Abstract
Objective:
To use childhood cancer survivors as a novel model to study whether children who experience central nervous system (CNS) injury are at higher risk for neurocognitive impairment associated with subsequent late onset chronic health conditions (CHC).
Methods:
Adult survivors of childhood cancer (n=2,859, ≥10 years from diagnosis, ≥18 years old) completed a comprehensive neurocognitive battery and clinical exam. Neurocognitive impairment was defined as age-adjusted Z-score <10th percentile. Participants impaired on ≥3 tests had global impairment. CHC were graded using the Common Terminology Criteria for Adverse Events v4.3 (grade 1:mild, 2:moderate, 3:severe/disabling), 4:life-threatening), and were combined into a severity/burden score by frequency and grade (none/low, medium, high, and very high). 1,598 survivors received CNS directed therapy including cranial radiation, intrathecal methotrexate, or neurosurgery. Logistic regression estimated the odds of neurocognitive impairment associated with severity/burden score and grade 2–4 conditions, stratified by CNS treatment.
Results:
CNS-treated survivors performed worse than non-CNS-treated survivors on all neurocognitive tests and were more likely to have global neurocognitive impairment (46.9% vs 35.3%, p<0.001). After adjusting for demographic and treatment factors, there was a dose-response association between severity/burden score and global neurocognitive impairment, but only among CNS-treated survivors (high OR=2.24, 95%CI 1.42–3.53; very high OR=4.07, 95%CI 2.30–7.17). Cardiovascular and pulmonary conditions were associated with processing speed, executive function, and memory impairments in CNS-treated but not non-CNS-treated survivors, who were impacted by neurologic conditions.
Interpretation:
Reduced cognitive/brain reserve associated with CNS-directed therapy during childhood may make survivors vulnerable to adverse cognitive effects of cardiopulmonary conditions during adulthood.
Introduction
There is significant variability in the onset and severity of dementia that can be influenced by premorbid environmental and health related experiences. Cognitive/brain reserve is one process proposed to explain these differences and refers to the individual cognitive variation that influences our ability to compensate for injury/pathology.1 Higher education and occupational attainment have been used as surrogates for cognitive/brain reserve in adult populations, demonstrating lower risk for dementia and less severe clinical courses in traumatic brain injury, Parkinson’s, and multiple sclerosis.2–6 However, when examining the impact of childhood neurologic injury on adult neurocognitive function, these constructs are not useful because childhood injury directly influences educational and occupational attainment. We propose here to use childhood cancer survivors to demonstrate how diminished brain plasticity and cognitive/brain reserve after cancer therapy may weaken the brain’s ability to compensate for additional stress/damage in mid-life such as that from chronic health conditions.7–10
Neurocognitive dysfunction in childhood cancer survivors is frequently associated with central nervous system (CNS) tumors and CNS-directed therapies including cranial radiation, neurosurgery, and intrathecal chemotherapy.11 The impact of these therapies is immediate and may alter brain development, functional integrity, and cognitive/brain reserve.7–10 Childhood cancer patients are also exposed to systemic therapies that are associated with high rates of adult-onset physical morbidity, including cardiovascular, pulmonary, and endocrine chronic health conditions.12 Sixty-eight percent of adult survivors of childhood cancer have at least one serious, life-threatening, or disabling condition.12 Many of the chronic health conditions childhood cancer survivors experience, such as diabetes, pulmonary dysfunction, and heart failure, have been associated with neurocognitive impairment in non-cancer populations.13–15 We propose that the impact of childhood cancer therapy is progressive and may only become fully apparent as other stress/damage is placed on the brain, such as that from various chronic health conditions, and that those with diminished cognitive/brain reserve from CNS-directed therapy will be more vulnerable.16
The aim of this study was to characterize the risk of neurocognitive impairment associated with chronic health conditions in adult survivors of childhood cancers who did or did not received CNS-directed therapy. We hypothesized that survivors treated with CNS-directed therapies will be more susceptible to the impact of chronic health conditions on neurocognitive function compared to those treated without CNS-directed therapies due to diminished cognitive/brain reserve. These findings may inform on the impact of injury during brain development from cancer treatment, traumatic brain injury, and other life-threatening health conditions, on the brain’s ability to compensate for additional aging-related pathophysiologic injury and stress.
Methods
Participants
Participants were survivors of childhood cancer treated at St. Jude Children’s Research Hospital (SJCRH) enrolled in the St. Jude Lifetime Cohort Study (SJLIFE), a dynamic cohort established to facilitate prospective assessment of health outcomes among long-term childhood cancer survivors.17 For the current analyses, survivors were followed through July 2017 and must have been treated at SJCRH, be at least ten years from diagnosis, and at least 18 years of age. Survivors who were non-English speaking, had a genetic or neurodevelopmental syndrome associated with cognitive impairment, or neurologic injury unrelated to cancer treatment (e.g. traumatic brain injury) were excluded. Hematopoietic stem cell transplantation survivors (n=219) were excluded because of unknown exposure to the CNS from varying intensity of conditioning regimens and immunologic changes associated with the transplantation. Of 3,181 potentially eligible survivors, 2,859 (89.9%) were eligible and included in the analyses (Figure 1). All participants provided written informed consent and the study was approved by the institutional review board.
Figure 1:
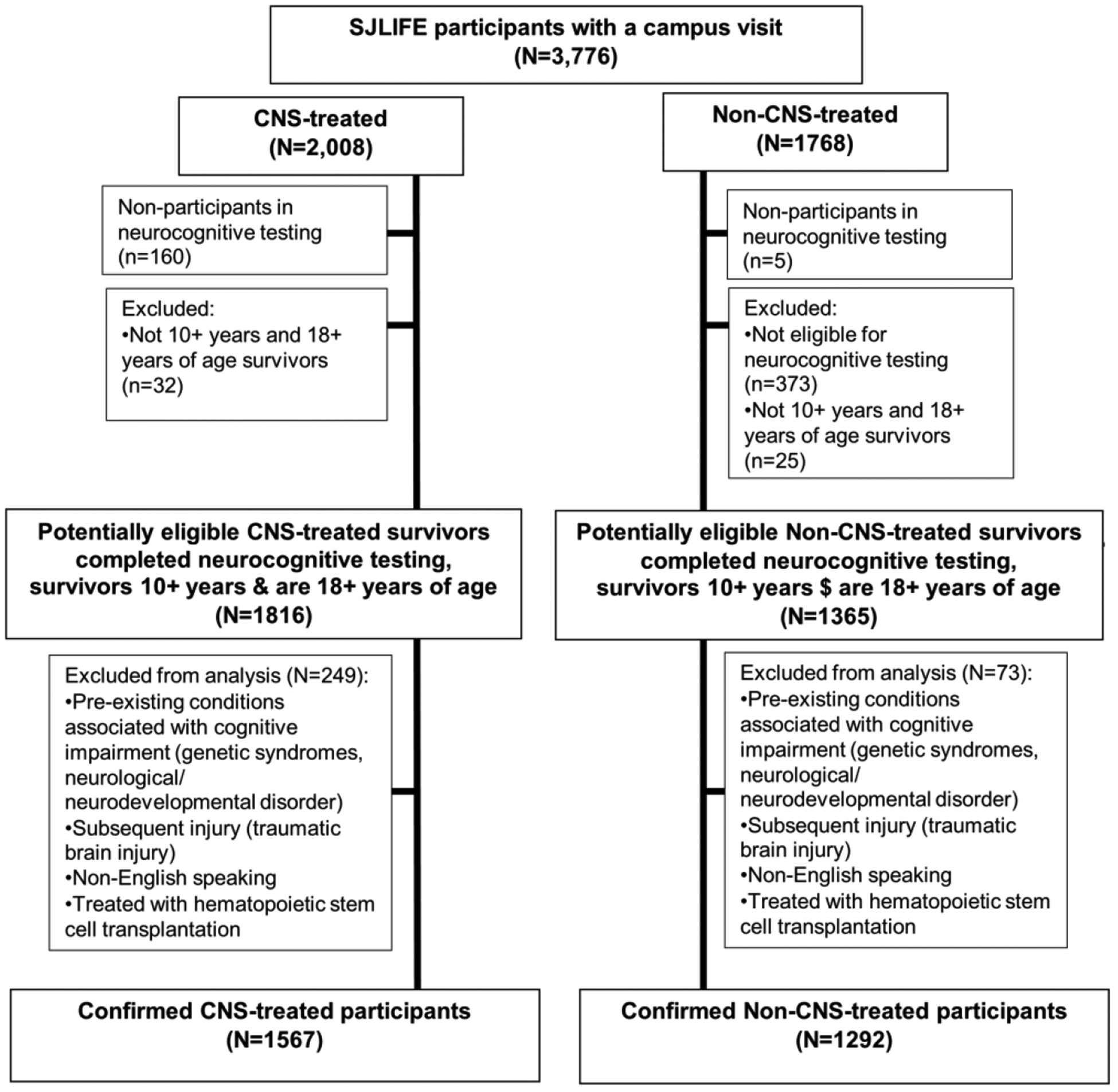
Participant enrollment and completion flowchart
Procedures
Treatment history including chemotherapy (cumulative doses), surgical procedures, and radiation therapy (fields and doses) were abstracted from medical records. We were specifically interested in characterizing participants who received cancer treatment physically directed to the CNS. Therefore, any participant who received cranial radiation therapy, intrathecal methotrexate, or underwent neurosurgery was classified as having received CNS-directed therapy (CNS-treated survivor). Along with neurosurgery, cumulative doses of cranial radiation and intrathecal methotrexate were adjusted for in multivariate analyses to account for the variability in these exposures as well as their known impact on neurocognitive function.18 Other treatments that are not directed to the CNS but are risk factors for neurocognitive impairment in childhood cancer survivors (e.g. intravenous methotrexate and cytarabine) were also adjusted for in multivariable analyses.
