28; DT score = 5; IQR, 0-7) and Hispanic patients (n = 23; DT score = 5; IQR, 1-9) had the highest median DT scores (P = .01). In addition to these differences in overall distress, there were also significant differences between racial/ethnic groups with regard to having a physical or spiritual stressor (Fig. 2, Table 1). Despite these variations, however, there was no significant difference in the number of individual stressors reported by Asian/PI, Black, White, and Hispanic patients (P= .07).
Figure 2.
Levels and causes of patient-reported distress among patients newly diagnosed with breast cancer between January 2014 and July 2016 (n = 1029). DT, distress thermometer; IQR, interquartile range; PI, Pacific Islander. *Statistically significant difference between racial/ethnic groups.
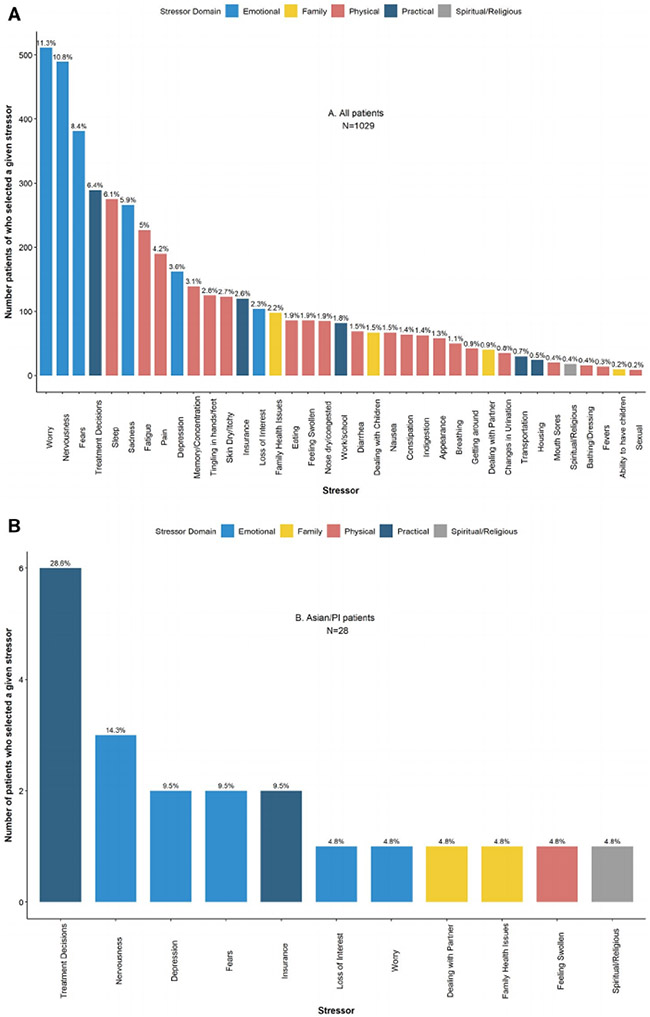
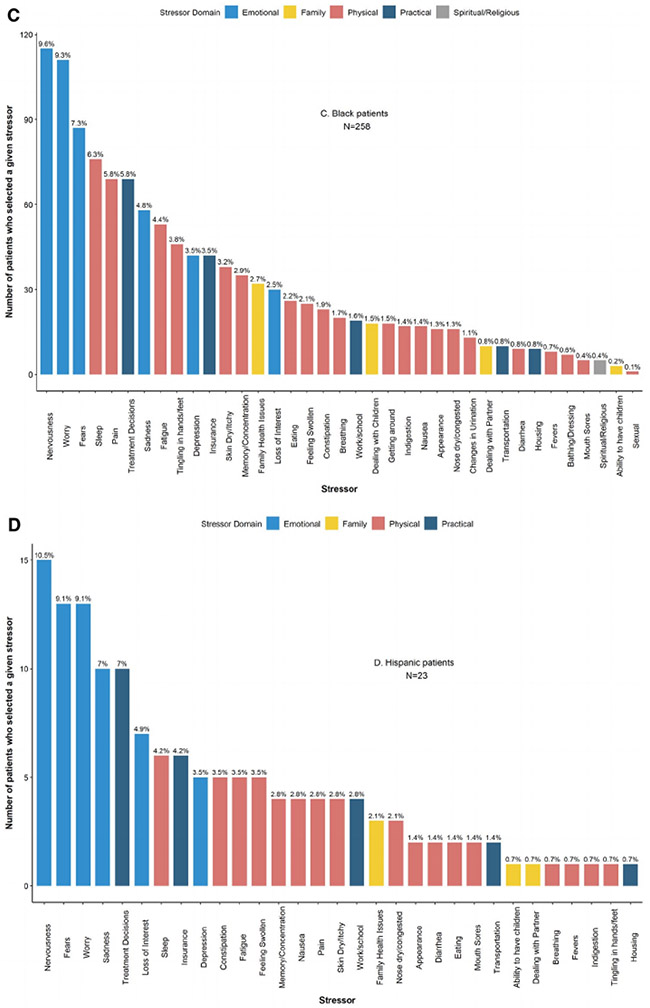
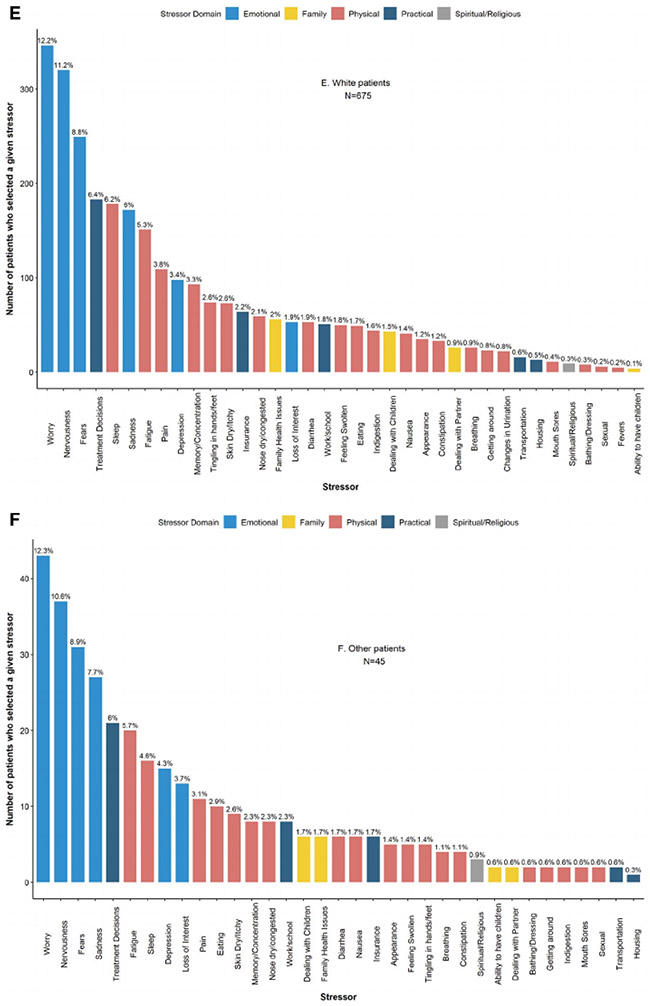
When patients were considered in aggregate, the 3 most commonly reported stressors were in the emotional domain: worry (n = 511 [49.7%]), nervousness (n = 489 [47.5%]), and fears (n = 381 [37%]), a pattern that persisted within every racial/ethnic group except for Asian/PI, for whom the primary stressor was treatment decisions within the practical domain (Fig. 3).
Figure 3.
Frequency of patient-reported stressors among patients newly diagnosed with breast cancer between January 2014 and July 2016 (n = 1029). (A) All patients. (B) Asian/PI patients. (C) Black patients. (D) Hispanic patients. (E) White patients. (F) Patients of other races/ethnicities. PI, Pacific Islander.
