Abstract
Lignocellulosic biofuels and chemicals have great potential to reduce our dependence on fossil fuels and mitigate air pollution by cutting down on greenhouse gas emissions. Chemical, thermal, and enzymatic processes are used to release the sugars from the lignocellulosic biomass for conversion to biofuels. These processes often operate at extreme pH conditions, high salt concentrations, and/or high temperature. These harsh treatments add to the cost of the biofuels, as most known biocatalysts do not operate at these conditions. To increase the economic feasibility of biofuel production, microorganisms that thrive in extreme conditions are considered as ideal resources to generate biofuels and value-added products. Halophilic archaea (haloarchaea) are isolated from hypersaline ecosystems with high salt concentrations approaching saturation (1.5 to 5 M salt concentration) including environments with extremes in pH and/or temperature. The unique traits of haloarchaea and their enzymes that enable them to sustain catalytic activity in these environments make them attractive resources for use in bioconversion processes that must occur across a wide range of industrial conditions. Biocatalysts (enzymes) derived from haloarchaea occupy a unique niche in organic solvent, salt-based and detergent industries. This review focuses on the use of haloarchaea and their enzymes to develop and improve biofuel production. The review also highlights how haloarchaea produce value added products, such as antibiotics, carotenoids, and bioplastic precursors, and can do so using feedstocks considered ‘too salty’ for most microbial processes including wastes from the olive-mill, shell fish, and biodiesel industries.
Keywords: Bio-compounds, Biodiesel, Extremophiles, Hydrolytic enzymes, Lignocellulose, Saccharification
Graphical Abstract
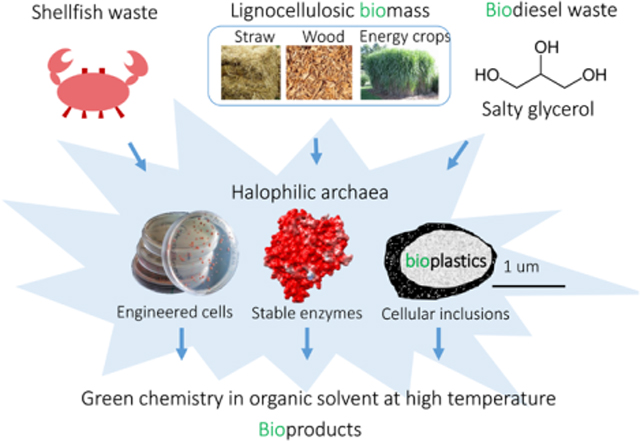
Introduction
As the world’s population continues to grow so has its per capita energy consumption. The depletion of reserves, volatility in price, and negative impact of fossil fuels on the environment has led researchers to seek alternative and renewable energy supplies. Biomass is an abundant and renewable resource of carbon-based material that can be converted into biofuels and chemicals to meet the world’s future energy demand and mitigate climate change (C. M. Anderson et al., 2019). The utilization of lignocellulosic biomass for biofuel production and its transformation into other desirable bio-chemicals is environmentally beneficial and would be more economically feasible if certain hurdles could be overcome. One such limitation is in the expense of the depolymerization and saccharification processes that are used to release the sugars and other building blocks from the biomass to generate the bio-products (Figure 1). Thermo-chemical processes that include high temperature, alkaline or acidic pH, organic solvents, or other harsh conditions are often used to depolymerize the lignocellulosic biomass. Identifying microbial and/or enzyme biocatalysts that operate under these harsh conditions would be beneficial to reduce the expense of the bioconversion processes (Barnard, Casanueva, Tuffin, & Cowan, 2010). While enzymatic hydrolysis has been combined with microbial fermentation to obtain value-added products from biomass in a single step by simultaneous saccharification and fermentation (SSF) (Olofsson, Bertilsson, & Lidén, 2008), progress is still needed in this area to improve cost-effectiveness.
Figure 1.
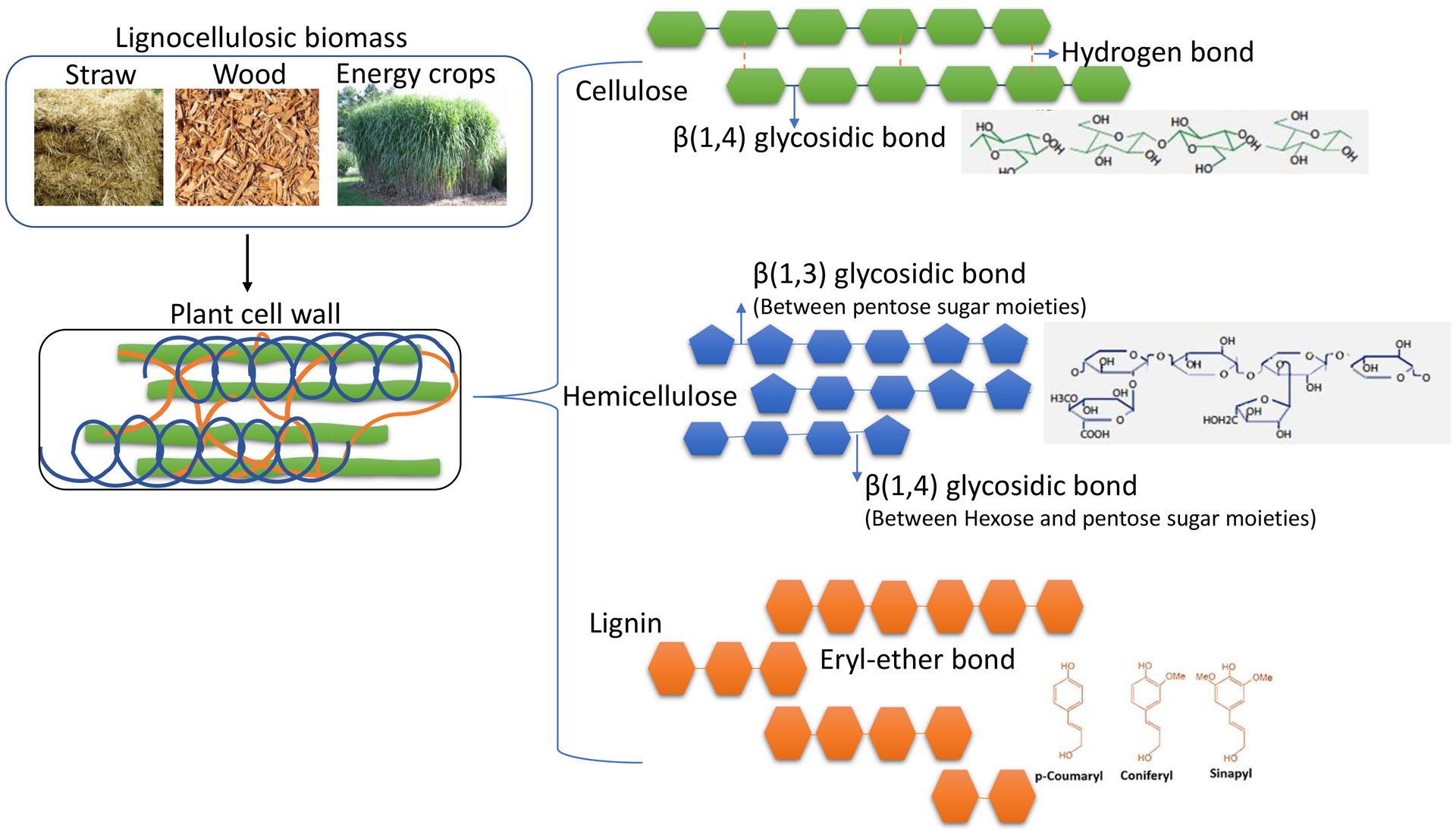
Structure of lignocellulosic biomass and its components.
Halophilic microorganisms are producers of antimicrobials, bioplastics, biofuel, extremozymes, extracellular polysaccharides, retinal proteins (bacteriorhodopsin), colored pigments and compatible solutes which has led to their application in agriculture, food and nutrition, biofuel and many more industrial processes such as treatment of saline wastewaters (Alsafadi, Khalili, Juwhari, & Lahlouh, 2018; de Lourdes Moreno, Pérez, García, & Mellado, 2013; Kargi & Dinçer, 2000; Lee, 2013; Rodrigo-Baños, Garbayo, Vílchez, Bonete, & Martínez-Espinosa, 2015). Halophilic microorganisms are found in the three domains of life: Eukarya, Bacteria, and Archaea (Kamekura, 1998; Oren, 2013). These microorganisms live in hypersaline environments such as the Dead Sea, hypersaline lakes, salt pans, saline soils, and salt marshes where the salt concentrations are generally at 3.5% dissolved NaCl (higher than sea water) (Rothschild & Mancinelli, 2001; Woese, Kandler, & Wheelis, 1990). In addition to their stability in high salt, halophilic microorganisms can withstand other harsh conditions such as extremes in pH, temperature, and organic solvent (Rampelotto, 2013; Usami et al., 2003; Usami et al., 2005). Based on their optimum growth parameters, halophiles are categorized as extreme at 2.5–5.2 M NaCl, moderate at 0.5–2.5 M, mild at 0.2–0.5 M and are considered extremely halotolerant when they do not require high concentrations (>2.5 M) of salt but grow well under these conditions (Amoozegar, Siroosi, Atashgahi, Smidt, & Ventosa, 2017; de Lourdes Moreno et al., 2013; Kushner, 1978; Oren, 2008). Adaptation of halophiles to withstand the adverse consequences when exposed to hypersaline conditions is based on either: 1) an osmoadaptation strategy (da Costa, Santos, & Galinski, 1998; Galinski, 1993) or 2) a salt-in-cytoplasm strategy (Oren, 2008). Cells that use the salt-out strategy accumulate compatible solutes or osmolytes, such as amino acids, glycine betaine, sugars, and polyols, intracellularly to overcome salt stress. By contrast, in the salt-in-cytoplasm strategy, KCl is accumulated inside the cell at concentrations equal to the NaCl that is present extracellularly. This latter strategy is common to haloarchaea and has interesting consequences for their proteins (Oren, 2008).
Haloarchaea are a target of the scientific community, as these microorganisms produce enzymes, exopolysaccharides, and pigments that may be applied in industrial biotechnology (Andrei, Banciu, & Oren, 2012). Due to the salt-in-cytoplasm strategy, haloarchaeal enzymes/biocatalysts are adapted to remain stable and catalytically active at high ionic strength (Timpson et al., 2013). As hypersaline and organic solvent conditions share common properties including low water activity, haloarchaeal proteins are reported to tolerate organic solvents (Alsafadi & Paradisi, 2013; McMillan, Hepowit, & Maupin-Furlow, 2016). In addition to their halo adaptation properties, haloenzymes can also withstand other conditions such as extreme pH and temperature (Paggi, Madrid, D’Alessandro, Cerletti, & De Castro, 2010; Wilson, Aldrich, & Maupin-Furlow, 1999). Haloarchaeal enzymes, such as cellulases, xylanases, pectinases, esterases, lipases, catalases, chitinase, amylases, β‐galactosidase, β‐glucosidases, and laccases, are useful for biomass conversion and herein reviewed. Haloarchaea are also found to glycosylate certain extracellular and surface structural proteins that may facilitate the stability of these proteins in harsh conditions (Eichler & Maupin-Furlow, 2013). Thus, haloenzymes have the potential to enhance the production of renewable fuels and chemicals. This review will focus on the structural components of the various types of biomass, the role of extremophiles, particularly those of haloarchaea, in generating products and/or enzymes that may be used towards biofuels and chemical production.
Lignocellulosic components and depolymerization
Lignocellulosic plant-based biomass is mainly comprised of aromatic polymers of lignin, polysaccharides (cellulose and hemicellulose) and pectin (Glass, Schmoll, Cate, & Coradetti, 2013; Popper et al., 2011). Plant cell walls are classified to the primary cell wall and the secondary cell wall based on structural and functional differences. The primary cell wall is made of cellulose, hemicellulose, and pectin. The synthesis of this wall occurs against the plasma membrane during the growing phase of the cell (Cosgrove, 1997). The secondary cell wall contains lignin along with cellulose and hemicellulose; usually, lignification of this cell wall occurs in specific cell tissue types after cessation of cell growth (Robert et al., 2005). Other useful chemicals, such as furans that have a circular structure consisting of four carbon atoms and an oxygen atom, are released from lignocellulosic biomass that could serve as alternative, high-energy-density fuels (Zoghlami & Paës, 2019). However, many factors still limit the conversion of lignocellulosic biomass to biofuels and bioproducts including: 1) the need for a pretreatment step to split the biomass components (cellulose, hemicellulose, and lignin) and to reduce/remove the recalcitrant nature of these plant cell wall components, 2) the high cost and low efficiency of current lignocellulolytic enzymes, 3) the degree of crystallinity and polymerization of cellulose, 4) the acetyl groups in hemicelluloses, and 5) the composition and content of the phenyl-propene polymer lignin (Zoghlami & Paës, 2019).
Cellulose
Cellulose is an organic linear polymer formed through intra- and inter-chain hydrogen bonding of β (1→4) linked D-glucose units crystalized to form cellulose microfibrils; the glucose released from this polymer can be fermented to bioethanol and biobutanol or other bioproducts (Gillis, 1969). Cellulose is synthesized by a membrane-bound cellulose synthase (CESA) encoded by 29 CESAs genes to form a complex of hexameric units called “rosette” (Mueller & Brown, 1980). CESA1, CASE3, and CESA6 constitute the catalytic hexameric unit, which is involved in the formation of the primary cell wall, while the isoforms CESA4, CESA7, and CESA8 are associated with synthesis of the secondary wall (Hill, Hammudi, & Tien, 2014). The catalytic domains of cellulose synthases are conserved for all cellulose-synthesizing organisms (bacteria, algae, and higher plants), but differ in the associated regulatory proteins and the molecular mechanisms of cellulose synthesis (Crowell, Gonneau, Stierhof, Höfte, & Vernhettes, 2010; S. Li, Lei, & Gu, 2013).
Cellulases
Cellulases are a general group of cellulose-hydrolyzing enzymes. Cellobiohydrolases are a type of cellulase that acts increasingly on cellulose and generates short cellooligosaccharides or cellobiose. Endoglucanases randomly cleave the internal bonds of cellulose, releasing oligosaccharides with new ends of the polysaccharide chains. Endoglucanases belong to the glycosyl hydrolase (GH) family of enzymes and amongst them the GH5, GH7, GH8, GH9 and GH44 proteins are the ones most active in cellulose hydrolysis (Yennamalli, Rader, Wolt, & Sen, 2011).
Cellulose degradation by haloarchaea
Haloarchaea produce cellulases that are active and stable under harsh conditions. These cellulases hold promise for use in optimizing biofuel production from lignocellulosic wastes, as these processes include de-crystallizing pretreatment steps that occur at extreme pH, temperature and/or ionic strength including ionic liquids. Genomics of haloarchaea indicate the presence of cellulase or related glycoside hydrolase (GH) gene homologs (Table 1). Functional cellulases and/or cellulase optimal activity conditions have been reported in haloarchaea as summarized below and in Table 2 (Begemann, Mormile, Paul, & Vidt, 2011).
Table 1.
Glycoside hydrolase gene homologs identified from CAZy database in haloarchaea (I. Anderson et al., 2011).
| Gene name | UniProtKB or Accession No | Organism |
|---|---|---|
| GH3 | D8J9S1_HALJB | Halalkalicoccus jeotgali B3 |
| AEM59010.1 | Haloarcula hispanica ATCC 33960 | |
| AHB65348.1 | Haloarcula hispanica N601 | |
| Q5V5G3_HALMA | Haloarcula marismortui ATCC 43049 | |
| AJF26480.1 | Haloarcula sp. CBA1115 | |
| D4GUN5_HALVD | Haloferax volcanii DS2 | |
| AEH38975.1 | Halopiger xanaduensis SH-6 | |
| AHZ24265.1 | Haloferax mediterranei ATCC 33500 | |
| C7P2G4_HALMD | Halomicrobium mukohataei DSM 12286 | |
| E4NVK1_HALBP | Halogeometricum borinquense DSM 11551 | |
| GH5, GH9, GH12, GH3, GH94 | CCQ32630.1, CCQ34238.1, CCQ34232.1, CCQ32864.1, CCQ33042.1 | Halorhabdus tiamatea SARL4B |
| GH3, GH5, GH9, GH94 |
C7NNE6_HALUD, C7NQI0_HALUD C7NMM6_HALUD C7NVE6_HALUD |
Halorhabdus utahensis DSM 12940 |
| GH3, GH5 | AZQ15935.1, AHG02057.1 | Halostagnicola larsenii XH-48 |
| GH3, GH16 | AGB38896.1, AGB37354.1 | Natronococcus occultus SP4 |
Table 2.
Cellulases and other glycoside hydrolases produced by haloarchaea.
| Enzyme | Haloarchaea | Enzyme activitya | Specific Activityb | Protein molecular mass | Ref. | ||
|---|---|---|---|---|---|---|---|
| pH | Temp. | NaCl | |||||
| Cellulase (Hu-CBH1) | Halorhabdus utahensis | 9.5 | 80 °C | 5 M | 4.1 U/mg | 76 kDa | (Zhang T., 2011) |
| Cellulase | Haloarcula sp. 2TK2 | 7 | 25 °C | 2.5 M | 1.8 U/mgc | Cell culture | (Ogan, Danis, Gozuacik, Cakmar, & Birbir, 2012) |
| Cellulase (endoglucanase) | Haloarcula sp G10 | 9 | 60 °C | 3 M | 71.2 U/mg | 36 kDa | (X. Li & Yu, 2013a) |
| β-Galactosidase | Halorubrum lacusprofundi | 6.5 | 50 °C | 4 M | 18 U/mg | 78 kDa | (Karan, Capes, DasSarma, & DasSarma, 2013) |
| Cellulase | Haloarcula vallismortis | n/ad | (Nercessian, Di Meglio, De Castro, & Paggi, 2015) | ||||
| Cellulase | Natronobiforma cellulositropha | 8.5 | 40 °C | 4 M | n/a | Cell culture | (Sorokin et al., 2018) |
| Cellulase | Haloarcula sp. LLSG7 | 8.0 | 50 °C | 3.5 M | 0.36 U/mg | Cell culture | (X. Li & Yu, 2013b) |
| Cellulase | Haloferax sulfurifontis GUMFAZ2 | 7.0 | 40 °C | 3.5 M | 150 U/mgc | 46 kDa | (Malik & Furtado, 2019) |
Enzyme activity was optimal for catalysis at these conditions.
U, unit defined as 1 μmol of glucose released per min for cellulase and 1 μmol of o-nitrophenol per min for β-galactosidase.
U, units recalculated to conform to table and enable comparison.
n/a, quantitative value not available.
In the quest to obtain cellulases that maintain activity in ionic liquids, a cellobiohydrolase that was stable and active in high salt, ionic liquids and high temperature was identified in Halorhabdus utahensis and was named Hu-CBH1 (Zhang T., 2011). Sequence analysis of a 37 kb genomic locus from this haloarchaeon revealed seven putative cellulose genes, including the gene encoding Hu-CBH1 that was in synteny with gene homologs of xylanase, mannase and pectinase enzymes that would be useful in depolymerization processes (Zhang T., 2011). These genes were organized in 5’-3’ orientation in an apparent operon suggesting coordinated biological function (Zhang T., 2011). The researchers expressed the Hu-CBH1 gene in Haloferax volcanii, a haloarchaeon amenable to genetic engineering, and used this modified organism as a host to purify the enzyme (Zhang T., 2011). Based on assay, the Hu-CBH1 enzyme was found to be haloalkaliphilic and heat tolerant in its ability to hydrolyze cellulose. Consistent with these properties, the enzyme was predicted by 3D homology modeling to be enriched in acidic amino acids with strong negative charges on its surface. Salt was found essential for the stability and function of the protein (Zhang T., 2011). Moreover, Hu-CBH1 was resistant to ionic liquids and could tolerate up to 20 % (w/w) of ionic liquids, including 1-ethyl-3-methylimidazolium acetate ([Emim]Ac), 1-ethyl-3-methylimidazolium chloride ([Emim]Cl), 1-butyl-3-methylimidazolium chloride ([Bmim]Cl) and 1-allyl3-methylimidazolium chloride ([Amim]Cl) (Zhang T., 2011). These findings demonstrate that haloarchaeal cellulases can be resistant to ionic liquids and, thus, may serve as biocatalysts to release sugars from cellulose during ionic liquid-based processes that depolymerize biomass.
