Abstract
An adverse intrauterine environment is associated with the future risk of obesity and type 2 diabetes. Changes in placental function may underpin the intrauterine origins of adult disease, but longitudinal studies linking placental function with childhood outcomes are rare. Here, we determined the abundance and phosphorylation of protein intermediates involved in insulin signaling, inflammation, cortisol metabolism, protein glycosylation, and mitochondrial biogenesis in placental villus samples from healthy mothers from the Healthy Start cohort. Using MANOVA, we tested the association between placental proteins and offspring adiposity (fat mass percentage) at birth (n = 109) and infancy (4–6 months, n = 104), and adiposity, skinfold thickness, triglycerides, and insulin in children (4–6 years, n = 66). Placental IGF-1 receptor protein was positively associated with serum triglycerides in children. GSK3β phosphorylation at serine 9, a readout of insulin and growth factor signaling, and the ratio of phosphorylated to total JNK2 were both positively associated with midthigh skinfold thickness in children. Moreover, peroxisome proliferator–activated receptor γ coactivator (PGC)-1α abundance was positively associated with insulin in children. In conclusion, placental insulin/IGF-1 signaling, PGC-1α, and inflammation pathways were positively associated with metabolic outcomes in 4- to 6-year-old children, identifying a novel link between placental function and long-term metabolic outcomes.
Introduction
A range of noncommunicable diseases, including obesity and type 2 diabetes, have intrauterine origins (1–3). The intrauterine environment is largely determined by the function of the placenta, the maternal-fetal interface performing an array of functions critical for normal fetal growth and development, including nutrient transport and hormone synthesis. Emerging evidence suggests that placental structure and function impact life-long health (4–7). We recently demonstrated that the activity of placental mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) is associated with cardiometabolic risk factors at 4–6 years (8). Thus, a better understanding of the mechanisms linking placental function to childhood and adult metabolic disease risk may offer innovative avenues to preventing them in future generations.
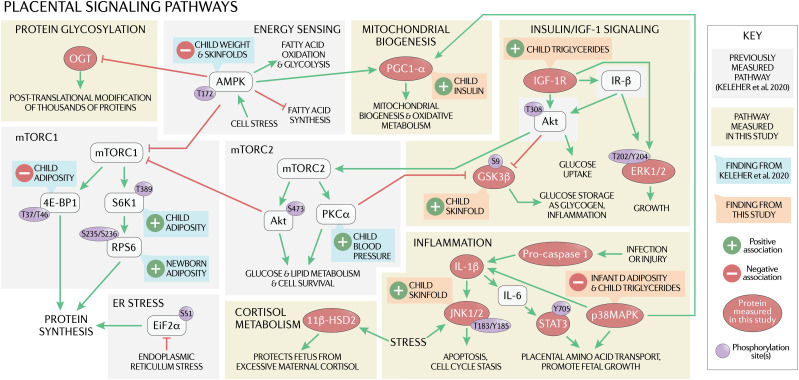
Here, we tested the hypothesis that altered abundance and phosphorylation of key proteins involved in placental insulin/IGF-1 and inflammation signaling, cortisol metabolism, protein glycosylation, and mitochondrial biogenesis (Fig. 1) are associated with infant and child markers of cardiometabolic health. We obtained placentas from 109 mother/infant pairs from the larger Environmental Influences on Child Health Outcomes (ECHO) Healthy Start prebirth cohort and determined protein levels, testing their association with metabolic outcomes measured in the children at birth, infancy (4–6 months), and childhood (4–6 years).
Figure 1.
