Abstract
Metastasis is a multistep process that accounts for the majority of cancer-related death. By the end of metastasize dissemination, circulating tumor cells (CTCs) need to extravasate the blood vessels at metastatic sites to form new colonization. Although cancer cell extravasation is a crucial step in cancer metastasis, it has not been successfully targeted by current anti-metastasis strategies due to the lack of a thorough understanding of the molecular mechanisms that regulate this process. This review focuses on recent progress in cancer extravasation visualization techniques, including the development of both in vitro and in vivo cancer extravasation models, that shed light on the underlying mechanisms. Specifically, multiple cancer extravasation stages, such as the adhesion to the endothelium and transendothelial migration, are successfully probed using these technologies. Moreover, the roles of different cell adhesive molecules, chemokines, growth factors, as well as the mechanical factors in these stages are well illustrated. Deeper understandings of cancer extravasation mechanisms offer us new opportunities to escalate the discovery of anti-extravasation drugs and therapies and improve the prognosis of cancer patients.
Keywords: Cancer metastasis, Cancer cell extravasation, Cancer models, Cancer therapies
1. Introduction
Metastasis is the most life-threatening aspect of cancer that roughly accounts for 90% of cancer-related death [1]. During metastasize dissemination, metastatic cancer cells from primary sites need to complete multiple steps to form metastases, including 1. go through the epithelial-to-mesenchymal transition (EMT) to acquire enhanced motility and resistance to apoptosis; 2. intravasate tumor blood vessels and enter the circulation; 3. survive from attacks of the immune cells and mechanical stress in the bloodstream; 4. extravasate the endothelium as single cell or clusters, and; 5. form new colonization [1–5].
Among these steps, most of them have been successfully targeted in the past decades. For example, EMT can be inhibited by several non-coding RNAs (miR200 and miR205). The inhibition is achieved by regulating the expression of EMT-related transcription factors ZEB1, ZEB2, and Twist1/2 [6]. The intravasation of metastatic cancer cells can be restricted by interfering with the transforming growth factor-beta (TGF-β) [7] or the epidermal growth factor receptor (EGRF) signaling pathways [8]. Despite all the progress made in regulating one or more essential steps to attenuate metastasis, no strategies have been focusing on targeting cancer cell extravasation, with the main reason being that the cancer cell extravasation is not yet well understood and the molecular mechanisms that regulate this step are still missing [5].
In general, circulating tumor cells can extravasate the blood vessel using either diapedesis or angiopellosis [9–12]. The former approach requires the cancer cells to squeeze through the endothelial junction and only one single circulating tumor cell (CTC) is allowed to pass through the endothelial cell (EC) barrier at a time [9,13]; while the latter approach, angiopellosis, involves active remodeling of vascular ECs to form a pocket around a single CTC or a CTC cluster and the following expulsion to push the CTCs out of the blood vessels [9,14,15]. Regardless of the extravasation approaches, accumulating data suggest that cancer cell extravasation is a complicated multi-step process consisting of adhesion of CTCs onto the endothelium and the following transendothelial migration (TEM) [4,5]. Recent data also prove that various biochemical and physical factors are participating in the cancer cell extravasation process in an intercorrelated manner, including multiple cell adhesive molecules (selectins, cadherins, and integrins), chemokines, growth factors, and mechanical factors [4,5].
To resolve the complexity involved in the cancer cell extravasation process and shed light on the underlying molecular mechanisms, and to develop more effective treatment methods that target cancer metastasis, a huge effort has been put into building platforms that can combine the advanced in vitro vasculature or in vivo animal model and the currently available high-resolution imaging techniques [16,17]. In this review, we focus on the recent advances made in cancer cell extravasation visualization techniques, including the development of microfluidic platforms to reproduce and probe extravasation in vitro and the use of several animal models for extravasation mechanism studies. In particular, we describe how these novel platforms help us gain deeper insight into the molecular mechanisms that regulate cancer cell extravasation. These cancer extravasation mechanisms can rapidly promote the development of anti-extravasation drugs and therapies and extend the life of cancer patients.
2. Current methods for cancer extravasation study:
2.1. In vitro extravasation assays:
In recent years, various in vitro tools have been newly developed or modified to capture the extravasation events. These tools include the cancer-endothelium adhesion and invasion assay, the Boyden chamber/Transwell assay, and microfluidic platforms with engineered blood vessels. The development of these in vitro cancer extravasation assays helps us gain deeper understanding of the molecular mechanisms that regulate the extravasation process, and facilitates the anti-metastasis drug discovery. The advantages and drawbacks of each assay are summarized in Table 1.
Table 1.
| Advantages | Disadvantages | ||
|---|---|---|---|
| Cancer-endothelium adhesion and invasion assay [18–23] |
|
|
|
| Boyden chamber/Transwell assay [24–36] |
|
|
|
| Pre-patterned substrate-based [47–50] |
|
|
|
| Self-assembled vascular network-based [16,51–55] |
|
|
|
2.1.1. Cancer-endothelium adhesion and invasion assay
The cancer-endothelium adhesion and invasion assay (Fig. 1a) is the simplest approach to investigate the interactions between cancer cells and ECs during the extravasation process. In general, ECs in this assay are plated on a gelatin-coated surface to form a confluent EC monolayer. Thereafter, cancer cells are directly added onto the EC monolayer and incubated for several hours, and the non-adherent cancer cells will then be removed with proper washings. Fluorescence microscopy or electron microscopy is conducted at several different time points post co-culture to examine the cancer cell-EC interactions [18–20]. Kramer and Nicolson used this assay to successfully reveal the sequential events involved in cancer cell TEM, including the initial attachment, cancer-cell induced EC retraction, complete penetration through the EC monolayer, and the following spreading and migration over forty years ago [19,20,21]. And more recently, this assay is further accommodated to investigate the effect of drugs, nanoparticles, and mechanical flow stress on cancer-endothelium adhesion due to its great simplicity and compatibility [12,18,22,23]. However, the two-dimensional (2D) nature of this assay restricts our observation and understanding of cancer cell invasion in a 3D physiological environment. Moreover, the transmigrated cancer cells can hardly be fully imaged, collected, and quantified. As a result, this assay is often limited to probing the cancer-endothelial adhesion in cancer extravasation research.
Fig. 1.

In vitro models for cancer cell extravasation study. a) Cancer-endothelium adhesion and invasion assay; b) Transendothelial migration assay using Transwell chambers that mimic the crossing of cancer cells through the endothelium; c) Cancer-vessel model in the pre-patterned substrate [49]; d) Self-assembled microvascular network in ECM hydrogel [16].
2.1.2. Boyden chamber/Transwell assay
The most commonly used model to investigate cancer extravasation is the trans-endothelial assay built on the Boyden chamber/Transwell platform (Fig. 1b) [24–26]. In this assay, endothelial cells are seeded on the inserted porous membrane (diameter ~3–12 μm) and cultured in the upper chamber, and condition medium containing specific chemokines or cytokines to attract cancer cells is placed in the lower chamber. Similar to the cancer-endothelium adhesion and invasion assay, after the ECs form a confluent monolayer, cancer cells are seeded on the top of the monolayer. The transmigrated cancer cells in the lower chamber within the following 48 hours will be collected and quantified for further analysis [24–27]. More recently, a 3D extracellular matrix (ECM) such as Matrigel is often incorporated beneath the endothelial monolayer in this assay, which offers more physiological relevance by recapitulating the invasion of cancer cells into ECM following the extravasation [28].
The key advantages of the trans-endothelial assay are its simplicity and great adjustability. The protocols for operating such assays have been fully illustrated in literature with great details [29]. Commercialized kits of the assay (e.g., Tumor Transendothelial Migration Assay kit from Cell Biolabs and QCM™ tumor cell trans-endothelial migration assay from Millipore) have also been proved to be efficient and robust [30,31]. Unlike the in vivo models in which the local biochemical environment is often hard to modulate, the high adjustability of this trans-endothelial migration assay allows us to determine the roles of specific cell types [32–34] and non-cell factors [35,36] under various biochemical and genetic settings in the cancer transendothelial migration. Moreover, as this assay can be easily scaled up, it is often preferred when conducting high-throughput drug screening for metastasis inhibitors [24].
However, this 2-dimensional (2D) monolayer-based system has less physiological relevance with the real 3D biological systems. As accumulating evidence point out the essential role of the spatial morphology in regulating the function of blood vessels, more advanced tools are needed to mimic the interactions between the cancer cells and the vascular ECs in 3D during the extravasation process. Another drawback of the trans-endothelial migration assay comes from its application aspect: the dynamic process of cancer cells can hardly be monitored in real-time or in high resolution, which largely limits the application of this assay to end-point quantitative measurements and analyses [25,37]. Moreover, most cancer cells in this 2D monolayer-based assay will eventually translocate across the EC monolayer within the 48-hour incubation period, which is inconsistent with the phenomenon observed in vivo where only less than 50% of CTCs can successfully extravasate [9,37]. A possible contributor to this inconsistency is the static, but not dynamic as what it should be in vivo, environment in this assay, which renders much more tumor-EC adhesion compared to the in vivo context [12] and thus more EC deaths induced by the cancer cells [38,39]. Therefore, microfluidic chips with controllable flow rates are recently introduced for better investigating cancer extravasation [40–45].
