Abstract
Background
Women with polycystic ovary syndrome (PCOS) are at a higher risk of cardiometabolic and psychiatric comorbidities and preconception and antepartum complications, but the impact of PCOS during the postpartum period is unknown.
Objective
To investigate the risk of postpartum cardiovascular disease (CVD) complications and perinatal and postpartum depression.
Study Design
This was a retrospective cohort study conducted using a US insurance claims database. Women with and without PCOS aged 18–50 years enrolled continuously in a single health plan during the preconception, antepartum and postpartum period between 2000–2016 were included. The primary outcome was postpartum CVD and depression (perinatal and postpartum). Multivariable logistic regression was used to adjust for covariates including age, geographic location, preterm delivery, ART use, multiple births, pre-pregnancy depression, pre-pregnancy diabetes, pre-pregnancy hypertension, gestational diabetes, gestational hypertension, obesity, history of hyperlipidemia, smoking and race.
Results
We identified 42,391 unique women with PCOS and 795,480 women without PCOS. In multivariable models, women with PCOS had significantly higher odds of CVD complications including postpartum preeclampsia (aOR: 1.30, 95% CI: 1.17–1.45), eclampsia (aOR: 1.45, 95%CI: 1.14–1.86) cardiomyopathy (aOR: 1.26, 95% CI: 1.03–1.54), hypertensive heart disease (aOR: 1.32, 95% CI: 1.07–1.64), thrombotic disease (aOR: 1.50, 95%CI: 1.20–1.87), congestive heart failure (aOR: 1.35, 95%CI: 1.13–1.61) and cerebrovascular accidents (aOR: 1.21, 95%CI: 1.14–1.29) compared to those without PCOS as well as both perinatal (aOR: 1.27; 95% CI: 1.22–1.33) and postpartum depression (aOR 1.46; 95% CI: 1.36–1.57). Non-obese women with PCOS had higher odds of postpartum eclampsia (aOR 1.72, 95%CI: 1.31–2.26), peripartum cardiomyopathy (aOR 1.43, 95%CI: 1.14–1.79) and cerebrovascular accidents (aOR 1.28, 95%CI: 1.19–1.38) compared to non-obese women without PCOS. In women without pre-pregnancy depression, the odds of perinatal depression (aOR 1.32, 95% CI: 1.26–1.39) and postpartum depression (aOR 1.50, 95% CI: 1.39–1.62) was higher in women with PCOS compared to those without PCOS.
Conclusions
In a large US cohort, our study found that women with PCOS are at increased risk of both cardiovascular and psychiatric complications during the postpartum period. PCOS should be recognized as an at-risk condition; our findings underscore the need for routine screening and early interventions for these major comorbidities.
Keywords: Cardiomyopathy, Cardiovascular, Claims database, Depression, Fourth trimester, Hypertension, Optum, PCOS, Postpartum, Preeclampsia
Condensation
Women with polycystic ovary syndrome are at higher risk of cardiovascular and psychiatric postpartum complications, highlighting the need to increase focus on the fourth trimester.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting reproductive age women.1 It is characterized by the presence of 2 of 3 diagnostic criteria: irregular menses, clinical or biochemical hyperandrogenism and polycystic appearing ovaries.2, 3 The worldwide prevalence of PCOS is 5–7%, but can be as high as 8–13% depending on the definition used.4, 5 The association between PCOS and several cardiovascular disease (CVD) risk factors such as type 2 diabetes6, obesity and lipid abnormalities7 is well recognized.8 Additionally, there is growing evidence for increased risk of psychiatric disorders and pregnancy-related complications in PCOS.9, 10 Meta-analyses show abnormal depression scores (OR 3.78, 95% CI: 3.03–4.72) and anxiety symptoms (OR: 5.62, 95% CI: 3.22–9.80)10, while large registries show increased prevalence of psychiatric morbidities.11, 12 Obstetric complications including gestational diabetes (OR 3.43; 95% CI: 2.49–4.74), pregnancy-induced hypertension (PIH) (OR 3.07, 95% CI: 1.81–5.18) and preeclampsia (OR 3.28, 95% CI: 2.06–5.22) are also increased in women with PCOS,13 further contributing to long term cardiometabolic risk in this population.
Despite extensive research in the non-pregnancy related comorbidities as well as obstetric complications,7, 14, 15 there are no studies in women with PCOS specifically targeting the postpartum period, typically defined as 4–6 weeks after delivery. According to the Centers for Disease Control and Prevention (CDC) report, nearly 70 percent of pregnancy-related deaths in the general population occur at delivery or within the first year postpartum, with CVD and stroke accounting for over a third of the causes.16, 17
Women presenting with postpartum preeclampsia are at increased risk of postpartum eclampsia and stroke and fifty percent of eclamptic episodes occur after delivery.18 Preeclampsia and PIH are also risk factors for peripartum cardiomyopathy19, which accounts for 23% of maternal cardiovascular related deaths.20 Given that CVD is the number one killer of women, the American Heart Association (AHA) has partnered with the American College of Obstetrics and Gynecology (ACOG) to identify female-specific CVD risk factors, recently adding hypertensive disease of pregnancy and PCOS.21, 22 Given the high prevalence of cardiometabolic risk factors in young women with PCOS during pregnancy6, 14, it is imperative that we understand if similar risks exist during the postpartum period.
Further, perinatal and postpartum depression are common conditions associated with significant adverse infant and maternal outcomes.23 The prevalence of postpartum depressive symptoms across 27 states in the US was reported to be as high as 11.5%23. A history of depression prior to pregnancy is a recognized risk factor for perinatal depression, the most common medical problem during pregnancy occurring in one in seven patients.23, 24 Although suicide is excluded from several definitions assessing maternal mortality rates, there is increasing evidence that maternal suicide is rising, highlighting the importance of identifying risk factors.25, 26 Despite the importance of mental health, no studies have evaluated the risk for perinatal and postpartum depression in women with PCOS.