Haloarcula species appear a rich resource of cellulases. In addition to Hu-CBH1, H. utahensis genome has other cellulase gene homologs including those of the GH5 and GH9 families. Biochemical study demonstrates the H. utahensis GH5 family member is an endoglucanase, with thermo and alkali-stable properties at hypersaline conditions (Zhang T., 2011). The investigators could not culture H. utahensis on insoluble celluloses, hence, the ability of this archaeon to hydrolyze insoluble celluloses was not tested (Zhang T., 2011). In another lab, two species of Haloarcula (sp. LLSG7 and sp. G10) from the saline soil of Yuncheng Salt Lake, China were identified to secrete endoglucanases (X. Li & Yu, 2013a, 2013b). These endoglucanase enzymes were shown to hydrolyze the soluble cellulose analogue carboxymethylcellulose (CMC) at 25 % NaCl, which is at an ionic strength higher than typically reported. The crude cellulase from Haloarcula sp. LLSG7 showed maximal activity with soluble cellulosic substrate (CMC-Na salt), 4-nitrophenyl-β-D-glucopyranoside (pNPG), and cellobiose indicating that the crude enzyme had endoglucanase activity, exoglucanase, and β-glucosidase activities (X. Li & Yu, 2013b). Haloarcula sp. LLSG7 also synthesized a multicomponent enzyme system with relationship to cellulosomes (X. Li & Yu, 2013b), which typically coordinate multiple types of hydrolytic activities that contribute towards efficient degradation of cellulose. The crude cellulase of Haloarcula sp. LLSG7 was further examined for hydrolysis of alkali-pretreated rice straw (pH 7.0 to 11.0), which was later used as the substrate for bioethanol production. A maximal yield of 10.7 g/L of bioethanol was obtained over the 30 h period of fermentation. The reduced sugar substrates derived from the pretreated rice straw material, 50 g/L at the onset of fermentation, were consumed to a concentration of 1.4 g/L by the end of incubation. The ethanol yield was 0.18 g/g of dry substrate, with a conversion efficiency of 42 %; these results reported by (X. Li & Yu, 2013b) were much higher than the earlier report in which fungal cellulases were used against three different feed stocks, i.e., sugar cane bagasse, rice straw and water hyacinth biomass, for bioethanol production (Sukumaran, Singhania, Mathew, & Pandey, 2009).
A cellulase of the haloarchaeon Natronobiforma cellulositropha was identified through isolation of six strains of extremely halophilic euryarchaea from surface brines and sediments of hypersaline alkaline lakes representing various geographical locations (Sorokin et al., 2018). The isolated strains were designated AArcel and were found to grow on insoluble celluloses (cellobiose), with a few also able to grown on soluble cellulose (glucans and xylan) as their carbon and energy source (Sorokin et al., 2018). All isolated microorganisms were extreme halophiles with optimum growth at 4 M NaCl and pH 8.5–9. Based on the 16S rRNA gene analysis and the phylogenetic distance with two conservative phylogenetic markers, the AArcel group was concluded to represent strains of the novel genus and species level branch N. cellulositropha within the family Natrialbaceae, with AArcel5 as the type strain (Sorokin et al., 2018), and two strains of the genera Halorhabdus and Haloarcula. This finding of haloarchaeal species that hydrolyze insoluble celluloses holds promise for identification of new enzymes with useful properties in processing of lignocellulosics.
A new haloarchaeal strain, Haloferax sulfurifontis GUMFAZ2, has been isolated that produces extracellular cellulase activity (Malik & Furtado, 2019). This strain was found associated with marine demosponges of the Haliclona sp., grown on rocky intertidal region of Anjuna, Goa. Interestingly, H. sulfurifontis GUMFAZ2 was able to produce xylanase‐free cellulase, degrade insoluble cellulose debris to soluble oligosaccharides, and produce other nonhazardous by‐products. The haloarchaeon was found to be extremely halophilic since it grew optimally at 3.4 M NaCl with no growth below 0.5 M NaCl. Maximum cellulase activity (11.7 U/mL) was observed in the extracellular fraction when cells were grown at 40°C on carboxymethylcellulose-Na (CMC-Na), as a sole source of carbon, in 3.5 M NaCl containing medium buffered to pH 7. This crude enzyme active fraction showed a protein band of 46 kDa by SDS-PAGE analysis (Malik & Furtado, 2019) suggesting a single enzyme was responsible for the cellulose activity. The 16S rRNA sequence of the GUMFAZ2 strain showed that it shared 98.7% identity to other isolates including H. sulfurifontis JCM 12327 and Haloferax lucentense DSM 14919T. Moreover, the GUMFAZ2 strain showed growth on gelatin, sucrose, galactose, and maltose, in which it varied from H. lucentense, and hence was classified as H. sulfurifontis GUMFAZ2. The enzyme fractions from haloarchaea isolated from the sponge Haliclona sp. are worth pursuing because of their capacity for cellulose degradation and stability at high salinity (5 M NaCl). Similarly, cellulases have been isolated and purified from bacterial halophiles, such as Bacillus sp. BG-CS10 (Zhang, Li, Xue, Mao, & Ma, 2012), Thalassobacillus sp. LY18 3 (X. Li, Wang, Li, & Yu, 2012) and Alkalilimnicola sp. NMDCM1 (Mesbah & Wiegel, 2017); however, these cellulases tolerated only 1 to 2.5 M NaCl, which is not as impressive as the haloarchaeal cellulases.
Hemicellulose
Hemicellulose, also known as polyose, is amorphous in structure and found in association with cellulose. The types of building blocks that are used to form hemicellulose can vary including the hexoses (glucose, galactose, mannose), pentoses (xylose, arabinose), sugar acids (glucuronic acid) and phenolic acids (ferulic acid, p-coumaric acid) (Scheller & Ulvskov, 2010). Based on the frequency of the monosaccharide in the main polymeric chain, hemicelluloses are classified into: xylans, mannans, β(1,3)- and β(1,4)-linked glucans, and xyloglucans. The hemicellulose polysaccharides that consist of β(1,4)-linked glycans interact non-covalently via H-bonds among themselves and with the cellulose chains (Scheller & Ulvskov, 2010). Structural similarities between the cellulose and the hemicellulosic polysaccharides have led to the prediction that “CESA like” (CSL) genes encode glycan synthases (Scheller & Ulvskov, 2010). Homologs that cluster to the CSL gene family are reported in all plant genomes. Based on amino acid sequence identity, the CSL homologs are divided into subgroups A–H and J. Groups B and G are specific to non-grass species, whereas groups F, H, and J are found only in grasses (Sandhu, Randhawa, & Dhugga, 2009).
Our present knowledge on genetic control of hemicellulose production in plants is limited in comparison with the biosynthesis of cellulose. However, based on the bioinformatic analysis of the gene expression patterns on tissue specific libraries of rice and Arabidopsis, a team has identified genes coding for xylan synthase, feruloyl transferase, and arabinosyl transferase (Mitchell, Dupree, & Shewry, 2007). Hemicelluloses are composed of glucose residues with β(1,4)-linkage with interlinked β(1,3)-linkages known as mixed linkage glucan (MLG). The enzymes involved in glycan chain formation belong to the CSL family, while CslF and CslH are exclusively responsible for the biosynthesis of β(1,3)- and β(1,4)-linked D-glucans (Carpita, 2012; Sandhu et al., 2009). (Burton et al., 2006) showed D-glucan accumulation in Arabidopsis sp. due to the heterologous expression of a rice CslF. The molecular knowledge on the synthesis and deposition of hemicellulose polymer is likely to guide the identification of appropriate and efficient hydrolytic enzymes to depolymerize biomass for fuels and chemicals. As hemicelluloses are mixtures of polysaccharides with high molecular mass, multiple types of hemicellulases are needed for depolymerization. Conversion of hemicelluloses requires esterases to hydrolyze acetyl and ferulic acids, and glycoside hydrolases to break the sugar backbone. Therefore, for a microorganism to efficiently degrade hemicellulose, it must produce multiple enzymes with distinct specificity and function (Shallom & Shoham, 2003). Recently, haloarchaea with unusual halotolerant hemicellulases have been identified and the enzymes have been purified. These enzymes are reviewed below.
Hemicellulose hydrolysis
Hydrolytic enzymes that degrade hemicelluloses are referred as hemicellulases (glycan hydrolases, EC 3.2.1) or hemicellulolytic enzymes including D-xylanases, D-galactanases, D-mannanases, and L-arabinases. These enzymes are of great interest in paper and pulp industry due to their bleach-boosting properties (Walia, Guleria, Mehta, Chauhan, & Parkash, 2017). Hemicellulases can be classified into two hydrolytic reaction types: exo that promote successive degradation of the polysaccharide from the nonreducing end and/or endo that catalyze random degradation causing multiple scissions. Most of the extracellular xylanases reported are from plants, psychrophilic fungi, mesophilic bacteria and marine algae (Bradner, Gillings, & Nevalainen, 1999; Dekker & Richards, 1976). Reports of thermophilic (Lüthi, Jasmat, & Bergquist, 1990; Winterhalter & Liebl, 1995) and alkalophilic (Honda, Kudo, & Horikoshi, 1985) bacteria producing xylanases are also described. Among all hemicelluloses, xylan is a heterogeneous polysaccharide frequently found as hemicellulose which is composed of D-xylose linked by β-1,4 glycosidic bonds (Jensen et al., 2018). Xylan degradation requires endo-β-xylanases for fragmentation of the chain, and β-xylosidases for converting the xylo oligomers to monomers (Kulkarni et al., 1999). In addition to these two enzymes, other auxiliary enzymes like α-glucuronidase, acetyl xylan esterase, and ferulic and p-coumaric acid esterases are needed for the removal of the side residues (Begemann et al., 2011).
Xylanases and xylosidases
Hydrolysis of xylan, the major constituent of hemicellulose is catalyzed by xylanases. Overall economics of processing lignocellulosic materials for the generation of liquid fuels and chemicals could be improved by optimized use of these enzymes. Early studies of archaea reported the production of xylanolytic enzymes and/or activity by hyperthermophilic archaea including Pyrodictium abyssi (Andrade, Aguiar, & Antranikian, 2001) and Thermococcus zilligii strain AN1 (Uhl & Daniel, 1999). More recently, haloarchaea, such as H. utahensis are identified to produce xylanases (Wainø & Ingvorsen, 2003). Isolation and characterization of the xylanase and xylosidase enzymes produced by H. utahensis, isolated from the sediments of the Great Salt Lake in Utah, USA was the first report of these types of enzyme activities from a haloarchaeon (Wainø & Ingvorsen, 2003). The H. utahensis strain produced β-xylanase and β-xylosidase enzymes that were thermophilic and thermostable, maintaining activity at 55–70°C. The strain differed from other related haloarchaea by tolerating not only high salt (3.5 M NaCl) but also conditions of low ionic strength (0.002% NaCl). SDS-PAGE and zymogram analyses of the culture supernatants revealed that the two xylan-degrading activities (β-xylanase and β-xylosidase) were related to proteins estimated at 45 and 67 kDa molecular mass. Salt stable xylanases and xylosidases have also been identified in halotolerant bacteria (Wang, Chan, Lin, & Shyu, 2010; Wejse, Ingvorsen, & Mortensen, 2003).
Metabolic conversion of the pentose sugars L-arabinose and D-xylose
Besides D-xylose, L-arabinose is also a component of the hemicellulose plant cell wall material. The biosynthesis and metabolic conversion of these pentose sugars (L-arabinose and D-xylose) are well studied in bacteria with limited information in archaea. The first D-xylose metabolic pathway described for archaea was well elucidated in the haloarchaeon H. volcanii (Johnsen et al., 2009; Johnsen, Sutter, Zaiß, & Schönheit, 2013) (Figure 2).This organism can convert D-xylose to α-ketoglutarate in a catabolic pathway initiated by the oxidation of D-xylose to D-xylono-γ-lactone catalyzed by NADP+-dependent xylose dehydrogenase (XDH). A lactonase hydrates this intermediate to D-xylonate followed by dehydration to 2-keto-3-deoxy-D-xylonate (KDX) by xylonate dehydratase (XAD). The KDX is further dehydrated by KDX-dehydratase (KDXD) to α-ketoglutarate semialdehyde and finally oxidized to α-ketoglutarate by NADP+-dependent α-ketoglutarate semialdehyde dehydrogenase (KGSADH) (Figure 2) (Johnsen et al., 2009). Later during 2013, the metabolism of L-arabinose to α-ketoglutarate by an highly specific L-arabinose dehydrogenase (L-AraDH) in H. volcanii was studied (Johnsen et al., 2013). Subsequent metabolism of the L-arabinoate intermediate to α-ketoglutarate was found to involve the same set of enzymes (XAD and KDXD) (Johnsen et al., 2013), as reported for D-xylonate conversion to α-ketoglutarate (Johnsen et al., 2009). The L-AraDH gene was identified as HVO_B0032 coding for a 130-kDa homotetrameric enzyme that catalyzed the oxidation of L-arabinose. Growth of an L-AraDH deletion mutant was observed on glucose and D-xylose, but not on L-arabinose indicating that L-AraDH in H. volcanii is involved in L-arabinose catabolism but not in D-xylose degradation. Thus, the L-arabinose and D-xylose degradation pathways in H. volcanii are initiated by distinct dehydrogenases, L-AraDH and XDH, catalyzing the specific oxidation of L-arabinose or D-xylose, respectively. By contrast, the conversion of the lactone derivatives to α-ketoglutarate involves common enzymes (lacontase, XAD and KDXD) indicating partial pathway promiscuity of pentose degradation in H. volcanii (Johnsen et al., 2013). The α-ketoglutarate generated from these pathways can feed into the TCA cycle or serve as a 5-carbon skeleton for bioproduct formation.
Figure 2.
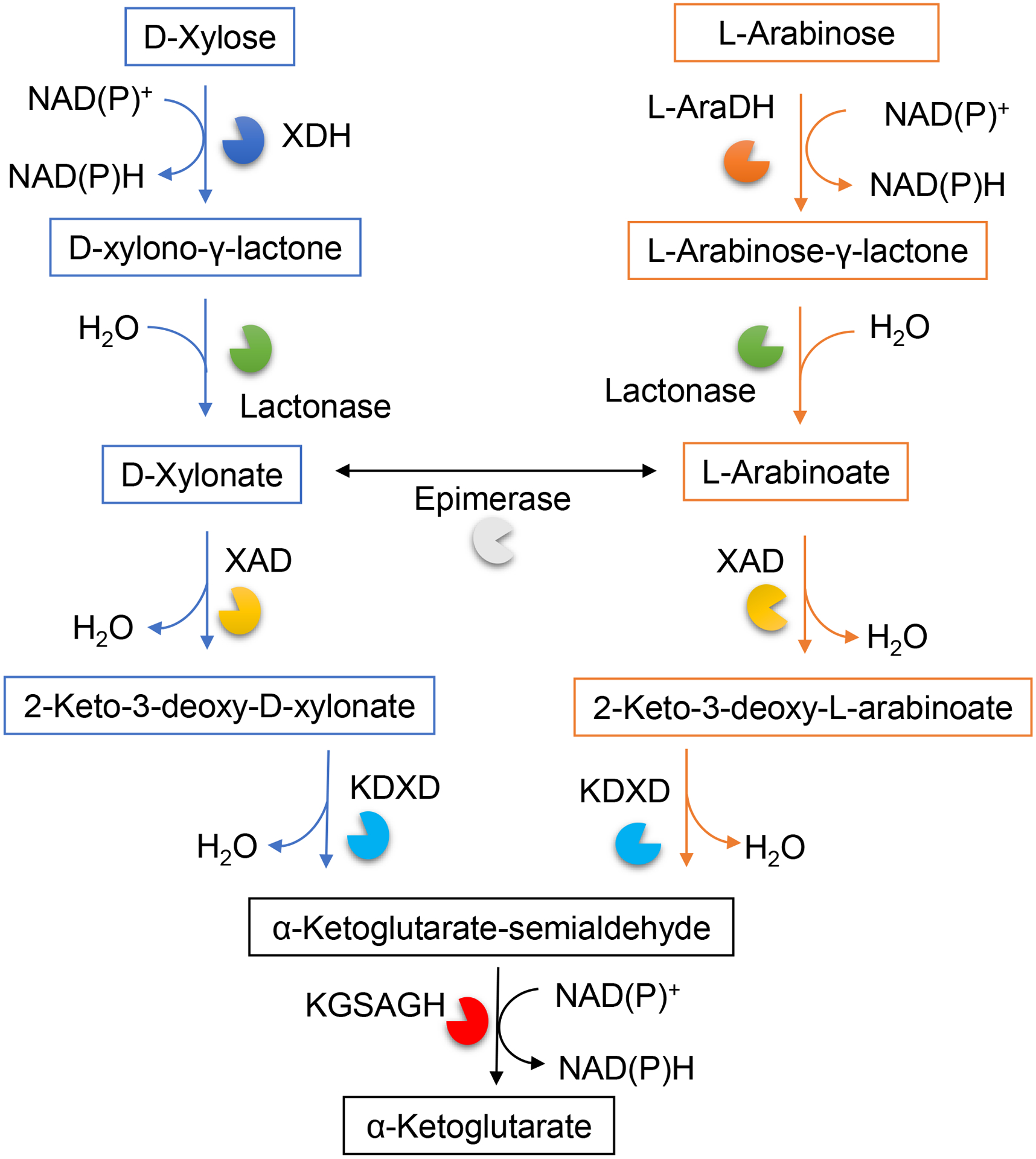
Metabolic conversion of L-arabinose and D-xylose to α-ketoglutarate in the model haloarchaeon Haloferax volcanii. XDH, D-xylose dehydrogenase (EC:1.1.1.179); L-AraDH, L-arabinose dehydrogenase (EC:1.1.1.376); Lactonase (EC 3.1.1.110); XAD, D-xylonate dehydratase (EC 4.2.1.82); KDXD, 2-keto-3-deoxyxylonate dehydratase (EC:4.2.1.141); KGSADH, α-ketoglutarate semialdehyde dehydrogenase (EC:1.2.1.26). Figure adapted from (Johnsen et al., 2009).
Lignin
Lignin is the major structural component of plant cell walls. This complex organic polymer provides mechanical strength and rigidity in plants making it recalcitrant to depolymerization (Kim & Shoda, 1999). Under aerobic conditions lignin can be degraded by peroxidases and phenol oxidases (including laccases and polyphenol oxidases) (Datta et al., 2017). Lignin and lignin derivatives are released in the waste streams of many industries such as paper and pulp industries, tanneries, textile mills, and molasses-based distilleries. These waste streams are considered as serious contamination due to the intense color of the lignin (Raghukumar, D’Souza-Ticlo, & Verma, 2008). 2008). Even in the new promising pretreatment method, which is based on the application of ionic liquids, the enzymatic modification of the lignocellulosic material cannot be directly performed in the ionic liquids (S. Sun, Cao, & Sun, 2016). Moreover, the waste streams usually contain high amounts of salts and organic solvents making it difficult to remove the lignin through biodegradation. Thus, lignin-degrading enzymes from halophilic microbes have gained interest in the bioindustry. Halotolerant enzymes could play important roles in biopulping and biobleaching processes (Geng & Li, 2002). Moreover, halophilic, and halotolerant microorganisms could have important roles in lignin degradation and modification for producing biofuels and high value chemicals (e.g., the valuable food/flavor chemical vanillin).
Lignin modification by laccases
White-rot fungi produce many extracellular enzymes such as laccases and peroxidases that can directly decompose lignin, cellulose, and hemicellulose components of the plant cell wall. These enzymes degrade lignin through free radicals such as hydroxyl radicals (‧OH), depolymerize the phenolic and non-phenolic lignin polymer, and mineralize the insoluble lignin (Datta et al., 2017). Laccases are multicopper oxidases (MCOs) in the phenoloxidase enzyme group that couple the oxidation of organic and/or inorganic substrates to the reduction of molecular oxygen to water. Laccases were first identified in the exudates of the Japanese lacquer (Rhus vernicifera), and extensive studies have been performed on fungi laccases in the past decades (Mikolasch & Schauer, 2009). Laccases are some of the most important enzymes in lignin degradation/modification (Sitarz, Mikkelsen, & Meyer, 2016) and do not require additional components such as manganese or hydrogen peroxide for activity (Molitoris, 1970). Laccase activities are useful in food industry (Osma, Toca-Herrera, & Rodríguez-Couto, 2010), in the pulp and paper industry (Virk, Sharma, & Capalash, 2012), in the forest product industry (Widsten, 2008), in grafting reactions (Kudanga, Prasetyo, Sipilä, Guebitz, & Nyanhongo, 2010), in organic synthesis (Mikolasch & Schauer, 2009; Witayakran & Ragauskas, 2009), and in bioremediation (Viswanath, Rajesh, Janardhan, Kumar, & Narasimha, 2014; Zucca et al., 2016). Laccases of eukaryotes and bacteria are well characterized, while knowledge of these enzymes from archaea is less studied.
Haloarchaeal laccase
In 2010, a laccase (LccA) from the haloarchaeon H. volcanii was purified and characterized (Uthandi, Saad, Humbard, & Maupin-Furlow, 2010). This laccase was found to be an extracellular multicopper oxidase that was secreted at high levels into the culture medium of an H. volcanii strain genetically engineered to overexpress the lccA gene (Uthandi et al., 2010). The LccA isolated from the spent culture broth could withstand prolonged exposure to extreme conditions such as high temperature, high salt concentration and organic solvent (Uthandi et al., 2010). When purified from stationary phase cells (72–80 h), LccA was shown to have a specific activity of 33 U/mg−1 in the oxidation of syringaldazine (SGZ) (Uthandi et al., 2010). Further examination revealed LccA to oxidize not only SGZ but also 2,2,-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), bilirubin, and dimethoxyphenol (DMP) with optimal activity at 45°C (Uthandi et al., 2010) The enzyme kinetics showed Km and kcat values for SGZ, bilirubin, and ABTS were 35, 236, 670 μM, and 22, 29, and 10 s−1 respectively. The purified LccA was stable while exposed for a prolonged period (31.5 h) to high temperature (50°C), wide range of salt concentrations from 100 mM to at least 1.4 M NaCl, and various organo solvents (Uthandi et al., 2010). The enzyme apparently underwent specific cleavage and removal of an N-terminal twin-arginine translocation (Tat) signal sequence and glycosylation during its secretion into the medium, as the purified protein had Ala32 as its N-terminal amino acid and was glycosylated to 6.9% carbohydrate content (Uthandi et al., 2010).
