Abstract
In type 1 diabetes (T1D), a lifelong autoimmune disease, T cells infiltrate the islets and the exocrine pancreas in high numbers. CD8+ T cells are the main cell type found in the insulitic lesion, and CD8+ T cells reactive against β-cell antigens have been detected in peripheral blood and in the pancreas of patients with short- or long-term disease. In the Diabetes Virus Detection (DiViD) study, researchers collected pancreatic tissue, by pancreatic tail resection, from living patients with recent-onset T1D. These tissues have been extensively studied by the scientific community, but the autoreactive nature of the T-cell infiltrate has remained unexplored. Our objective was to determine the number and localization of these cells in pancreas samples obtained through the DiViD study. Here, we demonstrate the presence of high frequencies of CD8+ T cells reactive against a highly relevant epitope derived from the preproinsulin signal peptide in pancreatic tissue samples from these donors. We also show the heterogeneity of islet distribution and CD8+ T-cell infiltration. Our findings contribute to the current limited existing knowledge of T-cell reactivity in the pancreas of donors with recent-onset T1D and indicate that antigen-specific therapies directed toward preproinsulin could have high clinical impact.
Introduction
Researchers involved in the Diabetes Virus Detection (DiViD) study collected samples of pancreatic tissue by pancreatic tail resection from six living, adult individuals newly diagnosed with type 1 diabetes (T1D) (1). These pancreatic biopsy specimens were laparoscopically obtained 3–9 weeks after diagnosis of T1D. The DiViD study was the first of its kind, and pancreatic tissue from all the individuals has been widely shared with the scientific community. Numerous studies of these extremely valuable samples have provided seminal contributions to our understanding of T1D. Notably, a detailed analysis of these samples showed the presence of a high number of remaining insulin-containing islets often in proximity to insulin-deficient islets (2). These islet pools were highly heterogenous; some seemed to be unaffected by the disease process, whereas others presented evident peri-islet and intraislet insulitis (2). This observation confirmed, on one hand, the presence of apparently intact β-cell mass left shortly after diagnosis and, on the other hand, the heterogenous distribution of the insulitic lesion in humans.
CD8+ T cells are the most abundant cell type in islets with insulitis and are associated with β-cell destruction (3). In the human diabetic pancreas, CD8+ T cells infiltrate the exocrine tissue as well as the islets (4), where they are most frequently found in the periphery of, rather than within, the islet parenchyma. Complete understanding of their phenotype, specificity, and function is still lacking. CD8+ T cells reactive against β-cell antigens can be detected in the blood of patients with T1D. However, similar cell frequencies of autoreactive T cells have been reported in people without diabetes (5), further underlining the importance of identifying these cells in the target tissue, the pancreas. Previous studies have demonstrated the presence of cells reactive against β-cell antigens in infiltrates from isolated islets (6) and in the pancreas from organ donors with short and long duration of T1D (7,8). These cells seem to be antigen experienced and sequestered in the pancreas in high numbers in patients with T1D (5,8).
Preproinsulin (PPI) is considered a prominent source of epitopes for multiple HLA class I alleles and a major autoantigen in T1D (9). β-cells undergo substantial endoplasmic reticulum stress in response to glucose stimulation (10), increasing PPI translation 50-fold and reaching a production rate of 1 million PPI molecules/min (11). This increase in production upregulates PPI presentation, increasing the likelihood of immune recognition in the context of autoimmunity (12). Consequently, cloned PPI-specific CD8+ T cells can kill human β-cells in vitro, especially if they are exposed to high glucose concentrations (12). We and others have described the presence of β-cell reactive CD8+ T cells in the pancreas of individuals with T1D (7,8,13). However, until recently, to our knowledge, the number and distribution of these cells had not been studied. Therefore, our objective was to determine the number and localization of these cells in pancreas samples obtained through the DiViD study. Here, we demonstrate the presence of high frequencies of CD8+ T cells reactive against a highly relevant epitope derived from the PPI signal peptide in the pancreas of living individuals with recent-onset T1D.