Participants underwent a comprehensive neurocognitive assessment that included tests of intelligence/academics,19, 20 attention,21, 22 processing speed,23, 24 memory,25 and executive function.22, 24, 25 Testing was completed in standardized order and administered by certified neurocognitive examiners under the supervision of a board-certified clinical neuropsychologist. Neurocognitive examiners were not blinded to cancer treatment exposures; however, they were blind to the study hypothesis and any chronic health conditions the participant may have had. Scores were referenced to national normative data to generate age-adjusted Z-scores for each neurocognitive outcome. As previously done in studies using this cohort, neurocognitive impairment was defined as a Z-score below −1.28 (10th percentile).10, 26 If a participant was impaired on three or more neurocognitive tests they were classified as having global neurocognitive impairment.. Results are discussed in the text for global impairment and one test representative of each cognitive domain; results from the entire battery are available in the tables and supplementary materials.
Participants also underwent a comprehensive health assessment, including a complete medical history, physical examination (including resting heart rate, blood pressure, and electrocardiography), laboratory evaluation (including complete blood count, comprehensive metabolic panel, fasting lipid profile, insulin, and hemoglobin A1c), physical performance assessment (including body composition measurement), echocardiography and pulmonary function tests.17
Chronic health conditions (CHC) were graded using the SJLIFE-modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) version 4.3 (Grade 1 = mild, Grade 2 = moderate, Grade 3 = severe or disabling, Grade 4 = life-threatening).27 Consent for external medical record review was obtained to validate CHC diagnosed prior to the SJLIFE visit. CHCs could have occurred anytime between diagnosis and the date of the neurocognitive exam. Thirty-eight different composite groups of CHC28 were included in this analysis due to their potential to influence neurocognitive functioning. These groups were then classified by organ system (cardiovascular, pulmonary, endocrine, neurologic, or other). For multiple cases of CHC, the highest-grade within a group or organ system was used. To estimate the impact of multiple CHC of varying severity, a severity/burden score was calculated according to previously published methods that take into account the frequency and grade of conditions.29 Survivors were grouped into hierarchical categories of none/low, medium, high, and very high severity/burden score. Categories were defined as “none/low” being grade 1 conditions only; “medium” being ≥1 grade 2 and/or 1 grade 3 condition(s); “high” being ≥2 grade 3, or 1 grade 4 and 1 grade 3 conditions; and “very high” being ≥2 grade 4 or ≥2 grade 3 and 1 grade 4 condition(s).
Statistical Analysis
A priori we hypothesized that neurocognitive function of survivors treated with CNS-directed therapies would be differentially affected by CHC. Therefore, analyses were stratified by whether or not survivors received CNS-directed therapies. Descriptive characteristics for demographic and treatment variables as well as CHC were calculated. One-sample t-tests compared mean age-adjusted Z-scores to the normative mean (mean=0 and SD=1) for each neurocognitive test. Neurocognitive test scores were compared between CNS- and non-CNS-treated survivors using generalized linear models adjusted for age at diagnosis, sex, and race. For multiple hypothesis testing adjustment, the FDR-controlling p-values with linear step-up method are reported (MULTTEST procedure in SAS).30 Associations with CHC were examined for any neurocognitive test that met the following criteria for either the CNS- or non-CNS-treated survivors: 1) more than 10% of survivors were impaired; and, 2) survivors performed statistically significantly worse than population mean.
Logistic regression was used to estimate the odds of global neurocognitive impairment associated with the CHC severity/burden score. An interaction term between severity/burden score and CNS group was added to the model in order to test if the association between severity/burden score and global impairment differed in CNS- and non-CNS-treated survivors. Logistic regression also estimated the odds of impairment on each neurocognitive test associated with associated with any grade 2–4 condition within each system (cardiovascular, respiratory, endocrine, and neurologic). In exploratory analyses, we also estimated the odds of impairment associated with any specific CHC that has been previously associated with neurocognitive impairment in the general population.13–15, 31–33 All models were adjusted for a priori defined confounders: age at diagnosis, sex, race, time since diagnosis, cumulative dose of high-dose intravenous cytarabine and high-dose intravenous methotrexate.18 Models in CNS-treated survivors were further adjusted for neurosurgery, cumulative cranial radiation dose, and cumulative intrathecal methotrexate dose.18 All p-values and confidence intervals were corrected for false discovery rate.30, 34
Path analysis was used to explore associations between treatment factors, mediated by CHC (grade 2–4, by system), with impairment in a neurocognitive domain (processing speed, memory, executive function, and attention). A hypothesized a priori model was generated based on existing literature and findings of our primary analysis for CNS- and non-CNS-treated survivors. The model was expanded by adding clinically meaningful paths, one at a time, with a modification index of 3.6 or greater, beginning with the largest index value. The model was then reduced by removing paths with non-significant coefficients, beginning with the largest p-value. This process was iterative until the best model-fitting criteria were achieved; a good fitting model includes a comparative fit index (CFI) and Tucker Lewis Index (TLI) ≥0.95, and the Root Mean Square Error of Approximation (RMSEA) <0.05.35 Analyses were again stratified by whether or not participants received CNS-directed therapy.
There were no missing data for independent variables. Some participants did not complete a neurocognitive test based on sensory limitations (e.g. vision impairment) or refusal of a specific test. Missingness for neurocognitive outcomes ranged from 1.6% to 5.6% except for the TOMAL-2 Visual Selective Reminding test of visual memory (14.7%) because this test was added to the battery after the study was underway. Missing neurocognitive outcomes were not imputed and the sample was not restricted to survivors with complete testing as to not bias the overall findings. All hypotheses testing was 2-sided and considered statistically significant with an FDR adjusted p-value <0.05. All analyses were completed using SAS 9.4 (SAS Institute, Cary, N.C.) and MPLUS version 7.11.
Results
Demographic and clinical data for survivors treated with and without CNS-directed therapy are presented in Table 1. CHC severity/burden scores were similar among CNS-treated (8.0% none/low, 48.6% medium, 32.3% high, 11.4% very high) and non-CNS-treated (6.8% none/low, 50.5% medium, 32.9% high, 9.8% very high) survivors (Table 1). Non-CNS-treated survivors experienced grade 2–4 cardiovascular (47.9% vs. 40.2%, p<0.001) and pulmonary (34.7% vs. 21.4 %, p<0.001) CHC more frequently than CNS-treated survivors (Table 2, Supplemental Table 1).