Hence the same team (Uthandi et al., 2012) modified the encoding lccA gene by either altering the Tat motif from arginine to lysine residues or removing the coding sequence for the N-terminal pro-peptide to purify LccA from recombinant Escherichia coli. LccA was readily purified as a 74-kDa monomer (yield of 54 %) from the soluble fraction of E. coli with SGZ oxidizing activity of 33 U mg−1 (Uthandi et al., 2012), a specific activity comparable to that of the enzyme secreted from H. volcanii. Consistent with this finding, the LccA proteins extracted from the culture broth of H. volcanii and the soluble fraction of recombinant E. coli cells proved to have a similar folding pattern with and a rich β-sheet structure analogous to other multicopper oxidases (Uthandi et al., 2012). However, the loaded copper was not fully inserted into the type-I Cu center of the E. coli produced LccA, thus, providing insight into avenues for further optimization (Uthandi et al., 2012). Overall, the LccA enzyme is a target for industrial applications based on its robust bilirubin oxidase and laccase activities.
Starch and its hydrolysis
Most plants store their carbohydrates in the form of starch, a form of biomass readily available on earth. Starch can be hydrolyzed to produce glucose and high-fructose corn syrups and the resulting sugars can be fermented to bioethanol (Bai et al., 2019). To do so, the starch industries depend on hydrolytic enzymes such as α-amylases (EC:3.2.1.1), β-amylases (EC:3.2.1.2), glucoamylases (EC 3.2.1.3) and pullulanases (EC 3.2.1.41) for release of the sugars (Bertoldo & Antranikian, 2002). D-glucose isomerases (D-xylose isomerases; EC 5.3.1.5) are used to convert the sugars to sweeteners (Zeikus, Vieille, & Savchenko, 1998).
α-Amylases are starch-degrading enzymes belonging to the family of glycoside hydrolases that that hydrolyze 1–4 glycosidic bonds of polysaccharides (starch and glycogen) to yield oligosaccharides like glucose and maltose (Sunna, Moracci, Rossi, & Antranikian, 1997). Alpha-amylases are identified in haloarchaea with details of the enzymes shown in Table 3. Of these, the extracellular α-amylase produced by Haloferax sp. HA10 was the first report of detergent-stable α-amylase isolated from a haloarchaeon (Bajpai, Chaudhary, & Saxena, 2015). This 66-kDa enzyme is calcium-dependent with maximum activity at 3 M NaCl, 37 °C, pH 7 and 1 % starch content (Bajpai et al., 2015). In another interesting study (Pérez-Pomares et al., 2003), H. mediterranei was shown to grow in a minimal medium with ammonium acetate as a carbon and nitrogen source and produce low levels of α-amylase activity when the medium was further enriched with starch. The researchers (Pérez-Pomares et al., 2003) found that this α-amylase was a monomeric enzyme that was stable from 2 to 4 M NaCl, with maximal activity at 3 M NaCl, pH from 7 to 8, and temperature from 50°C to 60°C. Other starch hydrolyzing haloarchaea are also described including Haloterrigena turkmenica, which secretes a salt tolerant α-amylase (AmyA) when grown in medium enriched with 0.2% (w/v) starch (Santorelli et al., 2016). SDS-PAGE and size exclusion chromatography (SEC) analyses of this extracellular fraction revealed the enzyme was a monomer of 54–66 kDa. The α-amylase activity of AmyA was optimal at 55 °C, pH 8.5 and 2 M NaCl and was enhanced by the addition of Triton X-100, but not by n-hexane or chloroform. The demonstrated efficiency of AmyA in degrading starch of grape cane (Santorelli et al., 2016) proved it a promising candidate for the utilization of agro-industrial waste in fuel and chemicals production. Unlike α-amylase, β-amylase is an exoenzyme that hydrolyzes starch by removing maltose from the non-reducing end of the starch. While not common among prokaryotes, β-amylase activity has been reported in halophilic bacteria (Kawazu et al., 1987). In addition, an organic-solvent-tolerant halophilic α-amylase is described from the moderately halophilic gram-positive bacterium Nesterenkonia sp. strain F (Shafiei, Ziaee, & Amoozegar, 2011).
Table 3.
Starch degrading α-amylases produced by haloarchaea.
| source | Enzyme activity optimum | Protein molecular mass (kDa) | Ref. | ||
|---|---|---|---|---|---|
| NaCl | Temp. | pH | |||
| Hfx. mediterranei | 3 M | 50–60 °C | 7.8 | 50 | (Pérez-Pomares et al., 2003) |
| Hrr. xinjiangense | 4 M | 70 °C | 8.5 | 60 | (Moshfegh, Shahverdi, Zarrini, & Faramarzi, 2013) |
| Har. hispanica | 4 M | 50 °C | 6.5 | 50 | (Hutcheon, Vasisht, & Bolhuis, 2005) |
| Haloarcula sp. | 4.3 M | 50 °C | 7 | 70 | (Fukushima, Mizuki, Echigo, Inoue, & Usami, 2005) |
| Ncc. amylolyticus | 2.5 M | 55 °C | 8.7 | 74 | (Kobayashi, Kanai, Aono, Horikoshi, & Kudo, 1994) |
| Haloarcula japonica | 4.2 M | 45 °C | 6.5 | 83 | (Onodera et al., 2013) |
| Haloferax sp. HA10 | 3 M | 37 °C | 7 | 66 | (Bajpai et al., 2015) |
| Haloterrigena turkmenica | 2 M | 55 °C | 8.5 | 54–66 | (Santorelli et al., 2016) |
Pullulanases and glucoamylases are also used in the starch industry. Glucoamylases catalyze the sequential cleavage of α-(1,4) and α-(1,6) glycosidic bonds within starch and produce glucose as the sole end-product (Xu, Yan, & Feng, 2016). Pullulanases widely serve as important debranching enzymes in the hydrolysis of starch, amylopectin, pullulan, and related oligosaccharides, thus, enabling a complete and efficient conversion of the branched polysaccharides into fermentable sugars during saccharification process (Läufer, 2019). Along with glucoamylases, pullulanases are used to improve the efficiency of starch saccharification. The halophilic archaeon Halorubrum sp. strain Ha25 was reported to produce an extracellular, halophilic, and organic solvent tolerant amylopullulanase (Siroosi, 2014). The molecular mass of the purified enzyme was 140 kDa by SDS–PAGE. The kinetic experiments showed a higher affinity and rate of conversion for soluble starch, with a Km of 1.8 mg/mL and Vmax of 25.2 μmol/min, when compared to pullulan at a Km of 4 mg/mL and Vmax of 9.5 μmol/min. The amylolytic and pullulytic activities for this enzyme were optimal at 50°C and pH 7 and 7.5, respectively. Furthermore, the amylopullulanase was highly stable in the presence of non-polar organic solvents.
Cyclodextrin glycosyltransferases (CGTases, EC 2.4.1.19) are α-amylases of the GH13 family that can convert starch, amyloses or other polysaccharides to products including cyclized dextrins or cyclodextrins (Graebin et al., 2016). Cyclodextrins are useful for their hydrophobic interior and hydrophilic exterior in drug delivery, food and other industrial applications (Graebin et al., 2016). (Bautista et al., 2012) identified, cloned, and characterized with biochemical and molecular techniques the first haloarchaeal CGTase. This enzyme was purified from the extracellular fraction of H. mediterranei as a 77-kDa monomer with optimum activity at 55°C, pH 7.5 and 1.5 M NaCl. The CGTase had demonstrated activity in the degradation and transformation of starch. The CGTase gene was identified as a 2,142 bp DNA sequence coding for a 713 amino acid protein that displays high similarity with α-amylase family enzymes. Conservation of an N-terminal Tat motif suggests the CGTase is secreted outside the cell by the Tat pathway. Upstream of the CGTase gene are four maltose ABC transporter (malE, malF, malG, and malK) gene homologs, which may be used to transport metabolites associated with this system.
Chitin as an abundant polysaccharide
Next to cellulose, chitin is the most abundant natural polysaccharide. Globally, around 6 to 8 million tons of chitin-containing seafood waste (crab, shrimp, and lobster shells) are produced (Elieh-Ali-Komi & Hamblin, 2016). While often just dumped in landfill or the sea, these crustacean shells harbor useful starting material for green chemistry including 20–40% protein, 20–50% calcium carbonate and 15–40% chitin, a polymer like cellulose, rich in nitrogen (Liao, Liu, Frear, & Chen, 2008). (Ning & Chen., 2015) comments that converting this sea waste into nitrogen-rich chemicals would benefit economies and the environment. Chitin is a β1–4-linked homopolymer of N-acetylglucosamine residues that is not only found in seafood waste, but is also a major (10%) and minor (1–2%) component of the cell walls of fungi and yeast, respectively (Elieh-Ali-Komi & Hamblin, 2016). The nitrogen-containing chemicals that could be derived from the chitin waste are widely used in: 1) the pharmaceutical industry for drugs, such as eszopiclone for sleeping difficulties and varenicline to treat nicotine addiction, 2) CO2 sequestration, such as ethanolamine (ETA) that is used to fix CO2 in power plants, 3) textiles industries, and 4) household cleansers and surfactants (Hamed, Özogul, & Regenstein, 2016). Establishing a profitable, sustainable industry from shell waste needs a viable fractionation method to separate proteins, calcium carbonate and chitin under harsh reaction conditions. Once separated from the crustacean waste, one must also consider that chitin is a crystalline material that prevents reagents from easily accessing the polymer chains. Enzymes that facilitate this depolymerization process would be useful to the chitin-based bioindustry.
Chitinases and chitin conversion by haloarchaea
Haloarchaea are identified to produce chitinases and convert chitin to useful products. Included among these haloarchaeal chitinases is the GH family 18 ChiN1 of Halobacterium salinarum sp. NRC-1; this enzyme was engineered to be secreted at high levels from Haloarcula japonicum using a strong glycoprotein (CSG) promoter (Yatsunami et al., 2010). By this approach, the chitinase was found active (8.9 × 10−3 U/mg, where 1 U is 1 μmol of reducing sugar per min) and stable over a wide range of salt concentrations with optimal activity at 55 °C at pH 6.0 in the presence of 1.0 M NaCl. (Yatsunami et al., 2010). A related chitinase from the marine haloarchaeon H. salinarum CECT 395 is produced and purified from recombinant E. coli with limited activity (García-Fraga, da Silva, López-Seijas, & Sieiro, 2014). A study by (Hou et al., 2014) has shown that H. mediterranei can grow on colloidal or powdered chitin and accumulate poly(3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBV). The researchers identified the gene cluster (HFX_5025 to HVO_5039) responsible for the pathway of chitin catabolism. Addition of colloidal or powdered chitin induced the expression of four putative chitinases (ChiAHme, ChiBHme, ChiCHme, and ChiDHme, HFX_5036–5039), the LmbE-like deacetylase (DacHme, HFX_5027), and the glycosidase (GlyAHme, HFX_5029) associated with chitin metabolism. ChiABCHme and ChiDHme mutant strains were unable to utilize chitin as the sole carbon source and lost the ability to synthesize PHBV. Similarly, deletion mutants of DacHme or GlyAHme did not produce PHBV on chitin, suggesting that ChiABCDHme, DacHme, and GlyAHme had a major role in chitin degradation in H. mediterranei. Assay for chitinase activity for the genes depleted of the signal sequences for intracellular expression, showed that the colloidal chitin was hydrolyzed into diacetylchitobiose by ChiABCDHme, which was further degraded to monosaccharides by DacHme and GlyAHme. Altogether, this study revealed the genes and enzymes involved in chitin catabolism in H. mediterranei might be used as whole-cell biocatalyst in chitin bioconversion.
Esterase and lipase enzymes
Deconstruction of the plant cell wall network, which is an interlinkage of cellulose fibers, hemicelluloses, and lignin, is highly recalcitrant to enzymatic attack and is the most challenging task of biofuel production. The recalcitrant challenge is due to the ester, ether, and glycosidic covalent bonds between polysaccharides (cellulose and hemicellulose) and lignin which forms the lignin–carbohydrate complexes (LCCs) (Björkman, 1954). The hydrolytic enzymes identified to cleave these bonds are glucuronoyl esterases (GEs), grouped into carbohydrate esterase family 15 (CE15). These enzymes are subdivided into two major classes of hydrolases: 1) esterases (EC 3.1.1.1, carboxyl ester hydrolases) and 2) lipases (EC 3.1.1.3, triacylglycerol hydrolases) (Bornscheuer, Bessler, Srinivas, & Krishna, 2002).
Esterases hydrolyze esters of short-chain fatty acids, while lipases are active on water as well as lipid interfaced triglycerides (water-insoluble long-chain fatty acids). Naturally, lipase activity is detected in milk, fruits, vegetables cereals, oilseeds and in the digestive system of mammals. As a ubiquitous hydrolytic enzyme, lipases are especially used in biodiesel production (Hama, Noda, & Kondo, 2018). These enzymes can hydrolyze triglycerides into glycerol and fatty even in a non-aqueous solvent background (Teo, Zhang, & Poh, 2003). Lipases are also used to remove the hydrophobic components of wood in pulp and paper industries (Fischer & Messner, 1992), used to synthesis biopolymeric (polyesters and polysaccharides), which are biodegradable and eco-friendly (Sandoval, Rivera, Barrera‐Rivera, & Martínez‐Richa, 2010). One of the most important application is it is used for transesterification of plant fats for biodiesel production (Fukuda, Kondo, & Noda, 2001). Many microorganisms release lipase-type enzymes into their culture media; however, halophilic lipases have their own valuable characteristics to work properly in the harsh conditions in most industrial processes (Sharma, Soni, Nazir, Oberoi, & Chadha, 2011). Esterase and lipase enzymes from halophilic organisms are considered important biocatalysts as they are stable in organic solvents (DasSarma & DasSarma, 2015).
Esterases and lipases are two well-known members of a larger group of enzymes denoted as carboxylic ester hydrolases (CEHs, EC 3.1.1.-). CEHs are a diverse group of enzymes that attack substrates with an ester bond, hydrolyzing this bond in the presence of water to form an alcohol and a carboxylic acid (Y. Chen, Black, & Reilly, 2016). In the presence of organic solvents, these enzymes can also catalyze the reverse reaction or a trans-esterification reaction (Levisson, van der Oost, & Kengen, 2009). Applications of carboxylic ester hydrolases are found in biodiesel production, detergent production, organic synthesis, and food related processes for flavor and aroma synthesis (Panda & Gowrishankar, 2005).
Haloarchaeal esterase and lipase enzymes
A first report on the ability of Haloarcula marismortui to synthesize esterases and lipases and the effect of physicochemical conditions on the production of these enzymes was investigated by (Camacho, Mateos, González-Reynoso, Prado, & Córdova, 2009). Based on the genomic predictions of esterase and lipase gene homologs, H. marismortui was cultured on a rich medium and the intra/extracellular crude extracts were assayed for these enzyme activities using p-nitrophenyl (pNP) esters and triacylglycerides as substrates. Under these conditions, H. marismortui was found to reach a specific growth rate of 0.053 h−1 and intracellular esterase and lipase activities of 2.1 and 0.72 U‧L−1 with p-nitrophenyl valerate (pNPV, C5 chain length) and p-nitrophenyl laurate (pNPL, C12 chain length) as substrates, respectively. When assayed on triacylglycerides, the enzyme activities were 3.5 U‧L−1 on trioctanoin (TC8) and 10.1 U L−1 on tributyrin (TC4). These experimental results demonstrate the H. marismortui enzymes have a selective affinity in conversion of triacylglycerides compared to the pNP esters. In this same study, (Camacho et al., 2009) found H. marismortui also produced the esterase and lipase activities in a bioreactor. These enzyme activities were maximal during the deceleration phase (after 48 h) and remained constant during the stationary phase of growth, with a specific growth rate of 0.052 h−1 and final biomass of 2.35 g L−1. The esterase and lipase activities were maximal at 45°C and were inactive at 75°C. Interestingly, the esterase activity of the lysate was optimal at two distinct salt concentrations (0.5 and 5 M NaCl) with 50% residual activity retained even in the absence of salt. Thus, two enzymes were proposed responsible for the esterase activity.
H. salinarum has also been investigated for the synthesis of carboxyl ester hydrolases and found to produce esterase activity (Camacho, Mateos-Díaz, Diaz-Montaño, González-Reynoso, & Córdova, 2010). Enzyme activities were monitored using pNP esters and triacylglycerides as substrates. Similarly, to H. marismortui, H. salinarum was found to display esterase activity with a preference for TC4 over pNP-esters. The researchers also determined how physicochemical conditions could affect enzyme production and the specific growth rate. From this approach, H. salinarum was found to have a maximum specific growth rate of 0.136 h−1 at 4.2 M NaCl, pH 6 and 44°C, and a maximal esterase production of 1.64 U‧L1 when cells were grown suboptimal at 4.6 M NaCl, pH 6 and 30°C. Further analysis revealed the esterase activity in the H. salinarum cell lysate to be optimal at 5 M NaCl and 80°C. This temperature optimum was notably higher than other halophilic enzymes. These extreme properties make this esterase activity of interest to catalyze transesterification reactions in non-aqueous conditions.
A lipase has been purified from Haloarcula sp. G41, isolated from the saline soil of Yuncheng Salt Lake, China (X. Li & Yu, 2014). The salinity of the medium strongly affected the production of the lipase with maximum yield achieved in the presence of 20% NaCl or 15% Na2SO4. The lipase was of 45 kDa molecular mass and preferred long- over short-chained pNP esters with highest activity for p-nitrophenyl myristate (pNPM, C14 chain length). Activity assay with this substrate revealed the enzyme had thermostable, alkali-stable, and halostable properties with optimum activity at 70°C, pH 8.0, and 15% NaCl. Activity and stability of the enzyme was observed over a broad range of temperature (30–80°C), pH (6.0–11.0), and NaCl concentration (10–25%). Application of free and immobilized forms of the lipase derived from strain G41 was assayed in biodiesel production from soybean oil, which showed a yield of 80.5 and 89.2%, respectively for lipase-catalyzed transesterification.
Additional types of lipases and esterases are discovered through screening haloarchaea. (Ozcan, Ozyilmaz, Cokmus, & Caliskan, 2009) screened 118 haloarchaeal strains for lipolytic activity. Of these strains, 18 isolates were identified to be positive on rhodamine agar plates containing lipase substrates. The plates are designed to identify lipase producing microorganisms, as the enzymes act upon the substrates to liberate free fatty acids that form complexes with the rhodamine dye and produce orange fluorescence (Jette & Ziomek, 1994). Hence, rhodamine agar plates are effective in identifying lipase-producing microorganisms. The haloarchaeal isolates that tested positive on the plates were further characterized for esterase and lipase activities when subjected to various salt concentrations, temperature, and pH. Esterase and lipase activities were determined by the hydrolysis of p-nitrophenol butyrate (pNPB, C4 chain length) and p-nitrophenol palmitate (pNPP, C16 chain length), respectively. The supernatants of isolates grown on medium with 25% NaCl and 1% gum arabic produced the maximum hydrolytic activities. Maximum esterase and lipase activities were observed at pH 8–8.5, 45–65°C and 3–4.5 M NaCl. The results indicated that a variety of haloarchaeal strains do produce salt- and temperature-tolerant lipolytic enzymes. The kinetic parameters of Vmax/Km were higher for the esterase than the lipase activities for all isolates examined (Ozcan et al., 2009). In addition to these studies, other halophiles, including haloarchaea, have been screened for novel lipase and/or esterase activities by many researchers (Ameri et al., 2017; Esakkiraj et al., 2017; Gutiérrez-Arnillas, Arellano, Deive, Rodríguez, & Sanromán, 2017; Moreno, Márquez, García, & Mellado, 2016).
Antifreeze and anti-desiccation activity
Production of advanced materials, such as antifreeze and anti-desiccants, can add value and expand the repertoire of products available in a bio-based economy. Recently, a number of haloarchaea were isolated from fish sauce fermentation broth that produced significant levels of antifreeze protein. The researchers (Desai, Patel, Markande, Kamala, & Sivaperumal, 2020) isolated Halorubrum sp. SS1 (KY053875.1), Halobacterium sp. SS2 (KY053876.1), Halococcus sp. AMS1 (KU995303.1), Halorhabdus sp. AMS6 (KU995310.1) and Halobacterium sp. SFF3 (KY053871.1) from the broth and then screened these haloarchaea for various activities that may be useful in biotechnology. The researchers found the isolates to have negligible levels of protease, amylase and surfactant activities with some differences in amylase production based on biofilm vs. planktonic growth. The most notable products, however, were antifreeze proteins that were desiccation tolerant for up to 4 h and stable for up to 8 days. Halobacterium sp. SS2 was the best candidate for the formation of this product, while Halorubrum sp. SS1 and Halococcus sp. AMS1 were least the desiccation tolerant and Halorhabdus sp. AMS6 exhibited a steady rate of anti-desiccation activity. This report is the first to describe archaea for their ability to produce antifreeze and anti-desiccation proteins (Desai et al., 2020). These findings highlight the biotechnological potential of haloarchaea in food industry where antifreeze and antidesiccation proteins are in demand.