2.1.3. Microfluidic platform with engineered blood vessels
Recently, microfluidic chips with functional microvascular networks in 3D extracellular matrix (ECM) constructs have been widely used for investigating cancer cell extravasation. According to the approach used to construct the microvascular networks, these chips can be classified into two categories: pre-patterned substrate-based (Fig. 1c), or self-assembled microvascular networks based (Fig. 1d) [10,25,46]. The former category requires the pre-construction of a cylindrical pipe-like pattern on the substrate, and the vascular networks are formed by injecting endothelial cells into the hollow of the pattern [47–50]. Wang et al. reported a 3D microfluidic chip with artificial blood vessels for recapturing the trans-vascular migration of cancer cells in vitro (Fig. 1c) [49]. The artificial vascular microtubes in this model were produced by seeding endothelial cells into the patterned cellulose/collagen scaffold. Thereafter, cancer cells were perfused into the system. Cancer cell adhesion and the following trans-vascular migration were reconstituted and recorded using live-cell fluorescence imaging. The major advantage of this type of chips is that their size and the vascular network pattern can be precisely controlled, which gives it great consistency and reproducibility [10]. The flow rates inside the blood vessels can also be well controlled in these pre-patterned chips [12]. In addition, high-resolution imaging of the cancer cell extravasation process is also enabled by such chips since the vascular networks can be patterned into a single focal plane [50]. However, since this technique did not follow the natural endothelial morphogenesis in making the 3D vascular networks with the lumen, it could result in losing some of the important biological features and functions of the blood vessels. Therefore, microfluidic chips with self-assembled vascular networks were developed and have been frequently reported in recent years [16,51–55]. This technique relies on the assistance of other participating cells (e.g. human lung fibroblasts) that are co-cultured with ECs in the 3D ECM hydrogel to facilitate the controlled heterotypic cell-cell interactions that will stimulate the endothelial cells to form lumenized vascular networks. The first microfluidic chip containing self-assembled microvascular networks was reported by Chen, X et al in 2009 [53], and was then revised and repurposed by Chen, M. B. et al in 2013 to visualize cancer cell extravasation [54]. In the model described by Chen, M. B., human umbilical vein endothelial cells (HUVECs) and human lung fibroblasts (HLFBs) were loaded into different hydrogel regions separated by media channels (Fig. 1d). During the device culture, HUVECs interacted with each other to form vasculature with the lumen, meanwhile, the paracrine signaling from fibroblasts prevented the premature regression of the microvascular network. After 4–5 days, cancer cells were then perfused into the microvascular network, and cancer cell extravasation events could be captured and quantified using confocal microscopy after the cell perfusion. Compared with the vascular networks obtained by pre-patterned substrate methods, this self-assembly technique closely mimics the natural endothelial morphogenesis to make a self-developed microvascular network. Therefore, the as-obtained vessels have similar sizes, mechanical properties, and biological functions as the natural blood vessels [16]. Besides, the small size of these chips renders the low drug or antibody amount per experiment, which makes them economically suitable for high-throughput screenings. However, since the microvascular networks are not pre-patterned, batch effect exists among independent samples and different research labs. Thus, during the production of such chips, extreme caution is needed to ensure quality control.
In general, microfluidic chips are promising tools to investigate cancer cell extravasation due to the advantage that they can better mimic the CTC-blood vessel interactions in vivo. To make this technique more robust while maintaining its great physiological relevance to the in vivo scenario, novel techniques are applied to combine the advantages from both pre-patterned ECM based and self-assembled microvascular networks. A good example of this is the bioprinting of vascular tissues [56,57]. For example, Kolesky et al reported a vascularized tissue with more than 1 cm in thickness using 3D bioprinting technology [56]. This vascularized tissue can incorporate multiple cell types including human mesenchymal stem cells (hMSCs) and human neonatal dermal fibroblasts (hNDFs) into the extracellular matrix. A potential drawback of the current vascularized microfluidic chips is the low drug screening capability since current techniques only allow us to test one drug per chip and image one chip per time, which cannot satisfy the needs of high content screening for anti-metastasis drugs. To solve this issue, multi-unit microfluidic chips are needed to enlarge the throughput of these model chips.
2.2. In vivo extravasation models:
The mouse model has been referred as the “golden standard” for cancer metastasis research for the last few decades. Recently, chorioallantoic membrane assays and the zebrafish model are also frequently used for visualizing cancer cell extravasation due to their great visibility and accessibility. The advantages and disadvantages of each animal model are summarized in Table 2.
Table 2.
| Advantages | Disadvantages | |
|---|---|---|
| Mouse [17,58–65] |
|
|
| Chorioallantoic membrane (CAM) assays [37,66,67] | ||
| Zebrafish [68–72] |
|
|
2.2.1. Mouse model
The mouse model is a widely used tool to reveal the complexity involved in the cancer cell extravasation process since it possesses great similarities to the human model in terms of anatomy, physiology, and genetics (Fig. 2a). In a mouse model, cancer cells expressing fluorescent proteins are injected into the blood vessels, then, to visualize the extravasation events, an imaging window needs to be implanted by surgery due to the opacity of mouse tissue and the inaccessibility of internal organs (e.g., lung and liver) in which the cancer cell extravasation takes place [17,58,59]. Multiple imaging platforms including confocal and multiphoton imaging techniques have been applied for tracking the extravasating cancer cells with high spatial resolution [60]. However, the limited imaging depth (1–2 mm) and the auto-fluorescence emitted by tissue in the visible wavelength (400–700 nm) make it hard to capture the metastasis events in deep tissues [61]. Recently, the second region near-infrared (NIR-II, 1,000–1,700 nm) bioimaging has been explored to visualize the metastatic cancer invasion in real-time [62–64]. The key to the highly selective NIR-II bioimaging is the NIR-II contrast agent, such as carbon nanotubes, semiconductor quantum dots, and organic small molecular dyes [64–68]. In recent years, quantum dots have been widely used in NIR-II cell imaging and tumor detection because of their strong NIR-II fluorescence and low toxicity. Surface modification with polyethylene glycol (PEG) or tumor‐seeking donor-acceptor-donor (D‐A‐D) dye significantly increased their water solubility and broadened their applications in tracking the lymph node metastasis in tumor-bearing mice [67,69,70]. Apart from NIR-II bio-imaging, mouse intravital imaging is another technique that has been rapidly advanced these years. The quick development and advancement of imaging technologies such as two-photon microscopies and fiber-optic fluorescence microendoscopies further potentiated our ability to use intravital imaging to visualize the cancer metastasis and metastatic growth at a cellular level in real-time [71,72]. However, since continuous intravital imaging in mouse models is generally limited to several hours, characterization and study of the whole metastasis process have not yet been achieved. So far, the mouse model has been reported to be capable of revealing the single steps of metastasis formation in the brain, lung, and liver [58,59,73,74]. More advanced technologies are needed to reduce the technical complexities involved in intravital imaging of mice and to prolong its duration. Tissue clearing is a promising technology that renders direct fluorescent imaging of intact organs without surgically creating an imaging window. Recently, Pan et al. established an integrated and highly automated cancer metastasis 3D imaging and characterization platform through combining the tissue clearing method and the vDISCO whole-body immunolabeling technology that was also newly developed in the same research group (Fig. 2a) [17,75]. Together with the convolutional neural networks (CNN)-based deep learning approach, they were able to use this platform to monitor cancer metastasis and metastatic growth in the whole mouse body, and identify even individual disseminated cancer cells. This platform also potentiated detailed feature analysis of metastases such as their size, shape, and spatial distribution which could not be achieved before. Tissue clearing, combined with modern immunolabeling and imaging techniques, is expected to provide new possibilities to the cancer metastasis study.
Fig. 2.
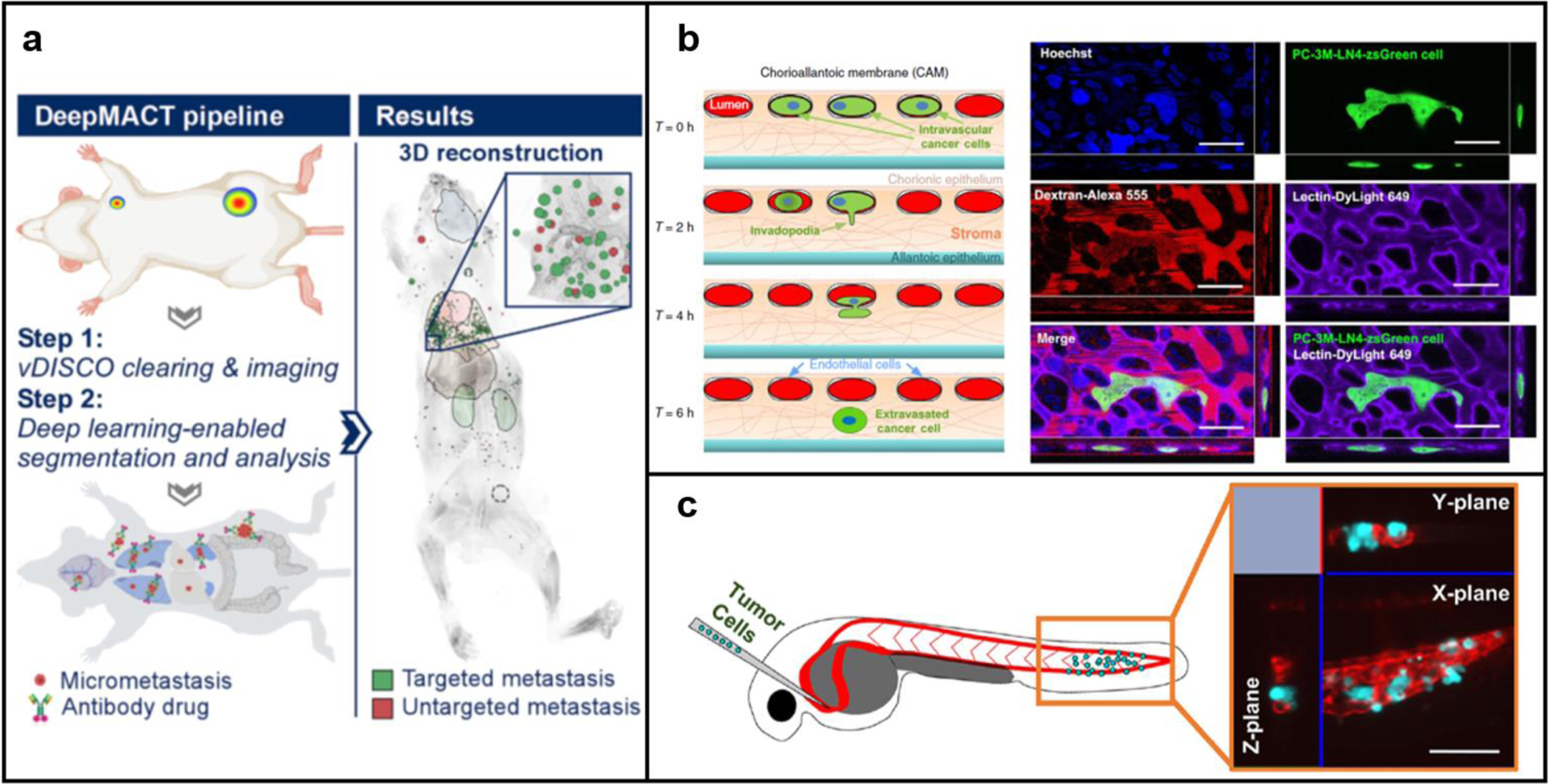
In vivo models for cancer cell extravasation study. a) Mouse model [17]; b) Chorioallantoic membrane (CAM) assay [37]; c) Zebrafish model [9].
