Abstract
((S)-(+)/(R)-(−)) vigabatrin (SabrilR; γ-vinyl GABA), an antiepileptic irreversibly inactivating GABA-transaminase, was administered to male C57Bl6J mice via continuous infusion (0, 40, 80 mg/kg/d) for 12 days. Our study design pooled retina, eye (minus retina), whole brain and plasma from n=24 animals for each dose to provide n=8 triplicates per treatment group. Hypothesizing that (S)-(+) VGB (active isomer) would preferentially accumulate in retina, we determined VGB isomers, comprehensive amino acids, and pharmacokinetic parameters. In brain, eye and plasma, the ((S)-(+)/(R)-(−)) ratio varied from 0.73–1.29 and 13.3 in retina, accompanied by a partition coefficient (tissue/plasma, ((S)-(+);(R)-(−))) of 5.8;0.34, 0.63;0.49, and 0.51;0.34 in retina, eye and brain, respectively. Racemic VGB (nmol/g; plasma, nmol/ml, range of means for dose) content was: retina, 25–36; eye (minus retina), 4.8–8.0; brain, 3.1–6.8 and plasma, 8.7–14.9. GABA tissue content (nmol/g) was 1246–3335, 18–64 and 2615–3200 as a function of VGB dose for retina, eye (minus retina) and brain, respectively. The retinal glial cell toxin 2-aminoadipic acid also increased with VGB dose (76–96 nmol/g). Partitioning of active (S)-(+) VGB to retina suggests the involvement of a stereospecific transporter, the identification of which could reveal new therapeutic paradigms that might mitigate VGB’s well-known retinal toxicity and expand its clinical utility.
Keywords: GABA, vigabatrin, retina, isomers, 2-aminoadipic acid
1. Introduction
In 2019, the World Health Organization estimated that ~ 50 million people suffer from epilepsy, making it one of the most common globally-identified neurological disorders (https://www.who.int/news-room/fact-sheets/detail/epilepsy). Many of the approximately thirty clinically available antiepileptic drugs (AEDs) alter ion channel function that seeks to counterbalance the excitatory/inhibitory neurotransmission that is offset in epilepsy (Vossler et al, 2018). Vigabatrin (VGB; γ-vinyl GABA; (S)-(+)/R-(−) 4-aminohex-5-enoic acid), however, represents an AED that directly targets the catabolic pathway of the major inhibitory neurotransmitter GABA (γ-aminobutyric acid) through irreversible inactivation of GABA-transaminase and resulting accumulation of GABA (Ben-Menachem, 2011). Therapeutic indications for VGB include infantile spasms (West Syndrome), focal-onset seizures, and an inherited disorder of GABA metabolism, succinic semialdehyde dehydrogenase deficiency (SSADHD) (Chiron, 2016; Malaspina et al 2016).
Despite its clinical utility, VGB carries a black box warning (https://www.sabril.net/) highlighting the risk of permanent bilateral peripheral visual field constriction (pVFC) with continued use. Earlier studies from our laboratory, under steady-state conditions, examined the distribution of VGB isomers ((S)-(+)/(R)-(−)); the S isomer inactivates GABA-transaminase; Walters et al 2019a) in whole eye, liver, prefrontal and visual cortex, brain (minus cortices) and plasma from C57BL/6J mice (Walters et al 2019a). We observed that the highest S/R ratios were observed in eye and visual cortex. Here, we have extended those studies and tested the hypothesis that the S isomer of VGB accumulates preferentially in the retina with accompanying elevation of GABA.
2. Materials and Methods
2.1. Vigabatrin preparation, infusion characteristics, quantification of isomers, and amino acid analyses
Animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as described by the NIH (OLAW; Office of Laboratory Animal Welfare) and approved by the Washington State University Institutional Animal Care and use Committee (Protocols ASAF 4232- 2 and 6134). Animals were prepared for surgery by shaving the incision site and scrubbing with alcohol/betadine. Animals were anesthetized by isoflurane inhalation, and osmotic pumps were inserted through a small incision in the neck/shoulder region. The incisions were subsequently closed with wound staples. Animals were given buprenorphine SR and carprofen post-surgery. Osmotic minipumps delivered racemic VGB (0, 40, 80 mg/kg/d, delivered in sterile phosphate-buffered saline) to male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) that were 8 weeks of age and 20.8–26.1 g in weight at the time of minipump insertion (Walters et al 2019a). These daily dosages were chosen to parallel both pediatric and adult clinical dosing. After 12 days of treatment, animals were euthanized for tissue harvesting. Tissues isolated for analysis included retina isolated from eye, whole brain, eye (minus retina) and plasma. Tissue extracts were prepared in 0.1 mol/L perchloric acid; plasma was analyzed without modification. All other methods were as reported (Walters et al 2019a; 2019b).
