Summary
Astrocytes represent a large and diverse population of morphologically complex cells that exist throughout nervous systems of multiple species. Progress over the last two decades has shown that astrocytes mediate developmental, physiological, and pathological processes. However, a longstanding open question concerns how astrocytes regulate neural circuits in ways that are behaviorally consequential. In these regards, we summarize recent studies from Caenorhabditis elegans, Drosophila melanogaster, Danio rerio, and Mus musculus. The data reveal diverse astrocyte mechanisms operating on seconds or much longer time scales within neural circuits and shaping multiple behavioral outputs. We also refer to human diseases that have a known primary astrocytic basis. We suggest that including astrocytes in mechanistic, theoretical, and computational studies of neural circuits provides new perspectives to understand behavior, its regulation, and its disease-related manifestations.
Keywords: astrocyte, behavior, neuronal circuit, glia, microcircuit, Caenorhabditis elegans, Drosophila melanogaster, Danio rerio, Mus musculus, genetic disorders
Introduction
Understanding how the brain works is arguably one of the last frontiers of currently conceivable biology. This is an important goal with societal relevance, because disorders of the nervous system represent a major and increasing health burden. There is also an expectation that deeper understanding of the brain will inspire new types of biological computing and artificial intelligence. To meet these quests, impressive strides have been made over the last 150 years since the building blocks of the nervous system, the neuronal and glial cells, were discovered.
Considerable effort has been devoted to the study of neurons as the excitable cells of the nervous system. Advances made using model organisms, electrophysiology, genetics, neuroanatomy, and imaging at multiple scales are now providing mechanistic understanding of neurons, neuronal circuits, and their contributions to complex behaviors (Luo et al., 2008, 2018). In contrast, our understanding of glia and how they contribute to the functions of neural circuits and behavior is comparatively primitive, even though glia were discovered in parallel with neurons (Kettenmann and Verkhratsky, 2008). For many researchers, CNS circuits can be simplified as comprising neurons, perhaps forestalling the necessity to consider glia. However, a more useful definition of a neural microcircuit is that “comprising neurons and associated cells such as glia, organized to carry out specific operations within a region of the nervous system” (Shepherd and Grillner, 2010), reflecting the anatomical and evolutionary reality that glia and neurons have coexisted since the Palaeozoic era (Freeman and Rowitch, 2013).
In this review, we explore four experimental model organisms that represent the vanguard in efforts to understand how astrocytes (or astrocyte-like cells) contribute to neural circuit function and behavior. Each section begins with a brief introduction to the key features of astrocytes in each organism (Figure 1) and then describes recently identified functions and mechanisms by which they guide behavior (Figures 2 and 3).
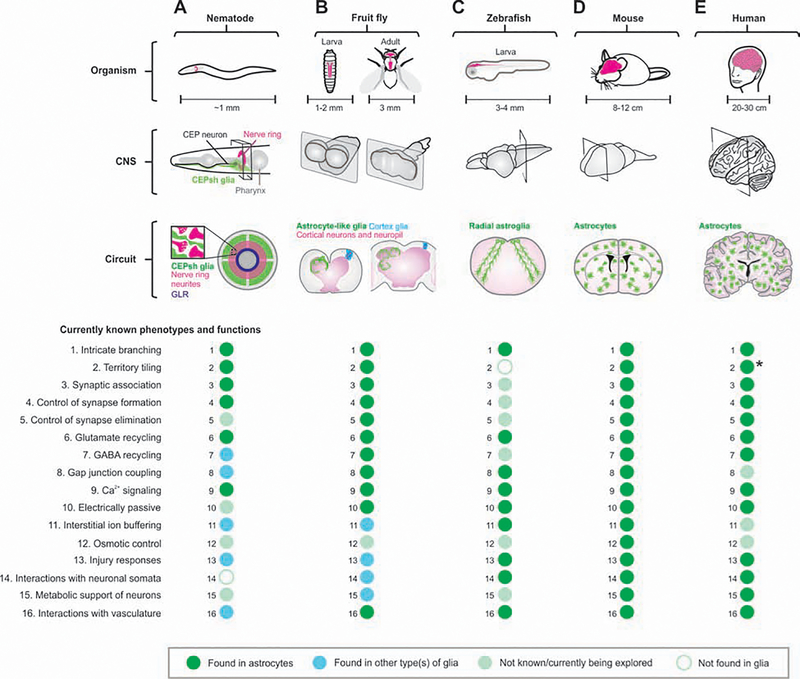
Figure 1: Phenotypes and functions of astrocytes from different species discussed herein.
The schematics illustrate the locations of CNS (gray), neuropil (purple) and astrocytes (green) in nematode (A), fruit fly (B), zebrafish (C), mouse (D), and human (E) at the level of organism, CNS and circuit. Dot plots summarize that 16 well-defined cellular phenotypes/functions of astrocytes are either found in astrocytes (green), found in other type(s) of glia (blue), not known or currently being explored (light green), or not found in glia (white) in the relevant organism indicated. Some human astrocytes project long, unbranched processes that cross cortical laminae (asterisk).
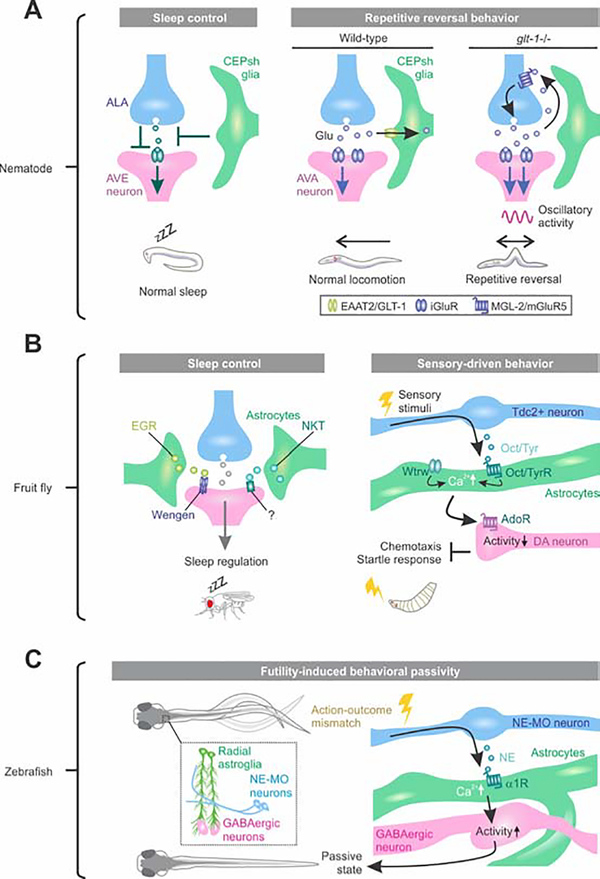
Figure 2: Neural circuit and behavioral functions of astrocyte-like cells in nematode, fruit fly and zebrafish.
(A) Nematode CEPsh glia are required for normal sleep and locomotion. Left: The oscillatory activity of AVE neuron correlates with head retraction of worm and regulates locomotion. ALA neurons are active during sleep and synaptically inhibit AVE. The working model from ablation experiments suggests that CEPsh glia tune the ALA-AVE synapse in proper behavioral state transition from wakefulness to sleep. Right: AVA neurons are a major class of interneuron driving backward locomotion. CEPsh glia uptake glutamate (Glu) from synaptic cleft at excitatory synapses onto AVA. Deletion of glutamate transporter GLT-1 from CEPsh glia results in spillover of Glu from the synapses, activating presynaptic mGluR5 to cause repetitive excitation of AVA and reversal in worm locomotion. (B) Fly astrocytes regulate sleep and sensory-driven behavior. Left: Drosophila TNFα homologue Eiger (EGR), expressed in fly astrocyte-like cells (green), acts on Wengen a receptor of EGR on neurons to regulate normal sleep. An astrocyte-enriched small secreted immunoglobulin (Ig)-domain protein Noktochor (NKT) exhibit reduction and fragmentation in night sleep, but not in day sleep. Right: upon sensory stimuli, the Tdc2-expressing neurons release neuromodulator tyramine (Tyr) or octopamine (Oct), the invertebrate analogues of norepinephrine, to increase Ca2+ in fly astrocytes in ventral nerve cord. Waterwitch (Wtrw)/TRP channel also produce Ca2+ in the same type of astrocytes. The astrocyte Ca2+ signaling inhibits dopaminergic neuron firing via ATP/adenosine and is required for olfactory-driven chemotaxis and touch-induced startle responses. (C) Fish radial astroglia play causal roles in behavioral passivity triggered by futility. When fish recognize an accumulated unsuccessful attempt, noradrenergic neurons in norepinephrine cluster of the medulla oblongata (NE-MO) become active and released NE activates α1-adenoceptor (AR) in radial astroglia. The radial astroglia Ca2+ signaling in turn enhances activity of GABAergic neurons in the lateral hindbrain to cause behavioral passivity.
At this stage, there is no common set of approaches that have been used across all the model organisms that we consider. Instead, we highlight the most informative studies pertinent to the topic of this review that employ methods best suited to the organism and the question at hand. For example, in mice many studies have employed chemogenetics, whereas in C. elegans, Drosophila, and zebrafish additional genetic interventions and screens have been very informative. In the case of mice, we have mainly focused on studies showing how acute astrocyte signaling can regulate behavior (seconds to hours) and have not focused on many studies where behavioral alterations result over longer periods such as following the deletion of a critical gene within astrocytes or during development and aging. In the case of C. elegans, Drosophila, and zebrafish, we have focused only on the most informative studies related to astrocytes and behavior. We finish by briefly mentioning human disorders with a known primary astrocytic basis. In the summary comments, we draw on common themes across species, interpretations, and key open questions.
Astrocytes: background narrative and core features
Rudolf Virchow proposed neuroglia as a type of “nerve cement” in 1858. The cellular elements comprising neuroglia were subsequently identified and named as astrocytes by Michael von Lenhossek (1893), and as microglia and oligodendrocytes by Pio del Rio-Hortega (1919) [reviewed in (Kettenmann and Verkhratsky, 2008)]. Astrocytes, microglia, and oligodendrocytes, collectively glia, probably represent no more than 50% of CNS cells.
Astrocytes are far more complex than first envisioned in the 1890s by their star-shaped morphology. Astrocytes vary morphologically between species and brain areas, but one feature that sets astrocytes apart from microglia and oligodendrocytes is their highly complex anatomy, which has been described as bushy and sponge-like. The finest processes of astrocytes extensively contact synapses and other cells, but seem relatively stable compared to those of microglia, which display spatial dynamics at the cellular and submicrometer scale over minutes (Bernier et al., 2019; Davalos et al., 2005; Nimmerjahn et al., 2005). Astrocytes do, however, undergo extensive structural remodeling during injury and display more subtle alterations during synaptic plasticity (Adams and Gallo, 2018; Henneberger et al., 2020). The complex anatomical features of astrocytes suggest that, whereas most neurons evolved to perform their core functions at distance through dendritic and axonal arbors, most astrocytes evolved to perform their core functions locally through complex, compact morphological forms impinging upon other cellular elements of the neuropil. Signaling of astrocytes at distances beyond their own territories can occur, however, via low resistance diffusive pathways formed by gap junctions between neighboring astrocytes (Giaume et al., 2010).
In terms of signaling, astrocytes are mostly electrically silent (Kuffler, 1967): their resting membrane potential rarely deviates from near the K+ equilibrium potential by more than a few millivolts and there is no evidence of any propagated or graded electrical signals that function in a manner analogous to those in neurons (Savtchenko et al., 2018). Indeed, astrocytes seem to be a poor substrate for propagating electrical signals owing to their extremely low membrane resistance, highly branched processes, high surface area, multiple consecutive failure and divisive points for current flow, and the fact that they lack predominant voltage-dependent channels. In the absence of an obvious electrical signal, the discovery of astrocyte intracellular Ca2+ dynamics (Charles et al., 1991; Cornell-Bell et al., 1990) provided great impetus to explore such slower biochemical signals as a means by which astrocytes communicate to other cells. Much in vivo work over that last few years has shown that astrocyte Ca2+ dynamics are extremely rich, occur locally in fine processes as well as globally, can be triggered by neuromodulators and neurotransmitters, occur in a behaviourally relevant manner, and are altered in disease (Bazargani and Attwell, 2016; Shigetomi et al., 2016).
Several known mechanisms allow astrocytes to regulate synapses and neurons. These include changes in neurotransmitter and ion homeostasis, release of neuromodulators and synaptogenic cues from astrocytes, movement of astrocyte processes relative to synapses to alter synaptic transmission, and contributions to multicellular neuroinflammatory responses (Allen and Lyons, 2018; Khakh and Sofroniew, 2015). We consider some of these mechanisms in the following sections in relation to behaviour. Furthermore, recent discoveries show that astrocytes display diversity within and between brain areas. This work is beyond the scope of this review, but has been summarized (Haim and Rowitch, 2017; Khakh and Deneen, 2019).
Insights from C. elegans
CEPsh glia: worm astrocytes
The nematode C. elegans is an important setting for glia studies. The hermaphrodite and male nervous systems are fully mapped connectomes of 302 and 391 neurons, and 56 and 92 glia, respectively, mediating locomotion, sleep, mating, decision-making, memory, and other behaviors (Doroquez et al., 2014; Jarrell et al., 2012; White et al., 1986). Powerful molecular-genetic tools, coupled with in vivo imaging and optogenetics, allow visualization and manipulation of any glial cell or neuron (Singhvi and Shaham, 2019). As C. elegans cell survival is generally programmed by lineage (Sulston and Horvitz, 1977; Sulston et al., 1983), glia are not required for neuron viability. Thus, primary effects of glia manipulation on neuron activity can be distinguished from confounding secondary causes (Shaham, 2005).