Research Design and Methods
Patient Characteristics
Four individuals (DiViD patients 1, 2, 4, and 5; age range, 24–31 years) were included in this study. Patients were selected on the basis of the presence of the HLA-A*02:01 haplotype. Detailed information is listed in Table 1. Pancreatic biopsy samples were collected 3–5 weeks after diagnosis of T1D as part of the DiViD study (1). HbA1c at biopsy ranged from 6.7% to 10.3%. All donors were autoantibody-positive as shown in Table 1. Previous basic histologic characterization showed the presence of remaining insulin-containing islets, some of them with insulitis. The DiViD study was approved by the Norwegian Government’s Regional Ethics Committee (Oslo, Norway) and written informed consent was obtained from participants. All experimental procedures were approved by the La Jolla Institute for Immunology Institutional Review Board (protocol no. DI-054–0218).
Table 1.
Patient characteristics
| DiViD-1 | DiViD-2 | DiViD-4 | DiViD-5 | |
|---|---|---|---|---|
| Age, years | 25 | 24 | 31 | 24 |
| BMI, kg/m2 | 21.0 | 20.9 | 25.6 | 28.6 |
| HbA1c at biopsy, % (mmol/mol) | 6.7 (49.7) | 10.3 (89.1) | 7.4 (57.4) | 7.4 (57.4) |
| Diabetes duration, weeks | 4 | 3 | 5 | 5 |
| T1D-associated autoantibodies | IA-2A+ZnT8A+GADA+mIAA+ | IA-2A+ZnT8A+GADA+ | IA-2A+GADA+mIAA+ | IA-2A+GADA+mIAA+ |
| HLA risk for T1D | A*01:01, 02:01 B*08:01, 40:01 DRB1*01:03, 03:01 DQB1*05:01, 02:01 | A*02:01, 11:01 B*18:01, 40:01 DRB1* 04:01, 13:01 DQB1*03:02, 06:03 | A*02:01 B*15:01, 35:01 DRB1*04:01 DQB1*03:02 | A*02:01, 03:01, B*18:01, 40:01 DRB1*03:01, 04:01 DQB1*02:01, 03:02 |
GADA, GAD antibodies; IA-2A, insulinoma-associated protein antibodies; mIAA, insulin antibodies; ZnT8A, islet-specific zinc transporter 8 antibodies.
Tissue Processing and Immunofluorescence Staining
Pancreatic tissues were embedded in optimal cutting temperature compound, and 5 μm–thick sections were processed and labeled using in situ tetramer and immunofluorescence staining. Tetramers were obtained from the Fred Hutchinson Cancer Research Center, Immune Monitoring Core (Seattle, WA). Quality control of the tetramers was done using peripheral blood mononuclear cells spiked with cytomegalovirus (CMV)495–503 or PPI15–24 T-cell clones and have been shown elsewhere (13). Tyramide Signal Amplification was used for tetramer visualization (Thermo Fisher Scientific, Waltham, MA). Briefly, 1 μg of allophycocyanin (APC)-labeled tetramer was diluted in 500 μL of PBS and then spun at 15,800g to avoid aggregation. After overnight incubation at 4°C and gentle washing, tissue sections were fixed with 1% paraformaldehyde for 15 min at room temperature and incubated with 3% H2O2 for 15 min. Sections were then washed and blocked with 10% goat serum for 30 min at room temperature.
After washing, sections were incubated with a purified mouse–anti-APC antibody (1/50, clone APC003; BioLegend) and rabbit–anti-glucagon (1/300; ab10988; Abcam) for 1 h at room temperature. Then, sections were washed and incubated with poly-goat–anti-mouse–horseradish peroxidase (TSA SuperBoost Kit; Thermo Fisher Scientific) and goat–anti-rabbit Alexa Fluor 488 (1/1,000; catalog no. A11070; Thermo Fisher Scientific). After a blocking step using 2% mouse serum, sections were washed and incubated with mouse–anti-human CD8 conjugated in house to Alexa Fluor 555 (1/100; catalog no. 555631; BD). Sections were then washed and incubated with tyramide–Alexa Fluor 647 following the manufacturer’s instructions (TSA SuperBoost Kit; Thermo Fisher Scientific). Finally, sections were counterstained with Hoechst (1/5,000; Life Technologies) and mounted with Prolong Gold antifade mounting medium (Invitrogen, Carlsbad, CA).
