Abstract
Background:
Obesity is a well-known risk factor for endometrial cancer, yet mechanisms of obesity-related carcinogenesis are not well-defined, particularly for premenopausal women. With the continuing obesity epidemic, increases in endometrial cancer incidence and younger age of diagnosis are often attributed to a hyper-estrogenic state created by hormone production in adipose tissue, but significant knowledge gaps remain. The balance of estrogen-responsive signals has not been defined in the endometrium of obese premenopausal women, where obesity may not create hyper-estrogenism in the context of a primarily ovarian source of estrogen production. Obesity is associated with a state of low-grade, chronic inflammation that can promote tumorigenesis, and it is known that hormonal changes alter the immune microenvironment of the endometrium. However, limited research has been conducted on endometrial immune-response differences in obese women at increased risk for cancer.
Objective:
Endometrial estrogen-regulated biomarkers previously shown to be dysregulated in endometrial cancer were evaluated in a cohort of premenopausal women to determine if obesity is associated with differences that reflect alterations in cancer risk. A multiplexed panel of immune-related genes was also evaluated for differences related to obesity.
Study Design:
Premenopausal women with Body Mass Index ≥30 kg/m2 (n=97) or Body Mass Index ≤25 kg/m2 (n=33) were prospectively enrolled in a cross-sectional study, including assessment of serum metabolic markers and timed endometrial biopsy for pathology evaluation, hormone-regulated biomarker analysis, and immune response gene expression analysis. Medical and gynecologic history was obtained. Endometrial gene expression markers were also compared across Body Mass Index groups in a previous cohort of premenopausal women with inherited cancer risk (Lynch syndrome).
Results:
In addition to known systemic metabolic differences, histologically normal endometrium from obese women showed decreased gene expression of progesterone receptor (p=0.0027) and estrogen-induced genes- RALDH2 (p=0.008), IGF-1 (p=0.016), and survivin (p=0.042) compared to non-obese women. Endometrial biomarkers IGF-1, survivin, and progesterone receptor remained statistically significant in multivariate linear regression models. In contrast, obese women with Lynch syndrome had increased IGF-1 (p=0.017). There were no differences in proliferation and limited endometrial immune differences were observed.
Conclusions:
When comparing obese and non-obese premenopausal women without endometrial pathology or inherited risk, endometrial biomarkers do not reflect a local hyper-estrogenic environment, but instead reflect a decreased risk profile that may be indicative of a compensated state. In describing premenopausal endometrial cancer risk, it may be insufficient to describe obesity alone as a high-risk state and further studies are warranted to evaluate individualized biomarker profiles reflecting a disturbance in hormone-responsive signals or immune response. In patients with Lynch syndrome, the endometrial biomarker profile suggests that obesity further increases risk.
Keywords: Endometrial cancer, Lynch syndrome, obesity, premenopausal, prevention
Introduction
Endometrial cancer incidence and mortality have increased substantially in the United States in recent years, linked to parallel increases in obesity1. With the continuing obesity epidemic, increases in endometrial cancer incidence and younger age of diagnosis2 are often attributed to a hyper-estrogenic state created by hormone production in adipose tissue, but significant knowledge gaps remain3. Research has generally focused on obesity-mediated increased cancer risk via increased estrogen action causing endometrial proliferation. Hyperinsulinemia, elevated systemic levels of insulin-like growth factors, and elevated levels of both anti- and pro-inflammatory adipokines have also been linked to carcinogenesis4. Despite the substantial increased risk at the population level, most obese women do not develop endometrial cancer. The molecular determinants for the development of cancer are unknown for this high-risk cohort. Identifying early molecular changes in histologically normal endometrium can improve risk stratification and prevention efforts. Of all types of cancer that affect women, obesity is most strongly associated with endometrial cancer 5. With a well-defined precancer lesion (complex atypical hyperplasia), endometrial cancer is also an ideal model to study obesity-related carcinogenesis.
Overall, epidemiologic studies show that hormonal factors associated with unopposed estrogen increase endometrial cancer risk6. Estrogen stimulation promotes endometrial cell proliferation while progesterone acts to suppress proliferation, and presumably prevent accumulation of oncogenic mutations3, 4. The use of estrogen-only hormone therapy, early age of menarche, late age of menopause, and nulliparity have been associated with increased risk of endometrial cancer in the general population7. Progestin-based contraceptive use, later age of menarche, and parity are associated with reduced endometrial cancer risk6. A multitude of rodent models support this association8–11. In addition to the reproductive and hormonal factors described above, genetic factors also increase endometrial cancer risk. Women with Lynch syndrome (inherited DNA mismatch repair defects) have an estimated 40–60% cumulative lifetime risk for endometrial cancer12–15. Epidemiologic studies show that reproductive factors also alter endometrial cancer risk in women with Lynch syndrome, similar to average-risk women16. Again, specific molecular mechanisms underlying these changes remain unknown.
The local immune environment contributes to either promoting cancer development or supporting surveillance to recognize and eliminate early preneoplastic changes17. Obesity is associated with a state of low-grade, chronic inflammation, which is associated with tumor-promoting inflammation18, 19. Limited research has been conducted on immune-response changes in normal at-risk endometrium. Furthermore, the immune microenvironment of the endometrium is modified by changes in estrogen and progesterone20, 21.
Our group has previously defined a panel of estrogen-regulated genes expressed in the endometrium that are also differentially expressed in hyperplasia or cancer. These represent pro-proliferative/anti-apoptotic genes (IGF-1, survivin)22, 23 and inhibitors of proliferation (SFRP4, RALDH2) 24, 25 that contribute to the counterbalance response (“brakes”) induced by estrogen. EIG121 reflects estrogen excess26 and regulates pro-survival signaling during cellular stress27. These genes have also been evaluated under cancer risk-reducing strategies. In a premenopausal cohort of women with Lynch Syndrome, progestin-based contraceptives reduced expression of many of these markers similar to the response in average-risk women28.
We hypothesized that obese premenopausal women have an altered estrogen-regulated endometrial biomarker profile. Beyond circulating hormone markers, expression of genes involved in the counterbalance to estrogen stimulation could become disturbed in the endometrium, contributing to increased cancer risk. Altered hormone signaling could also contribute to immune-related changes. To evaluate biomarker-based differences associated with obesity, microscopically benign endometrium was prospectively acquired from obese premenopausal women unselected for additional genetic risk. A retrospective analysis of endometrial biomarkers in obese versus non-obese women with Lynch syndrome was also conducted to determine if additional cancer risk-related aberrations influence obesity-associated differences. With increasing incidence of endometrial cancer and earlier age at diagnosis, an improved understanding of obesity-associated molecular aberrations in the endometrium of premenopausal women is essential to developing preventive interventions.
Materials and Methods
Participants
This biomarker analysis is embedded within a cross-sectional trial that was designed to evaluate prevalence of endometrial histologic abnormalities and additional clinical characteristics in obese and non-obese women. As such, the biomarker evaluation was conducted in the total sample recruited to the trial. The parent trial was registered on clinicaltrials.gov (NCT00500591) and approved by the Institutional Review Board at MD Anderson Cancer Center. Subjects were recruited via advertisement across MD Anderson Cancer Center including cancer prevention clinics, public areas, and employee work areas. Premenopausal women 30–55 years old with BMI ≥30 kg/m2 or ≤25 kg/m2 were recruited, unselected for genetic risk. Informed consent was obtained. Exclusion criteria: current use of hormonal agents (within three months of enrollment), personal history of endometrial hyperplasia or cancer, prior pelvic radiation, history of metastatic cancer, non-metastatic cancer that received hormonal therapy, or non-metastatic cancer receiving treatment ≤6 months prior to enrollment.
