Abstract
Phthalates are reproductive toxicants in experimental animal studies and exposure has been associated with infertility in human populations, although the results have been inconsistent. To help to address the data gap, we conducted a hypothesis-generating investigation of associations between urinary phthalate metabolites and reproductive outcomes among women (n = 56) and their male partners (n = 43) undergoing in vitro fertilization (IVF). Urine was collected from participants on the day of oocyte retrieval. Samples were analyzed for a series of phthalates, MEP, MBP, MPP, MHxP, MEHP, MEHHP, MECPP, MiNP, MiDP, MCHP, and MBzP, using liquid chromatography-tandem mass spectrometry. We employed Poisson regression with robust variance estimation to estimate associations between urinary phthalate levels and biochemical pregnancy and live birth, adjusted for partner’s concentration and confounding factors. Doublings in women’s MBP (relative risk (RR) = 0.32, 95% CI: 0.13, 0.78), and men’s MEHP (RR = 0.28, 95% CI: 0.09, 0.83), were associated with a lower likelihood for pregnancy. Doublings in women’s (RR = 0.08, 95% CI: 0.01, 0.67) and men’s (RR = 0.13, 95% CI: 0.02, 0.92) MHxP were associated with a lower likelihood of live birth. Our results suggest that phthalate exposure may impact IVF outcomes, and underscore the importance of including male partners when investigating the impact of phthalate exposure on IVF. These results also suggest that clinical recommendations should include male partners for limiting phthalate exposure. Still, a larger and more comprehensive investigation is necessary to more definitively assess the risks.
Keywords: assisted reproduction, endocrine disruptors, female infertility, male infertility, phthalates, reproductive outcomes
1. Introduction
Phthalates are found in plastics, building materials, pharmaceuticals, and personal care products. Their nearly ubiquitous use in consumer goods has led to widespread human exposure [1, 2]. Experimental studies, in vitro and in vivo, have identified phthalates as endocrine-disrupting compounds that interfere with sex-steroid hormone activities, possibly having important implications for reproduction [3, 4]. Infertility, defined as the inability to conceive a pregnancy after 12 months of unprotected sexual intercourse, is a concern for approximately 9.4 million U.S. women [5]. The potential role of environmental pollutants, including phthalates, in the pathogenesis of infertility, is an increasing concern among clinicians and investigators worldwide [6, 7].
Phthalates are mixed into, rather than covalently bound to products, including plastics to impart flexibility, and are used as solvents and fragrance carriers in personal care products [8]. They can leach from plastics into the environment or foods and be absorbed and inhaled from personal care products, resulting in episodic, yet ongoing exposures [9, 10]. Food [11] and indoor dust inhalation [10] are primary sources of exposure to high molecular weight phthalates (HMW), such as di-2-ethylhexyl phthalate (DEHP), which are found in polyvinyl chloride (PVC) plastics. Exposure to low molecular weight phthalates (LMW), such as diethyl phthalate (DEP) and dibutyl phthalate (DBP), predominantly occurs from absorption or inhalation when using fragrances, lotions, and other personal care products [2, 8]. Ingestion of time-release capsules is another source of exposure to DBP, found in the enteric coating of some medications [2].
Several previous epidemiologic studies have estimated associations between phthalate exposures and reproductive outcomes, among populations conceiving with [12–17] and without assistance [18–22], although the results have been inconsistent. For example, a study of phthalate exposure among fertility compromised women using in vitro fertilization (IVF) found an inverse association between the sum of four urinary DEHP metabolites (ΣDEHP) and the probabilities of clinical pregnancy and live birth [15]. However, another study reported no statistically significant associations between urinary phthalate metabolites and pregnancy or live birth from IVF, although they found negative associations between urinary phthalate metabolites and numbers of total, mature, and fertilized oocytes [16]. Greater phthalates exposure, including DEHP, DBP, and DEP, has also been associated with poorer semen quality in general [23–26] and infertile populations [27–29], with potentially deleterious effects on fertility [13].
However, very few studies have assessed associations for female IVF patients and their male partners simultaneously [12, 13, 30]. This study adds data to the scant literature on the couple-based impacts of phthalate exposures on IVF outcomes, in a diverse U.S. population. In this hypothesis generating investigation, we examined associations between urinary phthalate metabolites and reproductive outcomes among couples using IVF, to help to guide the design of larger and more definitive future studies.
2. Methods
2.1. Sample selection and clinical protocol
Sixty-nine women and 56 male partners completing a first IVF cycle, using fresh non-donor oocytes and intending embryo transfer, were recruited at the University of California at San Francisco (UCSF) from 2015 to 2016. Patients received physical and gynecologic examinations and completed a fertility questionnaire to ascertain reproductive and medical histories. Following the baseline infertility evaluation, patients underwent gonadotropin-induced ovarian follicle stimulation per clinic protocols. Nearly two weeks later, when a sufficient number of follicles had developed to ≥ 17 mm diameter, human chorionic gonadotropin (hCG) was administered to trigger ovulation, and oocytes were retrieved within 36 hours. Women were advised to fast for at least eight hours to facilitate conscious sedation during oocyte retrieval. A fresh semen specimen was collected from the male partner on the same day as oocytes. Oocytes retrieved in metaphase-2 arrest were incubated (conventional IVF) or injected (intracytoplasmic sperm injection) with sperm and fertilization was confirmed by the appearance of 2 pronuclei approximately 24 hours later. Embryos were transferred 2–5 days later. Biochemical pregnancy (i.e., embryo implantation) was confirmed using a quantitative serum beta hCG ELISA test 14 days following the embryo transfer and women were contacted by mail nine months later to capture live birth.
Urine samples were collected from 56 female patients and 43 male partners on the day of oocyte retrieval and semen collection, prior to the procedure. Of the 56 female patients, 36 had male partners who elected to participate in this study, leaving 36 female-male couples. Spot urine specimens were collected in polypropylene urine cups and stored at −80 °C until transfer to the laboratory for analysis. We used an Atago “Pocket” handheld digital refractometer (Atago Co., LTD, Bellevue, WA USA) to measure urine specific gravity. The laboratory acid washed all consumables and samples from each lot were screened negative for phthalate contamination. The UCSF Committee on Human Research approved the study protocol. All participants completed informed consent at study enrollment.
2.2. Urinary phthalate metabolites analysis
Urine analysis was completed by the Clinical Toxicology and Environmental Biomonitoring Laboratory at UCSF, using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with an LC 1260-AB Sciex 5500 (Agilent, Santa. Clara, CA, USA), as previously described in detail [31]. Briefly, we quantified each detected analyte via an isotope dilution method, using a 10-point calibration curve (0.1–100 ng/mL). Observations below the limits of detection (LOD), 0.01 to 2.00 ng/mL, were included as directly measured from the instrument to limit bias in regression models; we did not impute values below the LOD [32,33]. Eleven phthalate monoester metabolites were analyzed in urine, including monoethyl phthalate (MEP), a metabolite of DEP; monobutyl phthalate (MBP), a metabolite of DBP; mono-n-pentyl phthalate (MPP), a metabolite of di-n-pentyl-phthalate (DPP); mono-n-hexyl phthalate (MHxP), a metabolite of di-n-hexyl phthalate (DnHP); mono-2-ethylhexyl phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), metabolites of DEHP; mono-isononyl phthalate (MiNP), a metabolite of diisononyl phthalate (DiNP); mono-isodecyl phthalate (MiDP), a metabolite of diisodecyl phthalate (DiDP); monocyclohexyl phthalate (MCHP), a metabolite of dicyclohexyl phthalate (DCHP); and monobenzyl phthalate (MBzP), a metabolite of benzylbutyl phthalate (BzBP). We calculated ΣDEHP as the molar sum of MEHP, MEHHP, and MECPP; ΣLMW as the molar sum of MEP, MBP, and MPP; and ΣHMW as the molar sum of MHxP, MEHP, MEHHP, MECPP, MiNP, and MBzP. U.S. biomonitoring studies showed widespread exposure to these phthalate monoesters [1] and previous experimental studies have indicated their potential for reproductive toxicity [3, 4].
2.3. Statistical analysis
Distributions of all covariates and clinical factors were characterized. We examined urinary phthalate distributions and natural log-transformed the variables to normalize the distributions before analysis. The Wilcoxon signed-rank test was used to assess the paired differences in urinary phthalate metabolite concentrations between women and men. We assessed associations between urinary phthalates and covariates using Pearson correlation coefficients, Student’s t-test, and ANOVA as appropriate.