After adjustment, the presence of 1 or more emotional, physical, or practical stressors was significantly associated with distress in both the ZINB and logistic models (all P < .05) (Supporting Table 1). Black patients were significantly more likely to report no distress than Whites (ZINB zero model OR, 2.72; 95% CI, 1.68-4.40; P < .001) and less likely to report clinically significant distress (ie, a DT score ≥4 [logistic OR, 0.59; 95% CI, 0.41-0.83; P = .003]). Of all the covariates included in the logistic regression model, having any emotional stressors (OR, 4.50; 95% CI, 3.30-6.15; P < .001) was associated with the highest odds of reporting clinically significant distress (Supporting Table 1). We examined both the logistic and ZINB models with the 5 more granular racial groups, and the behavior of the coefficients in the models was essentially the same.
Time to Evaluation and Time to Treatment
Time-to-evaluation differed significantly across racial/ethnic groups, with Hispanic patients having the longest median length of time between diagnosis and first cancer-center evaluation (Asian/PI, 15 days; Black, 21 days; Hispanic, 23 days; White, 17 days [P < .001]) (Table 1). As with time to evaluation, Hispanic patients had the longest median length of time between diagnosis and treatment initiation (Asian/PI, 51 days; Black, 47 days; Hispanic, 71 days; White, 45 days [P < .001]) (Table 1), a pattern that persisted even among patients undergoing surgery first. Notably, there was no difference in time to evaluation and time to treatment between Hispanic patients who reported English versus Spanish as their primary language.
After adjusting for other covariates, higher DT score was associated with shorter time to evaluation (EE, 0.97; 95% CI, 0.96-0.99; P < .001) (Supporting Table 2). Being non-White (Black: EE, 1.16; 95% CI, 1.06-1.28; P= .002; other: EE, 1.30; 95% CI, 1.13-1.49; P < .001) or divorced (EE, 1.14; 95% CI, 1.00-1.29; P =.045) was associated with longer time to evaluation, as was having any physical (EE, 1.12; 95% CI, 1.03-1.22; P = .01) or practical stressors (EE, 1.21; 95% CI, 1.11-1.33; P < .001).
When we split the racial/ethnic groups into more granular categories (Asian/PI, Black, Hispanic, White, and other [Table 2]), being Black (EE, 1.22; 95% CI, 1.09-1.37; P < .001) was associated with longer time to evaluation, as was having any physical stressors (EE, 1.11; 95% CI, 1.02-1.21; P = .02) or practical stressors (EE, 1.26, 95% CI, 1.13-1.40; P < .001). There was also a significant interaction between race/ethnicity and having any practical stressors (P = .03): for Hispanic patients, having a practical stressor significantly delayed time to evaluation compared with White patients who had a practical stressor (EE, 1.74; 95% CI, 1.02-2.96; P = .04).
TABLE 2.
Days to First Evaluation and Days to First Treatment: Linear Regression Models With Log Transformation, 5-Level Race/Ethnicity Variable, and Race/Ethnicity*Any Practical Stressor Interaction Among Newly Diagnosed Breast Cancer Patients With Baseline Visit Between January 2014 and July 2016 (n = 1029)
| Days to First Evaluationa |
Days to First Treatmentb |
|||||
|---|---|---|---|---|---|---|