Most of these associations between PCOS and its co-morbidities in the non-pregnant state and during pregnancy are based on small cross-sectional studies, and large-scale studies in the US are lacking. In addition, there is no data on postpartum cardiovascular or psychiatric complications in this population. The objectives of this study were therefore to evaluate for significant differences in: 1. the prevalence of postpartum cardiovascular complications (preeclampsia, eclampsia, peripartum cardiomyopathy, hypertensive heart disease, thrombotic disease, congestive heart failure (CHF), cerebrovascular accidents and ischemic heart disease) and 2. the prevalence of perinatal and postpartum depression in women with PCOS compared to women without PCOS in a large US nation-wide cohort.
Materials and Methods
Cohort construction
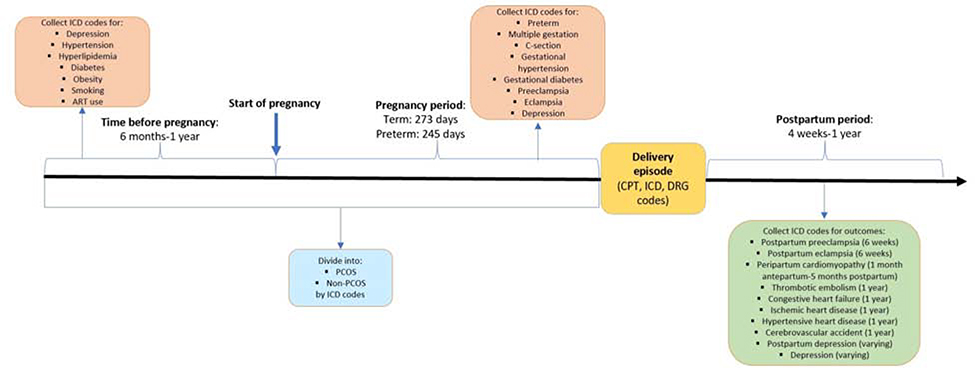
We conducted a retrospective cohort study using Optum’s de-identified Clinformatics® Data Mart database containing approximately 13 million unique individuals per year from 2000–2016 from all 50 US states. The dataset included outpatient and inpatient services. Subjects were initially identified using International Classification of Diseases (ICD) 9/10 codes, Current Procedural Terminology (CPT) codes and Diagnosis Related Group (DRG) delivery codes. (Supplemental Table 1) Date of delivery was defined as the date of filing the first delivery code. To ensure availability of data on comorbidities, we only included women with continuous enrollment in Optum for a minimum of 6 months prior to conception, throughout pregnancy and a minimum of 6 weeks post-delivery. (See Figure 1A) To keep missing data on co-morbidities to a minimum, we also collected data up to one year prior to and after date of delivery when available. For women who had multiple pregnancies with complete data, the first pregnancy was selected. For women with preterm delivery codes, the period of confinement (i.e. the pregnancy length) was defined as 245 days whereas for women without preterm delivery codes, 273 days was used.27 Women under the age of 18 and over the age of 50 years were excluded. From this eligible cohort, we identified women with PCOS using ICD codes for PCOS (256.4, E282.2). Given that there may be under reporting of PCOS in claims datasets, we also included women who had codes for both hirsutism (704.1, L68.0) and irregular menses (626.1, 626.4, 628.0, N97.0, N91.0-.5, N92.5-.6). Women who were not identified as PCOS were included in the non-PCOS group.
Figure 1A:
Diagram of cohort construction
Covariate identification
Covariates were identified using ICD codes either prior to pregnancy (depression, type 2 diabetes, hypertension, hyperlipidemia, obesity, assisted reproductive technology use (ART)) or during pregnancy (multiple gestation, gestational hypertension and gestational diabetes). Information on age, geographic division, smoking and socioeconomic status including race, education and net worth were also obtained.
Outcome identification and definition
The definitions of the outcomes were based on the current recommendations in the literature. Postpartum preeclampsia/eclampsia was defined based on ICD codes from date of delivery to 6 weeks after delivery.28 (Supplemental Table 2) Peripartum cardiomyopathy was defined as occurring within 1 month prior to delivery and up to 5 months after date of delivery.29 The remaining postpartum cardiovascular complications (hypertensive heart disease, thrombotic disease, CHF, cerebrovascular accidents and ischemic heart disease) were defined as occurring from date of delivery to one year after delivery, adapted from outcome definitions for pregnancy mortality data.30 Perinatal depression was defined as occurring during pregnancy and up to 3 months after delivery.23, 24 Postpartum depression was defined as occurring after date of delivery up to three months postpartum.23, 24
Statistical analysis
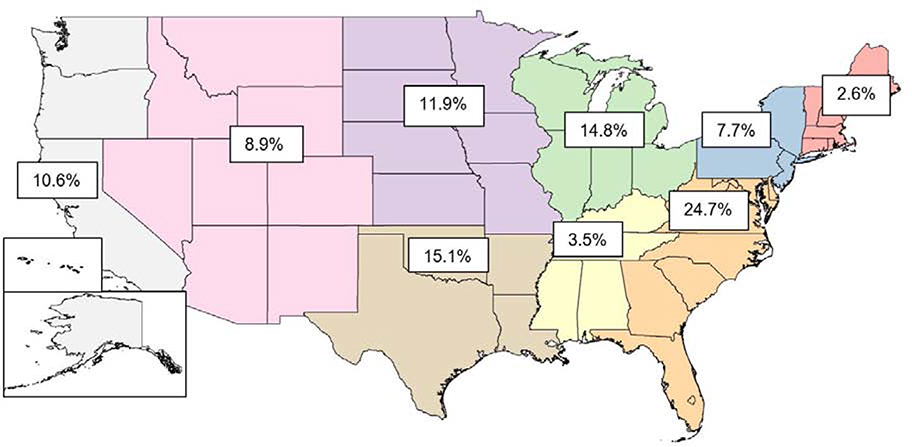
Continuous variables were compared between groups using student t-test and categorical variables were compared using Pearson chi-square tests. Separate multivariable logistic regression models were developed to evaluate the odds of developing each primary and secondary outcome. Racial differences in outcomes between black women and a reference group of white women were also assessed. Variables were identified a priori first and the final set of covariates was then selected using a backwards elimination strategy removing redundant variables. Variables evaluated included patient age, geographic division (See Figure 2), obesity, smoking, race, ART use, history of pre-pregnancy depression, history of pre-pregnancy hypertension, history of pre-pregnancy diabetes, hyperlipidemia, gestational hypertension in current pregnancy, gestational diabetes in current pregnancy, preterm delivery of current pregnancy, multiple gestation and socioeconomic factors including personal net worth and highest education level.