However, although these novel technologies possess the possibility of detailed observation of the extravasation process using the mouse model, some drawbacks still exist including the relatively high cost and high technological prerequisite [17]. Therefore, mouse models are generally considered to be more suitable for end-point ex vivo analyses in most cancer metastasis studies.
2.2.2. Chorioallantoic membrane assays
The chicken chorioallantoic membrane (CAM) is a highly vascularized extra-embryonic membrane. The great visibility and accessibility of the CAM make it a good candidate for evaluating cancer cell extravasation in vivo (Fig. 2b) [37,76]. After being injected into CAM veins, a large portion of the fluorescence-labeled cancer cells will physically be trapped in the capillary bed and then translocate into the underlying stroma layer [37]. Unlike murine models, the capillary bed of CAM is flat and can be easily labeled by intravenous injection of fluorescently conjugated lectins or dextran, which enables easy distinguish between the intravascular cancer cells and the extravasated counterparts using normal confocal microscopy [37,77]. Moreover, the blood volume of chicken embryos is much smaller (1.3–3.4 ml in total) compared to the mouse model [37], which means the consumption of labeling agents and therapeutic compounds in CAM assay will be lower. Given that the chicken embryos themselves are also cheaper than any other animal model, it enables users to economically perform high-content drug screening in vivo. However, since CAM is continuously undergoing rapid morphological alterations even during the short experimental period, it will be difficult to identify cancer cell-induced vascular remodeling during the extravasation process [76]. Also, the chicken-origin nature of the CAM assay may limit the feasibility of reagents compared to murine models [76].
2.2.3. Zebrafish model
Zebrafish is an emerging vertebrate model for cancer cell extravasation studies these years (Fig. 2c). The embryo zebrafish immune system is not fully developed, which allows easy xenotransplantation of human cancer cells [78–80]. Unlike mouse models or CAM models, the zebrafish embryos are naturally optical transparent, thus xenotransplanted fluorescence-labeled cancer cells can be easily visualized in real-time using a microscope. Also, since their vasculature is well patterned and is less subject to dynamic changes, cancer cells are often trapped in the capillaries and their extravasation requires interaction with the surrounding endothelium and the active remodeling of endothelial structure, which closely mimic the cancer cell extravasation process in human patients [78,81]. Thus, in a zebrafish model, the highly dynamic tumor-EC interactions and flow conditions inside blood vessels can be clearly imaged and evaluated at the cellular or even subcellular level by confocal or light-sheet microscopy in real-time [9,78]. Moreover, benefit from the fact that zebrafish embryos develop and mature externally, transgenic and chimeric zebrafish can be created by direct microinjection of genetic materials into the single-cell embryos, which allows easy identification of the dominant genes and signaling pathways involved in the cancer cell extravasation process [78]. For example, the essential role of a pro-metastatic gene, Twist, in facilitating intravascular migration and extravasation of cancer cells has been identified by Stoletov et al using intravital confocal microscopy and a transgenic zebrafish model [78]. In addition, only very low quantities of expensive test drugs and staining reagents are needed for a zebrafish, which makes it a suitable model for high throughput screening. The main drawbacks of the zebrafish model include the physiology of zebrafish is not identical to humans, the chorion presented in the zebrafish model can interfere with drug diffusion [82], and also, some technical challenges associated with cancer cell injection and live imaging in intact organisms might exist.
3. Cancer cell extravasation mechanisms
Cancer cell extravasation is a multistep process, which starts with the adhesion of CTCs to the luminal side of the blood vessel endothelium and ends with the transendothelial migration of the arrest cancer cells (Fig. 3). Novel cancer extravasation visualization platforms and characterization techniques have helped us gain deeper understandings of the underlying mechanisms and offered us more potential therapeutic targets to efficiently block cancer cell extravasation and cancer metastasis.
Fig. 3.
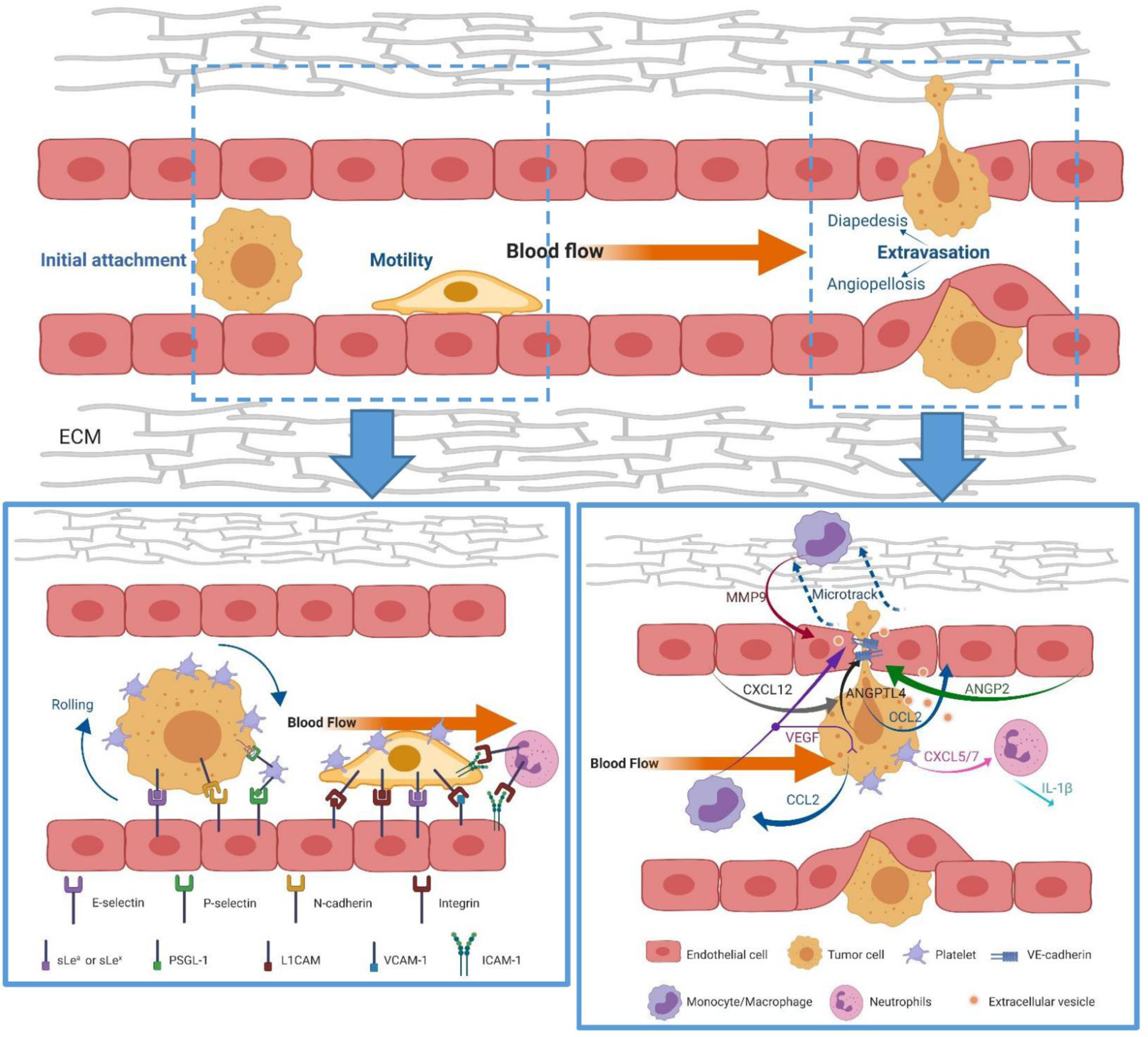
General steps and key contributors involved in cancer extravasation. Bottom left: cell adhesive molecules (selectins, N-cadherins, integrins) mediate the adhesion of cancer cells to the endothelium; Bottom right: Cancer cells form protrusion structure (invadopodia) to transmigrate the endothelial barrier, multiple growth factors, chemokines, proteases, and extracellular vesicles secreted by either cancer cells or cancer-associated leukocytes can facilitate the transendothelial migration of cancer by disrupting the integrity of endothelial cells or increasing the motility of cancer cells.
3.1. Adhesion of cancer cells to the endothelium
Adhesion to the endothelium is the first step in cancer cell extravasation. After being physically trapped in small capillaries, CTCs first form weak and intermittent adhesions to the endothelium. These weak attachments are then gradually replaced by high-affinity binding followed by transendothelial migration [4,83]. Accumulating data suggest that many pairs of cell ligands and receptors, including selectins, cadherins, integrins, and immunoglobulins, as well as several mechanical factors, e.g. blood flow rate, are required for tumor cell-endothelial cell adhesion [4,12].
3.1.1. Selectins and Neuronal cadherin (N-cadherin) contribute to the initial attachment
Selectins are a family of cell adhesion molecules consisting of E(ndothelial)-, P(latelet)-and L(ymphocyte)- selectin, among which E- and P-selectin have been shown to facilitate the adhesion of cancer cells to the endothelium [84]. The synthesis of E-selectin is induced by inflammatory cytokines like IL-1, TNFα, and interferon-γ secreted by cancer cells or cancer-associated leukocytes [83,85]; while P-selectin is pre-synthesized and stored in the granules of platelets (α-granules) and endothelial cells (Weibel-Palade bodies) and can rapidly translocate to the cell surface after cytokine activation [85,86]. Cancer cells from different cancer types can express various selectin-specific ligands such as hematopoietic cell E-selectin/L-selectin ligand (HCELL) and P-selectin glycoprotein ligand 1 (PSGL-1) to bind with selectin-expressing endothelium [87,84]. Among these selectin ligands, most of them contain a sialyl Lewisx/a (sLex/sLea) on their glycoproteins and glycolipids, which is a tetrasaccharide structure that facilitates CTC-endothelium adhesion and the increased level of which often results in poor clinical diagnosis [4,88,89,90]. Besides, the binding between P-selectins expressed by platelets and selectin ligands on cancer cells can bridge the CTCs and the platelets, enabling in-direct adhesion of cancer cells to endothelial cells [84]. The role of selectins in inducing the initial adhesion of CTCs to endothelium was confirmed by Shin et al using microfluidic chips [40]. The chips were coated with endothelial cells and the metastatic LoVo colon cancer cells were co-cultured in the chips. TNF-α was used to stimulate the endothelial cells and induce the expression of E-selectin on their surface. Initially, the LoVo cells failed to adhere to the unstimulated endothelium, however, after the TNF-α stimulation, the E-selectin expression on the endothelium induced the adherence of LoVo cells depending on the microfluidic flow rate. The further treatment of the LoVo-endothelium coculture with a broad-spectrum matrix metalloproteinase inhibitor, GM 6001, and an antibody to sLea, CA19–9, reduced the number of cancer cells that adhere to the endothelium, illustrating the crucial role of selectins and their ligands in mediating CTC-endothelium interaction and adhesion.