The C. elegans brain, a nerve ring composed of processes and synapses of ~180 neurons, is ensheathed by two glial types (Figure 1A). Four astrocyte-like CEPsh glia cover the outer surface and penetrate the structure, and six GLR glia abut the inner border (Singhvi and Shaham, 2019; White et al., 1986). CEPsh glia development suggests homology to vertebrate astrocytes (Rapti et al., 2017). The nerve ring is anatomically similar to the vertebrate spinal cord, with neural tissue surrounding a fluid-filled space and midline-crossing commissural tracts projecting rostrocaudally. In vertebrate development, radial glia extend processes from ventricular to pial surfaces, where they branch and guide commissural axons (Dominici et al., 2017; Varadarajan et al., 2017). Likewise, embryonic C. elegans CEPsh glia extend a neuron-guiding process (Rapti et al., 2017). Netrin, from radial glia pial branches or from C. elegans CEPsh glia processes, hones axon guidance. Semaphorins and Flamingo/CELSR, also do so in C. elegans.
Once neurogenesis is complete, some vertebrate radial glia transform into astrocytes (Noctor et al., 2008; Schmechel and Rakic, 1979). CEPsh glia undergo a similar cell division-independent transformation (Rapti et al., 2017), and in both settings glial branches contact synapses (White et al., 1986). Like astrocytes, CEPsh glia influence synaptogenesis (Allen et al., 2012; Christopherson et al., 2005; Colon-Ramos et al., 2007; Eroglu et al., 2009; Shao et al., 2013). Furthermore, both astrocytes and CEPsh glia cover non-overlapping neural domains, respecting yet unknown tiling rules (Bushong et al., 2002; White et al., 1986).
Gene-expression profiles support the homology of CEPsh glia and mammalian astrocytes. Quantitative comparisons reveal that CEPsh glia are more similar to mouse astrocytes than any other brain cell (Katz et al., 2019). For example, CEPsh glia express homologs of astrocytic glutamate transporter GLT-1 and glial fibrillary acidic protein (GFAP). Olig2 transcription factor, expressed by some mature vertebrate astrocytes (Tatsumi et al., 2018), is also expressed in CEPsh glia (McMiller and Johnson, 2005; Yoshimura et al., 2008). Like astrocytes, CEPsh glia are diverse, with Netrin, Pax6, and Hmx expression segregated dorsoventrally (Wadsworth et al., 1996; Yoshimura et al., 2008), impacting functional diversity and developmental potential (Mizeracka and Heiman, 2015). Finally, vertebrate astrocytes exhibit Ca2+ transients, whose purpose is debated (Shigetomi et al., 2016; Yu et al., 2020a), and gap junctions allow Ca2+ flow to adjacent astrocytes. CEPsh glia exhibit similar Ca2+ responses (M. Katz and S. Shaham, unpublished), and also express gap junction proteins (Altun et al., 2015), whose functional coupling is not yet demonstrated.
CEPsh astrocytes modulate synapses to control C. elegans behavior
Post-embryonic ablation of CEPsh glia does not perturb nerve-ring structure, but animals exhibit several independent locomotory defects (Katz et al., 2018; Katz et al., 2019). Ablated animals move slowly and follow abnormal circular paths. Ablation also affects sleep. Unlike most C. elegans neurons, the ALA neuron, which forms inhibitory synapses onto locomotory AVE interneurons, exhibits frequent Ca2+ transients during sleep (Nichols et al., 2017). In wakefulness these synapses are inactivated by CEPsh glia (Katz et al., 2018). CEPsh glia ablation uncovers ALA-AVE inhibition, and uncouples AVE firing from movement. Strikingly, CEPsh glia-ablated adults exhibit narcoleptic-like locomotory pauses and prolonged sleep bouts (Figure 2A). Importantly, astrocyte regulation of sleep is conserved in Drosophila and in mice, as discussed later.
Animals lacking CEPsh astrocytes also exhibit defects in the balance of forward and backward locomotion. Off food, adults mostly move forward, reversing infrequently. Reversal initiation events follow a Poisson distribution with a fixed temporal probability. Animals lacking CEPsh glia, or glt-1 mutants, instead exhibit repeated reversal initiation bouts (Katz et al., 2019; Mano et al., 2007). This repetitive behavior can be spontaneous, or elicited. In vivo dual-imaging of extracellular glutamate and intracellular Ca2+ in AVA, a backward locomotion interneuron, revealed surprising dynamics in glt-1 mutants. While wild-type animals occasionally exhibit spontaneous glutamate release onto AVA and subsequent AVA firing, glt-1 mutants exhibit oscillations of glutamate release near AVA and of AVA firing. Oscillation distribution matches repetitive behavior statistics, suggesting a causal role. Circuit analysis suggests that repetitive AVA firing originates in presynaptic neurons. In the absence of glial GLT-1, glutamate diffuses from AVA postsynaptic sites, engaging an extrasynaptic glutamate receptor, MGL-2, homologous to vertebrate mGluR5, on presynaptic neurons. This leads to un-evoked EGL-30/Gαq-dependent glutamate release, driving an autocrine feed-forward loop causing repetitive AVA firing and repetitive reversal behavior (Katz et al., 2019). Thus, CEPsh astrocytes are critical for restricting reversal motor program initiation (Figure 2A).
GLT1 conditional knockout in mouse astrocytes yields repetitive grooming (Aida et al., 2015), and mGluR5 inhibition prevents repetitive behaviors in mouse autism and repetitive behavior disorder models (Silverman et al., 2010). Thus, the C. elegans studies suggest that mammalian repetitive behavior, thought to involve inhibition defects among multiple brain regions (Nikolaus et al., 2010), may at least in part originate from defects in synaptic glutamate dynamics.
C. elegans glia control neuron-receptive-ending cell biology and physiology
Since C. elegans contains so few cells, individual cells often assume multiple functions. CEPsh astrocytes are a striking example, with each cell also sending a process to the nose, wrapping around sensory neuron receptive endings (NREs) (Doroquez et al., 2014; Perkins et al., 1986; Ward et al., 1975). Sensory NREs and their associated glia/glia-like cells, resemble synapses in which presynaptic signaling is replaced by environmental cues. Glia at both sites secrete thrombospondin TSP1 domain proteins, and the same receptor families (GPCRs, AChRs, iGRs) engage presynaptic cues (Shaham, 2010). CEPsh glia ablation early in development results in truncated sensory neurons dendrites (Yoshimura et al., 2008). A more severe dendrite extension defect is seen in animals lacking AMsh glia, a sensory-organ glial cell that does not interact with the nerve ring (Heiman and Shaham, 2009; Singhal and Shaham, 2017).
Studies of C. elegans sensory organ glia have unmasked how glia are specified, how glial compartments surrounding NREs are generated, how their size is determined, how glia control NRE shape, how glia modulate NRE structural plasticity, how glia control the NRE micro-environment, how glia affect age-dependent changes in neuronal structure and function, how a glial cell distinguishes among its associated neuron, and how glia integrate associated neuron activities (Bacaj et al., 2008; Grant et al., 2015; Han et al., 2013; Huang et al., 2020b; Johnson et al., 2020; Labouesse et al., 1994; Melkman and Sengupta, 2005; Oikonomou et al., 2012; Oikonomou et al., 2011; Perens and Shaham, 2005; Procko et al., 2011, 2012; Singhvi et al., 2016; Tucker et al., 2005; Wallace et al., 2016; Wang et al., 2017; Wang et al., 2008; Yoshimura et al., 2008; Zhang et al., 2020). C. elegans sensory organ glia also display Ca2+ signals following behaviorally relevant stimuli (Ding et al., 2015), perhaps providing a setting for understanding Ca2+ transients, their relevance to synaptic control, and their behavioral implications.
Insights from Drosophila
The fruit fly Drosophila melanogaster is a well-characterized invertebrate model organism for investigating the roles of glia in the CNS. The ease of powerful genetic manipulations, along with a large collection of openly available transgenic fly lines render it straightforward to specifically label small subsets of CNS cells, including astrocyte-like glia (Freeman, 2015; Yildirim et al., 2019). Using Drosophila, it is possible to genetically manipulate, visualize and electrophysiologically examine the CNS in behaving individuals, including at the single cell level.
Despite their small number (~10% of all CNS cells), glial cells display surprising morphological and functional diversity in Drosophila (Figure 1B). Among seven morphologically defined types (Bittern et al., 2020; Yildirim et al., 2019), two appear to share roles attributed to astrocytes in mammals: these – due to historical precedent – are called astrocyte-like glia (here referred to as astrocytes) and cortex glia.
In the Drosophila larval brain, neuronal cell bodies lie in the outer cortical region and extend their processes into the neuropil where all CNS synapses form. Cortex glia envelop neuronal cell bodies and proximal neurites throughout the synapse-deficient cortical regions, and there they provide trophic support for neurons, buffer extracellular ions, and monitor the extracellular environment. In contrast, astrocytes extend highly branched projections throughout the entirety of the neuropil where they interact with neural circuits via synapses (Figure 1B). Fly astrocytes are electrically non-excitable, utilize intracellular Ca2+ signaling to regulate communication, form gap junction-coupled networks, establish tight associations with tracheal elements (the fly vasculature), and organize in a tiled fashion to cover the neuropil with a modest overlap at astrocyte-astrocyte boundaries (Bittern et al., 2020; Ma et al., 2016; Yildirim et al., 2019)(Stork et al., 2014). As in mammals, well-studied functional roles for Drosophila astrocytes include modulation of synapse formation and plasticity, and circuit remodeling. For instance, ablation of astrocytes reduced the numbers of synapses that formed in developing circuits (Muthukumar et al., 2014), and fly astrocytes engulf and clear pruned synapses and other neuronal debris via pathways such as Draper/MEGF10 that are conserved in vertebrates (Tasdemir-Yilmaz and Freeman, 2014)(Awasaki et al., 2006; Chung et al., 2013; Hakim et al., 2014).
Electron microscopy studies show that astrocytes do not entirely ensheath synapses during the larval or adult stage, and the distance of astrocytic processes from synapses (e.g., of a looper neuron) in a third instar larva is ~375 nm (Macnamee et al., 2016; Muthukumar et al., 2014; Stork et al., 2014). These values are comparable to the spatial interactions of rodent astrocyte processes with synaptic elements associated with dopamine release (~300 nm), but slightly longer than those associated with fast excitatory synapses (Chai et al., 2017; Haustein et al., 2014; Octeau et al., 2018). It is feasible that the differences in the spatial relationships between astrocyte processes and synapses between species affect neuron-astrocyte interaction mechanisms, but the reality is that further detailed anatomical work is required at the synaptic scale with methods that have sufficient resolution. Such methods are becoming routine and their use has the potential to advance our understanding of astrocyte-synapse interactions, including how the ultrastructural organization of astrocytes modulates synaptic activity.
Functions of astrocytes in the fruit fly nervous system and for behavior
Selective Gal4 drivers for astrocytes enable in vivo characterization and manipulations of genes in order to explore their impacts on behavior (Stork et al., 2014)(Li et al., 2014). A recent study employing Translating Ribosome Affinity Purification (TRAP) RNA-sequencing has shown that in flies and mammals, astrocytes have substantially overlapping gene expression profiles (Ng et al., 2016). In this study, genetic screening using RNA interference (RNAi) for 318 targets identified multiple genes that were required for behavior (locomotive activity, circadian rhythmicity or vibration sensitivity). Such rapid and inexpensive in vivo forward genetic approaches to identify genes of interest are a key advantage of this system over the use of rodents. The genes identified included many transporters, metabolic support proteins, and secreted proteins. One type of secreted proteins were the thrombospondins whose contributions to synapse formation and locomotor behavior have been documented in mice (Christopherson et al., 2005; Eroglu et al., 2009; Eroglu and Barres, 2010; Nagai et al., 2019).
Secreted factors for sleep regulation
Several astrocyte-secreted factors have been shown to control sleep in fruit flies. An astrocyte-enriched small secreted immunoglobulin (Ig)-domain protein, Noktochor (NKT, fly CG14141), was identified to promote sleep (Ng et al., 2016; Sengupta et al., 2019). Drosophila sleep in the middle of the night and the day. Adult flies lacking NKT exhibited reduced and fragmented night sleep, but day sleep was normal, consistent with the hypothesis that different pathways regulate each sleep phase. Cellular and molecular targets of NKT remain to be elucidated. Furthermore, as for vertebrates (Shoham et al., 1987; Stellwagen and Malenka, 2006), cytokine signaling affects sleep behavior. The Drosophila tumor necrosis factor-alpha (TNFα) homologue, Eiger (EGR), is expressed in astrocytes (Ng et al., 2016) and controls sleep duration (Vanderheyden et al., 2018). Astrocytic, but not neuronal, EGR RNAi decreased baseline sleep during the day and night. Knockdown of Wengen, a receptor of EGR on neurons had no effect, however, on baseline sleep, but dramatically blunted recovery sleep after deprivation. The authors suggested that the discrepancy in outcomes between the two mutants (effects on sleep amount vs sleep homeostasis) may be explained by additional TNFα receptors for EGR (Figure 2B). Recent studies also show that increased astrocyte Ca2+ dynamics correlate with sleep need and contribute causally to sleep in Drosophila via the release of Spätzle, the interleukin-1 analogue. Spätzle then acts on specific neurons (R5) to regulate sleep (Blum et al., 2020).
Neurotransmitter uptake to control circuit activity
Drosophila astrocytes participate in neurotransmitter homeostasis by expressing a set of transporter proteins such as the excitatory amino acid transporter 1 (EAAT1/GLAST) and the GABA transporter (GAT) (Muthukumar et al., 2014; Stork et al., 2014) to ensure the balance of excitation and inhibition. Loss of EAAT1 resulted in shortened lifespan, neuropil degeneration that could be suppressed by drugs used in the clinic to suppress seizure activity (Rival et al., 2004), and extended glutamatergic interneuron-evoked inhibitory postsynaptic currents in motor neurons – even in synapses that lacked astrocytic contacts (Macnamee et al., 2016). Elimination of GAT led to early embryonic lethality, while partial loss led to strong defects in locomotor behavior, both of which can be rescued by astrocyte-specific expression of GAT (Stork et al., 2014). It has recently been suggested that EAAT1 plays a key role in long-term memory (LTM). Fruit flies form LTM in 24-hour spaced training paradigms (Matsuno et al., 2019), and during training, astrocyte EAAT1 expression was induced via the glial transcription factor Repo and the homophilic cell adhesion molecule Klingon (Klg). Age-related memory impairment in LTM (AMI-LTM) in Repo and Klg null mutants was rescued by EAAT1 overexpression. How each of these phenotypes relates to changes in extracellular glutamate levels was not directly measured. Stimulating astrocytic Ca2+ influx through activation of a TrpA1 channel led to rapid endocytosis of GAT from astrocytic membranes, behavioral paralysis, and termination of neuronal activity (Zhang et al., 2017), providing a potential mechanism for how astrocyte Ca2+ signaling might regulate neurophysiology.