In total, five consecutive sections were used to detect antigen-specific cells against PPI (PPI15–24), CMV (CMV495–503), Epstein-Barr virus (EBV; EBV426–434), influenza A virus (FLU; FLU matrix protein 58–66), and the enterovirus coxsackievirus B3 (EV; EV1,587–1,596). An additional section was included as a negative control and incubated without tetramer. Stained tissue sections were scanned using a Zeiss AxioScan.Z1 slide scanner and images were analyzed using Zen software (Zeiss, Jena, Germany).
Data and Resource Availability
The data sets generated during and/or analyzed during this study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
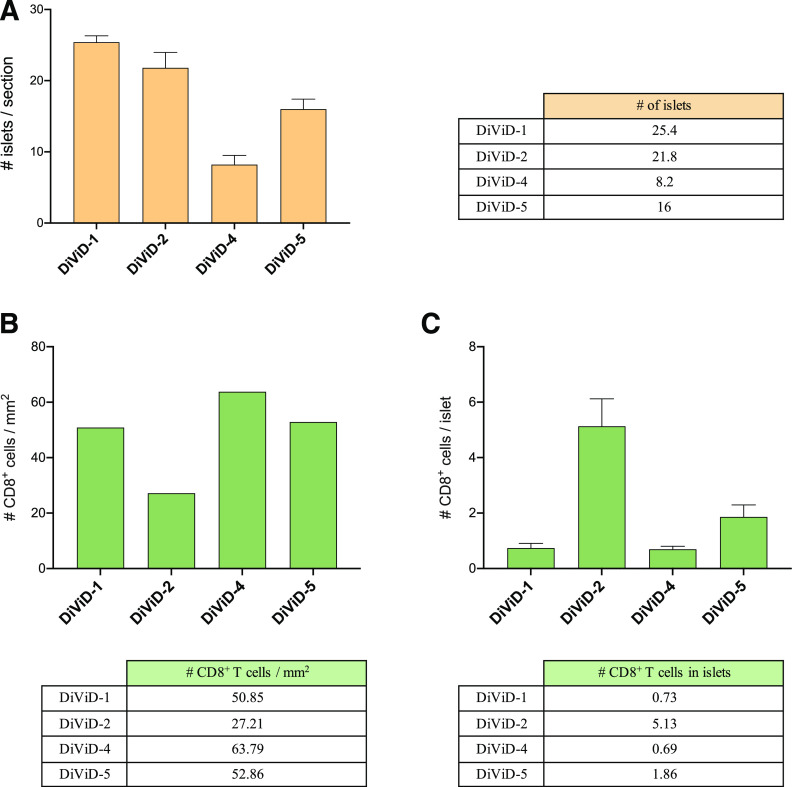
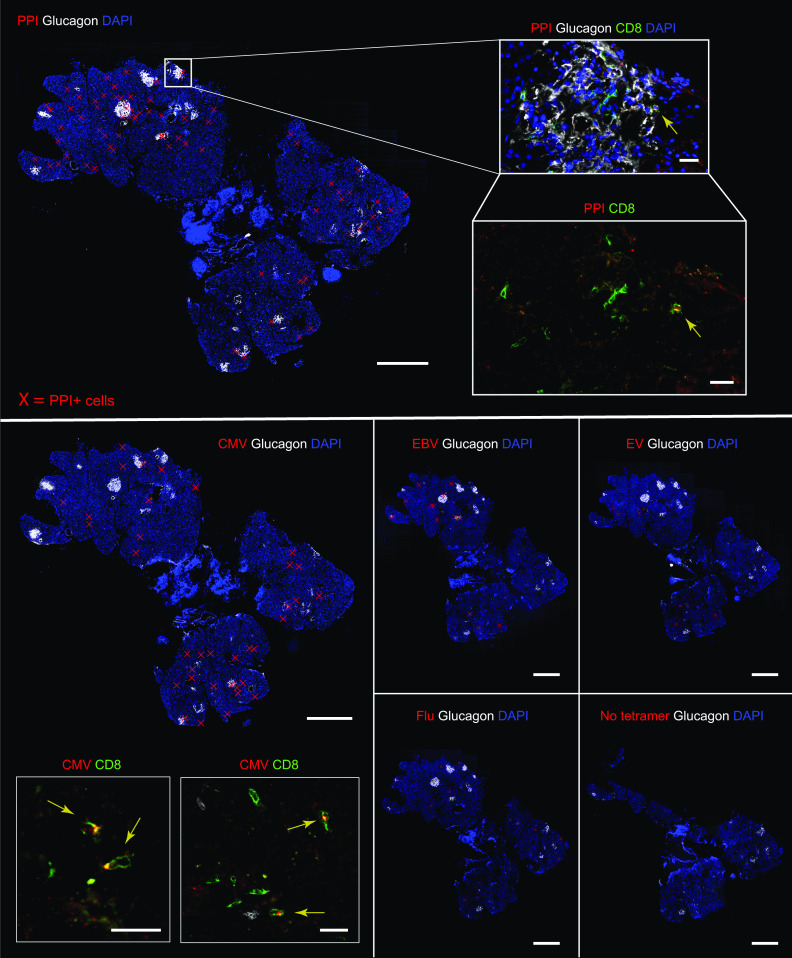
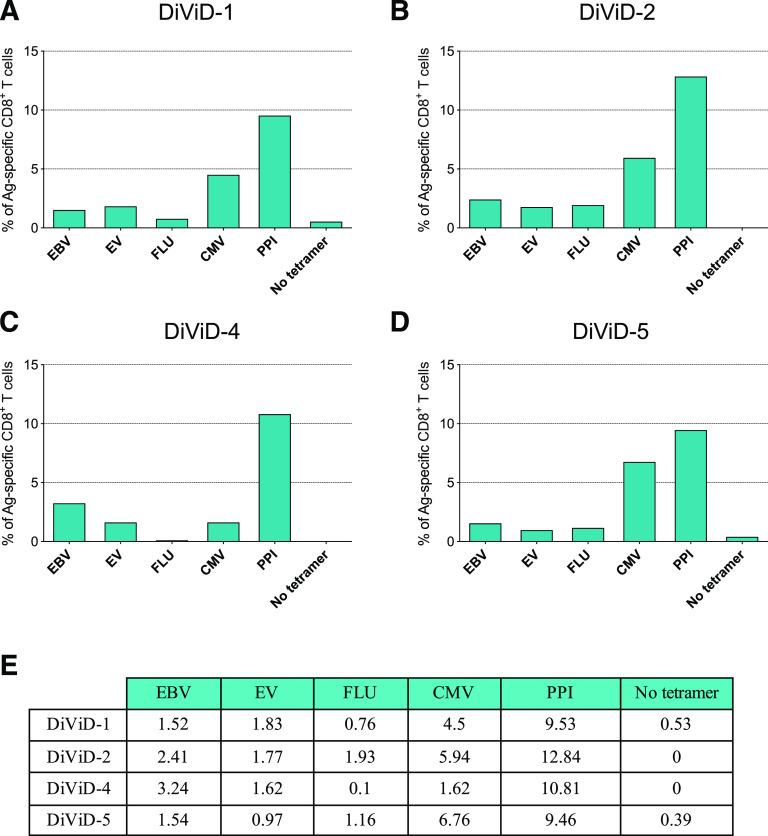
First, for each section, the tissue area was measured and the islets were identified and counted on the basis of glucagon staining (representative images are shown in Supplementary Fig. 2). The number of islets was highly variable between patients, and ranged from 8.2 to 25.4 islets per section (Fig. 1A). Then, the total number of CD8+ cells per section (expressed as CD8 T-cell density), and the number of CD8+ cells located in the islet periphery or inside the islets were calculated (Fig. 1 and Supplementary Fig. 1). CD8 T-cell density ranged from 27.2 to 63.8 cells/mm2 (Fig. 1B). The number of CD8+ cells per islet was also heterogenous. It varied between islets and between patients despite the small difference in disease duration (3–5 weeks) and