Neuronal cadherin (N-cadherin), expressed by both cancer cells and endothelial cells, is another type of receptor that mediates the initial attachment of cancer cells to the endothelium [91,92]. It was reported that the attachment of cancer cells to the endothelium could induce a two-fold increase in N-cadherin expression on the cancer cell surface and a quick redistribution of N-cadherin to the cell-cell contact region [92]. Knocking down N-cadherin expression on cancer cells can reduce the interaction of cancer cells with ECs as well as the subsequent transendothelial migration [92]. Although N-cadherin is clearly involved in tumor-EC interaction in vitro, no direct evidence has been shown that it plays the same role in vivo. Thus, more efforts need to be put into mechanism studies using advanced in vivo cancer extravasation models.
3.1.2. Integrins mediate stable attachment
Integrins are a family of transmembrane cell adhesion proteins consisting of α/β heterodimers. Integrins play an important role in both cell-cell and cell-ECM interactions and have been reported to be critical for cancer cells-EC adhesion. For example, the α4β1 integrins expressed by cancer cells can serve as an alternative ligand for VCAM-1 (vascular cellular adhesion molecule 1, CD106) to mediate firm adhesion of cancer cells to the endothelium [13,93]. The interactions between the αVβ1 or αVβ3 integrins and L1-CAM (neuronal cell adhesion molecule) can also contribute to tumor cell-EC adhesion [94]. Additionally, leukocytes that express αLβ2 integrins can function as linker cells to bind cancer cells and EC that express ICAM-1 (intercellular adhesion molecule 1, CD54) [95]. Thus, inhibiting integrin expression on EC surface can result in a reduction of protrusion and extravasation of cancer cells [96]. To illustrate the role of integrins in mediating cancer cell extravasation, Chen et al. built a 3D microfluidic model of human vasculature which could finely recapitulate the in vivo endothelium microenvironment and flow dynamics [97]. Combined with confocal imaging, their microfluidic model enabled visualization of single-cell extravasation events at a high spatial resolution that could not be achieved previously in any in vivo models. Benefited from this powerful platform, they demonstrated the essential role of integrin β1 in facilitating MDA-MB-231 cancer cell protrusion and trans-endothelium translocation by temporarily knocking it down using shRNAs. Loss of integrin β1 resulted in a significant reduction in cancer cell TEM rates. Moreover, recently, single-molecule atomic force microscopy (AFM) enabled us to directly characterize the force interaction between the integrin α5β1 and fibronectin in cancer cell-endothelium interaction [98]. All these findings demonstrate the essential role of integrins in mediating firm cancer cells-EC attachment and suggest that blocking integrins can be a promising approach to inhibit cancer cell extravasation and therefore metastasis.
3.2. Trans-endothelial migration
After stable attachment has formed, cancer cells will gradually pass the vascular endothelial barrier to evade the ECM surrounding the vasculature, after which the extravasated cancer cells will either form secondary tumors or remain dormant in the tissue [4,60,99]. To cross the endothelial barrier, cancer cells will form a protrusive structure, invadopodia, and squeeze out of the EC tight junctions [100,101]. Meanwhile, the vascular permeability can be modulated by cancer cells and cancer-associated leukocytes through either direct contact or secreted growth factors, chemokines, and small extracellular vesicles [102–104]. Recently, mechanical factors such as flow rates and shear forces have also been shown to lead to the remodeling of ECs and the promotion of cancer cell extravasation [12,105,106].
3.2.1. Cancer cells form invadopodia to mediate trans-endothelial migration
Invadopodia are actin-based dynamic protrusions of the plasma membrane that are formed actively by cancer cells [101,107,108]. These protrusions consist of structural proteins including cortactin, N-WASP, Tks4, Tks5, as well as pericellular proteases such as MT1-MMP, MMP9, and MMP2 [77,100,107,109]. The enriched proteases allow invadopodia to degrade the extracellular matrix, favoring the invasion and TEM of cancer cells [77]. Recently, invadopodia formation on cancer cells prior to and during the extravasation process has been visualized using chicken embryo chorioallantoic membrane (CAM) models [77], zebrafish models [110], and vascularized microfluidic systems [111,112]. In the work of Leong et al., they visualized the invadopodia formation and functioning during cancer cell transendothelial migration in a CAM model using intravital imaging [77]. Their CAM intravital imaging system enabled direct visualization and characterization of cancer cell invadopodia, and more importantly, provided direct in vivo evidence for the key functional role of invadopodia in the extravasation of CTCs and metastasis of cancer. Further, they inhibited the invadopodia assembly using both genetic methods (through shRNA knockdown of cortactin, Tks4, and Tks5) and pharmacological interruption (using a Src Kinase inhibitor, Saracatinib, which disrupts the phosphorylation of cortactin to its active state) and demonstrated that the major structural proteins of invadopodia such as cortactin could serve as potential therapeutic targets in disrupting CTC extravasation and abrogating metastasis. These along with other findings using alternative in vivo and in vitro models support the contribution of invadopodia in mediating cancer extravasation, and provide us new therapeutic targets to inhibit cancer metastasis [77,113].
3.2.2. Vascular integrity can be regulated by cancer cells or cancer-associated leukocytes and platelets during TEM
The endothelial barrier is a dynamic structure that constantly undergoes remodeling. During the extravasation process, cancer cells can destabilize the endothelial barrier to facilitate their TEM [5]. It has been shown that cancer cells can disrupt the integrity of endothelium by inducing endothelial necroptosis through interacting with the death receptor 6 (DR6) expressed on the surface of ECs [39]. Knocking down the DR6 ligand expressed by cancer cells, amyloid precursor protein (APP), can significantly reduce EC death and abrogate the cancer cell TEM [39]. In addition to the endothelial necroptosis and the barrier disruption resulted directly from the cell death, the endothelial cells that undergo necroptosis can also release damage-associated molecular patterns (DAMPs, e.g., ATP and HMGB1), which will induce the opening of the endothelial barrier and facilitate the extravasation of cancer cells [114].
Vascular integrity can also be regulated by multiple growth factors, chemokines, or proteases secreted by either cancer cells or cancer-associated leukocytes [4,5,115]. For example, VEGF and TGFβ1 secreted by cancer cells or their associated macrophages can induce the EC junction opening by interfering with the VE-cadherin–β-catenin complex [116–119]; angiopoietin-like 4 (ANGPTL4), and CC-chemokine ligand 2 (CCL2) that are secreted by cancer cells were shown to antagonize vascular endothelial tight junctions, and hence promote cancer cell extravasation [120,121]. Interestingly, CXC-chemokine ligand 12 (CXCL12), a chemokine that is secreted by endothelial cells and stromal cells in distant organs was shown to promote cancer cells TEM by interacting with its receptors CXCR4 and CXCR7 expressed on cancer cells [122]. Extravasation of cancer cells can be blocked upon the use of an antibody to CXCR4 or CXCR4 depletion on cancer cells [35]. Matrix metallopeptidase 9 (MMP9) highly expressed in monocytes was shown to facilitate cancer TEM by disrupting tight junction proteins [115]. The different expression patterns of chemokine and chemokine receptors in different organ vasculatures can partly explain why different types of cancer cells preferentially extravasate the blood vessels of different organs.
During cancer progression, various cell types can release membrane vesicles containing oxidized phospholipids that function as pro-inflammatory mediators [123]. These lipid mediators can modulate the vascular integrity by altering the profiles of cell surface adhesive molecules, regulating signaling pathways (Rho and Rac), and promoting cytoskeleton remodeling for endothelial retraction [123–125]. Among these pro-inflammatory mediators, sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) derived from α granules in activated platelets were reported to have a major role in regulating the vascular permeability [123–125]. S1P can bind to five G-protein coupled receptor isoforms S1P1 to S1P5 depending on its concentration. The binding of S1P with different receptor isoforms will result in different effects on endothelial cells. At physiological concentrations, S1P bound to S1P1 coupled to Gαi and shows a protective effect to maintain the endothelium integrity by activating Rac, while a high concentration of S1P binds to S1P2 and S1P3 coupled to Gα12/13 or Gαq and disrupts vascular integrity through the activation of Rho [123,126,127]. In addition to S1P, LPA is another lipid mediator secreted by platelets. LPA can stabilize the integrity of corneal endothelial types but will notably increase the permeability of brain microvascular endothelial cells [128,129]. All of these findings suggest the important role of platelets in regulating vascular integrity by secreting pro-inflammatory lipid mediators.
Besides functioning as pro-inflammatory mediators, extracellular vesicles, especially exosomes, have been reported to modulate the vascular permeability in many other approaches [130–132]. Exosomes secreted by cancer cells can be rapidly uptake by adjacent endothelial cells and can break down the vascular integrity by inducing endoplasmic reticulum stress in endothelial cells [131]. Additionally, the exosomal miRNAs can interfere with the expression of multiple vascular permeability-related proteins in endothelial cells through either directly targeting the protein-expressing genes or regulating the transcription factors [130,133]. For example, in colorectal cancer, the cancer cell-derived exosomes contain miRNAs (including miR-23a and miR-105) that can downregulate the tight junction protein ZO-1, resulting in loss of tight junctions in the endothelium and destroyed endothelial barrier [130,134].
3.3. Mechanical factors contribute to TEM
Biomechanical forces have been shown to have a crucial impact on cancer metastasis progression. Recent advances in cancer cell extravasation model and assay development, cooperating with multiple imaging and force-sensing tools, enabled us to investigate the roles of multiple mechanical factors in cancer extravasation with proper controls [135]. For example, the recent use of multiphoton laser scanning microscopy empowered us with an unprecedented capability to achieve real-time monitoring of the individual cells and extracellular matrix in cancer metastasis models in vivo [59]. And using the recently developed blot rolling assays and flow-based adhesion assays, Thomas et al. successfully characterized the influence of shear stresses on the adhesion of cancer cells to the selectins that were present on the endothelium [136]. Similarly, using parallel plate flow chambers, shear stress was found to induce the endothelial cell polarization through the mediation by Rho and Rac proteins [137]. All these models and technologies provided direct evidence that cancer cells and ECs could sense and respond to the surrounding mechanical changes, and advanced our understanding of how mechanical forces could regulate cancer cell mobility and TEM potential.