In a first set of multivariable models, we used Poisson regression with robust variance estimation [34], to evaluate associations between pregnancy and live birth as outcomes, and individual urinary phthalate metabolite as predictors, adjusted for specific gravity as a covariate [35, 36]. We estimated the associations among female patients, male partners, and simultaneously among patients and partners (couples). In a second set of models, we further adjusted for confounding variables selected a priori as predictors of both phthalate exposure and IVF outcomes based on the literature, including: age in years [37], body mass index in kg/m2 (BMI) (for women) [37,38], and “ever” vs. “never” history of cigarette smoking [39]. We employed multiple imputation with fully conditional specification to impute missing values for some covariates (12.5% of women and 9.3% of men) [40]. We examined model residuals to assess the adequacy of models.
We used principal component analysis (PCA) to characterize the mixture of urinary phthalate metabolites among couples (i.e., simultaneously including all phthalate metabolites measured in female patients and their male partners). We retained two factors to use as predictors of pregnancy and live birth, based on scree plots and eigenvalues >3.0 [41]. We retained Varimax rotated factor loadings that had correlations between individual phthalate metabolite concentrations and factors greater than |0.5| and corresponded to at least 10% of the total variability [42]. We multiplied each participant’s factor value by the factor loading to emphasize those phthalates more closely related to the summary factor.
We set significance as α=0.05 for a 2-tailed test. We did not correct for multiple testing to increase sensitivity for detecting associations to be confirmed by a future investigation. We used SAS v.9.4 (SAS Institute Inc., Cary, NC, USA) for the statistical analysis.
3. Results
3.1. Distribution of demographics, clinical factors, and urinary phthalate metabolites among women and men
Table 1 describes the demographic and clinical factors for 56 women and 43 men undergoing IVF. Women were approximately 38 years old on average, and male partners were slightly older. Women’s BMI ranged from 18.2 kg/m2 to 38.6 kg/m2 with an average of 24 kg/m2. Approximately, 28.6% of women (n=16) and 23.3% of men (n=10) were Asian, with the remainder primarily white (i.e., n=26 women and n=19 men).
Table 1.
Distribution of demographic and clinical factors among women (n=56) and men (n=43) undergoing IVF
| Factors | Women | Men |
|---|---|---|
| Age, years (mean + SD)a | 38.1 ± 3.34 | 39.6 ± 4.29 |
| BMI (kg/m2)b | 24.0 ± 4.59 | - |
| Race, n (%) | ||
| Asian | 16 (28.6) | 10 (23.3) |
| Other | 40 (71.4) | 26 (60.5) |
| Missing | - | 7 (16.3) |
| Ever smoking, n (%) | ||
| Yes | 11 (19.6) | 1 (2.3) |
| No | 45 (80.4) | 42 (97.7) |
| Diagnosis, n (%) | ||
| DOR | 20 (35.7) | - |
| Unexplained | 16 (28.6) | - |
| Malec | 7 (12.5) | 10 (23.3) |
| Tubal | 5 (8.93) | - |
| Other | 5 (8.93) | - |
| PCOS | 3 (5.36) | - |
| Missing | - | 1 (2.3) |
| Treatment, n (%)d | ||
| Gonadotropin antagonist protocols | 39 (69.6) | - |
| Lupron down-regulated protocols | 12 (21.4) | - |
| Flare protocols | 3 (5.36) | - |
| Missing | 2 (3.57) | - |
| Outcomes, n (%) | ||
| Pregnant | 17 (30.4) | - |
| Live birth | 12 (21.4) | - |
n=1 missing man;
n=4 missing women;
includes 7 couples without a primary female factor infertility diagnosis and 10 couples with a primary or secondary male factor infertility diagnosis;
Gonadotropin antagonist protocols include IVF antagonist (n=21), IVF E2-priming antagonist (n=14), NEP antagonist (n=3), and IVF antagonist-LP start (n=1); Lupron down-regulated protocols include IVF long luteal (n=2) and IVF demi-halt (no OCP) (n=10); Flare protocols include Aygestin priming CC flare antagonist (n=1), IVF Clomid flare-FSH/HMG (n=1), and Clomid only (n=1).
Abbreviations: BMI, body mass index; CC, Clomiphene Citrate; DOR, diminished ovarian reserve; E2, estradiol; FSH, follicle stimulating hormone; HMG, human menopausal gonadotrophin; IVF, in vitro fertilization; LP, luteal phase; NEP, neutral endopeptidase; OCP, oral contraceptives; PCOS, polycystic ovary syndrome
Table 2 describes the distribution of urinary phthalate metabolite concentrations among 56 women and 43 men. More than 97% of participants had urinary MEP, MBP, MHxP, MEHP, MEHHP, MECPP, and MBzP concentrations greater than the detection limits. We excluded MPP, MiNP, MiDP, and MCHP from further analysis as few values (<15%) were measured above the LODs. Supplemental Table 1 provides the specific gravity corrected urinary phthalate metabolite concentrations among 51 women and 40 men (n=5 women and n=3 men were missing specific gravity). Supplemental Table 2 examines correlations between individual and couple’s urinary phthalate metabolites. HMW phthalate metabolites were moderately and positively correlated among women and among men. However, there were few significant correlations in urinary phthalate metabolites measured between women and men.
Table 2.
Distribution of urinary phthalate metabolite concentrations (ng/mL) among women (n=56) and mean (n=43) undergoing IVF
| Phthalate Metabolite | Geometric Mean | Geometric SD | Minimum | 25th Percentile | 50th Percentile | 75th Percentile | Maximum | ≥LOD (%) |
|---|---|---|---|---|---|---|---|---|
| MEP | ||||||||
| Women | 30.0 | 3.66 | 1.44 | 10.8 | 29.5 | 72.6 | 971 | 100 |
| Men | 18.9 | 2.87 | <LOD | 8.96 | 15.7 | 42.2 | 601 | 98.0 |
| MBP | ||||||||
| Women | 16.1 | 2.82 | <LOD | 7.55 | 14.1 | 26.9 | 324 | 98.0 |
| Men | 9.72 | 2.59 | 1.75 | 4.97 | 7.59 | 17.0 | 252 | 100 |
| ΣLMWa | ||||||||
| Women | 258 | 3.19 | 18.4 | 120 | 240 | 511 | 5242 | - |
| Men | 155 | 2.82 | 13.5 | 83.2 | 125 | 330 | 3118 | - |
| MPP | ||||||||
| Women | <LOD | 1.05 | <LOD | <LOD | <LOD | <LOD | 0.36 | 5.0 |
| Men | <LOD | 1.14 | <LOD | <LOD | <LOD | <LOD | 1.15 | 9.0 |
| MHxP | ||||||||
| Women | 3.46 | 1.56 | 0.78 | 2.38 | 3.53 | 4.96 | 10.7 | 100 |
| Men | 2.05 | 1.64 | 0.35 | 1.26 | 1.76 | 3.46 | 10.0 | 100 |
| MEHP | ||||||||
| Women | 3.08 | 3.58 | <LOD | 0.66 | 2.03 | 3.50 | 114 | 98.0 |
| Men | 1.86 | 2.60 | 0.13 | 0.50 | 1.42 | 2.68 | 325 | 100 |
| MEHHP | ||||||||
| Women | 2.24 | 2.53 | 0.79 | 3.88 | 6.87 | 14.4 | 105 | 100 |
| Men | 6.17 | 2.78 | 0.69 | 2.56 | 4.30 | 9.64 | 315 | 100 |
| MECPP * | ||||||||
| Women | 19.5 | 2.94 | 2.39 | 8.35 | 16.4 | 46.5 | 373 | 100 |
| Men | R.7 | 3.33 | 1.24 | 5.05 | 11.2 | 20.5 | 448 | 100 |
| ΣDEHPb * | ||||||||
| Women | 108 | 3.06 | 16.3 | 44.3 | 86.6 | 223 | 1895 | 98.0 |
| Men | 69.0 | 3.28 | 6.87 | 31.2 | 56.7 | 108 | 3673 | 100 |
| MiNP | ||||||||
| Women | <LOD | 1.69 | <LOD | <LOD | <LOD | <LOD | 10.5 | 9.0 |
| Men | <LOD | 1.68 | <LOD | <LOD | <LOD | <LOD | 5.13 | 14.0 |
| MiDP | ||||||||
| Women | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 9.64 | 4.0 |
| Men | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.0 |
| MCHP | ||||||||
| Women | <LOD | 1.06 | <LOD | <LOD | <LOD | <LOD | 0.54 | 2.0 |
| Men | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 0.0 |
| MBzP * | ||||||||
| Women | 3.10 | 4.10 | <LOD | 0.99 | 2.31 | 5.61 | 52.3 | 98.0 |
| Men | 1.85 | 2.85 | 0.29 | 0.66 | 1.40 | 2.94 | 38.4 | 100 |
| ΣHMWc * | ||||||||
| Women | 148 | 2.72 | 24.3 | 67.3 | 131 | 365 | 1936 | - |
| Men | 89.5 | 3.01 | 13.2 | 40.5 | 74.7 | 143 | 3848 | - |
ΣLMW (nmol/mL) as the molar sum of MEP, MBP, and MPP;
ΣDEHP (nmol/mL) as the molar sum of MEHP, MEHHP, and MECPP;
ΣHMW (nmol/mL) as the molar sum of MHxP, MEHP, MEHHP, MECPP, MiNP, and MBzP;
p<0.05 for the difference between women and men using Wilcoxon signed rank test.