Height and weight were measured for eligibility. Participants completed a questionnaire for demographics, gynecologic/reproductive history and social history. Participants provided a fasting blood sample and underwent endometrial biopsy timed to days 6 – 11 of the menstrual cycle (proliferative phase). Endometrial biopsies were examined by a clinical anatomic pathologist. Biopsy was either formalin-fixed for routine histological processing or frozen for RNA extraction.
Serum Biomarkers
Total testosterone, free testosterone, sex hormone binding globulin (SHBG), follicle stimulating hormone (FSH), luteinizing hormone (LH), and glucose were measured by BioPharma Services, EMD Millipore (St. Charles, MO). IGF1, IGFBP1, and IGFBP2 protein levels were measured using MilliPlex MAP (Millipore). Insulin was measured by ELISA (Crystal Chem, Elk Grove Village, IL). Total estradiol (radioimmunoassay) and progesterone (Siemens Immulite 2000) were analyzed through University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by NICHD/NIH R24HD102061.
Immunohistochemistry
Primary antibodies for Ki67 (Clone 35, BD Biosciences, San Jose, CA) and PR (clone D8Q2J, Cell Signaling, Danvers, MA) were used according to manufacturers’ instructions. Ki67 was scored as percentage of cells in glands with nuclear staining. PR was semi-quantitated with modified H-score29.
Gene expression
Our previous studies22–28, 30 characterized a panel of endometrial estrogen-regulated genes, including insulin-like growth factor 1 (IGF-1), estrogen-induced gene 121 (EIG121), retinaldehyde dehydrogenase 2 (RALDH2), secreted frizzled related protein 4 (SFRP4), and survivin (BIRC5, baculoviral IAP repeat containing 5). RNA was isolated from endometrial biopsies and gene expression measured for Progesterone Receptor (PR), IGF-1, EIG121 (a.k.a. ELAPOR1 or KIAA1324), RALDH2 (a.k.a. ALDH1A2), SFRP4, and survivin28. Transcripts were normalized using “gNORM” with 18S rRNA, beta-actin (ACTB), and RPLP031. PR is a classical estrogen-regulated gene. Estrogen and progesterone signaling have crosstalk with the insulin/IGF-1 axis in both normal endometrium and cancer, linking hormonal and metabolic disruptions associated with obesity32, 33,34. IGF-1 is a pro-proliferative uterine growth factor linked with diabetes, polycystic ovarian syndrome (PCOS), and obesity, which is increased in hyperplasia and during neoplastic transformation22, 35, 36. EIG121 expression is linked with excess estrogen and increased in complex atypical hyperplasia and endometrioid endometrial cancers26, and regulates pro-survival signaling during cellular stress27. RALDH2 is an enzyme that catalyzes synthesis of retinoic acid, an inhibitor of endometrial proliferation25. SFRP4 acts as an antagonist to ligands in the Wnt signaling pathway and decreases Wnt-associated endometrial proliferation24,37. Survivin, an inhibitor of apoptosis, is increased in endometrial cancers23, 38.
An exploratory subgroup analysis was conducted within the obese cohort to determine impact of additional factors on endometrial biomarkers. Additional factors evaluated in obese women included extreme obesity (BMI 30–40 kg/m2 vs BMI >40 kg/m2), insulin resistance (IR), nulliparity, smoking status, and daily use of anti-inflammatory medications. Quantitative insulin sensitivity check index (QUICKI) was evaluated as 1/[log fasting insulin + log fasting glucose] 39. A QUICKI value of less than 0.357 defined insulin resistance (IR)40.
Multiplexed immune-related gene expression was measured for participants with BMI ≤25 kg/m2 versus BMI ≥35 kg/m2. NanoString PanCancer Immune Profiling Panel (730 immune-related genes plus 40 reference genes) was analyzed according to manufacturer procedures (NanoString, Inc, Seattle, WA) at the Genomic and RNA Profiling Core at Baylor College of Medicine (Houston, TX).
For comparison in a cohort with additional risk, hormone-regulated gene expression biomarkers were retrospectively analyzed based on BMI categories from a previous study of premenopausal women with inherited cancer risk due to Lynch syndrome28. Women with Lynch syndrome (n=51) were enrolled for a study of progestin-containing oral contraceptives or medroxyprogesterone acetate. Inclusion criteria required no use of hormonal agents for 4 months prior to study. Endometrial biopsies were collected on days 5–10 of the menstrual cycle. For the current analysis, only pre-treatment endometrial biopsies were evaluated. Only women with BMI ≥30 kg/m2 or ≤25 kg/m2 and histologically normal endometrium were included for the current analysis.
Statistical analysis
Given the sample size (33 non-obese and 97 obese women), the study has 80% power to detect standardized effect size of 0.569 for comparing biomarker values between non-obese and obese woman. Demographic and clinical characteristics were compared by BMI status using either t-test, Wilcoxon rank-sum test, chi-square test, or Fisher’s exact test, as appropriate. Individual linear regression models were conducted with serum and tissue biomarkers that were significant in initial comparisons. Biomarkers was regressed onto BMI status to assess the difference between obese and non-obese subjects. These models were then adjusted for other factors associated with BMI (race/ethnicity, education, age at menarche, parity, history of oral contraceptive use, and daily use of anti-inflammatory medications) to generate individual multivariable models to assess the change in the effect of BMI on biomarkers. For NanoString analysis, a Wilcoxon rank-sum test was used to determine if gene expression differed by BMI status. With a total of 730 genes tested, the beta-uniform mixture model (BUM) was used to control false discovery rate (FDR). Stata/MP v15.0 (College Station, TX) was used for statistical analysis. Box plots extend to 25th and 75th percentiles and whiskers indicate 10th and 90th percentiles, with values outside this range also indicated.
Results
Of the 130 women meeting inclusion criteria, 97 participants were obese (BMI ≥30.0 kg/m2). Of 97 obese study participants, one case of complex atypical hyperplasia was identified in a patient with BMI of 45.3 kg/m2, which is considered morbidly obese. This patient was referred for appropriate treatment and excluded from further molecular analysis due to endometrial pathology. No endometrial cancers were identified. No endometrial abnormalities were detected in non-obese women.
Clinical characteristics for obese (n=96) and non-obese (n=33) women are described in Table 1. Age was similar in both groups. Mean BMI in the non-obese cohort was 21.79 kg/m2 compared with 37.44 kg/m2, with BMI ranging from 30 – 55 kg/m2 in the obese cohort. Race differed by group with fewer Asian women (2.1%) and more Black women (45.8%) in the obese cohort compared to 30.3% and 12.1% in the non-obese cohort (p<0.001). Obese women were more likely to experience younger age at menarche and to have previously used oral contraceptives. A similar percentage of women reported regular monthly periods in both groups. There was no difference in diagnosis of PCOS. Obese women were more likely to report daily use of non-steroidal anti-inflammatory medications (12.5% vs 48.42%, p<0.001).
Table 1.