The placenta may play a role in the intrauterine origin of adult metabolic disease through several mechanisms, such as by determining the flux of oxygen, nutrients, and methyl donors to the fetus, thereby regulating fetal growth and development. The activity of placental insulin/IGF-1 signaling is positively correlated with fetal growth and regulates multiple downstream signaling pathways, including mTOR and critical cellular processes such as mitochondrial biogenesis. Placental inflammation, which is activated in maternal obesity, may also impact short- and long-term fetal outcomes, as increased p38MAPK and STAT3 signaling promote placental amino acid transport, which is associated with increased fetal growth. Placental 11β-HSD2 protects the fetus from excessive maternal cortisol, which causes fetal growth restriction and predisposes the offspring to metabolic disease later in life. OGT, a broad marker of nutrient status and downstream target of AMPK, is elevated in human skeletal muscle in type 2 diabetes, and placental OGT has been linked to poor neurodevelopment in mice. We previously demonstrated that placental proteins involved in the mTOR and energy-sensing pathways (shown in gray) are associated with child outcomes at 4–6 years of age (shown in green). Here, we investigated additional pathways (shown in yellow) and found that child outcomes (shown in orange) are also linked to placental proteins involved in inflammation, mitochondrial biogenesis, and insulin signaling. ER, endoplasmic reticulum; IL, interleukin.
Research Design and Methods
Ethics Statement
We used placental samples, body composition data, and serum biomarker data collected by the Healthy Start Study (ClinicalTrials.gov, NCT02273297). This study was approved by the Colorado Multiple Institutional Review Board at the University of Colorado Hospital (Aurora, CO). All participants gave written, informed consent at enrollment.
Participant Measures and Sample Collection
Pregnant Women
The Healthy Start longitudinal prebirth cohort study comprises 1,410 women aged ≥16 years who enrolled in the study between 2010 and 2014 at ≤23 weeks’ gestation. We recruited women from the University of Colorado Hospital’s obstetrics clinics and excluded participants if they currently had a multiple pregnancy or had a prior history of premature birth, diabetes, or serious psychiatric illness. Data collection included maternal height, weight, demographics, and a fasting blood sample between 24 and 32 weeks’ gestation (median 27 weeks), and is reported in detail elsewhere (9,10).
Placental Samples
In a convenience subsample of the Healthy Start cohort, as part of the ancillary Healthy Start BabyBUMP (biology of intrauterine metabolic programming) Project, we collected trophoblast villi samples from placentas after delivery (n = 111), snap froze them in liquid nitrogen, and stored them at −80°C. We excluded samples from two subjects because they were from preterm births (<37 weeks), resulting in a sample size of 109 for analysis.
Placental Signaling
We homogenized ∼20 mg frozen placental villus tissue in 75 μL ice-cold buffer D (250 mmol/L sucrose, 10 mmol/L HEPES, pH 7.4) with a 1:100 dilution of protease and phosphatase inhibitors. Subsequently, we used Wes (ProteinSimple, Santa Clara, CA) to perform Simple Western assays to measure the phosphorylated and total abundance of key proteins (Table 1). We ran the Wes plates according to the manufacturer’s instructions, with minor modification (200 V, 55 m separation time) using a 0.1 mg/mL total protein concentration (Supplementary Table 1 and Supplementary Figs. 1 and 2). To control for batch variation, we included an equalizer sample on each plate that had clean median values for each protein. We also multiplexed a loading control (vinculin or β-actin) in each capillary to normalize the protein levels to.
Table 1.