Abbreviations: IVF, in vitro fertilization; LOD, limit of detection; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MCHP, monocyclohexyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEP, monoethyl phthalate; MHxP, monohexyl phthalate; MiDP, mono-isodecyl phthalate; MiNP, mono-isononyl phthalate; MPP, mono-n-pentyl phthalate
Supplemental Table 3 describes the associations between individual urinary phthalate metabolite concentrations and demographic and clinical factors. We detected statistically significant associations between greater age and lower urinary MEP and ΣLMW concentrations among men. We did not find significant differences in urinary phthalate metabolites according to race or cigarette smoking history. Similarly, there were no significant differences in urinary phthalate metabolites according to infertility diagnosis or treatment protocol.
3.2. Associations between individual urinary phthalate metabolite concentrations and IVF outcomes among women and men
Table 3 shows the results of the Poisson regression analysis between individual urinary phthalate metabolites in 56 women and 43 men, and IVF outcomes, adjusted for covariates. We found a pattern of mostly inverse associations between urinary phthalates and reproductive outcomes.
Table 3.
Relative risks (95% confidence intervals) for IVF outcomes associated with a doubling in women’s (n=56) and men’s (n=43) individual urinary phthalate concentrations (ng/mL), adjusted for covariates
| Phthalate Metabolite | Pregnant | p-value | Live Birth | p-value |
|---|---|---|---|---|
| MEP | ||||
| Women | 0.83 (0.58, 1.19) | 0.32 | 0.72 (0.48, 1.09) | 0.12 |
| Men | 0.87 (0.53, 1.44) | 0.59 | 1.09 (0.54, 2.20) | 0.82 |
| MBP | ||||
| Women | 0.65 (0.44, 0.97) | 0.04 | 0.61 (0.35, 1.05) | 0.07 |
| Men | 0.77 (0.41, 1.46) | 0.42 | 0.68 (0.25, 1.85) | 0.45 |
| ΣLMW | ||||
| Women | 0.73 (0.49, 1.09) | 0.13 | 0.66 (0.44, 0.99) | 0.04 |
| Men | 0.82 (0.49, 1.37) | 0.45 | 1.00 (0.46, 2.18) | 0.99 |
| MHxP | ||||
| Women | 0.57 (0.19, 1.73) | 0.32 | 0.22 (0.05, 0.92) | 0.04 |
| Men | 0.80 (0.21, 3.08) | 0.74 | 0.48 (0.07, 3.46) | 0.46 |
| MEHP | ||||
| Women | 1.14 (0.85, 1.53) | 0.37 | 0.99 (0.60, 1.63) | 0.97 |
| Men | 0.42 (0.19, 0.92) | 0.03 | 0.52 (0.20, 1.36) | 0.18 |
| MEHHP | ||||
| Women | 1.15 (0.70, 1.90) | 0.59 | 1.15 (0.55, 2.43) | 0.71 |
| Men | 0.95 (0.61, 1.47) | 0.81 | 0.70 (0.32, 1.55) | 0.38 |
| MECPP | ||||
| Women | 0.98 (0.60, 1.60) | 0.94 | 0.96 (0.49, 1.91) | 0.92 |
| Men | 0.87 (0.56, 1.35) | 0.53 | 0.60 (0.26, 1.38) | 0.23 |
| ΣDEHP | ||||
| Women | 1.02 (0.66, 1.59) | 0.91 | 0.95 (0.48, 1.87) | 0.87 |
| Men | 0.82 (0.53, 1.29) | 0.40 | 0.54 (0.21, 1.40) | 0.20 |
| MBzP | ||||
| Women | 0.90 (0.61, 1.33) | 0.59 | 0.93 (0.54, 1.63) | 0.81 |
| Men | 1.32 (0.73, 2.40) | 0.36 | 1.38 (0.61, 3.14) | 0.44 |
| ΣHMW | ||||
| Women | 1.00 (0.61, 1.65) | 0.99 | 0.88 (0.41, 1.90) | 0.75 |
| Men | 0.82 (0.50, 1.35) | 0.44 | 0.57 (0.22, 1.53) | 0.26 |
NOTE: Adjusted for age (years),, urinary specific gravity, history of smoking (“ever” vs. “never”) and body mass index (kg/m2) in women using individual Poisson models, and multiple imputation for participants with missing values for some covariates (n=5 women missing specific gravity, n=3 men missing specific gravity, n=4 missing BMI, n=1 man missing age). P<0.05 in bold type.
Abbreviations: DEHP, di-2-ethylhexyl phthalate; HMW, high molecular weight phthalates; IVF, in vitro fertilization; LMW, low molecular weight phthalates; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEP, monoethyl phthalate; MHxP, monohexyl phthalate
Women’s MBP and men’s MEHP concentrations were statistically significantly associated with a lower likelihood of pregnancy. In addition, women’s urinary ΣLMW and MHxP were statistically significantly associated with a lower likelihood of live birth. Supplemental Table 4 shows the results adjusted only for urinary specific gravity.
3.3. Summary measures of couples’ urinary phthalate metabolite concentrations
Supplemental Table 5 describes the results of our PCA among 56 women and 43 men. Factor 1 characterized the collective distribution of men’s urinary phthalate concentrations, with all measured phthalate metabolites contributing to the loadings except MEP. Factor 2 did the same for women, but all measured phthalates contributed to the loadings, including MEP.
3.4. Associations between couples’ urinary phthalate metabolite concentrations and IVF outcomes
Table 4 and Figure 1 show the results of the Poisson regression analysis between urinary phthalate metabolites and pregnancy for 36 couples, adjusted for partner’s urinary phthalate concentration. We detected a significantly lower likelihood for pregnancy associated with doublings in women’s MBP (relative risk (RR) = 0.32, 95% CI: 0.13, 0.78) and men’s MEHP (RR = 0.28, 95% CI: 0.09, 0.83). Doublings in PCA Factor 1 (RR = 0.82, 95% CI: 0.64, 1.05), corresponding to men’s overall urinary phthalates, and Factor 2 (RR = 0.76, 95% CI: 0.53, 1.09), corresponding to women’s overall urinary phthalates, were also associated with a lower likelihood of pregnancy, albeit not statistically significant. Supplemental Table 6 shows the pregnancy results adjusted only for urinary specific gravity.
Table 4.