Figure 2:
Geographic distribution of study cohort by regions
We conducted the following sensitivity analyses: 1) including the date of delivery for appropriate outcomes, 2) using the DSM-V definition of perinatal depression up to 4 weeks postpartum and 3) expanding the definition of postpartum depression to up to 1 year after delivery. As we captured outcomes occurring in the postpartum period, it is possible that outcomes occurring after delivery but on the same day were filed along with other ICD codes as a package, making it difficult to distinguish if they were intrapartum or postpartum complications. Therefore, we conducted the first sensitivity analysis including date of delivery in outcome definitions. The last two analyses were conducted in order to account for varying definitions of peripartum depression using DSM-V criteria and those recommended by national organizations.31–33 Tests for interaction were also performed between obesity, pre-pregnancy depression and the outcomes. Interaction was evaluated by adding multiplicative terms to the multivariable logistic regression models. The statistical significance of the interaction term was determined by a wald based test where p <0.05 was considered significant. Joint wald tests were employed for interactions with more than 1 term. If statistically significant, linear combinations were then calculated to determine coefficients and appropriate confidence intervals for those with and without variable of interest. Data were extracted from Optum files using R while statistical analysis was performed using STATA version 14.2 (Stata-Corp). This study was deemed exempt after review by the University of Pennsylvania’s Institutional Review Board.
Results
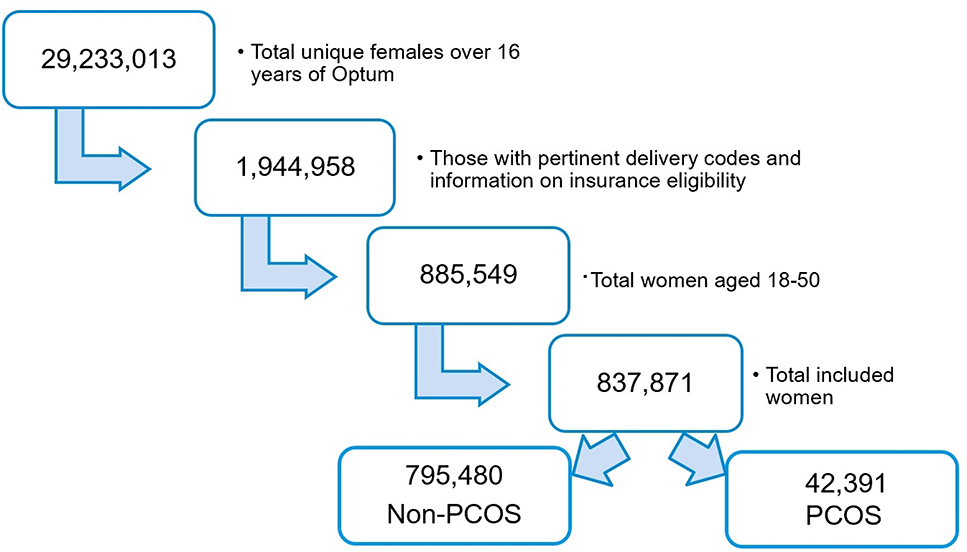
A total of 29,233,013 unique females were present over the 16 years of Optum data collection (See Figure 1B). After excluding ineligible patients there were 42,391 women with PCOS and 795,480 without PCOS who had a delivery and were continuously enrolled in the Optum dataset resulting in a prevalence of 5.06% for PCOS. Majority of these patients (97.0%) met criteria by a PCOS diagnosis code as compared to a combination of codes. Overall, prior to pregnancy women with PCOS were more likely to be obese, have a history of depression, hypertension, diabetes and hyperlipidemia (Table 1). They were more likely to have used ART to achieve pregnancy and had a significantly higher prevalence of gestational hypertension, gestational diabetes, preterm delivery, multiple births, stillbirth and C-section in the current pregnancy (p<0.001).