Demographic and clinical characteristics of study population
| Characteristic | Non-Obese (BMI ≤25 kg/m2) (n=33) | Obese (BMI ≥30 kg/m2) (n=96) | p-value |
|---|---|---|---|
| Age (mean, years) | 43.03 | 40.94 | 0.064 |
| BMI (mean, kg/m2) range | 21.79 (18.46 – 24.76) | 37.34 (30.04 – 54.73) | <0.001 |
| Race or Ethnicity (%) | <0.001 | ||
| Asian | 30.30 | 2.08 | |
| Black | 12.12 | 45.83 | |
| Hispanic | 24.24 | 20.83 | |
| Non-Hispanic White | 30.30 | 31.25 | |
| Other | 3.03 | 0.00 | |
| Education (%) | 0.007 | ||
| 8th grade or less | 0.00 | 1.04 | |
| High School or GED | 3.03 | 21.88 | |
| Vocational School | 3.03 | 14.58 | |
| College | 57.58 | 42.71 | |
| Graduate/professional school | 36.36 | 19.79 | |
| Age at menarche (mean, years) | 13.09 | 11.84 | <0.001 |
| Total number of live births (mean) | 1.27 | 2.00 | 0.005 |
| Personal history of PCOS (%) | >0.999 | ||
| Yes | 6.06 | 8.51 | |
| No | 93.94 | 91.49 | |
| History of oral contraceptive use (%) | 0.032 | ||
| Yes | 72.73 | 88.42 | |
| No | 27.27 | 11.58 | |
| Current menstrual cycle status (%) | 0.840 | ||
| Irregular periods | 18.18 | 19.79 | |
| Regular monthly periods | 81.82 | 80.21 | |
| Current regular use of anti-inflammatory medication at least once a day (%) | <0.001 | ||
| Yes | 12.50 | 48.42 | |
| No | 87.50 | 51.58 | |
| Smoked more than 100 cigarettes in your lifetime (%) | 0.080 | ||
| No | 90.91 | 75.79 | |
| Yes | 9.09 | 24.21 | |
Bold indicates statistical significance with p value <0.05.
Blood levels of systemic hormonal and endocrine markers (Table 2) showed increased leptin and insulin, and decreased sex hormone binding globulin (SHBG) and adiponectin in obese women. Total estradiol and testosterone (free and total) were similar between cohorts. Serum progesterone levels were below the limit of detection (0.1 ng/ml) for 24 out of 33 non-obese participants and 81 out of 96 obese participants, precluding statistical comparison.
Table 2.
Fasting serum hormonal and endocrine markers
| Serum Marker (median) | Non-Obese (BMI ≤25 kg/m2) | Obese (BMI ≥30 kg/m2) | p-value |
|---|---|---|---|
| Total Estradiol (pg/ml) | 76.76 | 74.59 | 0.608 |
| Total Testosterone (ng/ml) | 0.65 | 0.60 | 0.678 |
| Free Testosterone (nM) | 0.45 | 0.51 | 0.779 |
| Sex Hormone Binding Globulin (mIU/ml) | 84.84 | 50.71 | <0.001 |
| Follicle Stimulating Hormone (mg/dl) | 10.23 | 9.11 | 0.241 |
| Luteinizing Hormone (mIU/ml) | 4.85 | 3.78 | 0.127 |
| Adiponectin (ng/ml) | 806.58 | 588.77 | <0.001 |
| Leptin (ng/ml) | 10.04 | 37.69 | <0.001 |
| Glucose (mg/dl) | 122.00 | 117.00 | 0.793 |
| Insulin (μU/ml) | 2.3 | 6.5 | <0.001 |
| IGF-1 (ng/ml) | 114.95 | 93.00 | 0.092 |
| IGFBP1 (ng/ml) | 4.18 | 1.18 | <0.001 |
| IGFBP2 (ng/ml) | 23.52 | 8.50 | 0.003 |
Bold indicates statistical significance with p value <0.05.
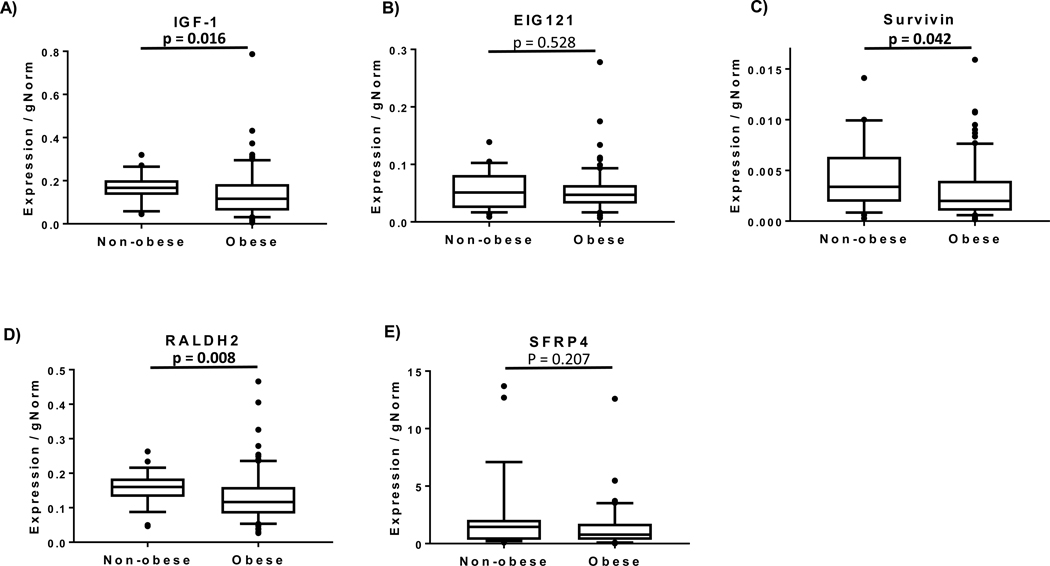
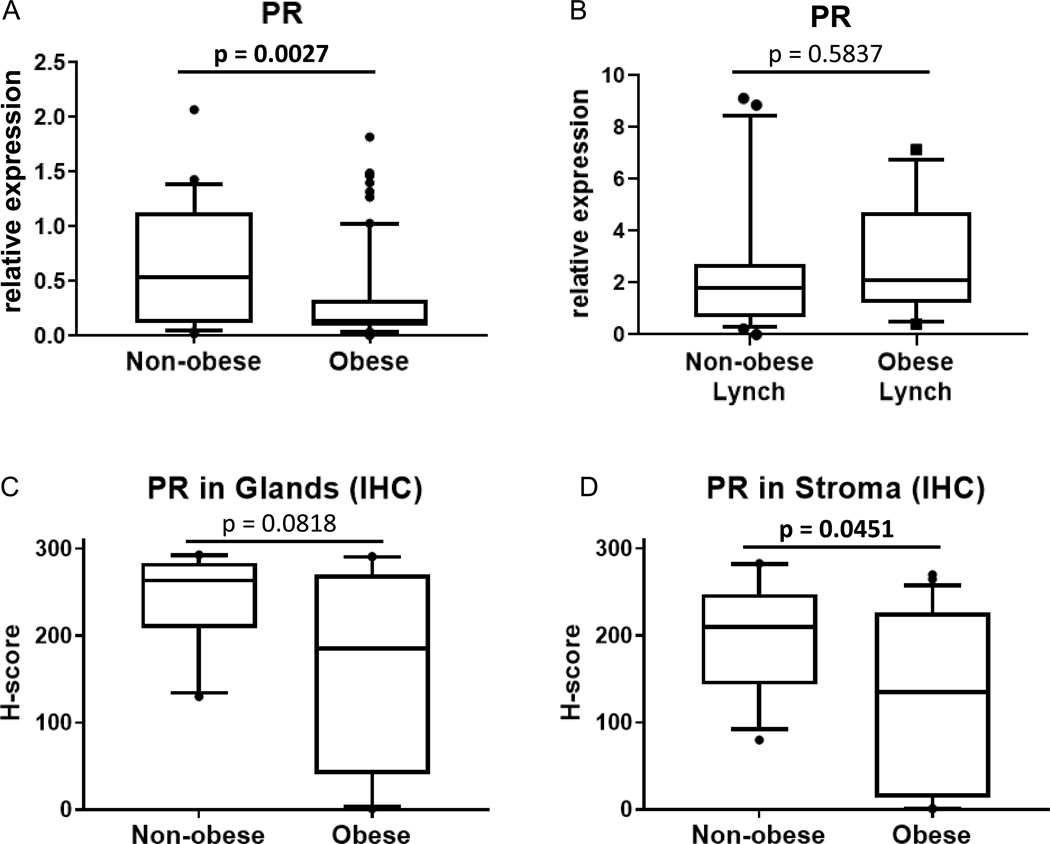
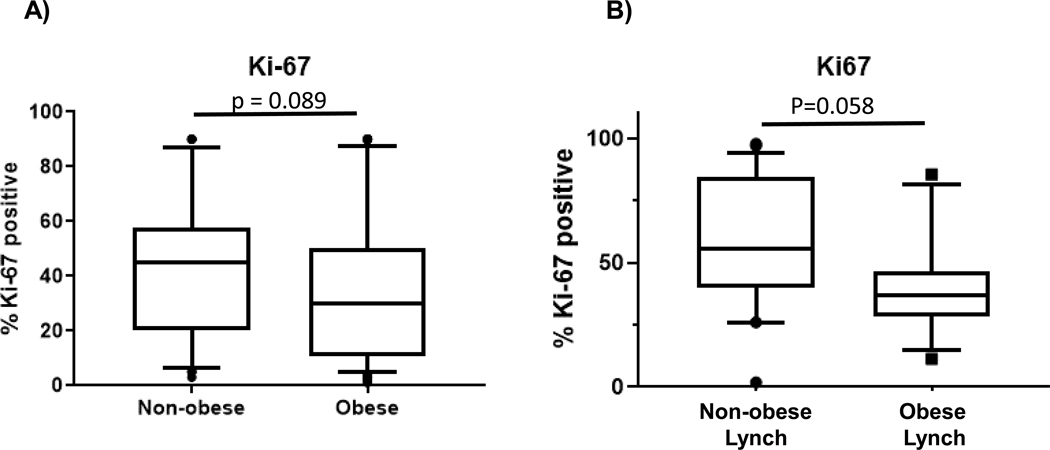
Biomarkers in endometrial biopsy samples (Figure 1) demonstrated significantly decreased gene expression of IGF-1, RALDH2, and survivin in the obese cohort. PR gene expression was significantly reduced (Figure 2A). Assessment of protein levels also show reduced expression of PR in the stroma of obese women versus non-obese, but no change in glands (Figure 2C-D). Proliferation rates of endometrial glands were not significantly different between the groups (Figure 3A).
Figure 1.
Obesity-related differences in estrogen-regulated gene expression levels in endometrial biopsy specimens. Non-obese women with BMI ≤25 kg/m2 (n=33) and obese women with BMI≥30 kg/m2 (n=96) were included. Box plots extend to 25th and 75th percentile with whiskers indicate 10th and 90th percentiles. All transcripts were normalized to “gNORM”, calculated as the geometric mean of the normalizer transcripts.
Figure 2.