| EE (95% CI) | P | Overall P | EE (95% CI) | P | Overall P | |
| Agec | 1.00 (0.99-1.00) | .63 | .63 | 0.99 (0.99-1.00) | .004 | .004 |
| DT scorec,d | 0.97 (0.96-0.99) | <.001 | <.001 | 0.98 (0.97-1.00) | .02 | .02 |
| Race/Ethnicity (median days to evaluation/median days to treatment) | ||||||
| Non-Hispanic White (17/45) | Reference | .001 | Reference | .007 | ||
| Asian/PI (15/51) | 1.18 (0.89-1.57) | .24 | 1.09 (0.85-1.40) | .48 | ||
| Non-Hispanic Black (21/47) | 1.22 (1.09-1.37) | <.001 | 1.19 (1.07-1.32) | .001 | ||
| Hispanic (23/71) | 1.05 (0.72-1.53) | .81 | 0.82 (0.58-1.14) | .23 | ||
| Other (21/67) | 1.65 (1.27-2.15) | <.001 | 1.47 (1.17-1.86) | .001 | ||
| Marital status | ||||||
| Married | Reference | .30 | Reference | .92 | ||
| Divorced | 1.13 (0.99-1.28) | .07 | 0.97 (0.87-1.08) | .56 | ||
| Single | 1.00 (0.89-1.12) | .99 | 1.01 (0.92-1.12) | .80 | ||
| Unknown | 1.08 (0.90-1.30) | .42 | 0.97 (0.82-1.14) | .70 | ||
| Widowed | 0.96 (0.82-1.12) | .57 | 0.96 (0.84-1.10) | .54 | ||
| Treatment groupc | ||||||
| Surgery | Not adjusted | Reference | <.001 | |||
| Neoadjuvant | – | – | 0.49 (0.44-0.54) | <.001 | ||
| Surgery type | ||||||
| Mastectomy without reconstruction | Not adjusted | Reference | .57 | |||
| Lumpectomy | – | 0.96 (0.88-1.04) | .32 | |||
| Mastectomy with reconstruction | – | 0.95 (0.84-1.08) | .42 | |||
| Insurance | ||||||
| Private | Reference | .26 | Reference | .36 | ||
| Medicaid | 0.85 (0.59-1.21) | .33 | 0.83 (0.60-1.13) | .24 | ||
| Medicare | 1.05 (0.94-1.17) | .41 | 1.04 (0.94-1.15) | .41 | ||
| Other | 1.27 (0.98-1.65) | .07 | 1.19 (0.95-1.50) | .13 | ||
| Unknown | 0.95 (0.81-1.12) | .58 | 1.00 (0.87-1.16) | .95 | ||
| Clinical disease stagec | ||||||
| 1 | Reference | .64 | Reference | <.001 | ||
| 0 | 0.98 (0.87-1.10) | .72 | 1.07 (0.97-1.19) | .18 | ||
| 2 | 1.04 (0.95-1.14) | .41 | 1.36 (1.24-1.48) | <.001 | ||
| 3 | 1.07 (0.92-1.25) | .37 | 2.01 (1.72-2.34) | <.001 | ||
| 4 | 0.87 (0.61-1.23) | .43 | 2.30 (1.68-3.15) | <.001 | ||
| Problem list items | ||||||
| ≥1 Emotional stressor | ||||||
| No | Reference | .14 | Reference | .51 | ||
| Yes | 0.93 (0.85-1.02) | .14 | 0.97 (0.89-1.06) | .51 | ||
| ≥1 Family stressor | ||||||
| No | Reference | .38 | Reference | .21 | ||
| Yes | 0.95 (0.85-1.06) | .38 | 0.94 (0.85-1.04) | .21 | ||
| ≥1 Physical stressord | ||||||
| No | Reference | .02 | Reference | .08 | ||
| Yes | 1.11 (1.02-1.21) | .02 | 1.07 (0.99-1.15) | .08 | ||
| ≥1 Spiritual stressor | ||||||
| No | Reference | .92 | Reference | .56 | ||
| Yes | 1.02 (0.75-1.37) | .92 | 0.92 (0.71-1.20) | .56 | ||
| ≥1 Practical stressorc,d | ||||||
| No | Reference | <.001 | Reference | <.001 | ||
| Yes | 1.26 (1.13-1.40) | <.001 | 1.23 (1.12-1.35) | <.001 | ||
| Race/Ethnicity (any practical stressor interaction)c,d | ||||||
| Non-Hispanic White | Reference | 0.03 | Reference | .002 | ||
| Asian/PI | 0.78 (0.46-1.32) | .35 | 1.18 (0.74-1.87) | .49 | ||
| Non-Hispanic Black | 0.87 (0.72-1.06) | .17 | 0.85 (0.72-1.01) | .06 | ||
| Hispanic | 1.74 (1.02-2.96) | .04 | 2.14 (1.34-3.41) | .002 | ||
| Other | 0.69 (0.47-1.00) | .05 | 0.79 (0.56-1.10) | .17 | ||
Abbreviations: DT, distress thermometer; EE, exponentiated estimate; PI, Pacific Islander.