Table 1:
Demographic, clinical and treatment characteristics.
| CNS-Treated Survivors (N=1567) | Non-CNS-Treated Survivors (N=1292) | ||||||
|---|---|---|---|---|---|---|---|
| N (%) | M (SD) | N (%) | M (SD) | ||||
| Age at evaluation, years | 33.0 (8.84) | 36.2 (9.84) | |||||
| Age at diagnosis, years | 7.6 (4.80) | 9.1 (6.33) | |||||
| Time since diagnosis, years | 25.5 (9.05) | 27.1 (9.57) | |||||
| Male | 835 (53.30) | 653 (50.54) | |||||
| Race/Ethnicity | |||||||
| White, non-Hispanic | 1345 (85.83) | 1012 (78.33) | |||||
| Black, non-Hispanic | 185 (11.81) | 258 (19.97) | |||||
| Hispanic | 20 (1.28) | 14 (1.08) | |||||
| Other | 17 (1.08) | 8 (0.62) | |||||
| Diagnosis | |||||||
| Acute lymphoblastic leukemia | 977 (62.35) | 1 (0.08) | |||||
| CNS tumor | 324 (20.68) | 22 (1.70) | |||||
| Ewing sarcoma | 6 (0.38) | 72 (5.57) | |||||
| Hodgkin lymphoma | 6 (0.38) | 330 (25.54) | |||||
| Neuroblastoma | 0 (0.00) | 106 (8.20) | |||||
| Non-Hodgkin lymphoma | 162 (10.34) | 43 (3.33) | |||||
| Osteosarcoma | 2 (0.13) | 119 (9.21) | |||||
| Retinoblastoma | 3 (0.19) | 101 (7.82) | |||||
| Rhabdomyosarcoma | 17 (1.08) | 81 (6.27) | |||||
| Soft tissue sarcoma | 6 (0.38) | 64 (4.95) | |||||
| Wilms tumor | 2 (0.13) | 183 (14.16) | |||||
| Othersa | 62 (3.96) | 170 (13.16) | |||||
| Radiation | |||||||
| Any radiation treatment | 867 (55.33) | 749 (57.97) | |||||
| Cranial radiation (Gy) (yes) | 822 (52.46) | 32.1 (19.4) | |||||
| ≤ 20 | 227 (27.53) | 17.7 (2.0) | |||||
| > 20 to 30 | 321 (39.10) | 24.2 (1.2) | |||||
| >30 | 274 (33.37) | 53.2 (21.0) | |||||
| Chest (Gy) (yes) | 27 (1.72) | 46.7 (3.5) | 380 (29.40) | 36.7(25.7) | |||
| ≤ 35 | 13 (48.15) | 23.3 (9.7) | 243(63.95) | 23.1 (6.5) | |||
| > 35 | 14 (51.85) | 68.5 (35.2) | 137(36.04) | 61.1 (28.6) | |||
| Chemotherapy (mg/m2) | |||||||
| High-dose IV cytarabineb | 123 (7.85) | 16053 (12945) | 6 (0.46) | 25647 (18394) | |||
| Standard-dose IV cytarabine | 898 (57.31) | 6284 (5157) | 9 (0.70) | 1473 (720) | |||
| High-dose IV methotrexateb | 721 (46.01) | 14112 (9694) | 101 (7.82) | 86920 (40971) | |||
| Standard-dose IV methotrexate | 642 (40.97) | 2334 (1634) | 136 (10.53) | 278 (606) | |||
| Intrathecal methotrexate | 1156 (73.77) | 174 (112) | |||||
| IV Vincristine | 1256 (80.15) | 36 (27) | 716 (55.42) | 26 (19) | |||
| Anthracyclinec | 931 (59.41) | 158 (116) | 733 (56.73) | 243 (111) | |||
| Cyclophosphamided | 902 (57.56) | 9459 (6516) | 732 (56.66) | 10530 (7453) | |||
| Cisplatin | 55 (3.51) | 343 (216) | 153 (11.84) | 451 (205) | |||
| Corticosteroid (All) (Yes/No) | 1132 (72.24) | 249 (19.27) | |||||
| Neurosurgery (yes) | 370 (23.61) | ||||||
| Chronic Health Condition Severity/Burden Scoref | |||||||
| None/Low | 126 (8.0) | 88 (6.8) | |||||
| Medium | 762 (48.6) | 652 (50.5) | |||||
| High | 506 (32.3) | 425 (32.9) | |||||
| Very High | 173 (11.1) | 127 (9.8) | |||||
CNS: central nervous system; M(SD): mean and standard deviation; IV: intravenous; Gray empty cells are not applicable.
Acute myeloid leukemia, chronic myeloid leukemia, colon carcinoma, histiocytosis, germ cell tumors, liver malignancies, myelodysplastic syndrome, nasopharyngeal carcinoma, other carcinoma, other leukemia, and other malignancy,
high dose: 1g/m2 per treatment,
based on COG Long-Term Follow Up Guidelines 2018,
Green at al Pediatr Blood Cancer 2014 Jan; 6(61(1):53–67,
methylprednisolone, prednisolone, and prednisone,
Low: having only grade 1 conditions; Medium: having ≥1 grade 2 and/or 1 grade 3 condition; High: having ≥ 2 grade 3 conditions or 1 grade 4 and 1 grade 3 conditions; Very High: ≥2 grade 4 events or ≥ 2 grade 3 conditions and a grade 4 condition.
Table 2:
Frequency of none, grade 1, and 2–4 chronic health conditions1 in CNS- and non-CNS-treated survivors.
| CNS-Treated | Non-CNS-Treated | p-value | |
|---|---|---|---|
| Any Cardiovascular | |||
| None/Grade 1 | 937 (59.8) | 6736 (52.1) | <0.001 |
| Grade 2–4 | 630 (40.2) | 619 (47.9) | |
| Any Respiratory | |||
| None/Grade 1 | 1231 (78.6) | 843 (65.3) | 0.001 |
| Grade 2–4 | 336 (21.4) | 449 (34.7) | |
| Any Endocrine | |||
| None/Grade 1 | 295 (18.8) | 289 (22.4) | 0.020 |
| Grade 2–4 | 1272 (81.2) | 1003 (77.6) | |
| Any Neurologic | |||
| None/Grade 1 | 942 (60.1) | 932 (72.1) | 0.001 |
| Grade 2–4 | 625 (39.9) | 360 (27.9) |
Chronic health conditions classified based on Bhakta et al Lancet 2017{Bhakta, 2017 #340},
dyslipidemia includes hypertriglyceridemia or hypercholesterolemia,
functional pulmonary deficits include obstructive, restrictive or diffusion abnormalities on pulmonary function tests,
chronic respiratory disorder included asthma or chronic obstructive pulmonary disease.
As expected, CNS-treated survivors were more likely to experience global neurocognitive impairment (46.9% vs. 35.3%, p<0.001). A significant proportion of both non-CNS- and CNS-treated survivors were impaired on each neurocognitive test (Table 3). Additionally, CNS-treated survivors performed significantly worse on all neurocognitive tests compared to non-CNS-treated survivors (Table 3).
Table 3:
Comparison of CNS- and non-CNS-treated survivors on neurocognitive outcomes.