Participant characteristics
| Characteristics | |
|---|---|
| Maternal characteristics | n = 109 |
| Prepregnancy BMI (kg/m2) | 25.0 (23.8–26.0) |
| Gestational weight gain (kg) | 13.5 (12.2–15.1) |
| Cesarean delivery, n (%) | 23 (21.1) |
| Maternal insulin at 24–32 weeks’ gestation (μIU/mL) | 12.9 (11.6–14.2) |
| Cord blood insulin (μIU/mL) | 7.27 (6.20–8.34) |
| Cord blood glucose (mg/dL) | 80.6 (77.3–84.0) |
| Newborn characteristics | n = 109 |
| Gestational age at delivery (weeks) | 39.5 (39.3–39.8) |
| Sex, n | |
| Female | 58 |
| Male | 51 |
| Adiposity (FM %) | 9.0 (8.4–9.9) |
| Body weight (kg) | 3.1 (3.0–3.2) |
| BMI z-score | −0.49 (−2.39 to 1.42) |
| Infant characteristics (4–6 months) | n = 104 |
| Age (months) | 4.50 (4.36–4.63) |
| Adiposity (%) | 24.38 (23.4–25.6) |
| Body weight (kg) | 6.6 (6.5–6.8) |
| BMI z-score | −0.35 (−2.32 to 1.61) |
| Child characteristics (4–6 years) | n = 66 |
| Age (years) | 4.62 (4.56–4.68) |
| Adiposity (%) | 18.2 (16.7–19.8) |
| Body weight (kg) | 17.2 (16.6–17.8) |
| BMI z-score | −0.06 (−1.72 to 1.61) |
| Midthigh skinfold (mm) | 14.4 (13.3–15.5) |
| Triceps skinfold (mm) | 10.3 (9.5–11.1) |
| Subscapular skinfold (mm) | 5.1 (4.7–5.5) |
| Triglycerides (mg/dL) | 59.8 (51.4–68.2) |
| Insulin (μIU/mL) | 5.5 (4.8–6.2) |
| Protein pathway | Protein measured |
| Insulin/IGF-1 signaling | IGF-1r |
| GSK3βSer9 | |
| GSK3βSer9/GSK3 | |
| ERK(1/2)Thr202/Tyr204 | |
| ERK(1/2)Thr202/Tyr204-to-ERK(1/2) | |
| Inflammation | Procaspase 1 |
| STAT3Tyr705 | |
| STAT3Tyr705-to-STAT3 | |
| JNK1Thr183/Tyr185 | |
| JNK1Thr183/Tyr185-to-JNK1 | |
| JNK2Thr183/Tyr185 | |
| JNK2Thr183/Tyr185-to-JNK2 | |
| IL-1β | |
| p38MAPK | |
| Cortisol metabolism | 11β-HSD2 |
| Protein glycosylation | OGT |
| Mitochondrial biogenesis | PGC-1α |
Data are presented as mean with 95% CI in parentheses, unless otherwise stated. 11β-HSD2, 11β-hydroxysteroid dehydrogenase type 2; ERK, extracellular signal-regulated kinase; GSK3β, glycogen synthase kinase-3β; IGF-1r, IGF-1 receptor; IL-1β, interleukin-1β; JNK, c-Jun N-terminal kinase; OGT, O-linked N-acetylglucosamine transferase.
Offspring Outcomes
At birth, we obtained birth weight and length from medical records (n = 105) and saved a cord blood sample to measure insulin, glucose, free fatty acids, cholesterol, and triglycerides. We measured body composition (fat mass [FM] and fat-free mass [FFM]) with whole-body air displacement plethysmography (PEA POD; COSMED) and calculated adiposity as FM/(FM + FFM) ∗ 100. We measured body composition again in infancy, between 4 and 6 months (PEA POD, n = 101). When the children reached 4–6 years (n = 66, median age 4.54 years), we assessed body composition (BOD POD; COSMED, n = 62), as previously described (10), and skinfold thickness twice at the triceps, subscapular, and midthigh points using skinfold calipers, with a third measurement if the first two were off by >1 cm. We collected a fasted venous blood sample from consenting children to measure circulating levels of insulin, triglycerides, cholesterol, adiponectin, and glucose.
Statistical Analysis
We analyzed the protein data as the phosphorylated form or as the ratio of phosphorylated to total. We analyzed only total protein in cases where the phosphorylated form was not measured or not relevant. We performed all statistical analyses in R (version 4.0.2). We tested the normality of the data (Shapiro-Wilk test) and used Box-Cox transformations to normalize variables that were not normal. In the one case where the transformed variable still did not pass the Shapiro-Wilk test, we used an inverse normal transformation. We performed the statistical tests on the transformed values (Supplementary Table 2) but presented the untransformed values in the graphs for interpretation. We identified outliers with the Grubbs test and removed one outlier for one subject (for serum insulin).