Figure 1B:
Flow diagram showing selection of the study population
Table 1:
Demographic characteristics of women with and without PCOS
| PCOSa (n=42,391) | Non-PCOS (n=795,480) | |
|---|---|---|
| Age at delivery, years (±SD) | 31.2 ± 4.7 | 30.9 ± 5.5 |
| Race, n (%) | ||
| White | 26, 832 (68.0%) | 499, 534 (69.7%) |
| Black | 3, 403 (8.6%) | 67,756 (9.4%) |
| Asian | 3, 967 (10.1%) | 53, 596 (7.5%) |
| Hispanic | 5, 262 (13.3%) | 96, 549 (13.5%) |
| Geographic division, n (%) | ||
| New England | 852 (2.0%) | 21, 063 (2.7%) |
| East North Central | 5, 865 (13.9%) | 118, 163 (14.9%) |
| West North Central | 4, 536 (10.7%) | 95, 208 (12.0%) |
| East South Central | 1, 434 (3.4%) | 28, 227 (3.6%) |
| West South Central | 7, 417 (17.5%) | 118, 933 (15.0%) |
| Mid-Atlantic | 4, 000 (9.4%) | 60, 381 (7.6%) |
| South Atlantic | 11, 110 (26.2%) | 195, 568 (24.7%) |
| Mountain | 3, 890 (9.2%) | 70, 546 (8.9%) |
| Pacific | 3, 250 (7.7%) | 85, 937 (10.8%) |
| Education level, n (%) | ||
| Less than 12th grade | 206 (0.5%) | 6,635 (0.9%) |
| High school diploma | 9,261 (22.4%) | 175, 150 (23.3%) |
| Less than bachelor degree | 22, 073 (53.4%) | 396, 908 (52.9%) |
| Bachelor degree plus | 9, 809 (23.7%) | 171, 996 (22.9%) |
| Home owner, n (%) | 28, 689 (69.1%) | 519, 758 (68.9%) |
| Net worth of individual, n (%) | ||
| >$500K | 3, 129 (9.0%) | 64, 772 (10.2%) |
| $250–499K | 6, 166 (17.8%) | 119, 548 (18.8%) |
| $150–249K | 5, 241 (15.1%) | 95, 646 (15.1%) |
| $25–149K | 11, 616 (33.5%) | 204, 493 (32.2%) |
| <$25K | 8, 505 (24.5%) | 150, 234 (23.7%) |
| Smokers, n (%) | 1, 342 (3.2%) | 23, 008 (2.9%) |
| Obese, n (%) | 6, 225 (14.7%) | 37, 089 (4.7%) |
| History of hyperlipidemia, n (%) | 4, 787 (11.3%) | 41, 960 (5.3%) |
| History of pre-pregnancy hypertension, n (%) | 2, 628 (6.2%) | 19, 829 (2.5%) |
| History of pre-pregnancy diabetes, n (%) | 2,239 (5.3%) | 9, 196 (1.2%) |
| History of ART use, n (%) | 2, 205 (5.2%) | 7, 865 (1.0%) |
| History of depression pre-pregnancy, n (%) | 1, 824 (4.3%) | 24, 915 (3.1%) |
| Gestational hypertension in current pregnancy, n (%) | 5, 787 (13.7%) | 61, 000 (7.7%) |
| Gestational diabetes in current pregnancy, n (%) | 10,279 (24.3%) | 105, 845 (13.3%) |
| Pre-eclampsia in current pregnancy, n (%) | 2, 128 (5.0%) | 20, 544 (2.6%) |
| Preterm delivery in current pregnancy, n (%) | 7, 158 (16.9%) | 96, 765 (12.2%) |
| Multiple gestation in current pregnancy, n (%) | 2, 796 (6.6%) | 19,668 (2.5%) |
| C-section delivery, n (%) | 19, 116 (45.1%) | 261, 196 (32.8%) |
| Stillbirth of current pregnancy, n (%) | 563 (1.33%) | 5, 507 (0.69%) |
PCOS=Polycystic Ovary Syndrome
Postpartum cardiovascular complications
Women with PCOS had a significantly higher prevalence of postpartum preeclampsia (OR 1.81, 95%CI: 1.63–2.01), eclampsia (OR 2.27, 95%CI: 1.81–2.84), peripartum cardiomyopathy (OR 1.84, 95%CI: 1.52–2.23), hypertensive heart disease (OR 2.09, 95%CI: 1.71–2.55), thrombotic disease (OR 1.85, 95%CI: 1.50–2.28), CHF (OR 2.00, 95%CI: 1.69–2.35) and cerebrovascular accidents (OR 1.56, 95% CI: 1.47–1.65) (Table 2). In adjusted analyses controlling for age, history of hypertension, history of diabetes, ART use, preterm delivery, multiple pregnancy, gestational diabetes, gestational hypertension, hyperlipidemia, obesity, smoking, geographic division and socioeconomic factors including education, net worth and race, women with PCOS had increased odds of postpartum preeclampsia (aOR 1.30, 95% CI: 1.17–1.45), eclampsia (aOR 1.45, 95%CI: 1.14–1.86), peripartum cardiomyopathy (aOR 1.26, 95%CI: 1.03–1.54), hypertensive heart disease (aOR 1.32, 95% CI: 1.07–1.64), thrombotic disease (aOR 1.50, 95%CI: 1.20–1.87), CHF (aOR 1.35, 95%CI: 1.13–1.61) and cerebrovascular accidents (aOR 1.21, 95%CI: 1.14–1.29). (Table 2)
Table 2:
Postpartum complications in women with and without PCOS
| PCOS (n=42,391) | Non-PCOS (n=795,480) | OR | aOR (95% CI) | |
|---|---|---|---|---|
| Cardiovascular outcomesa | ||||
| Postpartum preeclampsia (%) | 390 (0.92) | 4, 060 (0.51) | 1.81 (1.63–2.01) | 1.30 (1.17–1.45) |
| Postpartum eclampsia (%) | 85 (0.2) | 705 (0.09) | 2.27 (1.81–2.84) | 1.45 (1.14–1.86) |
| Peripartum cardiomyopathy (%) | 116 (0.27) | 1, 182 (0.15) | 1.84 (1.52–2.23) | 1.26 (1.03–1.54) |
| Hypertensive heart disease (%) | 109 (0.26) | 979 (0.12) | 2.09 (1.71–2.55) | 1.32 (1.07–1.64) |
| Thrombotic disease (%) | 98 (0.23) | 995 (0.13) | 1.85 (1.50–2.28) | 1.50 (1.20–1.87) |
| Congestive heart failure (%) | 157 (0.37) | 1,479 (0.19) | 2.00 (1.69–2.35) | 1.35 (1.13–1.61) |
| Cerebrovascular accidents (%) | 1, 263 (2.98) | 15, 366 (1.93) | 1.56 (1.47–1.65) | 1.21 (1.14–1.29) |
| Ischemic heart disease (%) | 11 (0.03) | 166 (0.02) | 1.24 (0.68–2.29) | -- |
| Depression outcomesb | ||||
| Perinatal depression (%) | 2, 956 (6.97) | 40, 311 (5.07) | 1.40 (1.35–1.46) | 1.27 (1.22–1.33) |
| Postpartum depression (%) | 899 (2.12) | 10, 815 (1.36) | 1.57 (1.47–1.68) | 1.46 (1.36–1.57) |
Adjusted analyses controlled for age, history of hypertension, history of diabetes, ART use, preterm delivery, multiple pregnancy, gestational diabetes, gestational hypertension, hyperlipidemia, obesity, smoking, geographic division and socioeconomic factors including education, net worth and race
Adjusted analyses controlled for age, history of pre-pregnancy depression, obesity, smoking, geographic division and socioeconomic factors including education, net worth and race
In our study, black women had significantly higher odds of postpartum preeclampsia (aOR: 1.59, 95%CI: 1.45–1.74), peripartum cardiomyopathy (aOR: 1.95, 95%CI: 1.67–2.29), hypertensive heart disease (aOR: 2.00, 95%CI: 1.70–2.37) and CHF (aOR: 2.02, 95%CI: 1.76–2.32) compared to white women, adjusted for PCOS status.