| Neurocognitive Outcomes^ | CNS-Treated (N=1597) | Non-CNS-Treated (N=1292) | ||||
|---|---|---|---|---|---|---|
| Global Cognition | Mean (SD) | Impairment1 % (95% Cl) | Mean (SD) | Impairment1 % (95% Cl) | P2 | |
| Verbal reasoning | −0.471* (1.15) | 21.64 (19.60, 23.68) | −0.223* (1.06) | 15.04 (13.09,17.00) | <0.001 | |
| Non-Verbal reasoning | −0.116 (1.04) | 12.03 (10.45, 13.75) | 0.118* (0.91) | 7.12 (5.77,8.67) | <0.001 | |
| Academics | ||||||
| Word reading | −0.394* (0.77) | 10.11 (8.63, 11.74) | −0.234* (0.63) | 4.31 (3.17,5.44) | <0.001 | |
| Mathematics | −0.697* (1.10) | 24.02 (21.88, 26.26) | −0.445* (0.92) | 14.95 (12.95,16.95) | <0.001 | |
| Attention | ||||||
| Sustained Attention | −0.327* (1.40) | 16.70 (14.86, 18.67) | −0.052* (1.21) | 10.33 (8.70,12.14) | <0.001 | |
| Variability | −0.352* (1.25) | 17.74 (15.85,19.75) | −0.268 (1.20) | 15.51 (13.55,17.63) | 0.023 | |
| Commissions | −0.092 (1.11) | 12.97 (11.32,14.76) | 0.095* (1.04) | 10.33 (8.70,12.14) | <0.001 | |
| Focused attention | −0.252* (1.44) | 17.99 (16.12,20.00) | 0.240* (1.13) | 8.95 (7.45,10.64) | <0.001 | |
| Processing speed | ||||||
| Visual-motor processing speed | −0.417* (1.14) | 26.08 (23.91,28.34) | −0.018 (1.00) | 12.13 (10.38,14.05) | <0.001 | |
| Motor processing speed | −1.044* (1.52) | 36.40 (34.01,38.85) | −0.584* (1.31) | 23.24 (20.95,25.65) | <0.001 | |
| Memory | ||||||
| Short-term memory | −0.290* (1.03) | 12.36 (10.77,14.10) | −0.042* (0.994) | 7.38 (6.01,8.94) | <0.001 | |
| Verbal Learning | −0.192* (1.27) | 17.31 (15.46,19.28) | −0.005 (1.14) | 12.68 (10.90,14.63) | <0.001 | |
| Short-term verbal recall | −0.236* (1.27) | 18.60 (16.69,20.62) | −0.082 (1.10) | 13.23 (11.42,15.22) | <0.001 | |
| Long-term verbal recall | −0.341* (1.30) | 22.34 (20.29,24.50) | −0.201* (1.16) | 18.20 (16.11,20.43) | <0.001 | |
| Visual memory3 | −0.588* (1.22) | 31.16 (28.66,33.75) | −0.251* (1.12) | 21.60 (19.27,24.08) | <0.001 | |
| Executive function | ||||||
| Perseveration | −0.354* (1.36) | 18.53 (16.61,20.57) | −0.163* (1.20) | 14.61 (12.71, 16.69) | <0.001 | |
| Working memory | −0.318* (0.97) | 9.80 (8.37,11.38) | −0.156* (0.90) | 4.20 (3.17,5.44) | <0.001 | |
| Cognitive flexibility | −0.793* (1.71) | 30.87 (28.58,33.23) | −0.262* (1.48) | 20.34 (18.17,22.65) | <0.001 | |
| Verbal fluency | −0.418* (1.16) | 27.23 (25.03,29.51) | −0.166* (1.10) | 17.77 (15.72,19.96) | <0.001 | |
CNS: central nervous system.
Higher scores for these measures are indicative of better functioning,
indicates mean is statistically significantly (P<0.05) different from population mean of 0 (SD=1).
Impairment defined as having a Z-score below the 10th percentile compared to national norms,
linear regression models to compare neurocognitive performance in survivors treated with and without direct CNS-targeted therapies adjusted for age at diagnosis, sex, and race, corrected for the false discovery rate,
15% of participants missing this test due to late addition to study protocol.
High and very high severity/burden scores were significantly associated with higher risk of global neurocognitive impairment in both groups, with a dose response effect observed among CNS-treated survivors (high OR=2.11, 95%CI 1.33–3.33; very high OR=4.22, 95%CI 2.40–7.43; Figure 2). The risk of global neurocognitive impairment associated with a very-high severity/burden score was significantly greater among CNS compared to non-CNS-treated survivors (OR 1.78, 95%CI 1.01,3.11) while the risk associated with high (OR 0.85, 95%CI 0.61,1.2) and moderate (1.01, 95%CI 0.74, 1.37) severity/burden score was similar. When specific CNS exposures were examined, survivors who received >30 Gy of cranial radiation were at highest risk for global impairment associated with a very high severity burden score (OR 12. 73, 95%CI 2.74,59.18; Figure 2).
Figure 2: Risk of Global Neurocognitive Impairment Associated with Severity/Burden Score Among A) CNS Treated Survivors and Non-CNS-Treated survivors and B) Specific CNS Exposures.
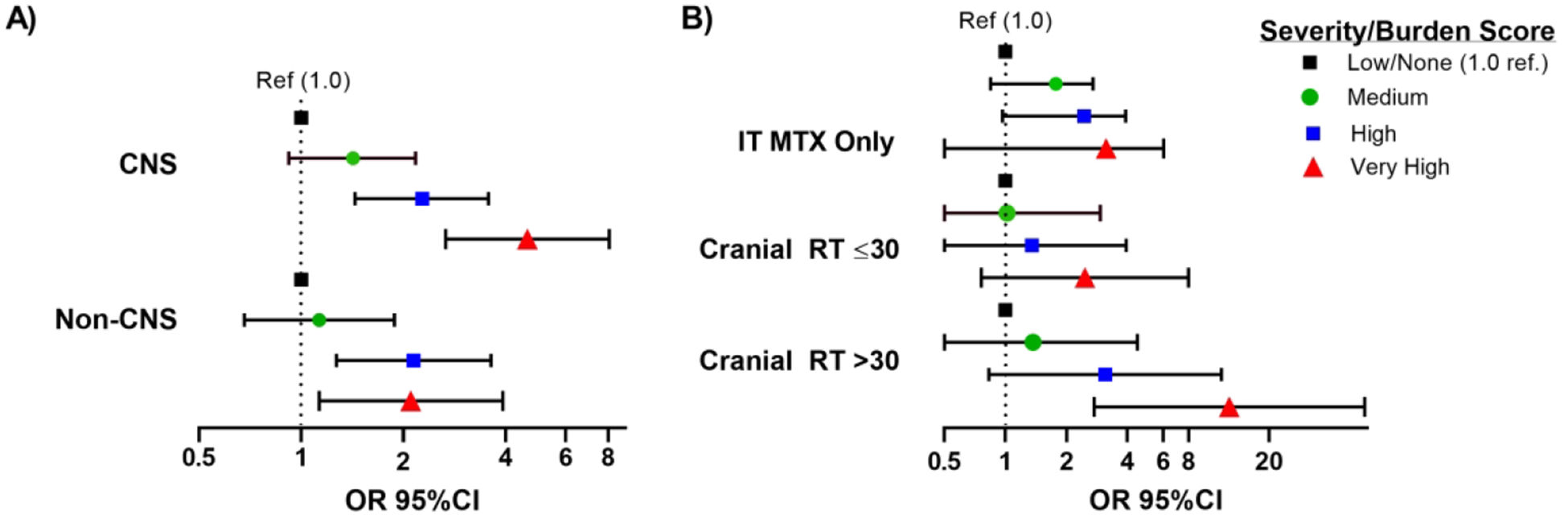
The bars and error bars below represent odds ratios and 95% confidence intervals for the risk of global neurocognitive impairment (age adjusted Z-score <10th percentile on ≥3 tests) associated with severity/burden score. The black dotted line represents the null reference association (OR=1, none/low burden score). In both CNS- and non-CNS-treated survivors, a high or very high severity/burden score was associated with an increased risk of global neurocognitive impairment compared to those with no conditions or a low burden score. A dose response relationship between burden score and risk of global neurocognitive impairment exists among CNS-treated survivors. Those with a low severity/burden score have only grade 1 condition, those with a medium score have ≥1 grade 2 and/or 1 grade 3 condition, those with a high score have ≥ 2 grade 3 conditions or 1 grade 4 and 1 grade 3 conditions, those with a very high score have ≥ 2 grade 4 events or ≥ 2 grade 3 conditions and a grade 4 condition. Logistic regression models are adjusted for age at diagnosis, gender, race, time since diagnosis, any high-dose cytarabine and cumulative dose of high-dose methotrexate. The CNS model is further adjusted for neurosurgery, cumulative cranial radiation dose, and intrathecal methotrexate dose.
Among CNS-treated survivors, any moderate, severe, disabling, or life-threatening (grade 2+) cardiovascular condition was associated with a higher risk of impairment on tests of verbal reasoning, mathematics, motor-processing speed, and cognitive flexibility, however only motor-processing speed was statistically significant among non-CNS-treated survivors (Figure 3, Supplemental Table 2). These associations appeared to be driven by hypertension and dyslipidemia in both groups. In both groups, hypertension was associated with impaired motor-processing speed (CNS OR 1.49, 95%CI 1.13–1.96, non-CNS OR 2.16 95%CI 1.52–3.08) and cognitive flexibility (CNS OR 1.57 95%CI 1.15–2.14, non-CNS OR 1.54 95%CI 1.04–2.28) while dyslipidemia was associated with impaired motor-processing speed (CNS OR 1.85 95%CI 1.36–2.52, non-CNS OR 1.70 95%CI 1.12–2.57).