We performed MANOVA to test for a relationship between the protein pathways and offspring body composition and cardiometabolic traits (Supplementary Table 3). When MANOVA showed a significant overall association, we performed multivariate multiple regression (MMR) to evaluate each dependent variable. Sex and age affected the offspring cardiometabolic outcomes, so they were included as covariates. Where indicated, linear regression and correlation were used post hoc to further explore complex relationships. All associations remained unchanged after reanalyzing data using BMI z-scores instead of the actual BMI (data not shown).
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Subject Characteristics
Our analysis included 109 mother/infant pairs at birth, 104 infants at 4–6 months, and 66 children at 4–6 years (Table 1). Mothers had prepregnancy BMIs ranging from 19.9 to 45.8 kg/m2. Infants had an even sex distribution (53% female), their gestational age at delivery ranged from 37 to 42 weeks, and two infants were born with macrosomia (>4 kg).
No Effect of Maternal Prepregnancy BMI, 11β-HSD2, or OGT
Maternal BMI did not significantly affect any of the signaling pathways (Supplementary Table 4). MANOVA did not detect any association between placental proteins involved in cortisol metabolism (Supplementary Table 5) or protein glycosylation (Supplementary Table 6) on child outcomes.
Proteins in the Insulin/IGF-1 Signaling Pathway Are Associated With Child Outcomes at Birth and 4–6 Years
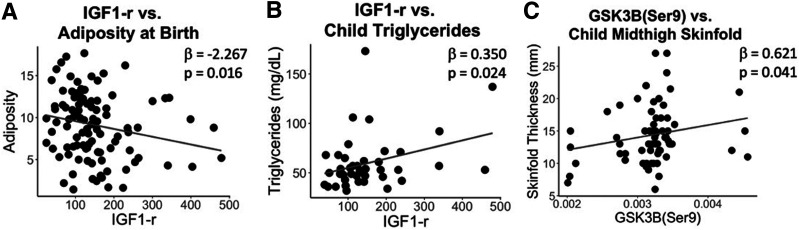
MANOVA of the insulin/IGF-1 signaling pathway proteins (Supplementary Table 7) revealed a significant association between placental IGF-1 receptor (IGF-1r) abundance and body composition at birth (P = 0.045), with MMR showing a negative association with adiposity (β = −2.267, P = 0.016) (Fig. 2A). By childhood, that association was no longer significant; however, placental IGF-1r expression was positively associated with child triglycerides (β = 0.350, P = 0.024) (Fig. 2B). At that same age, placental GSK3βSer9 was associated with skinfolds (P = 0.009), with a positive association with midthigh skinfold thickness (β = 0.621 P = 0.041) (Fig. 2C). Previous research has shown a positive association between maternal insulin levels and placental GSK3βSer9 (11), prompting our post hoc linear regression showing a nonsignificant positive trend between maternal insulin and placental GSK3βSer9 (β = 0.162, P = 0.112) and a significant positive association between cord blood insulin and GSK3βSer9 (β = 0.245, P = 0.036). ERK(1/2)Thr202/Tyr204-to-ERK(1/2) had a significant relationship with skinfolds overall; however, it did not have a clear relationship with any individual skinfold measurements.
Figure 2.
Placental insulin signaling proteins and child outcomes. MANOVA and MMR were performed for placental proteins in the growth factor/insulin signaling pathway vs. child outcomes, adjusting for child sex and age. A–C: Scatter plots are shown for significant MMR results that first passed significance with MANOVA.