Ca2+ signaling, circuits and behavior
Using a forward genetic approach to identify Ca2+ signaling-related genes that functioned in astrocytes to regulate a simple olfactory-driven behavior, the transient receptor potential (TRP) channel Water witch (Wtrw) was identified as an in vivo regulator of whole-cell changes in astrocytic Ca2+ in Drosophila larval astrocytes (Ma et al., 2016). Whole-cell astrocyte Ca2+ signaling in the larval CNS was elicited by the activity of Tdc2 neurons, which release octopamine and tyramine, the invertebrate analogues of norepinephrine (NE) that evokes similar whole-cell astrocyte Ca2+ increases in mice (Ding et al., 2013; Paukert et al., 2014). Dual-color Ca2+ imaging of Tdc2 neurons and astrocytes revealed that Tdc2 neurons show oscillatory Ca2+ signals which are followed by astrocyte activities with a delay of tens of seconds, which could be blocked by silencing Tdc2 neurons, or by genetic elimination of octopamine and tyramine. Astrocytes sensed octopamine/tyramine through the dual-specificity Oct-TyrR receptor expressed on astrocytes, which, through the PLCβ NorpA, was proposed to activate the Trp channel Wtrw and drive Ca2+ influx. Strikingly, cell-specific manipulations and pharmacological experiments revealed that olfactory-driven chemotaxis and touch-induced startle responses require this astrocyte signaling pathway, and that it likely acts by inhibiting dopaminergic neuron firing by increasing extracellular ATP/adenosine (Ma et al., 2016) (Figure 2B).
Insights from zebrafish
The zebrafish is a powerful vertebrate model system that offers unique experimental advantages for glial physiology and behavior. In particular, the small size and near transparency of zebrafish embryos and young larvae permit brain-wide cellular-resolution imaging of activity while the organism displays relevant naturalistic behaviors (Ahrens et al., 2012; Vladimirov et al., 2014). Importantly, zebrafish brains contain conserved regions associated with cognition in mammals (Jurisch-Yaksi et al., 2020) and so there has been an awareness that zebrafish may provide a quantitative handle on higher order functions not readily assessed in worms and flies.
On radial astroglia, radial astrocytes, and astrocytes
Until recently, one striking difference between the zebrafish and mammalian CNS, or more specifically between anamniotes (including fish) and amniotes (including mammals), was thought to be the absence of stellate (protoplasmic) astrocytes in anamniotes (Lyons and Talbot, 2015). In the developing and mature zebrafish CNS, GFAP labels a prominent type of glial cell that has radial morphology, typically spanning the entire width from the ependymal coating of the ventricles to the pial surface of the brain (Figure 1C). These GFAP+ radial glial cells serve as progenitor cells throughout life (Goldshmit et al., 2012; Kroehne et al., 2011; Kyritsis et al., 2012), whereas in mammals, radial glial cells serve as progenitor cells during development of the CNS and functionally and morphologically transform mainly to astrocytes at the end of embryonic development (Malatesta et al., 2008), except for a few locations such as the retina and the cerebellum, where the radial morphology of glia persists throughout life.
In zebrafish, the GFAP+ cells have often also been referred to as radial astroglia (Cuoghi and Mola, 2009) when they express astrocyte markers e.g., glutamine synthetase (GS), aquaporin-4 (AQP4), EAAT2b/GLT-1, S100β (Grupp et al., 2010; Lange et al., 2020; McKeown et al., 2012; Raj et al., 2018). They also display intricate branching of processes associated with neurons (Freifeld et al., 2017; Jurisch-Yaksi et al., 2020), and/or form glial networks through gap junctions (Diaz Verdugo et al., 2019). Recent single cell RNA-seq analyses of zebrafish brain (Cosacak et al., 2019; Lange et al., 2020; Raj et al., 2018) revealed that zebrafish GFAP+ cells have molecular diversity and a subset of those cells share close transcriptomic signatures with murine astrocytes. Therefore, studies have raised the possibility that GFAP+ radial glia and/or radial astroglia in zebrafish may perform and/or sub-serve many tasks ascribed to mammalian astrocytes (Lyons and Talbot, 2015).
In addition to radial glia and radial astroglia, a zebrafish cell type remarkably similar to mammalian astrocytes has recently been described (Chen et al., 2020). The authors generated transgenic lines to label Glast+ cells and found cells with dense cellular processes in the developing zebrafish CNS. With single-cell resolution imaging, these cells were shown to transform from radial glia into astrocyte-like cells that elaborated a dense meshwork of fine cellular processes, morphologically similar to astrocytes in Drosophila and mammals. These cells exhibited additional defining features of mammalian astrocytes including: expression of GS, close association with synapses, astrocyte tiling, and spontaneous microdomain Ca2+ transients that respond to NE (Chen et al., 2020). Finally, a cell-specific CRISPR/Cas9 approach demonstrated a functional role for Fgf receptors 3 and 4 in vertebrate astrocyte morphogenesis, as was found to be the case in Drosophila (Stork et al., 2014).
Functions of radial astrocytes and astroglia in zebrafish nervous system and behavior
Following extensive research of radial glia and radial astroglia in regenerative responses after tissue injury, their physiological roles in neural circuit function have begun to be revealed recently. First, a pioneering study identified a subset of GFAP+ cells termed “radial astrocytes” in the zebrafish medulla oblongata with long processes that ramify at distal ends and suggested that these cells play causal roles for information processing in failure of intended actions and triggering behavioral passivity (Mu et al., 2019). In the study, the authors designed a virtual reality environment where zebrafish larvae fictively swam with realistic visual feedback that was given during attempted swimming (closed-loop). Once such feedback was suddenly withheld to render swim efforts ineffective (open-loop), fish increased their swim vigor for tens of seconds, but then abruptly stopped swimming and became passive. This futility-induced passivity appears to be caused by decoupling of motor action and sensory feedback, reminiscent of highly conserved adaptive behaviors such as passive coping and learned helplessness in mammals. Combining this behavioral paradigm, whole-brain dual-color Ca2+ imaging using light sheet microscopy and cell-specific perturbations, the Ahrens lab discovered bi-directional interactions between neurons and radial astrocytes. The noradrenergic system, known to encode action-outcome mismatch, initially became activated ~10 s before the onset of the passive state. Within a few seconds of activation of the noradrenergic system, radial astrocyte Ca2+ signaling via α1-adrenergic receptors ramped up and activated GABAergic neurons in the brain stem to trigger behavioral passivity. These findings suggest that radial astrocytes convert information that actions are futile and thus accumulate evidence for behaviorally relevant decision making (Figure 2C).
Insights from mice
A typical grey matter mouse astrocyte comprises a cell body, one or two end feet bearing processes that contact blood vessels, six or seven thick primary branches that split into secondary and tertiary branches, and thousands of branchlets and leaflets that form highly complex sponge-like territories throughout the CNS (Sun and Jakobs, 2012). The finest astrocyte leaflets extensively contact synapses and perhaps all other CNS cell types. The processes of one astrocyte do not encroach onto that of its neighbor, causing astrocytes to evenly tile the CNS in non-overlapping territories (Bushong et al., 2002). Exploration of astrocytes and how they regulate neuronal circuits is advanced in mice (Figure 1D). There is a huge amount of data, but as stated at the outset, we restrict our summary mainly to acute astrocytic regulation of circuits and behavior (Figure 3). We have not considered in depth the wealth of studies where behavioral alterations result over longer periods such as following the deletion of critical genes within astrocytes, except for studies of circadian and sleep/wake behaviors, which occur over the timescale of days by definition. Since astrocytes express a rich variety of GPCRs, DREADDs (designer receptors exclusively activated by designer drugs) have been widely used to explore astrocyte biology in brain preparations such as slices, and in vivo. DREADDs are engineered GPCRs that have attenuated responses to their endogenous ligands and have been engineered to respond to synthetic, biologically inert chemical ligands, which are delivered in the animal’s water or food supply, or by systemic injection (Roth, 2016). DREADDs enable relatively non-invasive stimulation of GPCR pathways in a genetically targeted manner in vivo. Several types of DREADDs have been developed to target major Gα-protein signaling pathways: Gq-coupled hM3D, Gi-coupled hM4D, and Gs-coupled rM3D are most used in astrocyte studies (Yu et al., 2020a). As discussed in the finishing section of this review, such stimulations, although revealing in a causal sense do not, however, indicate that the pathways are activated physiologically.
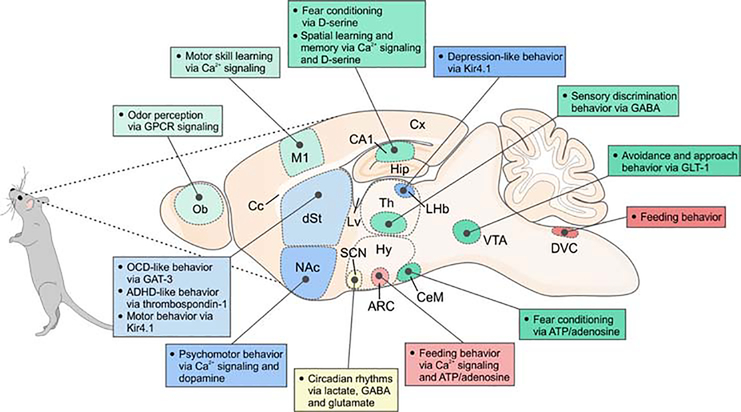
Figure 3. Summary illustrating acute astrocytic regulation of neuronal circuits and behaviors relevant to different regions of the mouse brain.
Schematic of a sagittal section of a mouse brain where various regions and nuclei as well as associated behaviors that were shown to be regulated by acute astrocytic mechanisms are depicted. Ob, olfactory bulb; Cx, cerebral cortex; M1, primary motor cortex; Lv, lateral ventricle; Cc, corpus callosum; dSt, dorsal striatum; NAc, nucleus accumbens; Hip, hippocampus; Th, thalamus; LHb, lateral habenula; Hy, hypothalamus; ARC, arcuate nucleus; SCN, suprachiasmatic nucleus; CeM, central amygdala; VTA, ventral tegmental area; DVC, dorsal vagal complex. Note: in the text we also consider sleep, but this is not illustrated here because it involves multiple brain nuclei. Furthermore, the cartoon does not include studies where behavioral alterations result over longer periods such as following the deletion of a critical gene within astrocytes or during development and aging.
Feeding behavior
Increased firing of hypothalamic arcuate nucleus agouti-related peptide (AGRP) neurons evokes food intake, whereas firing of pro-opiomelanocortin (POMC) neurons suppresses feeding. As with all other areas of the brain, astrocytes are intermingled with AGRP and POMC neurons and form close spatial interactions with them (Fuente-Martín et al., 2012). In light of the well-established roles of AGRP and POMC neurons in the regulation of feeding, it was natural to ask how astrocytes regulate AGRP and POMC neurons as well as feeding. We summarize two studies that used hM3Dq DREADDs in astrocytes. In one study, activation of hM3Dq in arcuate astrocytes resulted in decreased firing of AGRP neurons through astrocytic ATP/adenosine release and also decreased basal and ghrelin-evoked food intake (Yang et al., 2015). Opposing effects observed because of astrocyte hM4Di activation were attributed to a decrease in astrocyte Ca2+ activity, leading to the suggestion that astrocyte signaling controls feeding bidirectionally. It should be noted, however, that several studies have shown that Gi-GPCR activation in astrocytes actually elevates intracellular Ca2+ levels (Yu et al., 2020a), which echoes early pharmacological studies (Porter and McCarthy, 1997). Subsequently, activation of astrocyte hM3Dq DREADDs in arcuate astrocytes was shown to increase the activity of AGRP neurons and increased food intake during the dark phase (Chen et al., 2016). In this study, a causal role for Ca2+ was also explored by using a genetically encoded IP3 sponge that resulted in decreased Ca2+ signaling and food intake (Chen et al., 2016). Thus, these studies concluded that astrocytes in the arcuate nucleus bidirectionally control neuronal activity that regulates feeding, but with effects that are somewhat discordant. Technicalities such as the concentration of CNO used to activate the DREADDs may explain these differences, as could specificity of hM3Dq delivery to astrocytes within the arcuate nucleus relative to surrounding tissue. Furthermore, since AGRP and POMC neurons exert opposing effects on feeding, and are interspersed, it may be possible that the genetic strategies targeted subpopulations of astrocytes that affected either AGRP or POMC neurons preferentially.
Olfactory behavior
It has been recently reported that activation of hM3Dq DREADD in olfactory bulb (Ob) astrocytes in vivo decreased neuronal Ca2+ responses to odor stimulation and improved performance in an olfactory learning task (Ung et al., 2020). In contrast, stimulation of hM4Di DREADD in Ob astrocytes caused an increase in neuronal Ca2+ odor responses, but resulted in less accurate odor detection performance (Ung et al., 2020). Ob astrocytes were reported to respond to the neurotransmitters glutamate, GABA as well as dopamine (De Saint Jan and Westbrook, 2005; Doengi et al., 2009; Fischer et al., 2020), but it remains unknown which mechanisms are physiologically exploited by Ob astrocytes for fine-tuning neuronal activity and odor perception. Furthermore, transcriptomic analyses have revealed distinct molecular profiles of Ob astrocytes in relation to other brain regions (John Lin et al., 2017; Lozzi et al., 2020).
Circadian behavior
Circadian rhythms are molecular, physiological, and behavioral changes that synchronize living organisms to daily environmental cycles. Dysfunctions of circadian rhythms are associated with aging and frequently present as comorbidities with neurodegenerative diseases. Although elucidation of the molecular and neural circuit basis of circadian rhythms represents a pinnacle of modern physiology and neuroscience (Sehgal, 2017), recent accumulating evidence has uncovered new and previously unappreciated roles for astrocytes that function autonomously as a central circadian clock in mice.