Figure 3: Risk of Neurocognitive Impairment Associated with A)Cardiovascular or Pulmonary Conditions and B) Endocrine or Neurologic Conditions.
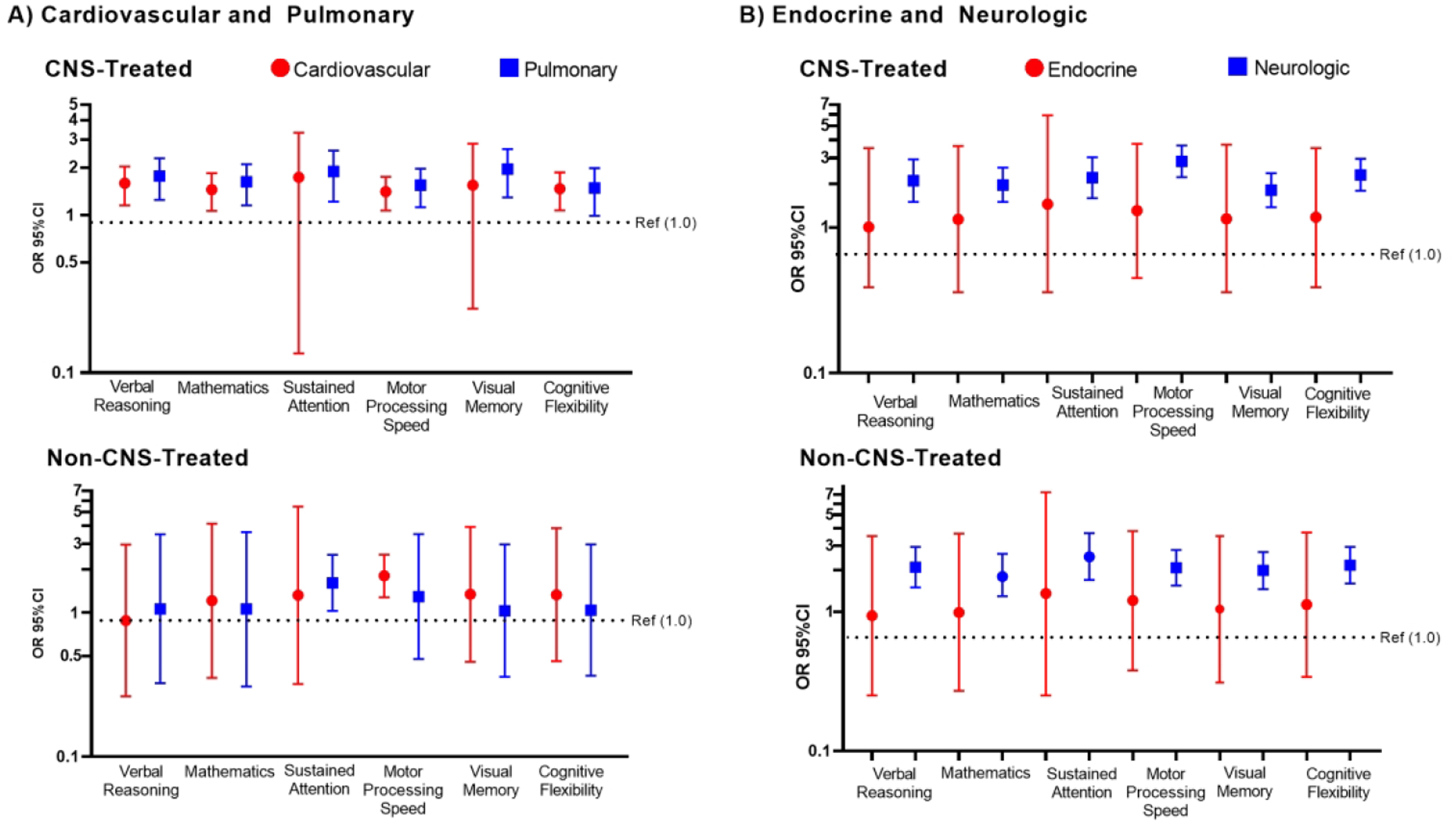
The bars and error bars below represent odds ratios (OR) and 95% confidence intervals for the risk of neurocognitive impairment associated with any grade 2 or higher condition. The black dotted line represents the null reference association (OR=1, no grade 2 or higher condition). Logistic regression models are adjusted for age at diagnosis, gender, race, time since diagnosis, any high-dose cytarabine and cumulative dose of high-dose methotrexate. Central nervous system (CNS) models are further adjusted for neurosurgery, cumulative cranial radiation dose, and intrathecal methotrexate dose.
Among CNS-treated survivors, grade 2+ pulmonary conditions were associated with a higher risk of impairment on tests of verbal reasoning, mathematics, attention, motor processing speed, and visual memory (Figure 3, Supplemental Table 2), and appeared to be attributable to functional pulmonary deficits rather than chronic respiratory disease. Functional pulmonary deficits were associated with an increased risk of impairment in verbal reasoning (OR=2.26, 95%CI 1.62–3.13), mathematics (OR=1.98 95%CI 1.42–2.74), attention (OR=1.93, 95%CI 1.23–3.02), motor processing speed (OR=1.56, 95%CI 1.13–2.15), and visual memory (OR=1.79, 95%CI 1.22–2.53) among CNS-treated survivors but not among non-CNS-treated survivors. No statistically significant associations with chronic respiratory disease were observed.
Although no statistically significant associations with grade 2+ endocrine conditions were noted in either group (Figure 3, Supplemental Table 2), abnormal glucose metabolism was associated with impaired motor processing speed among CNS-treated (OR=2.09, 95%CI 1.48–2.95) and non CNS-treated survivors (OR=1.79, 95%CI 1.05–3.05). Among CNS-treated survivors abnormal glucose metabolism was also associated with impaired cognitive flexibility (OR=1.56, 95%CI 1.06–2.31) and hypothyroidism was associated with increased risk of impairment on mathematics (OR=1.44, 95%CI 1.02–2.03) and cognitive flexibility (OR=1.51, 95%CI 1.10–2.08). Interestingly, hypothyroidism appeared protective for impairment on cognitive flexibility among non-CNS-treated survivors (OR=0.59, 95%CI 0.36–0.96).
Any grade 2+ neurologic condition was associated with an increased risk of neurocognitive impairment on all neurocognitive tests in both non-CNS- and CNS-treated survivors (Figure 3, Supplemental Table 2). Among CNS-treated survivors, cerebrovascular disease and peripheral neuropathy contributed similarly to risk of neurocognitive impairment (Figure 4). Notably, among non-CNS-treated survivors, peripheral neuropathy was significantly associated with neurocognitive impairment in all domains (Figure 4, all p<0.001).
Figure 4: Risk of Neurocognitive Impairment Associated with Cerebrovascular Conditions or Peripheral Neuropathy.