2.2. Study design
We estimated that 3 mouse retina would be required per dose (0, 40 and 80 mg/kg/d) and data point (to achieve a final n=8 values of pooled triplicates). Since retina would be pooled from individual animals, we chose to consistently pool eye (minus retina), whole brain and plasma in a similar fashion. Thus, for the three dosages employed, 72 animals were required.
2.3. Statistical and data analysis
Data analysis was performed using GraphPad Prism (version 8.0; San Diego, CA). Statistical analyses employed either two-tailed t test or one-way ANOVA with Tukey post hocanalysis. For determination of VGB isomers and amino acid concentrations, operators were blinded to the study groups and only provided raw data based upon animal study number. All data was presented as mean (n=8 of 3 each pooled replicates) ± SEM (standard error of the mean) and assessed for normal distribution prior to statistical evaluation. The threshold for significance was set at α = 0.05. Determination of VGB pool size was achieved by multiplying tissue VGB concentration (S and R) by tissue weight. Tissue partition coefficients (Kp) were calculated as the ratios of tissue to plasma VGB concentrations for both VGB isomers (Walters et al 2019a).
3. Results
3.1. Quantitative amino acids in tissues and plasma
Seventeen of thirty amino acids quantified in tissue extracts and plasma showed no significant differences in any tissue compartment, while eight revealed a statistically significant change only in a single compartment (serine, glutamine, aspartate and glutamate in eye; ethanolamine, tryptophan, the GABA analogue β-alanine, and the GABA-β alanine dipeptide carnosine in brain) (data not shown).
Results for physiological/non-physiological amino acids previously implicated in the retinal toxicity of VGB, including taurine, ornithine, 2-aminoadipic acid (2-AADA), and GABA (Tao et al 2016; Sorri et al 2010; Walters et al 2019b), are displayed in Fig. 1. For the glutamate homologue 2-AADA, a trend toward significance was observed in retina in addition to a significant increase with VGB dose in brain (F (2, 21)=8.223; p=0.0023) and non-detectable levels in eye following retina removal. GABA in both retina (F (2, 20)=20.49; p<0.0001), eye (F (2, 20)=3.861; p=0.0382) and brain (F (2, 21)=15.75; p<0.0001) increased significantly with VGB dose, with the level of retinal GABA being equivalent to, or surpassing, the brain content. The ratios of amino acids in retina and eye under vehicle administration are depicted in Fig. 2. Most displayed a ratio of ~0.5; the amide amino acids (asparagine, glutamine) displayed a ratio of ~1; taurine, phosphoethanolamine, and neurotransmitter amino acids (aspartate and glutamate, glycine) displayed ratios of ~1.5 to 4. This same ratio was ~70 for GABA, revealing a strong localization of the inhibitory neurotransmitter in retina.
Fig. 1. Concentration of selected amino acids in retina (R), eye (E), brain (B) and plasma (P) as a function of VGB dose (40 mg/kg/d, 80 mg/kg/d).
Note that taurine represents a sulfonic amino acid, and like 2-aminoadipic acid and ornithine (a component of the urea cycle), is not found in proteins. Statistical analysis employed a one way ANOVA in each tissue with Tukey post-hoc analysis (*p<0.05; **p<0.01; ****p<0.0001). ND, not detected.
Fig. 2. Ratio of amino acids in retina/eye under untreated (vehicle) conditions.
Standard three letter abbreviations are employed for amino acids. A ratio is not shown for those amino acids that were not detected in either compartment. Additional abbreviations: tau, taurine; PEA, phosphoethanolamine. Note the differential y-axis scale for the GABA ratio (red arrow).