ranged from 0.7 to 5.1 CD8+ cells/islet, reflecting the presence or absence of islets with insulitis (Fig. 1C). Then, cells positive for CD8 and tetramer signal were identified, counted, and the percentage of tetramer-positive CD8+ cells was calculated, as illustrated in Fig. 2 and Supplementary Fig. 2. First, the frequency of CD8+ T cells reactive against PPI (PPI15–24), a highly relevant antigen in T1D, was analyzed. The mean percentage of PPI+ CD8+ T cells was 10.4% (patient 1 in the DiViD study [DiViD-1] = 9.5% vs. DiViD-2 = 12.8% vs. DiViD-4 = 10.8% vs. DiViD-5 = 9.5%) (Fig. 3). Positive cells were observed mainly in the exocrine compartment, but they could also be found near or infiltrating the islets (Supplementary Figs. 1 and 2). To assess the relative abundance of PPI+ CD8+ T cells, their frequency was compared with that of relevant viral specificities. The mean percentage of CD8+ cells reactive against CMV (CMV495–503) was 5.1% (DiViD-1 = 4.5% vs. DiViD-2 = 5.9% vs. DiViD-4 = 1.6% vs. DiViD-5 = 6.7%), and was lower than the frequency of PPI+ CD8+ cells (Fig. 3). As with PPI+ cells, CMV+ cells were found predominantly in the exocrine tissue. Conversely, their presence around or inside the islets was rare (≤4 CMV+ CD8+ cells) (Supplementary Fig. 2). Last, the frequency of cells reactive against EBV (EBV426–434; mean percentage EBV+, 1.8), FLU (FLU58–66; mean percentage FLU+, 1.1), and the EV (EV1,587–1,596; mean percentage EV+, 1.6) was low in the islets and exocrine tissue for all the individuals compared with CMV- or PPI-specific CD8+ T cells (Fig. 3 and Supplementary Fig. 1).