Bioprospecting of extremely haloarchaea for hydrolytic enzymes
Haloarchaea are resistant to extreme environments and their extracellular hydrolytic enzymes are of great interest as they are generally stable and functional under extreme conditions. The bioprospecting for novel extremozymes is ongoing, as these enzymes are expected to be powerful biocatalysts in industrial biotransformation processes performed under harsh conditions. A total of 68 haloarchaea isolated from saline soils were subjected to phylogenetic analysis, using Blast database (NCBI) and EzTaxon-server (Menasria et al., 2018). The phylogenetic study revealed that the strains with 98–99% of sequence similarity grouped into seven different Halobacteria genera. Among the archaeal isolates, Haloferax spp. dominated the cluster with 30 isolates (44 % of total) including H. alexandrinus, H. larsenii, H. lucentensis, H. mediterranei and H. volcanii and representing high species diversity. This group was followed by Halococcus spp. with 9 isolates (13 % of total), Halogeometricum spp. and Natrinema spp. with eight isolates each (12 % each of total). The remainder of the archaeal groups were Haloarcula, Haloterrigena, and Natrialba, representing 8 % of the isolates (Menasria et al., 2018). Consistent with these findings a dominant occurrence of Haloferax and Halococcus spp. is generally observed in saline ecosystems (Oren, Elevi, Watanabe, Ihara, & Corcelli, 2002). All 68 haloarchaea isolates of the (Menasria et al., 2018) study were screened for extracellular halophilic active enzymes. From this analysis, 90 % of the strains were found to produce esterase, gelatinase, inulinase, and cellulase, while 53 % of the strains produced nuclease, pectinase, and xylanase. Among the 68 total isolates, 21 isolates (12 Haloferax, three Haococcus, three Haloarcula, two Haloterrigina and one Natrialba) produced extracellular amylase activity. Esterase and inulinase activities were frequently observed (46 and 37 isolates, respectively), while extracellular protease and nuclease activities were not as commonly detected (21 and 22 isolates respectively) (Menasria et al., 2018).
Other screening studies also show promise for the discovery of new salt-tolerant biocatalysts. (Karray et al., 2018) screened bacteria and archaea from Chott El Jerid, a hypersaline lake in the south of Tunisia and identified 68 isolates, which were able to grow on media with 15–25% of NaCl. Among the isolates studied, a number were identified that produced amylase (4 isolates), lipase (7 isolates), cellulase (11 isolates), xylanase (11 isolates), pectinase (6 isolates), and protease (6 isolates) activities; however, none of the isolates were found to produce chitinase or laccase activity. The researchers also identified that the haloarchaeal strains were able to secrete five types of enzyme activities including cellulase, xylanase, amylase, pectinase, and lipase (Karray et al., 2018). A similar screening approach was used by (Kakhki, 2011) who report that nine hydrolytic enzymes (i.e., DNase, amylase, lipase, inulinase, pullulanase, protease, cellulase, chitinase, and xylanase) were produced by archaeal isolates from Aran-Bidgol Hypersaline Lake in Iran. Likewise,(Birbir, Ogan, Calli, & Mertoglu, 2004) has determined the amylase, lipase, cellulase, β-galactosidase, gelatinase, caseinase, and DNase production among haloarchaeal strains isolated from a salt mine in Turkey.
Solid-state fermentation
Solid-state fermentation (SSF) has gained significant attention as a common technique to produce microbial metabolites with the advantage of a high product concentration but only a relatively low energy being required (Srivastava, Srivastava, Ramteke, & Mishra, 2019). In addition, SSF is ecofriendly, mostly utilizing solid agro-industrial wastes as the substrate and releases less wastewater with lesser risk of bacterial contamination. SSF processes have enormous potential for many new applications using the bioconversion of agro-industrial residues into biofuels and other high value–added products. SSF offers potential benefits on economic and environmental fronts, with sustainability.
Solid state fermentation (SSF) is growing microorganisms on moist solid particles with free oxygen available; the spaces between the particles are filled with continuous gas and thin films of water (Viniegra-Gonzàlez, 1997). Mostly SSF is used to culture filamentous fungi for their enzymes and secondary metabolites (Barrios-Gonzalez, 2012). A typical example is in optimizing the culturing of Aspergillus oryzae to secrete hydrolases (amylases and proteases) in the production of the Japanese seasoning ingredient koji. Likewise, synthesis of the lipase activity of Rhizopus delemar is five times more productive in SSF than submerged fermentation (SmF) (Narahara et al., 1982). Yeast, bacteria and archaea are less cultivated by SSF in comparison with fungi (Rodriguez-Couto S, 2006), in fact, most of the bacteria are preferably cultivated in SmF, as they require high (>0.9) water activity. Later when bacterial cultures were standardized to this solid medium, the production of metabolites or enzyme activity was normally high. For example, Bacillus subtilis is reported to generate 45 % more protease activity by SSF using soybean cake in comparison to SmF (Bhargav, Panda, Ali, & Javed, 2008). Generally, growth of halophilic microorganisms has been extensively examined through submerged fermentation (SmF) (Litchfield, 2011). Until now, there are only few reports about haloarchaea cultivation by SSF. Long ago halophilic microorganisms were demonstrated to multiply on a support (ZoBell, 1937). Recently, haloarchaea were found to survive for thousands of years in fluid inclusions at saturating concentrations of NaCl (Lowenstein, Schubert, & Timofeeff, 2011) suggesting SSF is a promising approach for optimizing bioproduct formation by haloarchaea.
Recently, (Martin del Campo et al., 2015) have successfully cultured three haloarchaea by SSF. In this study, four inert supports (perlite, vermiculite, high-density polyurethane foam and glass fiber) were examined. Esterase/lipase activity and biomass produced by SSF were evaluated along the time and compared with those generated by SmF, making this study the first report related to haloarchaea culture by SSF. When perlite, vermiculite and polyurethane were used as supports, low esterase activity with high-water activity was observed. By contrast, when polyurethane and glass fiber were mixed, the moisture retention capacity was improved, and maximal biomass and esterase production were achieved. Three haloarchaea (Natronococcus sp. TC6, Halobacterium sp. NRC-1 and H. marismortui) were grown by SmF and SSF and compared. An improvement of 1.3- to 6.2-fold in the biomass and esterase production was observed in the SSF vs. SmF grown cultures. This study is the first report on cultivation of haloarchaea on SSF with different inert support for improving biomass and esterase/lipase activity production (Martin del Campo et al., 2015). Conventional bioreactors systems used for mesophilic microorganisms are not optimized for the growth conditions of these esterase and lipase producing haloarchaea. Therefore, to over-come these difficulties, novel reactor systems and solid-state fermentation (SSF) should be considered to circumvent possible issues with oxygen transfer and salt corrosion (Bustard, Burgess, Meeyoo, & Wright, 2000).
Biodiesel waste, a resource of salty glycerol feedstock
More than 90 % of the biofuels produced are biodiesel or bioethanol (Oh, Hwang, Kim, Kim, & Lee, 2018). Biodiesel is generated through the transesterification and esterification of edible oils (soybean oil, palm oil, and rapeseed oil) and animal fats. One downside of biodiesel production is that it releases large amounts of crude glycerol as a major by-product, which is impure and of little to no economic value. Globally the annual production, of glycerol from biodiesel industries production is estimated to reach 4.2 million tons by 2020 (Viana, Viana, Vasconcelos, Santaella, & Leitao, 2014). Hence, the need of the hour is to explore novel strategies for the biotransformation of glycerol into value-added compounds, to sustain our environment during climate change. Until now researchers have progressed in producing value added products from crude glycerol as a feed for the production of propanediols, ethanol, hydrogen, methane, methanol, single cell oil, biosurfactants, and organic acids (M. Li, Zheng, Chen, & Zhu, 2014; Nicol, Marchand, & Lubitz, 2012)
Haloarchaea may offer a solution to the conversion of crude glycerol to useful products, for example they utilize glycerol as inexpensive carbon resources for useful products such as biopolymer polyhydroxyalkanoates (Hermann-Krauss et al., 2013). Glycerol is a highly abundant carbon and energy source in hypersaline environments, as it is produced in large quantities as an organic osmolyte by Dunaliella salina, a green microalga, that blooms and recedes with fluctuating salt concentrations in these ecosystems (Elevi Bardavid, Khristo, & Oren, 2008). Glycerol is readily used as a carbon and energy source by haloarchaea (Halobacteria class) (Williams, Allen, Tschitschko, & Cavicchioli, 2017). Haloarchaea metabolize glycerol in two different pathways (T. J. Williams et al., 2017). In the first, glycerol is phosphorylated by glycerol kinase to sn-glycerol-3-phosphate (G3P) and then oxidized by G3P dehydrogenase (G3PDH) to dihydroxyacetone phosphate (DHAP). Alternatively, glycerol can be oxidized by glycerol dehydrogenase to dihydroxyacetone (DHA) which is then phosphorylated by DHA kinase to DHAP. These pathways are referred to as the G3P and DHA pathway, respectively (Figure 3). Haloarchaea (Haloferax and Haloarcula spp.) grown on glycerol release lactate from DHAP via the methylglyoxal bypass (Oren & Gurevich, 1994) (Figure 3).
Figure 3.
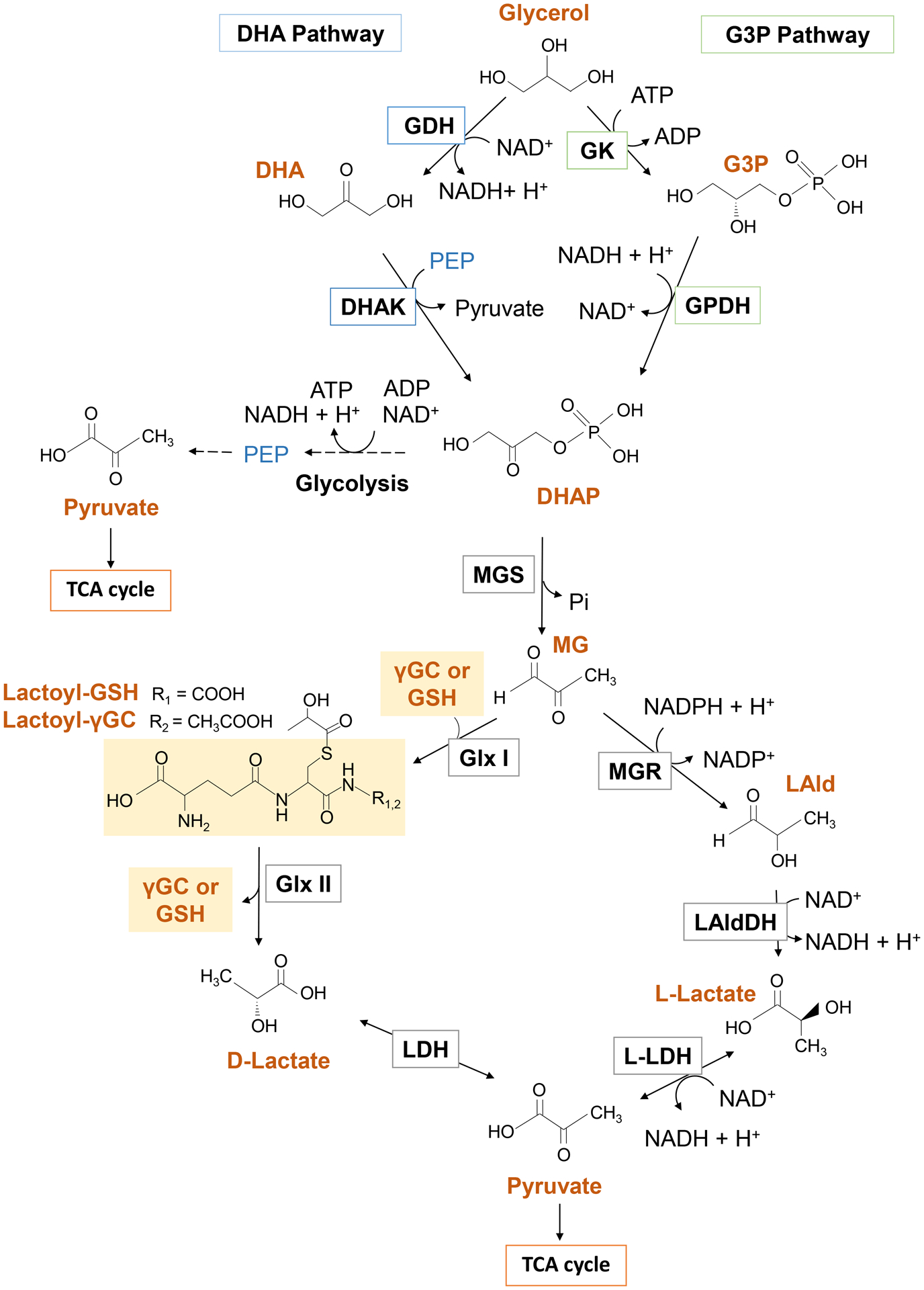
Catabolism of glycerol by haloarchaea. Abbreviations: G3P, sn-glycerol-3-phosphate; DHAP, dihydroxyacetone phosphate; DHA, dihydroxyacetone; MG, methylglyoxal; Pi, inorganic phosphate; LAld, lactaldehyde; GK, glycerol kinase (EC 2.7.1.30); G3PDH, G3P dehydrogenase (EC:1.1.5.3); GDH, glycerol dehydrogenase (EC 1.1.1.6); MGS, methylglyoxal synthase (EC 4.2.3.3); MGR, methylglyoxal reductase (NADPH-dependent) (EC 1.1.1.283); DHAK, DHA kinase (EC:2.7.1.29) linked to the PEP:phosphotransferase system;. LAldDH, lactaldehyde dehydrogenase (EC 1.2.1.22) (rare in haloarchaea); L-LDH, L-lactate dehydrogenase (EC 1.1.1.27); D-LDH, D-lactate dehydrogenase (EC 1.1.1.28. no examples apparent in haloarchaea); GlxI, glyoxalase I (S-lactoylglutathione methylglyoxal-lyase; EC 4.4.1.5); GlxII, glyoxylase II (S-2-hydroxyacylglutathione hydrolase; EC 3.1.2.6); γGC and GSH, γ-glutamylcysteine and glutathione (low molecular weight thiol di- and tripeptides, respectively; γGC abundant in haloarchaea)(Rawat & Maupin-Furlow, 2020).
The G3P pathway is common among haloarchaea. In H. volcanii, (Sherwood, Cano, & Maupin-Furlow, 2009) demonstrate that glycerol kinase (glpK) is required for the catabolism of glycerol but not for phospholipid (PL) biosynthesis as the latter uses glycerol-1-phosphate as the PL backbone. Another study by (Rawls, Martin, & Maupin-Furlow, 2011) investigated the oxidation of G3P to DHAP by G3PDH homologs in haloarchaea. Archaea of the Halobacteria class were commonly found to encode homologs of all three subunits of the anaerobic G3PDH complex (GlpA, GlpB, and GlpC) of bacteria. Homologs of the G3PDH catalytic subunit A, GlpA, were also identified in archaea of the classes Thermoplasmata and Thermoprotei. H. volcanii has two putative G3PDH operons: (i) glpA1B1C1, on the main chromosome in synteny within glpK, and (ii) glpA2B2C2, on megaplasmid pHV4. Analysis of H. volcanii mutant strains revealed that glpA1 alone is necessary for growth on glycerol. However, when glpA1 or glpA2 were ectopically expressed from a strong promoter, they alone could complement the glpA1 mutant for growth on glycerol and could restore the G3PDH activity of cell lysates. The genes encoding a putative glycerol facilitator (glpF) and a gene homolog to bacterial phosphotransferase protein Hpr (ptsH2) were found to be transcriptionally linked and expressed under a G3P-inducible promoter in an operon with the glpA1B1C1 (GPDH) genes. This study has provided the knowledge on the genetic control and metabolism of glycerol in H. volcanii. Based on analysis of 32 haloarchaeal genome sequences, (T. A. Williams et al., 2017) predicted at least 27 have genomic potential to utilize glycerol as a source of carbon and energy. These genomes encode glycerol kinase (GlpK), at least one form of G3PDH (GlpABC) and a putative glycerol facilitator (GlpF), thus, suggesting a complete pathway for the conversion of glycerol to DHAP.
PHA synthesis/degradation
PHAs (polyesters of hydroxy fatty acids) have gained global attention due to their biodegradability, biological, biobased biocompatible alternative to petrochemicals plastics, with melting temperature from 40 to 180 °C (Cornibert & Marchessault, 1972; Koller, Maršálek, de Sousa Dias, & Braunegg, 2017; Kourmentza et al., 2017). Certain haloarchaea, when cultured under unbalanced growth conditions (excess carbon and depletion of other nutrients) (Alsafadi, Al-Mashaqbeh, Mansour, & Alsaad, 2020) store carbon, energy, and reducing power in the form of accumulated PHAs that are synthesized as insoluble inclusions intracellularly (Grage et al., 2009). PHAs are classified into two distinct groups based on the type of monomeric units used to form the polymer, such as short-chain length PHA units (3–5 carbon atoms) and medium chain length PHA units (6–14 carbon atoms) (Meng et al., 2014). The β-ketothiolases and PHA synthases are central to PHA biosynthesis and extensively studied in bacteria (Slater et al., 1998; Zou, Shi, Zhang, Li, & Xian, 2017). Haloarchaea are identified to accumulate PHA including organisms of the genera Haloferax, Haloarcula, Natrialba, Haloterrigena, Halococcus, Haloquadratum, Halorubrum, Natronobacterium, Natronococcus, and Halobacterium (Poli, Di Donato, Abbamondi, & Nicolaus, 2011) (Table 4).
Table 4.
Haloarchaeal with polyhydroxyalkanoate (PHA)-accumulating ability.
| Haloarchaea | Carbon source | PHA typea | PHA content (wt%) | Ref. |
|---|---|---|---|---|
| Haloterrigena hispanica | Glucose | PHBV | 2.26% (wt) | (Romano et al., 2007) |
| Haloarcula marismortui | Pre-treated vinasse | PHB | 30% (wt) | (Kirk & Ginzburg, 1972) |
| Halogeometricum borinquense | Sugarcane bagasse hydrolysate | PHBV | 45.7% (wt) | (Salgaonkar, 2015) |
| Halogranum amylolyticum | Glucose | PHBV | 26.1% (wt) | (Liu et al., 2015) |
| Natrinema ajinwuensis | Glucose | PHBV | 61.02% (wt) | (Mahansaria, Dhara, Saha, Haldar, & Mukherjee, 2018) |
| Haloferax mediterranei | Glucose | PHBV | 16.4% (wt) | (Han et al., 2010) |
PHBV, poly(3-hydroxybutyrate-co-3-hydroxyvalerate); PHB, polyhydroxybutyrate.
wt, (wet weight)
Producing PHAs using haloarchaea has certain advantages, as these microorganisms have simple growth requirements and grow in high salt concentrations, which minimizes contamination and the need for sterilization (Alsafadi & Al-Mashaqbeh, 2017; Don, Chen, & Chan, 2006). Moreover, the biopolymers synthesized by haloarchaea are extracted by simply exposing the cells to osmotic shock (reduced salt concentration), thus, overcoming the need for solvent extraction. Using different carbon sources and fermentation techniques, numerous haloarchaea strains have been identified that produce PHAs including those of the polyhydroxybutyrate (PHB) type. The two most studied genera of haloarchaea for PHA production are Haloarcula and Haloferax. Haloarcula sp. IRU1 has proven to be the most productive strain, for obtaining PHB as biopolymer with a PHB accumulation of 63 % (w/w) of cell dry weight (CDW) in comparison to H. marismortui and H. hispanica, with yields of 21 and 2.4 % (w/w) CDW, respectively with glucose as carbon source (Han, Lu, Zhou, Zhou, & Xiang, 2007; Taran & Amirkhani, 2010). H. mediterranei is proposed as a model system for investigation of the PHA metabolism in haloarchaea due to its clear and well-established genetic background (Zhao et al., 2013). H. mediterranei accumulates PHAs of the PHBV-type that contain 3-hydroxyvalerate (3HV) monomeric units when grown on medium supplemented with different substrates as the carbon source (Table 5). Using H. mediterranei as a model system, the PHA synthase (PhaEC) and acetoacetyl-CoA reductases (PhaB1 and PhaB2) for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) biosynthesis (Cai et al., 2012) and the PHA granule-associated phasin protein (PhaP) and regulatory protein (PhaR) for PHA accumulation and granule formation (Han et al., 2007) have been identified. Likewise, a novel PHA depolymerase (PhaZh1, a patatin-like protein) on the PHA granules has been identified that has no relationship to the bacterial enzyme (Liu et al., 2015). The PhaZh1 function has been determined through gene knockout and complementation, protein activity assays, and site-directed mutagenesis analysis. The results reveal that PhaZh1 hydrolyzes the primary product of the native PHBV granule into 3-hydroxybutyrate (3HB) monomers, which are subsequently converted into acetoacetate by a 3HB dehydrogenase homolog (BdhA) (Liu et al., 2015). PhaZh1 may provide a novel strategy for synthesizing the chiral compound (R)-3HB from PHB accumulated in haloarchaea due to its efficient activity in native PHA hydrolysis. Regarding Haloferax spp., H. volcanii and H. gibbonsii also produce PHB as a biopolymer with yields of 7 and 1.2% (w/w) CDW in the presence of glucose and yeast extract as carbon and nitrogen sources (Fernandez-Castillo, Rodriguez-Valera, Gonzalez-Ramos, & Ruiz-Berraquero, 1986).