A log(x + 0.5) transformation was used to ensure normality and to compensate occurrence of 0 days.
A log transformation was used to ensure normality.
Type 3 overall P < .05 for days to first treatment.
Type 3 overall P < .05 for days to first evaluation.
Higher DT score was associated with shorter time to treatment after adjustment for other covariates (EE, 0.98; 95% 0.97-1; P = .02) (Supporting Table 2). Receiving neoadjuvant treatment rather than surgery first was also associated with shorter time to treatment (EE, 0.49; 95% CI, 0.44-0.54; P < .001), but there was no significant difference among those receiving different types of surgery, including those undergoing reconstruction. Being non-White (Black: EE, 1.12; 95% Cl, 1.03-1.22; P = .008; other: EE, 1.24; 95% CI, 1.09-1.40; P < .001) and having higher stage disease (vs stage I; all P < .001) were associated with longer time to first treatment. As with time to evaluation, having physical stressors (EE, 1.08; 95% CI, 1.00-1.16, P = .005) or practical stressors (EE, 1.20; 95% CI, 1.10-1.30; P < .001) was associated with longer time to treatment.
When we used more granular racial/ethnic categories (Table 2), being Black (EE, 1.19; 95% CI, 1.07-1.32; P = .001), having a higher stage of disease (vs stage I; all P < .001), and having a practical stressor (EE, 1.23; 95% CI, 1.12-1.35; P < .001) continued to be associated with longer time to treatment, but having a physical stressor was not. As with time to evaluation, a significant interaction between race/ethnicity and having any practical stressors was observed: Hispanic patients who had a practical stressor had longer time to treatment compared with White patients who had a practical stressor (EE, 2.14; 95% CI, 1.34-3.41; P = .002).
DISCUSSION
In our analysis of distress after breast cancer diagnosis, having physical or practical stressors predicted delays in time to evaluation and time to treatment, particularly for Hispanic patients, who represent only a small proportion of our cohort but had the longest delays to evaluation (23 days) and treatment (71 days) of any group and some of the highest median self-reported distress levels. Being Black (vs White) was associated with longer time to both evaluation and treatment, despite the fact that Black patients had the lowest self-reported levels of distress and reported a similar number of stressors compared with other groups of patients. Indeed, we found that higher distress scores were unexpectedly associated with shorter time to evaluation and time to treatment.
Thus, the NCCN DT can potentially be used to identify factors that contribute to disparities in time to care among breast cancer patients, but not by using a rigid threshold, as is currently done at many institutions (including our own). Our findings suggest that the types of stressors contributing to patient-reported distress predict which patients are at risk for disparate care, but that the summative self-assessed levels of distress reported by patients do not. Indeed, using summative distress scores rather than individual stressors to initiate interventions may prevent potentially vulnerable patients from being connected with services that could facilitate more timely care.
Accordingly, we must collectively reconsider how the NCCN DT is currently used to address patient-reported concerns, and this re-examination must go beyond simply shifting the threshold for clinical significance as has been suggested by some.11,12 At many institutions, including ours, pathological diagnosis of breast cancer is typically communicated over the phone by the radiologist or surgeon who performed the diagnostic biopsy. However, patients may benefit from a more extensive conversation that includes needs assessment and psychosocial evaluation at the same time these diagnoses are being communicated to address potentially modifiable barriers to care initiation at the point of diagnosis.