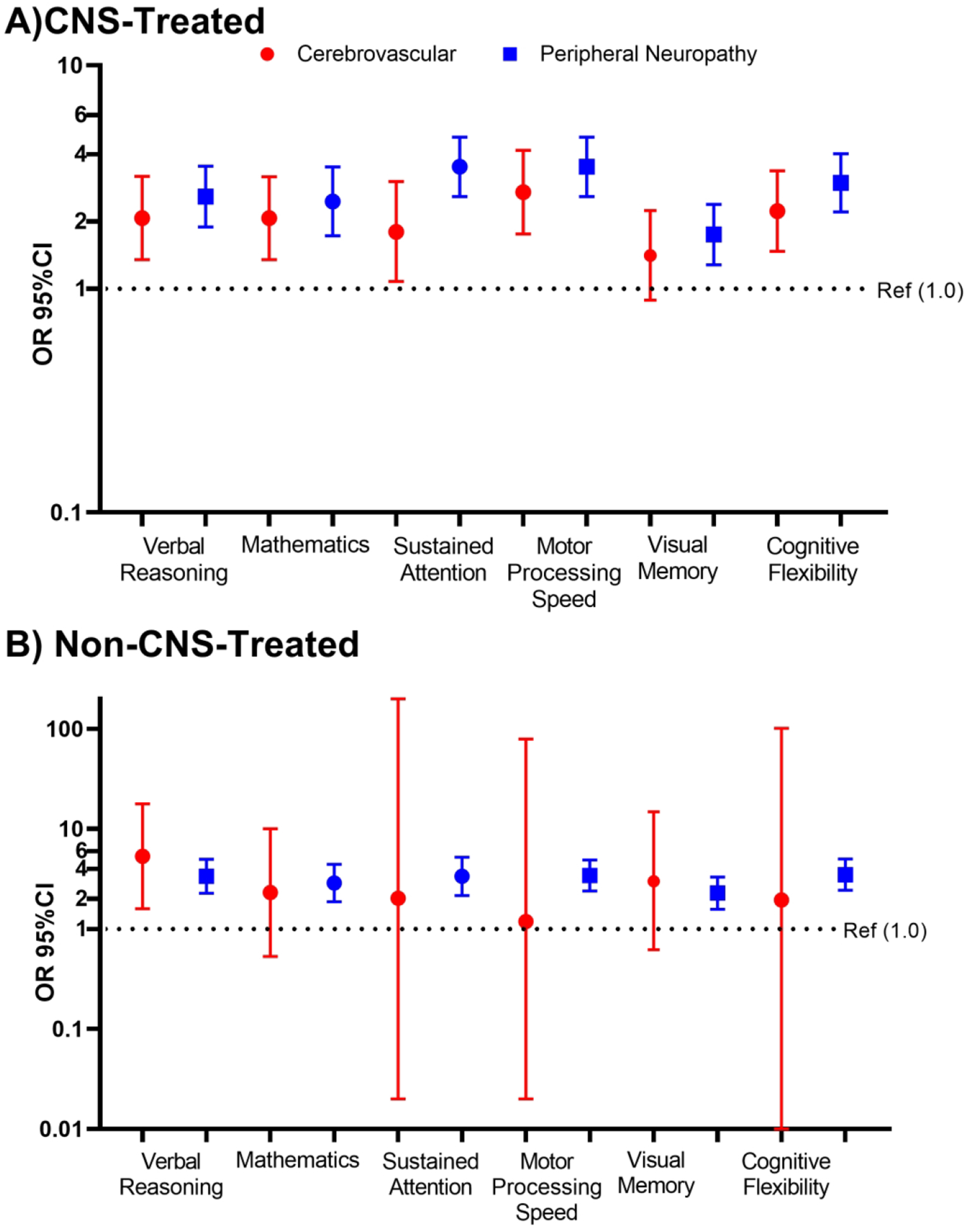
The bars and error bars below represent odds ratios (OR) and 95% confidence intervals for the risk of neurocognitive impairment associated with any grade 2 or higher cerebrovascular conditions (red) or peripheral neuropathy (blue) condition among A) CNS-treated and B) non-CNS-treated survivors. Cerebrovascular conditions included cerebrovascular disease or accidents and intracranial hemorrhage. The black dotted line represents the null reference association (OR=1, no grade 2 or higher condition). Logistic regression models are adjusted for age at diagnosis, gender, race, time since diagnosis, any high-dose cytarabine and cumulative dose of high-dose methotrexate. CNS models are further adjusted for neurosurgery, cumulative cranial radiation dose, and intrathecal methotrexate dose.
Exploratory path analyses revealed cranial radiation dose had significant indirect effects through neurologic conditions on attention and executive function, as well as through pulmonary conditions for a higher risk of memory impairment (Figure 5). Significant indirect associations of neurosurgery through neurologic conditions on impairment in memory, attention, processing speed, and executive function were also observed. Among survivors treated without CNS therapy, anthracycline dose and chest radiation dose had an indirect effect on processing speed through cardiovascular conditions (Figure 5).
Figure 5: Structural Paths for the Mediation of Treatment Effects on Neurocognitive Function.
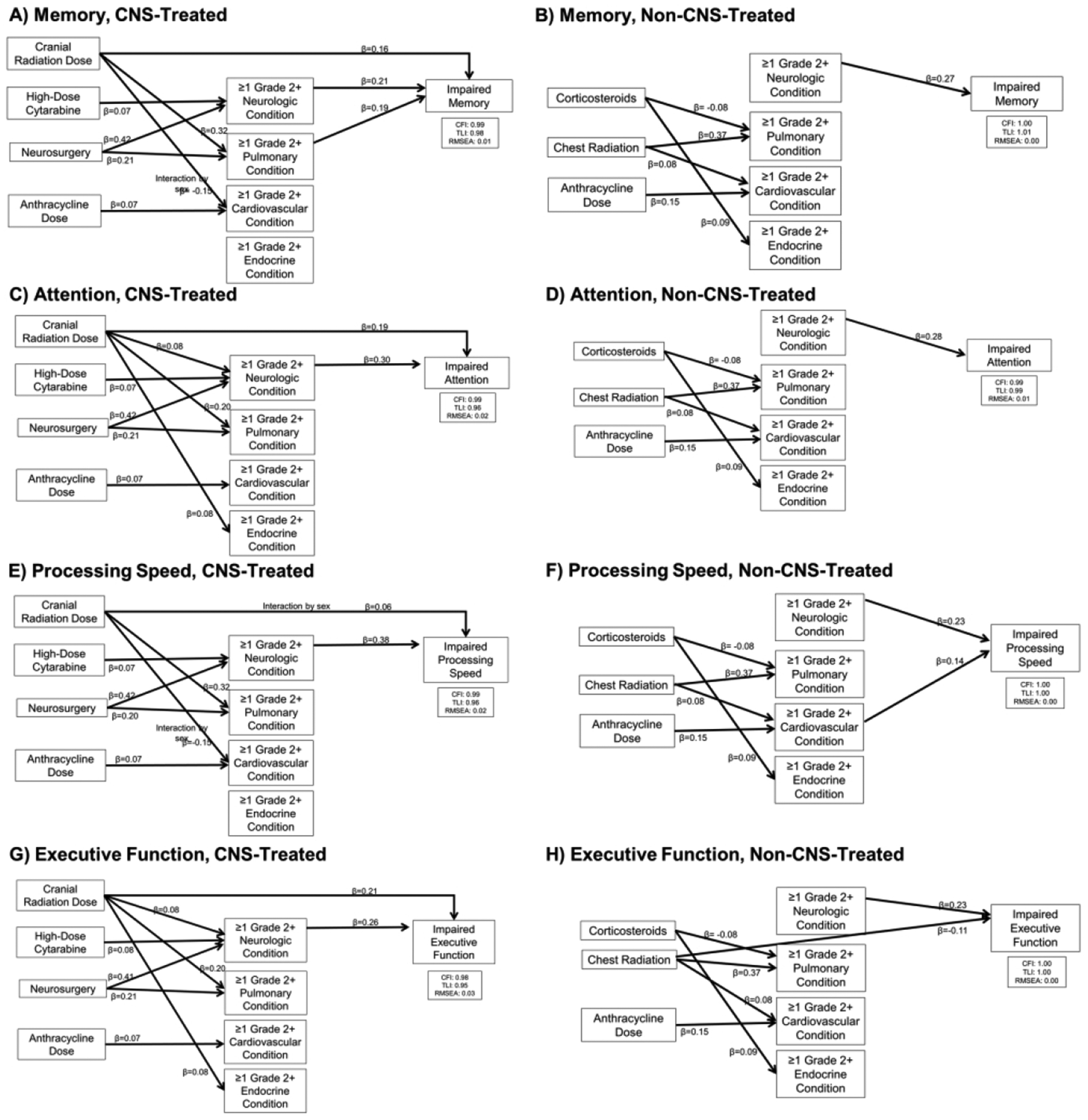
Final path models are presented for each outcome. Models are adjusted for sex, age at diagnosis, and time since diagnosis. Model fit indices are represented by the comparative fit index (CFI), Tucker-Lewis index (TLI), and the root mean square error of approximation (RMSEA). Each model includes only significant paths and is labeled with a standardized beta. Curved lines with double arrows indicate covariances between chronic health conditions. Neurologic, pulmonary, cardiovascular, and endocrine conditions were significantly associated with one another and allowed to covary.