3.4. Angiopellosis is an alternative cancer extravasation mechanism distinct from diapedesis
In recent years, it has been reported that cancer cells possess the ability to cross the endothelial barrier through a new mechanism, termed “angiopellosis” [12,15]. Unlike the diapedesis-like process that requires cells to squeeze through the junctions between ECs, the angiopellosis process relies on the active remodeling of ECs to cover the extravasating cells and then push these cells out of the blood vessels. Due to this fact, multicellular cancer clusters, which possess a higher metastatic potential, can also extravasate using this mechanism. This new mechanism was identified independently by Allen et al in 2017 and Follain et al in 2018 as the dominant mechanism for both cancer cells and stem cells extravasation using intravital imaging of a zebrafish vasculature model [12,15]. Before these two independent identifications, earlier work had also eluded that cancer cells trapped in pulmonary capillary would become physically covered by endothelial cells during lung metastasis, and the phenomenon was termed as “endothelization” [11,138,139]. Altogether, these findings provide solid evidence to support that endothelial remodeling plays an essential role in cancer extravasation. Yet, the underlying molecular mechanisms behind this phenomenon are poorly understood. Further investigation of angiopellosis will expand our knowledge in the relationship between vascular functions and cancer cell dissemination and offer us new therapeutic targets to treat metastatic cancer.
It is known that endothelial cells comprise a diverse population of cells [140]. For example, endothelial cells can be briefly classified as continuous, fenestrated, or discontinuous endothelial cells depending on the size of intercellular junctions. Continuous endothelial cells are found in most arteries, veins, and capillaries of the muscle, skin, brain, lung, heart, and connective tissues. They anchor to a continuous basal membrane with only tight junctions between the adjacent cells. Fenestrated endothelial cells are often present in endocrine and exocrine glands, kidney, and villi of the intestine. They usually contain 50–60 nm transcellular pores sealed by 5- to 6-nm-thick diaphragms. Discontinuous endothelial cells have larger fenestrations with up to 200 nm without diaphragm. They are frequently observed in the liver, spleen, and bone marrow [141]. The heterogeneity of endothelial cells renders their distinct behaviors in different organs. For example, brain capillary endothelial cells are able to form the blood-brain barrier (BBB), which regulates selective transportation from blood to the brain and protects the brain from being infected by circulating toxins or pathogens [142]. Except for the barrier functions, endothelial cells are also associated with the clearance of blood clots through a process termed “angiophagy”. Endothelial cells in the brain, heart, lung, and kidney can engulf large emboli and deliver them into the perivascular space [143,144]. Furthermore, it has been observed that endothelial cells can internalize apoptotic bodies, cell debris, and even pathogens through the phagocytosis-like process, and subsequently shutter them into the lysosome or out of the blood vessels [145–148]. The escort function of blood vessels allows endothelial cells to expel clogged cancer cells through the angiopellosis-like manner.
The angiopellosis process can reflect an early attempt of CTCs to initiate a tumor-based vessel. Identified by Maniotis et al. in 1999, vasculogenic mimicry is a process of micro-blood vessels formation by metastatic cancer cells without the presence of endothelial cells [149,150]. Vasculogenic mimicry is made possible by the diversified gene profile of metastatic cancer cells. Transcriptomic analysis reveals that tumor endothelial cell cluster released from tumor vasculature is one of the main components of CTC clusters. These circulating endothelial clusters, which exhibit high-level expression of endothelial markers including VE-Cadherin, CD31, and VWF, can potentially form new vasculatures and support the growth of tumors at a distant site [151]. Similar to vasculogenic mimicry, the angiopellosis-participating tumor cell clusters also possess diversified gene profiles. This genetic diversity not only contributes to the high survival ability of the extravasated tumor cells clusters in different organs, but also facilitates the communications between tumor cells and endothelial cells during the extravasation process. To some extent, the angiopellosis process reflects the attempt of CTCs to utilize the existing vascular endothelium to help themselves survive and form new metastatic sites. Blocking the interactions between CTCs and endothelium is therefore a potential target to inhibit distant tumor formation.
However, the molecular mechanisms that control the angiopellosis process are largely unknown. The research by Follain et al. revealed the contribution of flow forces in facilitating the arrest, adhesion, and successful extravasation steps during angiopellosis. Using a zebrafish cancer extravasation model, Follain et al. reported that a threshold flow velocity value of 400–600 μm/s elevates cancer cell adhesion to the endothelium and favors the endothelial-pocketing extravasation [12]. The switch of the extravasation approach partially results from the altered ligand and receptor expression levels on the surfaces of both cancer cells and ECs under the influence of the flow rates. Further studies are needed to specify these pairs of ligands and receptors that are responsible for the angiopellosis process and design relevant pharmacological therapeutic strategies to control the process.
4. Potential drugs for treating cancer extravasation:
Although it is widely acknowledged that metastasis is the leading cause of cancer-associated death and that cancer cell extravasation is a pivotal step in metastasis, no drugs or therapies that specifically target cancer cell extravasation have been approved so far. In the last decade, multiple molecular targets for impeding cancer cell extravasation have been newly revealed or further validified using novel cancer extravasation models and imaging techniques [77,78]. These targets allow us to make a step forward to explore extravasation-inhibitory drugs, and improve the effectiveness of current cancer therapies.
Cell adhesion molecules, including selectins and integrins, play an essential role in cancer cells-EC adhesion [84,96]. Disrupting the interaction between these adhesive molecules was shown to possess the power to control and reduce metastasis spreading. GMI-1271, a small molecule E-selectin antagonist, can bind to E-selectin expressed by ECs. The administration of GMI-1271 was proved to result in reduced attachment of cancer cells to the endothelium and the following TEM process [152,153]. Heparin, low molecular weight heparin, and non-anticoagulant heparin derivatives were reported to possess the capability of inhibiting multiple cell adhesion molecules (P- and L-selectin, VLA-4 integrin) [154–157]. And similar to the effect of GMI-1271, treatment by heparin significantly reduced the cancer cell adhesion to endothelium in vitro and attenuated the metastasis in vivo [158].
In addition to regulating cell adhesion molecules, enhancing the endothelial barrier is also a promising approach to reduce cancer cell extravasation. Angiopoietin (ANGPT)–Tie signaling plays an important role in vessel maturation [159,160]. Activation of Tie2 receptors on ECs by Angiopoietin-1 (Ang1) can significantly reduce the EC tight junction opening, and thus inhibiting TEM. Wu et al reported that the use of vasculotide (VT)-a purported Ang1 mimetic, Tie2 agonist, could stabilize the host vasculature and delay the distant metastatic dissemination to the lung [161]. Moreover, considering the contribution of chemokines in the TEM of cancer cells, metastasis inhibition can also be achieved by blocking the interaction between chemokines and their receptors. Mifepristone, a progesterone blocker that has been commonly used for terminating the pregnancy, was demonstrated to have metastasis inhibiting functions through altering the SDF-1(CXCL12) /CXCR4 signaling. Mifepristone can reduce the expression of CXCR4 on the cancer cell surface in a dose-dependent manner and thus suppress SDF-1-induced metastasis [162]. Similar strategies were also used by Uchida et al. and their findings suggested that oral administration of AMD-070, a CXCR4 antagonist, could significantly reduce SDF‑1/CXCR4‑dependent migration and invasion of oral cancer cells [163].
Cancer-associated platelets, which can shelter the CTCs from being attacked by hemodynamic shear forces and natural killer (NK) cells, can also promote cancer cell extravasation by indirectly inducing EC barrier opening or recruiting cancer-associated leukocytes via the secretion of CXCL5/7 [164,33,165]. Therefore, using anti-platelet therapeutics is another promising approach to inhibit cancer cell TEM [165]. Clinical data have shown that daily administration of a low dose aspirin can reduce the risk of distant metastasis in cancer patients by inhibiting the function of platelets [166].
Despite the exciting progress made in the anti-extravasation drug investigation, some obstacles still exist and remain to be tackled. For example, well developed preclinical murine models and rational clinical designs that are needed for evaluating and validating the efficacy of extravasation inhibitors are currently absent [167,168]. Also, although the drug repurposing using existing FDA-approved drugs is an increasing trend for anti-metastasis drug development [169,170], we are still lacking the in vitro platforms with high physiological relevance to the in vivo vasculature to perform high-throughput identification of the cancer cell extravasation inhibitors under proper pathological environments [171].