Endometrial progesterone receptor (PR) gene expression and protein analyses. PR gene expression in endometrial biopsies from non-obese versus obese premenopausal women in women without inherited cancer risk (A) and women with Lynch syndrome (B) were measured by quantitative real-time PCR. Protein levels were assessed by immunohistochemistry (IHC) in women without inherited risk and quantitated for endometrial glands or stroma (C-D). Box plots extend to 25th and 75th percentile with whiskers indicate 10th and 90th percentiles.
Figure 3.
Endometrial proliferation in obese and non-obese cohorts. Proliferation rates evaluated using immunohistochemistry for Ki67 in non-obese and obese women without inherited cancer risk (A) and in women with Lynch syndrome (B).
Linear regression models (Table 3) confirmed that differences in serum markers SHBG, adiponectin, leptin, insulin, and IGFBP1 remained statistically significant after adjusting by race/ethnicity, education, age at menarche, parity, use of oral contraceptives, and use of daily anti-inflammatory medication. Differences in endometrial biomarkers IGF-1, survivin, and PR remained statistically significant in the adjusted models (Table 3).
Table 3.
Linear regression models for BMI status and biomarkers
| Univariate | Multivariable* | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta | 95% LB | 95% UB | p-value | Beta | 95% LB | 95% UB | p-value | |
| Serum markers | ||||||||
| SHBG | −30.61 | −46.66 | −14.56 | <0.001 | −39.43 | −62.84 | −16.02 | 0.001 |
| Adiponectin | −279.05 | −449.89 | −108.22 | 0.002 | −239.79 | −478.61 | −0.97 | 0.049 |
| Leptin | 36.63 | 17.75 | 55.51 | <0.001 | 40.73 | 13.49 | 67.96 | 0.004 |
| Insulin | 6.74 | 3.98 | 9.49 | <0.001 | 6.71 | 3.45 | 9.97 | <0.001 |
| IGFBP1 | −2.64 | −3.58 | −1.70 | <0.001 | −3.38 | −4.79 | −1.97 | <0.001 |
| Log(IGFBP2) | −0.50 | −0.90 | −0.11 | 0.014 | −0.59 | −1.20 | 0.02 | 0.057 |
| Endometrial biomarkers | ||||||||
| Log(IGF-1) | −0.39 | −0.74 | −0.03 | 0.032 | −0.59 | −1.14 | −0.03 | 0.039 |
| Log(Survivin) | −0.45 | −0.90 | 0.00 | 0.049 | −0.72 | −1.42 | −0.02 | 0.045 |
| Log(RALDH2) | −0.24 | −0.48 | 0.00 | 0.049 | −0.26 | −0.63 | 0.10 | 0.154 |
| PR | −0.35 | −0.56 | −0.14 | 0.002 | −0.49 | −0.81 | −0.16 | 0.004 |
models adjusted by race/ethnicity, education, age at menarche, number of live births, history of oral contraceptive use, daily anti-inflammatory medication use; log transformations made on specific markers to ensure regression assumptions were met. Bold indicates statistical significance with p-value <0.05.
An exploratory subgroup analysis showed that obese women with insulin resistance (IR) had significantly higher relative expression of survivin (0.0019 vs 0.0037, p=0.041). Obese women with extreme obesity (BMI>40 kg/m2) had higher relative expression of EIG121 compared to women with BMI 30–40 kg/m2 (0.06 vs 0.04, p=0.003). Nulliparous obese women had higher PR expression compared to parous obese women (0.39 vs 0.12, p=0.008). Expression of other markers were not significantly different between obese women with these combined factors. No significant differences were observed for obese women that smoked cigarettes versus women that did not or women that used daily anti-inflammatory medications versus those that did not.
NanoString immune-related gene expression analysis was conducted in obese versus non-obese cohorts. When controlling FDR at the 5% level, 28 statistically significant (p<0.005) differentially expressed genes were identified (Table 4). Broadly, altered genes were related to innate immune response, adaptive immune response, regulation of immune response, and cluster of differentiation (CD) molecules. However, fold-change values were very small. T cell-related molecules were upregulated, including immune checkpoints PD-1 [PDCD1] (1.6-fold) and TNFRSF18 (2.1-fold); and T cell and B cell signaling mediator, CARD11 (1.5-fold). CCL15, which encodes for a chemoattractant of monocytes, macrophages and lymphocytes, was upregulated 1.5-fold.
Table 4.