The transcription-translation negative feedback loops that drive circadian rhythms in mammals exist in both neurons and astrocytes of the suprachiasmatic nucleus (SCN) of the hypothalamus, which is the master circadian pacemaker. In accord, both SCN neurons and astrocytes exhibit oscillations of clock gene expression and intracellular Ca2+ levels (Brancaccio et al., 2019; Brancaccio et al., 2017; Tso et al., 2017). Importantly, the two cell populations are active at opposite phases, with neuronal activity in circadian day and astrocyte activity during night. Deletion of the clock gene Bma1 or the CK1εTau mutant allele specifically in SCN astrocytes significantly lengthened the period of wheel-running locomotor activity during darkness: wheel running is a readily observable behavior that faithfully reflects circadian biology (Brancaccio et al., 2017; Tso et al., 2017). Restored expression of another clock gene, Cry1, in SCN astrocytes alone was sufficient to initiate and sustain circadian patterns of clock gene expression and locomotor activity in otherwise arrhythmic mice that lacked Cry1/2 (Brancaccio et al., 2019). This local astrocytic control of the circadian activity of SCN neurons was mediated by circadian changes in extracellular glutamate. Specifically, astrocytically released glutamate, which was mediated by Cx43 hemichannels, acted on presynaptic neuronal NMDAR receptors to regulate GABAergic tone across the SCN circuit (Brancaccio et al., 2019; Brancaccio et al., 2017). Interestingly, deletion of Bmal1 in GLAST+ astrocytes throughout the brain using the GLAST-Cre/ERT2 mouse line (rather than local manipulations in the SCN) was found to alter neuronal GABA signaling, but with only a mild impact on rhythmic locomotor activity (Barca-Mayo et al., 2017). Nevertheless, both cognition and lifespan of mice were reduced (Barca-Mayo et al., 2020; Barca-Mayo et al., 2017). These differences between SCN and global effects may highlight circuit-specific roles of astrocytes within the SCN or possibly differences between types of astrocytes that were variably targeted with existing genetic strategies. Besides key clock genes, astrocyte signature genes have also been suggested to contribute to circadian behaviors. For example, the expression of astrocyte intermediate filament protein GFAP was significantly changed under constant lighting conditions or in the absence of Bmal1 due to altered glutathionylation (Lananna et al., 2018; Moriya et al., 2000). Furthermore, mice lacking GFAP display altered circadian activity rhythms in constant light (Moriya et al., 2000). Together, these studies suggest that astrocytes are potent regulators and determinants of physiological and behavioral rhythms mediated by the SCN. More broadly, the SCN represents an important nucleus to systematically explore astrocyte biology and its relevance to behavior in a manner that has clear relevance to human biology.
Sleep/wake behavior
Accumulating data have suggested that astrocytes play an essential regulatory role in the physiology of sleep and wakefulness (Haydon, 2017). Sleep and wakefulness are conserved behaviors across species and are regulated by coordinated interactions from multiple neural circuits. There are three well-characterized vigilance states in mammals, consisting of non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep and wakefulness. Different sleep/wakefulness states are identified by distinct characteristics of the brain’s electrical activity and muscle movement measured by electroencephalographic (EEG) and electromyographic (EMG) recordings, which have been utilized in studies involving astrocytes.
In one approach, dnSNARE was used to abrogate astrocyte release of gliotransmitters by expressing the dominant negative construct selectively in astrocytes using a GFAP-tTA mouse line. This prevented exocytotic release of ATP, which reduced the accumulation of extracellular adenosine, a degradation product of ATP and known neuromodulator that activates adenosine A1 receptors to regulate sleep homeostasis (Halassa et al., 2009). Different lines of dnSNARE mice are available, but in these astrocyte-specific mice, the authors found significantly reduced EEG slow wave activity (with a frequency range 0.5–4.0 Hz) during NREM sleep, which represented decreased sleep pressure following wakefulness periods (Halassa et al., 2009). Furthermore, in normal mice, sleep deprivation is well known to impair recognition memory (for example in the novel object recognition task), but in dnSNARE mice this response was significantly attenuated likely because of reduced adenosine levels and consequently reduced activation of adenosine A1 receptors (Halassa et al., 2009). Roles for adenosine in such responses were supported by the finding that selective A1 adenosine receptor antagonists recapitulated the findings in wild type mice, i.e. impairment of recognition memory mediated by sleep deprivation was observed with A1 receptor antagonists. This study represents initial evidence supporting astrocytic regulation of sleep homeostasis and its related cognitive functions via purinergic signaling in vivo. Subsequently, additional studies have supported the importance of astrocytes in adenosine-regulated sleep homeostasis in different settings (Clasadonte et al., 2014; Florian et al., 2011; Schmitt et al., 2012).
Microarray based gene expression analysis has revealed molecular changes in cortical astrocytes that were associated with sleep and wakefulness (Bellesi et al., 2015). Sleep deprivation was found to alter astrocytic pathways including those related to purine nucleotide binding, phagocytosis and lactate metabolism (Bellesi et al., 2017; Bellesi et al., 2015; Petit et al., 2013). In accord, deletion of gap junction subunit Cx43 in astrocytes using the hGFAP-Cre mouse line limited lactate diffusion within the astrocytic network and resulted in reduced excitability of orexin neurons in the lateral hypothalamus (LHA) (Clasadonte et al., 2017). Mice lacking Cx43 in all astrocytes or predominantly within LHA astrocytes specifically displayed increased sleep time and fragmented wakefulness during the active (dark) phase – effects that were ameliorated by lactate administration in vivo (Clasadonte et al., 2017). Whether this astrocytic lactate dependent regulation of neuronal excitability is a region-specific mechanism remains to be determined. However, one study has suggested that the anticonvulsive effects of the ketogenic diet, which limits glucose and lactate supply, results from reduced pyramidal neuron excitability because of limited astrocytic supply of lactate (Sada et al., 2015). Furthermore, an anti-convulsant, stiripentol, has as an off-target action the inhibition of lactate dehydrogenase and one of the known side effects of the drug is drowsiness (Sada et al., 2015).
Additional mechanisms regulating sleep and wakefulness that are mediated by astrocytes continue to be discovered. For instance, a mutation of the Fabp7 gene, which encodes a fatty acid binding protein expressed in astrocytes, was shown to be associated with fragmented sleep in humans (Gerstner et al., 2017). Moreover, mice with Fabp7 deficiency and Drosophila expressing mutant human FABP7 selectively in astrocytes both exhibited similar sleep alterations (Gerstner et al., 2017), suggesting an evolutionarily conserved mechanism by astrocyte lipid signaling in sleep. Astrocyte intracellular Ca2+ signaling was also found to be critical in modulating sleep and neuronal synchronization. It has been found that intracellular Ca2+ signals of cortical astrocytes were less active during sleep but were enhanced preceding transitions to wakefulness in vivo (Bojarskaite et al., 2020; Ingiosi et al., 2020). Furthermore, attenuation of Ca2+ signaling in astrocytes either using IP3 receptor KO mice or astrocyte specific conditional STIM1 KO mice altered the architecture of NREM and REM sleep as well as associated brain rhythms (Bojarskaite et al., 2020; Foley et al., 2017) as well as impaired the homeostatic response to sleep deprivation (Ingiosi et al., 2020). Collectively, these studies have highlighted astrocytes as an integrative component in sleep/wake behavior. Such responses may be reliant on extensive astrocytic networks and their ability to coordinate neuronal activity in large volumes of tissue. Several brain regions have emerged to be critical in the induction and maintenance of different vigilance states (Chowdhury et al., 2019; Liu et al., 2017; Oishi et al., 2017; Yu et al., 2019), and it will be interesting to find out whether astrocytes have ubiquitous or diverse regulatory roles in different sleep circuitry.
Learning and memory-related behaviors
The activation of Gi or Gq-GPCR signaling with hM4Di and hM3Dq DREADDs or Gq-coupled melanopsin is commonly used to stimulate astrocyte Ca2+ dynamics and probe downstream effects on learning and memory. For instance, a melanopsin-based method to temporally trigger Gq activation in astrocytes provided evidence for a role of hippocampal astrocytes in spatial episodic memory (Mederos et al., 2019), and use of hM3Dq DREADD suggested that enhancing astrocyte Ca2+ signaling augmented spatial and contextual memory formation in T-maze and fear conditioning assays (Adamsky et al., 2018). This latter finding coincided with de novo synaptic plasticity in vitro and involved the control of NMDAR function at CA3-CA1 synapses by D-serine an obligatory co-agonist (Papouin et al., 2017; Papouin et al., 2012). In this circuit, the authors concluded that astrocyte activation enabled a local and task-specific increase in neuronal activity, restricted to the ensemble active during memory allocation (Adamsky et al., 2018). Conversely, stimulating the Gq-GPCR pathway in central amygdala (CeM) astrocytes, using the same hM3Dq approach, reduced neuronal firing through astrocyte-derived ATP/adenosine release and was accompanied by decreased fear responses in a cued fear conditioning task of associative learning (Martin-Fernandez et al., 2017). While these studies may appear to contradict each other, they may point to the diverse nature of astrocytes wherein the same stimulus delivered to two distinct networks of astrocytes (CA1 vs CeM) is transduced differently onto the local circuit, yielding region-specific effects on behavior. Furthermore, such discrepancies may be resolved by identifying and then manipulating critical endogenous signaling pathways and genes.
Selective activation of astrocytic Gi-coupled μ-opioid receptors (MOR) in the hippocampus elicited conditioned place preference, suggesting a possible role of astrocytes in positive emotional valence (Nam et al., 2019). The authors suggested that this pathway triggered astrocyte-derived glutamate release, which acted on presynaptic mGluR1 to facilitate LTP induction at CA3-CA1 synapses, and the activation of hM4Di in astrocytes mimicked both the behavioral effect of MOR activation and the facilitation of LTP. Although in this and other reports, hM4Di activation in astrocytes increased intracellular Ca2+ signaling (Chai et al., 2017; Durkee et al., 2019; Nagai et al., 2019), a more complex bimodal response wherein the initial elevation is followed by a modest taming of astrocyte Ca2+ events has also been reported (Kol et al., 2020). Exploiting this indirect reduction of astrocyte signaling, it was suggested that activation of hM4Di during learning impaired the retrieval of remote, but not recent memories (Kol et al., 2020), in line with earlier work (Pinto-Duarte et al., 2019). Mechanistically, Gi-GPCR activation in CA1 astrocytes was found to inhibit neuronal activity in the anterior cingulate cortex (ACC) and attenuated excitatory postsynaptic potentials, suggesting a selective astrocytic modulation of CA1-to-ACC projecting pyramidal neurons that support the formation of remote memories. How astrocytes distinguish distant neuronal projections at a local level within the hippocampus remains unclear, as does the causal link by which the acute activation of astrocyte Gi-GPCR signaling affects long-term memory storage.
Suppression of function approaches have also provided strong grounds to support astrocyte contributions to cognitive behavior. The deletion of cannabinoid receptor 1 (CB1R) selectively from astrocytes, for instance, impairs object recognition memory (Robin et al., 2018) and deleting the transcription factor NF1A in adult astrocytes reduced astrocyte Ca2+ signaling, D-serine levels and synaptic plasticity in the hippocampal CA1 (Huang et al., 2020a). Suppressing Ca2+ dynamics to probe the role of astrocytes in behavior proved deceptive at first, because mice lacking IP3R2 receptors (IP3R2 KO), once thought to be pivotal for astrocyte Ca2+ signaling, exhibited normal behavior (Agulhon et al., 2010; Petravicz et al., 2014). However, NMDAR/D-serine dependent remote memory deficits have been reported in these mice (Pinto-Duarte et al., 2019), while mice with conditional deletion of IP3R2 in GLAST+ astrocytes showed partial reduction of astrocyte Ca2+ signaling in the primary motor cortex and mild impairment in a forelimb motor learning task (Padmashri et al., 2015). Similarly, mice expressing the glutathione-S-transferase (GST)-IP3 sponge in astrocytes showed modest memory impairments in the Morris water maze and in a contextual fear memory task (Tanaka et al., 2013). The fact that altering IP3R2 signaling yields modest effects on behavior seems consistent with the notion that some astrocyte Ca2+ dynamics are independent from IP3R2s (Srinivasan et al., 2015). The dnSNARE mouse line (Pascual et al., 2005) was also used to show the role of astrocyte-derived transmitter release in spatial learning, recognition memory and working memory (Sardinha et al., 2017). In this latter study, the range of behavioral alterations coincided with desynchronization of neural theta oscillations from the dorsal hippocampus to the prefrontal cortex, both of which are normalized by D-serine administration. In a similar mouse model, in which tetanus toxin is conditionally expressed in astrocytes, object recognition memory was disabled (Lee et al., 2014). Together, these findings illustrate diverse pathways and mechanisms through which astrocytes dynamically optimize synaptic properties and functional connectivity in local circuits responsible for learning and memory.
The contribution of astrocyte Gs-GPCR signaling to learning and memory has also been explored in the context of Alzheimer’s disease (AD) and aging in mice. Both AD patients and AD model mice were found to exhibit increased levels of adenosine receptor A2A in hippocampal astrocytes, which was accompanied with memory deficits (Orr et al., 2015). When human Gs-coupled 5-HT4b serotonin receptor Rs1 was expressed in astrocytes and activated, long-term memory was impaired without affecting learning in young and aging mice. In contrast, genetic reduction of astrocytic A2A receptors improved memory in adult wild-type mice as well as in aged AD mice (Orr et al., 2015). This finding suggests astrocyte Gs-GPCR signaling as a mechanism and potential therapeutic target for memory loss in AD.