Perinatal & postpartum depression
Women with PCOS had an increased risk of perinatal depression (OR 1.40, 95% CI: 1.35–1.46) and postpartum depression (OR 1.57, 95% CI: 1.47v1.68). After controlling for age, history of pre-pregnancy depression, obesity, smoking, geographic division and socioeconomic factors including education, net worth and race, women with PCOS had higher odds of perinatal depression (aOR 1.27, 95% CI: 1.22–1.33) and postpartum depression (aOR 1.46, 95% CI: 1.36–1.57). (Table 2)
Interestingly, black women had lower odds of perinatal (aOR: 0.78, 95%CI: 0.75–0.81) and postpartum (aOR: 0.91, 95%CI: 0.85–0.97) depression.
Sensitivity analyses
When using the definition of a given outcome first occurring on the date of delivery as compared to the day after delivery, the odds of postpartum preeclampsia (aOR 1.19, 95%CI: 1.14–1.25), eclampsia (aOR 1.35, 95%CI: 1.14–1.60), hypertensive heart disease (aOR 1.35, 95%CI: 1.10–1.66), thrombotic disease (aOR 1.50, 95%CI: 1.23–1.83), CHF (aOR 1.23, 95%CI: 1.04–1.46), cerebrovascular accidents (aOR 1.17, 95%CI: 1.11–1.25) and postpartum depression (aOR 1.27, 95%CI: 1.21–1.34) remained elevated. As the definition of perinatal depression and peripartum cardiomyopathy includes the date of delivery, sensitivity analysis was not performed for these outcomes. The odds of perinatal depression remained higher when using the DSM-V definition (aOR 1.19, 95% CI: 1.14–1.25) and the odds of postpartum depression remained higher (aOR 1.40, 95% CI: 1.33–1.48) when including episodes occurring after date of delivery and up to one year.
Interaction between pre-pregnancy obesity and outcomes
Approximately 70% of women with PCOS in the US are obese6, and obesity is also associated with depression and CVD,14, 34 therefore the interaction between obesity and outcomes was studied. In our dataset, the interaction between PCOS and obesity was statistically significant for the following CVD related outcomes; postpartum eclampsia (p=0.032), peripartum cardiomyopathy (p=0.039) and cerebrovascular accidents (p=0.002). When we included this interaction in the multivariable model non-obese women with PCOS had higher odds of postpartum eclampsia (aOR 1.72, 95%CI: 1.31–2.26), peripartum cardiomyopathy (aOR 1.43, 95%CI: 1.14–1.79) and cerebrovascular accidents (aOR 1.28, 95%CI: 1.19–1.38) compared to non-obese women without PCOS. Obese women with PCOS did not have a significantly higher odds of postpartum eclampsia (aOR 0.93, 95%CI:0.56–1.53), peripartum cardiomyopathy (aOR 0.86, 95%CI:0.56–1.32) or cerebrovascular accidents (aOR 1.02, 95%CI: 0.90–1.16) compared to those without PCOS. (Supplemental Table 3)
Interaction between history of pre-pregnancy depression with outcomes
Given the association between PCOS and depression in the non-pregnant state, we examined the impact of depression prior to the incident pregnancy on the outcomes. The interaction between pre-pregnancy depression and PCOS was significant for perinatal depression (p<0.001) and postpartum depression (p=0.024). Including this interaction in the multivariable model in women without pre-pregnancy depression, the odds of perinatal depression (aOR 1.32, 95% CI: 1.26–1.39) and postpartum depression (aOR 1.50, 95% CI: 1.39–1.62) was higher in women with PCOS compared to those without PCOS. However, in women with history of pre-pregnancy depression, women with PCOS did not have higher odds of either perinatal depression (aOR 1.06, 95%CI: 0.96–1.18) or postpartum depression (aOR 1.13, 95%CI: 0.90–1.43). (Supplemental Table 4)
Discussion
Principal Findings
This is the largest study using a nation-wide cohort to examine the risk of perinatal and postpartum comorbidities in women with PCOS residing in the US. Our study, including over 42,000 women with PCOS, showed a higher prevalence of cardiometabolic and depression complications both before and during pregnancy.