Statistically significant immune-related gene expression differences in obese women
| Gene Annotation | Gene name | Gene symbol | BMI ≤25 kg/m2 (n=21) normalized counts | BMI ≥35 kg/m2 (n=26) normalized counts | Fold change | p-value |
|---|---|---|---|---|---|---|
| Innate immune response | Activating transcription factor 2 | ATF2 | 4081.7 | 2769.5 | 0.68 | 0.0029 |
| Autophagy related 5 | ATG5 | 273.6 | 318.5 | 1.16 | 0.0019 | |
| Complement C8 gamma chain | C8G | 465.8 | 363.5 | 0.78 | 0.0054 | |
| Collectin subfamily member 12 | COLEC12 | 178.2 | 139.2 | 0.78 | 0.0026 | |
| Interleukin 1 receptor accessory protein like 2 | IL1RAPL2 | 18.8 | 26.8 | 1.42 | 0.0056 | |
| Janus kinase 2 | JAK2 | 150.0 | 181.0 | 1.21 | 0.0054 | |
| S100 calcium binding protein A12 | S100A12 | 49.3 | 41.2 | 0.83 | 0.0040 | |
| Toll like receptor 3 | TLR5 | 162887.4 | 122280.2 | 0.75 | 0.0013 | |
| Adaptive immune response | C-C motif chemokine ligand 15 | CCL15 | 8.6 | 13.1 | 1.52 | 0.0022 |
| CD5 molecule | CD5 | 4457.4 | 3552.6 | 0.80 | 0.0007 | |
| Junctional adhesion molecule 3 | JAM3 | 662.6 | 536.2 | 0.81 | 0.0024 | |
| Programmed cell death 1 | PDCD1 | 9.6 | 15.0 | 1.56 | 0.0033 | |
| Regulation of immune response | Caspase recruitment domain family member 11 | CARD11 | 105.1 | 158.0 | 1.50 | 0.0041 |
| H2B adaptor protein 2 | SH2B2 | 58.1 | 65.5 | 1.13 | 0.0029 | |
| Cluster of differentiation (CD) molecules | Integrin subunit beta 1 | ITGB1 | 11342.2 | 10220.6 | 0.90 | 0.0041 |
| Integrin subunit beta 4 | ITGB4 | 329.5 | 518.8 | 1.57 | 0.0029 | |
| Lysosomal associated membrane protein 2 | LAMP2 | 58.2 | 72.9 | 1.25 | 0.0021 | |
| Natural cytotoxicity triggering receptor 1 | NCR1 | 34.8 | 48.1 | 1.38 | 0.0012 | |
| Toll like receptor 3 | TLR3 | 1019.8 | 906.3 | 0.89 | 0.0022 | |
| TNF receptor superfamily member 14 | TNFRSF14 | 497.8 | 593.0 | 1.19 | 0.0029 | |
| Triggering receptor expressed on myeloid cells 1 | TREM1 | 4269.1 | 3838.1 | 0.90 | 0.0002 | |
| Miscellaneous | ||||||
| Leukocyte migration | Fucosyltransferase 7 | FUT7 | 711.9 | 633.9 | 0.89 | 0.0014 |
| Humoral immune response | Paired box 5 | PAX5 | 2481.6 | 3219.3 | 1.30 | 0.0011 |
| Antigen processing and presentation | Proteasome subunit beta 7 | PSMB7 | 2242.1 | 1981.9 | 0.88 | 0.0007 |
| Transcriptional repressor | SSX family member 4 | SSX4 | 1981.9 | 1823.9 | 0.92 | 0.0022 |
| Basic cell functions | Synaptotagmin 17 | SYT17 | 30.9 | 47.9 | 1.55 | 0.0003 |
| TNF superfamily members and their receptors | TNF receptor superfamily member 18 | TNFRSF18 | 55.8 | 117.3 | 2.10 | 0.0058 |
| T-cell proliferation | Tumor protein p53 | TP53 | 23.2 | 33.0 | 1.42 | 0.0054 |
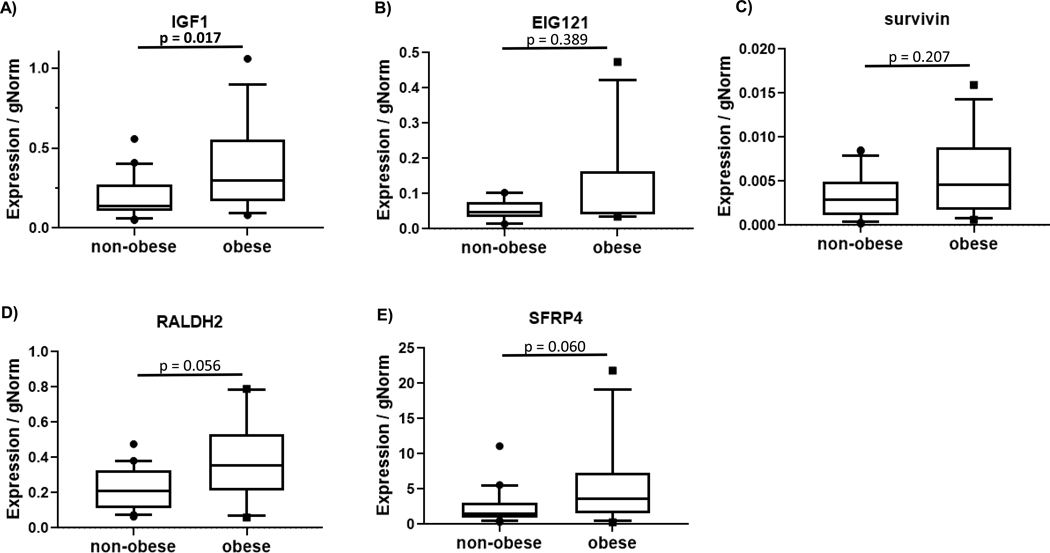
Gene expression biomarkers and proliferation were also compared in non-obese (n=22) and obese (n=13) premenopausal women with inherited endometrial cancer risk due to Lynch syndrome (Figure 4). Endometrial gene expression showed significantly higher IGF-1 in the obese group (p=0.017). Higher median expression of SFRP4 and RALDH2 did not reach statistical significance (p=0.060 and p=0.056, respectively). Endometrial proliferation was not significantly different (Figure 3B, p=0.058). No significant difference was observed for gene expression of PR in women with Lynch syndrome who were obese (Figure 2B).
Figure 4.
Obesity-related differences in estrogen-regulated gene expression levels in endometrial biopsy specimens in women with inherited cancer risk associated with Lynch syndrome. Non-obese women with BMI ≤25 kg/m2 (n=22) and obese women with BMI≥30 kg/m2 (n=13) were included. Box plots extend to 25th and 75th percentile with whiskers indicate 10th and 90th percentiles.
Structured Discussion
Principal Findings
Circulating total estradiol levels were not significantly different between obese and non-obese premenopausal women. Estrogen-regulated endometrial biomarkers (IGF-1, survivin, RALDH2) were decreased in obesity. PR gene expression and stromal PR protein level were decreased in obese women. Multivariable linear regression models for endometrial gene expression biomarkers showed that IGF1, survivin, and PR remain statistically significant in the adjusted models. No proliferation differences and limited immune-related differences were identified. A separate retrospective analysis of endometrial biomarkers from women with inherited cancer risk (Lynch syndrome) showed increased expression of IGF-1 in obesity and an opposite biomarker trend contrasting with observations in women unselected for inherited cancer risk.
Comment
Although it is well-known that obesity represents a significant increase in endometrial cancer risk in epidemiologic studies, in this study we found that estrogen-regulated biomarkers do not reflect estrogen dominance in the endometrium of obese premenopausal women and instead reflect a decreased risk environment. We hypothesize that potential compensatory or protective mechanisms may be mounted against pro-tumorigenic changes in the endometrium of obese premenopausal women. Potential compensatory mechanisms may be suppressed or exhausted under multiple risk factors, such as obesity plus inherited cancer risk or obesity plus insulin resistance. Given the large range of biomarker values observed (Figures 1 and 4), women on the extremes of these measures (or extremes of BMI) may be at especially increased risk.