3.2. Distribution of VGB isomers in tissues and plasma, partition coefficients, and tissue pools
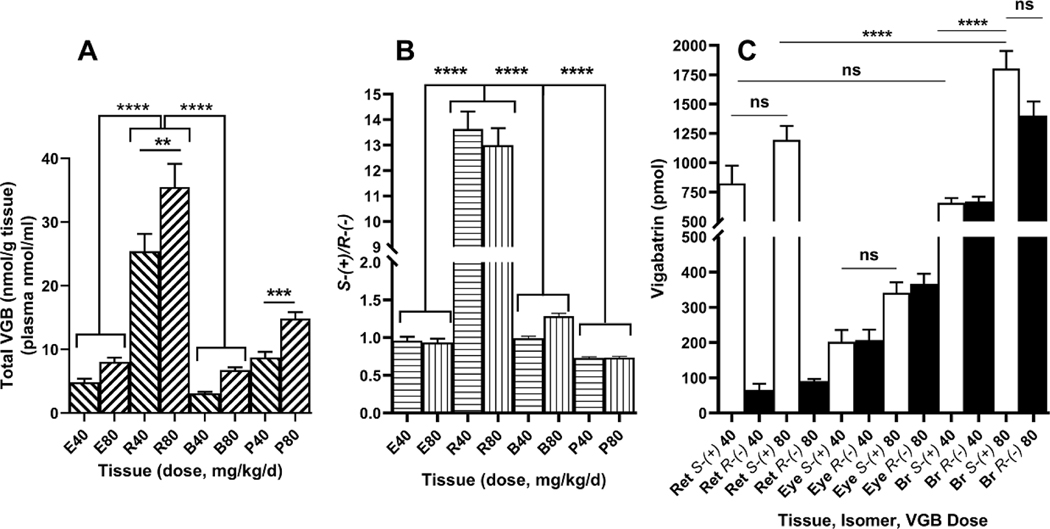
The concentration of racemic VGB is displayed in Fig. 3A. The concentration of VGB in retina was significantly higher than the same values in both eye and brain, and for both doses (F (5, 42)=49.12; p<0.0001). The ratios of active S-(+) to inactive R-(+) VGB are displayed in Fig. 3B, showing a striking increase in retina (~13) while ratios varied between ~1–1.5 in eye and brain (F (7, 56)=286.2; p<0.0001). Conversely, the ratio of VGB isomers in plasma was ~0.75, consistent with earlier studies revealing a mean plasma ratio of 0.74 (Walters et al 2019a). Total tissue pools of VGB isomers is displayed in Fig. 3C. It was noteworthy that the tissue pool for retina at the low dose of VGB was comparable to that of brain, and significantly higher than eye, although the tissue pool for brain at the higher dose of VGB was significantly higher than that for retina at the same dose (F (11, 84)=46.28; p<0.0001). The partition coefficients (tissue/plasma, based on a mean plasma ratio of 0.75) of VGB isomers for dosages (40, 80 mg/kg/d) were: retina, 6.4 (S); 0.35 (R) and 5.2 (S); 0.30 (R); eye (minus retina), 0.64 (S); 0.49 (R) and 0.61 (S); 0.48 (R); and brain, 0.42 (S); 0.31 (R) and 0.60 (S); 0.35 (R). Partitioning of S-(+) VGB was 17–18 fold higher than that of R-(−) VGB in retina. Partitioning of the S-(+) isomer into retina exceeded that of brain and eye by 9–15 fold, whereas partitioning of the inactive R-(−) isomer from plasma to all tissues examined was comparable between tissue beds (0.3–0.49).
Fig. 3. Total racemic VGB (A), S-(+)/R-(−) ratio (B) and tissue pools (C) for animals dosed with 0, 40 and 80 mg/kg/d VGB.
Abbreviations: E, eye (minus retina); R, retina; B, brain; P, plasma. Statistical analysis, one-way ANOVA with Tukey post-hoc analysis (**p<0.01; ***p<0.001; ****p<0.0001; ns, not significant).
4. Discussion
The current study extends our earlier work in intact eye (Walters et al 2019a), and reveals that the elevated ratio of S-(+)/R-(−) VGB in whole eye, and the concomitant significant accumulation of GABA, was almost entirely contributed by the retinal contribution of the (S-(+)) enantiomer (Fig. 1, 2 and 3B). Moreover, the retinal content of GABA (total retinal tissue pool) was comparable to that of brain (Fig. 3C). Our work also further underscores the association of dysregulated taurine and ornithine with VGB intake, and confirms that these amino acid abnormalities are confined to eye and absent in retina (Fig. 1) (Walters et al 2019b; Daune and Seiler 1988; Hisama et al 2001; Rasmussen et al 2015; Gaucher et al 2012). We further found that the gliotoxin 2-AADA was completely localized to retina and was absent from eye (Fig. 1) (Walters, 2019b).