Relative risks (95% confidence intervals) for IVF outcomes associated with a doubling in couples’ individual or mixed urinary phthalate concentrations (ng/mL), adjusted for covariates and using multiple imputation for missing values (n=36)
| Phthalate Metabolite | Pregnant | p-value | Live Birth | p-value |
|---|---|---|---|---|
| MEP | ||||
| Women | 0.86 (0.52, 1.43) | 0.56 | 0.65 (0.36, 1.17) | 0.15 |
| Men | 0.63 (0.28, 1.44) | 0.27 | 0.83 (0.31, 2.27) | 0.72 |
| MBP | ||||
| Women | 0.32 (0.13, 0.78) | 0.01 | 0.16 (0.02, 1.19) | 0.07 |
| Men | 0.59 (0.29, 1.18) | 0.13 | 0.50 (0.21, 1.20) | 0.12 |
| ΣLMW | ||||
| Women | 0.75 (0.39, 1.43) | 0.38 | 0.55 (0.27, 1.14) | 0.11 |
| Men | 0.63 (0.25, 1.57) | 0.32 | 0.83 (0.26, 2.67) | 0.76 |
| MHxP | ||||
| Women | 0.43 (0.12, 1.53) | 0.19 | 0.08 (0.01, 0.67) | 0.02 |
| Men | 0.53 (0.13, 2.21) | 0.38 | 0.13 (0.0, 0.92) | 0.04 |
| MEHP | ||||
| Women | 0.85 (0.52, 1.40) | 0.53 | 0.75 (0.27, 2.03) | 0.57 |
| Men | 0.28 (0.09, 0.83) | 0.02 | 0.38 (0.10, 1.39) | 0.15 |
| MEHHP | ||||
| Women | 0.81 (0.37, 1.79) | 0.60 | 0.97 (0.32, 2.97) | 0.95 |
| Men | 0.82 (0.39, 1.73) | 0.60 | 0.67 (0.15, 2.90) | 0.59 |
| MECPP | ||||
| Women | 0.62 (0.29, 1.31) | 0.21 | 0.75 (0.26, 2.15) | 0.59 |
| Men | 0.71 (0.41, 1.22) | 0.22 | 0.61 (0.24, 1.54) | 0.30 |
| ΣDEHP | ||||
| Women | 0.68 (0.35, 1.32) | 0.26 | 0.68 (0.21, 2.24) | 0.53 |
| Men | 0.67 (0.40, 1.11) | 0.12 | 0.50 (0.22, 1.14) | 0.10 |
| MBzP | ||||
| Women | 0.64 (0.32, 1.27) | 0.20 | 0.50 (0.17, 1.44) | 0.20 |
| Men | 0.97 (0.29, 3.25) | 0.97 | 0.79 (0.18, 3.36) | 0.75 |
| ΣHMW | ||||
| Women | 0.63 (0.29, 1.37) | 0.24 | 0.56 (0.10, 3.14) | 0.51 |
| Men | 0.59 (0.32, 1.11) | 0.10 | 0.45 (0.18, 1.12) | 0.09 |
| PCA | ||||
| Factor 1a | 0.82 (0.64, 1.05) | 0.11 | 0.76 (0.56, 1.04) | 0.09 |
| Factor 2b | 0.76 (0.53, 1.09) | 0.14 | 0.72 (0.35, 1.49) | 0.38 |
NOTE: Adjusted for partners’ ages (years), urinary specific gravities, histories of smoking (“ever” vs. “never”), women’s body mass index (kg/m2), and partner’s urinary phthalates using individual Poisson models, and multiple imputation for participants with missing values for some covariates (n=2 women missing specific gravity, n=3 men missing specific gravity, n=1 missing BMI, n=1 man missing age). P<0.05 in bold type.
Factor 1 describes men with higher urinary MBP, MHxP, MEHP, MEHHP, MECPP, and MBzP concentrations;
Factor 2 describes women with higher urinary MEP, MBP, MHxP, MEHP, MEHHP, MECPP, and MBzP concentrations.
Abbreviations: DEHP, di-2-ethylhexyl phthalate; HMW, high molecular weight phthalates; IVF, in vitro fertilization; LMW, low molecular weight phthalates; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEP, monoethyl phthalate; MHxP, monohexyl phthalate; PCA, principal component analysis
Figure 1.
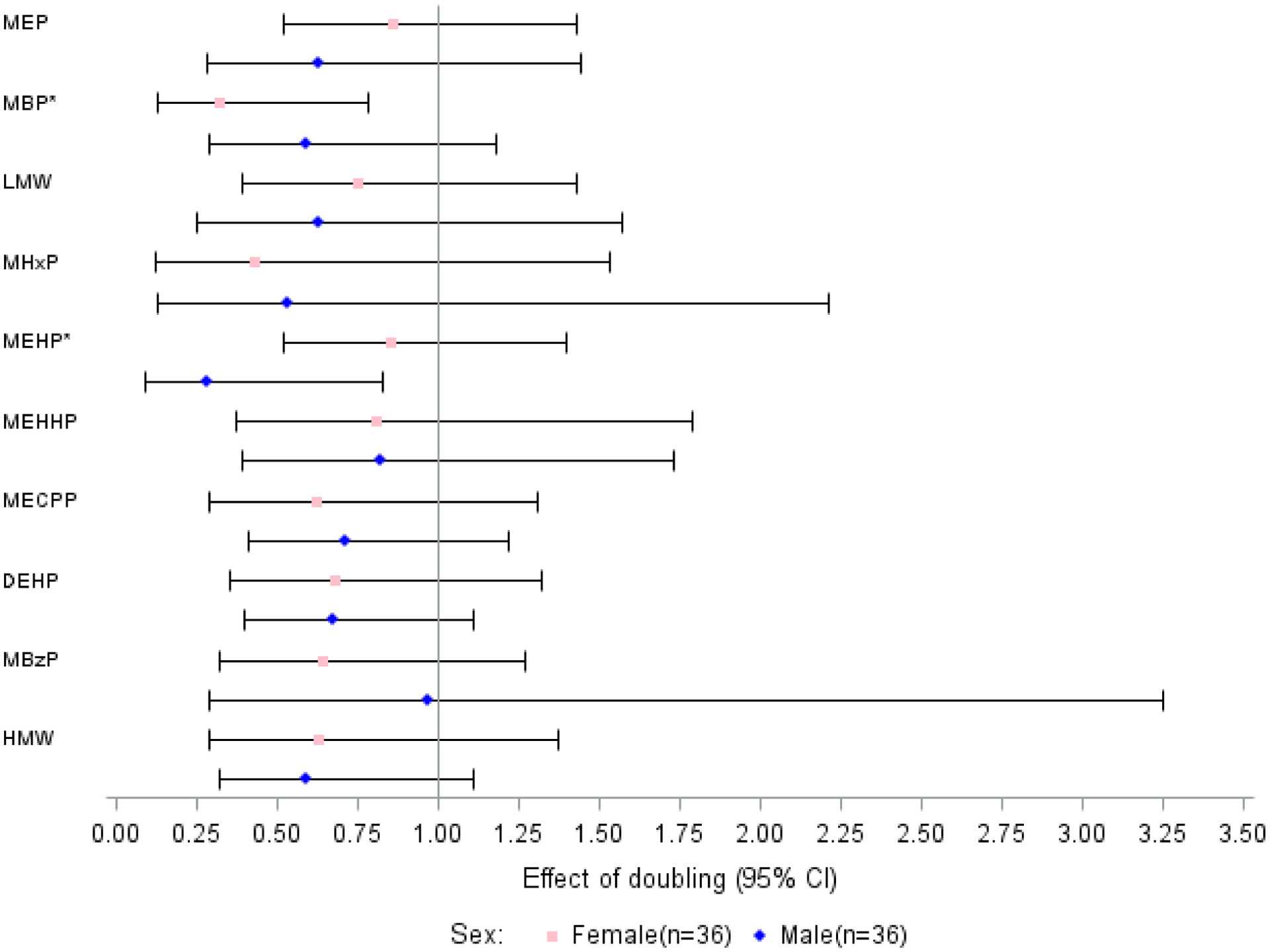
Relative risks (95% confidence intervals) for an IVF pregnancy associated with a doubling in couples’ urinary phthalate concentrations (ng/mL), adjusted for covariates (n=36)
NOTE: Adjusted for partners’ ages (years), urinary specific gravities, histories of smoking (“ever” vs. “never”), women’s body mass index (kg/m2), and partner’s urinary phthalates using individual Poisson models, and multiple imputation for participants with missing values for some covariates (n=2 women missing specific gravity, n=3 men missing specific gravity, n=1 missing BMI, n=1 man missing age).
* p<0.05.