Placental JNK2, p38 Mitogen-Activated Protein Kinase, and PGC-1α Are Associated With Child Outcomes at 4–6 Years
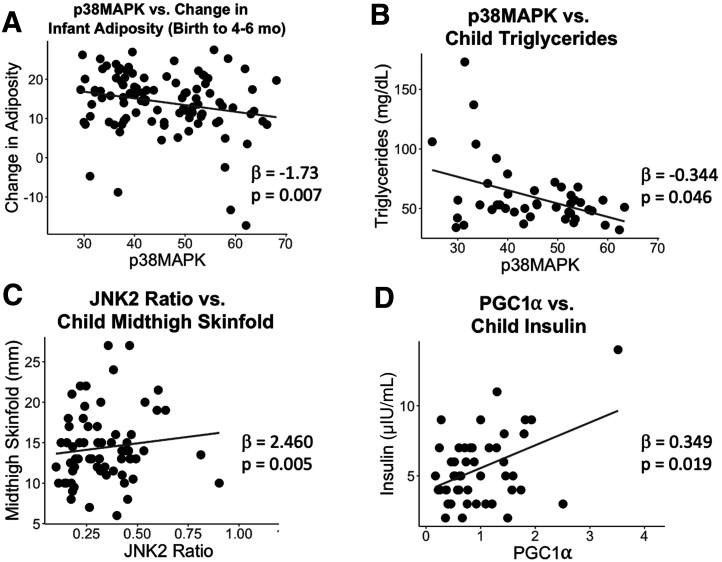
MANOVA on the inflammatory pathway (Supplementary Table 8) revealed a significant relationship between placental p38 mitogen-activated protein kinase (MAPK) abundance and growth trajectory from birth to 4–6 months (P = 0.008), driven by a negative association with change in adiposity from birth to 4–6 months (β = −1.73, P = 0.007) (Fig. 3A). Similarly, p38MAPK had a negative association with child triglycerides at 4–6 years of age (β = −0.344, P = 0.046) (Fig. 3B). JNK2Thr183/Tyr185-to-JNK2 was also associated with child skinfolds (P = 0.037), with a positive association with midthigh skinfold thickness (β = 2.460, P = 0.005) (Fig. 3C). At 4–6 months, there was a significant effect of both JNK2Thr183/Tyr185-to-JNK2 (P = 0.006) and p38MAPK (P = 0.0124) on infant body composition. Although the univariate tests were not significant for either protein, there was a positive correlation between JNK2Thr183/Tyr185-to-JNK2 and 4–6 month adiposity (r = 0.253, P = 0.0115) (Supplementary Fig. 3A). Similarly, MANOVA revealed a significant effect of STAT3Tyr705 on child body composition at 4–6 years (P = 0.047), without significant associations with adiposity or body weight, although there was a positive correlation between STAT3Tyr705 and child adiposity (r = 0.324, P = 0.010) (Supplementary Fig. 3B). The mitochondrial biogenesis pathway analysis (Supplementary Table 9) revealed a significant positive association between placental peroxisome proliferator–activated receptor γ coactivator (PGC)-1α and child insulin at 4–6 years (β = 0.349, P = 0.019) (Fig. 3D). In a post hoc analysis, PGC-1α was positively correlated with IGF-1r (r = 0.39, P < 0.001) and OGT (r = 0.27, P = 0.005).
Figure 3.
Placental inflammation and mitochondrial biogenesis proteins and child outcomes. MANOVA and MMR were performed for placental proteins in the growth factor/insulin signaling pathway vs. child outcomes, adjusting for child sex and age. A–D: Scatter plots are shown for significant MMR results that first passed significance with MANOVA.
Discussion
A better understanding of the mechanisms linking placental function to childhood and adult disease will allow us to develop innovative avenues to prevent obesity and diabetes in future generations. Here, we report that the activity of placental pathways involving inflammation and growth factor/insulin signaling were positively associated with midthigh skinfold thickness and serum triglycerides in healthy 4- to 6-year-old children. Additionally, PGC-1α, which regulates mitochondrial biogenesis, was positively associated with child insulin. Collectively, these findings suggest that activation of placental signaling pathways promoting nutrient transport, protein synthesis, and oxidative phosphorylation are related to growth and development of offspring born to healthy pregnant mothers.