Telehealth via a patient’s or family member’s computer, smartphone, or other device with internet connectivity could serve as an excellent modality through which a patient with a new diagnosis of cancer could not only have a face-to-face conversation with a clinician to communicate pathology results but also complete psychosocial screening via the NCCN DT. In response to that screening, referrals to support services could be made immediately on the patients behalf, thereby preempting potential barriers to timely care initiation. Additional in-person follow-up could subsequently take place in coordination with the patient’s initial oncologic consultations. There are, however, significant age-, ethnicity-, and race-related disparities regarding mobile device ownership and internet connectivity.13,14 Accordingly, we must collectively prioritize the thoughtful dissemination of both telehealth and the technological resources required to facilitate its equitable implementation and avoid the risk of exacerbating extant disparities in patient access. The COVID-19 pandemic has unexpectedly fast-tracked our collective use of telehealth, and we hope that a silver lining of this otherwise challenging time is that the accelerated evolution and deployment of telehealth may potentially improve access to care for some of our more vulnerable patients.15
Using patient-reported DT scores to trigger support service referrals may be especially misleading for patients who have several stressors before breast cancer diagnosis. Black and Hispanic women had longer time to evaluation and time to treatment compared with White women, and there is evidence that women of color may have more logistical, social, and psychological barriers to breast cancer care initiation than their White peers.16-18 Black patients had the highest rate of neoadjuvant systemic therapy (27.9%) of any racial/ethnic group in our cohort (Table 1), reflecting the higher rates of triple-negative, HER2+, and locally advanced breast cancer historically observed in this group compared with White patients.19 However, despite the fact that neoadjuvant treatment was independently associated with shorter time to treatment and that Black patients received it more frequently than any other group, Black patients still experienced delays in treatment overall, demonstrating how entrenched barriers to care initiation can be.
The relatively low DT scores reported by Black women in our study do not necessarily represent a failure of self-perception, though we do acknowledge that denial may be a contributor to these lower scores and even to the higher rates of late-stage presentation observed among Black women. Rather, this tendency to report lower levels of distress compared with their peers of different races may actually reflect greater levels of resilience, or the ability to rebound from adversity.20
At-risk populations, including people of color who are stigmatized because of their race/ethnicity, often develop a combination of internalized and culturally specific mechanisms that help them to persevere in the face of adversity and bias. Among Black women, resilience has been identified as a protective factor with regard to cardiovascular disease, HIV/AIDS, and pregnancy outcomes.20-24 The resilience of already stressed patients who receive the additional burden of a breast cancer diagnosis is not to be minimized. However, it is imperative that we avoid penalizing patients who have been forced to embrace this coping strategy. Institutions must prioritize recruitment of diverse support staff as well iterative clinician training in culturally responsive needs assessment to better address the modifiable challenges of potentially vulnerable patients.
Our study limitations include those inherent to retrospective reviews. Because this analysis was performed using data from a clinical electronic health record, several patients were excluded due to missing data. In addition, records for 31 of the 96 patients in the race/ethnicity category “other” did not contain any meaningful racial/ethnic data. Furthermore, the sample sizes for our Hispanic and Asian/PI patients were small, thus we urge caution in extrapolating results for these groups. Nevertheless, we felt it was important to report the findings derived from the analyses with the more granular racial/ethnic categories to ensure that potentially significant differences between Asian/PI and Hispanic patients were not obscured by funneling them into a single heterogeneous pool. We recognize that some of our comparisons are based on small sample sizes with multiple comparisons, and we consider this portion of our work to be hypothesis-generating. However, we also recognize that this work presents an opportunity to shift supportive care from an individual- to a population-level approach that has the potential to maximize the efficacy of an important element of clinical care. Finally, we describe our experience at a university-based health system and acknowledge that our conclusions might not be generalizable to clinical oncology practices different from our own.
In conclusion, our analysis of a contemporary cohort of women with breast cancer revealed that patient-reported causes of distress predicted delays in time to evaluation and time to treatment, but patient-reported levels of distress did not, with Black patients reporting lower levels of distress than White patients, despite having longer time to evaluation and time to treatment. We recommend that assessments of distress be performed at the time cancer diagnoses are communicated. This early psychosocial evaluation—in combination with a more nu-anced application of the NCCN DT—could potentially address delays in time to care and mitigate disparities for vulnerable patients through targeted and culturally responsive interventions.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Oluwadamilola M. Fayanju is supported by the National Institutes of Health (NIH) under award 1K08CA241390. This work was also supported by the Duke Cancer Institute through NIH grant P30CA014236 and philanthropic funds provided by Sara and Bruce Brandaleone.