Results
We show that women with PCOS are at increased risk of cardiovascular and psychiatric conditions during the postpartum period including preeclampsia and eclampsia, peripartum cardiomyopathy, hypertensive heart disease, thrombotic disease, CHF, cerebrovascular accidents and perinatal and postpartum depression, compared to women without PCOS. On performing sensitivity analyses, we found that non-obese women with PCOS appeared to drive the increased risk observed for postpartum eclampsia, cardiomyopathy and cerebrovascular accidents. Further, in the subgroup without pre-pregnancy depression, women with PCOS had an increased risk of perinatal and postpartum depression. When evaluating the strength of variables that drive the risk we observed that gestational hypertension strongly affected several cardiovascular complications including preeclampsia, eclampsia, peripartum cardiomyopathy, hypertensive heart disease and CHF; obesity strongly affected odds of eclampsia, peripartum cardiomyopathy, thrombotic disease, CHF, cerebrovascular accidents, perinatal and postpartum depression whereas history of depression impacted perinatal depression. (Data not shown). The postpartum findings are novel, while findings regarding co-morbidities prior to pregnancy and in the antepartum period have previously been described in smaller cohort studies primarily outside the US.11,35
Clinical Implications
Pregnancy is recognized as a ‘stress test,’ and hypertensive diseases in pregnancy are linked to significant long term consequences, such as chronic hypertension, CVD, heart failure and stroke.36–41 In 2018, the AHA teamed with ACOG to release a Presidential Advisory highlighting the importance of multidisciplinary care in identifying, understanding and preventing risk factors for CVD in women.42 In addition to the higher prevalence of hypertensive disease during pregnancy, our study found increased risk of hypertensive disease in the postpartum period. Although the prevalence of postpartum preeclampsia and eclampsia was lower than in the antepartum period, the diagnosis can be missed due to inconsistent follow up during the postpartum period.43 ACOG has redefined the postpartum period as the “fourth trimester” of pregnancy43 and new guidelines recommend early contact with the patient in the first 3 weeks after delivery and then an individualized comprehensive visit no later than 12 weeks postpartum.
Untreated perinatal and postpartum depression have been linked to increased risk of maternal psychiatric disease and suicide, low breastfeeding rates and lower IQ in offspring.44–47 The United States Preventative Task Force recommends screening for depression in pregnant and postpartum women and then referring those at increased risk for first line therapy.48 In March 2019, the FDA approved the first medication specifically for use in postpartum depression, Brexanolone, adding to non-pharmacological options such as cognitive behavioral therapy.49, 50
Racial disparities in perinatal complications have been described in the literature. Lower risk of depression in black women with PCOS may be related to decreased reporting of symptoms due to stigma, reporting somatic symptoms more than mood symptoms and protective factors such as strong family ties and religion.51, 52 These findings need to be confirmed in prospectively collected datasets.
Research Implications
Current guidelines for preeclampsia and perinatal depression risk assessment do not include women with PCOS as an at-risk population.31, 53 Given the heterogeneity of PCOS, future studies should estimate the prevalence of perinatal and postpartum depression in different PCOS phenotypes. In accordance with ACOG recommendations for the general population, all women with PCOS should be screened for depression both in the prenatal and postpartum period. The findings of our study underscore the need to evaluate the impact of early interventions on risk of long term CVD complications and depression specifically in women with PCOS.48, 54
Strengths
Although our study has inherent limitations associated with a health insurance database, it has several strengths. First, we found similar increases in PCOS related pre-pregnancy comorbidities as described in other population-based datasets.11,35, 55 Second, this study includes the largest cohort of women with PCOS residing in the US. Our large sample size compared to other studies11, 56 allowed us to capture a racially and geographically diverse population and include significant postpartum complications that typically have a relatively low prevalence. Moreover, we were able to conduct sensitivity analyses varying outcome definitions adding to the robustness of our findings.
Limitations
Claims databases lack temporality to evaluate if certain variables are confounders versus mediators in a causal pathway. In addition, causality cannot be concluded, only associations. We recognize that both obesity and depression are under coded in claims datasets,23 and women with billable codes likely have more severe symptoms. These limitations precluded reliable assessment of women with morbid obesity, although this subset of women would likely have greater risk of complications. The low prevalence of these conditions could have contributed to some of the findings in the interaction analyses, although our results indicate that non-obese women with PCOS are also at an increased risk for some CVD related outcomes. Nevertheless, the prevalence of chronic hypertension, gestational hypertension, preeclampsia, preterm delivery and C-section delivery mirror rates that have been reported in the general US population,40, 57–60 adding to the generalizability of our findings. We chose to restrict our analyses to the first pregnancy included in the database, as the risk of several of our outcomes is greater in subsequent pregnancies. As we did not have data on gravidity and parity, we were unable to account for pregnancies that occurred prior to the start of data collection in 2000 or pregnancies in women whose insurance may have changed during this timeframe.
It has been reported that as many as 40% of women in the US do not attend a postpartum visit43, thereby decreasing the availability of postpartum data in claims databases61. As we only included women with continuous eligibility during the study period, this may result in a selection bias towards women who seek postpartum care. The 2 cohorts were unlikely to be selected differentially for inclusion in the Optum database, therefore minimizing the impact of selection bias. Misclassification bias could be present if women are incorrectly coded as having PCOS. However, regardless of the direction of misclassification, this would bias results towards the null and provide a more conservative estimate of increased risk. Despite these limitations, this is the largest cohort of women with PCOS in the US, allowing sufficient power to study postpartum outcomes.
Conclusion
To our knowledge, this study is the largest and first to describe an increased risk of both cardiovascular and psychiatric postpartum complications in women with PCOS.