Clinical Implications
This study prospectively enrolled women in a cross-sectional study and defined obesity-associated estrogen-regulated biomarker differences in the endometrium. Results suggest that obesity alone may not increase premenopausal endometrial cancer risk. Biomarker patterns from the current study suggest that more detailed risk stratification, including the combined impact of multiple risk factors, must be evaluated. Additional studies are needed to translate population-based observations to individualized endometrial cancer risk assessment and prevention strategies. If early markers of risk are identified or perhaps a risk score developed using multiple factors, obese patients at greatest risk could be offered cancer prevention strategies. Importantly, targeting the highest risk populations confers the additional benefit of potential cost-effectiveness compared to a strategy in a large, mixed-risk cohort where effectiveness may be diluted41.
Research Implications
In the postmenopausal setting where estrogen production occurs primarily in peripheral adipose tissue, the estrogen-dominant context of obesity is well-documented. However, up to 25% of endometrial cancers are diagnosed in premenopausal women42, where obesity is not strongly associated with hyper-estrogenism due to the primarily ovarian source of estrogen43, 44. While SHBG, which binds and inhibits estrogen activity, was decreased in obese women consistent with previous studies45, 46,44, bioavailable estrogen levels have not been compared in large cohort studies for these groups. Alternatively, reduced progesterone is another potential disruption to normal estrogen balance in obesity47, 48,49. Future studies in premenopausal women must look beyond obesity-mediated hyper-estrogenism and evaluate these measures across the post-ovulatory phase. Biomarker evaluation in the secretory phase should also include additional biomarkers reflecting progesterone-specific action to provide insight on whether increased risk associated with obesity may be due to a disturbance or imbalance in estrogen/progesterone signaling.
Decreased expression of survivin and IGF-1 in the endometrium of obese women may be unexpected based on previous studies showing similar decreases following administration of progestin-based contraceptives in a biomarker-based endometrial cancer chemoprevention study28; however, it is important to note that cancer development is still a relatively rare event for obese women. The markers evaluated here included both pro-proliferative genes and inhibitors of proliferation, with previous studies suggesting that both are upregulated in endometrial proliferation as a biologic counterbalance (increasing the “brakes” as the “accelerator” increases). In the current study, RALDH2 (one of the “brakes”) was decreased along with pro-proliferative IGF-1 and survivin. Reduced expression of PR in in the overall obese cohort contrasts with increased PR in endometrial cancers in obese women versus non-obese50, 51. Interestingly, the exploratory analysis of combined factors showed that PR expression is higher in obese nulliparous women compared to obese parous women. However, reduced PR in the overall cohort is consistent with lack of hyper-estrogenism observed in this obese cohort without endometrial pathology. Longitudinal studies to evaluate whether these endometrial counterbalance genes become decoupled during various risk conditions would provide further insight into mechanisms of cancer risk. The increased biomarker expression pattern observed in obese premenopausal women with Lynch syndrome reflects a pattern suggestive of a local pro-estrogenic environment, more typically associated with obesity and cancer risk, including increased IGF-1. In addition to human studies showing increased expression in hyperplasia and cancer, several transgenic mouse studies confirm that IGF-1 plays a central role in estrogen-induced proliferative response in normal uterine biology52–55. IGF-1 activation may be a key indicator of the switch from a normal counterbalance response to aberrant pro-tumorigenic endometrial environment.
Given the pattern of lower expression of endometrial biomarkers in obese premenopausal women, we have proposed that this may be indicative of a compensated state. While further studies are certainly required to evaluate this hypothesis, the observation of a compensated state or adaptive mechanisms during progression of obesity-associated chronic diseases is well-known, particularly in cardiovascular disease 56, 57 and conditions known to further increase cancer risk (liver cirrhosis 58 and chronic kidney disease 59, 60). Studies of these chronic diseases may more readily identify compensatory changes due to a reliance on function or molecular biomarkers, whereas cancer pathogenesis studies relying on histologic precursor lesions may miss compensatory changes occurring prior to histologic abnormalities.
The current study shows limited endometrial immune-related gene expression differences due to obesity in the proliferative phase. It must be noted that obese individuals use non-steroidal anti-inflammatory drugs (NSAIDs) at higher rates than non-obese individuals61. NSAIDs reduce inflammation and disrupt immune cell function by curbing immune cell proliferation, decreasing antibody production62, and increasing immune tolerance in dendritic cells63. It is not possible to separate NSAID-induced and obesity-related immunomodulatory effects in this study. Further studies should evaluate immune response changes in the endometrium due to obesity and the effect of NSAIDs on endometrial immune cells. With increased understanding that inflammation is a driver of premalignant conditions64, characterization of immune-related and inflammatory genes in benign, premalignant, and malignant endometrium is needed.
Previous literature evaluating biomarker changes in obese women (BMI > 40 kg/m2) undergoing bariatric surgery provides an important reference for several observations reported here. While comparisons are complicated by the inclusion of premenopausal and postmenopausal women and use of exogeneous hormonal agents, bariatric surgery studies report that endometrial tissue biomarkers are modulated by weight loss. In particular, women with hyperplasia showed reduced endometrial hormone receptor (ER and PR) expression following weight loss due to bariatric surgery 65 and normalization of PTEN and phospho-AKT expression have been observed in normal endometrium after weight loss66. While bariatric surgery studies include obese women with generally higher BMI than the current report, weight loss did not result in modulation of circulating estrogen, progesterone, or testosterone levels67, in line with the observations reported here. Systemic inflammatory markers (hsCRP, IL-6) were reduced by weight loss after bariatric surgery (tissue-based inflammatory markers were not evaluated) and metabolic function was improved66, 67. Each of these studies also highlight the substantially increased risk of endometrial pathology for women at these extremes of obesity, with up to 14% showing abnormalities and nearly 6% diagnosed with endometrial cancer at baseline 66. Interestingly, one of these studies also reports higher levels of amenorrhea or irregular menstrual cycles (42% and 26%, respectively)66 than the obese cohort described here.
Strengths and Limitations
The strengths of this study include prospective enrollment of premenopausal women without endometrial pathology, a large cohort of obese women, robust estrogen-regulated biomarker characterization paired with serum endocrine markers, and a unique retrospective cohort analysis in Lynch syndrome. Differences in race/ethnicity between groups is a limitation of the study population; however, models were adjusted for race/ethnicity in the multivariate analyses. Other than educational attainment, this study did not define participant characteristics related to social, environmental, or cultural factors that could provide more insight instead of the social construct of race. This study focused on the estrogen-driven differences during the proliferative phase; however, evaluation in the post-ovulatory phase would be valuable to evaluate progesterone differences. The cohort of obese women in this study included only 20% with irregular menstrual cycles; however, selecting for obese women with irregular cycles could define a group at additional increased risk, as previous studies show higher rates of irregular menstrual cycles in premenopausal women with endometrial cancer68.
Conclusions
In describing premenopausal endometrial cancer risk, it may be insufficient to describe obesity alone as a high-risk state and further studies are warranted to define individualized biomarker profiles for cancer risk. Evaluation of additional cohorts will be required to determine if compensatory mechanisms are at play in the endometrium of obese premenopausal women. Additional events to overcome this compensatory process may distinguish obese women who will develop complex atypical hyperplasia and cancer compared to those that do not. Biomarker differences in obese women with Lynch syndrome or insulin resistance suggest that these combined aberrations may result in an imbalance or disturbance in estrogen-responsive signals.
Condensation:
Endometrial biomarkers in obese premenopausal women do not reflect hyper-estrogenism, but instead reflect a decreased risk profile that may be indicative of a compensated state.
AJOG at a Glance:
- Why was this study conducted?