Retinal Mueller cells are highly sensitive to 2-AADA toxicity, the latter selectively transported into retina via the cystine/glutamate antiporter xCT (SLC7a11) (Kato et al 1993; Murphy et al 1989; Sturman et al 1989). In carp and rat retina, 2-AADA inhibits cystine uptake in glial cells via this antiporter, leading to reduction of retinal GSH levels (Pow, 2001). The role of endogenous 2-AADA (a glutamate homologue) in retina remains unknown (Vallat et al 1996). Recent studies in retinal pigment epithelial cells provided evidence for the involvement of the taurine transporter (SLC6a6) in retinal accumulation of VGB, but it is unknown if this transporter is active on one specific isomer, or transports both isomers equally well (Police et al 2020). In addition to SLC7a11 and SLC6a6, other potential stereoselective VGB transporters could include SLC36a1 (hPAT-1; proton coupled amino acid transporter; Abbott et al 2006) and SLC6a13 (GABA transporter inhibited by tiagabine; Sills, 2003). Identification of an enantiomeric-specific VGB transporter in retina, should such a transporter exist, would provide novel opportunities for the development of pharmaceutics that could block VGB’s retinal accumulation and mitigate its retinal toxicity that would vastly extend its clinical utility.
Highlights.
(S)-(+) vigabatrin (VGB) preferentially accumulates (~14-fold) in mouse retina
Partitioning of (S)-(+) VGB exceeds that of (R)-(−) by ~ 18 fold in retina
Retinal partitioning of (S)-(+) VGB exceeded that of brain/eye by 9–15 fold
(S)-(+) VGB may undergo stereospecific partitioning into mammalian retina
Retinal accumulation of the active VGB isomer may underlie its retinal toxicity
Acknowledgements:
Supported by R01EY027476 from the National Eye Institute, National Institutes of Health (KMG).
Footnotes
Statement on Conflict of Interest:
Walters DC, Jansen EEW, Salomons GS, Arning E, Ashcraft P, Bottiglieri T, Roullet J-B and Gibson KM all declare that they have no conflict of interest in submission of this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbot EL, Grenade DS, Kennedy DJ, Gatfield KM, Thwaites D, 2006. Vigabatrin transport across the human intestinal epithelial (Caco-2) brush-border membrane is via the H+ -coupled amino-acid transporter hPAT1. Br J Pharmacol 147, 298–306. doi: 10.1038/sj.bjp.0706557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Menachem E, 2011. Mechanism of action of vigabatrin: correcting misperceptions. Acta Neurol Scand Suppl 192:5–15. doi: 10.1111/j.1600-0404.2011.01596.x. [DOI] [PubMed] [Google Scholar]
- Chiron C (2016) Stiripentol and vigabatrin current roles in the treatment of epilepsy. Expert Opin Pharmacother 17, 1091–101. doi: 10.1517/14656566.2016.1161026. [DOI] [PubMed] [Google Scholar]
- Daune G, Seiler N, 1988. Interrelationships between ornithine, glutamate, and GABA. II. Consequences of inhibition of GABA-T and ornithine aminotransferase in brain. Neurochem Res 13, 69–75. doi: 10.1007/BF00971857. [DOI] [PubMed] [Google Scholar]
- Gaucher D, Arnault E, Husson Z, Froger N, Dubus E, Gondouin P, Dherbécourt D, Degardin J, Simonutti M, Fouquet S, Benahmed MA, Elbayed K, Namer IJ, Massin P, Sahel JA, Picaud S, 2012. Taurine deficiency damages retinal neurones: cone photoreceptors and retinal ganglion cells. Amino Acids 43, 1979–93. doi: 10.1007/s00726-012-1273-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisama FM, Mattson RH, Lee HH, Felice K, Petroff OA, 2001. GABA and the ornithine delta-aminotransferase gene in vigabatrin-associated visual field defects. Seizure 10, 505–7. doi: 10.1053/seiz.2001.0524 [DOI] [PubMed] [Google Scholar]