Discussion
This large prospective cohort of clinically evaluated survivors of childhood cancer provides novel data on the potential susceptibility of children who experience brain injury during childhood to future adverse effects of CHC on neurocognitive functioning during adulthood. While both groups experienced an increased risk of neurocognitive impairment associated with high and very high severity/burden CHC scores, a dose response relationship was in CNS-treated survivors only. Further, the risk global neurocognitive impairment associated with a very-high severity/burden score was greater among CNS-survivors compared to non-CNS-treated survivors. Among CNS-treated survivors, those with cardiovascular or pulmonary CHC were at significantly higher risk of neurocognitive impairment compared to CNS-treated survivors without these CHC. Among non-CNS-treated survivors, primarily neurologic conditions were associated with a significantly higher risk of domain specific neurocognitive impairment. These compelling data suggest that survivors treated with therapy directly affecting the CNS may be more vulnerable to the effects of accumulating CHC on neurocognitive functioning as they age.
Childhood cancer survivors experience significantly greater morbidity from CHC as they age. We demonstrate that increasing number and severity of CHC conditions is associated with an increased risk of neurocognitive impairment, which appeared greater in magnitude among CNS-treated survivors. The accumulation of co-existing CHC is a second neurologic insult, added in aggregate to the initial insult to cognitive/brain reserve and plasticity from direct therapy to the CNS during childhood.1, 36 Similar “double hit” hypotheses have been proposed to explain slower recovery after traumatic brain injury in children with attention deficit hyperactivity disorder or worse neurocognitive function in IV drug users who are seropositive for HIV.37–39 The dose response effect of chronic health burden suggests an aggregating pathology that accumulates over time resulting in premature or exacerbated neurocognitive dysfunction. These effects are more pronounced in CNS-treated survivors, suggesting that the CHC are a second hit in CNS-treated survivors that produces greater neurocognitive functional impairment. Non-CNS-treated survivors, who presumably have greater cognitive/brain reserve following treatment and experience their first “hit” to cognitive/brain reserve with the development of chronic health conditions, are able to remain above the threshold for functional neurocognitive impairment, which may help to explain why some estimates were similar in magnitude to estimates from the CNS-treated group but did not reach statistical significance.40 Differences in the age at treatment and brain plasticity may influence how the child’s brain rebuilds and responds after treatment and how susceptible they may be to the second hit of CHCs. Similarly, evidence suggest changes in connectivity after childhood cancer that may have differing ability to compensate for the original and subsequent injuries.41 Future research is warranted to examine the neuropathological effects of chronic health conditions in this population.
Similar to our findings, a study from the Childhood Cancer Survivor Study found that cardiopulmonary conditions were associated with an increased risk of impairment in self-reported task efficiency and memory.31 We have expanded on these findings using prospectively collected, objective data that permitted evaluation of the independent effects of cardiovascular and pulmonary events and as well as specific health conditions in survivors who did and did not receive CNS-directed therapy. We have demonstrated that the risk of objective neurocognitive impairment associated with cardiopulmonary conditions is more frequently associated with pulmonary dysfunction and that magnitude of these effects appeared larger, and more often statistically significant, in CNS-treated survivors. Pulmonary function deficits may result in neurocognitive impairment through altered cerebral perfusion, reduced blood oxygenation, and altered neurotransmitter metabolism.42–44 Impaired lung function and cardiovascular disease are also associated with increased oxidative stress, inflammation, and a pro-coagulant state that places patients at risk for cerebrovascular disease which was significantly associated with neurocognitive impairment in this study.44–46 We also report that hypertension, another risk factor for cerebrovascular disease and decreased cerebral perfusion, was associated with impairment in executive function and processing speed domains. Hypertension has long been recognized as a common risk factor for cognitive decline and dementia as well as cerebrovascular disease across various age groups in the general population.47 Hypertension and cerebrovascular disease are preventable and treatable risk factors that childhood cancer survivors experience at rates higher than expected in their peers with increasing risk the further they are from treatment.48, 49 Thus, future research is warranted to examine how interventions tailored to childhood cancer survivors that improve cardiovascular, pulmonary, and cerebrovascular health impact neurocognitive functioning.
In both CNS and non-CNS-treated survivors, neurologic conditions were associated with a greater risk of neurocognitive impairment in all domains. The magnitude of the effect appeared greater among non-CNS-treated survivors for tests of memory. In CNS-treated survivors neurologic conditions mediated the effects of cranial radiation and neurosurgery on neurocognitive function, which suggests that prevention or improved management of these conditions (e.g., seizures) may maintain or improve neurocognitive functioning. Large effect sizes were seen in both groups for peripheral neuropathy (which includes both motor and sensory neuropathy). Previous studies have associated pain with memory impairments50 and animal models suggest that peripheral nerve injury increases the concentration of tumor necrosis factor-alpha in peripheral blood, cerebrospinal fluid, and the hippocampus suggesting that peripheral neuropathy may contribute to neuroinflammation and subsequent neurocognitive impairment.51 Additional research is needed to understand the biopsychosocial components of peripheral neuropathy and its effects on neurocognitive functioning in this population.
This study has several notable strengths including objective systematically and prospectively collected clinical data on neurocognitive functioning and 38 different CHC. We were also able to examine effect modification by CNS-directed treatment history, a surrogate for damage to cognitive/brain reserve, and examine both organ system level and specific CHC. An important limitation of this study is the lack of neurocognitive data on survivors immediately after the completion of treatment. However, in sensitivity analyses that excluded patients with CNS tumors, arguably those most immediately affected by treatment, results were similar. Further, in path analyses the effects of both cranial radiation and neurosurgery were mediated by neurologic conditions for each neurocognitive domain. Nonetheless, while our data were collected prospectively, in our sample the frequency of incident chronic health conditions between two subsequent neurocognitive exams was too small to evaluate a true longitudinal effect. There were 22 survivors of CNS tumors who did not receive CNS-directed treatments included in the non-CNS-treated group. Recognizing the potential for the tumor itself to influence cognitive/brain reserve, we conducted a sensitivity analyses omitting these survivors which yielded very similar results. Additionally, there may be residual confounding by factors related to diagnosis group that influenced treatment choices and subsequent outcomes, chronic health conditions, and neurocognitive function that we were unable to account for. Lastly, some of the modified CTCAE grading criteria utilized to classify CHC do consider treatment of the CHC, however, we were not able to fully characterize all potential medications/lifestyle interventions that may have influenced these associations. We will continue to follow these patients in order to examine the effects of incident CHC and medical or lifestyle intervention on neurocognitive improvement/decline over time.
In summary, we demonstrate that increased severity and burden of CHC is associated with an increased risk for global neurocognitive impairment and that survivors treated with direct therapy to the CNS during childhood and adolescence appear more vulnerable to the effects of very-high severity/burden of CHC on neurocognitive function during adulthood. These findings may be considered as a model to examine the potential chronic health condition-related exacerbation of neurocognitive impairment in other populations with early life brain injury, such as traumatic brain injury, organ failure/transplant, hydrocephalus, epilepsy, and other neurodevelopmental disorders. Future research is warranted to examine how interventions tailored to childhood cancer survivors that improve cardiopulmonary and neurologic health impact neurocognitive functioning. In addition, modification of treatment paradigms, tailored to those patients who have had direct therapy or injury to the CNS, that decrease the risk for cardiopulmonary and neurologic conditions, may also improve neurocognitive functioning and should be considered.
Supplementary Material
Acknowledgements
We would like to thank the participants of SJLIFE for their time and Dr. Sedigheh Mirzaei Salehabadi for her help correcting confidence intervals for multiple comparisons. This work was supported by NIH grants K00CA222742 (Dr. Williams) and U01CA195547 (Drs. Hudson and Robison) from the National Cancer Institute and the American Lebanese Syrian Associated Charities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare no conflicts of interested. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decisions to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record.