Tactile sensory acuity
Via the actions of diamine oxidase (DAO) and aldehyde dehydrogenase 1a1 (Aldh1a1) thalamic astrocytes convert putrescine into GABA, which is then released via Best1 channels (Kwak et al., 2020). The use of DAO to synthesize GABA is distinct from astrocytes in other brain regions such as hippocampus and cerebellum (Jo et al., 2014)(Lee et al., 2010), suggesting regional diversity of astrocytic mechanisms. Once released, such tonic GABA activates high affinity extrasynaptic GABAA receptors containing δ subunits to mediate tonic inhibition in thalamocortical (TC) neurons (Kwak et al., 2020). The immediate action of astrocytic GABA was to inhibit synaptically evoked action potential probability of thalamocortical neurons via postsynaptic shunting inhibition. Shunting inhibition by tonic GABA reduced the membrane input resistance and the time constant of TC neurons, reducing both the amplitude and width of EPSPs. The decrease in EPSP amplitude rendered synaptic integration less saturable leading to enhanced dynamic range, i.e. greater linearity of the stimulus-response relations of TC neurons. Faster EPSP kinetics narrowed the time window of synaptic integration resulting in high temporal fidelity of TC neurons, which are capable of distinguishing two independent inputs to them. Next, the authors explored in vivo consequences by using the tactile-based novel object recognition test (Wu et al., 2013). Low tonic GABA conditions following astrocytic knockdown of Best1, DAO or Aldh1a1 lowered discrimination indices whereas high tonic GABA conditions improved tactile discriminability. By showing that norepinephrine induced Ca2+ transients in thalamic astrocytes enhanced tonic GABA, this study proposed a model whereby astrocytic derived tonic GABA tunes sensory acuity following activity of locus coeruleus projections during attentive states. This model supports previous studies reporting that norepinephrine from locus coeruleus modulates feature selectivity and sensory discrimination in mice (Hirata et al., 2006; Rodenkirch et al., 2019).
Striatum-dependent behaviors
Several studies have provided links between astrocyte function and dysfunction to animal behavioral alterations reminiscent of phenotypes seen in mouse models of human psychiatric disorders. In the striatum, astrocytes sit within a brain area where ~95% of the neurons are GABAergic medium spiny neurons (MSNs), which they contact extensively (Chai et al., 2017; Octeau et al., 2018). Astrocytes also express G-protein coupled GABAB receptors, which elevate Ca2+ levels even though they couple to Gi proteins (Chai et al., 2017; Porter and McCarthy, 1997). When Ca2+ signaling of astrocytes in the dorsal striatum was attenuated by heterologously overexpressing a Ca2+ pump, the mice exhibited an obsessive compulsive disorder-like behavior (excessive self-grooming) via a mechanism that was modulated by astrocyte GABA transporter GAT-3 (Yu et al., 2018). Furthermore, activation of Gi-GPCR signaling in astrocytes from the dorsal striatum by hM4Di DREADDs triggered upregulation of the gene for a synaptogenic cue, Thrombospondin-1, which resulted in enhanced excitatory synaptic transmission onto MSNs and behavioral phenotypes related to hyperactivity with disrupted attention (Nagai et al., 2019). Astrocytes from the nucleus accumbens (NAc) in the ventral striatum responded to dopamine release from ventral tegmental area (VTA) neurons by elevating intracellular Ca2+ levels (Corkrum et al., 2020). When dopamine D1Rs or intracellular IP3R2s were deleted from astrocytes in the NAc, the mice displayed damped locomotor responses to amphetamine, suggesting involvement of NAc astrocytes in reward signaling and addiction behaviors. Furthermore, cocaine increased NAc astrocyte Ca2+ signaling, whereas attenuating astrocyte Ca2+ signaling decreased the number of silent synapses in the NAc shell in response to cocaine. Such mechanisms, operating via thrombospondin 2, contribute to cue-induced cocaine seeking after withdrawal, and cue-induced reinstatement of cocaine seeking after extinction (Wang et al.). Furthermore, careful analyses of gene expression changes in striatal astrocytes following multiple experimental perturbations suggested that astrocytes responded in a highly context-specific manner, that such responses could be teased apart and understood in molecular terms to devise astrocyte GPCR-based strategies in order to modify disease-related responses (Yu et al., 2020b).
Lateral habenula-regulated behavior
Detailed experiments implicate astrocytes in depression-like behaviors in rodents. Increased Kir4.1 in astrocytes of the lateral habenula (LHb) increased LHb neuronal firing and resulted in depression-like behaviors (Cui et al., 2018). Moreover, several genetic strategies that reduced Kir4.1 function in the LHb reduced depression-like behaviors in rodents. In this instance, astrocyte-mediated K+ buffering was proposed to regulate the intrinsic excitability and action potential firing properties of LHb neurons, which by virtue of their synaptic connectivity regulate behaviors such as anhedonia and giving up (immobility) in the forced swim test (Cui et al., 2018). More specifically, increased Kir4.1 is proposed to decrease extracellular K+ levels around LHb neuron somata, leading to hyperpolarization and de-inactivation of a T-type voltage-gated Ca2+ channel that subsequently evoked LHb neuron burst firing. If true in humans, the implication of this study is that a partial blocker of Kir4.1 may display anti depressive properties. In accord, expression levels of Kir4.1 were upregulated in the parietal cortex of patients with major depressive disorder, but not in those with schizophrenia (SCZ) or bipolar disorder (Xiong et al., 2019b), and several antidepressants are known to interact with Kir4.1 channel pore residues and inhibit Kir4.1 K+ currents (Furutani et al., 2009).
Human diseases with a primary astrocytic basis
Extensively ramified human astrocytes display unique morphological features such as varicosities and processes that cross between cortical layers (Figure 1E). Gene expression analyses show that human astrocytes differ from those in rodents in accord with expectations of human-mouse differences (Zhang et al., 2016). It has been suggested that greater astrocyte complexity may contribute to the higher cognitive abilities of hominids (Bush and Nedergaard, 2017; Oberheim et al., 2009), and chimeric mice harboring human glial cell progenitors (many of which become astrocytes) exhibited improved performance in learning and memory tasks relative to the control immunocompromised mice (Han et al., 2013). However, hypothesis testing and detailed physiology of human astrocytes is in its infancy, and emerging evidence indicates that human astrocytes also exhibit heterogeneous regional gene expression (Kelley et al., 2018). Although it is not possible to link dynamic astrocyte signaling to specific human behaviors, we briefly mention disorders that have a primary basis in astrocyte-enriched proteins and result in profound behavioral alterations (Table 1).
Table 1:
Examples of astrocyte enriched genes associated with CNS disorders in humans.
| Disease or clinical presentation | Gene HUMAN, mouse | Protein | 1 Mutation type | 2 Astrocyte enrichment versus [other brain cell type] human; mouse |
|---|---|---|---|---|
| Alexander disease | GFAP, Gfap | Glial fibrillary acidic protein:
GFAP |
Gain-of-function | [Neurons]: 19; 22
[Oligodendrocytes]: 8;7 [Microglia/macrophages]: 70; 18 [Endothelia]: 27;36 |
| Autism-epilepsy, sleep disorder,
EAST syndrome, SeSAME syndrome |
KCNJ10, Kcnj10 | Inwardly-rectifying potassium channel: Kir4.1 | Gain or loss-offunction | [Neurons]: 14;23 [Oligodendrocytes]: 4;3 [Microglia/Macrophages]: 207;73 [Endothelia]: 48;395 |
| Ataxia, migraine | SLC1A3, Slc1a3 | Glutamate transporter: EAAT1 | Loss | [Neuron]:12;25
[Oligodendrocytes]: 22;61 [Microglia/Macrophages]: 7;65 [Endothelia]: 55; 296 |
| Epilepsy, bipolar disorder, schizophrenia | SLC1A2, Slc1a2 | Glutamate transporter: EAAT2 | Probably loss-offunction | [Neuron]: 12;25
[Oligodendrocytes]: 39;32 [Microglia/Macrophages]: 452;93 [Endothelia]: 62;423 |
| Familial hemiplegic migraine type 2 | ATP1A2, Atp1a2 | Na+/K+-ATPase α2subunit | Loss-of-function and
gain-of-function |
[Neuron]: 17;29 [Oligodendrocytes]: 33;31 [Microglia/Macrophages]: 598;51 [Endothelia]: 4;30 |
| neuromyelitis optica spectrum disorder (Devic’s disease) |
AQP4 Aqp4 | Water channel: aquaporin 4 | Relapsingremitting autoimmune disease producing antibodies against aquaporin 4 |
[Neurons]: 13;27
[Oligodendrocytes]: 54;224 [Microglia/Macrophages]: 395;374 [Endothelial]: 71;265 |
| Vanishing white matter disease |
EIF2B1–5,
Eif2b1–5 |
Guanine nucleotide exchange factor: eIF2B | Probably loss-offunction | [Neurons]: *0.9, 1.2, 0.5, 0.9, 0.5;0.7, 0.8, 0.8, 0.8,
0.6 [Oligodendrocytes]: 1.1, 1.6, 1.0, 1.0, 0.9;0.5, 0.7, 0.6, 1.0, 0.5 [Microglia/Macrophages]: 1.3, 3.0, 1.0, 1.8, 0.7;1.1, 0.9, 1.2, 0.7, 0.4 [Endothelial]: 1.4, 4.0, 2.4, 1.8, 2.2;0.6, 0.4, 0.4, 0.9, 0.3 |
In each case, multiple mutations have been identified, which cannot be listed due to space limits.
The astrocyte enrichment score was estimated from https://www.brainrnaseq.org/ and represents the (astrocyte/other cell type) gene expression (FPKM) ratio for human (blue) and mouse (red) cells.
Values shown in the order of EIF2B1, EIF2B2, EIF2B3, EIF2B4 and EIF2B5 for each cell type.
Mutation of GFAP causes a rare progressive form of leukodystrophy called Alexander disease (AxD). GFAP is an intermediate filament protein expressed by astrocytes and is a prototypical astrocytic marker (Messing and Brenner, 2020). Patients with AxD display seizures, ataxia, and psychomotor retardation. The pathogenic variants of the GFAP gene are considered to cause a gain-of-function phenotype, which leads to abnormal protein aggregates in astrocytes termed Rosenthal fibers, as well as altered astrocyte physiology (Saito et al., 2018).
Kir4.1 weakly inwardly-rectifying potassium channels contribute to passive electrical properties and to extracellular K+ homeostasis by astrocytes and oligodendrocytes (Chever et al., 2010; Djukic et al., 2007). Kir4.1 also facilitates glutamate transport, regulates cell volume and water content. Loss-of-function mutations in KCNJ10 are linked to autosomal recessive disorders called EAST syndrome (Epilepsy, Ataxia, Sensorineural deafness, and (a renal salt-wasting) Tubulopathy) and SeSAME syndrome (Seizures, Sensorineural deafness, Ataxia (lack of muscle coordination), intellectual (Mental) disability, and Electrolyte imbalance) (Bockenhauer et al., 2009; Scholl et al., 2009). Both syndromes include epilepsy, ataxia, sensorineural deafness and tubulopathy. SeSAME EAST mutations result in reduced or abolished Kir4.1 K+ currents (Williams et al., 2010). In contrast, gain-of-function mutations in KCNJ10 were correlated with an autism and epilepsy phenotype, and with sleep alterations in children (Cucchiara et al., 2020; Reichold et al., 2010; Sicca et al., 2016; Sicca et al., 2011).
EAAT1 and EAAT2 are predominantly expressed in astrocytes and are responsible for removing excessive glutamate from the synaptic cleft. Heterozygous mutations in the SLC1A3 gene cause episodic ataxia (Choi et al., 2017; Jen et al., 2005; Pyle et al., 2015; De Vries et al., 2009). Recently, another loss-of-function mutation of the SLC1A3 gene was identified in a patient with migraine, but without ataxia (Kovermann et al., 2017). This mutation impaired K+ binding to EAAT1 and diminished glutamate uptake. Mutations in SLC1A2 (encoding EAAT2/GLT-1) have been linked to epileptic encephalopathies, which are a group of early-onset epilepsies with severe cognitive and behavioral impairments (Epi4Kconsortium, 2016).
The transmembrane Na+/K+-ATPase pump is crucial for maintaining ionic gradients as well as for regulating cell volume and signaling pathways. Out of the four α isoforms, the α2-subunit is encoded by the ATP1A2 gene and is mainly expressed by astrocytes. Autosomal dominant mutations in the ATP1A2 gene cause an inherited form of migraine called familial hemiplegic migraine type 2 (FHM2), characterized by aura, hemiparesis and dysphasia (Bøttger et al., 2012; Carreño et al., 2013; Hiekkala et al., 2018). FHM2 patients can also display seizures, cognitive impairments, and rare manifestations of psychiatric symptoms. The underlying mechanisms of how ATP1A2 mutations lead to FHM remain mysterious. However, dysfunctions of Na+/K+-ATPase and its coupled proteins result in cortical spreading depression that triggers migraine aura (Friedrich et al., 2016). Mice carrying a missense mutation in Atp1a2 displayed increased cortical dendritic excitability and sensitivity to head pain induction (Romanos et al., 2020).
AQP4 is a water channel located at the peri-vascular and peri-ventricular processes of astrocytes. Serum immunoglobulin G autoantibodies against AQP4 (AQP4-IgG) were described in patients with the rare idiopathic inflammatory demyelinating disease, neuromyelitis optica (NMO) (Lennon et al., 2005; Lennon et al., 2004). NMO primarily affects the optic nerves and spinal cord of individuals and causes clinical manifestations involving visual impairment, weakness, paralysis, numbness or increased sensitivity in the legs or arms, painful spasms, uncontrollable vomiting and hiccups, and bladder or bowel problems. AQP4-IgG binding to AQP4 on astrocytes causes complement-dependent multicellular cytotoxicity without altering AQP4 water permeability (Papadopoulos and Verkman, 2012; Saadoun et al., 2010).
We have restricted our discussion to causal molecular changes in astrocyte-enriched genes that lead to neurological or psychiatric behavioral readouts (Table 1). It is noteworthy, however, that vanishing white matter disease, which is the result of mutations in eukaryotic translation initiation factor 2B (eIF2B), appears to have a predominantly astrocytic basis even though eIF2B is expressed ubiquitously (Bugiani et al., 2018; Dooves et al., 2016; van der Knaap et al., 2006). Hence, it is possible that mechanistic studies will reveal that astrocytes are critical drivers of pathology in other diseases even when the underlying mutation exists in multiple cell types.