- Kato S, Ishita S, Sugawara K, Mawatari K, 1993. Cystine/glutamate Antiporter Expression in Retinal Müller Glial Cells: Implications for DL-alpha-aminoadipate Toxicity. Neuroscience 57, 473–82. doi: 10.1016/0306-4522(93)90080-y. [DOI] [PubMed] [Google Scholar]
- Malaspina P, Roullet JB, Pearl PL, Ainslie GR, Vogel KR, Gibson KM, 2016. Succinic semialdehyde dehydrogenase deficiency (SSADHD): Pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism. Neurochem Int 99, 72–84. doi: 10.1016/j.neuint.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TH, Miyamoto M, Sastre A, Schnaar RL, Coyle JT, 1989. Glutamate Toxicity in a Neuronal Cell Line Involves Inhibition of Cystine Transport Leading to Oxidative Stress. Neuron 2, 1547–58. doi: 10.1016/0896-6273(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Police A, Shankar VK, Murthy SN, 2020. Role of Taurine Transporter in the Retinal Uptake of Vigabatrin. AAPS PharmSciTech 21, 196. doi: 10.1208/s12249-020-01736-7. [DOI] [PubMed] [Google Scholar]
- Pow DV, 2001. Visualising the activity of the cystine-glutamate antiporter in glial cells using antibodies to aminoadipic acid, a selectively transported substrate. Glia 34, 27–38. doi: 10.1002/glia.1037. [DOI] [PubMed] [Google Scholar]
- Rasmussen AD, Truchot N, Pickersgill N, Thale ZI, Rosolen SG, Botteron C, 2015. The effects of taurine on vigabatrin, high light intensity and mydriasis induced retinal toxicity in the pigmented rat. Exp Toxicol Pathol 67, 13–20. doi: 10.1016/j.etp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- Sills GJ, 2003. Pre-clinical studies with the GABAergic compounds vigabatrin and tiagabine. Epileptic Disord. 5, 51–6. PMID: 12773297 [PubMed] [Google Scholar]
- Sorri I, Brigell MG, Mályusz M, Mahlamäki E, de Meynard C, Kälviäinen R, 2010. Is reduced ornithine-δ-aminotransferase activity the cause of vigabatrin-associated visual field defects? Epilepsy Res 92, 48–53. doi: 10.1016/j.eplepsyres.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Sturman JA, Messing JM, Gargano AD, Rerecich M, Imaki H, Rudelli R, 1989. Cystine neurotoxicity is increased by taurine deficiency. Neurotoxicology 10, 15–28. PMID: 2570388 [PubMed] [Google Scholar]
- Tao Y, Yang J, Ma Z, Yan Z, Liu C, Ma J, Wang Y, Yang Z, Huang YF, 2016. The Vigabatrin Induced Retinal Toxicity is Associated with Photopic Exposure and Taurine Deficiency: An In Vivo Study. Cell Physiol Biochem 40, 831–846. doi: 10.1159/000453143 [DOI] [PubMed] [Google Scholar]
- Vallat C, Rivier F, Bellet H, Magnan de Bornier B, Mion H, Echenne B, 1996. Treatment with vigabatrin may mimic alpha-aminoadipic aciduria. Epilepsia. 37, 803–5. doi: 10.1111/j.1528-1157.1996.tb00655.x [DOI] [PubMed] [Google Scholar]
- Vossler DG, Weingarten M, Gidal BE, American Epilepsy Society Treatments Committee, 2018. Summary of Antiepileptic Drugs Available in the United States of America: WORKING TOWARD A WORLD WITHOUT EPILEPSY. Epilepsy Curr 18(4 Suppl 1):1–26. doi: 10.5698/1535-7597.18.4s1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DC, Jansen EEW, Ainslie GR, Salomons GS, Brown MN, Schmidt MA, Roullet JB, Gibson KM, 2019a. Preclinical tissue distribution and metabolic correlations of vigabatrin, an antiepileptic drug associated with potential use-limiting visual field defects. Pharmacol Res Perspect 7, e00456. doi: 10.1002/prp2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters DC, Arning E, Bottiglieri T, Jansen EEW, Salomons GS, Brown MN, Schmidt MA, Ainslie GR, Roullet JB, Gibson KM, 2019b. Metabolomic analyses of vigabatrin (VGB)-treated mice: GABA-transaminase inhibition significantly alters amino acid profiles in murine neural and non-neural tissues. Neurochem Int 125, 151–162. doi: 10.1016/j.neuint.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]