Table 5.
Haloferax mediterranei with PHA-accumulating ability using different substrates.
| Carbon source | Product* | Yield | Ref. |
|---|---|---|---|
| Extruded starch | P(3HB-co-10.4 mol% 3HV) | 50.8% (wt) | (Don et al., 2006) |
| 25% pre-treated vinasse | P(3HB-co-12.36 mol% 3HV) | 70% (wt) | (Bhattacharyya et al., 2012) |
| 50% pre-treated vinasse | P(3HB-co-14.09 mol% 3HV) | 66% (wt) | (Bhattacharyya et al., 2012) |
| Olive mill wastewater | P(3HB-co-6.5 mol% 3HV) | 43% (wt) | (Alsafadi & Al-Mashaqbeh, 2017) |
| Enzymatic hydrolysate of cheese whey | P(3HB-co-6 mol% 3HV) | 72.8% (wt) | (Koller et al., 2007) |
| Chemical hydrolysate of cheese whey | P(3HB-co-1.5 mol% 3HV) | 53% (wt) | (Pais, Serafim, Freitas, & Reis, 2016) |
| Waste stillage | P(3HB-co-15.4 mol% 3HV) | 71% (wt) | (Bhattacharyya et al., 2014) |
| Waste stillage | P(3HB-co-17.9 mol% 3HV) | 63% (wt) | (Bhattacharyya et al., 2015) |
| Crude glycerol | P(3HB-co-10 mol% 3HV) | 76% (wt) | (Hermann-Krauss et al., 2013) |
| Ulva sp. hydrolysate | P(3HB-co-8 mol% 3HV) | 55% (wt) | (Ghosh, 2019) |
| Whey sugars, sodium valerate and γ-butyrolactone | P(3HB-co-21.8 mol% 3HVco-5.1 mo% 4HB) | 87.5% (wt) | (Koller et al., 2007) |
| Date waste | PHBV (3 HV- 18.0 mol%) | 25% (wt) | (Alsafadi, Ibrahim, Alamry, Hussein, & Mansour, 2020) |
P(3HB-co-3HV)- (poly(3-hydroxybutyrate-co-3-hydroxyvalerate)) PHB with inserting hydroxyvalerate units (HV) between polymeric chain and obtained as a co-polymer.
Generation of PHA from olive mill wastewater
The major olive oil producing countries (Spain, Italy, and Greece) generate an abundance of olive-mill wastes (OMWs) in a short time period leading to a huge environmental problem for the Mediterranean basin. OMWs are characterized by acidic pH, high salt concentration, unpleasant odor and color, high chemical oxygen demand (COD) values, and high polyphenolic content. OMWs are significant sources of environmental pollution, due to the elevated organic load, salt and polyphenols, and are found to create reducing conditions that affect microbial diversity by altering the soil characteristics (Ben Sassi, Boularbah, Jaouad, Walker, & Boussaid, 2006). Inhibition of seed germination and early plant growth is also observed (Saez, 1992). In contrast, phenolics of OMWs if properly separated and modified may be used as natural antioxidants and disinfectants in food and chemical industries (Ntougias, Bourtzis, & Tsiamis, 2013; Yangui, 2009). Moreover, the organic and inorganic nutrients of OMWs, if properly treated, may be recycled or used as compost or substrates for cultivating edible fungi as well as for biopolymer and biogas production (Ntougias, Zervakis, Ehaliotis, Kavroulakis, & Papadopoulou, 2006; Ouzounidou, Zervakis, & Gaitis, 2010). Hence, formulating different biodegradation options and strategies for valorization and for the effective management of OMWs are important and needing solutions.
As OMW is high in salt content, haloarchaea may serve as a useful resource for mitigating and converting this waste into higher value bioproducts. In pursuit of this goal, H. mediterranei was shown to use OMW as a sole carbon source to generate PHAs by a one-stage cultivation step (Alsafadi & Al-Mashaqbeh, 2017). H. mediterranei grew and tolerated the inhibitory effect of the polyphenols present in the cultivation medium containing 25% OMW. Optimization of growth conditions revealed a PHA polymer yield of 0.2 g/L and content of 43% PHA/cell dry mass could be achieved from 15% OMW when H. mediterranei was grown at 37°C and 170 rpm (rotary shaking) with 22 % NaCl. However, this PHA yield was less than the 0.35 g/L obtained when OMW was anaerobically fermented by high storage capacity mixed cultures, enriched under feast and famine conditions (Dionisi et al., 2005). Analysis of the PHA extracted from H. mediterranei (Alsafadi & Al-Mashaqbeh, 2017) revealed it was a co-polyester of poly(3-hydroxybutyrate-co-3-hydroxyvalerate)(PHBHV) containing 6.5 mol% 3-hydroxyvalerate (3HV). In comparison to the homopolymeric PHB, which is brittle and has a melting temperature close to its degradation temperature making it difficult to process, the PHBHV copolymer is more flexible and has a lower melting temperature that broadens the window for processing the polymer without degrading the material (Alavi et al., 2014). Thus, the physiochemical properties of PHBHV are compatible with the processing conditions typically used in large-scale plastic production making H. mediterranei a promising biocatalyst this industry.
Carotenoids produced by haloarchaea
Carotenoids are isoprenoid pigments synthesized by bacteria, plants, algae, yeasts and archaea including the haloarchaea (Mata-Gómez, Montañez, Méndez-Zavala, & Aguilar, 2014). Carotenoid production from haloarchaea has been reported since the 1960s (Kelly & Jensen, 1967; Schwieter, Rüegg, & Isler, 1966). C50 carotenoids, such as bacterioruberin and its precursors 2-isopentenyl-3,4-dehydrorhodopin (IDR), bis-anhydrobacterioruberin (BABR), and mono-anhydrobacterioruberin (MABR), are the main carotenoids synthetized by haloarchaea (Abbes et al., 2013; Giani, Garbayo, Vílchez, & Martínez-Espinosa, 2019). Through gene deletion analysis of the haloarchaeon H. japonica JCM 7785T, several genes were identified to be required for the conversion of lycopene to bacterioruberin (Yang et al., 2015). Bacterioruberin is generated from lycopene by the addition of prenyl groups at each end of the lycopene chain along with the addition of hydroxyl groups (Pfander, 1994). Thus, a hypothetical metabolic map of carotenogenesis was reconstructed in Haloferax spp. through comparative genomics (Giani, Miralles-Robledillo, Peiró, Pire, & Martínez-Espinosa, 2020). Overall, haloarchaea are predicted to use an alternative mevalonic acid (MVA) pathway to produce isopentenyl pyrophosphate (IPP) as the carotenoid precursor. IPP is further modified to lycopene, which serves as an apparent branch point for the production of β-carotene, bacterioruberin and other related bioproducts. Exclusive reviews summarize the effect of different culture conditions on carotenoid production by haloarchaea as well as their applications in biotechnology and biomedicine (Calegari-Santos, Diogo, Fontana, & Bonfim, 2016; Giani et al., 2019).
High value products such as antibiotics
Halophiles are promising candidates for the generation of high value products like antimicrobials, pigments, compatible solutes, and lipids that may be applied to industries associated with pharmaceuticals, laundry, food coloring, food formulation and anti-adhesives (Litchfield, 2011; Tango & Islam, 2002). Haloarchaea produce peptide/protein-based antibiotics named halocins that are secreted into the environment to kill or inhibit other microorganisms in the same niche, much like the bacterial produced bacteriocins (Rodriguez-Valera, Juez, & Kushner, 1982; C. Sun et al., 2005). Although the variety of halocins seems extensive, little attention has been paid to these peptidic antibiotics and few of them are studied in detail (Atanasova, Roine, Oren, Bamford, & Oksanen, 2012; Litchfield, 2011). Halocins are active at high temperature and NaCl concentration making them attractive candidates for applications that require high peptide stability (Besse, Peduzzi, Rebuffat, & Carré-Mlouka, 2015; Shand & Leyva, 2008). Rod-shaped haloarchaea commonly produce halocins and the effects can be wide reaching in terms of the species that are sensitive to the halocin (Quadri et al., 2016; Torreblanca, Meseguer, & Ventosa, 1994). For example, halocins produced by the rod-shaped Natrinema spp. impair growth of a diversity of haloarchaea including those from the genera Halorubrum, Halobacterium, Haloarcula, Haloferax, Natronobacterium, and Natronomonas (Imadalou-Idres et al., 2013; Karthikeyan, Bhat, & Chandrasekaran, 2013; Y. Li, Xiang, Liu, Zhou, & Tan, 2003).
At least 11 types of halocins are produced (HalHA1, HalHA3, HalA4, HalH1, HalH4, HalH6/H7, HalR1, HalSech7A, HalSH10, HalS8, and HalC8) with many differing in the type molecular mechanism used to inhibit or impair growth (Besse et al., 2015; Karthikeyan et al., 2013; Kumar, Saxena, & Tiwari, 2016; Kumar & Tiwari, 2017; Y. Li et al., 2003; Mazguene et al., 2018; O’Connor & Shand, 2002; Pasić, Velikonja, & Ulrih, 2008). One of the more extensively characterized halocins is C8 is purified and characterized from Natrinema sp. strain AS7092 (formerly Halobacterium sp. strain AS7092) (Y. Li et al., 2003; Mei et al., 2008). Halocin C8 exhibits strong antimicrobial activity and inhibits the growth of at least 16 haloarchaeal strains (Y. Li et al., 2003). The halC8 gene encodes a 283-amino acid preproprotein (ProC8) that is processed into two functional peptides: the C-terminal peptide antibiotic HalC8 (76 amino acids) and the N-terminal immunity protein HalI (Mei et al., 2008; C. Sun et al., 2005). Having a single gene encode the peptide antibiotic and its immunity protein differs from other systems like bacteriocins and their associated immunity protein that are encoded by separate genes (Siegers & Entian, 1995). Regarding HalI, this immunity protein appears to orient itself to the outside of the host cell membrane and bind HalC8, thus, sequestering and preventing HalC8 from its antimicrobial activity (C. Sun et al., 2005). In sensitive neighboring strains, HalC8 (similarly to H4) is found to alter cells from rods into spheres, ultimately leading to cellular lysis (Y. Li et al., 2003; Meseguer & Rodriguez-Valera, 1986). Other mechanisms include that of HalH6 produced by Haloferax gibbonsii that inhibits Na+/H+ antiport in H. salinarum (Meseguer, Torreblanca, & Konishi, 1995). Surprisingly, HalH4 secreted by Haloferax mediterranei appears to benefit the producer by promoting DNA uptake by these cells (S. Chen, Sun, Korfanty, Liu, & Xiang, 2019).
Clearly, future research will be focused on understanding the diversity of molecular mechanisms of halocin production and function, as well as the identification of the growth factors responsive for halocin gene expression, maturation, and their target molecules. However, the application potential is widespread from using halocins as Na+/H+ antiporter inhibitors in human medicine (Lequerica et al., 2006; Such et al., 1998) to preventing microbial growth while curing hides in the leather industry (Birbir et al., 2004).
Haloarchaeal enzymes as high value products
The next generation biorefineries are aimed at managing the production of biofuels with as many added-value products as technically and economically feasible (Escamilla‐Alvarado, Pérez‐Pimienta, Ponce‐Noyola, & Poggi‐Varaldo, 2017). Integrative approaches to the biofuel industry that include enzyme and non-fuel based chemicals in the product portfolio are proposed to provide a solid workflow to withstand fluctuating oil prices and other market conditions. Both the extracellular and intracellular enzymes of haloarchaea are active and stable at high salinity conditions which are adverse environments generally to other enzymes (Torregrosa-Crespo, Galiana, & Martínez-Espinosa, 2017). Enzymes with these types of extreme properties can add high value to bioprocessing systems.
One such example of a haloarchaeal enzyme that fills a niche where enzymes from other sources could not withstand the concentration of organic solvents needed for certain biotechnology applications is the enzyme inorganic pyrophosphatase (PPA) (EC 3.6.1.1). PPA catalyzes the hydrolysis of the phosphor anhydride bond of inorganic pyrophosphate (PPi; P2O74−) to form two orthophosphates (Pi; PO43−) (Heinonen, 2001; Lahti, 1983). PPi is a common by-product of biochemical reactions like biosynthesis of nucleic acids, protein and post-translational modifications (adenylation, uridylation and ubiquitylation) lipids, cellulose, starch, peptidoglycan synthesis. The hydrolysis of PPi by PPAs releases a considerable amount of energy, which is used for completion of the unfavorable biochemical transformations. During in vitro transcription, PPAs are used to remove PPi contaminant, increase the yield of nucleic acid synthesis in DNA sequencing (Tabor & Richardson, 1990; Vander Horn et al., 1997), polymerase chain reaction (Park et al., 2010), single-base extension (SBE) reactions (Xiao et al., 2004). PPAs also facilitate the enzymatic synthesis of guanosine 5′-diphosphate (GDP)-sugars and derivatives (L. Li et al., 2013). PPAs are also used to measure the kinetics of reactions (SNP genotyping, RNA synthesis by viral RNA-dependent RNA polymerases and aminoacyl-tRNA synthetase activity) that release PPi as a by-product, as PPA can convert this to 2Pi which is readily detected by a colorimetric assay (Geladopoulos, Sotiroudis, & Evangelopoulos, 1991; Itaya & Ui, 1966). Most PPAs are inactive at high salt concentrations and organic solvents, thereby limiting their usage. PPAs that function in a wide range of organic solvents and salt concentrations are desirable in bioindustry to increase the solubility of hydrophobic substrates, which has advantages towards synthetic chemistry, to alter substrate specificity, ease product recovery, and reduce microbial contamination (Grazinoli-Garrido & Sola-Penna, 2004).
Haloarchaea produce PPAs that are thermostable and organic solvent tolerant. (McMillan et al., 2016) reported a class A type PPA purified from H. volcanii (HvPPA) that has a robust PPi -hydrolyzing activity in organic solvent of 25% (vol/vol) and salt concentrations of 25 mM to 3 M. HvPPA was readily purified by a rapid two-step method as a 134 kDa homohexamer. The enzyme displayed non-Michaelis-Menten kinetics with a Vmax of 465 U‧mg−1 for PPi hydrolysis (optimal at 42°C and pH 8.5) and Hill coefficients indicative of cooperative binding to PPi and Mg2+. HvPPA was inhibited by sodium fluoride like other class A PPAs, but hierarchical clustering and 3D homology modeling studies showed HvPPA to be distinct in structure and harbor a high concentration of acidic residues on its surface. In application, HvPPA was demonstrated to hydrolyze the PPi by-product generated during the ATP-dependent E1 mediated adenylation of a ubiquitin-like protein that required 2 M NaCl for activity, which had been an obstacle for the previous PPA-based technologies.
Conclusion
The excessive utilization of fossil fuels and their negative consequences such as global warming and environmental pollutions have spurred the need for renewable biofuels and chemicals. Lignocellulosic biomass represents the most abundant carbon-based source of energy. Woody biomass, agricultural residues, and perennial grasses have emerged as potential feedstocks of choice, as they do not compete with the food crops. The production of fuels, biomaterials, and other value-added products from biomass will lower our dependence on fossil fuels and reduce greenhouse gas emissions. Using microbes for the conversion of biomass to biofuels has facilitated the development of industrial strategies that are economically viable. As the processes used to depolymerize the biomass and release the sugars for microbial conversion to bioproducts are carried out in the presence of harsh environments, researchers have focused on extremophiles. Extremophiles and their enzymes are regarded as good choices for biofuel production and other biotechnological applications that require the biocatalyst to be active and/or stable in conditions of high temperature, high salt, organic solvent, and/or extreme pH (acidic or alkali). Haloarchaea are salt-loving extremophiles that inhabit hypersaline environments including soda (alkali) lakes. These microorganisms are attractive for biotechnological and industrial applications, as they produce biomolecules and enzymes, such as cellulases, amylases, lipases, laccases and chitinases, which are active in the presence of high salt concentrations and other extreme conditions. Many haloarchaea and their potential applications in biomass degradation for the consequent production of biofuels, bioproducts from industrial waste have been investigated. The expanding biofuel industries has initiated dumping huge surplus of glycerol-derived waste, which has raised environmental concerns regarding its disposal or biotransformation. However, researchers have developed synthetic biosystems consisting of three enzymatic cascades for the biotransformation of glycerol into valuable chemicals of which haloarchaeal biocatalysts may contribute. Moreover, microbial synthesis of biodegradable plastics to address the global environmental threat due to extensive plastic usage has been explored in haloarchaea that synthesize PHA.
Acknowledgements
This work was supported in part by funds awarded to LK by the Bioenergy-Awards for Cutting Edge Research (B-Acer) Fellowship sponsored by Indo-US Science & Technology Forum, New Delhi, India. The work was also supported by funds awarded to JM-F by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences, Physical Biosciences Program [grant number DOE DE-FG02-05ER15650] to advance applications of archaea in bioenergy as well as the National Institutes of Health [grant number NIH R01 GM057498] to improve human health through study of archaea.
List of Abbreviations
- ABTS
2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)
- AmyA
α-amylase
- BABR
bis-anhydrobacterioruberin
- CBH
cellobiohydrolase
- CESA
cellulose synthase
- CGTases
Cyclodextrin glycosyltransferases
- CMC
carboxymethylcellulose
- CSL
cellulose synthase like - CSL
- DH
dihydroxyacetone
- DHAP
dihydroxyacetone phosphate
- DMP
dimethoxyphenol
- ETA
ethanolamine
- G3P
glycerol-3-phosphate
- G3PDH
G3P dehydrogenase
- GEs
glucuronoyl esterases
- GH
glycoside hydrolase
- 3HB
3-hydroxybutyrate
- IDR
2-isopentenyl-3,4-dehydrorhodopin
- IPP
isopentenyl pyrophosphate
- KDX
2-keto-3-deoxy-D-xylonate
- KGSADH
ketoglutarate semialdehyde dehydrogenase
- L-AraDH
L-arabinose dehydrogenase
- LccA
laccase
- LCCs
lignin–carbohydrate complexes
- MABR
mono-anhydrobacterioruberin
- MVA
mevalonic acid
- PHAs
polyhydroxyalkanoates
- PHBV
poly(3-hydroxybutyrate-co-3-hydroxyvalerate)
- PL
phospholipid
- pNP
p-nitrophenyl
- pNPB
p-nitrophenol butyrate
- pNPG
p-nitrophenyl-β-D-glucopyranoside
- pNPL
p-nitrophenyl laurate
- pNPM
p-nitrophenyl myristate
- pNPP
p-nitrophenol palmitate
- pNPV
p-nitrophenyl valerate
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- SGZ
syringaldazine
- SmF
submerged fermentation
- SSF
solid-state fermentation
- TC4
tributyrin
- TC8
trioctanoin
- XAD
xylonate dehydratase
- XDH
xylose dehydrogenase
Footnotes
CONFLICT OF INTEREST
The authors do not have a conflict of interest to declare.