Abbreviations: DEHP, di-2-ethylhexyl phthalate; HMW, high molecular weight phthalates; IVF, in vitro fertilization; LMW, low molecular weight phthalates; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEP, monoethyl phthalate; MHxP, monohexyl phthalate
Table 4 and Figure 2 also describe associations for couples’ (n=36) urinary phthalate metabolite concentrations with live birth, adjusted for partner’s urinary phthalate concentration and other covariates. Doublings in women’s urinary MBP concentration (RR = 0.16, 95% CI: 0.02, 1.19) and men’s MEHP concentration (RR = 0.38, 95% CI: 0.10, 1.39) were associated with a lower likelihood of live birth, albeit not statistically significant. However, doublings in women’s (RR = 0.08, 95% CI: 0.01, 0.67) and men’s (RR = 0.13, 95% CI: 0.02, 0.92) urinary MHxP concentrations were statistically significantly associated with lower likelihoods of a live birth. Doublings in PCA Factors 1 (RR = 0.76, 95% CI: 0.56, 1.04) and 2 (RR = 0.72, 95% CI: 0.35, 1.49) were also associated with a lower likelihood of live birth, though neither was statistically significant. Supplemental Table 6 shows the live birth results adjusted only for urinary specific gravity.
Figure 2.
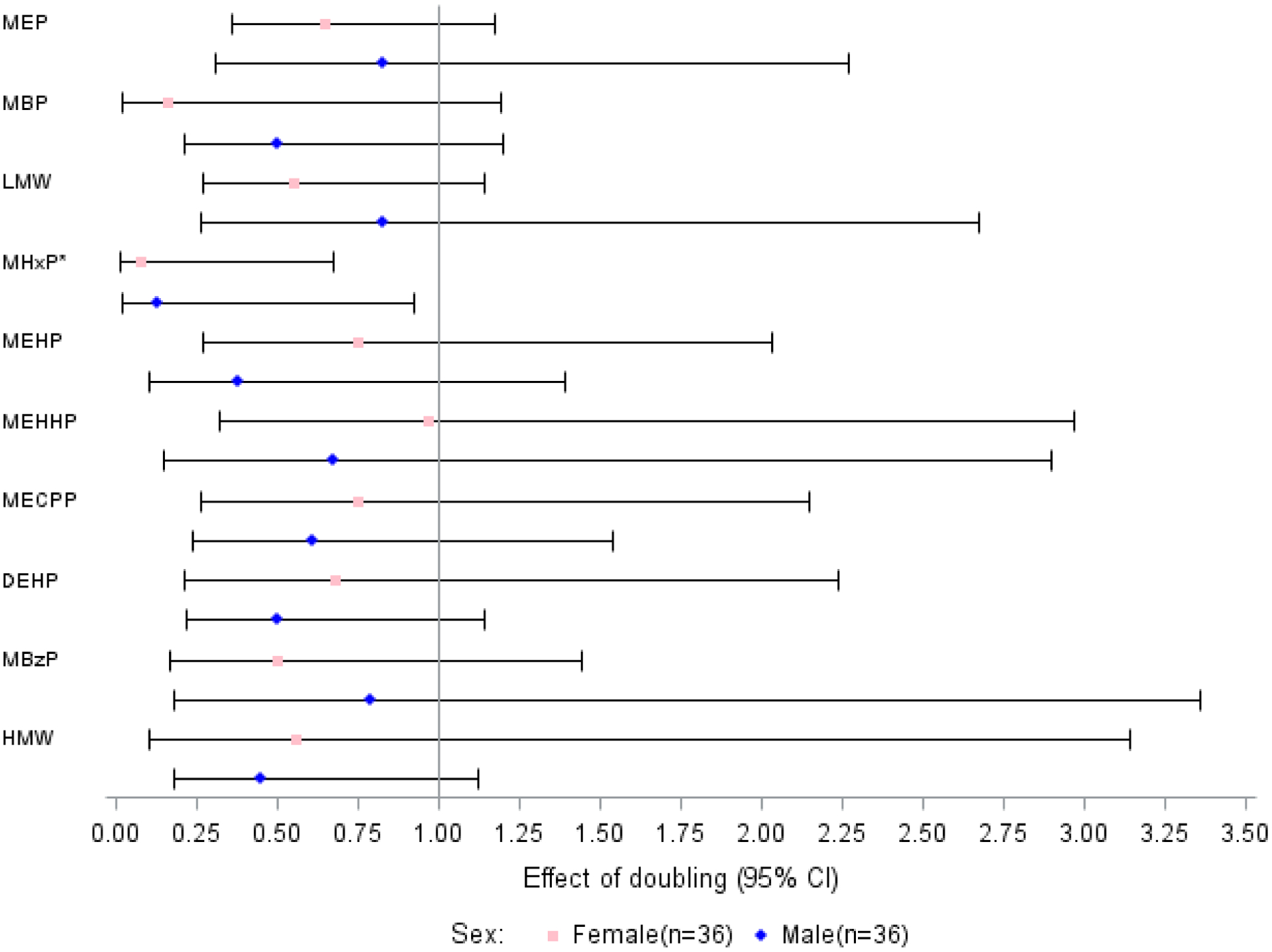
Relative risks (95% confidence intervals) for an IVF live birth associated with a doubling in couples’ urinary phthalate concentrations (ng/mL), adjusted for covariates (n=36)
NOTE: Adjusted for partners’ ages (years), urinary specific gravities, histories of smoking (“ever” vs. “never”), women’s body mass index (kg/m2), and partner’s urinary phthalates using individual Poisson models, and multiple imputation for participants with missing values for some covariates (n=2 women missing specific gravity, n=3 men missing specific gravity, n=1 missing BMI, n=1 man missing age).
* p<0.05.
Abbreviations: DEHP, di-2-ethylhexyl phthalate; HMW, high molecular weight phthalates; IVF, in vitro fertilization; LMW, low molecular weight phthalates; MBP, monobutyl phthalate; MBzP, monobenzyl phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono (2-ethyl-5-hydroxyhexyl) phthalate; MEHP, mono-(2-ethylhexyl) phthalate; MEP, monoethyl phthalate; MHxP, monohexyl phthalate
4. Discussion
In this prospective pilot cohort study, we estimated associations between couples’ urinary phthalates on the day of oocyte retrieval and IVF outcomes. We found that urinary MBP, MHxP, and MEHP, were associated with lower likelihoods of biochemical pregnancy and live birth when simultaneously considering exposure in the female patient and her male partner. We found a similar pattern of associations when estimating exposure only in women or only in men. In fact, using PCA, in a “mixtures-based” approach, we found that men’s phthalate summary measures were similarly important as women’s in associations with pregnancy and live birth. The results, concordant with previous studies reporting associations between phthalates and poorer sperm quality [17, 27, 29], underscore the importance of a “couples-based” approach to risk assessment.
4.1. Comparison to similar studies
Several groups previously reported on associations between urinary phthalates and reproductive outcomes among couples undergoing IVF [12–17]. In a large Saudi Arabian cohort (n=599) that measured eight urinary phthalates, higher concentrations of women’s MEP and MEHP were associated with a probability of failed live birth after IVF, adjusted for male exposures [12]. In addition, this study also found that higher women’s urinary MEP and MEHP were associated with a greater risk of a failed clinical pregnancy, when adjusted for men’s concentrations. The geometric mean phthalate concentrations measured in that study were much higher than ours (e.g., women’s MEP = 9137 ng/mL; women’s MEHP = 388 ng/mL), possibly due to differences in exposure profiles attributed to country-specific lifestyles. Similar to their results, we also found that IVF couples with higher urinary MEHP concentrations were less likely to become pregnant. A smaller cohort from Wuhan, China (n=112), reported no association between eight follicular fluid or urinary phthalate metabolites, including MBP, MBzP, MEHHP, MEHP, and MEP, and IVF outcomes [14].
A long running Boston, Massachusetts study (n=218), measured 11 urinary phthalate metabolites and found inverse associations between male partners’ urinary mono (3-carboxypropyl) phthalate (MCPP), mono carboxyoctyl phthalate (MCOP), and mono-isobutyl phthalate (MiBP) concentrations and embryo implantation after IVF, adjusted for female urinary phthalates [13]. The highest quartile of paternal urinary MCPP concentrations was associated with lower odds of a live birth (odds ratio (OR) = 0.42, 95% CI: 0.17, 1.07). While we did not measure MCPP, MCOP, or MiBP in our study, concordant with our findings, this study also reported lower odds for live birth in association with greater paternal urinary MBP concentrations. An additional analysis of women (n=256) from the same cohort, reported a greater risk of biochemical pregnancy loss for women in the highest relative to the lowest quartile of urinary ΣDEHP (i.e., MEHP, MEHHP, MEOHP, and MECPP) [17]. The authors speculated that some DEHP metabolites might associate with implantation, decidualization, placentation, or embryogenesis, possibly altering hormonal signaling and secretion of endogenous hormones such estrogen and progesterone. Another analysis of the women in this cohort reported associations between greater ΣDEHP and lower probabilities of clinical pregnancy and live birth [15]. We also found an inverse association between greater urinary ΣDEHP among women and pregnancy and live birth from IVF, although not statistically significant.