Figure 1.
Heterogeneity of islet distribution and CD8+ T-cell infiltration in the DiViD cases. Bar graphs and tables show: the mean number of islets per section (# islets/section) (A); CD8 T-cell density in the whole tissue section expressed as number of CD8+ cells/mm2 (B); and mean number of CD8+ cells per islet (# CD8+ cells/islet) (C). Error bars represent the SD.
Figure 2.
Representative images from immunofluorescence in situ tetramer staining of pancreas sections from DiViD-2. Staining was performed for the indicated tetramer (positive cells are marked with red crosses in whole-slide images), glucagon (white staining), and DAPI (blue staining). Consecutive sections are shown to illustrate the staining and quantification of antigen-specific cells reactive against PPI, CMV, EBV, FLU, EV, and a negative control without tetramer (no tetramer). Scale bars, 1,000 µm in whole-slide images. Magnified images show staining for tetramer (red) and CD8 (green). Yellow arrows indicate tetramer-positive cells. Scale bars, 25 µm for PPI; 20 µm for CMV.
Figure 3.
Quantification of tetramer-positive cells in the entire tissue section. A–D: Bar graphs show the percentage of antigen (Ag)-specific CD8+ T cells for each tissue section and individual (DiViD-1, 2, 4, and 5). PPI, CMV, EBV, FLU, EV, and negative control without tetramer (no tetramer). E: The summary table shows the percentage of Ag-specific CD8+ cells for each antigen and patient.