References
- Abbes M, Baati H, Guermazi S, Messina C, Santulli A, Gharsallah N, & Ammar E (2013). Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complement Altern Med, 13, 255. doi: 10.1186/1472-6882-13-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi S, Thomas S, Sandeep KP, Kalarikkal N, Varghese J, & Yaragalla S (2014). Polymers for Packaging Applications (1st ed.). New York: Apple Academic Press. [Google Scholar]
- Alsafadi D, & Al-Mashaqbeh O (2017). A one-stage cultivation process for the production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) from olive mill wastewater by Haloferax mediterranei. N Biotechnol, 34, 47–53. doi: 10.1016/j.nbt.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Alsafadi D, Al-Mashaqbeh O, Mansour A, & Alsaad M (2020). Optimization of nitrogen source supply for enhanced biosynthesis and quality of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by extremely halophilic archaeon Haloferax mediterranei. MicrobiologyOpen, 9(8), e1055. doi: 10.1002/mbo3.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsafadi D, Ibrahim MI, Alamry KA, Hussein MA, & Mansour A (2020). Utilizing the crop waste of date palm fruit to biosynthesize polyhydroxyalkanoate bioplastics with favorable properties. Sci Total Environ, 737, 139716. doi: 10.1016/j.scitotenv.2020.139716 [DOI] [PubMed] [Google Scholar]
- Alsafadi D, Khalili FI, Juwhari H, & Lahlouh B (2018). Purification and biochemical characterization of photo-active membrane protein bacteriorhodopsin from Haloarcula marismortui, an extreme halophile from the Dead Sea. Int J Biol Macromol, 118(Pt B), 1942–1947. doi: 10.1016/j.ijbiomac.2018.07.045 [DOI] [PubMed] [Google Scholar]
- Alsafadi D, & Paradisi F (2013). Effect of organic solvents on the activity and stability of halophilic alcohol dehydrogenase (ADH2) from Haloferax volcanii. Extremophiles, 17(1), 115–122. doi: 10.1007/s00792-012-0498-0 [DOI] [PubMed] [Google Scholar]
- Ameri A, Shakibaie M, Faramarzi MA, Ameri A, Amirpour-Rostami S, Reza-Rahimi H, & Forootanfar H (2017). Thermoalkalophilic lipase from an extremely halophilic bacterial strain Bacillus atrophaeus FSHM2: Purification, biochemical characterization and application. Biocatal Biotransformation, 35(3), 151–160. [Google Scholar]
- Amoozegar MA, Siroosi M, Atashgahi S, Smidt H, & Ventosa A (2017). Systematics of haloarchaea and biotechnological potential of their hydrolytic enzymes. Microbiology, 163(5), 623–645. doi: 10.1099/mic.0.000463 [DOI] [PubMed] [Google Scholar]
- Anderson CM, DeFries RS, Litterman R, Matson PA, Nepstad DC, Pacala S, … Field CB (2019). Natural climate solutions are not enough. Science, 363(6430), 933–934. doi: 10.1126/science.aaw2741 [DOI] [PubMed] [Google Scholar]
- Anderson I, Scheuner C, Göker M, Mavromatis K, Hooper SD, Porat I, … Kyrpides N (2011). Novel insights into the diversity of catabolic metabolism from ten haloarchaeal genomes. PLoS One, 6(5), e20237. doi: 10.1371/journal.pone.0020237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade CM, Aguiar WB, & Antranikian G (2001). Physiological aspects involved in production of xylanolytic enzymes by deep-sea hyperthermophilic archaeon Pyrodictium abyssi. Appl Biochem Biotech, 91–93, 655–669. doi: 10.1385/abab:91-93:1-9:655 [DOI] [PubMed] [Google Scholar]
- Andrei A, Banciu HL, & Oren A (2012). Living with salt: metabolic and phylogenetic diversity of archaea inhabiting saline ecosystems. FEMS Microbiol Lett, 330(1), 1–9. doi: 10.1111/j.1574-6968.2012.02526.x [DOI] [PubMed] [Google Scholar]
- Atanasova NS, Roine E, Oren A, Bamford DH, & Oksanen HM (2012). Global network of specific virus-host interactions in hypersaline environments. Environ Microbiol, 14(2), 426–440. doi: 10.1111/j.1462-2920.2011.02603.x [DOI] [PubMed] [Google Scholar]
- Bai L, Greca LG, Xiang W, Lehtonen J, Huan S, Nugroho RWN, … Rojas OJ (2019). Adsorption and assembly of cellulosic and lignin colloids at oil/water interfaces. Langmuir, 35(3), 571–588. doi: 10.1021/acs.langmuir.8b01288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai B, Chaudhary M, & Saxena J (2015). Production and characterization of α-amylase from an extremely halophilic archaeon, Haloferax sp. HA10. Food Technol Biotechnol, 53(1), 11–17. doi: 10.17113/ftb.53.01.15.3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D, Casanueva A, Tuffin M, & Cowan D (2010). Extremophiles in biofuel synthesis. Environ Technol, 31(8–9), 871–888. doi: 10.1080/09593331003710236 [DOI] [PubMed] [Google Scholar]
- Barrios-Gonzalez. (2012). Solid-state fermentation: physiology of solid medium, its molecular basis and applications. Process Biochem, 47, 175–185. [Google Scholar]
- Bautista V, Esclapez J, Pérez-Pomares F, Martínez-Espinosa RM, Camacho M, & Bonete MJ (2012). Cyclodextrin glycosyltransferase: a key enzyme in the assimilation of starch by the halophilic archaeon Haloferax mediterranei. Extremophiles, 16(1), 147–159. doi: 10.1007/s00792-011-0414-z [DOI] [PubMed] [Google Scholar]
- Begemann MB, Mormile MR, Paul VG, & Vidt DJ (2011). Potential enhancement of biofuel production through enzymatic biomass degradation activity and biodiesel production by halophilic microorganisms In Ventosa A. OA, Ma Y (Berlin: Springer; ) (Ed.), Halophiles and Hypersaline Environments. Current Research and Future Trends (pp. 341–357). [Google Scholar]
- Ben Sassi A, Boularbah A, Jaouad A, Walker G, & Boussaid A (2006). A comparison of Olive oil Mill Wastewaters (OMW) from three different processes in Morocco. Process Biochem, 41(1), 74–78. doi: 10.1016/j.procbio.2005.03.074 [DOI] [Google Scholar]
- Bertoldo C, & Antranikian G (2002). Starch-hydrolyzing enzymes from thermophilic archaea and bacteria. Curr Opin Chem Biol, 6(2), 151–160. doi: 10.1016/s1367-5931(02)00311-3 [DOI] [PubMed] [Google Scholar]
- Besse A, Peduzzi J, Rebuffat S, & Carré-Mlouka A (2015). Antimicrobial peptides and proteins in the face of extremes: Lessons from archaeocins. Biochimie, 118, 344–355. doi: 10.1016/j.biochi.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Bhargav S, Panda BP, Ali M, & Javed S (2008). Solid-state fermentation: an overview. Chem Biochem Eng Q, 22, 49–70. [Google Scholar]
- Bhattacharyya A, Jana K, Haldar S, Bhowmic A, Mukhopadhyay UK, De S, & Mukherjee J (2015). Integration of poly-3-(hydroxybutyrate-co-hydroxyvalerate) production by Haloferax mediterranei through utilization of stillage from rice-based ethanol manufacture in India and its techno-economic analysis. World J Microb Biot, 31(5), 717–727. doi: 10.1007/s11274-015-1823-4 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya A, Pramanik A, Maji SK, Haldar S, Mukhopadhyay UK, & Mukherjee J (2012). Utilization of vinasse for production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei. AMB Express, 2(1), 34. doi: 10.1186/2191-0855-2-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A, Saha J, Haldar S, Bhowmic A, Mukhopadhyay UK, & Mukherjee J (2014). Production of poly-3-(hydroxybutyrate-co-hydroxyvalerate) by Haloferax mediterranei using rice-based ethanol stillage with simultaneous recovery and re-use of medium salts. Extremophiles, 18(2), 463–470. doi: 10.1007/s00792-013-0622-9 [DOI] [PubMed] [Google Scholar]
- Birbir M, Ogan A, Calli B, & Mertoglu B (2004). Enzyme characteristics of extremely halophilic archaeal community in Tuzkoy Salt Mine, Turkey. World J Microb Biot, 20, 613–621. [Google Scholar]
- Björkman. (1954). Isolation of lignin from finely divided wood with neutral solvents. Nature, 174, 1057–1058. [Google Scholar]
- Bornscheuer UT, Bessler C, Srinivas R, & Krishna SH (2002). Optimizing lipases and related enzymes for efficient application. Trends Biotechnol, 20(10), 433–437. doi: 10.1016/s0167-7799(02)02046-2 [DOI] [PubMed] [Google Scholar]
- Bradner J, Gillings M, & Nevalainen K (1999). Qualitative assessment of hydrolytic activities in Antarctic microfungi grown at different temperatures on solid media. World J Microb Biot, 15, 131–132. [Google Scholar]
- Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Medhurst A, … Fincher GB (2006). Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-β-D-glucans. Science, 311(5769), 1940–1942. doi: 10.1126/science.1122975 [DOI] [PubMed] [Google Scholar]
- Bustard MT, Burgess JG, Meeyoo V, & Wright PC (2000). Novel opportunities for marine hyperthermophiles in emerging biotechnology and engineering industries. J Chem Technol Biotechnol 75, 1095–1109. [Google Scholar]
- Cai S, Cai L, Liu H, Liu X, Han J, Zhou J, & Xiang H (2012). Identification of the haloarchaeal phasin (PhaP) that functions in polyhydroxyalkanoate accumulation and granule formation in Haloferax mediterranei. Appl Environ Microbiol, 78(6), 1946–1952. doi: 10.1128/AEM.07114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calegari-Santos R, Diogo RA, Fontana JD, & Bonfim TM (2016). Carotenoid production by halophilic archaea under different culture conditions. Curr Microbiol, 72(5), 641–651. doi: 10.1007/s00284-015-0974-8 [DOI] [PubMed] [Google Scholar]
- Camacho RM, Mateos JC, González-Reynoso O, Prado LA, & Córdova J (2009). Production and characterization of esterase and lipase from Haloarcula marismortui. J Ind Microbiol Biotechnol, 36(7), 901–909. doi: 10.1007/s10295-009-0568-1 [DOI] [PubMed] [Google Scholar]
- Camacho RM, Mateos-Díaz JC, Diaz-Montaño DM, González-Reynoso O, & Córdova J (2010). Carboxyl ester hydrolases production and growth of a halophilic archaeon, Halobacterium sp. NRC-1. Extremophiles, 14(1), 99–106. doi: 10.1007/s00792-009-0291-x [DOI] [PubMed] [Google Scholar]
- Carpita NC (2012). Progress in the biological synthesis of the plant cell wall: new ideas for improving biomass for bioenergy. Curr Opin Biotech, 23(3), 330–337. doi: 10.1016/j.copbio.2011.12.003 [DOI] [PubMed] [Google Scholar]
- Chen S, Sun S, Korfanty GA, Liu J, & Xiang H (2019). A halocin promotes DNA uptake in Haloferax mediterranei. Front Microbiol, 10, 1960. doi: 10.3389/fmicb.2019.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Black DS, & Reilly PJ (2016). Carboxylic ester hydrolases: Classification and database derived from their primary, secondary, and tertiary structures. Protein Sci, 25(11), 1942–1953. doi: 10.1002/pro.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornibert J, & Marchessault RH (1972). Physical properties of poly-β-hydroxybutyrate. IV. Conformational analysis and crystalline structure. J Mol Biol, 71(3), 735–756. doi: 10.1016/s0022-2836(72)80035-4 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (1997). Assembly and enlargement of the primary cell wall in plants. Annu Rev Cell Dev Bi, 13, 171–201. doi: 10.1146/annurev.cellbio.13.1.171 [DOI] [PubMed] [Google Scholar]
- Crowell EF, Gonneau M, Stierhof YD, Höfte H, & Vernhettes S (2010). Regulated trafficking of cellulose synthases. Curr Opin Plant Biol, 13(6), 700–705. doi: 10.1016/j.pbi.2010.07.005 [DOI] [PubMed] [Google Scholar]
- da Costa MS, Santos H, & Galinski EA (1998). An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv Biochem Eng Biotechnol, 61, 117–153. doi: 10.1007/BFb0102291 [DOI] [PubMed] [Google Scholar]
- DasSarma S, & DasSarma P (2015). Halophiles and their enzymes: negativity put to good use. Curr Opin Microbiol, 25, 120–126. doi: 10.1016/j.mib.2015.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Kelkar A, Baraniya D, Molaei A, Moulick A, Meena RS, & Formanek P (2017). Enzymatic degradation of lignin in soil: a review. Sustainability, 9(7), 1163. doi: 10.3390/su9071163 [DOI] [Google Scholar]
- de Lourdes Moreno M, Pérez D, García MT, & Mellado E (2013). Halophilic bacteria as a source of novel hydrolytic enzymes. Life (Basel), 3(1), 38–51. doi: 10.3390/life3010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker RF, & Richards GN (1976). Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem, 32, 277–352. doi: 10.1016/s0065-2318(08)60339-x [DOI] [PubMed] [Google Scholar]
- Desai C, Patel P, Markande AR, Kamala K, & Sivaperumal P (2020). Exploration of haloarchaea for their potential applications in food industry. Int. J. Environ. Sci. Technol doi: 10.1007/s13762-020-02773-2 [DOI] [Google Scholar]
- Dionisi D, Carucci G, Papini MP, Riccardi C, Majone M, & Carrasco F (2005). Olive oil mill effluents as a feedstock for production of biodegradable polymers. Water Res, 39(10), 2076–2084. doi: 10.1016/j.watres.2005.03.011 [DOI] [PubMed] [Google Scholar]
- Don TM, Chen CW, & Chan TH (2006). Preparation and characterization of poly(hydroxyalkanoate) from the fermentation of Haloferax mediterranei. J Biomater Sci Polym Ed, 17(12), 1425–1438. doi: 10.1163/156856206778937208 [DOI] [PubMed] [Google Scholar]
- Eichler J, & Maupin-Furlow J (2013). Post-translation modification in Archaea: lessons from Haloferax volcanii and other haloarchaea. FEMS Microbiol Rev, 37(4), 583–606. doi: 10.1111/1574-6976.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elevi Bardavid R, Khristo P, & Oren A (2008). Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds. Extremophiles, 12(1), 5–14. doi: 10.1007/s00792-006-0053-y [DOI] [PubMed] [Google Scholar]
- Elieh-Ali-Komi D, & Hamblin MR (2016). Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int J Adv Res (Indore), 4(3), 411–427. [PMC free article] [PubMed] [Google Scholar]
- Esakkiraj P, Antonyraj CB, Meleppat B, Ankaiah D, Ayyanna R, Ahamed SIB, & Arul V (2017). Molecular characterization and application of lipase from Bacillus sp. PU1 and investigation of structural changes based on pH and temperature using MD simulation. Int J Biol Macromol, 103, 47–56. doi: 10.1016/j.ijbiomac.2017.04.111 [DOI] [PubMed] [Google Scholar]
- Escamilla‐Alvarado C, Pérez‐Pimienta JA, Ponce‐Noyola T, & Poggi‐Varaldo HM (2017). An overview of the enzyme potential in bioenergy‐producing biorefineries. J. Chem. Technol. Biotechnol, 92, 906–924. [Google Scholar]
- Fernandez-Castillo R, Rodriguez-Valera F, Gonzalez-Ramos J, & Ruiz-Berraquero F (1986). Accumulation of poly (β-hydroxybutyrate) by halobacteria. Appl Environ Microbiol, 51(1), 214–216. doi: 10.1128/AEM.51.1.214-216.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, & Messner K (1992). Reducing troublesome pitch in pulp mills by lipolytic enzymes. TAPPI J, 75(2), 130–135. [Google Scholar]
- Fukuda H, Kondo A, & Noda H (2001). Biodiesel fuel production by transesterification of oils. J Biosci Bioeng, 92(5), 405–416. doi: 10.1263/jbb.92.405 [DOI] [PubMed] [Google Scholar]
- Fukushima T, Mizuki T, Echigo A, Inoue A, & Usami R (2005). Organic solvent tolerance of halophilic α-amylase from a haloarchaeon, Haloarcula sp. strain S-1. Extremophiles, 9(1), 85–89. doi: 10.1007/s00792-004-0423-2 [DOI] [PubMed] [Google Scholar]
- Galinski EA (1993). Compatible solutes of halophilic eubacteria: molecular principles, water-solute interaction, stress protection. Experientia, 49, 487–496 doi: 10.1007/BF01955150 [DOI] [Google Scholar]
- García-Fraga B, da Silva AF, López-Seijas J, & Sieiro C (2014). Functional expression and characterization of a chitinase from the marine archaeon Halobacterium salinarum CECT 395 in Escherichia coli. Appl Microbiol Biotechnol, 98(5), 2133–2143. doi: 10.1007/s00253-013-5124-2 [DOI] [PubMed] [Google Scholar]
- Geladopoulos TP, Sotiroudis TG, & Evangelopoulos AE (1991). A malachite green colorimetric assay for protein phosphatase activity. Anal Biochem, 192(1), 112–116. doi: 10.1016/0003-2697(91)90194-x [DOI] [PubMed] [Google Scholar]
- Geng X, & Li K (2002). Degradation of non-phenolic lignin by the white-rot fungus Pycnoporus cinnabarinus. Appl Microbiol Biot, 60, 342–346. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Gnaim R, Greiserman S, Fadeev L, Gozin M, Golberg A (2019). Macroalgal biomass subcritical hydrolysates for the production of polyhydroxyalkanoate (PHA) by Haloferax mediterranei. Bioresour Technol, 271, 166–173. doi: 10.1016/j.biortech.2018.09.108 [DOI] [PubMed] [Google Scholar]
- Giani M, Garbayo I, Vílchez C, & Martínez-Espinosa RM (2019). Haloarchaeal carotenoids: Healthy novel compounds from extreme environments. Mar Drugs, 17(9). doi: 10.3390/md17090524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani M, Miralles-Robledillo JM, Peiró G, Pire C, & Martínez-Espinosa RM (2020). Deciphering pathways for carotenogenesis in haloarchaea. Molecules, 25(5). doi: 10.3390/molecules25051197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis PP, Mark RE & Tang R (1969). Elastic stiffness of crystalline cellulose in the folded-chain solid state. J Mater Sci, 4, 1003–1007. [Google Scholar]
- Glass NL, Schmoll M, Cate JH, & Coradetti S (2013). Plant cell wall deconstruction by ascomycete fungi. Annu Rev Microbiol, 67, 477–498. doi: 10.1146/annurev-micro-092611-150044 [DOI] [PubMed] [Google Scholar]
- Graebin NG, Schöffer JN, Andrades D, Hertz PF, Ayub MA, & Rodrigues RC (2016). Immobilization of glycoside hydrolase families GH1, GH13, and GH70: State of the art and perspectives. Molecules, 21(8). doi: 10.3390/molecules21081074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grage K, Jahns AC, Parlane N, Palanisamy R, Rasiah IA, Atwood JA, & Rehm BH (2009). Bacterial polyhydroxyalkanoate granules: biogenesis, structure, and potential use as nano-/micro-beads in biotechnological and biomedical applications. Biomacromolecules, 10(4), 660–669. doi: 10.1021/bm801394s [DOI] [PubMed] [Google Scholar]
- Grazinoli-Garrido R, & Sola-Penna M (2004). Inactivation of yeast inorganic pyrophosphatase by organic solvents. An Acad Bras Cienc, 76(4), 699–705. doi: 10.1590/s0001-37652004000400006 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Arnillas E, Arellano M, Deive FJ, Rodríguez A, & Sanromán M (2017). Unravelling the suitability of biological induction for halophilic lipase production by Halomonas sp. LM1C cultures. Bioresour Technol, 239, 368–377. doi: 10.1016/j.biortech.2017.04.128 [DOI] [PubMed] [Google Scholar]
- Hama S, Noda H, & Kondo A (2018). How lipase technology contributes to evolution of biodiesel production using multiple feedstocks. Curr Opin Biot, 50, 57–64. doi: 10.1016/j.copbio.2017.11.001 [DOI] [PubMed] [Google Scholar]
- Hamed I, Özogul F, & Regenstein J (2016). Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci Technol, 48, 40–50. doi: 10.1016/j.tifs.2015.11.007 [DOI] [Google Scholar]
- Han J, Hou J, Liu H, Cai S, Feng B, Zhou J, & Xiang H (2010). Wide distribution among halophilic archaea of a novel polyhydroxyalkanoate synthase subtype with homology to bacterial type III synthases. Appl Environ Microbiol, 76(23), 7811–7819. doi: 10.1128/AEM.01117-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Lu Q, Zhou L, Zhou J, & Xiang H (2007). Molecular characterization of the phaECHm genes, required for biosynthesis of poly(3-hydroxybutyrate) in the extremely halophilic archaeon Haloarcula marismortui. Appl Environ Microbiol, 73(19), 6058–6065. doi: 10.1128/AEM.00953-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen JK (2001). Biological Role of Inorganic Pyrophosphate. Boston: Kluwer Academic Publishers. [Google Scholar]
- Hermann-Krauss C, Koller M, Muhr A, Fasl H, Stelzer F, & Braunegg G (2013). Archaeal production of polyhydroxyalkanoate (PHA) co- and terpolyesters from biodiesel industry-derived by-products. Archaea, 2013, 129268. doi: 10.1155/2013/129268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JL, Hammudi MB, & Tien M (2014). The Arabidopsis cellulose synthase complex: a proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell, 26(12), 4834–4842. doi: 10.1105/tpc.114.131193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda H, Kudo T, & Horikoshi K (1985). Molecular cloning and expression of the xylanase gene of alkalophilic Bacillus sp. strain C-125 in Escherichia coli. J Bacteriol, 161(2), 784–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Han J, Cai L, Zhou J, Lü Y, Jin C, … Xiang H (2014). Characterization of genes for chitin catabolism in Haloferax mediterranei. Appl Microbiol Biot, 98(3), 1185–1194. doi: 10.1007/s00253-013-4969-8 [DOI] [PubMed] [Google Scholar]