A study of 17 urinary phthalate metabolites measured in n = 136 Israeli IVF patients with a male factor or unexplained infertility diagnosis, reported associations between greater urinary ΣDEHP metabolites (i.e., MEHHP, MEOHP, and MECPP) and lower numbers of oocytes retrieved, mature oocytes, fertilized oocytes, and top-quality embryos [16]. Women in the highest quartile of ΣDEHP concentrations, had 2.9 fewer oocytes, 1.7 fewer mature oocytes, and 1.2 fewer fertilized oocytes on average than women in the lowest quartile, factors associated with poorer IVF outcomes. However, there was no association with pregnancy or live birth. Although inconsistent with our results, phthalate exposures were generally higher in that study, which had a larger sample size than our own.
Although conceiving without assistance, associations were reported for couples’ pre-pregnancy urinary phthalates and pregnancy in a large prospective investigation of 14 urinary phthalate metabolites in couples [19]. In single partner models, men’s urinary mono-methyl phthalate (MMP) (fecundability odds ratio (FOR) = 0.80, 95% CI: 70, 0.93), MBP (FOR = 0.82, 95% CI: 0.70. 0.97), and MBzP (FOR = 0.77, 95% CI: 0.65, 0.92) were associated with a longer time to conceive a pregnancy. In couples, greater men’s urinary MMP (FOR = 0.81, 95% CI: 0.70, 0.94) and MBzP (FOR = 0.80, 95% CI 0.67, 0.97) concentrations were also associated with longer time to pregnancy. In contrast, our results did not indicate an association for men’s urinary MBzP with pregnancy, adjusted for women’s, and we did not measure MMP. Yet, we identified statistically significant inverse associations for women’s and men’s urinary MBP and MEHP with pregnancy, respectively. The discordant results might be related to higher MBzP in the previous study and higher MBP concentrations in our study. The different time windows for exposure (i.e., preconception in the previous study vs. completion of the 1st meiosis after administering the hCG trigger in the current study) and different study populations (i.e., unassisted conception in the previous study vs. assisted conception in the current study) may also be important. Still, the previous results reinforce our findings suggesting that the male’s phthalate exposures may play an important role in pregnancy.
4.2. Study strengths and limitations
The results of this hypothesis-generating investigation have several limitations; therefore, our results should be interpreted with caution. The small sample size may have undermined our ability to detect modest associations and led to imprecise effect estimates in some regression models. Nevertheless, we retained sufficient statistical power to estimate joint associations between women’s and men’s urinary phthalates in single regression models adjusted for important confounding variables, and to identify hypotheses for future confirmation. We had few missing data points and our results were robust using a multiple imputation procedure to impute missing covariate information for some couples. Consistent with the exploratory nature of our study, to maximize detection of potential associations, we did not correct for multiple testing error [43]. Still, some associations may be chance findings given the large number of hypothesis tests and so our results require confirmation in a larger investigation.
Our reliance on a single spot urine sample may have misclassified phthalates exposure for some couples, given their short in vivo half-lives and substantial within-individual variabilities [44]. However, the habitual nature of exposure to LMW phthalates, such as DEP through use of personal care products, confers moderate reliability within-individual over time [12, 57], and previous studies report that a single spot urine sample is sufficient to describe average daily phthalate exposures [45]. Still, we expect that exposure misclassification was non-differential among women and so any bias was likely towards the null hypothesis. Furthermore, our fasting urine samples from women may have underestimated exposure to phthalates commonly associated with food products and food packaging materials, including metabolites of DEHP [46], which may also have underestimated associations among women. In fact, we detected very few values for MPP, MiNP, MiDP, and MCHP and we were unable to investigate them further. Nevertheless, we jointly modeled exposures from female patients and male partners in the same models, to more closely approximate the “couple-based” nature of reproduction.
We used PCA to generate “summary measures” for assessing associations between mixtures of phthalates, to more closely represent a real-life scenario [47]. The aforementioned Boston study also described associations between IVF outcomes and a mixture of eight urinary phthalate metabolites, bisphenol A, and parabens measured in women [28]. Women in the highest urinary DEHP metabolite concentration quartile had lower likelihoods of implantation (−22%), clinical pregnancy (−24%), and live birth (−38%) than women in the lowest quartile, although without statistical significance. A larger future investigation that uses a couples-based approach to more comprehensively integrate multiple phthalates in both partners will be necessary to interpret our results in a more definitive fashion.
Finally, we measured only urinary phthalate metabolites and so we cannot preclude confounding by other reproductive toxicants with similar sources of exposure, such as environmental phenols and parabens [48–50]. Similarly, we were unable to adjust for male BMI, a potentially important confounder when assessing IVF outcomes [51]. An E-value equal to 10.3 indicates that very strong unmeasured confounding could account for the observed association between men’s MHxP and live birth among couples in our study [52]. Although this was unlikely, a future paternal BMI-adjusted analysis is necessary to confirm the findings. We also did not adjust for socioeconomic status. However, couples initiating IVF treatment are often more educated and more affluent than the general population in U.S. states without mandated IVF treatment insurance coverage, like California [53–55]. Nevertheless, our study population is unlikely to represent all couples planning a pregnancy and may not be generalizable to infertile couples not using IVF [56].
5. Conclusions
Among couples undergoing infertility treatment, greater urinary phthalate metabolite concentrations were associated with lower likelihoods of pregnancy and live birth. In particular, exposures to MBP, MHxP, and MEHP were more strongly associated with pregnancy and live birth than other phthalate metabolites. Our results suggest that both female and male phthalate exposure may be similarly associated with IVF outcomes and so future investigations should consider couple-level exposure. Our results also suggest the importance of including the male partner in clinical recommendations for lifestyle and/or behavioral interventions with the aim to increase the chance of a live birth from IVF. However, given the exploratory nature of this study, these results will require confirmation in a larger and more comprehensive investigation.
Supplementary Material
Highlights:
Urinary phthalate metabolites were measured in IVF patients and their male partners
MBP, MEHP, and MHxP were inversely associated with biochemical pregnancy and live birth
Associations for men were of similar importance as associations for women
Clinical recommendations for interventions should consider both partners
Acknowledgements:
We are indebted to the study participants for making this work possible.
Funding:
This work was funded in part by the National Institute of Environmental Health Sciences (NIEHS) grant number R56ES023886 (MSB), and a grant to the Center for Social and Demographic Analysis (CSDA) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R24HD044943; MSB).
The study protocol was approved by the UCSF Committee for Human Research, and the Institutional Review Board at the University at Albany, State University of New York.