Summary comments
Exploring how astrocytes regulate neuronal circuits in vivo was the logical extension of anatomical reality and several years of pioneering studies that showed that astrocytes displayed physiological responses in vivo during diverse types of sensory and behavioral paradigms. In this review, we have summarized recent studies from diverse species suggesting that astrocytes also contribute consequentially to the functions of neural circuits and the behaviors they encode. These data reveal an under recognized richness in nervous system function and suggest neurons and astrocytes as determinants of complex brain functions, and therefore also as targets to be exploited in order to correct behavioral dysfunctions associated with disease.
How does one interpret the behaviorally relevant responses ascribed to astrocytes and discussed in this review? We suggest three types of interpretation are possible based on the available data and each should be considered in relation to future work.
Type 1 interpretations:
In this category are examples suggesting astrocytes integrate incoming neuronal signals over seconds and then switch to a mode resulting in altered neuronal function. Zebrafish radial astrocytes in a specific subregion of the brainstem temporally integrate noradrenergically encoded behavioral failures to accumulate evidence of futility over seconds before inducing a state of behavioral passivity (Mu et al., 2019). The ability of Drosophila astrocytes to regulate sleep via Spätzle and olfactory-driven chemotaxis and touch-induced startle via ATP/adenosine also require Type 1 interpretations (Blum et al., 2020; Ma et al., 2016). Furthermore, genetically impairing Ca2+ signaling in striatal astrocytes and restoring expression of a clock gene, Cry1, in SCN astrocytes alone were sufficient to guide very specific behaviors: highly stereotyped self-grooming (Yu et al., 2018) and circadian patterns of locomotor activity in otherwise arrhythmic mice (Brancaccio et al., 2019), respectively. Although the behavioral relevance is unclear, in the mouse hippocampus, long bouts of action potential firing in NPY positive interneurons results in the emergence of barrage firing (Deemyad et al., 2018). This switch to barrage firing may be explained by astrocytes functioning as leaky integrators of ongoing activity and then abruptly changing to allow interneurons to switch mode. Together, these studies suggest that astrocyte signaling performs specific functions that result in specific behavioral outcomes.
For Type 1 interpretations, understanding how astrocytes integrate information at a biophysical level requires quantitative measurements and modeling. In these regards, understanding the details of astrocyte Ca2+ signaling in subcellular compartments will be important. For example, recent studies suggest that basal Ca2+ levels determine the properties of diverse types of Ca2+ events in brain slices and in vivo (King et al., 2020). If basal Ca2+ levels are regulated, this could represent a substrate for a leaky integrator that shapes subsequent responses. Another possibility is that integration occurs as signals in distinct spatial locations, which also have different basal Ca2+ levels, summate during ongoing activity (Zheng et al., 2015). Further studies to quantify and model the potential ability of astrocytes to integrate or process activity within realistic cellular geometries or compartments are critical (Savtchenko et al., 2018). There are clear reasons to study Ca2+ signaling, but we ought to consider other intracellular signaling mechanisms as well.
Type 2 interpretations:
One interpretation of some studies that we have considered is that astrocytes are broadly important for brain homeostasis/function and therefore it is natural that some behaviors will be altered when astrocytes are changed. This type of explanation may be applicable in several cases and may be important and of particular interest in the context of how astrocytes contribute to disease phenotypes. An example of Type 2 explanations is Kir4.1 upregulation in the lateral habenula, which through altered K+ homeostasis drives behaviors associated with depression (Cui et al., 2018). Another example is how altered astrocyte-mediated glutamate homeostasis affects multiple aspects of brain function (Danbolt, 2001). In several cases, the use of DREADDs and other actuators could fall into responses requiring Type 2 interpretations when there is a lack of additional evidence for endogenous GPCR mechanisms that the actuator mimics. Responses requiring Type 2 interpretations may be particularly meaningful in disease related behaviors, either to understand the phenotypes or to modulate them for beneficial effect (Cui et al., 2018; Yu et al., 2020b).
Type 3 interpretations:
For some astrocyte responses, we consider a third explanation. In this case, a neural circuit or behavioral alteration may be best explained by a coincident effect that is unrelated to normal astrocyte biology. One example of responses needing Type 3 interpretations may be the use of Channelrhodopsin in astrocytes, which elevates extracellular K+ levels when activated for seconds or more (Octeau et al., 2019). In cases where this occurs, alterations in neural activity and behavior are expected, but these are no more insightful than saying K+ depolarizes neurons. Such responses tell us what astrocytes are capable of doing under artificial experimental settings, but not necessarily about what they actually do under normal settings. This distinction is important to recognize in order for the field to grow and capture new researchers. Another example of Type 3 responses is the outcome of the use of promoters and driver lines that are meant to be astrocyte selective, but result in alteration of neurons with resultant coincident changes in circuits and behavior that confound the interpretation of astrocytic effects that may occur in parallel. An example in this category may include the use of some mouse lines, which target populations of neurons as well as astrocytes making it problematic to interpret behavioral effects ascribed to astrocytes. Such issues have been widely discussed in the literature (Hirbec et al., 2020; Xie et al., 2015; Yu et al., 2020a).
More generally, although revealing in a causal sense, optogenetic and chemogenetic approaches nonetheless have general limitations. First, they often lack holistic context for the cellular effects making it problematic to separate direct and secondary astrocyte contributions to behavior. Second, exogenous stimulation may not faithfully recapitulate endogenous pathways used in vivo. In the future, such explorations could be aided with suppression of defined types of ongoing physiological activity as a complementary interventional approach. Moreover, the roles of striatal astrocyte Kir4.1 in the context of HD and in the LHb in the context of depression are illustrative of a general and important point. In the LHb, overexpression of Kir4.1 led to increased excitability of LHb neurons, whereas in the striatum downregulation of Kir4.1 led to increased MSN excitability (Cui et al., 2018; Tong et al., 2014). These data suggest that the circuit consequences of astrocyte mechanisms will be dictated by the biophysical properties of neurons within specific brain regions. Along with the evidence for region-specific astrocyte properties, there are likely to exist astrocyte mechanisms with differential effects between brain areas even when the underlying molecular change is related or the same (Huang et al., 2020a). In these regards, it will be important to expand the pallet of tools available to measure diverse astrocyte activity in parallel with neuronal function between brain areas. Improved tools to image ATP, metabolism, spatial dynamics, glycolysis, and ions such as K+ would be a valuable start.
It will also be necessary to explore astrocyte functions in circuits from diverse species at a mechanistic level based on data-driven insights regarding molecular causation. Conservation of glial cell types, anatomical simplicity, experimental accessibility, and new tools will continue to allow model organisms such as worms, flies, and zebrafish to unveil how astrocytes regulate neural circuit function and animal behavior at cellular and molecular resolution. In parallel, it will be critical to explore astrocytic contributions to ethologically natural behaviors and to those that accompany neurological and psychiatric disease states. Both types of exploration are necessary and a comprehensive body of such studies should reveal if astrocytes contribute to normal or disease phenotypes, or possibly to both. Another parallel direction is to combine behavioral paradigms with computational approaches to decipher the underlying logic of circuit mechanisms in relation to cognition, which relates to earlier discussion of how astrocytes integrate information. The fruit fly has contributed enormously to our understanding of many basic principles in animal development and physiology, and it seems poised to help understand astrocytes. The powerful array of molecular-genetic approaches available for Drosophila hold great promise for rapid identification of genes that are required to specify, build, and operate astrocytes in vivo within neural circuits. In zebrafish, it is expected that taxonomic analysis (Lange et al., 2020; Raj et al., 2018) will reveal the molecular identities of astrocytes and help design novel animal lines and/or reagents that are more specific to them (Chen et al., 2020). Employing rapidly evolving imaging tools for neurotransmitters, biomolecules and ions will also likely broaden the questions one can ask, because zebrafish provides unique opportunities for monitoring neuron-glia interactions, especially at a level of scale and detail that, currently, would be difficult in studies using mammals. Naturally, there will likely be some differences in the precise operational mechanisms employed in any one-model organism, but there is ample reason to believe that they will have many more things in common and irrespectively comparisons between species will prove fruitful and likely teach us important aspects of biology.
With recent progress, it now seems clear that whereas electrophysiology and the oscilloscope provided powerful early means to study fast electrical events in neurons and foreshadowed decades of insight and progress, experimental explorations of astrocytes have had to await the advent of genetic and imaging methods that capture the slower dynamics of these cells. Reassuringly, these approaches are now beginning to provide new and unexpected insights concerning astrocyte biology, nervous system function, and the regulation of behavior.
Acknowledgments
JN and XY were supported by Khakh lab funds during this work. Research in the Papouin lab is funded by a NARSAD Young Investigator Grant (Brain & Behavior Research Foundation, #28616), a McDonnell Center for Cellular and Molecular Neurobiology grant (#22-3930-26275U), and a Whitehall Foundation Inc. Research Grant (#2020-08-35). EC was supported by the National Research Foundation of Korea (NRF) (2017R1A2B3011098, 2017M3C7A1023471, 2020R1A4A1019009). MRF was supported by R01 NS053538. KRM is supported by the NIH (1R21NS115437). MHH was supported by the Medical Research Council, U.K. (MC_U105170643). PGH is supported by grants from the NIH 5RO1 NS037585-22, 5RO1 NS107315-03 and 1RO1 AG061838-01. DR is a Wellcome Trust Senior Investigator. SS was supported by NIH grant R35NS105094. BSK was supported by NIH (R35NS111583 and DA047444), CHDI Inc, by an Allen Distinguished Investigator Award, a Paul G. Allen Frontiers Group advised grant of the Paul G. Allen Family Foundation, and by the Ressler Family Foundation.
Footnotes
In this review, Nagai et al summarize recent findings and provide an interpretative framework for diverse astrocyte mechanisms regulating neural circuit functions and animal behaviors in multiple species, including C. elegans, Drosophila melanogaster, Danio rerio, and Mus musculus.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams KL, and Gallo V (2018). The diversity and disparity of the glial scar. Nat Neurosci 21, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, et al. (2018). Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 59–71. [DOI] [PubMed] [Google Scholar]
- Agulhon C, Fiacco TA, and McCarthy KD (2010). Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254. [DOI] [PubMed] [Google Scholar]
- Aida T, Yoshida J, Nomura M, Tanimura A, Iino Y, Soma M, Bai N, Ito Y, Cui W, Aizawa H, et al. (2015). Astroglial glutamate transporter deficiency increases synaptic excitability and leads to pathological repetitive behaviors in mice. Neuropsychopharmacology 40, 1569–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, and Barres BA (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, and Lyons DA (2018). Glia as architects of central nervous system formation and function. Science 362, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altun ZF, Chen B, Wang ZW, and Hall DH (2015). High resolution map of caenorhabditis elegans gap junction proteins. Dev Dyn 244, 903. doi: 910.1002/dvdy.24287. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, and Ito K (2006). Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50, 855–867. [DOI] [PubMed] [Google Scholar]
- Bacaj T, Tevlin M, Lu Y, and Shaham S (2008). Glia are essential for sensory organ function in C. elegans. Science 322, 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca-Mayo O, Boender AJ, Armirotti A, and De Pietri Tonelli D (2020). Deletion of astrocytic BMAL1 results in metabolic imbalance and shorter lifespan in mice. Glia 68, 1131–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barca-Mayo O, Pons-Espinal M, Follert P, Armirotti A, Berdondini L, and De Pietri Tonelli D (2017). Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun 8, 14336. doi: 14310.11038/ncomms14336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, and Attwell D (2016). Astrocyte calcium signalling: the third wave. Nat Neurosci 19, 182–189. [DOI] [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, and Cirelli C (2017). Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J Neurosci 37, 5263–5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellesi M, de Vivo L, Tononi G, and Cirelli C (2015). Transcriptome profiling of sleeping, waking, and sleep deprived adult heterozygous Aldh1L1 - eGFP-L10a mice. Genomics data 6, 114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier LP, Bohlen CJ, York EM, Choi HB, Kamyabi A, Dissing-Olesen L, Hefendehl JK, Collins HY, Stevens B, Barres BA, et al. (2019). Nanoscale Surveillance of the Brain by Microglia via cAMP-Regulated Filopodia. Cell Rep 27, 2895–2908.e2894. [DOI] [PubMed] [Google Scholar]
- Blum ID, Keleş MF, Baz E-S, Han E, Park K, Luu S, Issa H, Brown M, Ho MCW, Tabuchi M, et al. (2020). Astroglial Calcium Signaling Encodes Sleep Need in Drosophila. Current Biology 10.1016/j.cub.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojarskaite L, Bjørnstad DM, Pettersen KH, Cunen C, Hermansen GH, Åbjørsbråten KS, Chambers AR, Sprengel R, Vervaeke K, Tang W, et al. (2020). Astrocytic Ca(2+) signaling is reduced during sleep and is involved in the regulation of slow wave sleep. Nat Commun 11, 3240. doi: 3210.1038/s41467–41020-17062–41462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Edwards MD, Patton AP, Smyllie NJ, Chesham JE, Maywood ES, and Hastings MH (2019). Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science 363, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancaccio M, Patton AP, Chesham JE, Maywood ES, and Hastings MH (2017). Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron 93, 1420–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugiani M, Vuong C, Breur M, and van der Knaap MS (2018). Vanishing white matter: a leukodystrophy due to astrocytic dysfunction. Brain pathology (Zurich, Switzerland) 28, 408–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NAO, and Nedergaard M (2017). Do evolutionary changes in astrocytes contribute to the computational power of the hominid brain? Neurochem Res 42, 2577–2587. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, and Ellisman MH (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, Cohn W, Rajendran PS, Vondriska TM, Whitelegge JP, et al. (2017). Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, and Sanderson MJ (1991). Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6, 983–992. [DOI] [PubMed] [Google Scholar]