Placental IGF-1r expression was positively correlated with childhood serum triglycerides. Increased GSK3β phosphorylation—indicating activation of placental insulin signaling—was linked to child skinfold thickness. These findings are in general agreement with our previous observations that the activity of placental mTORC1, downstream of insulin signaling, is positively associated with child adiposity (8). The activation of placental insulin/IGF-1 and mTOR signaling may be mechanistically involved in the well-established link between maternal obesity/gestational diabetes mellitus (GDM) and metabolic disease in the offspring (12,13). Given that these signaling pathways promote placental nutrient transport, it is possible that increased fetal nutrient delivery contributes to this association by increasing fat deposition in utero and/or altering fetal nutrient sensing systems.
In addition to being linked to infant adiposity, JNK2 was positively associated with midthigh skinfold thickness in childhood. These findings are in general agreement with studies reporting activation of placental JNK2 in maternal obesity (14) and suggest that placental inflammation in maternal obesity may predispose the infant to future obesity. The inverse correlation between placental p38MAPK and child triglycerides is contradictory. However, we speculate that this may reflect a positive correlation between the ratio of phosphorylated to total p38MAPK (which we were unable to measure) and adiposity in childhood.
Placental PGC-1α has been reported to be decreased in GDM (15,16), possibly reflecting a homeostatic downregulation of mitochondrial respiration by glucose abundance. In our cohort consisting largely of healthy pregnant women and their children, placental PGC-1α was positively associated with child serum insulin. At first glance, this may be counterintuitive as PGC-1α is phosphorylated by AMPK, which we previously reported was negatively associated with child weight and skinfold thickness at 4–6 years (8). However, we found that PGC-1α was not correlated with the AMPK values measured in our previous study in the same individuals. Instead, PGC-1α was positively correlated with IGF-1r, a known stimulator of mitochondrial biogenesis. PGC-1α was also positively correlated with OGT. These two proteins form a complex that mediates inappropriate gluconeogenesis in the diabetic liver via PGC-1α recruiting OGT to activate FoxO via GlcNAcylation (17). Thus, greater IGF-1/insulin signaling in the placenta may underlay the positive association between placental PGC-1α and child insulin. Increased placental mitochondrial respiration in response to enhanced PGC-1α signaling may provide additional ATP for placental protein synthesis and active nutrient transport promoting fetal growth and fat deposition, predisposing the infant for future metabolic disease.
The strengths of the study include having a cohort of women across the BMI spectrum, a large sample size, and follow-up with the children from birth to 4–6 years of age. Because recruited pregnant women were largely healthy by design, limitations of the study include the lack of women with pregnancy complications, such as GDM and preterm birth, known to be associated with programming of offspring metabolic disease.
Our findings suggest that activation of signaling pathways promoting placental nutrient transport, protein synthesis, and oxidative phosphorylation could be involved in metabolic pathways that contribute to metabolic risk in young children. Our data provide additional evidence that changes in placental function are linked to long-term health in children and may serve as a foundation for mechanistic studies in animal models and the development of placental biomarkers and novel intervention strategies to prevent future cardiometabolic disease.
Article Information
Acknowledgments. The authors thank M. Martinez (the Healthy Start Study project coordinator, Colorado School of Public Health, University of Colorado), the Healthy Start team, and the Healthy Start participants for their hard work and dedication to this study. The authors thank KIMEN Design4Research for the graphic design of Fig. 1.
Funding. This work was supported by Colorado Clinical and Translational Sciences Institute UG3OD023248-03 (to D.D.). The Healthy Start BabyBUMP Project is supported by grants from the American Heart Association (predoctoral fellowship 14PRE18230008) and by the parent Healthy Start Study (to D.D.). The Healthy Start Study was supported by the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK076648 to D.D.) and the Colorado Clinical and Translational Sciences Institute via National Institutes of Health, National Center for Advancing Translational Sciences (UL1 TR001082) for maternal visits and collection of birth measures.