Abbreviations:
- BBzP
benzylbutyl phthalate
- BMI
body mass index
- CI
confidence interval
- DBP
dibutyl phthalate
- DCHP
dicyclohexyl phthalate
- DEHP
di-2-ethylhexyl phthalate
- DEP
diethyl phthalate
- DiDP
diisodecyl phthalate
- DiNP
di-isononyl phthalate
- DnHP
di-n-hexyl phthalate
- DPP
di-n-pentyl-phthalate
- FOR
fecundability odds ratio
- hCG
human chorionic gonadotropin
- HMW
high molecular weight
- IVF
in vitro fertilization
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- LMW
low molecular weight
- LOD
limit of detection
- MBP
monobutyl phthalate
- MBzP
monobenzyl phthalate
- MCHP
monocyclohexyl phthalate
- MCOP
mono carboxyoctyl phthalate
- MCPP
mono (3-carboxypropyl) phthalate
- MECPP
mono-(2-ethyl-5-carboxypentyl) phthalate
- MEHHP
mono(2-ethyl-5-hydroxyhexyl) phthalate
- MEHP
mono-ethylhexyl phthalate
- MEP
monoethyl phthalate
- MHxP
mono-n-hexyl phthalate
- MiBP
mono-isobutyl phthalate
- MiDP
mono-isodecyl phthalate
- MiNP
mono-isononyl phthalate
- MMP
mono-methyl phthalate
- MPP
mono-n-pentyl phthalate
- OR
odds ratio
- PCA
principal component analysis
- PVC
polyvinyl chloride
- RR
relative risk
- UCSF
University of California-San Francisco
- ΣDEHP
molar sum of MEHP, MEHHP, and MECPP
- ΣHMW
molar sum of MHxP, MEHP, MEHHP, MECPP, MiNP, and MBzP
- ΣLMW
molar sum of MEP, MBP, and MPP
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References:
- [1].CDC (Centers for Disease Control and prevention), The fourth national report on human exposure to environmental chemicals - January 2019. Update
- [2].Pacyga DC, Sathyanarayana S, Strakovsky RS, Dietary predictors of phthalate and bisphenol exposures in pregnant women, Adv. Nutr 10 (5) (2019) 803–815. 10.1093/advances/nmz029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kay VR, Chambers C, Foster WG, Reproductive and developmental effects of phthalate diesters in females, Crit. Rev. Toxicol 43 (3) (2013) 200–219. 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kay VR, Bloom MS, Foster WG, Reproductive and developmental effects of phthalate diesters in males, Crit. Rev. Toxicol 44 (6) (2014) 467–498. 10.3109/10408444.2013.875983. [DOI] [PubMed] [Google Scholar]
- [5].Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, Buck Louis GM, Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach, Fertil. Steril 99 (5) (2013) 1324–1331. 10.1016/j.fertnstert.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].The American College of Obstetricians and Gynecologists, Committee Opinion: Exposure to toxic environmental agents. Obstet. Gynecol (2013). 10.1097/01.aog.0000435416.21944.54 [DOI] [Google Scholar]
- [7].Di Renzo GC, Conry JA, Blake J, Defrancesco MS, Denicola N, Martin JN, McCue KA, Richmond D, Shah A, Sutton P, Woodruff TJ, Van Der Poel SZ, Giudice LC, International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals, Int. J. Gynecol. Obstet 131 (3) (2015) 219–225. 10.1016/j.ijgo.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Godwin A, Uses of phthalates and other plasticizers. Chronic Hazard Advisory Panel (CHAP) on Phthalates, ExxonMobil Chemical Company; Retrieved from https://www.cpsc.gov/s3fs-public/godwin.pdf/, 2010. (accessed 14 September 2020). [Google Scholar]
- [9].Begum TF, Gerona R, Melamed J, McGough A, Lenhart N, Wong R, Mok-Lin E, Butts CD, Feingold BJ, Romeiko XX, Fujimoto VY, Bloom MS, Sources of exposure to urinary phthalates among couples undergoing infertility treatment, Int. J. Hyg. Environ. Health 229 (2020) 113567 10.1016/j.ijheh.2020.113567. [DOI] [PubMed] [Google Scholar]
- [10].Heudorf U, Mersch-Sundermann V, Angerer J, Phthalates: Toxicology and exposure, Int. J. Hyg. Environ. Health 210 (5) (2007) 623–634. 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- [11].Zota AR, Calafat AM, Woodruff TJ, Temporal trends in phthalate exposures: Findings from the national health and nutrition examination survey, 2001–2010, Environ. Health Perspect 122 (3) (2014) 235–241. 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Al-Saleh I, Coskun S, Al-Doush I, Abduljabbar M, Al-Rouqi R, Al-Rajudi T, Al-Hassan S, Couples exposure to phthalates and its influence on in vitro fertilization outcomes, Chemosphere. 226 (2019) 597–606. 10.1016/j.chemosphere.2019.03.146. [DOI] [PubMed] [Google Scholar]
- [13].Dodge LE, Williams PL, Williams MA, Missmer SA, Souter I, Calafat AM, Hauser R, Associations between paternal urinary phthalate metabolite concentrations and reproductive outcomes among couples seeking fertility treatment, Reprod. Toxicol 58 (2015) 184–193. 10.1016/j.reprotox.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Du YY, Fang YL, Wang YX, Zeng Q, Guo N, Zhao H, Li YF, Follicular fluid and urinary concentrations of phthalate metabolites among infertile women and associations with in vitro fertilization parameters, Reprod. Toxicol 61 (2016) 142–150. 10.1016/j.reprotox.2016.04.005. [DOI] [PubMed] [Google Scholar]
- [15].Hauser R, Gaskins AJ, Souter I, Smith KW, Dodge LE, Ehrlich S, Meeker JD, Calafat AM, Williams PL, Urinary phthalate metabolite concentrations and reproductive outcomes among women undergoing in vitro fertilization: Results from the EARTH study, Environ. Health Perspect 124 (6) (2016) 831–839. 10.1289/ehp.1509760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Machtinger R, Gaskins AJ, Racowsky C, Mansur A, Adir M, Baccarelli AA, Calafat AM, Hauser R, Urinary concentrations of biomarkers of phthalates and phthalate alternatives and IVF outcomes, Environ. Int 111 (2018) 23–31. 10.1016/j.envint.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Messerlian C, Wylie BJ, Mínguez-Alarcón L, Williams PL, Ford JB, Souter IC, Calafat AM, Hauser R, Urinary concentrations of phthalate metabolites and pregnancy loss among women conceiving with medically assisted reproduction, Epidemiology. 27 (6) (2016) 879 10.1097/EDE.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, Gaudreau E, Foster WG, Choeurng V, Fraser WD, Phthalate and bisphenol A exposure among pregnant women in Canada - Results from the MIREC study, Environ. Int 68 (2014) 55–65. 10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- [19].Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K, Urinary bisphenol A, phthalates, and couple fecundity: The Longitudinal Investigation of Fertility and the Environment (LIFE) Study, Fertil. Steril 101 (5) (2014) 1359–1366. 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chin HB, Jukic AM, Wilcox AJ, Weinberg CR, Ferguson KK, Calafat AM, McConnaughey DR, Baird DD, Association of urinary concentrations of phthalate metabolites and bisphenol A with early pregnancy endpoints, Environ. Res 168 (2019) 254–260. 10.1016/j.envres.2018.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jukic AM, Calafat AM, Robert McConnaughey D, Longnecker MP, Hoppin JA, Weinberg CR, Wilcox AJ, Baird DD, Urinary concentrations of phthalate metabolites and bisphenol a and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss, Environ. Health Perspect 124 (3) (2016) 321–328. 10.1289/ehp.1408164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yi H, Gu H, Zhou T, Chen Y, Wang G, Jin Y, Yuan W, Zhao H, Zhang L, A pilot study on association between phthalate exposure and missed miscarriage, Eur. Rev. Med. Pharmacol. Sci 20 (9) (2016) 1894–1902. [PubMed] [Google Scholar]
- [23].Axelsson J, Rylander L, Rignell-Hydbom A, Jönsson BAG, Lindh CH, Giwercman A, Phthalate exposure and reproductive parameters in young men from the general Swedish population, Environ. Int 85 (2015) 54–60. 10.1016/j.envint.2015.07.005. [DOI] [PubMed] [Google Scholar]
- [24].Bloom MS, Whitcomb BW, Chen Z, Ye A, Kannan K, Buck Louis GM, Associations between urinary phthalate concentrations and semen quality parameters in a general population, Hum. Reprod 30 (11) (2015) 2645–2657. 10.1093/humrep/dev219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen Q, Yang H, Zhou N, Sun L, Bao H, Tan L, Chen H, Ling X, Zhang G, Huang L, Li L, Ma M, Yang H, Wang X, Zou P, Peng K, Liu T, Shi X, Feng D, Zhou Z, Ao L, Cui Z, Cao J, Phthalate exposure, even below US EPA reference doses, was associated with semen quality and reproductive hormones: Prospective MARHCS study in general population, Environ. Int 104 (2017) 58–68. 10.1016/j.envint.2017.04.005. [DOI] [PubMed] [Google Scholar]