Although the prevalence of individual postpartum cardiovascular outcomes is low, the collective burden and potential impact on long-term CVD risk supports need for closer surveillance of this population. Our study results also indicate that women with PCOS have a higher risk of perinatal and postpartum depression, and should be considered as a high risk group that would benefit from closer monitoring, early counselling and interventions to ameliorate long-term health risks.54
Supplementary Material
AJOG at a Glance.
A. Why was the study conducted?
There is a dearth of data on postpartum complications, including perinatal depression, in women with PCOS.
B. What are the key findings?
In a nationwide retrospective claims study, women with PCOS were at increased risk for postpartum preeclampsia, eclampsia, cardiomyopathy, hypertensive heart disease, thrombotic disease, congestive heart failure, cerebrovascular accidents and perinatal and postpartum depression compared to those without PCOS. Non-obese women with PCOS were at increased risk of postpartum eclampsia, cardiomyopathy and cerebrovascular accidents compared to non-obese women without PCOS.
C. What does this study add to what is already known?
Women with PCOS are at a higher risk of postpartum complications in addition to antepartum complications, highlighting the need to include these outcomes in pregnancy counseling and risk assessment.
Acknowledgements
The authors would like to thank Optum for access to the data. This source did not play any role in design and conduct of the study; collection, management, analysis, or interpretation of the data or in funding of the study.
Source of Funding: Snigdha Alur-Gupta is supported by the NIH T32 Training Grant: HD007440. This source did not play any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Conflicts of Interest: The authors report no conflict of interest.
Paper presentation information: Prize Paper Award winner at the 75th Annual Scientific Congress and Expo, The American Society for Reproductive Medicine, Philadelphia, Pennsylvania, October 12-16, 2019.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.De Leo V, Musacchio MC, Cappelli V, Massaro MG, Morgante G, Petraglia F. Genetic, hormonal and metabolic aspects of PCOS: an update. Reprod Biol Endocrinol 2016;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004;81:19–25. [DOI] [PubMed] [Google Scholar]
- 3.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and metaanalysis. Hum Reprod 2016;31:2841–55. [DOI] [PubMed] [Google Scholar]
- 5.Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nature reviews Disease primers 2016;2:16057. [DOI] [PubMed] [Google Scholar]
- 6.DUMESIC DA, OBERFIELD SE, STENER-VICTORIN E, MARSHALL JC, LAVEN JS, LEGRO RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev 2015;36:487–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roe A, Hillman J, Butts S, et al. Decreased cholesterol efflux capacity and atherogenic lipid profile in young women with PCOS. J Clin Endocrinol Metab 2014;99:E841–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update 2015;21:575–92. [DOI] [PubMed] [Google Scholar]
- 10.Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 2017;32:1075–91. [DOI] [PubMed] [Google Scholar]
- 11.Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab 2015;100:911–9. [DOI] [PubMed] [Google Scholar]
- 12.Cesta CE, Mansson M, Palm C, Lichtenstein P, Iliadou AN, Landen M. Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology 2016;73:196–203. [DOI] [PubMed] [Google Scholar]
- 13.Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol 2013;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dokras A Cardiovascular disease risk in women with PCOS. Steroids 2013;78:773–6. [DOI] [PubMed] [Google Scholar]
- 15.Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril 2011;95:1073– 9.e1–11. [DOI] [PubMed] [Google Scholar]
- 16.CDC. Pregnancy-related Deaths. In: Services UDoHH, ed. Vital Signs. cdc.gov/vitalsigns/maternal-deaths, 2019. [Google Scholar]
- 17.Petersen EE, Davis NL, Goodman D, et al. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep 2019;68:423–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilchez G, Hoyos LR, Leon-Peters J, Lagos M, Argoti P. Differences in clinical presentation and pregnancy outcomes in antepartum preeclampsia and new-onset postpartum preeclampsia: Are these the same disorder? Obstetrics & gynecology science 2016;59:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ARANY Z, EL KAYAM U. Peripartum Cardiomyopathy. Circulation 2016;133:1397–409. [DOI] [PubMed] [Google Scholar]
- 20.Main EK, McCain CL, Morton CH, Holtby S, Lawton ES. Pregnancy-related mortality in California: causes, characteristics, and improvement opportunities. Obstet Gynecol 2015;125:938–47. [DOI] [PubMed] [Google Scholar]
- 21.LU MC. Reducing Maternal Mortality in the United States. JAMA 2018;320:1237–38. [DOI] [PubMed] [Google Scholar]
- 22.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−−2011 update: a guideline from the american heart association. Circulation 2011;123:1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ko JY, Rockhill KM, Tong VT, Morrow B, Farr SL. Trends in Postpartum Depressive Symptoms - 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep 2017;66:153–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katon W, Russo J, Gavin A. Predictors of postpartum depression. Journal of women’s health (2002) 2014;23:753–9. [DOI] [PubMed] [Google Scholar]
- 25.Palladino CL, Singh V, Campbell J, Flynn H, Gold KJ. Homicide and suicide during the perinatal period: findings from the National Violent Death Reporting System. Obstet Gynecol 2011;118:1056–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangla K, Hoffman MC, T rumpff C, O’Grady S, Monk C. Maternal self-harm deaths: an unrecognized and preventable outcome. Am J Obstet Gynecol 2019. [DOI] [PubMed] [Google Scholar]