Discussion
Previous in situ studies have shown the presence of PPI-specific cells in the pancreas of donors with T1D, but the frequency of these cells, as well as relative abundance compared with other known viral specificities, has not been reported to date. Here, we show the presence of a high frequency of PPI-reactive CD8+ cells in the pancreas of living individuals whose tissue samples were collected shortly after diagnosis. PPI15–24 has been identified as one of the major known epitopes in T1D on the basis of well-defined criteria (14): 1) it is naturally processed and presented by β-cells; 2) reactivity against this epitope has been validated in human pancreatic lymph nodes or islet-infiltrating T cells, and 3) a confirmed recognition of this epitope exists through isolated T clones and lines. Furthermore, PPI-specific CD8+ T cells exhibit cytotoxic activity when exposed to human islets (12,15). Among the studied specificities, PPI-specific CD8+ T cells could be detected more frequently in islets but, as shown in Fig. 1, the heterogeneity in the number of islets and in the number of CD8+ T cells per islet limited our ability to form strong conclusions regarding islet infiltration. Conversely, quantification of the total number of cells in the entire tissue section provided robust data. The frequency of PPI-specific cells was close to 10% for most of the four patients. Even though this percentage could seem low, it has been shown in transgenic mouse models and virus-induced diabetes that only 1–2% of antigen-specific cells are sufficient to induce diabetes (16,17), thus highlighting the efficiency of these cells in killing their target.
The significance of PPI-specific CD8 T cells in the pancreas in the context of autoimmunity highlights the importance of PPI as a major antigen in T1D. β-cells have an extraordinary capacity to increase PPI translation in response to glucose stimulation, increasing its potential exposure to the immune system under conditions of high insulin demand and cellular stress. As mentioned, PPI is naturally processed and presented by β-cells (5,14), and it is a major source of epitopes for HLA-A and HLA-B alleles (9). Recently, no differences in the frequency of β-cell–reactive CD8 T cells against multiple antigens were reported in the blood of individuals with and without T1D. The notable exception was the epitope studied here, PPI15–24, whose frequency was higher in individuals with T1D (8). In agreement with the data presented here, an increased presence of PPI15–24 CD8 T cells has been reported recently in the pancreas of individuals with T1D up to 8 years postdiagnosis (13). In addition, multiple studies have shown the capacity of PPI-specific CD8 T cells to kill β-cells in vitro (12,15), highlighting their potential implication in disease pathogenesis. Taken together, data reported by us and others point to a scenario in which, in the context of autoimmunity, increased β-cell stress in response to insulin demand could have a strong impact on epitope generation, antigen presentation, and immune activation, making β-cells an attractive target for PPI-specific cells that could be probing for antigen in the pancreas.
A strength, and simultaneously a limitation, of our study is the uniqueness of the samples, which were collected from living individuals by pancreatic-tail resection. This precluded us from including samples from nondiabetic donors, which would have been collected and preserved in very different conditions and have been already studied elsewhere elsewhere (13). Instead, our objective was to compare the frequency of PPI-specific cells to the frequency of well-known viral reactivities within individuals with diabetes. Our study was not designed to target all the epitopes spanning multiple antigens but rather to offer an overview of what could be the tip of the autoreactive iceberg. Therefore, it is possible the data presented here underestimate the number of reactive cells against a given antigen, whether viral or from β-cells. This could be the case for EV-specific cells, which were not found in the islets despite the reported presence of a low-grade EV infection in these individuals (18). It is important, therefore, to foster epitope discovery efforts to sample a wider antigenic repertoire and to extract conclusions regarding antigen recognition in T1D.
Our study increases the amount of data from the relatively limited number of pancreas samples in which antigen-specific T cells have been studied in situ in donors with recent-onset T1D. Given the scientific value of these tissues and the current clinical relevance of immune interventions targeting T cells (19) and antigen-specific immunotherapies like oral insulin (20), our findings, together with previous reports, could inform future clinical trials. This study contributes to the current existing knowledge about PPI and indicates that antigen-specific therapies directed toward PPI could have substantial clinical impact.
Article Information
Acknowledgments. The authors thank Zbigniew Mikulski of La Jolla Institute for Immunology for his help and support with image acquisition.