5. Conclusion and future perspective
In the last decade, advances in both in vitro and in vivo cancer extravasation models have successfully revealed more details involved in the cancer cell extravasation process. Compared with diapedesis, the recently identified extravasation mechanism, “angiopellosis”, is relatively poorly understood and has not been captured in vitro [15]. Considering the higher metastasis potential enabled by this mechanism, more efforts are required to investigate its governing molecular mechanisms and potential therapeutic approaches. In recent years, various models and assays have been newly developed or modified to recapture the cancer cell extravasation events in vitro. Since a close mimicking of the in vivo cancer cell extravasation environment requires co-culturing multiple cell types including cancer cells, cancer associate immune cells, endothelial cells, and other stromal cells, one of the key challenges in designing these models and assays is to provide cell-specific environments, including a proper ECM coating and growth medium, to support the growth, proliferation, and differentiation of different cell types. Applying a physical barrier like a porous membrane to separate different types of cells is a commonly used approach for providing the cell-specific environment. However, the existence of physical barriers hinders the communications and restricts the movements among different types of cells. As a result, most of the in vitro cancer extravasation assays lack the physiological relevance to the in vivo scenario. Vascularized microfluidic chips are more advantageous for the real-time recapture of the dynamic interactions between cancer cells and 3D vasculature during the extravasation process and offer us a great opportunity to investigate the contribution of specific factors to the process. However, considering the complexity of designing, fabricating, and handling the microfluidic platforms, they are almost as time-consuming and labor-intensive as the in vivo animal models, and can be hardly scaled up to perform high-content drug screening. Since the successful modulation of an extravasation-contributing factor sometimes requires us to screen the library of thousands of drugs, multi-units vascularized microfluidic platforms are urgently needed to fit the requirement for identifying new targeting therapeutics. In addition, since the formation of metastases is organ-specific instead of randomized, vascularized organ-on-a-chip platforms are required to investigate the extravasation patterns in different organs. Tissue clearing technologies in mice combined with machine learning tools render us to directly visualize the metastases formation at a cellular level. To further mimic the pathological and physiological environments in human patients, preclinical in vivo murine models based on the technologies should be developed for the safety and efficacy evaluation of the selected anti-extravasation drugs.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declarations
Not applicable
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Eccles SA, & Welch DR (2007). Metastasis: recent discoveries and novel treatment strategies. The Lancet, 369(9574), 1742–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sahai E (2007). Illuminating the metastatic process. Nature Reviews Cancer, 7(10), 737–749. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS (2016). Targeting metastasis. Nature Reviews Cancer, 16(4), 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reymond N, d’Agua BB, & Ridley AJ (2013). Crossing the endothelial barrier during metastasis. Nature Reviews Cancer, 13(12), 858–870. [DOI] [PubMed] [Google Scholar]
- 5.Strilic B, & Offermanns S (2017). Intravascular survival and extravasation of tumor cells. Cancer Cell, 32(3), 282–293. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti R, Hwang J, Blanco MA, Wei Y, Lukačišin M, Romano R-A, et al. (2012). Elf5 inhibits the epithelial–mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nature cell biology, 14(11), 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, & Sahai E (2009). Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nature cell biology, 11(11), 1287–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai K-L, et al. (2006). Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer research, 66(1), 192–197. [DOI] [PubMed] [Google Scholar]
- 9.Allen TA, Asad D, Amu E, Hensley MT, Cores J, Vandergriff A, et al. (2019). Circulating tumor cells exit circulation while maintaining multicellularity, augmenting metastatic potential. Journal of Cell Science, 132(17), jcs231563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bittner KR, Jiménez JM, & Peyton SR (2020). Vascularized Biomaterials to Study Cancer Metastasis. Advanced Healthcare Materials, 9(8), 1901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paku S, Laszlo V, Dezso K, Nagy P, Hoda MA, Klepetko W, et al. (2017). The evidence for and against different modes of tumour cell extravasation in the lung: diapedesis, capillary destruction, necroptosis, and endothelialization. The Journal of pathology, 241(4), 441–447. [DOI] [PubMed] [Google Scholar]
- 12.Follain G, Osmani N, Azevedo AS, Allio G, Mercier L, Karreman MA, et al. (2018). Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Developmental cell, 45(1), 33–52. e12. [DOI] [PubMed] [Google Scholar]
- 13.Strell C, & Entschladen F (2008). Extravasation of leukocytes in comparison to tumor cells. Cell Communication and Signaling, 6(1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng K, Shen D, Xie Y, Cingolani E, Malliaras K, & Marbán E (2012). Brief report: mechanism of extravasation of infused stem cells. Stem Cells, 30(12), 2835–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen TA, Gracieux D, Talib M, Tokarz DA, Hensley MT, Cores J, et al. (2017). Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells, 35(1), 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MB, Whisler JA, Fröse J, Yu C, Shin Y, & Kamm RD (2017). On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nature protocols, 12(5), 865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan C, Schoppe O, Parra-Damas A, Cai R, Todorov MI, Gondi G, et al. (2019). Deep Learning Reveals Cancer Metastasis and Therapeutic Antibody Targeting in the Entire Body. Cell, 179(7), 1661–1676. e1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng F, Setyawati MI, Tee JK, Ding X, Wang J, Nga ME, et al. (2019). Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nature nanotechnology, 14(3), 279–286. [DOI] [PubMed] [Google Scholar]
- 19.Kramer RH, & Nicolson GL (1979). Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proceedings of the National Academy of Sciences, 76(11), 5704–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolson GL (1982). Metastatic tumor cell attachment and invasion assay utilizing vascular endothelial cell monolayers. Journal of Histochemistry & Cytochemistry, 30(3), 214–220. [DOI] [PubMed] [Google Scholar]
- 21.Kramer R, & Nicolson G (1981). Invasion of vascular endothelial cell monolayers and underlying matrix by metastatic human cancer cells In International Cell Biology 1980–1981 (pp. 794–799): Springer. [Google Scholar]
- 22.Kang S-A, Bajana S, & Tanaka T (2016). In vitro Flow Adhesion Assay for Analyzing Shear-resistant Adhesion of Metastatic Cancer Cells to Endothelial Cells. Bio-protocol, 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spencer A, Spruell C, Nandi S, Wong M, Creixell M, & Baker AB (2016). A high-throughput mechanofluidic screening platform for investigating tumor cell adhesion during metastasis. Lab on a chip, 16(1), 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pouliot N, Pearson HB, & Burrows A (2013). Investigating metastasis using in vitro platforms In Madame Curie Bioscience Database [Internet]: Landes Bioscience. [Google Scholar]
- 25.Katt ME, Placone AL, Wong AD, Xu ZS, & Searson PC (2016). In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Frontiers in bioengineering and biotechnology, 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon J, Zervantonakis I, Chung S, Kamm R, & Charest J (2013). In vitro model of tumor cell extravasation. PLoS ONE 8, e56910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y-HV, Middleton K, You L, & Sun Y (2018). A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsystems & Nanoengineering, 4(1), 1–13.31057891 [Google Scholar]
- 28.Mierke CT (2011). Cancer cells regulate biomechanical properties of human microvascular endothelial cells. Journal of Biological Chemistry, 286(46), 40025–40037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y-H, & Zhu C (1999). A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Clinical & experimental metastasis, 17(5), 423–429. [DOI] [PubMed] [Google Scholar]
- 30.Laferrière J, Houle F, Taher MM, Valerie K, & Huot J (2001). Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. Journal of Biological Chemistry, 276(36), 33762–33772. [DOI] [PubMed] [Google Scholar]
- 31.Lee W, Choong L, Jin T, Mon N, Chong S, Liew C, et al. (2017). TRPV4 plays a role in breast cancer cell migration via Ca 2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis, 6(5), e338–e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pignatelli J, Goswami S, Jones JG, Rohan TE, Pieri E, Chen X, et al. (2014). Invasive breast carcinoma cells from patients exhibit MenaINV-and macrophage-dependent transendothelial migration. Sci. Signal, 7(353), ra112–ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orellana R, Kato S, Erices R, Bravo ML, Gonzalez P, Oliva B, et al. (2015). Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC cancer, 15(1), 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter JC, & Church FC (2012). Mature breast adipocytes promote breast cancer cell motility. Experimental and molecular pathology, 92(3), 312–317. [DOI] [PubMed] [Google Scholar]
- 35.Gassmann P, Haier J, Schlüter K, Domikowsky B, Wendel C, Wiesner U, et al. (2009). CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia (New York, NY), 11(7), 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y, Hoeppner LH, Bach S, Guangqi E, Guo Y, Wang E, et al. (2013). Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial α5 integrin. Cancer research, 73(14), 4579–4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y, Williams KC, Gavin CT, Jardine E, Chambers AF, & Leong HS (2016). Quantification of cancer cell extravasation in vivo. Nature protocols, 11(5), 937. [DOI] [PubMed] [Google Scholar]
- 38.Heyder C, Gloria-Maercker E, Entschladen F, Hatzmann W, Niggemann B, Zänker K, et al. (2002). Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. Journal of cancer research and clinical oncology, 128(10), 533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strilic B, Yang L, Albarrán-Juárez J, Wachsmuth L, Han K, Müller UC, et al. (2016). Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature, 536(7615), 215–218. [DOI] [PubMed] [Google Scholar]
- 40.Shin MK, Kim SK, & Jung H (2011). Integration of intra-and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab on a chip, 11(22), 3880–3887. [DOI] [PubMed] [Google Scholar]
- 41.Cui X, Guo W, Sun Y, Sun B, Hu S, Sun D, et al. (2017). A microfluidic device for isolation and characterization of transendothelial migrating cancer cells. Biomicrofluidics, 11(1), 014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song JW, Cavnar SP, Walker AC, Luker KE, Gupta M, Tung Y-C, et al. (2009). Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PloS one, 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Li Z, Yu Y, Sizdahkhani S, Ho WS, Yin F, et al. (2016). A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Scientific reports, 6, 36670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen MB, Hajal C, Benjamin DC, Yu C, Azizgolshani H, Hynes RO, et al. (2018). Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proceedings of the National Academy of Sciences, 115(27), 7022–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, et al. (2015). Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proceedings of the National Academy of Sciences, 112(1), 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin MF, & Kamm RD (2020). The Use of Microfluidic Platforms to Probe the Mechanism of Cancer Cell Extravasation. Advanced Healthcare Materials, 9(8), 1901410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chrobak KM, Potter DR, & Tien J (2006). Formation of perfused, functional microvascular tubes in vitro. Microvascular research, 71(3), 185–196. [DOI] [PubMed] [Google Scholar]