- Chen J, Poskanzer KE, Freeman MR, and Monk KR (2020). Live-imaging of astrocyte morphogenesis and function in zebrafish neural circuits. Nat Neurosci 23, 1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Kim J, Fu Z, Barak B, Sur M, Feng G, and Han W (2016). Direct modulation of GFAP-expressing glia in the arcuate nucleus bi-directionally regulates feeding. Elife October 18;5:e18716. doi: 10.7554/eLife.18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chever O, Djukic B, McCarthy KD, and Amzica F (2010). Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J Neurosci 30, 15769–15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Matsubara T, Miyazaki T, Ono D, Fukatsu N, Abe M, Sakimura K, Sudo Y, and Yamanaka A (2019). GABA neurons in the ventral tegmental area regulate non-rapid eye movement sleep in mice. Elife 8 10.7554/eLife.44928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, and Barres BA (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. [DOI] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, McIver SR, Schmitt LI, Halassa MM, and Haydon PG (2014). Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J Neurosci 34, 1879–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, Scemes E, Wang Z, Boison D, and Haydon PG (2017). Connexin 43-mediated astroglial metabolic networks contribute to the regulation of the sleep-wake cycle. Neuron 95, 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colon-Ramos DA, Margeta MA, and Shen K (2007). Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, and Smith SJ (1990). Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247, 470–473. [DOI] [PubMed] [Google Scholar]
- Cucchiara F, Frumento P, Banfi T, Sesso G, Di Galante M, D’Ascanio P, Valvo G, Sicca F, and Faraguna U (2020). Electrophysiological features of sleep in children with Kir4.1 channel mutations and Autism-Epilepsy phenotype: a preliminary study. Sleep April 15;43(4):zsz255. doi: 10.1093/sleep/zsz255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y, et al. (2018). Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 554, 323–327. [DOI] [PubMed] [Google Scholar]
- Danbolt NC (2001). Glutamate uptake. Prog Neurobiol 65, 1–105. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, and Gan WB (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci 8, 752–755. [DOI] [PubMed] [Google Scholar]
- De Saint Jan D, and Westbrook GL (2005). Detecting activity in olfactory bulb glomeruli with astrocyte recording. J Neurosci 25, 2917–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deemyad T, Luthi J, and Spruston N (2018). Astrocytes integrate and drive action potential firing in inhibitory subnetworks. Nat Commun 9, 4336 4310.1038/s41467–41018-06338–41463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Zou W, Zhang H, Xue Y, Cai Y, Huang G, Chen L, Duan S, and Kang L (2015). In vivo tactile stimulation-evoked responses in Caenorhabditis elegans amphid sheath glia. PLoS One 10, e0117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, and McCarthy KD (2007). Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27, 11354–11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doengi M, Hirnet D, Coulon P, Pape HC, Deitmer JW, and Lohr C (2009). GABA uptake-dependent Ca(2+) signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci U S A 106, 17570–17575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, Bernet A, Mehlen P, and Chédotal A (2017). Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 545, 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooves S, Bugiani M, Postma NL, Polder E, Land N, Horan ST, van Deijk AL, van de Kreeke A, Jacobs G, Vuong C, et al. (2016). Astrocytes are central in the pathomechanisms of vanishing white matter. J Clin Invest 126, 1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroquez DB, Berciu C, Anderson JR, Sengupta P, and Nicastro D (2014). A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in Caenorhabditis elegans. eLife 3, e01948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkee CA, Covelo A, Lines J, Kofuji P, Aguilar J, and Araque A (2019). Gi/o protein-coupled receptors inhibit neurons but activate astrocytes and stimulate gliotransmission. Glia 67, 1076–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epi4Kconsortium (2016). De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet 99, 287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. (2009). Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 139, 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu C, and Barres BA (2010). Regulation of synaptic connectivity by glia. Nature 468, 223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T, Scheffler P, and Lohr C (2020). Dopamine-induced calcium signaling in olfactory bulb astrocytes. Sci Rep 10, 631 610.1038/s41598–41020-57462–41594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florian C, Vecsey CG, Halassa MM, Haydon PG, and Abel T (2011). Astrocyte-derived adenosine and A1 receptor activity contribute to sleep loss-induced deficits in hippocampal synaptic plasticity and memory in mice. J Neurosci 31, 6956–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Blutstein T, Lee S, Erneux C, Halassa MM, and Haydon P (2017). Astrocytic IP3/Ca(2+) signaling modulates theta rhythm and REM sleep. Front Neural Circuits 11, 3 10.3389/fncir.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman MR, and Rowitch DH (2013). Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron 80, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuente-Martín E, García-Cáceres C, Granado M, de Ceballos ML, Sánchez-Garrido MÁ, Sarman B, Liu ZW, Dietrich MO, Tena-Sempere M, Argente-Arizón P, et al. (2012). Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 122, 3900–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner JR, Perron IJ, Riedy SM, Yoshikawa T, Kadotani H, Owada Y, Van Dongen HPA, Galante RJ, Dickinson K, Yin JCP, et al. (2017). Normal sleep requires the astrocyte brain-type fatty acid binding protein FABP7. Sci Adv 3, e1602663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, and Rouach N (2010). Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci 11, 87–99. [DOI] [PubMed] [Google Scholar]
- Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen-Chi M, and Currie PD (2012). Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. J Neurosci 32, 7477–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J, Matthewman C, and Bianchi L (2015). A Novel Mechanism of pH Buffering in C. elegans Glia: Bicarbonate Transport via the Voltage-Gated ClC Cl-Channel CLH-1. J Neurosci 35, 16377–16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim LB, and Rowitch DH (2017). Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18, 31–41. [DOI] [PubMed] [Google Scholar]
- Hakim Y, Yaniv SP, and Schuldiner O (2014). Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS One 9, e86178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, and Frank MG (2009). Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61, 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Wang Y, Sangaletti R, D’Urso G, Lu Y, Shaham S, and Bianchi L (2013). Two novel DEG/ENaC channel subunits expressed in glia are needed for nose-touch sensitivity in Caenorhabditis elegans. J Neurosci 33, 936–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haustein MD, Kracun S, Lu XH, Shih T, Jackson-Weaver O, Tong X, Xu J, Yang XW, O’Dell TJ, Marvin JS, et al. (2014). Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 82, 413–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG (2017). Astrocytes and the modulation of sleep. Curr Opin Neurobiol 44, 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman MG, and Shaham S (2009). DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137, 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Bard L, Panatier A, Reynolds JP, Kopach O, Medvedev NI, Minge D, Herde MK, Anders S, Kraev I, et al. (2020). LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron September 21;S0896–6273(20)30661–9. doi: 10.1016/j.neuron.2020.08.030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, and Castro-Alamancos MA (2006). Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26, 4426–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H, Déglon N, Foo LC, Goshen I, Grutzendler J, Hangen E, Kreisel T, Linck N, Muffat J, Regio S, et al. (2020). Emerging technologies to study glial cells. Glia 68, 1692–1728. [DOI] [PubMed] [Google Scholar]
- Huang AY, Woo J, Sardar D, Lozzi B, Bosquez Huerta NA, Lin CJ, Felice D, Jain A, Paulucci-Holthauzen A, and Deneen B (2020a). Region-specific transcriptional control of astrocyte function oversees local circuit activities. Neuron 106, 992–1008 e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TT, Matsuyama HJ, Tsukada Y, Singhvi A, Syu RT, Lu Y, Shaham S, Mori I, and Pan CL (2020b). Age-dependent changes in response property and morphology of a thermosensory neuron and thermotaxis behavior in Caenorhabditis elegans. Aging cell 19, e13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingiosi AM, Hayworth CR, Harvey DO, Singletary KG, Rempe MJ, Wisor JP, and Frank MG (2020). A Role for Astroglial Calcium in Mammalian Sleep and Sleep Regulation. Curr Biol November 16;30(22):4373–4383.e7. doi: 10.1016/j.cub.2020.08.052.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell TA, Wang Y, Bloniarz AE, Brittin CA, Xu M, Thomson JN, Albertson DG, Hall DH, and Emmons SW (2012). The connectome of a decision-making neural network. Science 337, 437–444. [DOI] [PubMed] [Google Scholar]
- Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, Bae JY, Kim T, Lee J, Chun H, et al. (2014). GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med 20, 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, et al. (2017). Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CK, Fernandez-Abascal J, Wang Y, Wang L, and Bianchi L (2020). The Na(+)-K(+)-ATPase is needed in glia of touch receptors for responses to touch in C. elegans. J Neurophysiol 123, 2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Corson F, Iwanir S, Biron D, and Shaham S (2018). Glia modulate a neuronal circuit for locomotion suppression during sleep in C. elegans. Cell Rep 22, 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Corson F, Keil W, Singhal A, Bae A, Lu Y, Liang Y, and Shaham S (2019). Glutamate spillover in C. elegans triggers repetitive behavior through presynaptic activation of MGL-2/mGluR5. Nat Commun 10, 1882. April 1823;1810(1881):1882. doi: 1810.1038/s41467–41019-09581–41464.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Nakao-Inoue H, Molofsky AV, and Oldham MC (2018). Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat Neurosci 21, 1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, and Verkhratsky A (2008). Neuroglia: the 150 years after. Trends Neurosci 31, 653–659. [DOI] [PubMed] [Google Scholar]
- Khakh BS, and Deneen B (2019). The emerging nature of astrocyte diversity. Annu Rev Neurosci 42, 187–207. [DOI] [PubMed] [Google Scholar]
- Khakh BS, and Sofroniew MV (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CM, Bohmbach K, Minge D, Delekate A, Zheng K, Reynolds J, Rakers C, Zeug A, Petzold GC, Rusakov DA, et al. (2020). Local Resting Ca(2+) Controls the Scale of Astroglial Ca(2+) Signals. Cell Rep 30, 3466–3477.e3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kol A, Adamsky A, Groysman M, Kreisel T, London M, and Goshen I (2020). Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat Neurosci 23, 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, and Brand M (2011). Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 138, 4831–4841. [DOI] [PubMed] [Google Scholar]
- Kuffler SW (1967). Neuroglial cells: physiological properties and a potassium mediated effect of neuronal activity on the glial membrane potential. Proc R Soc Lond B Biol Sci 168, 1–21. [DOI] [PubMed] [Google Scholar]
- Kwak H, Koh W, Kim S, Song K, Shin JI, Lee JM, Lee EH, Bae JY, Ha GE, Oh JE, et al. (2020). Astrocytes Control Sensory Acuity via Tonic Inhibition in the Thalamus. Neuron August 30;S0896–6273(20)30644–9. doi: 10.1016/j.neuron.2020.08.013. [DOI] [PubMed] [Google Scholar]
- Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, and Brand M (2012). Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338, 1353–1359. [DOI] [PubMed] [Google Scholar]
- Labouesse M, Sookhareea S, and Horvitz HR (1994). The Caenorhabditis elegans gene lin-26 is required to specify the fates of hypodermal cells and encodes a presumptive zinc-finger transcription factor. Development 120, 2359–2368. [DOI] [PubMed] [Google Scholar]
- Lananna BV, Nadarajah CJ, Izumo M, Cedeno MR, Xiong DD, Dimitry J, Tso CF, McKee CA, Griffin P, Sheehan PW, et al. (2018). Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep 25, 1–9.e5. doi: 10.1016/j.celrep.2018.1009.1015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Rost F, Machate A, Reinhardt S, Lesche M, Weber A, Kuscha V, Dahl A, Rulands S, and Brand M (2020). Single cell sequencing of radial glia progeny reveals the diversity of newborn neurons in the adult zebrafish brain. Development 147, dev185595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Ghetti A, Pinto-Duarte A, Wang X, Dziewczapolski G, Galimi F, Huitron-Resendiz S, Piña-Crespo JC, Roberts AJ, Verma IM, et al. (2014). Astrocytes contribute to gamma oscillations and recognition memory. Proc Natl Acad Sci U S A August 12;111(32):E3343–52. doi: 10.1073/pnas.1410893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, and Hinson SR (2005). IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202, 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, and Weinshenker BG (2004). A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364, 2106–2112. [DOI] [PubMed] [Google Scholar]
- Liu K, Kim J, Kim DW, Zhang YS, Bao H, Denaxa M, Lim SA, Kim E, Liu C, Wickersham IR, et al. (2017). Lhx6-positive GABA-releasing neurons of the zona incerta promote sleep. Nature 548, 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzi B, Huang TW, Sardar D, Huang AY, and Deneen B (2020). Regionally Distinct Astrocytes Display Unique Transcription Factor Profiles in the Adult Brain. Front Neurosci 14, 61 10.3389/fnins.2020.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2008). Genetic dissection of neural circuits. Neuron 57, 634–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, and Svoboda K (2018). Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron. Neuron 98, 256–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Stork T, Bergles DE, and Freeman MR (2016). Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature 539, 428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano I, Straud S, and Driscoll M (2007). Caenorhabditis elegans glutamate transporters influence synaptic function and behavior at sites distant from the synapse. J Biol Chem 282, 34412–34419. [DOI] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J, Benneyworth MA, Marsicano G, and Araque A (2017). Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20, 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMiller TL, and Johnson CM (2005). Molecular characterization of HLH-17, a C. elegans bHLH protein required for normal larval development. Gene 356, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos S, Hernandez-Vivanco A, Ramirez-Franco J, Martin-Fernandez M, Navarrete M, Yang A, Boyden ES, and Perea G (2019). Melanopsin for precise optogenetic activation of astrocyte-neuron networks. Glia 67, 915–934. [DOI] [PubMed] [Google Scholar]
- Melkman T, and Sengupta P (2005). Regulation of chemosensory and GABAergic motor neuron development by the C. elegans Aristaless/Arx homolog alr-1. Development 132, 1935–1949. [DOI] [PubMed] [Google Scholar]
- Messing A, and Brenner M (2020). GFAP at 50. ASN Neuro Jan-Dec;12:1759091420949680. doi: 10.1177/1759091420949680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizeracka K, and Heiman MG (2015). The many glia of a tiny nematode: studying glial diversity using Caenorhabditis elegans. Wiley Interdiscip Rev Dev Biol 4, 151–160. [DOI] [PubMed] [Google Scholar]