- [26].Han X, Cui Z, Zhou N, Ma M, Li L, Li Y, Lin H, Ao L, Shu W, Liu J, Cao J, Urinary phthalate metabolites and male reproductive function parameters in Chongqing general population, China, Int. J. Hyg. Environ. Health 217 (2–3) (2014) 271–278. 10.1016/j.ijheh.2013.06.006. [DOI] [PubMed] [Google Scholar]
- [27].Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R, Phthalate exposure and reproductive hormones in adult men, Hum. Reprod 20 (3) (2005) 604–610. 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- [28].Mínguez-Alarcón L, Messerlian C, Bellavia A, Gaskins AJ, Chiu YH, Ford JB, Azevedo AR, Petrozza JC, Calafat AM, Hauser R, Williams PL, Urinary concentrations of bisphenol A, parabens and phthalate metabolite mixtures in relation to reproductive success among women undergoing in vitro fertilization, Environ. Int 126 (2019) 355–362. 10.1016/j.envint.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wirth JJ, Rossano MG, Potter R, Puscheck E, Daly DC, Paneth N, Krawetz SA, Protas BM, Diamond MP, A pilot study associating urinary concentrations of phthalate metabolites and semen quality, Syst. Biol. Reprod. Med 54 (3) (2008) 143–154. 10.1080/19396360802055921. [DOI] [PubMed] [Google Scholar]
- [30].Wu H, Ashcraft L, Whitcomb BW, Rahil T, Tougias E, Sites CK, Pilsner JR, Parental contributions to early embryo development: Influences of urinary phthalate and phthalate alternatives among couples undergoing IVF treatment, Hum. Reprod 32 (1) (2017) 65–75. 10.1093/humrep/dew301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gerona RR, Schwartz JM, Pan J, Friesen MM, Lin T, Woodruff TJ, Suspect screening of maternal serum to identify new environmental chemical biomonitoring targets using liquid chromatography-quadrupole time-of-flight mass spectrometry, J. Expo. Sci. Environ. Epidemiol 28 (2) (2018) 101–108. 10.1038/jes.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Richardson DB, Ciampi A, Effects of exposure measurement error when an exposure variable is constrained by a lower limit, Am. J. Epidemiol 157 (4) (2003) 355–363. 10.1093/aje/kwf217. [DOI] [PubMed] [Google Scholar]
- [33].Schisterman EF, Vexler A, Whitcomb BW, Liu A, The limitations due to exposure detection limits for regression models, Am. J. Epidemiol 163 (4) (2006) 374–383. 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zou G, A Modified Poisson Regression Approach to Prospective Studies with Binary Data, Am. J. Epidemiol 159 (7) (2004) 702–706. 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- [35].Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL, Urinary creatinine concentrations in the U.S. population: Implications for urinary biologic monitoring measurements, Environ. Health Perspect 113 (2) (2005) 192–200. 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kim K, Steuerwald AJ, Parsons PJ, Fujimoto VY, Browne RW, Bloom MS, Biomonitoring for exposure to multiple trace elements via analysis of urine from participants in the Study of Metals and Assisted Reproductive Technologies (SMART), J. Environ. Monit 13 (9) (2011) 2413–2419. 10.1039/c1em10341e. [DOI] [PubMed] [Google Scholar]
- [37].Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF, Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: A cross-sectional study of NHANES data, 1999–2002, Environ. Heal. A Glob. Access Sci. Source 7 (1) (2008) 27 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kudesia R, Wu H, Hunter Cohn K, Tan L, Lee JA, Copperman AB, Yurttas Beim P, The effect of female body mass index on in vitro fertilization cycle outcomes: a multi-center analysis, J. Assist. Reprod. Genet 35 (11) (2018) 2013–2023. 10.1007/s10815-018-1290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mlynarcikova A, Fickova M, Scsukova S, Ovarian intrafollicular processes as a target for cigarette smoke components and selected environmental reproductive disruptors, Endocr. Regul 39 (1) (2005) 21–32. [PubMed] [Google Scholar]
- [40].Lee KJ, Carlin JB, Multiple imputation for missing data: Fully conditional specification versus multivariate normal imputation. Am. J. Epidemiol 171 (5) (2010) 624–632. 10.1093/aje/kwp425 [DOI] [PubMed] [Google Scholar]
- [41].Yong AG, Pearce S, A Beginner’s Guide to Factor Analysis: Focusing on Exploratory Factor Analysis, Tutor. Quant. Methods Psychol 9 (2) (2013) 79–94. 10.20982/tqmp.09.2.p079. [DOI] [Google Scholar]
- [42].DiStefano C, Zhu M, Mîndrilǎ D, Understanding and using factor scores: Considerations for the applied researcher, Pract. Assessment, Res. Eval 14 (1) (2009) 20. [Google Scholar]
- [43].Goldberg M, Silbergeld E, On multiple comparisons and on the design and interpretation of epidemiological studies of many associations, Environ. Res 8 (11) (2011) 1007–1009. 10.1016/j.envres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- [44].Hauser R, Calafat AM, Phthalates and human health. Occup. Environ. Med 62 (2005) 806–818. 10.1136/oem.2004.017590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Christensen KLY, Lorber M, Koch HM, Kolossa-Gehring M, Morgan MK, Population variability of phthalate metabolites and bisphenol A concentrations in spot urine samples versus 24-or 48-h collections, J. Expo. Sci. Environ. Epidemiol 22 (6) (2012) 632–640. 10.1038/jes.2012.52. [DOI] [PubMed] [Google Scholar]
- [46].Zota AR, Phillips CA, Mitro SD, Recent fast food consumption and bisphenol A and phthalates exposures among the U.S. population in NHANES, 2003–2010, Environ. Health Perspect 124 (10) (2016) 1521–1528. 10.1289/ehp.1510803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].M Braun J, Gennings C, Hauser R, Webster TF, What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ. Health Perspect 124 (1) (2016) A6–A9. 10.1289/ehp.1510569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Calafat AM, Valentin-Blasini L, Ye X, Trends in exposure to chemicals in personal care and consumer products, Curr. Environ. Heal. Reports 2 (4) (2015) 348–355. 10.1007/s40572-015-0065-9. [DOI] [PubMed] [Google Scholar]
- [49].Koch HM, Aylward LL, Hays SM, Smolders R, Moos RK, Cocker J, Jones K, Warren N, Levy L, Bevan R, Inter- and intra-individual variation in urinary biomarker concentrations over a 6-day sampling period. Part 2: Personal care product ingredients, Toxicol. Lett 231 (2) (2014) 261–269. 10.1016/j.toxlet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- [50].Zota AR, Shamasunder B, The environmental injustice of beauty: framing chemical exposures from beauty products as a health disparities concern, Am. J. Obstet. Gynecol 217 (4) (2017) 418e1–418.e6. 10.1016/j.ajog.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Colaci DS, Afeiche M, Gaskins AJ, Wright DL, Toth TL, Tanrikut C, Hauser R, Chavarro JE, Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization, Fertil. Steril 98 (5) (2012) 1193–1199. 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].VanderWeele TJ, Ding P, Sensitivity analysis in observational research: Introducing the E-value, Ann. Intern. Med 167 (4) (2017) 268–274. 10.1016/j.envres.2011.08.010. [DOI] [PubMed] [Google Scholar]
- [53].Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, Glasier A, Sonnenberg P, Field N, Mercer CH, Johnson AM, Wellings K, Prevalence of infertility and help seeking among 15 000 women and men, Hum. Reprod 31 (9) (2016) 2108–2118. 10.1093/humrep/dew123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Farland LV, Collier ARY, Correia KF, Grodstein F, Chavarro JE, Rich-Edwards J, Missmer SA, Who receives a medical evaluation for infertility in the United States?, Fertil. Steril 105 (5) (2016) 1274–1280. 10.1016/j.fertnstert.2015.12.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Katz P, Showstack J, Smith JF, D Nachtigall R, G Millstein S, Wing H, L Eisenberg M, Pasch LA, Croughan MS, Adler N, Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil. Steril 95 (3) (2011) 915–921. 10.1016/j.fertnstert.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Den Hond E, Tournaye H, De Sutter P, Ombelet W, Baeyens W, Covaci A, Cox B, Nawrot TS, Van Larebeke N, D’Hooghe T, Human exposure to endocrine disrupting chemicals and fertility: A case-control study in male subfertility patients, Environ. Int 84 (2015) 154–160. 10.1016/j.envint.2015.07.017. [DOI] [PubMed] [Google Scholar]
- [57].Meeker JD, Calafat AM, Hauser R, Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women, J. Expo. Sci. Environ. Epidemiol 22 (4) (2012) 376–385, doi:https://doi-org.mutex.gmu.edu/10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