- 27.MARGULIS AV, SETOGUCHI S, MITTLEMAN MA, GLYNN RJ, DORMUTH CR, HERNANDEZ-DIAZ S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf 2013;22:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AL-SAFI Z, IMUDIA AN, FILETTI LC, HOBSON DT, BAHADO-SINGH RO, AWONUGA AO. Delayed postpartum preeclampsia and eclampsia: demographics, clinical course, and complications. Obstet Gynecol 2011;118:1102–7. [DOI] [PubMed] [Google Scholar]
- 29.Johnson-Coyle L, Jensen L, Sobey A. Peripartum cardiomyopathy: review and practice guidelines. Am J Crit Care 2012;21:89–98. [DOI] [PubMed] [Google Scholar]
- 30.Hirshberg A, Srinivas SK. Epidemiology of maternal morbidity and mortality. Semin Perinatol 2017;41:332–37. [DOI] [PubMed] [Google Scholar]
- 31.The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol 2015;125:1268–71. [DOI] [PubMed] [Google Scholar]
- 32.Heterogeneity of postpartum depression: a latent class analysis. The lancet Psychiatry 2015;2:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Association AP. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Publishing; Number of pages. [Google Scholar]
- 34.Jantaratnotai N, Mosikanon K, Lee Y, McIntyre RS. The interface of depression and obesity. Obes Res Clin Pract 2017;11:1–10. [DOI] [PubMed] [Google Scholar]
- 35.ROOS N, KlELER H, SAHLIN L, EKMAN-ORDEBERG G, FALCONER H, STEPHANSSON O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ 2011;343:d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.CAIN MA, Salemi JL, TANNER JP, KlRBY RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. Am J Obstet Gynecol 2016;215:484.e1–84.e14. [DOI] [PubMed] [Google Scholar]
- 37.Hauspurg A, Ying W, Hubel CA, Michos ED, Ouyang P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin Cardiol 2018;41:239–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neiger R Long-Term Effects of Pregnancy Complications on Maternal Health: A Review. J Clin Med 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leon LJ, McCarthy FP, Direk K, et al. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation 2019;140:1050–60. [DOI] [PubMed] [Google Scholar]
- 40.Ying W, Catov JM, Ouyang P. Hypertensive Disorders of Pregnancy and Future Maternal Cardiovascular Risk. Journal of the American Heart Association 2018;7:e009382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith GN, Louis JM, Saade GR. Pregnancy and the Postpartum Period as an Opportunity for Cardiovascular Risk Identification and Management. Obstet Gynecol 2019;134:851–62. [DOI] [PubMed] [Google Scholar]
- 42.Brown HL, Warner JJ, Gianos E, et al. Promoting Risk Identification and Reduction of Cardiovascular Disease in Women Through Collaboration With Obstetricians and Gynecologists: A Presidential Advisory From the American Heart Association and the American College of Obstetricians and Gynecologists. Circulation 2018;137:e843–e52. [DOI] [PubMed] [Google Scholar]
- 43.ACOG Committee Opinion No. 736: Optimizing Postpartum Care. Obstet Gynecol 2018;131 :e140–e50. [DOI] [PubMed] [Google Scholar]
- 44.ILIADIS SI, SKALKIDOU A, RANSTRAND H, GEORGAKIS MK, AXFORS C, PAPADOPOULOS FC. Self-Harm Thoughts Postpartum as a Marker for Long-Term Morbidity. Frontiers in public health 2018;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Machiyama K, Hirose A, Cresswell JA, et al. Consequences of maternal morbidity on health-related functioning: a systematic scoping review. BMJ open 2017;7:e013903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meltzer-Brody S, Stuebe A. The long-term psychiatric and medical prognosis of perinatal mental illness. Best Pract Res Clin Obstet Gynaecol 2014;28:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sui G, Pan B, Liu G, Liu G, Wang L. The Long-Term Effects of Maternal Postnatal Depression on a Child’s Intelligence Quotient: A Meta-Analysis of Prospective Cohort Studies Based on 974 Cases. J Clin Psychiatry 2016;77:e1474–e82. [DOI] [PubMed] [Google Scholar]
- 48.Curry SJ, Krist AH, Owens DK, et al. Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA 2019;321:580–87. [DOI] [PubMed] [Google Scholar]
- 49.Administration USFD. FDA approves first treatment for post-partum depression, 2019.
- 50.Yonkers KA, Vigod S, Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol 2011;117:961–77. [DOI] [PubMed] [Google Scholar]
- 51.Ertel KA, Rich-Edwards JW, Koenen KC. Maternal depression in the United States: nationally representative rates and risks. Journal of women’s health (2002) 2011;20:1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lara-Cinisomo S, Clark CT, Wood J. Increasing Diagnosis and Treatment of Perinatal Depression in Latinas and African American Women: Addressing Stigma Is Not Enough. Womens Health Issues 2018;28:201–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 54.Berks D, Hoedjes M, Raat H, et al. Feasibility and effectiveness of a lifestyle intervention after complicated pregnancies to improve risk factors for future cardiometabolic disease. Pregnancy Hypertens 2019;15:98–107. [DOI] [PubMed] [Google Scholar]
- 55.Lo JC, Feigenbaum SL, YANG J, PRESSMAN AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. J Clin Endocrinol Metab 2006;91:1357–63. [DOI] [PubMed] [Google Scholar]
- 56.Lovvik TS, Wikstrom AK, Neovius M, Stephansson O, Roos N, Vanky E. Pregnancy and perinatal outcomes in women with polycystic ovary syndrome and twin births: a population-based cohort study. BJOG 2015;122:1295–302. [DOI] [PubMed] [Google Scholar]
- 57.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clapp MA, Barth WH. The Future of Cesarean Delivery Rates in the United States. Clin Obstet Gynecol 2017;60:829–39. [DOI] [PubMed] [Google Scholar]
- 59.Duley L The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009;33:130–7. [DOI] [PubMed] [Google Scholar]
- 60.Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. Fertil Steril 2016;106:6–15. [DOI] [PubMed] [Google Scholar]
- 61.Madden JM, Lakoma MD, Rusinak D, Lu CY, Soumerai SB. Missing clinical and behavioral health data in a large electronic health record (EHR) system. J Am Med Inform Assoc 2016;23:1143–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.