Potential Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. 2018. September 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994. April 6;271(13):1004–10. [PubMed] [Google Scholar]
- 3.Sumowski JF, Chiaravalloti N, DeLuca J. Cognitive reserve protects against cognitive dysfunction in multiple sclerosis. J Clin Exp Neuropsychol. 2009. November;31(8):913–26. [DOI] [PubMed] [Google Scholar]
- 4.Poletti M, Emre M, Bonuccelli U. Mild cognitive impairment and cognitive reserve in Parkinson’s disease. Parkinsonism Relat Disord. 2011. September;17(8):579–86. [DOI] [PubMed] [Google Scholar]
- 5.Xu W, Tan L, Wang HF, et al. Education and Risk of Dementia: Dose-Response Meta-Analysis of Prospective Cohort Studies. Mol Neurobiol. 2016. July;53(5):3113–23. [DOI] [PubMed] [Google Scholar]
- 6.Fay TB, Yeates KO, Taylor HG, et al. Cognitive reserve as a moderator of postconcussive symptoms in children with complicated and uncomplicated mild traumatic brain injury. J Int Neuropsychol Soc. 2010. January;16(1):94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Billiet T, Elens I, Sleurs C, et al. Brain Connectivity and Cognitive Flexibility in Nonirradiated Adult Survivors of Childhood Leukemia. J Natl Cancer Inst. 2018. August 1;110(8):905–13. [DOI] [PubMed] [Google Scholar]
- 8.Brace KM, Lee WW, Cole PD, Sussman ES. Childhood leukemia survivors exhibit deficiencies in sensory and cognitive processes, as reflected by event-related brain potentials after completion of curative chemotherapy: A preliminary investigation. J Clin Exp Neuropsychol. 2019. October;41(8):814–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacola LM, Krull KR, Pui CH, et al. Longitudinal Assessment of Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia Treated on a Contemporary Chemotherapy Protocol. J Clin Oncol. 2016. April 10;34(11):1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Cheung YT, Conklin HM, et al. Evolution of neurocognitive function in long-term survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. J Cancer Surviv. 2018. June;12(3):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krull KR, Hardy KK, Kahalley LS, Schuitema I, Kesler SR. Neurocognitive Outcomes and Interventions in Long-Term Survivors of Childhood Cancer. J Clin Oncol. 2018. July 20;36(21):2181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). The Lancet. 2017;390(10112):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JH, Choi Y, Jun C, et al. Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinology and metabolism (Seoul, Korea). 2014. June;29(2):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews WD, Jefferson AL, Bolduc T, et al. Neuropsychological dysfunction in patients suffering from end-stage chronic obstructive pulmonary disease. Arch Clin Neuropsychol. 2001. October;16(7):643–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Vogels RL, Oosterman JM, van Harten B, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;24(6):418–23. [DOI] [PubMed] [Google Scholar]
- 16.Cheung YT, Brinkman TM, Li C, et al. Chronic Health Conditions and Neurocognitive Function in Aging Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2018. April 1;110(4):411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011. May;56(5):825–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Group CsO. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult cancers, Version 5.0 Monrovia, CA: Children’s Oncology Group; October 2018. [Google Scholar]
- 19.Wechsler D Abbreviated Scale of Intelligence. San Antonio, TX: Pyschological Corporation; 1999. [Google Scholar]
- 20.Woodcock RW, McGrew KS, Mather N Woodcock-Johnson III Tests of Acheivement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 21.Conners CK, Connelly V, Campbell S, MacLean M Conners’ Continuous Performance Test, 2nd ed. North Tonawanda, NY: Multi-Health Systems Inc.; 2003. [Google Scholar]
- 22.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004. March;19(2):203–14. [DOI] [PubMed] [Google Scholar]
- 23.Strauss E, Sherman EM, Spreen O A Compendium of Neuropsychological Tests: Adminstration, Norms, and Commentary, 3rd ed. London, UK: Oxford University Press; 2006. [Google Scholar]
- 24.Wechsler D Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: Pyschological Corporation; 2008. [Google Scholar]
- 25.Delis DC, Kramer JH, Kaplan E California Verbal Learning Test, 2nd ed. San Antonio, TX: 2000. [Google Scholar]
- 26.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol. 2013. December 10;31(35):4407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity Grading of Long-term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2017. May;26(5):666–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhakta N, Liu Q, Ness KK, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). The Lancet. 2017;390:2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. Jama. 2007. June 27;297(24):2705–15. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 31.Cheung YT, Brinkman TM, Li C, et al. Chronic health conditions and neurocognitive function in aging survivors of childhood cancer: A report from the childhood cancer survivor study. Journal of the National Cancer Institute. 2018;110:411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopman DS. Cerebrovascular disease and dementia. Br J Radiol. 2007. December;80 Spec No 2:S121–7. [DOI] [PubMed] [Google Scholar]
- 33.Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP. Cardiovascular Disease Risk Factors and Cognition in the Elderly. Curr Cardiovasc Risk Rep. 2011. October;5(5):407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Yekutieli D. False Discovery Rate–Adjusted Multiple Confidence Intervals for Selected Parameters. Journal of the American Statistical Association. 2005. 2005/03/01;100(469):71–81. [Google Scholar]
- 35.Schreiber JB, Nora A, Stage FK, Barlow EA, King J. Reporting Structural Equation Modeling and Confirmatory Factor Analysis Results: A Review. The Journal of Educational Research. 2006. 2006/07/01;99(6):323–38. [Google Scholar]
- 36.Satz P Brain reserve capacity on symptom onset after brain injury: A formulation and review of evidence for threshold theory. Neuropsychology. 1993;7(3):273–95. [Google Scholar]
- 37.Durvasula RS, Hinkin CH. Neuropsychological Dysfunction among HIV Infected Drug Abusers. Am J Infect Dis. 2006;2(2):67–73. [PMC free article] [PubMed] [Google Scholar]
- 38.Bonfield CM, Lam S, Lin Y, Greene S. The impact of attention deficit hyperactivity disorder on recovery from mild traumatic brain injury. J Neurosurg Pediatr. 2013. August;12(2):97–102. [DOI] [PubMed] [Google Scholar]
- 39.Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg Pediatr. 2016. April;17(4):491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stern Y What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002. March;8(3):448–60. [PubMed] [Google Scholar]
- 41.Kesler SR, Ogg R, Reddick WE, et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain Connect. 2018. August;8(6):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson GE, Pulsinelli W, Blass JP, Duffy TE. Brain dysfunction in mild to moderate hypoxia. Am J Med. 1981. June;70(6):1247–54. [DOI] [PubMed] [Google Scholar]
- 43.Hoiland RL, Bain AR, Rieger MG, Bailey DM, Ainslie PN. Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am J Physiol Regul Integr Comp Physiol. 2016. March 1;310(5):R398–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang R, Burke GL, Enright PL, et al. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008. September 15;168(6):602–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caprio FZ, Sorond FA. Cerebrovascular Disease: Primary and Secondary Stroke Prevention. Med Clin North Am. 2019. March;103(2):295–308. [DOI] [PubMed] [Google Scholar]
- 46.Thyagarajan B, Jacobs DR, Apostol GG, Smith LJ, Lewis CE, Williams OD. Plasma fibrinogen and lung function: the CARDIA Study. Int J Epidemiol. 2006. August;35(4):1001–8. [DOI] [PubMed] [Google Scholar]
- 47.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005. August;4(8):487–99. [DOI] [PubMed] [Google Scholar]
- 48.Gibson TM, Li Z, Green DM, et al. Blood Pressure Status in Adult Survivors of Childhood Cancer: A Report from the St. Jude Lifetime Cohort Study. Cancer Epidemiol Biomarkers Prev. 2017. December;26(12):1705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chow EJ, Chen Y, Hudson MM, et al. Prediction of Ischemic Heart Disease and Stroke in Survivors of Childhood Cancer. J Clin Oncol. 2018. January 1;36(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg. 2007. May;104(5):1223–9, tables of contents. [DOI] [PubMed] [Google Scholar]
- 51.Ren X, Boriero D, Chaiswing L, Bondada S, St. Clair DK, Butterfield DA. Plausible biochemical mechanisms of chemotherapy-induced cognitive impairment (“chemobrain”), a condition that significantly impairs the quality of life of many cancer survivors. Biochimica et Biophysica Acta - Molecular Basis of Disease. 2019;1865:1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