- 48.Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen D-HT, Cohen DM, et al. (2012). Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nature materials, 11(9), 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X-Y, Pei Y, Xie M, Jin Z-H, Xiao Y-S, Wang Y, et al. (2015). An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab on a chip, 15(4), 1178–1187. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y, Chen J, Craven M, Choi NW, Totorica S, Diaz-Santana A, et al. (2012). In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences, 109(24), 9342–9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moya ML, Hsu Y-H, Lee AP, Hughes CC, & George SC (2013). In vitro perfused human capillary networks. Tissue Engineering Part C: Methods, 19(9), 730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shirure VS, Bi Y, Curtis MB, Lezia A, Goedegebuure MM, Goedegebuure SP, et al. (2018). Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab on a chip, 18(23), 3687–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Aledia AS, Ghajar CM, Griffith CK, Putnam AJ, Hughes CC, et al. (2009). Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Engineering Part A, 15(6), 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen MB, Whisler JA, Jeon JS, & Kamm RD (2013). Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integrative Biology, 5(10), 1262–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paek J, Park SE, Lu Q, Park K-T, Cho M, Oh JM, et al. (2019). Microphysiological engineering of self-assembled and Perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS nano, 13(7), 7627–7643. [DOI] [PubMed] [Google Scholar]
- 56.Kolesky DB, Homan KA, Skylar-Scott MA, & Lewis JA (2016). Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences, 113(12), 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, et al. (2015). Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science advances, 1(9), e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, et al. (2018). A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nature methods, 15(1), 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kienast Y, Von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, et al. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nature medicine, 16(1), 116. [DOI] [PubMed] [Google Scholar]
- 60.Condeelis J, & Segall JE (2003). Intravital imaging of cell movement in tumours. Nature Reviews Cancer, 3(12), 921–930. [DOI] [PubMed] [Google Scholar]
- 61.Cao J, Zhu B, Zheng K, He S, Meng L, Song J, et al. (2019). Recent progress in NIR-II contrast agent for biological imaging. Frontiers in bioengineering and biotechnology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith AM, Mancini MC, & Nie S (2009). Second window for in vivo imaging. Nature nanotechnology, 4(11), 710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Welsher K, Sherlock SP, & Dai H (2011). Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proceedings of the National Academy of Sciences, 108(22), 8943–8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Welsher K, Liu Z, Sherlock SP, Robinson JT, Chen Z, Daranciang D, et al. (2009). A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature nanotechnology, 4(11), 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong G, Lee JC, Robinson JT, Raaz U, Xie L, Huang NF, et al. (2012). Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nature medicine, 18(12), 1841–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hong G, Robinson JT, Zhang Y, Diao S, Antaris AL, Wang Q, et al. (2012). In vivo fluorescence imaging with Ag2S quantum dots in the second near‐infrared region. Angewandte Chemie International Edition, 51(39), 9818–9821. [DOI] [PubMed] [Google Scholar]
- 67.Tian R, Ma H, Zhu S, Lau J, Ma R, Liu Y, et al. (2020). Multiplexed NIR‐II probes for lymph node‐invaded cancer detection and imaging‐guided surgery. Advanced Materials, 32(11), 1907365. [DOI] [PubMed] [Google Scholar]
- 68.Antaris AL, Chen H, Cheng K, Sun Y, Hong G, Qu C, et al. (2016). A small-molecule dye for NIR-II imaging. Nature materials, 15(2), 235–242. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Hong G, Zhang Y, Chen G, Li F, Dai H, et al. (2012). Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS nano, 6(5), 3695–3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Zhang Y, Hong G, He W, Zhou K, Yang K, et al. (2013). Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice. Biomaterials, 34(14), 3639–3646. [DOI] [PubMed] [Google Scholar]
- 71.Flusberg BA, Cocker ED, Piyawattanametha W, Jung JC, Cheung EL, & Schnitzer MJ (2005). Fiber-optic fluorescence imaging. Nature methods, 2(12), 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ritsma L, Steller EJ, Beerling E, Loomans CJ, Zomer A, Gerlach C, et al. (2012). Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Science translational medicine, 4(158), 158ra145–158ra145. [DOI] [PubMed] [Google Scholar]
- 73.Chambers AF, Groom AC, & MacDonald IC (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer, 2(8), 563–572. [DOI] [PubMed] [Google Scholar]
- 74.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, et al. (2006). Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer research, 66(8), 4208–4214. [DOI] [PubMed] [Google Scholar]
- 75.Cai R, Pan C, Ghasemigharagoz A, Todorov MI, Förstera B, Zhao S, et al. (2019). Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nature neuroscience, 22(2), 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nowak-Sliwinska P, Segura T, & Iruela-Arispe ML (2014). The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis, 17(4), 779–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leong HS, Robertson AE, Stoletov K, Leith SJ, Chin CA, Chien AE, et al. (2014). Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell reports, 8(5), 1558–1570. [DOI] [PubMed] [Google Scholar]
- 78.Stoletov K, Kato H, Zardouzian E, Kelber J, Yang J, Shattil S, et al. (2010). Visualizing extravasation dynamics of metastatic tumor cells. Journal of Cell Science, 123(13), 2332–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanada M, Zhang J, Yan L, Sakurai T, & Terakawa S (2014). Endothelial cell-initiated extravasation of cancer cells visualized in zebrafish. PeerJ, 2, e688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Baeten JT, & de Jong JL (2018). Genetic models of leukemia in zebrafish. Frontiers in cell and developmental biology, 6, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isogai S, Lawson ND, Torrealday S, Horiguchi M, & Weinstein BM (2003). Angiogenic network formation in the developing vertebrate trunk. Development, 130(21), 5281–5290. [DOI] [PubMed] [Google Scholar]
- 82.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, & Schilling TF (1995). Stages of embryonic development of the zebrafish. Developmental dynamics, 203(3), 253–310. [DOI] [PubMed] [Google Scholar]
- 83.Sökeland G, & Schumacher U (2019). The functional role of integrins during intra-and extravasation within the metastatic cascade. Molecular cancer, 18(1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barthel SR, Gavino JD, Descheny L, & Dimitroff CJ (2007). Targeting selectins and selectin ligands in inflammation and cancer. Expert opinion on therapeutic targets, 11(11), 1473–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, & Brodt P (2007). The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. The American journal of pathology, 170(5), 1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kansas GS (1996). Selectins and their ligands: current concepts and controversies. [PubMed]
- 87.Burdick MM, Chu JT, Godar S, & Sackstein R (2006). HCELL is the major E-and L-selectin ligand expressed on LS174T colon carcinoma cells. Journal of Biological Chemistry, 281(20), 13899–13905. [DOI] [PubMed] [Google Scholar]
- 88.Dimitroff CJ, Lechpammer M, Long-Woodward D, & Kutok JL (2004). Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer research, 64(15), 5261–5269. [DOI] [PubMed] [Google Scholar]
- 89.Läubli H, & Borsig L Selectins promote tumor metastasis In Seminars in cancer biology, 2010. (Vol. 20, pp. 169–177, Vol. 3): Elsevier; [DOI] [PubMed] [Google Scholar]
- 90.Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, et al. (2005). Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer research, 65(13), 5750–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shenoy AK, & Lu J (2016). Cancer cells remodel themselves and vasculature to overcome the endothelial barrier. Cancer letters, 380(2), 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G, Satyamoorthy K, & Herlyn M (2001). N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer research, 61(9), 3819–3825. [PubMed] [Google Scholar]
- 93.Garofalo A, Chirivi RG, Foglieni C, Pigott R, Mortarini R, Martin-Padura I, et al. (1995). Involvement of the very late antigen 4 integrin on melanoma in interleukin 1-augmented experimental metastases. Cancer research, 55(2), 414–419. [PubMed] [Google Scholar]
- 94.Kiefel H, Bondong S, Hazin J, Ridinger J, Schirmer U, Riedle S, et al. (2012). L1CAM: a major driver for tumor cell invasion and motility. Cell adhesion & migration, 6(4), 374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strell C, Lang K, Niggemann B, Zaenker K, & Entschladen F (2007). Surface molecules regulating rolling and adhesion to endothelium of neutrophil granulocytes and MDA-MB-468 breast carcinoma cells and their interaction. Cellular and Molecular Life Sciences, 64(24), 3306–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Desgrosellier JS, & Cheresh DA (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews Cancer, 10(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen MB, Lamar JM, Li R, Hynes RO, & Kamm RD (2016). Elucidation of the roles of tumor integrin β1 in the extravasation stage of the metastasis cascade. Cancer research, 76(9), 2513–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li F, Redick SD, Erickson HP, & Moy VT (2003). Force measurements of the α5β1 integrin–fibronectin interaction. Biophysical journal, 84(2), 1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kai F, Drain AP, & Weaver VM (2019). The extracellular matrix modulates the metastatic journey. Developmental cell, 49(3), 332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.CHEN WT, & WANG JY (1999). Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1‐MMP, MMP‐2, and TIMP‐2 localization. Annals of the New York Academy of Sciences, 878(1), 361–371. [DOI] [PubMed] [Google Scholar]
- 101.Paz H, Pathak N, & Yang J (2014). Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene, 33(33), 4193–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, & Simpson RJ (2018). Extracellular vesicles in cancer—implications for future improvements in cancer care. Nature reviews Clinical oncology, 15(10), 617. [DOI] [PubMed] [Google Scholar]
- 103.De Palma M, Biziato D, & Petrova TV (2017). Microenvironmental regulation of tumour angiogenesis. Nature Reviews Cancer, 17(8), 457. [DOI] [PubMed] [Google Scholar]
- 104.García-Román J, & Zentella-Dehesa A (2013). Vascular permeability changes involved in tumor metastasis. Cancer letters, 335(2), 259–269. [DOI] [PubMed] [Google Scholar]
- 105.Jain RK, Martin JD, & Stylianopoulos T (2014). The role of mechanical forces in tumor growth and therapy. Annual review of biomedical engineering, 16, 321–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stroka KM, & Aranda‐Espinoza H (2010). A biophysical view of the interplay between mechanical forces and signaling pathways during transendothelial cell migration. The FEBS journal, 277(5), 1145–1158. [DOI] [PubMed] [Google Scholar]
- 107.Weaver AM (2006). Invadopodia: specialized cell structures for cancer invasion. Clinical & experimental metastasis, 23(2), 97–105. [DOI] [PubMed] [Google Scholar]