- Obesity is a well-known risk factor for endometrial cancer, yet molecular determinants of disease are unknown, especially for premenopausal women.
- This study evaluated estrogen-regulated endometrial biomarkers to determine if obesity is associated with differences that reflect alterations in cancer risk.
- What are the key findings?
- Normal endometrium from obese premenopausal women showed decreased expression of estrogen-regulated biomarkers compared to non-obese women.
- However, obese women with Lynch syndrome (inherited cancer risk) showed an opposite pattern of increased biomarker expression.
- What does this study add to what is already known?
- Endometrial biomarkers do not reflect hyper-estrogenism in obese premenopausal women without endometrial pathology or inherited risk, but instead reflect a decreased risk profile that may be indicative of a compensated state.
- In describing premenopausal endometrial cancer risk, it may be insufficient to describe obesity alone as a high-risk state.
- In Lynch syndrome, endometrial biomarkers suggest that obesity further increases risk.
Acknowledgments
Funding: This work was supported in part by the MD Anderson Uterine Cancer SPORE (P50CA098258 to KHL and RRB) and the MD Anderson Cancer Center Support Grant (CA016672), which supports the Biostatistics Resource Group. A T32 training grant for gynecologic oncology (CA101642) provided support to JAD. University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by NICHD/NIH R24HD102061.
This study was presented in abstract form during Transdisciplinary Cancer Interception: Leveraging Biology to Improve Prevention and Detection, hosted by Huntsman Cancer Institute at the University of Utah and Nature Reviews Cancer, Salt Lake City, Utah, March 9–11, 2020.
Footnotes
Disclosure Statement
The authors have no disclosures relevant to the current study. P.T.S. receives unrelated research trial support from Novartis and Incyte, and serves on the advisory board for Tesaro. Y.Y. is a consultant to Boehringer Ingelheim Pharmaceuticals, Servier Pharmaceuticals, Amgen Inc., Deciphera Pharmaceuticals, Ono Pharmaceuticals, Citius Pharmaceuticals, and Midas Medical Technologies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.HENLEY SJ, MILLER JW, DOWLING NF, BENARD VB, RICHARDSON LC. Uterine Cancer Incidence and Mortality - United States, 1999–2016. MMWR Morbidity and mortality weekly report 2018;67:1333–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NEVADUNSKY NS, VAN ARSDALE A, STRICKLER HD, et al. Obesity and age at diagnosis of endometrial cancer. Obstet Gynecol 2014;124:300–6. [DOI] [PubMed] [Google Scholar]
- 3.ONSTAD MA, SCHMANDT RE, LU KH. Addressing the Role of Obesity in Endometrial Cancer Risk, Prevention, and Treatment. J Clin Oncol 2016;34:4225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SCHMANDT RE, IGLESIAS DA, CO NN, LU KH. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol 2011;205:518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.REEVES GK, PIRIE K, BERAL V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ 2007;335:1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FELIX AS, YANG HP, BELL DW, SHERMAN ME. Epidemiology of Endometrial Carcinoma: Etiologic Importance of Hormonal and Metabolic Influences. Adv Exp Med Biol 2017;943:3–46. [DOI] [PubMed] [Google Scholar]
- 7.KITSON SJ, EVANS DG, CROSBIE EJ. Identifying High-Risk Women for Endometrial Cancer Prevention Strategies: Proposal of an Endometrial Cancer Risk Prediction Model. Cancer Prev Res 2017;10:1–13. [DOI] [PubMed] [Google Scholar]
- 8.KIM HI, KIM TH, LIM JM, JEONG JW. Steroid hormone intervenes in the endometrial tumorigenesis of pten ablation. Journal of cancer prevention 2013;18:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KIM TH, WANG J, LEE KY, et al. The Synergistic Effect of Conditional Pten Loss and Oncogenic K-ras Mutation on Endometrial Cancer Development Occurs via Decreased Progesterone Receptor Action. J Oncol 2010;2010:139087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KIM TH, YOO JY, JEONG JW. Mig-6 Mouse Model of Endometrial Cancer. Adv Exp Med Biol 2017;943:243–59. [DOI] [PubMed] [Google Scholar]
- 11.GOAD J, KO YA, KUMAR M, JAMALUDDIN MFB, TANWAR PS. Oestrogen fuels the growth of endometrial hyperplastic lesions initiated by overactive Wnt/beta-catenin signalling. Carcinogenesis 2018;39:1105–16. [DOI] [PubMed] [Google Scholar]
- 12.BONADONA V, BONAITI B, OLSCHWANG S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA : the journal of the American Medical Association 2011;305:2304–10. [DOI] [PubMed] [Google Scholar]
- 13.LYNCH HT, LYNCH PM, LANSPA SJ, SNYDER CL, LYNCH JF, BOLAND CR. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009;76:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MEYER LA, BROADDUS RR, LU KH. Endometrial cancer and Lynch syndrome: clinical and pathologic considerations. Cancer Control 2009;16:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MOLLER P, SEPPALA TT, BERNSTEIN I, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut 2018;67:1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DASHTI SG, CHAU R, OUAKRIM DA, et al. Female Hormonal Factors and the Risk of Endometrial Cancer in Lynch Syndrome. JAMA : the journal of the American Medical Association 2015;314:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SAUTES-FRIDMAN C, CHERFILS-VICINI J, DAMOTTE D, et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev 2011;30:13–25. [DOI] [PubMed] [Google Scholar]
- 18.NAIR S, NGUYEN H, SALAMA S, AL-HENDY A. Obesity and the Endometrium: Adipocyte-Secreted Proinflammatory TNF alpha Cytokine Enhances the Proliferation of Human Endometrial Glandular Cells. Obstet Gynecol Int 2013;2013:368543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IYENGAR NM, GUCALP A, DANNENBERG AJ, HUDIS CA. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol 2016;34:4270–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GOLDFIEN GA, BARRAGAN F, CHEN J, et al. Progestin-Containing Contraceptives Alter Expression of Host Defense-Related Genes of the Endometrium and Cervix. Reproductive sciences 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LEE SK, KIM CJ, KIM DJ, KANG JH. Immune cells in the female reproductive tract. Immune network 2015;15:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MCCAMPBELL AS, BROADDUS RR, LOOSE DS, DAVIES PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res 2006;12:6373–8. [DOI] [PubMed] [Google Scholar]
- 23.NABILSI NH, BROADDUS RR, MCCAMPBELL AS, et al. Sex hormone regulation of survivin gene expression. The Journal of endocrinology 2010;207:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CARMON KS, LOOSE DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res 2008;6:1017–28. [DOI] [PubMed] [Google Scholar]
- 25.DENG L, SHIPLEY GL, LOOSE-MITCHELL DS, et al. Coordinate regulation of the production and signaling of retinoic acid by estrogen in the human endometrium. J Clin Endocrinol Metab 2003;88:2157–63. [DOI] [PubMed] [Google Scholar]
- 26.DENG L, BROADDUS RR, MCCAMPBELL A, et al. Identification of a novel estrogen-regulated gene, EIG121, induced by hormone replacement therapy and differentially expressed in type I and type II endometrial cancer. Clin Cancer Res 2005;11:8258–64. [DOI] [PubMed] [Google Scholar]
- 27.DENG L, FENG J, BROADDUS RR. The novel estrogen-induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Dis 2010;1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LU KH, LOOSE DS, YATES MS, et al. Prospective multicenter randomized intermediate biomarker study of oral contraceptive versus depo-provera for prevention of endometrial cancer in women with Lynch syndrome. Cancer Prev Res 2013;6:774–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.JOHNSTON SR, SACCANI-JOTTI G, SMITH IE, et al. Changes in estrogen receptor, progesterone receptor, and pS2 expression in tamoxifen-resistant human breast cancer. Cancer Res 1995;55:3331–8. [PubMed] [Google Scholar]