- Hutcheon GW, Vasisht N, & Bolhuis A (2005). Characterisation of a highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles, 9(6), 487–495. doi: 10.1007/s00792-005-0471-2 [DOI] [PubMed] [Google Scholar]
- Imadalou-Idres N, Carré-Mlouka A, Vandervennet M, Yahiaoui H, Peduzzi J, & Rebuffat S (2013). Diversity and antimicrobial activity of cultivable halophilic archaea from three Algerian sites. J Life Sci, 7(10), 1057–1069. [Google Scholar]
- Itaya K, & Ui M (1966). A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta, 14(3), 361–366. [DOI] [PubMed] [Google Scholar]
- Jensen JK, Busse-Wicher M, Poulsen CP, Fangel JU, Smith PJ, Yang JY, … Harholt J (2018). Identification of an algal xylan synthase indicates that there is functional orthology between algal and plant cell wall biosynthesis. New Phytol, 218(3), 1049–1060. doi: 10.1111/nph.15050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette JF, & Ziomek E (1994). Determination of lipase activity by a rhodamine-triglyceride-agarose assay. Anal Biochem, 219(2), 256–260. doi: 10.1006/abio.1994.1265 [DOI] [PubMed] [Google Scholar]
- Johnsen U, Dambeck M, Zaiss H, Fuhrer T, Soppa J, Sauer U, & Schönheit P (2009). D-xylose degradation pathway in the halophilic archaeon Haloferax volcanii. J Biol Chem, 284(40), 27290–27303. doi: 10.1074/jbc.M109.003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen U, Sutter JM, Zaiß H, & Schönheit P (2013). L-Arabinose degradation pathway in the haloarchaeon Haloferax volcanii involves a novel type of L-arabinose dehydrogenase. Extremophiles, 17(6), 897–909. doi: 10.1007/s00792-013-0572-2 [DOI] [PubMed] [Google Scholar]
- Kakhki AM, Amoozegar MA & Khaledi EM (2011). Diversity of hydrolytic enzymes in haloarchaeal strains isolated from salt lake.. Int. J. Environ. Sci. Technol, 8, 705–714. [Google Scholar]
- Kamekura M (1998). Diversity of extremely halophilic bacteria. Extremophiles, 2(3), 289–295. doi: 10.1007/s007920050071 [DOI] [PubMed] [Google Scholar]
- Karan R, Capes MD, DasSarma P, & DasSarma S (2013). Cloning, overexpression, purification, and characterization of a polyextremophilic β-galactosidase from the Antarctic haloarchaeon Halorubrum lacusprofundi. BMC Biotechnol, 13, 3. doi: 10.1186/1472-6750-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargi F, & Dinçer A (2000). Use of halophilic bacteria in biological treatment of saline wastewater by fed-batch operation. Water Environ Res, 72(2), 170–174. [Google Scholar]
- Karray F, Ben Abdallah M, Kallel N, Hamza M, Fakhfakh M, & Sayadi S (2018). Extracellular hydrolytic enzymes produced by halophilic bacteria and archaea isolated from hypersaline lake. Mol Biol Rep, 45(5), 1297–1309. doi: 10.1007/s11033-018-4286-5 [DOI] [PubMed] [Google Scholar]
- Karthikeyan P, Bhat SG, & Chandrasekaran M (2013). Halocin SH10 production by an extreme haloarchaeon Natrinema sp. BTSH10 isolated from salt pans of South India. Saudi J Biol Sci, 20(2), 205–212. doi: 10.1016/j.sjbs.2013.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazu T, Nakanishi Y, Uozumi N, Sasaki T, Yamagata H, Tsukagoshi N, & Udaka S (1987). Cloning and nucleotide sequence of the gene coding for enzymatically active fragments of the Bacillus polymyxa β-amylase. J Bacteriol, 169(4), 1564–1570. doi: 10.1128/jb.169.4.1564-1570.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M, & Jensen SL (1967). Bacterial carotenoids. XXVI. C50-carotenoids. 2. Bacterioruberin. Acta Chem Scand, 21(9), 2578–2580. doi: 10.3891/acta.chem.scand.21-2578 [DOI] [PubMed] [Google Scholar]
- Kim SJ, & Shoda M (1999). Purification and characterization of a novel peroxidase from Geotrichum candidum dec 1 involved in decolorization of dyes. Appl Environ Microbiol, 65(3), 1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk RG, & Ginzburg M (1972). Ultrastructure of two species of halobacterium. J Ultrastruct Res, 41(1), 80–94. doi: 10.1016/s0022-5320(72)90040-8 [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kanai H, Aono R, Horikoshi K, & Kudo T (1994). Cloning, expression, and nucleotide sequence of the α-amylase gene from the haloalkaliphilic archaeon Natronococcus sp. strain Ah-36. J Bacteriol, 176(16), 5131–5134. doi: 10.1128/jb.176.16.5131-5134.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller M, Hesse P, Bona R, Kutschera C, Atlić A, & Braunegg G (2007). Biosynthesis of high quality polyhydroxyalkanoate co‐ and terpolyesters for potential medical application by the archaeon Haloferax mediterranei. Macromol Symp, 253, 33–39. doi: 10.1002/masy.200750704 [DOI] [Google Scholar]
- Koller M, Maršálek L, de Sousa Dias MM, & Braunegg G (2017). Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. N Biotechnol, 37(Pt A), 24–38. doi: 10.1016/j.nbt.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Kourmentza C, Plácido J, Venetsaneas N, Burniol-Figols A, Varrone C, Gavala HN, & Reis MAM (2017). Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering (Basel), 4(2), 55. doi: 10.3390/bioengineering4020055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudanga T, Prasetyo EN, Sipilä J, Guebitz GM, & Nyanhongo GS (2010). Reactivity of long chain alkylamines to lignin moieties: implications on hydrophobicity of lignocellulose materials. J Biotechnol, 149(1–2), 81–87. doi: 10.1016/j.jbiotec.2010.06.020 [DOI] [PubMed] [Google Scholar]
- Kumar V, Saxena J, & Tiwari SK (2016). Description of a halocin-producing Haloferax larsenii HA1 isolated from Pachpadra salt lake in Rajasthan. Arch Microbiol, 198(2), 181–192. doi: 10.1007/s00203-015-1175-3 [DOI] [PubMed] [Google Scholar]
- Kumar V, & Tiwari SK (2017). Activity-guided separation and characterization of new halocin HA3 from fermented broth of Haloferax larsenii HA3. Extremophiles, 21(3), 609–621. doi: 10.1007/s00792-017-0930-6 [DOI] [PubMed] [Google Scholar]
- Kushner D (1978). Life in high salt and solute concentrations In Kushner D (Ed.), Microbial Life in Extreme Environments (pp. 317–368). London: Academic Press. [Google Scholar]
- Lahti R (1983). Microbial inorganic pyrophosphatases. Microbiol Rev, 47(2), 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS (2013). Diversity of halophilic archaea in fermented foods and human intestines and their application. J Microbiol Biotechn, 23(12), 1645–1653. doi: 10.4014/jmb.1308.08015 [DOI] [PubMed] [Google Scholar]
- Lequerica JL, O’Connor JE, Such L, Alberola A, Meseguer I, Dolz M, … Soria B (2006). A halocin acting on Na+/H+ exchanger of haloarchaea as a new type of inhibitor in NHE of mammals. J Physiol Biochem, 62(4), 253–262. doi: 10.1007/BF03165754 [DOI] [PubMed] [Google Scholar]
- Levisson M, van der Oost J, & Kengen SW (2009). Carboxylic ester hydrolases from hyperthermophiles. Extremophiles, 13(4), 567–581. doi: 10.1007/s00792-009-0260-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu Y, Wan Y, Li Y, Chen X, Zhao W, & Wang PG (2013). Efficient enzymatic synthesis of guanosine 5’-diphosphate-sugars and derivatives. Org Lett, 15(21), 5528–5530. doi: 10.1021/ol402585c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zheng Y, Chen Y, & Zhu X (2014). Biodiesel production from waste cooking oil using a heterogeneous catalyst from pyrolyzed rice husk. Bioresour Technol, 154, 345–348. doi: 10.1016/j.biortech.2013.12.070 [DOI] [PubMed] [Google Scholar]
- Li S, Lei L, & Gu Y (2013). Functional analysis of complexes with mixed primary and secondary cellulose synthases. Plant Signal Behav, 8(3), e23179. doi: 10.4161/psb.23179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang HL, Li T, & Yu HY (2012). Purification and characterization of an organic solvent-tolerant alkaline cellulase from a halophilic isolate of Thalassobacillus. Biotechnol Lett, 34(8), 1531–1536. doi: 10.1007/s10529-012-0938-z [DOI] [PubMed] [Google Scholar]
- Li X, & Yu HY (2013a). Characterization of a halostable endoglucanase with organic solvent-tolerant property from Haloarcula sp. G10. Int J Biol Macromol, 62, 101–106. doi: 10.1016/j.ijbiomac.2013.08.047 [DOI] [PubMed] [Google Scholar]
- Li X, & Yu HY (2013b). Halostable cellulase with organic solvent tolerance from Haloarcula sp. LLSG7 and its application in bioethanol fermentation using agricultural wastes. J Ind Microbiol Biotechnol, 40(12), 1357–1365. doi: 10.1007/s10295-013-1340-0 [DOI] [PubMed] [Google Scholar]
- Li X, & Yu HY (2014). Characterization of an organic solvent-tolerant lipase from Haloarcula sp. G41 and its application for biodiesel production. Folia Microbiol (Praha), 59(6), 455–463. doi: 10.1007/s12223-014-0320-8 [DOI] [PubMed] [Google Scholar]
- Li Y, Xiang H, Liu J, Zhou M, & Tan H (2003). Purification and biological characterization of halocin C8, a novel peptide antibiotic from Halobacterium strain AS7092. Extremophiles, 7(5), 401–407. doi: 10.1007/s00792-003-0335-6 [DOI] [PubMed] [Google Scholar]
- Liao W, Liu Y, Frear C, & Chen S (2008). Co-production of fumaric acid and chitin from a nitrogen-rich lignocellulosic material - dairy manure - using a pelletized filamentous fungus Rhizopus oryzae ATCC 20344. Bioresour Technol, 99(13), 5859–5866. doi: 10.1016/j.biortech.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Litchfield CD (2011). Potential for industrial products from the halophilic Archaea. J Ind Microbiol Biot, 38(10), 1635–1647. doi: 10.1007/s10295-011-1021-9 [DOI] [PubMed] [Google Scholar]
- Liu G, Hou J, Cai S, Zhao D, Cai L, Han J, … Xiang H (2015). A patatin-like protein associated with the polyhydroxyalkanoate (PHA) granules of Haloferax mediterranei acts as an efficient depolymerase in the degradation of native PHA. Appl Environ Microbiol, 81(9), 3029–3038. doi: 10.1128/AEM.04269-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein T, Schubert B, & Timofeeff M (2011). Microbial communities in fluid inclusions and long-term survival in halite. GSA Today, 1, 4–9. [Google Scholar]
- Läufer A (2019). Starch biorefinery enzymes. Adv Biochem Eng Biotechnol, 166, 137–152. doi: 10.1007/10_2016_60 [DOI] [PubMed] [Google Scholar]
- Lüthi E, Jasmat NB, & Bergquist PL (1990). Xylanase from the extremely thermophilic bacterium “Caldocellum saccharolyticum”: overexpression of the gene in Escherichia coli and characterization of the gene product. Appl Environ Microbiol, 56(9), 2677–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahansaria R, Dhara A, Saha A, Haldar S, & Mukherjee J (2018). Production enhancement and characterization of the polyhydroxyalkanoate produced by Natrinema ajinwuensis (as synonym) Natrinema altunense strain RM-G10. Int J Biol Macromol, 107(Pt B), 1480–1490. doi: 10.1016/j.ijbiomac.2017.10.009 [DOI] [PubMed] [Google Scholar]
- Malik AD, & Furtado IJ (2019). Haloferax sulfurifontis GUMFAZ2 producing xylanase-free cellulase retrieved from Haliclona sp. inhabiting rocky shore of Anjuna, Goa-India. J Basic Microbiol, 59(7), 692–700. doi: 10.1002/jobm.201800672 [DOI] [PubMed] [Google Scholar]
- Martin del Campo M, Camacho RM, Mateos-Díaz JC, Müller-Santos M, Córdova J, & Rodríguez JA (2015). Solid-state fermentation as a potential technique for esterase/lipase production by halophilic archaea. Extremophiles, 19(6), 1121–1132. doi: 10.1007/s00792-015-0784-8 [DOI] [PubMed] [Google Scholar]
- Mata-Gómez LC, Montañez JC, Méndez-Zavala A, & Aguilar CN (2014). Biotechnological production of carotenoids by yeasts: an overview. Microb Cell Fact, 13, 12. doi: 10.1186/1475-2859-13-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazguene S, Rossi M, Gogliettino M, Palmieri G, Cocca E, Mirino S, … Benallaoua S (2018). Isolation and characterization from solar salterns of North Algeria of a haloarchaeon producing a new halocin. Extremophiles, 22(2), 259–270. doi: 10.1007/s00792-017-0994-3 [DOI] [PubMed] [Google Scholar]
- McMillan LJ, Hepowit NL, & Maupin-Furlow JA (2016). Archaeal inorganic pyrophosphatase displays robust activity under high-salt conditions and in organic solvents. Appl Environ Microbiol, 82(2), 538–548. doi: 10.1128/AEM.03055-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S, Sun C, Liu X, Lu Q, Cai L, Li Y, & Xiang H (2008). The helix-loop-helix motif at the N terminus of HalI is essential for its immunity function against halocin C8. J Bacteriol, 190(19), 6501–6508. doi: 10.1128/JB.00665-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasria T, Aguilera M, Hocine H, Benammar L, Ayachi A, Si Bachir A, … Monteoliva-Sánchez M (2018). Diversity and bioprospecting of extremely halophilic archaea isolated from Algerian arid and semi-arid wetland ecosystems for halophilic-active hydrolytic enzymes. Microbiol Res, 207, 289–298. doi: 10.1016/j.micres.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Meng DC, Shen R, Yao H, Chen JC, Wu Q, & Chen GQ (2014). Engineering the diversity of polyesters. Curr Opin Biotech, 29, 24–33. doi: 10.1016/j.copbio.2014.02.013 [DOI] [PubMed] [Google Scholar]
- Mesbah NM, & Wiegel J (2017). A halophilic, alkali thermostable, ionic liquid-tolerant cellulase and its application in in situ saccharification of rice straw. Bioenergy Res, 10, 583–591. [Google Scholar]
- Meseguer I, & Rodriguez-Valera F (1986). Effect of halocin H4 on cells of Halobacterium halobium. Microbiology, 132(11), 3061–3068. doi: 10.1099/00221287-132-11-3061 [DOI] [Google Scholar]
- Meseguer I, Torreblanca M, & Konishi T (1995). Specific inhibition of the halobacterial Na+/H+ antiporter by halocin H6. J Biol Chem, 270(12), 6450–6455. doi: 10.1074/jbc.270.12.6450 [DOI] [PubMed] [Google Scholar]
- Mikolasch A, & Schauer F (2009). Fungal laccases as tools for the synthesis of new hybrid molecules and biomaterials. Appl Microbiol Biotechnol, 82(4), 605–624. doi: 10.1007/s00253-009-1869-z [DOI] [PubMed] [Google Scholar]
- Mitchell RA, Dupree P, & Shewry PR (2007). A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol, 144(1), 43–53. doi: 10.1104/pp.106.094995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris HP, Esser K (1970). The phenoloxidases of the ascomycete Podospora anserina. V. Properties of laccase I after further purification. Arch Mikrobiol, 72(3), 267–296. [PubMed] [Google Scholar]
- Moreno ML, Márquez MC, García MT, & Mellado E (2016). Halophilic bacteria and archaea as producers of lipolytic enzymes In Rampelotto P (Ed.), Biotechnology of Extremophiles: Grand Challenges in Biology and Biotechnology (Vol. 1): Springer, Cham. [Google Scholar]
- Moshfegh M, Shahverdi AR, Zarrini G, & Faramarzi MA (2013). Biochemical characterization of an extracellular polyextremophilic α-amylase from the halophilic archaeon Halorubrum xinjiangense. Extremophiles, 17(4), 677–687. doi: 10.1007/s00792-013-0551-7 [DOI] [PubMed] [Google Scholar]
- Mueller SC, & Brown RM (1980). Evidence for an intramembrane component associated with a cellulose microfibril-synthesizing complex in higher plants. J Cell Biol, 84(2), 315–326. doi: 10.1083/jcb.84.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahara H, Koyama Y, Yoshida T, Pichangkura S, Ueda R, & Yaguchi H (1982). Growth and enzyme production in a solid-culture of Aspergillus oryzae. J Ferment Technol, 60(4), 311–319. [Google Scholar]
- Nercessian D, Di Meglio L, De Castro R, & Paggi R (2015). Exploring the multiple biotechnological potential of halophilic microorganisms isolated from two Argentinean salterns. Extremophiles, 19(6), 1133–1143. doi: 10.1007/s00792-015-0785-7 [DOI] [PubMed] [Google Scholar]
- Nicol RW, Marchand K, & Lubitz WD (2012). Bioconversion of crude glycerol by fungi. Appl Microbiol Biot, 93(5), 1865–1875. doi: 10.1007/s00253-012-3921-7 [DOI] [PubMed] [Google Scholar]
- Ning Y, & Chen X (2015). Sustainability: Don’t waste seafood waste. Nature, 524, 155–157. [DOI] [PubMed] [Google Scholar]
- Ntougias S, Bourtzis K, & Tsiamis G (2013). The microbiology of olive mill wastes. Biomed Res Int, 2013, 784591. doi: 10.1155/2013/784591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntougias S, Zervakis GI, Ehaliotis C, Kavroulakis N, & Papadopoulou KK (2006). Ecophysiology and molecular phylogeny of bacteria isolated from alkaline two-phase olive mill wastes. Res Microbiol, 157(4), 376–385. doi: 10.1016/j.resmic.2005.09.010 [DOI] [PubMed] [Google Scholar]
- O’Connor EM, & Shand RF (2002). Halocins and sulfolobicins: the emerging story of archaeal protein and peptide antibiotics. J Ind Microbiol Biotechnol, 28(1), 23–31. doi: 10.1038/sj/jim/7000190 [DOI] [PubMed] [Google Scholar]
- Ogan A, Danis O, Gozuacik A, Cakmar E, & Birbir M (2012). Production of cellulase by immobilized whole cells of Haloarcula. Prikl Biokhim Mikrobiol, 48(5), 490–493. [PubMed] [Google Scholar]
- Oh YK, Hwang KR, Kim C, Kim JR, & Lee JS (2018). Recent developments and key barriers to advanced biofuels: A short review. Bioresour Technol, 257, 320–333. doi: 10.1016/j.biortech.2018.02.089 [DOI] [PubMed] [Google Scholar]
- Olofsson K, Bertilsson M, & Lidén G (2008). A short review on SSF - an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels, 1(1), 7. doi: 10.1186/1754-6834-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera M, Yatsunami R, Tsukimura W, Fukui T, Nakasone K, Takashina T, & Nakamura S (2013). Gene analysis, expression, and characterization of an intracellular α-amylase from the extremely halophilic archaeon Haloarcula japonica. Biosci Biotechnol Biochem, 77(2), 281–288. doi: 10.1271/bbb.120693 [DOI] [PubMed] [Google Scholar]
- Oren A (2008). Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Systems, 4, 2. doi: 10.1186/1746-1448-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A (2013). Life at high salt concentrations, intracellular KCl concentrations, and acidic proteomes. Front Microbiol, 4, 315. doi: 10.3389/fmicb.2013.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren A, Elevi R, Watanabe S, Ihara K, & Corcelli A (2002). Halomicrobium mukohataei gen. nov., comb. nov., and emended description of Halomicrobium mukohataei. Int J Syst Evol Microbiol, 52(Pt 5), 1831–1835. doi: 10.1099/00207713-52-5-1831 [DOI] [PubMed] [Google Scholar]
- Oren A, & Gurevich P (1994). Distribution of glycerol dehydrogenase and glycerol kinase activity in halophilic archaea. FEMS Microbiol Lett, 118(3), 311–315. doi: 10.1111/j.1574-6968.1994.tb06846.x [DOI] [Google Scholar]
- Osma JF, Toca-Herrera JL, & Rodríguez-Couto S (2010). Uses of laccases in the food industry. Enzyme Res, 2010, 918761. doi: 10.4061/2010/918761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouzounidou G, Zervakis GI, & Gaitis F (2010). Raw and microbiologically detoxified olive mill waste and their impact on plant growth. Terr Aquat Environ Toxicol, 4(1), 21–38. [Google Scholar]
- Ozcan B, Ozyilmaz G, Cokmus C, & Caliskan M (2009). Characterization of extracellular esterase and lipase activities from five halophilic archaeal strains. J Ind Microbiol Biot, 36(1), 105–110. doi: 10.1007/s10295-008-0477-8 [DOI] [PubMed] [Google Scholar]
- Paggi RA, Madrid EA, D’Alessandro CP, Cerletti M, & De Castro RE (2010). Growth phase-dependent biosynthesis of Nep, a halolysin-like protease secreted by the alkaliphilic haloarchaeon Natrialba magadii. Lett Appl Microbiol, 51(1), 36–41. doi: 10.1111/j.1472-765X.2010.02855.x [DOI] [PubMed] [Google Scholar]
- Pais J, Serafim LS, Freitas F, & Reis MA (2016). Conversion of cheese whey into poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Haloferax mediterranei. Nat Biotechnol, 33(1), 224–230. doi: 10.1016/j.nbt.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Panda T, & Gowrishankar BS (2005). Production and applications of esterases. Appl Microbiol Biot, 67(2), 160–169. doi: 10.1007/s00253-004-1840-y [DOI] [PubMed] [Google Scholar]