- 108.Yamaguchi H, & Condeelis J (2007). Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1773(5), 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jacob A, & Prekeris R (2015). The regulation of MMP targeting to invadopodia during cancer metastasis. Frontiers in cell and developmental biology, 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stoletov K, Montel V, Lester RD, Gonias SL, & Klemke R (2007). High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proceedings of the National Academy of Sciences, 104(44), 17406–17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang S, Li E, Gao Y, Wang Y, Guo Z, He J, et al. (2013). Study on invadopodia formation for lung carcinoma invasion with a microfluidic 3D culture device. PloS one, 8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu T, Li C, Li H, Zeng S, Qin J, & Lin B (2009). A microfluidic device for characterizing the invasion of cancer cells in 3‐D matrix. Electrophoresis, 30(24), 4285–4291. [DOI] [PubMed] [Google Scholar]
- 113.Eckert MA, & Yang J (2011). Targeting invadopodia to block breast cancer metastasis. Oncotarget, 2(7), 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaczmarek A, Vandenabeele P, & Krysko DV (2013). Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity, 38(2), 209–223. [DOI] [PubMed] [Google Scholar]
- 115.Kim H, Chung H, Kim J, Choi DH, Shin Y, Kang YG, et al. (2019). Macrophages‐Triggered Sequential Remodeling of Endothelium ‐ Interstitial Matrix to Form Pre ‐ Metastatic Niche in Microfluidic Tumor Microenvironment. Advanced Science, 6(11), 1900195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee T-H, Avraham HK, Jiang S, & Avraham S (2003). Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. Journal of Biological Chemistry, 278(7), 5277–5284. [DOI] [PubMed] [Google Scholar]
- 117.Weis S, Cui J, Barnes L, & Cheresh D (2004). Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. The Journal of cell biology, 167(2), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Anderberg C, Cunha SI, Zhai Z, Cortez E, Pardali E, Johnson JR, et al. (2013). Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. Journal of Experimental Medicine, 210(3), 563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roussos ET, Condeelis JS, & Patsialou A (2011). Chemotaxis in cancer. Nature Reviews Cancer, 11(8), 573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Padua D, Zhang XH-F, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. (2008). TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell, 133(1), 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, et al. (2012). Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell, 22(1), 91–105. [DOI] [PubMed] [Google Scholar]
- 122.Wendel C, Hemping-Bovenkerk A, Krasnyanska J, Mees ST, Kochetkova M, Stoeppeler S, et al. (2012). CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PloS one, 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Karki P, & Birukov KG (2018). Lipid mediators in the regulation of endothelial barriers. Tissue Barriers, 6(1), e1385573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Freyssinet J-M, & Toti F (2010). Formation of procoagulant microparticles and properties. Thrombosis research, 125, S46–S48. [DOI] [PubMed] [Google Scholar]
- 125.Gay LJ, & Felding-Habermann B (2011). Contribution of platelets to tumour metastasis. Nature Reviews Cancer, 11(2), 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Takuwa Y (2002). Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1582(1–3), 112–120. [DOI] [PubMed] [Google Scholar]
- 127.McVerry BJ, & Garcia JG (2005). In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cellular signalling, 17(2), 131–139. [DOI] [PubMed] [Google Scholar]
- 128.Yin F, & Watsky MA (2005). LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Investigative ophthalmology & visual science, 46(6), 1927–1933. [DOI] [PubMed] [Google Scholar]
- 129.Sarker MH, HU DE, & Fraser PA (2010). Regulation of cerebromicrovascular permeability by lysophosphatidic acid. Microcirculation, 17(1), 39–46. [DOI] [PubMed] [Google Scholar]
- 130.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25(4), 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin Y, Zhang C, Xiang P, Shen J, Sun W, & Yu H (2020). Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. Journal of extracellular vesicles, 9(1), 1722385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kikuchi S, Yoshioka Y, Prieto-Vila M, & Ochiya T (2019). Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. International journal of molecular sciences, 20(10), 2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. (2018). Cancer-derived exosomal miR-25–3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature communications, 9(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hsu Y, Hung J, Chang W, Lin Y, Pan Y, Tsai P, et al. (2017). Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene, 36(34), 4929–4942. [DOI] [PubMed] [Google Scholar]
- 135.Wirtz D, Konstantopoulos K, & Searson PC (2011). The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature Reviews Cancer, 11(7), 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thomas SN, Zhu F, Schnaar RL, Alves CS, & Konstantopoulos K (2008). Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E-and L-selectin in shear flow. Journal of Biological Chemistry, 283(23), 15647–15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wojciak-Stothard B, & Ridley AJ (2003). Shear stress–induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. The Journal of cell biology, 161(2), 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lapis K, Paku S, & Liotta L (1988). Endothelialization of embolized tumor cells during metastasis formation. Clinical & experimental metastasis, 6(1), 73–89. [DOI] [PubMed] [Google Scholar]
- 139.Paku S, Döme B, Tóth R, & Timár J (2000). Organ-specificity of the extravasation process: an ultrastructural study. Clinical & experimental metastasis, 18(6), 481–492. [DOI] [PubMed] [Google Scholar]
- 140.Krüger-Genge A, Blocki A, Franke R-P, & Jung F (2019). Vascular endothelial cell biology: An update. International journal of molecular sciences, 20(18), 4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Félétou M The endothelium, Part I: Multiple functions of the endothelial cells--focus on endothelium-derived vasoactive mediators In Colloquium Series on Integrated Systems Physiology: From Molecule to Function, 2011. (Vol. 3, pp. 1–306, Vol. 4): Morgan & Claypool Life Sciences; [PubMed] [Google Scholar]
- 142.Stamatovic SM, Keep RF, & Andjelkovic AV (2008). Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Current neuropharmacology, 6(3), 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lam CK, Yoo T, Hiner B, Liu Z, & Grutzendler J (2010). Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature, 465(7297), 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grutzendler J, Murikinati S, Hiner B, Ji L, Lam CK, Yoo T, et al. (2014). Angiophagy prevents early embolus washout but recanalizes microvessels through embolus extravasation. Science translational medicine, 6(226), 226ra231–226ra231. [DOI] [PubMed] [Google Scholar]
- 145.Zhou T, Zheng Y, Sun L, Badea SR, Jin Y, Liu Y, et al. (2019). Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nature neuroscience, 22(3), 421–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, et al. (1995). Phagocytosis of apoptotic bodies by liver endothelial cells. Journal of Cell Science, 108(3), 967–973. [DOI] [PubMed] [Google Scholar]
- 147.Steffan AM, Gendrault JL, McCuskey RS, McCuskey PA, & Kirn A (1986). Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology, 6(5), 830–836. [DOI] [PubMed] [Google Scholar]
- 148.Rengarajan M, Hayer A, & Theriot JA (2016). Endothelial cells use a formin-dependent phagocytosis-like process to internalize the bacterium Listeria monocytogenes. PLoS pathogens, 12(5), e1005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, et al. (1999). Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. The American journal of pathology, 155(3), 739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hendrix MJ, Seftor EA, Hess AR, & Seftor RE (2003). Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nature Reviews Cancer, 3(6), 411–421. [DOI] [PubMed] [Google Scholar]
- 151.Cima I, Kong SL, Sengupta D, Tan IB, Phyo WM, Lee D, et al. (2016). Tumor-derived circulating endothelial cell clusters in colorectal cancer. Science translational medicine, 8(345), 345ra389–345ra389. [DOI] [PubMed] [Google Scholar]
- 152.Esposito M, Magnani JL, & Kang Y (2014). Exploration of a potent E-selectin antagonist (GMI-1271) as a potential novel therapeutic for treating breast cancer metastasis to the bone and lung. AACR. [Google Scholar]
- 153.Steele MM, Radhakrishnan P, Magnani JL, & Hollingsworth MA (2014). A small molecule glycomimetic antagonist of E-selectin (GMI-1271) prevents pancreatic tumor metastasis and offers a novel treatment for improved efficacy of chemotherapy. AACR. [Google Scholar]
- 154.Bendas G, & Borsig L (2012). Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. International journal of cell biology, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hostettler N, Naggi A, Torri G, Ishai-Michaeli R, Casu B, Vlodavsky I, et al. (2007). P-selectin-and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. The FASEB Journal, 21(13), 3562–3572. [DOI] [PubMed] [Google Scholar]
- 156.Fritzsche J, Simonis D, & Bendas G (2008). Melanoma cell adhesion can be blocked by heparin in vitro: suggestion of VLA-4 as a novel target for antimetastatic approaches. Thrombosis and haemostasis, 100(12), 1166–1175. [PubMed] [Google Scholar]
- 157.Zhang C, Liu Y, Gao Y, Shen J, Zheng S, Wei M, et al. (2009). Modified heparins inhibit integrin αIIbβ3 mediated adhesion of melanoma cells to platelets in vitro and in vivo. International Journal of Cancer, 125(9), 2058–2065. [DOI] [PubMed] [Google Scholar]
- 158.Kakkar A, & Macbeth F (2010). Antithrombotic therapy and survival in patients with malignant disease. British journal of cancer, 102(1), S24–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang H, Bhat A, Woodnutt G, & Lappe R (2010). Targeting the ANGPT-TIE2 pathway in malignancy. Nature Reviews Cancer, 10(8), 575–585. [DOI] [PubMed] [Google Scholar]
- 160.Biel NM, & Siemann DW (2016). Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer letters, 380(2), 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu FT, Lee CR, Bogdanovic E, Prodeus A, Gariépy J, & Kerbel RS (2015). Vasculotide reduces endothelial permeability and tumor cell extravasation in the absence of binding to or agonistic activation of Tie2. EMBO molecular medicine, 7(6), 770–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng N, Chen J, Liu W, Liu J, Li T, Chen H, et al. (2017). Mifepristone inhibits ovarian cancer metastasis by intervening in SDF-1/CXCR4 chemokine axis. Oncotarget, 8(35), 59123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uchida D, Kuribayashi N, Kinouchi M, Sawatani Y, Shimura M, Mori T, et al. (2018). Effect of a novel orally bioavailable CXCR4 inhibitor, AMD070, on the metastasis of oral cancer cells. Oncology reports, 40(1), 303–308. [DOI] [PubMed] [Google Scholar]
- 164.Schlesinger M (2018). Role of platelets and platelet receptors in cancer metastasis. Journal of hematology & oncology, 11(1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Elaskalani O, Berndt MC, Falasca M, & Metharom P (2017). Targeting platelets for the treatment of cancer. Cancers, 9(7), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, & Mehta Z (2012). Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. The Lancet, 379(9826), 1591–1601. [DOI] [PubMed] [Google Scholar]
- 167.Lieu CH, Tan A-C, Leong S, Diamond JR, & Eckhardt SG (2013). From bench to bedside: lessons learned in translating preclinical studies in cancer drug development. Journal of the National Cancer Institute, 105(19), 1441–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gould SE, Junttila MR, & de Sauvage FJ (2015). Translational value of mouse models in oncology drug development. Nature medicine, 21(5), 431. [DOI] [PubMed] [Google Scholar]
- 169.Ozsvári B, Lamb R, & Lisanti MP (2016). Repurposing of FDA-approved drugs against cancer–Focus on metastasis. Aging (Albany NY), 8(4), 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Klangjorhor J, Chaiyawat P, Teeyakasem P, Sirikaew N, Phanphaisarn A, Settakorn J, et al. (2020). Mycophenolic acid is a drug with the potential to be repurposed for suppressing tumor growth and metastasis in osteosarcoma treatment. International Journal of Cancer, 146(12), 3397–3409. [DOI] [PubMed] [Google Scholar]
- 171.Hachey SJ, & Hughes CC (2018). Applications of tumor chip technology. Lab on a chip, 18(19), 2893–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]