The funders had no influence on the results of the study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.R.K., K.E., H.A.S., and K.J.K. contributed to the statistical analysis. M.R.K., I.V.Y., D.D., J.E.F., K.E.B., and T.J. contributed to the interpretation of the results. M.R.K., K.E.B., and T.J. drafted the manuscript. K.E., K.E.B., D.D., and T.J. contributed to data collection. T.J. contributed to the conception of the manuscript. All authors approved the final version of the manuscript. T.J. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13491417.
References
- 1.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab 2004;15:183–187 [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ The developmental origins of insulin resistance. Horm Res 2005;64(Suppl. 3):2–7 [DOI] [PubMed] [Google Scholar]
- 4.Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev 2016;96:1509–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJ, Gelow J, Thornburg K, Osmond C, Kajantie E, Eriksson JG. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur J Heart Fail 2010;12:819–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Larsen G, Osmond C, Thornburg KL, Kajantie E, Eriksson JG. The placental origins of sudden cardiac death. Int J Epidemiol 2012;41:1394–1399 [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Guilmette J, Luo Z-C, et al. Placental 11β-HSD2 and cardiometabolic health indicators in infancy. Diabetes Care 2019;42:964–971 [DOI] [PubMed] [Google Scholar]
- 8.Keleher MR, Erickson K, Kechris K, et al. Associations between the activity of placental nutrient-sensing pathways and neonatal and postnatal metabolic health: the ECHO Healthy Start cohort. Int J Obes (Lond) 2020;44:2203–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starling AP, Brinton JT, Glueck DH, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start Study. Am J Clin Nutr 2015;101:302–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sauder KA, Stamatoiu AV, Leshchinskaya E, Ringham BM, Glueck DH, Dabelea D. Cord blood vitamin D levels and early childhood blood pressure: the Healthy Start Study. J Am Heart Assoc 2019;8:e011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassance L, Miedl H, Absenger M, et al. Hyperinsulinemia stimulates angiogenesis of human fetoplacental endothelial cells: a possible role of insulin in placental hypervascularization in diabetes mellitus. J Clin Endocrinol Metab 2013;98:E1438–E1447 [DOI] [PubMed] [Google Scholar]
- 12.Lindell N, Carlsson A, Josefsson A, Samuelsson U. Maternal obesity as a risk factor for early childhood type 1 diabetes: a nationwide, prospective, population-based case-control study. Diabetologia 2018;61:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santangeli L, Sattar N, Huda SS. Impact of maternal obesity on perinatal and childhood outcomes. Best Pract Res Clin Obstet Gynaecol 2015;29:438–448 [DOI] [PubMed] [Google Scholar]
- 14.Saben J, Zhong Y, Gomez-Acevedo H, et al. Early growth response protein-1 mediates lipotoxicity-associated placental inflammation: role in maternal obesity. Am J Physiol Endocrinol Metab 2013;305:E1–E14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Fan H, Zhou L, Wu Y, Lu H, Luo J. Altered expression of PGC-1α and PDX1 and their methylation status are associated with fetal glucose metabolism in gestational diabetes mellitus. Biochem Biophys Res Commun 2018;501:300–306 [DOI] [PubMed] [Google Scholar]
- 16.Jiang S, Teague AM, Tryggestad JB, Aston CE, Lyons T, Chernausek SD. Effects of maternal diabetes and fetal sex on human placenta mitochondrial biogenesis. Placenta 2017;57:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Housley MP, Udeshi ND, Rodgers JT, et al. A PGC-1α-O-GlcNAc transferase complex regulates FoxO transcription factor activity in response to glucose. J Biol Chem 2009;284:5148–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]