- Park SY, Lee B, Park KS, Chong Y, Yoon MY, Jeon SJ, & Kim DE (2010). Facilitation of polymerase chain reaction with thermostable inorganic pyrophosphatase from hyperthermophilic archaeon Pyrococcus horikoshii. Appl Microbiol Biotechnol, 85(3), 807–812. doi: 10.1007/s00253-009-2314-z [DOI] [PubMed] [Google Scholar]
- Pasić L, Velikonja BH, & Ulrih NP (2008). Optimization of the culture conditions for the production of a bacteriocin from halophilic archaeon Sech7a. Prep Biochem Biotechnol, 38(3), 229–245. doi: 10.1080/10826060802164637 [DOI] [PubMed] [Google Scholar]
- Pfander H (1994). C45- and C50-carotenoids. Pure & Appl. Chem, 66(10–11), 2369–2374. doi: 10.1351/pac199466102369 [DOI] [Google Scholar]
- Poli A, Di Donato P, Abbamondi GR, & Nicolaus B (2011). Synthesis, production, and biotechnological applications of exopolysaccharides and polyhydroxyalkanoates by archaea. Archaea, 2011, 693253. doi: 10.1155/2011/693253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Michel G, Hervé C, Domozych DS, Willats WG, Tuohy MG, … Stengel DB (2011). Evolution and diversity of plant cell walls: from algae to flowering plants. Annu Rev Plant Biol, 62, 567–590. doi: 10.1146/annurev-arplant-042110-103809 [DOI] [PubMed] [Google Scholar]
- Pérez-Pomares F, Bautista V, Ferrer J, Pire C, Marhuenda-Egea FC, & Bonete MJ (2003). α-Amylase activity from the halophilic archaeon Haloferax mediterranei. Extremophiles, 7(4), 299–306. doi: 10.1007/s00792-003-0327-6 [DOI] [PubMed] [Google Scholar]
- Quadri I, Hassani II, l’Haridon S, Chalopin M, Hacène H, & Jebbar M (2016). Characterization and antimicrobial potential of extremely halophilic archaea isolated from hypersaline environments of the Algerian Sahara. Microbiol Res, 186–187, 119–131. doi: 10.1016/j.micres.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Raghukumar C, D’Souza-Ticlo D, & Verma AK (2008). Treatment of colored effluents with lignin-degrading enzymes: an emerging role of marine-derived fungi. Crit Rev Microbiol, 34(3–4), 189–206. doi: 10.1080/10408410802526044 [DOI] [PubMed] [Google Scholar]
- Rampelotto PH (2013). Extremophiles and extreme environments. Life (Basel), 3(3), 482–485. doi: 10.3390/life3030482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat M, & Maupin-Furlow JA (2020). Redox and thiols in archaea. Antioxidants (Basel), 9(5), 381. doi: 10.3390/antiox9050381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls KS, Martin JH, & Maupin-Furlow JA (2011). Activity and transcriptional regulation of bacterial protein-like glycerol-3-phosphate dehydrogenase of the haloarchaea in Haloferax volcanii. J Bacteriol, 193(17), 4469–4476. doi: 10.1128/JB.00276-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert S, Bichet A, Grandjean O, Kierzkowski D, Satiat-Jeunemaître B, Pelletier S, … Vernhettes S (2005). An Arabidopsis endo-1,4-β-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell, 17(12), 3378–3389. doi: 10.1105/tpc.105.036228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Baños M, Garbayo I, Vílchez C, Bonete MJ, & Martínez-Espinosa RM (2015). Carotenoids from haloarchaea and their potential in biotechnology. Mar Drugs, 13(9), 5508–5532. doi: 10.3390/md13095508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Couto S SM (2006). Application of solid-state fermentation to food industry—a review. J Food Eng, 76, 291–302. [Google Scholar]
- Rodriguez-Valera F, Juez G, & Kushner DJ (1982). Halocins: salt-dependent bacteriocins produced by extremely halophilic rods. Can J Microbiol, 28(1), 151–154. doi: 10.1139/m82-019 [DOI] [Google Scholar]
- Romano I, Poli A, Finore I, Huertas FJ, Gambacorta A, Pelliccione S, … Nicolaus B (2007). Haloterrigena hispanica sp. nov., an extremely halophilic archaeon from Fuente de Piedra, southern Spain. Int J Syst Evol Microbiol, 57(Pt 7), 1499–1503. doi: 10.1099/ijs.0.64895-0 [DOI] [PubMed] [Google Scholar]
- Rothschild LJ, & Mancinelli RL (2001). Life in extreme environments. Nature, 409(6823), 1092–1101. doi: 10.1038/35059215 [DOI] [PubMed] [Google Scholar]
- Saez L, Perez J and Martinez J,. (1992). Low molecular weight phenolics attenuation during simulated treatment of wastewaters from olive oil mills in evaporation ponds. Water Res, 26, 1261–1266. [Google Scholar]
- Salgaonkar BB, Bragança JM (2015). Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Halogeometricum borinquense strain E3. Int J Biol Macromol, 78, 339–346. doi: 10.1016/j.ijbiomac.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Sandhu AP, Randhawa GS, & Dhugga KS (2009). Plant cell wall matrix polysaccharide biosynthesis. Mol Plant, 2(5), 840–850. doi: 10.1093/mp/ssp056 [DOI] [PubMed] [Google Scholar]
- Sandoval G, Rivera I, Barrera‐Rivera KA, & Martínez‐Richa A (2010). Biopolymer synthesis catalyzed by tailored lipases. Macromol Symp, 289, 135–139. doi: 10.1002/masy.200900016 [DOI] [Google Scholar]
- Santorelli M, Maurelli L, Pocsfalvi G, Fiume I, Squillaci G, La Cara F, … Morana A (2016). Isolation and characterisation of a novel α-amylase from the extreme haloarchaeon Haloterrigena turkmenica. Int J Biol Macromol, 92, 174–184. doi: 10.1016/j.ijbiomac.2016.07.001 [DOI] [PubMed] [Google Scholar]
- Scheller HV, & Ulvskov P (2010). Hemicelluloses. Annu Rev Plant Biol, 61, 263–289. doi: 10.1146/annurev-arplant-042809-112315 [DOI] [PubMed] [Google Scholar]
- Schwieter U, Rüegg R, & Isler O (1966). [Syntheses in the carotinoid series. 21. Synthesis of 2,2’-diketo-spirilloxanthin (P 518) and 2,2’-diketo-bacterioruberin]. Helv Chim Acta, 49(2), 992–996. doi: 10.1002/hlca.19660490221 [DOI] [PubMed] [Google Scholar]
- Shafiei M, Ziaee AA, & Amoozegar MA (2011). Purification and characterization of an organic-solvent-tolerant halophilic α-amylase from the moderately halophilic Nesterenkonia sp. strain F. J Ind Microbiol Biotechnol, 38(2), 275–281. doi: 10.1007/s10295-010-0770-1 [DOI] [PubMed] [Google Scholar]
- Shallom D, & Shoham Y (2003). Microbial hemicellulases. Curr Opin Microbiol, 6(3), 219–228. doi: 10.1016/s1369-5274(03)00056-0 [DOI] [PubMed] [Google Scholar]
- Shand RF, & Leyva KJ (2008). Archaeal antimicrobials: An undiscovered country In Blum P (Ed.), Archaea: New Models for Prokaryotic Biology. Norfolk, U.K.: Caister Academic Press. [Google Scholar]
- Sharma M, Soni R, Nazir A, Oberoi HS, & Chadha BS (2011). Evaluation of glycosyl hydrolases in the secretome of Aspergillus fumigatus and saccharification of alkali-treated rice straw. Appl Biochem Biotechnol, 163(5), 577–591. doi: 10.1007/s12010-010-9064-3 [DOI] [PubMed] [Google Scholar]
- Sherwood KE, Cano DJ, & Maupin-Furlow JA (2009). Glycerol-mediated repression of glucose metabolism and glycerol kinase as the sole route of glycerol catabolism in the haloarchaeon Haloferax volcanii. J Bacteriol, 191(13), 4307–4315. doi: 10.1128/JB.00131-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegers K, & Entian KD (1995). Genes involved in immunity to the lantibiotic nisin produced by Lactococcus lactis 6F3. Appl Environ Microbiol, 61(3), 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroosi M, Amoozegar MA, Khajeh K, Fazeli M, Rezaei MH (2014). Purification and characterization of a novel extracellular halophilic and organic solvent-tolerant amylopullulanase from the haloarchaeon, Halorubrum sp. strain Ha25. Extremophiles, 18(1), 25–33. doi: 10.1007/s00792-013-0589-6 [DOI] [PubMed] [Google Scholar]
- Sitarz AK, Mikkelsen JD, & Meyer AS (2016). Structure, functionality and tuning up of laccases for lignocellulose and other industrial applications. Crit Rev Biotechnol, 36(1), 70–86. doi: 10.3109/07388551.2014.949617 [DOI] [PubMed] [Google Scholar]
- Slater S, Houmiel KL, Tran M, Mitsky TA, Taylor NB, Padgette SR, & Gruys KJ (1998). Multiple ß-ketothiolases mediate poly(ß-hydroxyalkanoate) copolymer synthesis in Ralstonia eutropha. J Bacteriol, 180(8), 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin DY, Khijniak TV, Kostrikina NA, Elcheninov AG, Toshchakov SV, Bale NJ, … Kublanov IV (2018). Natronobiforma cellulositropha gen. nov., sp. nov., a novel haloalkaliphilic member of the family Natrialbaceae (class Halobacteria) from hypersaline alkaline lakes. Syst Appl Microbiol, 41(4), 355–362. doi: 10.1016/j.syapm.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N, Srivastava M, Ramteke PW, & Mishra PK (2019). Solid-state fermentation strategy for microbial metabolites production: An overview In Gupta VK & Pandey A (Eds.), New and Future Developments in Microbial Biotechnology and Bioengineering (pp. 345–354). [Google Scholar]
- Such L, Chorro FJ, Colom F, Alba I, Secaduras A, Such LM, … Alberola A (1998). Effects of halocin H7 on A-V nodal conduction and heart rate in isolated rabbit heart. J Physiol, 509.P, 148–149P. [Google Scholar]
- Sukumaran RK, Singhania RR, Mathew GM, & Pandey A (2009). Cellulase production using biomass feed stock and its application in lignocellulose saccharification for bio-ethanol production. Renew. Energy, 34(2), 421–424. doi: 10.1016/j.renene.2008.05.008 [DOI] [Google Scholar]
- Sun C, Li Y, Mei S, Lu Q, Zhou L, & Xiang H (2005). A single gene directs both production and immunity of halocin C8 in a haloarchaeal strain AS7092. Mol Microbiol, 57(2), 537–549. doi: 10.1111/j.1365-2958.2005.04705.x [DOI] [PubMed] [Google Scholar]
- Sun S, Cao X, & Sun R (2016). The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol, 199, 49–58. doi: 10.1016/j.biortech.2015.08.061 [DOI] [PubMed] [Google Scholar]
- Sunna A, Moracci M, Rossi M, & Antranikian G (1997). Glycosyl hydrolases from hyperthermophiles. Extremophiles, 1(1), 2–13. doi: 10.1007/s007920050009 [DOI] [PubMed] [Google Scholar]
- Tabor S, & Richardson CC (1990). DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Effect of pyrophosphorolysis and metal ions. J Biol Chem, 265(14), 8322–8328. [PubMed] [Google Scholar]
- Tango MSA, & Islam MR (2002). Potential of extremophiles for biotechnological and petroleum applications. Energy Sources, 24(6), 543–559. doi: 10.1080/00908310290086554 [DOI] [Google Scholar]
- Taran M, & Amirkhani H (2010). Strategies of poly(3-hydroxybutyrate) synthesis by Haloarcula sp. IRU1 utilizing glucose as carbon source: Optimization of culture conditions by Taguchi methodology. Int J Biol Macromol, 47(5), 632–634. doi: 10.1016/j.ijbiomac.2010.08.008 [DOI] [PubMed] [Google Scholar]
- Teo JW, Zhang LH, & Poh CL (2003). Cloning and characterization of a novel lipase from Vibrio harveyi strain AP6. Gene, 312, 181–188. doi: 10.1016/s0378-1119(03)00615-2 [DOI] [PubMed] [Google Scholar]
- Timpson LM, Liliensiek AK, Alsafadi D, Cassidy J, Sharkey MA, Liddell S, … Paradisi F (2013). A comparison of two novel alcohol dehydrogenase enzymes (ADH1 and ADH2) from the extreme halophile Haloferax volcanii. Appl Microbiol Biotechnol, 97(1), 195–203. doi: 10.1007/s00253-012-4074-4 [DOI] [PubMed] [Google Scholar]
- Torreblanca M, Meseguer I, & Ventosa A (1994). Production of halocin is a practically universal feature of archaeal halophilic rods. Lett Appl Microbiol, 19(4), 201–205. doi: 10.1111/j.1472-765X.1994.tb00943.x [DOI] [Google Scholar]
- Torregrosa-Crespo J, Galiana CP, & Martínez-Espinosa RM (2017). Biocompounds from haloarchaea and their uses in biotechnology In Sghaier H (Ed.), Archaea - New Biocatalysts, Novel Pharmaceuticals and Various Biotechnological Applications (pp. 63–82): Intechopen. [Google Scholar]
- Uhl AM, & Daniel RM (1999). The first description of an archaeal hemicellulase: the xylanase from Thermococcus zilligii strain AN1. Extremophiles, 3(4), 263–267. doi: 10.1007/s007920050126 [DOI] [PubMed] [Google Scholar]
- Usami R, Fukushima T, Mizuki T, Inoue A, Yoshida Y, & Horikoshi K (2003). Organic solvent tolerance of halophilic archaea. Biosci Biotechnol Biochem, 67(8), 1809–1812. [DOI] [PubMed] [Google Scholar]
- Usami R, Fukushima T, Mizuki T, Yoshida Y, Inoue A, & Horikoshi K (2005). Organic solvent tolerance of halophilic archaea, Haloarcula strains: effects of NaCl concentration on the tolerance and polar lipid composition. J Biosci Bioeng, 99(2), 169–174. doi: 10.1263/jbb.99.169 [DOI] [PubMed] [Google Scholar]
- Uthandi S, Prunetti L, De Vera IM, Fanucci GE, Angerhofer A, & Maupin-Furlow JA (2012). Enhanced archaeal laccase production in recombinant Escherichia coli by modification of N-terminal propeptide and twin arginine translocation motifs. J Ind Microbiol Biotechnol, 39(10), 1523–1532. doi: 10.1007/s10295-012-1152-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthandi S, Saad B, Humbard MA, & Maupin-Furlow JA (2010). LccA, an archaeal laccase secreted as a highly stable glycoprotein into the extracellular medium by Haloferax volcanii. Appl Environ Microbiol, 76(3), 733–743. doi: 10.1128/AEM.01757-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Horn PB, Davis MC, Cunniff JJ, Ruan C, McArdle BF, Samols SB, … Fuller CW (1997). Thermo Sequenase DNA polymerase and T. acidophilum pyrophosphatase: new thermostable enzymes for DNA sequencing. Biotechniques, 22(4), 758–762, 764–755. [DOI] [PubMed] [Google Scholar]
- Viana QM, Viana MB, Vasconcelos EA, Santaella ST, & Leitao RC (2014). Fermentative H2 production from residual glycerol: a review. Biotechnol Lett, 36(7), 1381–1390. doi: 10.1007/s10529-014-1507-4 [DOI] [PubMed] [Google Scholar]
- Viniegra-Gonzàlez G (1997). Solid state fermentation: Definition, characteristics, limitations and monitoring In Roussos S, Lonsane BK, Raimbault M, & Viniegra-Gonzalez G (Eds.), Advances in Solid State Fermentation (pp. 5–22). Dordrecht: Springer. [Google Scholar]
- Virk AP, Sharma P, & Capalash N (2012). Use of laccase in pulp and paper industry. Biotechnol Prog, 28(1), 21–32. doi: 10.1002/btpr.727 [DOI] [PubMed] [Google Scholar]
- Viswanath B, Rajesh B, Janardhan A, Kumar AP, & Narasimha G (2014). Fungal laccases and their applications in bioremediation. Enzyme Res, 2014, 163242. doi: 10.1155/2014/163242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainø M, & Ingvorsen K (2003). Production of ß-xylanase and ß-xylosidase by the extremely halophilic archaeon Halorhabdus utahensis. Extremophiles, 7(2), 87–93. doi: 10.1007/s00792-002-0299-y [DOI] [PubMed] [Google Scholar]
- Walia A, Guleria S, Mehta P, Chauhan A, & Parkash J (2017). Microbial xylanases and their industrial application in pulp and paper biobleaching: a review. 3 Biotech, 7(1), 11. doi: 10.1007/s13205-016-0584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Chan H, Lin HT, & Shyu YT (2010). Production, purification and characterisation of a novel halostable xylanase from Bacillus sp. NTU‐06. Ann. Appl. Biol, 156, 187–197 doi: 10.1111/j.1744-7348.2009.00378.x [DOI] [Google Scholar]
- Wejse PL, Ingvorsen K, & Mortensen KK (2003). Purification and characterisation of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles, 7(5), 423–431. doi: 10.1007/s00792-003-0342-7 [DOI] [PubMed] [Google Scholar]
- Widsten P, and Kandelbauer A (2008). Laccase applications in the forest products industry: a review. Enzyme Microb Tech, 42, 293–307. [Google Scholar]
- Williams TA, Szöllősi GJ, Spang A, Foster PG, Heaps SE, Boussau B, … Embley TM (2017). Integrative modeling of gene and genome evolution roots the archaeal tree of life. Proc Natl Acad Sci U S A, 114(23), E4602–E4611. doi: 10.1073/pnas.1618463114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams TJ, Allen M, Tschitschko B, & Cavicchioli R (2017). Glycerol metabolism of haloarchaea. Environ Microbiol, 19(3), 864–877. doi: 10.1111/1462-2920.13580 [DOI] [PubMed] [Google Scholar]
- Wilson H, Aldrich H, & Maupin-Furlow J (1999). Halophilic 20S proteasomes of the archaeon Haloferax volcanii: Purification, characterization, and gene sequence analysis. J. Bacteriol, 181(18), 5814–5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhalter C, & Liebl W (1995). Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol, 61(5), 1810–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witayakran, & Ragauskas. (2009). Modification of high-lignin softwood kraft pulp with laccase and amino acids. Enzyme and Microb Tech, 44(3) 176–181. [Google Scholar]
- Woese CR, Kandler O, & Wheelis ML (1990). Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A, 87(12), 4576–4579. doi: 10.1073/pnas.87.12.4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Phong A, Lum KL, Greene RA, Buzby PR, & Kwok PY (2004). Role of excess inorganic pyrophosphate in primer-extension genotyping assays. Genome Res, 14(9), 1749–1755. doi: 10.1101/gr.2833204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu QS, Yan YS, & Feng JX (2016). Efficient hydrolysis of raw starch and ethanol fermentation: a novel raw starch-digesting glucoamylase from. Biotechnol Biofuels, 9, 216. doi: 10.1186/s13068-016-0636-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Yatsunami R, Ando A, Miyoko N, Fukui T, Takaichi S, & Nakamura S (2015). Complete biosynthetic pathway of the C50 carotenoid bacterioruberin from lycopene in the extremely halophilic archaeon Haloarcula japonica. J Bacteriol, 197(9), 1614–1623. doi: 10.1128/JB.02523-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yangui T, Dhouib A, Rhouma A, & Sayadi S (2009). Potential of hydroxytyrosol-rich composition from olive mill wastewater as a natural disinfectant and its effect on seeds vigour response. Food Chemistry, 117(1), 1–8. [Google Scholar]
- Yatsunami R, Sato M, Orishimo K, Hatori Y, Zhang Y, Takashina T, … Nakamura S (2010). Gene expression and characterization of a novel GH family 18 chitinase from extremely halophilic archaeon Halobacterium salinarum NRC-1. J. Jpn. Soc. Extremophiles, 9(1), 19–24. doi: 10.3118/jjse.9.19 [DOI] [Google Scholar]
- Yennamalli RM, Rader AJ, Wolt JD, & Sen TZ (2011). Thermostability in endoglucanases is fold-specific. BMC Struct Biol, 11, 10. doi: 10.1186/1472-6807-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus JG, Vieille C, & Savchenko A (1998). Thermozymes: biotechnology and structure-function relationships. Extremophiles, 2(3), 179–183. doi: 10.1007/s007920050058 [DOI] [PubMed] [Google Scholar]
- Zhang G, Li S, Xue Y, Mao L, & Ma Y (2012). Effects of salts on activity of halophilic cellulase with glucomannanase activity isolated from alkaliphilic and halophilic Bacillus sp. BG-CS10. Extremophiles, 16(1), 35–43. doi: 10.1007/s00792-011-0403-2 [DOI] [PubMed] [Google Scholar]
- Zhang T, D. S, Eichler J, Ivanova N, Axen SD, Kerfeld CA (2011). Identification of a haloalkaliphilic and thermostable cellulase with improved ionic liquid tolerance. Green Chemistry, 13, 2083–2090. [Google Scholar]
- Zhao D, Cai L, Wu J, Li M, Liu H, Han J, … Xiang H (2013). Improving polyhydroxyalkanoate production by knocking out the genes involved in exopolysaccharide biosynthesis in Haloferax mediterranei. Appl Microbiol Biot, 97(7), 3027–3036. doi: 10.1007/s00253-012-4415-3 [DOI] [PubMed] [Google Scholar]
- ZoBell CE (1937). Direct microscopic evidence of an autochthonous bacterial flora in the Grat Salt Lake. Ecology, 18, 453–458. [Google Scholar]
- Zoghlami A, & Paës G (2019). Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front Chem, 7, 874. doi: 10.3389/fchem.2019.00874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Shi M, Zhang T, Li L, & Xian M (2017). Natural and engineered polyhydroxyalkanoate (PHA) synthase: key enzyme in biopolyester production. Appl Microbiol Biotechnol, 101(20), 7417–7426. doi: 10.1007/s00253-017-8485-0 [DOI] [PubMed] [Google Scholar]
- Zucca P, Neves CM, Simões MM, Neves M. a. G., Cocco G, & Sanjust E (2016). Immobilized lignin peroxidase-like metalloporphyrins as reusable catalysts in oxidative bleaching of industrial dyes. Molecules, 21(7). doi: 10.3390/molecules21070964 [DOI] [PMC free article] [PubMed] [Google Scholar]