Funding. This work was supported by the National Institutes of Health grant R01 AI092453 to M.v.H. K.D.-J. is the principal investigator of the DiViD study and is funded by the South-Eastern Norway Regional Health Authority, the Novo Nordisk Foundation, and through the Persistent Virus Infection in Diabetes Network (PEVNET) Study Group funded by the European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 261441 PEVNET.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.R.-C. participated in project design, performed experiments, analyzed and interpreted data, and wrote the manuscript. N.A. performed experiments. L.K. and K.D.-J. provided samples from the DiViD study and participated in writing the manuscript. M.v.H. supervised the project, interpreted data, and participated in writing the manuscript. M.v.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
T.R.-C. is currently affiliated with the Institute of Diabetes Research, Helmholtz Diabetes Center at Helmholtz Zentrum München, Munich, Germany.
T.R.-C. and L.K. contributed equally to this study.
This article contains supplementary material online at https://doi.org/10.2337/figshare.13469904.
K.D.-J. and M.v.H. contributed equally as senior authors.
References
- 1.Krogvold L, Edwin B, Buanes T, et al. Pancreatic biopsy by minimal tail resection in live adult patients at the onset of type 1 diabetes: experiences from the DiViD study. Diabetologia 2014;57:841–843 [DOI] [PubMed] [Google Scholar]
- 2.Krogvold L, Wiberg A, Edwin B, et al. Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia 2016;59:492–501 [DOI] [PubMed] [Google Scholar]
- 3.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009;155:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Calvo T, Ekwall O, Amirian N, Zapardiel-Gonzalo J, von Herrath MG. Increased immune cell infiltration of the exocrine pancreas: a possible contribution to the pathogenesis of type 1 diabetes. Diabetes 2014;63:3880–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez-Duque S, Azoury ME, Colli ML, et al. Conventional and neo-antigenic peptides presented by β cells are targeted by circulating naïve CD8+ T cells in type 1 diabetic and healthy donors. Cell Metab 2018;28:946–960.e6 [DOI] [PubMed] [Google Scholar]
- 6.Babon JAB, DeNicola ME, Blodgett DM, et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat Med 2016;22:1482–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med 2012;209:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culina S, Lalanne AI, Afonso G, et al. Islet-reactive CD8 + T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci Immunol 2018;3:eaao4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronenberg-Versteeg D, Eichmann M, Russell MA, et al. Molecular pathways for immune recognition of preproinsulin signal peptide in type 1 diabetes. Diabetes 2018;67:687–696 [DOI] [PubMed] [Google Scholar]
- 10.Marré ML, James EA, Piganelli JD. β cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol 2015;3:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 2008;29:317–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skowera A, Ellis RJ, Varela-Calviño R, et al. CTLs are targeted to kill β cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope [published correction appears in J Clin Invest 2009;119:2844]. J Clin Invest 2008;118:3390–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bender C, Rodriguez-Calvo T, Amirian N, Coppieters KT, von Herrath MG. The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci Adv 2020;6:eabc5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James EA, Mallone R, Kent SC, DiLorenzo TP. T-cell epitopes and neo-epitopes in type 1 diabetes: a comprehensive update and reappraisal. Diabetes 2020;69:1311–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kronenberg D, Knight RR, Estorninho M, et al. Circulating preproinsulin signal peptide-specific CD8 T cells restricted by the susceptibility molecule HLA-A24 are expanded at onset of type 1 diabetes and kill β-cells. Diabetes 2012;61:1752–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldstone MBA, Edelmann KH, McGavern DB, Cruite JT, Welch MJ. Molecular anatomy and number of antigen specific CD8 T cells required to cause type 1 diabetes. PLoS Pathog 2012;8:e1003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevilla N, Homann D, von Herrath M, et al. Virus-induced diabetes in a transgenic model: role of cross-reacting viruses and quantitation of effector T cells needed to cause disease. J Virol 2000;74:3284–3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogvold L, Edwin B, Buanes T, et al. Detection of a low-grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015;64:1682–1687 [DOI] [PubMed] [Google Scholar]
- 19.Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019;381:603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziegler A-G, Achenbach P, Berner R, et al. Oral insulin therapy for primary prevention of T1D in infants with high genetic risk: the GPPAD-POInT (Global Platform for the Prevention of Autoimmune Diabetes Primary Oral Insulin Trial) study protocol. BMJ Open 2019;9:e028578. [DOI] [PMC free article] [PubMed] [Google Scholar]