- 30.WESTIN SN, BROADDUS RR, DENG L, et al. Molecular clustering of endometrial carcinoma based on estrogen-induced gene expression. Cancer biology & therapy 2009;8:2126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.VANDESOMPELE J, DE PRETER K, PATTYN F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome biology 2002;3:RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FLANNERY CA, SALEH FL, CHOE GH, et al. Differential Expression of IR-A, IR-B and IGF-1R in Endometrial Physiology and Distinct Signature in Adenocarcinoma. J Clin Endocrinol Metab 2016;101:2883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MERRITT MA, STRICKLER HD, EINSTEIN MH, et al. Insulin/IGF and sex hormone axes in human endometrium and associations with endometrial cancer risk factors. Cancer Causes Control 2016;27:737–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.KASHIMA H, SHIOZAWA T, MIYAMOTO T, et al. Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E. Endocrine-related cancer 2009;16:113–22. [DOI] [PubMed] [Google Scholar]
- 35.SHAFIEE MN, SEEDHOUSE C, MONGAN, et al. Up-regulation of genes involved in the insulin signalling pathway (IGF1, PTEN and IGFBP1) in the endometrium may link polycystic ovarian syndrome and endometrial cancer. Molecular and cellular endocrinology 2016;424:94–101. [DOI] [PubMed] [Google Scholar]
- 36.TALAVERA F, REYNOLDS RK, ROBERTS JA, MENON KM. Insulin-like growth factor I receptors in normal and neoplastic human endometrium. Cancer Res 1990;50:3019–24. [PubMed] [Google Scholar]
- 37.KAWANO Y, KYPTA R. Secreted antagonists of the Wnt signalling pathway. Journal of cell science 2003;116:2627–34. [DOI] [PubMed] [Google Scholar]
- 38.NABILSI NH, BROADDUS RR, LOOSE DS. DNA methylation inhibits p53-mediated survivin repression. Oncogene 2009;28:2046–50. [DOI] [PubMed] [Google Scholar]
- 39.KATZ A, NAMBI SS, MATHER K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–10. [DOI] [PubMed] [Google Scholar]
- 40.HREBICEK J, JANOUT V, MALINCIKOVA J, HORAKOVA D, CIZEK L. Detection of insulin resistance by simple quantitative insulin sensitivity check index QUICKI for epidemiological assessment and prevention. J Clin Endocrinol Metab 2002;87:144–7. [DOI] [PubMed] [Google Scholar]
- 41.DOTTINO JA, HASSELBLAD V, SECORD AA, MYERS ER, CHINO J, HAVRILESKY LJ. Levonorgestrel Intrauterine Device as an Endometrial Cancer Prevention Strategy in Obese Women: A Cost-Effectiveness Analysis. Obstet Gynecol 2016;128:747–53. [DOI] [PubMed] [Google Scholar]
- 42.GRESSEL GM, PARKASH V, PAL L. Management options and fertility-preserving therapy for premenopausal endometrial hyperplasia and early-stage endometrial cancer. Int J Gynaecol Obstet 2015;131:234–9. [DOI] [PubMed] [Google Scholar]
- 43.FREEMAN EW, SAMMEL MD, LIN H, GRACIA CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause 2010;17:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.RANDOLPH JF JR., SOWERS M, GOLD EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab 2003;88:1516–22. [DOI] [PubMed] [Google Scholar]
- 45.KIDDY DS, SHARP PS, WHITE DM, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin Endocrinol (Oxf) 1990;32:213–20. [DOI] [PubMed] [Google Scholar]
- 46.KOPELMAN PG, PILKINGTON TR, WHITE N, JEFFCOATE SL. Abnormal sex steroid secretion and binding in massively obese women. Clin Endocrinol (Oxf) 1980;12:363–9. [DOI] [PubMed] [Google Scholar]
- 47.DOWSETT M, FOLKERD E. Reduced progesterone levels explain the reduced risk of breast cancer in obese premenopausal women: a new hypothesis. Breast Cancer Res Treat 2015;149:1–4. [DOI] [PubMed] [Google Scholar]
- 48.SANTORO N, LASLEY B, MCCONNELL D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab 2004;89:2622–31. [DOI] [PubMed] [Google Scholar]
- 49.JAIN A, POLOTSKY AJ, ROCHESTER D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 2007;92:2468–73. [DOI] [PubMed] [Google Scholar]
- 50.MAULAND KK, TROVIK J, WIK E, et al. High BMI is significantly associated with positive progesterone receptor status and clinico-pathological markers for non-aggressive disease in endometrial cancer. Br J Cancer 2011;104:921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.BUSCH EL, CROUS-BOU M, PRESCOTT J, et al. Endometrial Cancer Risk Factors, Hormone Receptors, and Mortality Prediction. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2017;26:727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WINUTHAYANON W, HEWITT SC, ORVIS GD, BEHRINGER RR, KORACH KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA 2010;107:19272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.ZHU L, POLLARD JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA 2007;104:15847–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.HEWITT SC, LI Y, LI L, KORACH KS. Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem 2010;285:2676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.RAJKUMAR K, DHEEN T, KRSEK M, MURPHY LJ. Impaired estrogen action in the uterus of insulin-like growth factor binding protein-1 transgenic mice. Endocrinology 1996;137:1258–64. [DOI] [PubMed] [Google Scholar]
- 56.MILLANE T, JACKSON G, GIBBS CR, LIP GY. ABC of heart failure. Acute and chronic management strategies. BMJ 2000;320:559–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.INSULL W JR. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med 2009;122:S3–S14. [DOI] [PubMed] [Google Scholar]
- 58.SEN B, RASTOGI A, NATH R, et al. Senescent Hepatocytes in Decompensated Liver Show Reduced UPR(MT) and Its Key Player, CLPP, Attenuates Senescence In Vitro. Cell Mol Gastroenterol Hepatol 2019;8:73–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.BONVENTRE JV. Adaptation of the Kidney to Injury. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson JL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 19e. New York, NY: McGraw-Hill Education, 2014. [Google Scholar]
- 60.FOGO AB. Mechanisms of progression of chronic kidney disease. Pediatr Nephrol 2007;22:2011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DAVIS JS, LEE HY, KIM J, et al. Use of non-steroidal anti-inflammatory drugs in US adults: changes over time and by demographic. Open Heart 2017;4:e000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.BANCOS S, BERNARD MP, TOPHAM DJ, PHIPPS RP. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell Immunol 2009;258:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.BUCKLAND M, LOMBARDI G. Aspirin and the induction of tolerance by dendritic cells. Handb Exp Pharmacol 2009:197–213. [DOI] [PubMed] [Google Scholar]
- 64.KENSLER TW, SPIRA A, GARBER JE, et al. Transforming Cancer Prevention through Precision Medicine and Immune-oncology. Cancer Prev Res 2016;9:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ARGENTA P, SVENDSEN C, ELISHAEV E, et al. Hormone receptor expression patterns in the endometrium of asymptomatic morbidly obese women before and after bariatric surgery. Gynecol Oncol 2014;133:78–82. [DOI] [PubMed] [Google Scholar]
- 66.MACKINTOSH ML, DERBYSHIRE AE, MCVEY RJ, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer 2019;144:641–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MODESITT SC, HALLOWELL PT, SLACK-DAVIS JK, et al. Women at extreme risk for obesity-related carcinogenesis: Baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol Oncol 2015;138:238–45. [DOI] [PubMed] [Google Scholar]
- 68.SOLIMAN PT, OH JC, SCHMELER KM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol 2005;105:575–80. [DOI] [PubMed] [Google Scholar]