- Moriya T, Yoshinobu Y, Kouzu Y, Katoh A, Gomi H, Ikeda M, Yoshioka T, Itohara S, and Shibata S (2000). Involvement of glial fibrillary acidic protein (GFAP) expressed in astroglial cells in circadian rhythm under constant lighting conditions in mice. J Neurosci Res 60, 212–218. [DOI] [PubMed] [Google Scholar]
- Mu Y, Bennett DV, Rubinov M, Narayan S, Yang CT, Tanimoto M, Mensh BD, Looger LL, and Ahrens MB (2019). Glia Accumulate Evidence that Actions Are Futile and Suppress Unsuccessful Behavior. Cell 178, 27–43.e19. [DOI] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, Fanselow MS, and Khakh BS (2019). Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 177, 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam MH, Han KS, Lee J, Won W, Koh W, Bae JY, Woo J, Kim J, Kwong E, Choi TY, et al. (2019). Activation of Astrocytic μ-Opioid Receptor Causes Conditioned Place Preference. Cell Rep 28, 1154–1166.e1155.doi: 1110.1016/j.celrep.2019.1106.1071.. [DOI] [PubMed] [Google Scholar]
- Ng FS, Sengupta S, Huang Y, Yu AM, You S, Roberts MA, Iyer LK, Yang Y, and Jackson FR (2016). TRAP-seq profiling and RNAi-based genetic screens identify conserved glial genes required for adult drosophila behavior. Front Mol Neurosci 9, 146 110.3389/fnmol.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols ALA, Eichler T, Latham R, and Zimmer M (2017). A global brain state underlies C. elegans sleep behavior. Science 356, 1247–1256. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, and Müller HW (2010). Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Reviews in the neurosciences 21, 119–139. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, and Helmchen F (2005). Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, and Kriegstein AR (2008). Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol 508, 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. (2009). Uniquely hominid features of adult human astrocytes. J Neurosci 29, 3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octeau JC, Chai H, Jiang R, Bonanno SL, Martin KC, and Khakh BS (2018). An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron 98, 49–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Octeau JC, Gangwani MR, Allam SL, Tran D, Huang S, HoangTrong TM, Golshani P, Rumbell T, Kozloski JR, and Khakh BS (2019). Transient, consequential extarcellular potassium elevations accompany Channelrhodopsin excitation. Cell Reports May 21;27(8):2249–2261.e7. doi: 10.1016/j.celrep.2019.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou G, Perens EA, Lu Y, and Shaham S (2012). Some, but not all, retromer components promote morphogenesis of C. elegans sensory compartments. Dev Biol 362, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou G, Perens EA, Lu Y, Watanabe S, Jorgensen EM, and Shaham S (2011). Opposing activities of LIT-1/NLK and DAF-6/patched-related direct sensory compartment morphogenesis in C. elegans. PLoS Biol 9, e1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi Y, Xu Q, Wang L, Zhang BJ, Takahashi K, Takata Y, Luo YJ, Cherasse Y, Schiffmann SN, de Kerchove d’Exaerde A, et al. (2017). Slow-wave sleep is controlled by a subset of nucleus accumbens core neurons in mice. Nat Commun 8, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J, Yu GQ, Adame A, et al. (2015). Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashri R, Suresh A, Boska MD, and Dunaevsky A (2015). Motor-skill learning is dependent on astrocytic activity. Neural Plast 2015, 938023. doi: 938010.931155/932015/938023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos MC, and Verkman AS (2012). Aquaporin 4 and neuromyelitis optica. Lancet Neurol 11, 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Henneberger C, Rusakov DA, and Oliet SHR (2017). Astroglial versus Neuronal D-Serine: Fact Checking. Trends Neurosci 40, 517–520. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladépêche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, and Oliet SH (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, and Haydon PG (2005). Astrocytic purinergic signaling coordinates synaptic networks. Science 310, 113–116. [DOI] [PubMed] [Google Scholar]
- Perens EA, and Shaham S (2005). C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell 8, 893–906. [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, and Culotti JG (1986). Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117, 456–487. [DOI] [PubMed] [Google Scholar]
- Petit JM, Gyger J, Burlet-Godinot S, Fiumelli H, Martin JL, and Magistretti PJ (2013). Genes involved in the astrocyte-neuron lactate shuttle (ANLS) are specifically regulated in cortical astrocytes following sleep deprivation in mice. Sleep 36, 1445–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Boyt KM, and McCarthy KD (2014). Astrocyte IP3R2-dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci November 12;8:384. doi: 10.3389/fnbeh.2014.00384 eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Duarte A, Roberts AJ, Ouyang K, and Sejnowski TJ (2019). Impairments in remote memory caused by the lack of Type 2 IP(3) receptors. Glia 67, 1976–1989. [DOI] [PubMed] [Google Scholar]
- Porter JT, and McCarthy KD (1997). Astrocytic neurotransmitter receptors in situ and in vivo. Prog Neurobiol 51, 439–455. [DOI] [PubMed] [Google Scholar]
- Procko C, Lu Y, and Shaham S (2011). Glia delimit shape changes of sensory neuron receptive endings in C. elegans. Development 138, 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko C, Lu Y, and Shaham S (2012). Sensory organ remodeling in Caenorhabditis elegans requires the zinc-finger protein ZTF-16. Genetics 190, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B, Wagner DE, McKenna A, Pandey S, Klein AM, Shendure J, Gagnon JA, and Schier AF (2018). Simultaneous single-cell profiling of lin-eages and cell types in the vertebrate brain. Nat. Biotechnol. 36, 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapti G, Li C, Shan A, Lu Y, and Shaham S (2017). Glia initiate brain assembly through noncanonical Chimaerin-Furin axon guidance in C. elegans. Nat Neurosci 20, 1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, et al. (2010). KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A 107, 14490–14495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival T, Soustelle L, Strambi C, Besson MT, Iché M, and Birman S (2004). Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol 14, 599–605. [DOI] [PubMed] [Google Scholar]
- Robin LM, Oliveira da Cruz JF, Langlais VC, Martin-Fernandez M, Metna-Laurent M, Busquets-Garcia A, Bellocchio L, Soria-Gomez E, Papouin T, Varilh M, et al. (2018). Astroglial CB1 Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron 98, 935–944. [DOI] [PubMed] [Google Scholar]
- Rodenkirch C, Liu Y, Schriver BJ, and Wang Q (2019). Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat Neurosci 22, 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for neuroscientists. Neuron 89, 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, and Papadopoulos MC (2010). Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 133, 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada N, Lee S, Katsu T, Otsuki T, and Inoue T (2015). Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science 347, 1362–1367. [DOI] [PubMed] [Google Scholar]
- Sardinha VM, Guerra-Gomes S, Caetano I, Tavares G, Martins M, Reis JS, Correia JS, Teixeira-Castro A, Pinto L, Sousa N, et al. (2017). Astrocytic signaling supports hippocampal-prefrontal theta synchronization and cognitive function. Glia 65, 1944–1960. [DOI] [PubMed] [Google Scholar]
- Savtchenko LP, Bard L, Jensen TP, Reynolds JP, Kraev I, Medvedev N, Stewart MG, Henneberger C, and Rusakov DA (2018). Disentangling astroglial physiology with a realistic cell model in silico. Nat Commun 9, 3554 3510.1038/s41467–41018-05896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, and Rakic P (1979). A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anatomy and embryology 156, 115–152. [DOI] [PubMed] [Google Scholar]
- Schmitt LI, Sims RE, Dale N, and Haydon PG (2012). Wakefulness affects synaptic and network activity by increasing extracellular astrocyte-derived adenosine. J Neurosci 32, 4417–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A (2017). Physiology Flies with Time. Cell 171, 1232–1235. [DOI] [PubMed] [Google Scholar]
- Shaham S (2005). Glia-neuron interactions in nervous system function and development. Current topics in developmental biology 69, 39–66. [DOI] [PubMed] [Google Scholar]
- Shaham S (2010). Chemosensory organs as models of neuronal synapses. Nat Rev Neurosci 11, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Watanabe S, Christensen R, Jorgensen EM, and Colon-Ramos DA (2013). Synapse location during growth depends on glia location. Cell 154, 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, and Grillner S (2010). Handbook of brain microcircuits. Oxford University Press, p xvii. [Google Scholar]
- Shigetomi E, Patel S, and Khakh BS (2016). Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol 26, 300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicca F, Ambrosini E, Marchese M, Sforna L, Servettini I, Valvo G, Brignone MS, Lanciotti A, Moro F, Grottesi A, et al. (2016). Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci Rep 6, 34325 34310.31038/srep34325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicca F, Imbrici P, D’Adamo MC, Moro F, Bonatti F, Brovedani P, Grottesi A, Guerrini R, Masi G, Santorelli FM, et al. (2011). Autism with seizures and intellectual disability: possible causative role of gain-of-function of the inwardly-rectifying K+ channel Kir4.1. Neurobiol Dis 43, 239–247. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, and Crawley JN (2010). Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 35, 976–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal A, and Shaham S (2017). Infrared laser-induced gene expression for tracking development and function of single C. elegans embryonic neurons. Nat Commun 8, 14100 14110.11038/ncomms14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi A, Liu B, Friedman CJ, Fong J, Lu Y, Huang XY, and Shaham S (2016). A glial K/Cl transporter controls neuronal receptive ending shape by chloride inhibition of an rgc. Cell 165, 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi A, and Shaham S (2019). Glia-neuron interactions in Caenorhabditis elegans. Annu Rev Neurosci 42, 149–168. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P, and Khakh BS (2015). Ca(2+) signaling in astrocytes from Ip3r2(−/−) mice in brain slices and during startle responses in vivo. Nat Neurosci 18, 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Sheehan A, Tasdemir-Yilmaz OE, and Freeman MR (2014). Neuron-glia interactions through the Heartless FGF receptor signaling pathway mediate morphogenesis of Drosophila astrocytes. Neuron 83, 388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, and Horvitz HR (1977). Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56, 110–156. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, and Thomson JN (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100, 64–119. [DOI] [PubMed] [Google Scholar]
- Sun D, and Jakobs TC (2012). Structural remodeling of astrocytes in the injured CNS. Neuroscientist 18, 567–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Shih PY, Gomi H, Yoshida T, Nakai J, Ando R, Furuichi T, Mikoshiba K, Semyanov A, and Itohara S (2013). Astrocytic Ca2+ signals are required for the functional integrity of tripartite synapses. Mol Brain January 28;6:6. doi: 10.1186/1756-6606-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi K, Isonishi A, Yamasaki M, Kawabe Y, Morita-Takemura S, Nakahara K, Terada Y, Shinjo T, Okuda H, Tanaka T, et al. (2018). Olig2-lineage astrocytes: a distinct subtype of astrocytes that differs from GFAP astrocytes. Front Neuroanat 12, 8 10.3389/fnana.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, et al. (2014). Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci 17, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso CF, Simon T, Greenlaw AC, Puri T, Mieda M, and Herzog ED (2017). Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Curr Biol 27, 1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Sieber M, Morphew M, and Han M (2005). The Caenorhabditis elegans aristaless orthologue, alr-1, is required for maintaining the functional and structural integrity of the amphid sensory organs. Molecular biology of the cell 16, 4695–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K, Tepe B, Pekarek B, Arenkiel BR, and Deneen B (2020). Parallel astrocyte calcium signaling modulates olfactory bulb responses. J Neurosci Res 98, 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap MS, Pronk JC, and Scheper GC (2006). Vanishing white matter disease. Lancet Neurol 5, 413–423. [DOI] [PubMed] [Google Scholar]
- Varadarajan SG, Kong JH, Phan KD, Kao TJ, Panaitof SC, Cardin J, Eltzschig H, Kania A, Novitch BG, and Butler SJ (2017). Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 94, 790–799.e793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth WG, Bhatt H, and Hedgecock EM (1996). Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16, 35–46. [DOI] [PubMed] [Google Scholar]
- Wallace SW, Singhvi A, Liang Y, Lu Y, and Shaham S (2016). PROS-1/Prospero Is a Major Regulator of the Glia-Specific Secretome Controlling Sensory-Neuron Shape and Function in C. elegans. Cell Rep 15, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li K-L, Shukla A, Beroun A, Ishikawa M, Huang X, Wang Y, Wang YQ, Yue Y, Bastola ND, et al. (2020). Cocaine Triggers Astrocyte-Mediated Synaptogenesis. Biological Psychiatry 10.1016/j.biopsych.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Perens EA, Oikonomou G, Wallace SW, Lu Y, and Shaham S (2017). IGDB-2, an Ig/FNIII protein, binds the ion channel LGC-34 and controls sensory compartment morphogenesis in C. elegans. Dev Biol 430, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Apicella A Jr., Lee SK, Ezcurra M, Slone RD, Goldmit M, Schafer WR, Shaham S, Driscoll M, and Bianchi L (2008). A glial DEG/ENaC channel functions with neuronal channel DEG-1 to mediate specific sensory functions in C. elegans. The EMBO journal 27, 2388–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Thomson N, White JG, and Brenner S (1975). Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J Comp Neurol 160, 313–337. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, and Brenner S (1986). The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314, 1–340. [DOI] [PubMed] [Google Scholar]
- Wu HP, Ioffe JC, Iverson MM, Boon JM, and Dyck RH (2013). Novel, whisker-dependent texture discrimination task for mice. Behavioural brain research 237, 238–242. [DOI] [PubMed] [Google Scholar]
- Xie AX, Petravicz J, and McCarthy KD (2015). Molecular approaches for manipulating astrocytic signaling in vivo. Front Cell Neurosci 9, 144 110.3389/fncel.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qi Y, and Yang Y (2015). Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep 11, 798–807. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Murray JI, Lu Y, Waterston RH, and Shaham S (2008). mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development 135, 2263–2275. [DOI] [PubMed] [Google Scholar]
- Yu X, Li W, Ma Y, Tossell K, Harris JJ, Harding EC, Ba W, Miracca G, Wang D, Li L, et al. (2019). GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nat Neurosci 22, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Nagai J, and Khakh BS (2020a). Improved tools to study astrocytes. Nat Rev Neurosci 21, 121–138. [DOI] [PubMed] [Google Scholar]
- Yu X, Nagai J, Marti-Solano M, Soto JS, Coppola G, Babu MM, and Khakh BS (2020b). Context-specific striatal astrocyte molecular responses are phenotypically exploitable. Neuron October 9;S0896–6273(20)30745–5. doi: 10.1016/j.neuron.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, and Khakh BS (2018). Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99, 1170–1187 e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Noma K, and Yan D (2020). Regulation of Gliogenesis by lin-32/Atoh1 in Caenorhabditis elegans. G3 (Bethesda). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K, Bard L, Reynolds JP, King C, Jensen TP, Gourine AV, and Rusakov DA (2015). Time-Resolved Imaging Reveals Heterogeneous Landscapes of Nanomolar Ca(2+) in Neurons and Astroglia. Neuron 88, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]