Footnotes
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: distress management, version 2.2019. Accessed 20 May 2019 http://www.nccn.org/professionals/physician_gls/pdf/distress.pdf [DOI] [PMC free article] [PubMed]
- 2.Gessler S, Low J, Daniells E, et al. Screening for distress in cancer patients: is the distress thermometer a valid measure in the UK and does it measure change over time? A prospective validation study. Psycho-Oncology. 2008;17:538–547. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005; 103: 1494–1502. [DOI] [PubMed] [Google Scholar]
- 4.Fayanju OM, Yenokyan K, Ren Y, et al. The effect of treatment on patient-reported distress after breast cancer diagnosis. Cancer. 2019;125:3040–3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016;2:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupstas AR, Hoskin TL, Day CN, Habermann EB, Boughey JC. Effect of surgery type on time to adjuvant chemotherapy and impact of delay on breast cancer survival: a National Cancer Database analysis. Ann Surg Oncol. 2019;26:3240–3249. [DOI] [PubMed] [Google Scholar]
- 7.Polverini AC, Nelson RA, Marcinkowski E, et al. Time to treatment: measuring quality breast cancer care. Ann Surg Oncol. 2016;23:3392–3402. [DOI] [PubMed] [Google Scholar]
- 8.Prakash I, Thomas SM, Greenup RA, et al. Time to surgery among women treated with neoadjuvant systemic therapy and upfront surgery for breast cancer. Breast Cancer Res Treat. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feeley TW, Fly HS, Albright H, Walters R, Burke TW. A method for defining value in healthcare using cancer care as a model. J Healthc Manage. 2010;55:399–411; discussion 411–412. [PubMed] [Google Scholar]
- 10.Yau KKW, Wang K, Lee AH. Zero-inflated negative binomial mixed regression modeling of over-dispersed count data with extra zeros. Biom J. 2003;45:437–452. [Google Scholar]
- 11.Ploos van Amstel FK, Tol J, Sessink KH, van der Graaf WTA, Prins JB, Ottevanger PB. A specific distress cutoff score shortly after breast cancer diagnosis. Cancer Nurs. 2017;40:E35–E40. [DOI] [PubMed] [Google Scholar]
- 12.Cutillo A, O’Hea E, Person S, Lessard D, Harralson T, Boudreaux E. The distress thermometer: cutoff points and clinical use. Oncol Nurs Forum. 2017;44:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pew Research Center: Internet & Technology. Mobile fact sheet. Accessed March 30, 2020 https://www.pewresearch.org/internet/fact-sheet/mobile/
- 14.Pew Research Center: Internet & Technology. Internet/broadband fact sheet. Accessed March 30, 2020 https://wwwpewresearch.org/internet/fact-sheet/internet-broadband/
- 15.Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020;382:1679–1681. [DOI] [PubMed] [Google Scholar]
- 16.Kim SJ, Glassgow AE, Watson KS, Molina Y, Calhoun EA. Gendered and racialized social expectations, barriers, and delayed breast cancer diagnosis. Cancer. 2018;124:4350–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGee SA, Durham DD, Tse C-K, Millikan RC. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol Biomarkers Prev. 2013;22:1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheppard VB, Oppong BA, Hampton R, et al. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015;22:2902–2911. [DOI] [PubMed] [Google Scholar]
- 19.Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014; 106:dju055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungar M. Researching and theorizing resilience across cultures and contexts. Prev Med. 2012;55:387–389. [DOI] [PubMed] [Google Scholar]
- 21.Artinian NT, Abrams J, Keteyian SJ, et al. Correlates of depression at baseline among African Americans enrolled in cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2009;29:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felix AS, Lehman A, Nolan TS, et al. Stress, resilience, and cardiovascular disease risk among Black women. Circ Cardiovasc Qual Outcomes. 2019;12:e005284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grobman WA, Parker CB, Willinger M, et al. Racial disparities in adverse pregnancy outcomes and psychosocial stress. Obstet Gynecol. 2018;131:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurston IB, Howell KH, Kamody RC, Maclin-Akinyemi C, Mandell J. Resilience as a moderator between syndemics and depression in mothers living with HIV. AIDS Care. 2018;30:1257–1264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.